Abstract
Aims
Heart failure (HF) is accompanied by diminished cognitive, motor, learning, emotional, and planning deficits, which are associated with increased morbidity and mortality. A basal ganglia structure, the putamen, serves many functions that are affected in HF, but its global or localized structural integrity is unknown. Our aim was to evaluate global and regional putamen volume differences in HF over control subjects.
Methods and results
We collected two high-resolution T1-weighted scans from 16 HF patients (age, 54.1 ± 8.3 years; 12 males; left ventricular ejection fraction, 27.8 ± 6.8%) and 32 control subjects (52.4 ± 7.3 years; 24 males) using a 3.0 T magnetic resonance imaging scanner. After realigning, averaging, and reorienting the T1-weighted volumes into a common space, the structures were manually outlined, tracings were normalized for head size, volumes calculated, and surface models generated. Demographic data were compared between groups with χ2 and independent samples t-tests, global putamen volumes were evaluated using independent samples t-tests, and regional differences were examined with surface morphometry. No significant differences in age or sex appeared between groups, but body mass index differed significantly (P = 0.008). Heart failure patients showed significantly lower left (controls vs. HF; 4842.1 ± 740.0 vs. 4224.1 ± 894.4 mm3, P = 0.014) and right (4769.3 ± 651.9 vs. 4193.7 ± 876.2 mm3, P = 0.014) global putamen volumes than controls, with localized reductions in bilateral rostral, mid-dorsal, and medial-caudal regions (left, P < 0.003; right, P < 0.0002).
Conclusion
Putamen structures showed global and localized volume reductions in HF over controls. The localized volume losses suggest deficits in motor and neuropsychological functions, which are evident in HF subjects, and may be due to hypoxic and ischaemic processes targeting these areas.
Keywords: Magnetic resonance imaging, Brain, Heart failure, Three-dimensional surface morphometry, Basal ganglia
See page 597 for the editorial comment on this article (doi:10.1093/eurjhf/hfr055)
Introduction
Heart failure (HF) patients show multiple neuropsychological and motor impairments, including dysfunctions in affect, attention, complex planning, memory, motor speed, grip strength, and gait.1–4 A basal ganglia structure, the putamen, plays a significant role in motor planning,5 motivation,6 emotional regulation,7 language processing,8 and aspects of memory function,9 and shows injury in HF, as determined by voxel-based T2-relaxometry and grey matter assessment.10,11 However, the extent of putamen injury and localization of damage within the structure could not be evaluated from earlier assessments, since such procedures were intended to reveal whole-brain injury, not localized tissue changes. The extent and precise localization of injury in the putamen is of interest, since such determination may help reveal the functional outcomes that might be expected in patients with HF.
The putamen is topographically organized, and injury to specific sites within the structure may interrupt key pathways between brain regions. Certain subareas send dopaminergic projections to midbrain areas, including the substantia nigra and the ventrotegmental region,12,13 and receive inputs from the prefrontal cortex and thalamus;14,15 other subregions also send projections to the globus pallidus.16 Manual volumetric measurements, combined with three-dimensional surface morphometry, can assess global and regional tissue differences, and can demonstrate the extent of global injury and regional structural integrity of the putamen in HF patients.
Our aim was to evaluate global and regional putamen volume differences in HF vs. control subjects with manual volumetric and three-dimensional surface morphometry using high-resolution T1-weighted images, to better understand the basis of behavioural and motor deficits in the syndrome.
Methods
Subjects
We studied 16 clinically stable (not in congestive HF and no changes in medications in the previous 6 months) HF patients, recruited from the University of California at Los Angeles (UCLA) Cardiomyopathy Center and the Los Angeles community, and 32 control subjects, recruited from the greater Los Angeles area. Demographic data and other variables of HF and control subjects are summarized in Table 1. The diagnosis of HF was based on national diagnostic criteria,17 and all subjects were New York Heart Association Functional class II. Of the 16 HF subjects, 4 had ischaemic and 12 had idiopathic aetiology; none had alcohol-induced cardiomyopathy. Three HF subjects had type II diabetes, and none were diagnosed with any psychiatric disorder. All HF subjects were treated with similar drug therapies, including diuretics, angiotensin receptor blockers or angiotensin-converting enzyme inhibitors, and beta blockers to achieve specific haemodynamic parameters. Body weight and drug dosages for HF patients were stable for at least 6 months prior to the magnetic resonance imaging (MRI) scan. All control subjects were in good health, without any diagnosed cardiovascular disease, prior history of stroke, respiratory, or neurological disorder that might introduce brain injury. We excluded all HF and control subjects who were claustrophobic or had non-removable metal, such as braces, embolic coils, pacemakers/implantable cardioverter-defibrillators, and stents, as suggested by the MR safety website, the Institute for Magnetic Resonance Safety, Education, and Research (http://www.mrisafety.com/).
Table 1.
Demographics and characteristics of heart failure and control subjects
| Variables | Controls (n = 32) | HF (n = 16) | P-value |
|---|---|---|---|
| Age (mean ± SD, years) | 52.4 ± 7.3 | 54.1 ± 8.3 | 0.48 |
| Gender (male:female) | 24:8 | 12:4 | 1.0 |
| BMI (mean ± SD, kg/m2) | 24.6 ± 2.9 | 28.9 ± 5.6 | 0.008a |
| LVEF (mean ± SD, %) | – | 27.8 ± 6.8 | – |
SD, standard deviation; BMI, body mass index; LVEF, left ventricular ejection fraction.
aEqual variances not assumed.
All HF and control subjects gave written and informed consent before the study, and the research protocol was approved by the institutional review board at UCLA.
Magnetic resonance imaging
We used a 3.0 T MRI scanner (Magnetom Tim-Trio; Siemens, Erlangen, Germany), with a receive-only eight-channel phased-array head-coil, and a whole-body transmitter coil to perform brain studies. Subjects lay supine, and foam pads were used on both sides of the head to reduce motion during data acquisition. Two high-resolution T1-weighted image series, covering the whole brain, were collected using a magnetization prepared rapid acquisition gradient-echo pulse sequence [repetition-time (TR) = 2200 ms; echo-time (TE) = 2.2 ms; inversion time = 900 ms; flip angle (FA) = 9°; matrix size = 256 × 256; field-of-view (FOV) = 230× 230 mm; slice thickness = 1.0 mm]. Proton density (PD) and T2-weighted images were acquired using a dual-echo turbo spin-echo pulse sequence (TR = 10 000 ms; TE1, 2 = 17, 134 ms; FA = 130°; matrix size = 256 × 256; FOV = 230 × 230 mm; slice thickness = 4.0 mm; turbo factor = 5). A parallel imaging technique was used, called ‘generalized autocalibrating partially parallel acquisition', with an acceleration factor of two for both scans.
Data analysis
We used high-resolution T1-, PD-, and T2-weighted images for visual examination to detect any major brain pathology, such as cystic lesions, major infarcts, or other space occupying lesions. High-resolution T1-weighted images were also examined for any head motion-related or other imaging artefacts.
The statistical parametric mapping package SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), MRIcroN (http://www.cabiatl.com/mricro/mricron/index.html), and MATLAB-based (The MathWorks Inc, Natick, MA, USA) custom software were used to prepare the brain images, outline putamen regions, and calculate putamen volumes.
Realignment, averaging, and rigid-body transformation
Using SPM8 software, both high-resolution T1-weighted structural scans were realigned and averaged. Averaged images were bias-corrected for any potential image signal intensity variation using the unified segmentation approach, and image volumes were reoriented into a common Montreal Neurological Institute (MNI) space, using affine transformation (rigid body, six parameters), and resampled to voxel size 0.9 × 0.9 × 0.9 mm. Affine transformations (nine parameters; shift, rotate, scale) of each individual subject's image to the MNI space template were also calculated, and used to correct head size variations.
Putamen tracing and volume calculation
Reoriented and resampled brain image volumes were used to outline left and right putamen structures. An investigator, blinded to subject group assignments, delineated structures using MRIcroN software. Both left and right structures were outlined separately in axial views; coronal and sagittal views were used to verify structural boundaries and to assist any editing, if required. The delineated voxels in each structure were counted, and volumes calculated, based on affine-transformed images.
Statistical analysis
We used the Statistical Package for the Social Sciences (SPSS, V 18.0, Chicago, IL, USA) for demographic data evaluation. The numerical data (age and body mass index) were assessed with independent-samples t-tests, and categorical values were examined with χ2 tests. Global putamen volume data from HF and control subjects were assessed with Shapiro–Wilk W tests for distribution type. Global putamen volumes were assessed between HF and control groups using independent-samples t-tests, as Shapiro–Wilk W tests showed that the data were normally distributed. Intra-tracer reliability was established with an intra-class correlation (ICC) procedure.
Intra-tracer reliability
Intra-tracer reliability for putamen structure delineation was assessed by retracing those structures in 12 randomly chosen subjects. Of the 12 subjects, 3 were HF and 9 were control subjects. Intra-tracer reliability was high for putamen volume tracings (ICC = 0.89, P < 0.001), suggesting that initial assessments were consistent across the tracings.
Three-dimensional surface morphometry
Regional putamen volume differences between HF and control subjects were assessed with surface modelling and surface-based statistical procedures.18 Left and right surface models were derived from individual affine-transformed putamen tracings and three-dimensional meshes were created; each mesh consisted of a fixed number of equally spaced points.18 Based on each mesh, a medial curve was calculated in the anterior–posterior direction, and using the medial curve, radial distances were calculated for each surface point.
Two-sample t-tests were used to assess radial distances between HF and control subjects. Regional putamen sites showing significant group differences were colour-coded and overlaid on averaged three-dimensional putamen surface models, derived from all HF and control subjects. We performed permutation testing to control for multiple comparisons, and an overall P-value was calculated for the putamen on each side.
Results
Demographics
No significant differences in age or sex emerged between HF and control groups (Table 1). Body mass indices significantly differed between the groups (P = 0.008); HF subjects were heavier, on average, than control subjects.
Global putamen volumes changes
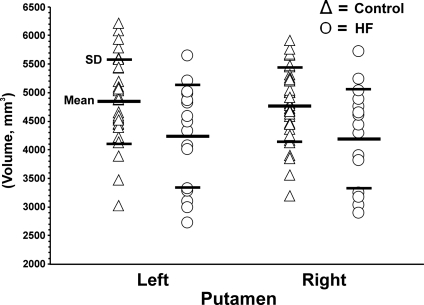
The Shapiro–Wilk W tests showed that both HF and control putamen data were normally distributed (HF: left putamen, P = 0.36, right putamen, P = 0.39; controls: left putamen, P = 0.89, right putamen, P = 0.91), which suggested the use of parametric tests for data evaluation (non-significant values indicative of normal distribution). The left and right putamen volumes from individual HF and control subjects are displayed in scatter plots (Figure 1). Both left (controls vs. HF; 4842.1 ± 740.0 vs. 4224.1 ± 894.4 mm3, P = 0.014) and right (4769.3 ± 651.9 vs. 4193.7 ± 876.2 mm3, P = 0.014) global putamen volumes were significantly lower in HF patients than in control subjects based on parametric statistical tests.
Figure 1.
Individual global putamen volumes from control (open triangle) and heart failure (open circle) subjects. Both left and right side structures showed significant volume reduction in heart failure patients over control subjects (left, P < 0.014; right, P < 0.014).
Regional putamen volume changes
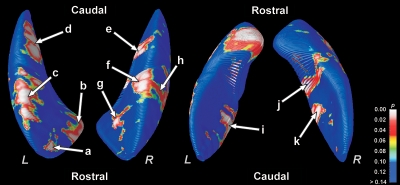
Significant regional putamen volume loss was detected in multiple sites for the HF compared with the control subjects. Regions with volume loss within the putamen included bilateral rostral (Figure 2a, b and g), mid-dorsal (Figure 2c, f and h), medial-caudal (Figure 2d and e), and mid-ventral (Figure 2i and k) areas. The volume loss on the right mid-dorsal portion extended to the ventral region (Figure 2j). Rostral and mid-dorsal portions showed more widespread injury on the left side compared with the right (Figure 2b–g). Permutation testing also confirmed significant bilateral putamen volume reduction in HF (left, P < 0.003; right, P < 0.0002).
Figure 2.
Sites of regional volume loss in the putamen of heart failure over control subjects. Bilateral volume reduction appears in rostral, mid-dorsal, and medial-caudal regions. Areas with significant differences are colour-coded and overlaid onto averaged three-dimensional surface models. The permutation test confirmed significant differences in putamen volumes between the groups (left, P < 0.003; right, P < 0.0002).
Discussion
Overview
Heart failure patients show lower global putamen volumes, with regional changes localized to bilateral rostral, mid-dorsal and ventral, and medial-caudal areas. Gross putamen injury was identified earlier, based on voxel-based T2-relaxometry and grey matter evaluation;10,11 however, regional differences within the structure were not identified due to limited spatial resolution. The specific sites with volume reduction within the putamen are implicated in motor functions, as well as neuropsychological characteristics; injury in these regions may contribute to the deficits found in HF.1–4
Global putamen volume changes
Both the left and right putamen showed reduced global volume in HF over control subjects. The putamen contains dopaminergic neurons, receives projections from the prefrontal cortex and thalamus,14,15 and sends dopaminergic projections to the midbrain, including the substantia nigra and the ventral tegmentum.12,13 These latter regions also show injury in HF subjects. The neurotransmitter dopamine released by these projections to the putamen serves multiple functions, especially behaviours associated with motor function and reward.19,20 Significant movement pathology, including impaired gait,3 as well as deficient sense of reward, impaired, for example, in depression are affected in HF.2
Regional putamen injury
Bilateral rostral, mid-dorsal and ventral, and medial-caudal regional areas showed volume loss in the putamen in HF subjects. The putamen receives projections from motor and somatosensory regions,21 with motor regulation being a primary role. The dorsolateral region of the putamen receives projections from the lower limbs, the ventromedial area from facial areas, and sites between the dorsolateral and ventromedial portions from upper limb areas of motor and somatosensory cortical representation sites.21,22 These projecting fields extend in a rostro-caudal direction. The premotor and supplementary motor regions also project to the putamen;23,24 the ventromedial putamen receives inputs from supplementary motor areas representing the face.25
The rostroventral putamen sends limited projections to the ventrolateral area of the globus pallidus and caudolateral areas of the substantia nigra.16 The ventrolateral region of the globus pallidus, in turn, projects to the ventrolateral nucleus of the thalamus, representing the oral region and upper airway,26 and projects to the supplementary motor area. The lateral substantia nigra, with significant projections to the putamen, is involved in orofacial roles, based on primate studies.27
Heart failure patients show various motor, movement, and neuropsychological irregularities, including motor speed, grip strength, gait, and affect functions.2–4 Regional sites with injury within the putamen found here are involved in motor deficits, especially in gait and grip strength, and affect regulation, and may contribute to deficient functions found in the syndrome.
Pathological mechanisms and other contributing factors
Hypoxic/ischaemic processes, which are common in HF, result from poor perfusion as a consequence of low cardiac output and poor cerebral autoregulation in HF, as well as from sleep-related breathing disorders accompanying the syndrome; both processes can introduce putamen injury in the condition. Both hypoxic and ischaemic events trigger a range of processes that are injurious to tissue, including oxidative and inflammatory mechanisms.28 Multiple brain regions, including putamen, cerebellum, and other limbic sites, show tissue injury in animal studies of intermittent hypoxic exposure,28 and, in a similar fashion, sleep-disordered breathing conditions may induce injury in the putamen.
Other factors that can exaggerate putamen injury in HF together with hypoxic/ischaemic processes include reduced neuro-protective nutrients. Hypoxic processes are more injurious in the presence of inadequate thiamine levels,29 which are significantly reduced in HF.30 Loss of this water-soluble nutrient likely results from use of diuretics and malabsorption issues. Thiamine, together with another nutrient, magnesium (essential for thiamine metabolic action), is a key component of carbohydrate metabolism; reduced levels, especially in conditions with high energy demand, may lead to neurotoxic injury.
Limitations
Limitations of this study include the small sample size. Moreover, confounding conditions may introduce brain injury independently, and such conditions should be partitioned in a future study. The finding of significant differences between our small sample of HF patients and the control subjects indicates the very large effect size (differences between groups). Independent confounding factors that can introduce brain injury include diabetes, hypertension, sleep-related breathing disorders, and dyslipidaemia, and these conditions could have contributed to the damage found here. The small sample size precluded partitioning of injury from such conditions. Differences in body mass index may also have contributed to the study findings. Global and regional grey matter volume losses occur in obese healthy males, but not in females,31 which suggest that body mass index should be partitioned during evaluations with only male subjects. However, our sample was composed of 25% females, and high body mass index is reflected partially in increased intra-cranial volume; adjustment for intra-cranial volumes was performed before calculation of global putamen volumes. Higher body mass can contribute to cardiovascular and respiratory conditions (such as sleep-disordered breathing) as a direct consequence, and secondary consequences of these conditions can introduce brain injury.
Conclusions
Heart failure patients showed significantly lower bilateral global putamen volumes over control subjects. These volume reductions were detected in rostral, mid-dorsal and ventral, and medial-caudal regions. The affected putamen subregions contribute to motor and neuropsychological functions, and regional volume losses may contribute to impaired functions in the HF syndrome, particularly those associated with dopamine as the underlying neurotransmitter. The precise pathological processes contributing to the putamen volume reduction are unknown, but likely derive from impaired perfusion accompanying low cardiac output from the primary condition or from disordered breathing during sleep, which is common in HF.
Funding
This research was supported by NR-009116. Additional support for morphometry algorithm development was provided by the NIA, NIBIB, and the National Center for Research Resources (AG016570, EB01651, and RR019771 to P.T.).
Conflict of interest: none declared.
Acknowledgements
We thank Ms Rebecca Harper, Mr Edwin Valladares, and Drs Rebecca Cross and Stacy Serber for assistance in data collection.
References
- 1.Almeida OP, Flicker L. The mind of a failing heart: a systematic review of the association between congestive heart failure and cognitive functioning. Intern Med J. 2001;31:290–295. doi: 10.1046/j.1445-5994.2001.00067.x. doi:10.1046/j.1445-5994.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O'Connor CM. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154:102–108. doi: 10.1016/j.ahj.2007.03.043. doi:10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Davies SW, Greig CA, Jordan SL, Grieve DW, Lipkin DP. Short-stepping gait in severe heart failure. Br Heart J. 1992;68:469–472. doi: 10.1136/hrt.68.11.469. doi:10.1136/hrt.68.11.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrucci RJ, Truesdell KC, Carter A, Goldstein NE, Russell MM, Dilkes D, Fitzpatrick JM, Thomas CE, Keenan ME, Lazarus LA, Chiaravalloti ND, Trunzo JJ, Verjans JW, Holmes EC, Samuels LE, Narula J. Cognitive dysfunction in advanced heart failure and prospective cardiac assist device patients. Ann Thorac Surg. 2006;81:1738–1744. doi: 10.1016/j.athoracsur.2005.12.010. doi:10.1016/j.athoracsur.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. doi: [DOI] [PubMed] [Google Scholar]
- 6.Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. doi: [DOI] [PubMed] [Google Scholar]
- 7.Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Na C, Escalona PR, Boyko OB, Nemeroff CB, Krishnan KR. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. doi:10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 8.Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. doi:10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badgaiyan RD, Fischman AJ, Alpert NM. Explicit motor memory activates the striatal dopamine system. Neuroreport. 2008;19:409–412. doi: 10.1097/WNR.0b013e3282f6435f. doi:10.1097/WNR.0b013e3282f6435f. [DOI] [PubMed] [Google Scholar]
- 10.Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15:214–223. doi: 10.1016/j.cardfail.2008.10.020. doi:10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. doi: [DOI] [PubMed] [Google Scholar]
- 12.Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol. 1987;57:201–217. doi: 10.1152/jn.1987.57.1.201. doi: [DOI] [PubMed] [Google Scholar]
- 13.Gorbachevskaya AI. Projections of the ventral tegmental area of the midbrain, the substantia nigra, and the amygdaloid body in different parts of the putamen in the dog. Neurosci Behav Physiol. 1997;27:496–502. doi: 10.1007/BF02463891. doi:10.1007/BF02463891. [DOI] [PubMed] [Google Scholar]
- 14.Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. doi:10.1002/1096-9861(20000925)425:3<447::AID-CNE9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Avendano C, de Las Heras S, Gimenez-Amaya JM. Striatal projections from the lateral and posterior thalamic complexes. An anterograde tracer study in the cat. Histochem Cell Biol. 2006;125:265–271. doi: 10.1007/s00418-005-0054-4. doi:10.1007/s00418-005-0054-4. [DOI] [PubMed] [Google Scholar]
- 16.DeVito JL, Anderson ME, Walsh KE. A horseradish peroxidase study of afferent connections of the globus pallidus in Macaca mulatta. Exp Brain Res. 1980;38:65–73. doi: 10.1007/BF00237932. doi: [DOI] [PubMed] [Google Scholar]
- 17.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JG, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, Goff DC, Grover FL, Malenka DJ, Peterson ED, Redberg RF. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Failure Society of America. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. doi:10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 18.Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. NeuroImage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. doi:10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. doi: [DOI] [PubMed] [Google Scholar]
- 20.Inase M, Li BM, Tanji J. Dopaminergic modulation of neuronal activity in the monkey putamen through D1 and D2 receptors during a delayed Go/Nogo task. Exp Brain Res. 1997;117:207–218. doi: 10.1007/s002210050217. doi:10.1007/s002210050217. [DOI] [PubMed] [Google Scholar]
- 21.Kunzle H. Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res. 1977;30:481–492. doi: 10.1007/BF00237639. doi: [DOI] [PubMed] [Google Scholar]
- 22.Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis{italicise if it is genus name} Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. doi:10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- 23.Miyata M, Sasaki K. Horseradish peroxidase studies on thalamic and striatal connections of the mesial part of area 6 in the monkey. Neurosci Lett. 1984;49:127–133. doi: 10.1016/0304-3940(84)90148-4. doi:10.1016/0304-3940(84)90148-4. [DOI] [PubMed] [Google Scholar]
- 24.Kunzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in Macaca fascicularis. Brain Behav Evol. 1978;15:185–234. doi: 10.1159/000123779. doi:10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- 25.Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. doi:10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- 26.DeVito JL, Anderson ME. An autoradiographic study of efferent connections of the globus pallidus in Macaca mulatta. Exp Brain Res. 1982;46:107–117. doi: 10.1007/BF00238104. doi: [DOI] [PubMed] [Google Scholar]
- 27.Boussaoud D, Joseph JP. Role of the cat substantia nigra pars reticulata in eye and head movements. II. Effects of local pharmacological injections. Exp Brain Res. 1985;57:297–304. doi: 10.1007/BF00236535. doi: [DOI] [PubMed] [Google Scholar]
- 28.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. doi: [DOI] [PubMed] [Google Scholar]
- 29.Shin BH, Choi SH, Cho EY, Shin MJ, Hwang KC, Cho HK, Chung JH, Jang Y. Thiamine attenuates hypoxia-induced cell death in cultured neonatal rat cardiomyocytes. Mol Cells. 2004;18:133–140. doi: [PubMed] [Google Scholar]
- 30.Hanninen SA, Darling PB, Sole MJ, Barr A, Keith ME. The prevalence of thiamin deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006;47:354–361. doi: 10.1016/j.jacc.2005.08.060. doi:10.1016/j.jacc.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 31.Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity. 2008;16:119–124. doi: 10.1038/oby.2007.4. doi:10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]