Abstract
Tensin1 is the archetype of a family of focal adhesion proteins. Tensin1 has a phosphotyrosine binding domain that binds the cytoplasmic tail of β-integrin, a Src homology 2 domain that binds focal adhesion kinase, p130Cas, and the RhoGAP called deleted in liver cancer-1, a phosphatase and tensin homology domain that binds protein phosphatase-1α and other regions that bind F-actin. The association between tensin1 and these partners affects cell polarization, migration, and invasion. In this study we analyzed the phosphorylation of human S-tag-tensin1 expressed in HEK293 cells by mass spectrometry. Peptides covering >90% of the sequence initially revealed 50 phosphorylated serine/phosphorylated threonine (pSer/pThr) but no phosphorylated tyrosine (pTyr) sites. Addition of peroxyvanadate to cells to inhibit protein tyrosine phosphatases exposed 10 pTyr sites and addition of calyculin A to cells to inhibit protein phosphatases type 1 and 2A gave a total of 62 pSer/pThr sites. We also characterized two sites modified by O-linked N-acetylglucosamine. Tensin1 F302A, which does not bind protein phosphatase-1, showed > twofold enhanced phosphorylation of seven sites. The majority of pSer/pThr have adjacent proline (Pro) residues and we show endogenous p38 mitogen activated protein kinase (MAPK) associated with and phosphorylated tensin1 in an in vitro kinase assay. Recombinant p38α MAPK also phosphorylated S-tag-tensin1, resulting in decreased binding with deleted in liver cancer-1. Activation of p38 MAPK in cells by sorbitol-induced hyperosmotic stress increased phosphorylation of S-tag-tensin1, which reduced binding to deleted in liver cancer-1 and increased binding to endogenous pTyr proteins, including p130Cas and focal adhesion kinase. These data demonstrate that tensin1 is extensively phosphorylated on Ser/Thr residues in cells and phosphorylation by p38 MAPK regulates the specificity of the tensin1 Src homology 2 domain for binding to different proteins. Tensin1 provides a hub for connecting signaling pathways involving p38 MAP kinase, tyrosine kinases and RhoGTPases.
Tensin1 is a protein localized at focal adhesions that acts as a scaffold for signaling (1). The tensin1 phosphotyrosine binding (PTB)1 domain binds the cytoplasmic tail of β-integrin (2), presumed to be the basis for focal adhesion localization. Human tensin1 interacts with actin by capping the barbed ends and cross-linking actin filaments through two different actin binding regions (3). Actin binding regions were identified in chicken tensin1 at residues 1–263, 263–463, and 889–1143 (4). The C terminus region of tensin1, as well as family members tensin2, tensin3, and c-ten, has adjacent Src homology 2 (SH2) and PTB domains that interact with the tyrosine phosphorylated proteins Dok2 and PDK1 (5) as well as PI3 kinase, p130Cas, and focal adhesion kinase (FAK) (6), thereby posing a role for tensin1 in multiple signal transduction pathways. The N-terminal region of tensin1 contains a domain that is related in sequence to the tumor suppressor protein and PIP3 phosphatase called phosphatase and tensin homologue (PTEN) (3). This domain of tensin1 binds the alpha isoform of protein phosphatase 1 (PP1) (7), the major protein Ser/Thr phosphatase in cells that regulates a variety of signaling pathways. The SH2 domain of tensin1 also associates with a RhoGAP protein called deleted in liver cancer-1 (DLC-1) but does not require Tyr phosphorylation of DLC-1 (8). DLC-1 has a role in cell migration and is a negative regulator of tumor formation (8–10). Human breast carcinoma, prostate carcinoma, head and neck squamous cell carcinoma, and melanoma all exhibit reduced expression of tensin1, suggesting a tumor suppressor action (11). In addition, various cancer cell lines do not express detectable levels of tensin1 protein relative to normal fibroblasts that have abundant expression (1, 7). Re-expression of tensin1 in cancer cells promoted formation of focal adhesions (4) and decreased migration and invasion of MDA MB 231 human breast cancer cells (12). Taken together, these studies support a model for tensin1 as a tumor suppressor that acts as a scaffold protein for various signaling enzymes.
Tensin1 was first shown to be tyrosine phosphorylated following concentration by immunoprecipitation and immunoblotting with a pTyr antibody (6). Tyrosine phosphorylation of tensin1 was only detected if fibroblasts were plated on fibronectin, laminin, or vitronectin (13), suggesting that tensin1 tyrosine phosphorylation depends on integrin-mediated signaling. Jiang et al. (14) showed increased tyrosine phosphorylation of tensin1 when cells were treated with platelet-derived growth factor. In addition, epidermal growth factor treatment of human gastric epithelial cells stimulated tyrosine phosphorylation of tensin1 and this stimulation was inhibited with the nonsteroidal anti-inflammatory drug indomethacin (15). Cells transformed by the oncogene p210BCR/ABL contained tyrosine phosphorylated tensin1 (16). Treatment of rat aortic smooth muscle cells with angiotensin or thrombin also showed an increase in tensin1 tyrosine phosphorylation (17). Rapid turnover of pTyr by phosphatases presumably keeps tensin1 pTyr levels low in cells following stimulation. Different publications report tensin1 is phosphorylated on Ser and Thr residues, but data supporting these claims was not shown (1, 3, 18, 19). Phosphoproteomics implementing shotgun mass spectrometry techniques have turned up as many as 20 pTyr, 30 pSer, and 8 pThr peptides from human tensin (www.phosphosite.org). However, to date no comprehensive analysis of tensin1 phosphorylation has been reported.
We previously identified residue F302 in the KVEF motif in tensin1 as necessary for PP1α binding (12). Tensin1 F302A showed a reduced electrophoretic mobility in SDS-PAGE compared with tensin1 wild type, suggesting an increase in tensin1 phosphorylation because of absence of bound PP1. We also observed less DLC-1 binding to tensin1 F302A, but it is not known whether this was because of an increase in tensin1 phosphorylation (12). The tensin1 F302A did not suppress cancer cell invasion like tensin1 wild type (12), and this could be because of loss of PP1 binding, or less DLC-1 binding, or changes in phosphorylation.
In the present study we comprehensively analyze the phosphorylation of human S-tag-tensin1. Addition of phosphatase inhibitors to cells is shown to enhance phosphorylation to yield a total of 62 Ser/Thr phosphorylation sites and expose 10 Tyr sites not otherwise seen. The majority of Ser/Thr sites have adjacent proline residues and we identify p38α MAPK activity associated with tensin1. The p38MAPK phosphorylation of tensin1 alters binding of DLC-1, p130Cas and FAK. Our results demonstrate that tensin1 is extensively phosphorylated on Ser/Thr residues in addition to Tyr residues and this phosphorylation alters association with its SH2 domain binding partners.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
HEK-293 and HEK293T cells were grown in modified Eagle's medium and Dulbecco's modified Eagle's medium, respectively, supplemented with 10% fetal bovine serum (Invitrogen). All cell cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2. S-protein agarose and S-TagTM monoclonal antibody were purchased from Novagen (Madison, WI). The anti-DLC-1 mouse monoclonal antibody was purchased from BD Transduction Laboratories, the anti-phospho-tyrosine (pTyr) mouse monoclonal antibody 4G10 was purchased from Millipore (Billerica, MA), and the p38MAPK, phospho-p38MAPK, and phospho-threonine-proline antibodies were purchased from Cell Signaling (Danvers, MA). The p130Cas and FAK antibodies were generous gifts from Dr. Amy Bouton (University of Virginia). The secondary antibody Alexa Fluor® 680 goat anti-mouse IgG was purchased from Invitrogen and the goat anti-mouse HRP was purchased from Thermo Scientific.
Full length pTriEX4-(S-tag)-tensin1 was mutated to F302A by polymerase chain reaction as previously described (12).
Affinity purification of S-tagged Tensin1 and Tensin1 F302A
For each experiment HEK293T cells were plated onto five 15-cm plates, grown to 80% confluency and transiently transfected with pTriEx4-tensin1 wild type (wt) or F302A according to manufacturer's instructions with Arrest-InTM Transfection Reagent (Open Biosystems). Cells were treated with DMSO as control or 25 nm calyculin A for 30 min. Calyculin caused the cells to detach and they were collected by centrifugation at 1500 × g for 5 min. For the identification of Tyr phosphorylation, cells were treated with 1 mm peroxyvanadate for 30 min prior to scraping in a total of 3 ml ice-cold MS lysis buffer (50 mm Tris-HCl, pH 7.9, 1% IGEPAL CA-630, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 20 mm β-glycerophosphate, 1× protease inhibitor mixture V (Calbiochem), and 1 mm dithiothreitol). The cell suspension was centrifuged at 16,000 × g for 15 min in a microcentrifuge and the supernatant (extract) incubated with 60 μl of S-protein agarose for 3 h. The agarose beads were washed twice by centrifugation with MS lysis buffer containing 500 mm NaCl and no protease inhibitors, twice with 50 mm Tris, pH 7.9, 500 mm NaCl, and once with Tris buffer, 150 mm NaCl.
LC-MS/MS Analysis of S-tagged Tensin1 and Tensin1 F302A
Each sample of purified tensin1 was reduced and carbamidomethylated at room temperature using dithiothreitol (Sigma Aldrich, St. Louis, MO) and iodoacetamide (Sigma Aldrich), respectively, then subjected to proteolytic digestion (1:20 enzyme to substrate) by trypsin (Promega, Madison, WI) as previously reported (20). An additional chymotrypsin (Roche, Penzberg, Germany) digest using similar conditions was used to characterize modified sites in tryptic peptide comprised of residues Asp1199 to Arg1248. For mass spectrometric analysis, a fraction of the digest was pressure loaded onto a precolumn (360 μm o.d. × 150 μm i.d. fused silica capillary) packed with 5 cm of C18 reverse-phase resin (5–20 μm diameter, 120 Å pore size, YMC Co., Ltd., Kyoto, Japan). Following a desalting rinse using 0.1 m acetic acid, the precolumn was connected to an analytical column (360 μm o.d. × 50 μm i.d.) packed with 8 cm of C18 reverse-phase resin (5 μm diameter, 120 Å pore size, YMC Co., Ltd.) and equipped with an electrospray emitter tip (21). For liquid chromatography-tandem MS (LC-MS/MS) analysis, the tryptic peptides were gradient eluted at a flow rate of 60 nL/min using an LC gradient previously described (22). MS mass analyses were acquired in high-resolution Fourier transform mass analyzers (FT or Orbitrap) whereas MS2 spectra were measured in linear ion traps of LTQ-FT-ion cyclotron resonance and LTQ-Orbitrap hybrid instruments (Thermo Fisher Scientific, Bremen, Germany) using collisionally activated dissociation (CAD) and front end electron transfer dissociation. Mass analyses were completed using a method consisting of one high resolution MS1 scan (resolving power of 60,000 at m/z 400) followed by 8 to 10 data dependent low resolution MS2 scans acquired in the LTQ ion trap. Data dependence parameters were set as follows: repeat count of 2, repeat duration of 30s, exclusion list duration of 20s. Electron transfer dissociation (ETD) MS2 parameters were set as follows: 30 ms reaction time, 3 m/z precursor isolation window, charge state rejection “on” for +1 and +2 charge state precursor ions, 2 × 105 FTMS full automated gain control target, 1 × 104 ITMSn automated gain control target, 2 × 105 reagent target with azulene as the electron transfer reagent.
Differential Phosphorylation Analysis
Prior to phosphopeptide enrichment using immobilized metal affinity chromatography (IMAC), tensin1 wt and tensin1 F302A tryptic peptides were differentially esterified on acidic residues using d0-methanolic HCl and d3-methanolic HCl, respectively to reduce nonspecific binding to the IMAC column (23). Esterified peptides from both tensin1 protein samples were then combined and simultaneously enriched for phosphorylated peptides using IMAC methods previously described (24). The LC-MS/MS methods were identical to those described above. Phosphorylation relative abundance comparisons between samples, namely the d3/d0 ratios, were calculated on the basis of the abundance of the C12 isotope peak within the charge envelope of each interrogated phosphorylated peptide.
Mass Spectrometry Data Analysis
Prior to using the Open Mass Spectrometry Search Algorithm (version 2.1.1) to search against a human tensin1 and tensin1 F302A protein sequence database (gi 187954597, Accession number: BC140942.1, NCBI), MS/MS peak lists were generated using Bioworks Browser (version 3.3.1 SP1). For both CAD (b- and y-type ions) and ETD (c- and z-type ions) data set searches, Open Mass Spectrometry Search Algorithm mass tolerances were ± 0.01 Da and ± 0.35 Da for precursor and product ion masses, respectively. The Open Mass Spectrometry Search Algorithm provided the removal of reduced charge species from ETD peak lists prior to searching. All database searches were completed selecting trypsin for enzyme specificity and included the following variable modifications: carbamidomethylation of Cys, oxidation of Met, phosphorylated Ser, Thr, and Tyr as well as O-GlcNAcylation of Ser and Thr. Missed cleavage count was set to three. The Open Mass Spectrometry Search Algorithm search results for complementary CAD and ETD data sets were confirmed by manual interpretation of the MS/MS spectra.
Analysis of S-tag-Tensin1 by Immunoblotting
HEK293 cells transfected with pTriEx4-tensin1 or tensin1 F302A plasmid were untreated, or treated with either dimethyl sulfoxide, or 25 nm calyculin A (Calbiochem), collected, and lysed on ice for 15 min in Golden lysis buffer (20 mm Tris-HCl, pH 7.9, 0.137 m NaCl, 1 mm EGTA, 5 mm EDTA, 10% glycerol, 1% Triton X-100, 10 mm NaF, 1 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, and 1× protease inhibitor mixture set V). Following centrifugation at 16,000 × g for 20 min whole cell extracts were resolved on a 4%–15% gradient Criterion Tris-HCl polyacrylamide gel (BioRad Laboratories), proteins transferred to a nitrocellulose membrane and immunoblotted anti-phospho-ThrPro, anti-phospho-Tyr, or anti-S-tag. Immunoblots were scanned by the Odyssey Infrared Imaging System (LiCor®) and analyzed with the Odyssey Application Software version 2.0.41 (LiCor®).
In Vitro Kinase Assays
HEK293 cells were transiently transfected with pTriEx4-tensin1 or mock transfected, the medium removed by aspiration, and cells lysed on ice for 15 min in 0.4 ml Golden lysis buffer or in RIPA buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% IGEPAL CA-630, 0.25% deoxycholate, 0.1% SDS, 20 mm β-glycerophosphate, 1 mm EDTA, and 0.4 mm Pefabloc). Cells were scraped from the plates and following centrifugation the extracts were incubated with S-protein agarose for 3 h and the beads recovered and washed with Golden buffer or RIPA buffer by centrifugation. S-protein agarose beads were incubated in kinase reaction buffer alone (50 mm Tris-HCl, pH 7.5, 0.1% 2-mercaptoethanol, 0.2 mm Na3VO4, and 1 mm dithiothreitol), with 10 μm kinase inhibitors (GSKβ3 inhibitor II, SB220025, or staurosporine), or with 100 ng of p38α MAPK purified kinase. The 32P-ATP was added in a concentrated Mg/Mn buffer to give final concentrations of 10 mm MgCl2, 1 mm MnCl2, 0.1 mm ATP, and 5 mm β-glycerophosphate. Samples were incubated at 30 °C for the specified time points, boiled for 5 min following adding SDS sample buffer, and proteins resolved by SDS-PAGE on 12% gels that were dried and imaged with the PhosphorImager 445S1 (Molecular Probes, Carlsbad, CA).
DLC-1 Binding Assays
For DLC-1 binding experiments S-tag-tensin1 was prepared as described above and incubated ± p38 MAPK with nonradioactive ATP. Separate plates of HEK293 cells transfected with pcDNA3-DLC-1 (a generous gift from Dr. D. Lowy, National Institutes of Health) were extracted with Golden lysis buffer and the extract incubated with the S-tag-tensin1 on beads for 2 h. Following incubation the S-protein agarose beads were washed, boiled in SDS sample buffer, and the proteins resolved a on a 4%–15% gradient gel, transferred to nitrocellulose and immunoblotted for DLC-1 and anti-S-tag. S-tag immunoblots were scanned by the Odyssey Infrared Imaging System (LiCor) and analyzed with the Odyssey Application Software version 2.0.41 (LiCor). DLC-1 immunoblots were detected with enhanced chemiluminescence, exposure of x-ray film and analyzed with Image J software.
Hyperosmotic Sorbitol Treatment of Cells
HEK293 cells were transfected with pTriEx4-tensin1 or mock transfected with reagent alone. Following 24 h cells were treated with 0.4 m sorbitol for 20 min to activate p38 MAPK (25), lysed on ice for 15 min in 400 μl Golden lysis buffer, and the lysates centrifuged at 16,000 × g for 15 min. Supernatants were incubated with S-protein agarose for 3 h at 4 °C, and the beads washed three times with Golden lysis buffer by cenrtrifugation. DLC-1 binding was assayed as described above. Samples were washed 3× in Golden lysis buffer and proteins eluted and resolved on 4%–15% Criterion precast gradient gels (BioRad) and transferred to a nitrocellulose membrane. Membranes were immunoblotted with anti-DLC-1 and anti-S-tag. Proteins bound to S-protein agarose were immunoblotted with anti-phospho-threonine-proline, anti-phospho-tyrosine, anti-p130Cas, or anti-FAK antibodies. Extracts from cells with and without sorbitol treatment were immunoblotted with p38 and phospho-p38 antibodies. All immunoblots except DLC-1 were scanned by the Odyssey Infrared Imaging System (LiCor) and analyzed with the Odyssey Application Software version 2.0.41 (LiCor).
RESULTS
Comprehensive Analysis of Human Tensin1 Phosphorylation
Initial purifications using green fluorescent protein-tagged tensin1 recovered from cell extracts by immunoprecipitation revealed elution of multiple contaminating proteins, in addition to the immunoglobulin itself, which frustrated attempts to achieve complete sequence coverage of tensin1 by mass spectrometry. As an alternative approach HEK293 cells were transfected with pTri-Ex4-(S-tag) tensin1 to express full length S-tag tensin1. Wash conditions were optimized for purification of S-tag tensin1 with minimal contaminants, as monitored by silver staining following SDS-PAGE. The tensin1 was reduced and alkylated, then subjected to trypsin digestion (20). Mass analyses were completed on LTQ-FT-ion cyclotron resonance and LTQ-Orbitrap hybrid instruments using collisionally activated dissociation (CAD) and front end electron transfer dissociation (FETD). We identified 102 unique tryptic peptides, which together accounted for 93% coverage of the tensin1 sequence and 97% coverage of all Ser/Thr/Tyr sites. This extent of coverage is quite uncommon for proteins as large as tensin1. Use of the S-tag resulted in an effective purification in high purity and yield, of nearly 100 pmol of protein, calculated from peptide recoveries relative to internal standards.
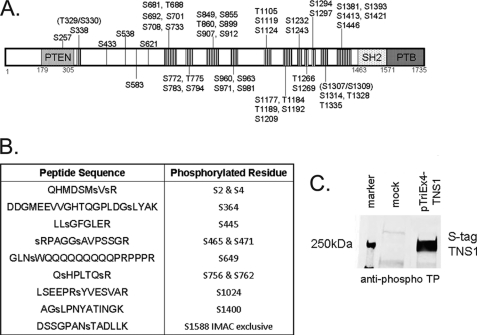
Among all spectra we identified 50 Ser/Thr phosphorylation sites in tensin1 (Fig. 1A, Supplemental Table S1). No tyrosine phosphorylated peptides were identified in this analysis, a surprising result because of several previous reports that tensin1 is tyrosine phosphorylated (3, 6, 13–16). The phospho-Ser/Thr sites are distributed in a series of a dozen clusters that lie in the central region of tensin1 between the N-terminal PTP and C-terminal SH2-PTB domains (3) (see Fig. 1A). It was remarkable that no phosphorylation was observed in the recognized structural domains, or in the N-terminal or C-terminal 300 residues at either end of tensin1. When cells expressing S-tag tensin1 were treated with calyculin A to inhibit Ser/Thr phosphatases, we identified 12 additional Ser/Thr phosphosites (Fig. 1B) for a total of 62 sites (see Supplemental Table 2). Treatment of cells with calyculin A produced a noticeable reduction in electrophoretic mobility of S-tag tensin1 in SDS-PAGE (see Fig. 3A), corresponding to phosphorylation of these additional sites. Human tensin1 is comprised of 1735 residues, with a total of 314 Ser/Thr residues; therefore up to 20% of the Ser/Thr residues are phosphorylated. We observed the majority of all the phosphorylated Ser/Thr residues (30 out of 50) were immediately adjacent to Pro residues (Supplemental Table S1). This reveals that tensin1 is primarily a substrate of Pro-directed protein kinases. As further evidence that tensin1 is phosphorylated at Thr-Pro sites, we immunoblotted S-tag-tensin1 pulled down from HEK293 cells with an anti-phospho Thr-Pro antibody. We observed a robust signal at 250 kDa corresponding to S-tag tensin1 and no corresponding band from mock transfected cells, as a control (Fig. 1C). We conclude that tensin1 is phosphorylated predominantly at Ser/Thr residues, catalyzed mostly by Pro-directed kinases.
Fig. 1.
Serine/threonine phosphorylation of human tensin1. A, Schematic showing the central location of the 50 S/T phosphorylation sites identified in human tensin1 by mass spectrometry, indicated by hatched segments and residue numbers. The PTP or PTEN homology, SH2 and PTB domains are indicated and approximate boundaries given by residue numbers. B, Peptide sequences and phosphorylated residues identified by mass spectrometry in tensin1 recovered from cells treated with calyculin A. C, HEK293 cells mock transfected or transfected to express S-tag tensin1. Proteins bound to S-protein agarose were immunoblotted with anti-phospho Thr-Pro antibody show phosphorylation of tensin1.
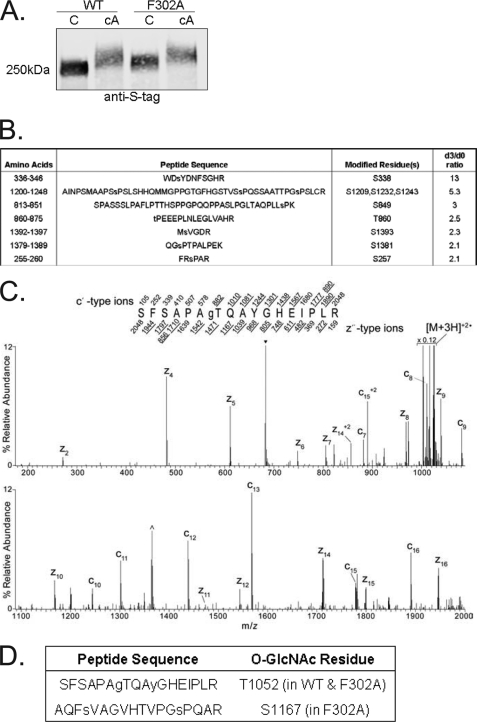
Fig. 3.
Phosphorylation and O-GlcNAcylation of tensin1 WT and F302A. A, HEK293 cells transfected to express either S-tag tensin1 wt (WT) or S-tag tensin1 F302A (F302A) were either not treated as control (C) or treated with 25 nm calyculin A (cA) for 30 min to enhance S/T phosphorylation. Whole-cell extracts were immunoblotted with anti-S-tag antibody to identify tensin1, which revealed reduced electrophoretic mobility of the F302A tensin1 versus wild type in untreated cells and in both samples from cells treated with calyculin A. B, Singly phosphorylated peptide sequences identified by mass spectrometry that showed > twofold enhanced phosphorylation (d3/d0 ratio) in the S-tag tensin1 F302A compared with wt. C, The MS/MS spectrum acquired in the ion trap of an Front End Electron Transfer Dissociation-enabled LTQ-Orbitrap on the [M+3H]+3 ions (m/z 683.6) of the singly O-GlcNAcylated tryptic tensin1 peptide, sequence SFSAPATQAYGHEIPLR. The predicted monoisotopic singly and selected average mass doubly charged c- and z-type fragment ion masses are listed above and below the peptide sequence, respectively. Fragment ions observed in the spectrum are underlined within the peptide sequence and allowed for the O-GlcNAc site localization at Thr, which corresponds to Thr1052 in full length tensin1. Charge-reduced species are labeled and the ions resulting from neutral losses are bracketed. The peak labeled with ▾ corresponds to ions that fall within the 3 m/z precursor isolation window prescribed in the instrument method. The peak labeled with ^ corresponds to charge-reduced ions resulting from a coeluting yet different species that falls within the same precursor isolation window. D, O-GlcNAc residues identified in tensin1 and tensin1 F302A.
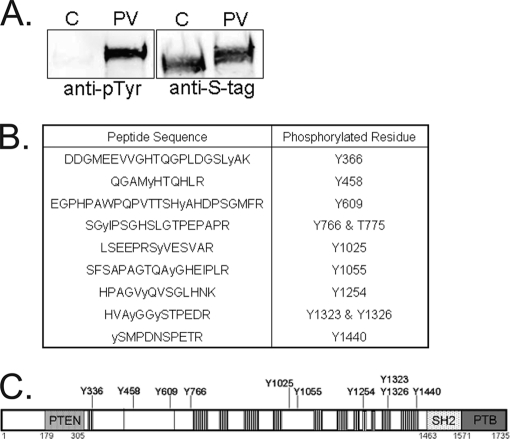
In our initial analyses no pTyr peptides were identified by mass spectrometric techniques. In fact, we also did not detect Tyr phosphorylation of tensin1 by immunoblotting S-pull downs from HEK293 cells with an anti-pTyr antibody (Fig. 2A). However, tyrosine phosphorylation was greatly enhanced when transfected cells were pretreated with peroxyvanadate, a potent tyrosine phosphatase inhibitor (Fig. 2A). We prepared S-tag tensin1 from peroxyvanadate pretreated HEK293 cells and determined the phosphorylation using mass spectrometry. We identified 10 phosphorylated tyrosine residues, two of which had not been previously reported (Fig. 2B). These sites of Tyr phosphorylation were not clustered together, but were widely dispersed across the central region of tensin1 (Fig. 2C). Most of the pTyr sites are well separated from the pSer/pThr sites in the primary structure of tensin1. Again, like with Ser/Thr, there was no Tyr phosphorylation in the PTEN, SH2 or PTB domains and none in the 300 residues at either end of the protein. In living cells tensin1 phosphorylation on Tyr is quite limited, but is detected if cells are treated with PTP inhibitor.
Fig. 2.
Tyrosine phosphorylation of human tensin1. A, HEK293 cells were transfected to express S-tag tensin1 and either not treated as control (C) or treated for 30 min with 1 mm peroxyvanadate (PV) to inhibit endogenous PTPs. S-protein beads were used to recover S-tag tensin1, which was immunoblotted with anti-pTyr antibody to show increased tyrosine phosphorylation with peroxyvanadate treatment and anti-S-tag antibody to show equal recovery of S-tag tensin1. B, Peptide sequences and phosphorylated residues in tensin1 recovered from cells pre-treated with peroxyvanadate, as identified by mass spectrometry. C, Schematic showing the central location of the 10 Tyr phosphorylation sites identified in human tensin1 by mass spectrometry.
Tensin1 WT and F302A: Phosphorylation and O-GlcNAcylation
We observed when tensin1 F302A (that does not bind PP1) was expressed in HEK293 cells it displayed reduced electrophoretic mobility in SDS-PAGE compared with tensin1 wild type (Fig. 3A, lanes 1 and 3). Treatment of the cells expressing S-tag tensin1 or tensin1 F302A with calyculin A caused an additional reduction in electrophoretic mobility of both proteins (Fig. 3A, lanes 2 and 4). Our hypothesis to account for this observation was that phosphorylated sites that appeared in response to calyculin A were not substrates for the PP1 bound to tensin1, because calyculin A affected both forms of tensin. Presumably other calyculin-sensitive protein phosphatases in proximity to tensin1 in cells effectively dephosphorylate these sites.
We expected if tensin1 was a substrate for bound PP1 the lack of PP1 binding would result in increased phosphorylation of some sites in tensin1 F302A compared with wt, consistent with the difference in electrophoretic mobility seen without added calyculin A. To compare phosphorylation sites tryptic peptides from tensin1 wt and tensin1 F302A were differentially esterified on acidic residues using d0-methanolic HCl and d3-methanolic HCl, respectively. The samples were combined and simultaneously enriched for phosphorylated peptides using IMAC methods previously described (24). The relative abundance of phosphorylation between samples, namely the d3/d0 ratios, were calculated on the basis of the abundance of the C12 isotope peak within the charge envelope for each interrogated phosphorylated peptide mass. We observed the same phosphopeptides separated by mass because of differential labeling in tensin1 wt and F302. For most of these peptides relative abundance between tensin1 wt and F302 was relatively unchanged, with d3/d0 ratios under 2.0. However, we did find seven phosphopeptides with higher d3/d0 ratios, indicative of increased phosphorylation in F302A relative to tensin1 wt (Fig. 3B). We propose that PP1α bound to tensin1 acts to dephosphorylate this subset of pSer/pThr sites in tensin1. The peptide with the highest d3/d0 ratio (13-fold) contains pS338, located closest to the primary PP1 binding site. We suspect this location makes it a preferred substrate for PP1α. On the other hand, the peptide with the second highest d3/d0 ratio is nearly 1000 residues distant from the primary PP1 site in the primary sequence.
In addition to phosphorylation, we identified O-GlcNAc at T1052 in tensin1. This site was found to be modified in tensin1 F302A as well. In addition a second O-GlcNAc site was located at S1167 (Fig. 3 C, D). The absence of PP1α binding to tensin1 resulted in increased phosphorylation and also additional O-GlcNAcylation.
p38 MAPK Associates with and Phosphorylates Tensin1
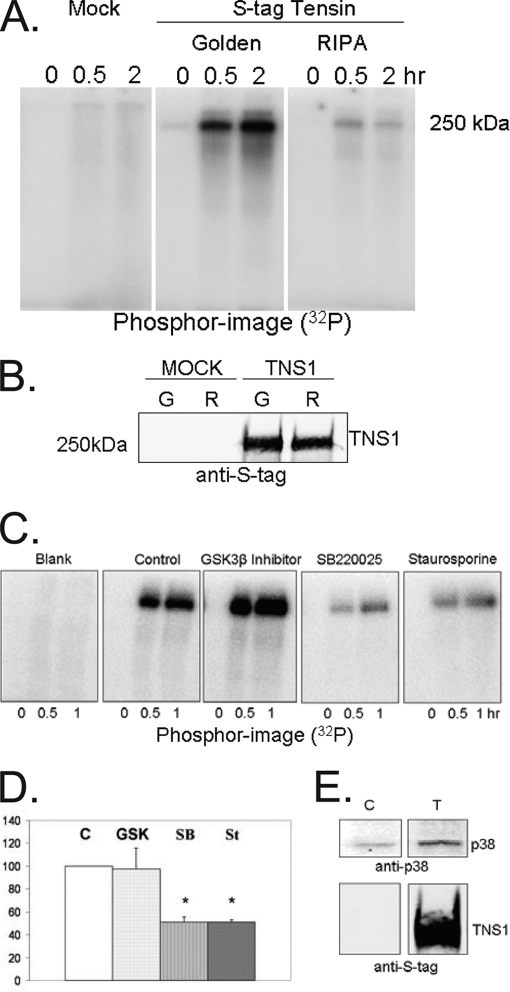
The majority (30 out of 50) of tensin1 Ser/Thr phosphorylation sites were Ser-Pro or Thr-Pro sequences. These sites are recognized by so called Pro-directed kinases: glycogen synthase kinase 3 (GSK3), mitogen-activated protein kinases (MAPK), or cyclin dependent kinases. Using an in vitro kinase assay with γ[32P]ATP and S-tag tensin1 bound to agarose beads we observed an endogenous kinase(s) survived coprecipitation from cells and catalyzed the time-dependent phosphorylation of tensin1 (Fig. 4A). This kinase was recovered only if the S tag tensin1 complexes were prepared with mild detergent conditions (Golden buffer), but not with more stringent conditions (RIPA buffer). Either condition yielded identical amounts of S-tag tensin1 (Fig. 4B) so the difference in phosphorylation could be attributed to removal of a tensin1-bound kinase.
Fig. 4.
Phosphorylation of tensin1 by associated p38 MAPK. A, HEK293 cells were mock transfected or transfected to express S-tag tensin1 and lysed in either Golden buffer or RIPA buffer. Proteins bound to S-protein agarose were subjected to an in vitro kinase assay with radioactive ATP for 0, 0.5 or 2.0 h. The phosphorimage of incorporated 32P demonstrated recovery of a kinase(s) that catalyzed tensin1 phosphorylation when cells were prepared in Golden buffer but not in RIPA buffer. B, Immunoblot of proteins bound to S-protein agarose in either Golden (G) or RIPA (R) buffer demonstrate equal recovery of S-tag tensin1. C, HEK293 cells mock transfected or transfected to express S-tag tensin1 were lysed in Golden buffer. Proteins bound to S-protein agarose were incubated with either buffer only (control), 10 μm GSK3β inhibitor (GSK), 10 μm SB220025 (SB, p38 MAPK inhibitor), or 10 μm staurosporine (St, a broad spectrum kinase inhibitor) and subjected to an in vitro kinase assay for 0, 0.5, or 1.0 h. The phosphorimage showed that addition of either SB220025 or staurosporine reduced tensin1 32P phosphorylation to the same extent, compared with reactions with buffer as control or with GSK3β inhibitor. D, SB220025 and staurosporine produced statistically significant (p ≤ 0.007, n = 3 independent experiments) and equal reduction in tensin1 phosphorylation at 30 min, suggesting that p38 MAPK is the dominant kinase associated with tensin1. E, HEK293 cells mock transfected as control (C) or transfected to express S-tag tensin1 (T) were lysed in Golden buffer and proteins bound to S-protein agarose were immunoblotted with anti-p38 antibody. Staining for S-tag shows tensin1 recovery in the pull down.
To identify the kinase bound to tensin1, we added to the in vitro kinase assay either: 1) buffer as control, 2) GSK3β inhibitor II, 3) SB220025 (a p38 MAPK inhibitor), or 4) staurosporine, as a broad spectrum kinase inhibitor (26). We observed the same level of tensin1 32P phosphorylation in the control and the assay with added GSK3β inhibitor. However, there was a statistically significant (p ≤ 0.007, n = 3) reduction of 32P phosphorylation in assays with either SB220025 or staurosporine added (Fig. 4C, D). The equivalent effects of these inhibitors indicate that p38 MAPK catalyzed tensin1 phosphorylation in this assay. Indeed, we detected by immunoblotting p38 MAPK in the S-tag tensin1 complexes used in these kinase assays (Fig. 4E). Together, these data show association of endogenous p38 MAPK with S-tag tensin1 and in vitro phosphorylation of tensin1 by this bound kinase.
Tensin1 Phosphorylation by p38α MAPK Affects Binding to the SH2 Domain
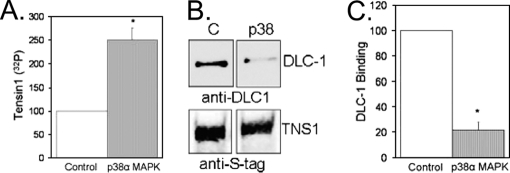
Incubation of S-tag tensin1 with purified recombinant p38 MAPK produced a 2.5-fold, significant (p ≤ 0.005) increase in tensin1 32P phosphorylation (Fig. 5A). Attempts to further increase the extent of S-tag tensin1 phosphorylation by pre-incubating with recombinant lambda phosphatase prior to the kinase assay were unsuccessful. We observed no 32P labeling at all in these assays, either because the lambda phosphatase was not removed, despite repeated washes with RIPA buffer, or the dephosphorylation of S-tag tensin 1 prevented its reaction with added p38 MAPK. Phosphorylation of S-tag tensin1 by recombinant p38α MAPK significantly (p ≤ 0.001) reduced binding of DLC-1, by nearly 80% (Fig. 5B). The results show that phosphorylation of tensin1 by p38 MAPK reduces the association of tensin1 and DLC-1.
Fig. 5.
Tensin1 phosphorylation by recombinant p38α MAPK decreases binding to DLC-1. A, HEK293 cells transfected to express S-tag tensin1 were lysed in RIPA buffer and proteins bound to S-protein agarose were subjected to an in vitro kinase assay with purified recombinant p38α MAPK that significantly increased (p ≤ 0.005, n = 3) tensin1 32P phosphorylation. B, Following phosphorylation by added p38α MAPK the S-tag tensin1 on beads was incubated with extracts of HEK293 transfected to express DLC-1. Immunoblot analysis with anti-DLC-1 antibody (insert) demonstrated a significant (p ≤ 0.001, n = 4) decrease in DLC-1 binding to tensin1. Staining for S-tag shows equal recovery of tensin1 in the binding assay.
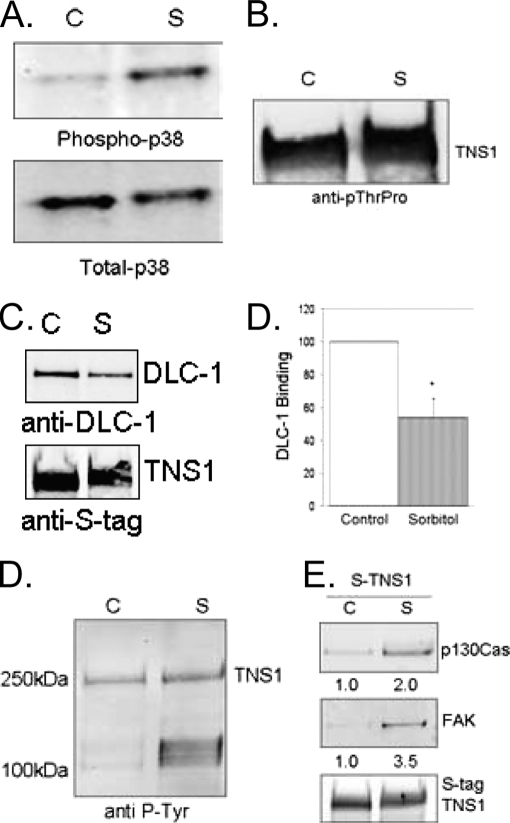
To test whether in cells activation of p38 MAPK would alter tensin1 to reduce binding to DLC-1, we expressed S-tag tensin1 in HEK293 cells and treated the cells with 0.4 m sorbitol as a hyperosmotic stress to activate endogenous p38 MAPK. This treatment was sufficient to activate the endogenous p38 MAPK on the basis of immunoblotting with a phosphosite specific antibody (Fig. 6A). Furthermore, we used the phosphosite pThr-Pro antibody to demonstrate the sorbitol-induced phosphorylation of S-tag tensin1 in these cells (Fig. 6B). The increase in S-tag tensin1 phosphorylation was evident by both increased intensity of phosphosite staining, as well as a reduction in electrophoretic mobility of tensin1 in SDS-PAGE. To assay DLC-1 binding cells expressing S-tag tensin1 were treated ± sorbitol, the S-tag tensin1 recovered with S-agarose, and the beads incubated with extracts from separate HEK293 cells over expressing DLC-1. We used extracts from non-treated cells as the source of DLC-1 to ensure that differences in binding were because of changes in tensin1, not in the DLC-1 itself. DLC-1 binding to S-tag tensin1 recovered from sorbitol treated cells was significantly (p ≤ 0.03) reduced to about half compared with the binding of DLC-1 to S-tag tensin1 recovered from cells not treated with sorbitol as control (Fig. 6C).
Fig. 6.
Sorbitol-induced tensin1 phosphorylation regulates binding to protein partners. A, HEK293 cells expressing S-tag tensin1 were untreated as control (C) or treated with a solution of 0.4 m sorbitol (S) for 20 min. Cells were lysed in Golden buffer and whole cell extracts immunoblotted with anti-phospho p38 to show activation of p38 MAPK. B, Proteins bound to S-protein agarose were immunoblotted with anti-pThr-Pro to demonstrate an increase in tensin1 phosphorylation and the reduced electrophoretic mobility in SDS-PAGE. C, HEK293 cells transfected to express S-tag tensin1 were untreated as control (C) or treated with 0.4 m sorbitol (S) for 20 min. Proteins bound to S-protein agarose were washed in RIPA buffer and incubated with extracts from HEK293 cells expressing DLC-1. Immunoblot with anti-DLC-1 antibody shows a significant (p ≤ 0.03, n = 3) decrease in DLC-1 binding. D, HEK293 cells transfected to express S-tag tensin1 were untreated as control (C) or treated with 0.4 m sorbitol (S) for 20 min. Proteins bound to S-protein agarose were immunoblotted with an anti-pTyr antibody to show an increase in endogenous phosphotyrosine proteins associated with tensin1 following treatment of cells with sorbitol. E, Proteins bound to S-protein agarose were immunoblotted with anti-p130Cas and anti-FAK to detect the endogenous proteins. The numbers give the -fold increase in recovery, on the basis of quantitative fluorescent imaging of immunoblots for p130Cas and FAK. Staining of S-tag as loading control shows equal recovery of tensin1.
To determine whether other tensin1 binding partners were affected by treating cells with sorbitol, we immunoblotted S-tag tensin1 complexes from cells not treated or treated with sorbitol with an anti-pTyr antibody. We observed equivalent recovery of S-tag tensin1, with an obvious increase in the amount of endogenous pTyr proteins (seen at 100–130 kDa) from cells treated with sorbitol as compared with control cells (Fig. 6D). Pretreatment of cells with SB220025 prevented the sorbitol-induced increase in binding of pTyr proteins to S-tag tensin1 (not shown). Immunoblot analysis of the endogenous proteins bound to tensin1 identified both p130Cas and FAK and showed > 2-fold increase in binding to tensin1 with sorbitol treatment (Fig. 6E). These data show that tensin1 phosphorylation in cells increases in response to sorbitol activation of p38 MAPK. Furthermore, increased Ser/Thr phosphorylation of tensin1 by p38 MAPK reduces association with DLC-1 but increases association with pTyr proteins, including p130Cas and FAK.
DISCUSSION
The results of this study show the unexpected extensive Ser/Thr phosphorylation and two sites of O-GlcNAcylation in addition to the reported tyrosine phosphorylation of the focal adhesion protein tensin1. Our analyses revealed a total of 62 Ser/Thr and 10 Tyr phosphorylation sites. Human tensin1 is comprised of 1735 residues, 314 of which are Ser/Thr and 48 are Tyr residues and of all these possible sites we found 19% of the Ser/Thr and 21% the Tyr to be phosphorylated. Of these, 41 Ser/Thr, two Tyr phosphorylation, and both O-GlcNAc sites are reported for the first time. Critical to these analyses was the preparation of highly purified protein using an S-tag fusion and S-protein agarose. This yielded a preparation with relatively few contaminating proteins on the basis of SDS-PAGE and mass spectrometry analyses, compared with use of an epitope tag and immunoprecipitation. The low background and effective cleavage by trypsin afforded 93% coverage of the entire tensin1 sequence. The initial analysis of tensin1 expressed in cells identified 50 Ser/Thr sites but zero Tyr phosphorylated sites. This was a surprising observation considering that tensin1 was initially described as a pTyr protein. However, in previous studies a relative increase in Tyr phosphorylation of tensin1 was detected following addition of growth factors such as epidermal growth factor receptor or PDGF or replating cells onto extracellular matrix proteins (13–15). We suspect that the Tyr phosphorylation of tensin1 is rapidly reversed and only in low abundance in cells because of the action of protein Tyr phosphatases. This idea was supported by results from addition of phosphatase inhibitor peroxyvanadate to cells expressing tensin1 that revealed 10 sites of Tyr phosphorylation not otherwise detected by mass spectrometry. Addition of calyculin A, a PP1/PP2A phosphatase inhibitor, to cells increased tensin1 phosphorylation with the appearance of 12 additional Ser/Thr sites. This indicates that tensin1 is a target for kinases and phosphatases and there is rapid turnover of phosphorylation at several sites. It was remarkable that phosphorylation was almost entirely excluded from the 300+ N- and C-terminal residues at the ends of the protein and was not observed at all in the PTEN, SH2, and PTB structural domains. We do not yet understand the mechanisms or the significance of the particular distribution of phosphorylation sites in tensin1.
Furthermore, we show Ser/Thr phosphorylation of tensin1 involves p38 MAPK, which modulates binding of DLC-1, FAK, and p130Cas to the SH2 domain. We recognized that 30 of the pSer/pThr residues were adjacent to Pro, which led us to test which Pro-directed kinases catalyze tensin1 phosphorylation. We used an in vitro assay to test the activity of a kinase that was bound to tensin1 prepared with mild detergents but not in RIPA buffer. Both SB220025 (p38 MAPK inhibitor) and staurosporine (a broad spectrum kinase inhibitor) were equally effective at inhibiting tensin1 in vitro phosphorylation. Phosphorylation of tensin1 in vitro also was catalyzed by purified recombinant p38α MAPK and we detected p38 MAPK from the HEK293 cells associated with S-tag tensin1 by immunoblotting. Katz et al. (27) showed that coexpression of chicken tensin1 with HA-JNK or HA-p38 in HEK293T cells activated these kinases without requiring the GTP binding proteins Rac and Cdc42. Perhaps the association we observe between tensin1 and p38 MAPK promotes activation of the kinase and phosphorylation of tensin1. Phosphorylation of tensin1 by p38 MAPK could provide another mechanistic link between stress signaling and reorganization of focal adhesions and the actin cytoskeleton.
We analyzed phosphorylation of tensin1 wt compared with tensin1 F302A that does not bind PP1 (12). There were no differences in the phosphorylation sites present, however, seven phosphosites in tensin1 F302A that had greater than a twofold higher level of phosphorylation, suggesting that PP1 bound to tensin1 did dephosphorylate sites in tensin1, and if PP1 was not bound then tensin1 phosphorylation is increased. The sites with the largest relative increases were S338 (13-fold increase) and a residue in the 1200–1248 region (fivefold increase). These results suggest that PP1 bound to tensin1 can dephosphorylate sites both in close proximity as well as those far away in the primary sequence, possibly because tensin1 forms dimers head to tail to present opposite ends for dephosphorylation by PP1 bound near the N terminus. There is physicochemical evidence for dimerization of chicken tensin (28). An alternative model would be that tensin1 is folded back on itself to present these distant sites to the bound PP1.
In this study we also show that tensin1 has an O-GlcNAc modification at T1052 and that tensin1 F302A has an additional O-GlcNAc moiety at S1167. It is now appreciated that many proteins are modified by GlcNAc (29). An interesting relationship exists with PP1, which forms protein complexes with O-GlcNAc transferase (OGT) (30). Binding of PP1 to tensin1 might facilitate GlcNAc modification by recruiting the OGT, however OGT reaction with tensin1 is not dependent on binding of PP1 because tensin1 F302A, which does not bind PP1, had two sites of O-GlcNAc. One possible explanation for this apparent contradiction is that only the PP1α isoform binds to tensin (4), whereas OGT binds other PP1 isoforms (27). The function of the GlcNAc modifications in tensin1 remains unknown.
Tensin1 acts as a scaffold to recruit and organize other enzymes at focal adhesions. Our hypothesis that in part motivated this study was that association with PP1 would regulate both Ser/Thr phosphorylation of tensin1 itself and the association with and/or phosphorylation of other proteins bound to tensin1. The mutation of tensin1 at F302A (previously shown to reduce binding of DLC-1(12)) was shown here to increase by greater than twofold the phosphorylation of seven sites throughout the length of the protein. Furthermore, we found that p38 MAPK enhanced Ser/Thr phosphorylation of tensin1 both in vitro and in cells, which reduced association with DLC-1. However, neither the phosphosites increased by F302A nor any of the 30 pSerPro/pThrPro sites potentially phosphorylated by p38 MAPK are located within the SH2 domain that associates with DLC-1. Therefore, we are forced to conclude that Ser/Thr phosphorylation of tensin1 in its central sequence region produces conformational changes that indirectly alter the binding specificity of the SH2 domain.
Sorbitol stress-induced activation of endogenous p38 MAPK increases tensin1 Ser/Thr phosphorylation and promotes recruitment of the endogenous pTyr proteins, including p130Cas and FAK. Both FAK and p130Cas have pTyr sites with sequences compatible with direct binding to the tensin1 SH2 domain (5). The unequal increase in association of FAK and p130Cas with tensin1 seen in response to sorbitol treatment supports the idea of separate FAK-tensin1 and p130Cas-tensin1 complexes. Alternatively, because FAK associates with p130Cas (31), these proteins may bind to tensin1 together as a complex, and immunoblotting detection is not strictly proportional. Association with tensin1 may facilitate Ser/Thr phosphorylation of p130Cas and FAK by p38 MAPK. We observed reduced migration in SDS-PAGE of all the endogenous p130Cas in cells in response to sorbitol addition, but this response was seen only in cells over expressing tensin1, consistent with a tensin1-assisted phosphorylation of p130Cas, possibly by p38 MAPK. FAK is phosphorylated on Ser-Pro sites, which has been attributed to reaction with GSK-3 (32), and to our knowledge p38 MAPK phosphorylation of FAK has not been reported.
Osmotic stress with sorbitol that activates p38MAPK reportedly requires upstream activity of both PI3K and p21-activated kinase (33). p21-Activated kinase activation triggers rapid disassembly of focal adhesions, which occurs even in cells pretreated with SB202580, indicating these effects are not dependent on p38MAPK (33). The adhesions affected were those stained brightly for paxillin, which contain some tensin1 (34), but have been distinguished from other adhesions with relatively less paxillin and more tensin1 (34). Therefore, p21-activated kinase and p38MAPK might act in concert in the same adhesions, or affect different focal adhesions in response to osmotic stress. Formation of tensin1-p130Cas-FAK complexes in response to osmotic stress activation of p38 MAPK is likely to modulate pTyr signaling and provides a new mechanism for cross talk between Ser/Thr and Tyr phosphorylation. The reciprocal effects of p38MAPK phosphorylation of tensin1 on binding of p130Cas-FAK versus the RhoGAP DLC-1 makes it possible that tensin1 serves as a phospho Ser/Thr-dependent switch for signals involving Tyr phosphorylation and Rho activation.
Supplementary Material
Acknowledgments
We thank D. Lowy for the DLC-1 expression vector and A. Bouton for antibodies.
Footnotes
* This work was supported in part by National Institutes of Health grants GM37537 and U54 GM064346 (to D. F. H.), CA40042 (to DLB), and T32 CA009109 (E. H. H.).
 This article contains supplemental Table1S, Table2S.
This article contains supplemental Table1S, Table2S.
1 The abbreviations used are:
- PTB
- Phosphotyrosine binding
- MAPK
- mitogen activated protein kinase
- SH2
- Src Homology 2
- FAK
- focal adhesion kinase
- DLC-1
- deleted in liver cancer-1
- PTEN
- phosphatase and tensin homologue
- PP1
- protein phosphatase 1
- Ser
- serine residue
- Thr
- threonine residue
- Tyr
- tyrosine residue
- Pro
- proline residue
- p
- phosphorylated residue
- wt
- wild-type
- IMAC
- immobilized metal affinity chromatography
- LC
- liquid chromatography
- MS
- mass spectrometry
- ICR
- ion cyclotron resonance
- CAD
- collisionally activated dissociation
- ETD
- electron transfer dissociation
- LTQ
- Linear Trap Quadrupole
- FT
- Fourier transform.
REFERENCES
- 1. Chen H., Ishii A., Wong W. K., Chen L. B., Lo S. H. (2000) Molecular characterization of human tensin. Biochem J 351 Pt 2, 403–411 [PMC free article] [PubMed] [Google Scholar]
- 2. Calderwood D. A., Fujioka Y., de Pereda J. M., Garcia-Alvarez B., Nakamoto T., Margolis B., McGlade C. J., Liddington R. C., Ginsberg M. H. (2003) Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A 100, 2272–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo S. H. (2004) Tensin. Int J Biochem Cell Biol 36, 31–34 [DOI] [PubMed] [Google Scholar]
- 4. Lo S. H., Janmey P. A., Hartwig J. H., Chen L. B. (1994) Interactions of tensin with actin and identification of its three distinct actin-binding domains. J Cell Biol 125, 1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wavreille A. S., Pei D. (2007) A chemical approach to the identification of tensin-binding proteins. ACS Chem Biol 2, 109–118 [DOI] [PubMed] [Google Scholar]
- 6. Davis S., Lu M. L., Lo S. H., Lin S., Butler J. A., Druker B. J., Roberts T. M., An Q., Chen L. B. (1991) Presence of an SH2 domain in the actin-binding protein tensin. Science 252, 712–715 [DOI] [PubMed] [Google Scholar]
- 7. Eto M., Kirkbride J., Elliott E., Lo S. H., Brautigan D. L. (2007) Association of the tensin N-terminal protein-tyrosine phosphatase domain with the alpha isoform of protein phosphatase-1 in focal adhesions. J Biol Chem 282, 17806–17815 [DOI] [PubMed] [Google Scholar]
- 8. Liao Y. C., Si L., deVere White R. W., Lo S. H. (2007) The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol 176, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liao Y. C., Lo S. H. (2008) Deleted in liver cancer-1 (DLC-1): a tumor suppressor not just for liver. Int J Biochem Cell Biol 40, 843–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qian X., Li G., Asmussen H. K., Asnaghi L., Vass W. C., Braverman R., Yamada K. M., Popescu N. C., Papageorge A. G., Lowy D. R. (2007) Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci U S A 104, 9012–9017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rhodes D. R., Yu J., Shanker K., Deshpande N., Varambally R., Ghosh D., Barrette T., Pandey A., Chinnaiyan A. M. (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall E. H., Daugherty A. E., Choi C. K., Horwitz A. F., Brautigan D. L. (2009) Tensin1 requires protein phosphatase-1alpha in addition to RhoGAP DLC-1 to control cell polarization, migration, and invasion. J Biol Chem 284, 34713–34722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bockholt S. M., Burridge K. (1993) Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J Biol Chem 268, 14565–14567 [PubMed] [Google Scholar]
- 14. Jiang B., Yamamura S., Nelson P. R., Mureebe L., Kent K. C. (1996) Differential effects of platelet-derived growth factor isotypes on human smooth muscle cell proliferation and migration are mediated by distinct signaling pathways. Surgery 120, 427–431; discussion 432 [DOI] [PubMed] [Google Scholar]
- 15. Szabo I. L., Pai R., Jones M. K., Ehring G. R., Kawanaka H., Tarnawski A. S. (2002) Indomethacin delays gastric restitution: association with the inhibition of focal adhesion kinase and tensin phosphorylation and reduced actin stress fibers. Exp Biol Med (Maywood) 227, 412–424 [DOI] [PubMed] [Google Scholar]
- 16. Salgia R., Brunkhorst B., Pisick E., Li J. L., Lo S. H., Chen L. B., Griffin J. D. (1995) Increased tyrosine phosphorylation of focal adhesion proteins in myeloid cell lines expressing p210BCR/ABL. Oncogene 11, 1149–1155 [PubMed] [Google Scholar]
- 17. Ishida T., Ishida M., Suero J., Takahashi M., Berk B. C. (1999) Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J Clin Invest 103, 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Auger K. R., Songyang Z., Lo S. H., Roberts T. M., Chen L. B. (1996) Platelet-derived growth factor-induced formation of tensin and phosphoinositide 3-kinase complexes. J Biol Chem 271, 23452–23457 [DOI] [PubMed] [Google Scholar]
- 19. Lee S. B., Cho K. S., Kim E., Chung J. (2003) blistery encodes Drosophila tensin protein and interacts with integrin and the JNK signaling pathway during wing development. Development 130, 4001–4010 [DOI] [PubMed] [Google Scholar]
- 20. Schroeder M. J., Shabanowitz J., Schwartz J. C., Hunt D. F., Coon J. J. (2004) A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal Chem 76, 3590–3598 [DOI] [PubMed] [Google Scholar]
- 21. Udeshi N. D., Compton P. D., Shabanowitz J., Hunt D. F., Rose K. L. (2008) Methods for analyzing peptides and proteins on a chromatographic timescale by electron-transfer dissociation mass spectrometry. Nat Protoc 3, 1709–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Syka J. E., Coon J. J., Schroeder M. J., Shabanowitz J., Hunt D. F. (2004) Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci U S A 101, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ficarro S., Chertihin O., Westbrook V. A., White F., Jayes F., Kalab P., Marto J. A., Shabanowitz J., Herr J. C., Hunt D. F., Visconti P. E. (2003) Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem 278, 11579–11589 [DOI] [PubMed] [Google Scholar]
- 24. Zarling A. L., Polefrone J. M., Evans A. M., Mikesh L. M., Shabanowitz J., Lewis S. T., Engelhard V. H., Hunt D. F. (2006) Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc Natl Acad Sci U S A 103, 14889–14894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prickett T. D., Brautigan D. L. (2007) Cytokine activation of p38 mitogen-activated protein kinase and apoptosis is opposed by alpha-4 targeting of protein phosphatase 2A for site-specific dephosphorylation of MEK3. Mol Cell Biol 27, 4217–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karaman M. W., Herrgard S., Treiber D. K., Gallant P., Atteridge C. E., Campbell B. T., Chan K. W., Ciceri P., Davis M. I., Edeen P. T., Faraoni R., Floyd M., Hunt J. P., Lockhart D. J., Milanov Z. V., Morrison M. J., Pallares G., Patel H. K., Pritchard S., Wodicka L. M., Zarrinkar P. P. (2008) A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 26, 127–132 [DOI] [PubMed] [Google Scholar]
- 27. Katz B. Z., Zohar M., Teramoto H., Matsumoto K., Gutkind J. S., Lin D. C., Lin S., Yamada K. M. (2000) Tensin can induce JNK and p38 activation. Biochem Biophys Res Commun 272, 717–720 [DOI] [PubMed] [Google Scholar]
- 28. Lo S. H., An Q., Bao S., Wong W. K., Liu Y., Janmey P. A., Hartwig J. H., Chen L. B. (1994) Molecular cloning of chick cardiac muscle tensin. Full-length cDNA sequence, expression, and characterization. J Biol Chem 269, 22310–22319 [PubMed] [Google Scholar]
- 29. Wang Z., Gucek M., Hart G. W. (2008) Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci U S A 105, 13793–13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wells L., Kreppel L. K., Comer F. I., Wadzinski B. E., Hart G. W. (2004) O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem 279, 38466–38470 [DOI] [PubMed] [Google Scholar]
- 31. Harte M. T., Hildebrand J. D., Burnham M. R., Bouton A. H., Parsons J. T. (1996) p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem 271, 13649–13655 [DOI] [PubMed] [Google Scholar]
- 32. Bianchi M., De Lucchini S., Marin O., Turner D. L., Hanks S. K., Villa-Moruzzi E. (2005) Regulation of FAK Ser-722 phosphorylation and kinase activity by GSK3 and PP1 during cell spreading and migration. Biochem J 391, 359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan P. M., Lim L., Manser E. (2008) PAK is regulated by PI3K, PIX, CDC42, and PP2Calpha and mediates focal adhesion turnover in the hyperosmotic stress-induced p38 pathway. J Biol Chem 283, 24949–24961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zamir E., Katz B. Z., Aota S., Yamada K. M., Geiger B., Kam Z. (1999) Molecular diversity of cell-matrix adhesions. J Cell Sci 112 (Pt 11), 1655–1669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.