Abstract
Dogs with hemophilia A, hemophilia B, von Willebrand disease (VWD), and factor VII deficiency faithfully recapitulate the severe bleeding phenotype that occurs in humans with these disorders. The first rational approach to diagnosing these bleeding disorders became possible with the development of reliable assays in the 1940s through research that used these dogs. For the next 60 years, treatment consisted of replacement of the associated missing or dysfunctional protein, first with plasma-derived products and subsequently with recombinant products. Research has consistently shown that replacement products that are safe and efficacious in these dogs prove to be safe and efficacious in humans. But these highly effective products require repeated administration and are limited in supply and expensive; in addition, plasma-derived products have transmitted bloodborne pathogens. Recombinant proteins have all but eliminated inadvertent transmission of bloodborne pathogens, but the other limitations persist. Thus, gene therapy is an attractive alternative strategy in these monogenic disorders and has been actively pursued since the early 1990s. To date, several modalities of gene transfer in canine hemophilia have proven to be safe, produced easily detectable levels of transgene products in plasma that have persisted for years in association with reduced bleeding, and correctly predicted the vector dose required in a human hemophilia B liver-based trial. Very recently, however, researchers have identified an immune response to adeno-associated viral gene transfer vector capsid proteins in a human liver-based trial that was not present in preclinical testing in rodents, dogs, or nonhuman primates. This article provides a review of the strengths and limitations of canine hemophilia, VWD, and factor VII deficiency models and of their historical and current role in the development of improved therapy for humans with these inherited bleeding disorders.
Keywords: coagulation assays, dog model, hemophilia, factor VII, factor VIII, factor IX, von Willebrand disease, von Willebrand factor
Introduction
Hemophilia A, hemophilia B, and von Willebrand disease (VWD1) are among the most common inherited bleeding disorders in dogs and humans. Each is characterized by quantitative or qualitative abnormalities of a single plasma coagulation protein, termed “factor” and designated by a Roman numeral (Table 1): factor VIII (F.VIII1) for hemophilia A, factor IX (F.IX1) for hemophilia B, and von Willebrand factor (VWF1) for VWD. There are also a number of rare bleeding disorders, including factor VII (F.VII1) deficiency.
Table 1.
Roman numeral designation and names of blood coagulant and hemostatic proteinsa
| Roman numeral | Name | Protein characteristics |
|---|---|---|
| Factor I | Fibrinogen | Dimeric, each monomer has 3 subchains |
| Factor II | Prothrombin | Vitamin K-dependent |
| Factor III | Tissue factor | Transmembrane protein |
| Factor IV | Calcium ions | |
| Factor V | Labile factor | Cofactor to factor Xa |
| (Factor VI) | (obsolete for Accelerin) | Now recognized as factor Va |
| Factor VII | Stable factor | Vitamin K-dependent |
| Factor VIII | Antihemophilic factor | Cofactor to factor IXa |
| Factor IX | Christmas factor | Vitamin K-dependent |
| Factor X | Stuart-Prower factor | Vitamin K-dependent |
| Factor XI | Plasma thromboplastin | Homodimeric |
| Factor XII | Hageman factor | Activates factor XI |
| Factor XIII | Fibrin-stabilizing factor | Tetrameric (A2B2) |
| VWF | von Willebrand factor | Carries F.VIII in plasma |
This list simply provides a frame of reference for the reader and does not list all names or known coagulation proteins. Historically, the same coagulation protein may have had many names that were based on the state of knowledge at the time (Monroe DM, Hoffman M, Roberts HR. 2007. Fathers of modern coagulation. Thromb Hæmost 98:3–5; Owen CA Jr. 2001. A History of Blood Coagulation. Nichols WL, Bowie EJW, eds. Rochester MN: Mayo Foundation for Medical Education and Research).
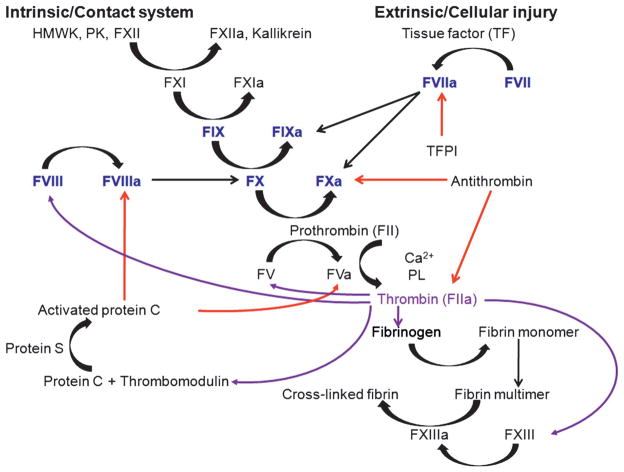
Recent reviews have described how these factors are activated, inhibited, and contribute mechanistically to normal hemostasis and blood coagulation as well as bleeding when these proteins are absent or dysfunctional (Gailani and Renne 2007; Mackman et al. 2007; Monroe et al. 2002). Briefly, the fundamental mechanism that causes bleeding in hemophilia is a defect in thrombin formation by activated factor X (F. Xa) when F.VIII or F.IX are deficient or dysfunctional. Two recognized pathways activate F.X (Figure 1). The first, the extrinsic or cellular injury pathway, is mediated by the binding of F.VIIa to tissue factor (TF); the TF:F.VIIa complex in turn activates both F.X and F.IX. The second, called the intrinsic or contact system pathway, is mediated by F.VIIIa, which functions as a cofactor and significantly accelerates activation of F.X by F.IXa. Once activated by either or both pathways, F.Xa participates in the prothrombinase complex (F.Xa:F.Va), which, in the presence of Ca2+ and phospholipids, converts prothrombin to thrombin. The latter, in turn, cleaves fibrinogen to soluble fibrin monomers that are cross linked by F.XIIIa to form a hemostatic plug or clot. VWF mediates platelet adhesion at sites of endothelial disruption, and bleeding in VWD results from the impaired inability of platelets to adhere to injured subendothelium in the absence of VWF (Sadler 2005; Sadler et al 2006). Because the bleeding phenotype in hemophilia and VWD is spontaneous, occurs stochastically, and is frequently severe, leading to significant morbidity, death, and enormous use of health care resources, these inherited bleeding disorders have been attractive targets for both replacement therapy for many decades and, relatively recently, gene transfer.
Figure 1. Coagulation cascade.
The extrinsic or cellular injury pathway is mediated by the binding of F.VIIa to tissue factor (TF) and the TF:F.VIIa complex in turn activates both F.X and F.IX. In the intrinsic or contact system, coagulation is initiated through high-molecular-weight kininogen (HMWK), prekallikrein (PK), and F.XII activation, which in turn activates F.XI, which then activates F.IX. F.VIIIa functions as a cofactor and significantly accelerates activation of F.X to F.Xa by F.IXa. Once activated, F.Xa participates in the prothrombinase complex (F.Xa:F.Va), which, in the presence of calcium (Ca2+) and phospholipids (PL), converts prothrombin (F.II) to thrombin. The latter, in turn, cleaves fibrinogen to soluble fibrin monomers that are cross linked by F.XIIIa to form a hemostatic plug or clot. Deficiency of F.VIII (hemophilia A) or F.IX (hemophilia B) results in impaired activation of F.X and downstream activation of thrombin and fibrin formation. Black arrow = conversion or activation of factor or cofactor function; red arrows = action of inhibitors; purple arrows = various functions of thrombin. TFPI, tissue factor pathway inhibitor.
Although inherited bleeding disorders have been recognized for many centuries, much of the basic understanding of hemophilia and VWD began to emerge in the 1940s and 1950s from canine models of these disorders. Affected dogs were key to researchers’ understanding of the hemophilic clotting defect, knowledge that led to the establishment of modern methods for diagnosis and treatment: the partial thromboplastin time test, the one-stage factor assay system for F.VIII and F.IX, and the “home” treatment management system consisting of an early alert at the first sign of hemorrhage and immediate intensive treatment with replacement products (Brinkhous et al. 1956, 1968, 1985, 1989a,b, 1996, 2002; Jones 1980; Langdell et al. 1953; Pemberton 2004; Webster et al. 1965). This work paralleled studies in humans that first demonstrated a rationale for replacement therapy for hemophilia and VWD during the first half of the 20th century, when clinicians recognized that bleeding events were partially corrected by whole blood transfusion (Graham et al. 1949; Macfarlane 1965; Owen 2001). This recognition sparked research that led to the identification, purification, and characterization of individual coagulation proteins (Biggs et al. 1952; Monroe et al. 2007; Patek and Taylor 1937). The resulting advances in detection, characterization, treatment, and prevention of inherited bleeding disorders dramatically improved the life expectancy of hemophilia and VWD patients. More recently, studies have shown that continuous prophylactic administration of replacement proteins in humans (Federici et al. 2006; Manco-Johnson et al. 2007) and canine models (Russell et al. 2003) corrects the hemophilic phenotype.
Thus over the past six decades, studies in animals and humans with these disorders have contributed to considerable progress in the development of safe and successful replacement products. But their development has not been without setbacks, and limitations persist. The products are expensive (typically $50,000 to $100,000 per year for an adult) and not readily available in less developed countries (Manco-Johnson et al. 2007; Roosendaal and Lafeber 2007; Van Cott et al. 2004), so for most patients worldwide, replacement therapy is usually used to treat acute bleeding on demand rather than to provide continuous prophylactic coverage. Furthermore, the products’ half-life in the circulation is relatively short and quite variable among patients (e.g., 8 to 23 hours for F.VIII), mandating repeated venipunctures or port implantation for prophylactic administration (Brinkhous et al. 1996; van den Berg et al. 2007). Last, although current versions of plasma-derived and recombinant replacement products are considered safe, extensive exposure to blood products has had tragic effects, such as the infection of this patient population with the human immunodeficiency and hepatitis C viruses through viral contamination of commercial plasma-derived products.
The experimental treatment of inherited bleeding disorders using gene transfer methods and modern molecular biological technology, which has only recently become available, presents a potentially attractive solution to problems with replacement therapy for several reasons. First, the severity of the bleeding disorder is usually inversely proportional to the plasma activity level of the involved protein, and only small amounts of the transgene product are required to change the hemophilia phenotype from severe to mild. For example, patients with F.VIII or F.IX levels greater than 5% of normal (i.e., more than ~5 to 10 ng/mL and 250 ng/ml, respectively) have a mild phenotype; patients whose levels are lower than 1% have a severe phenotype. Second, because F.VIII and F.IX have a broad therapeutic range, tight transcriptional control may not be necessary. Third, since these coagulation proteins are secreted, gene transfer is not restricted to the liver, the normal site of synthesis of most coagulation proteins. Fourth, only a portion of a given organ needs to be transduced to achieve some degree of therapeutic levels of protein secretion; this contrasts with many other genetic disorders that alter organ function (e.g., muscular dystrophy, cystic fibrosis). For these reasons, various vector systems—retroviral, recombinant adenoviral, recombinant adeno-associated viral (rAAV), lentiviral, and nonviral vectors as well as cell therapy—have been designed for expression of F.VIII, F.IX, VWF, and F.VII in a gene therapy setting.
Like all medications, viral vectors carry risks, including potentially harmful inflammatory reactions, germline transmission, and insertional mutagenesis (Arruda et al. 2001; Donsante et al. 2007; Hacein-Bey-Abina et al. 2003; Hasbrouck and High 2008; Mingozzi and High 2007). An important and relevant preclinical test model for assessment of these and other potential adverse outcomes is available in hemophilic dogs, which have a lifespan of 10 years or more (Arruda et al. 2001; Herzog et al. 2002). Thus the goal of producing a sustained, therapeutically useful elevation in circulating F.VIII or F.IX through a vector-mediated gene therapy strategy now more than ever appears within reach based on recent results in hemophilic animals.
In this review we present work done in hemophilia A, hemophilia B, VWD, and F.VII-deficient dogs and the contributions from that work that produced reliable assays for accurately diagnosing bleeding disorders and for monitoring correction of the hemostatic defect after replacement therapy and gene transfer. We review the history of testing the safety and efficacy of protein replacement and gene transfer. Finally, we discuss the strengths and limitations of these animal models and challenges to the development of improved therapy for patients with these inherited bleeding disorders.
Canine Hemophilia A
Hemophilia A is an inherited X-linked disorder caused by a deficiency of coagulation factor VIII (F.VIII) in humans and dogs (Hoyer 1994; Mannucci and Tuddenham 2001). A colony of hemophilia A dogs established in 1947 in Chapel Hill (North Carolina) appears to be the longest-maintained strain of a serious genetic disease in dogs (Graham et al. 1949). The prototype animals were Irish setters, but outbreeding was required to attain hybrid vigor. Both homozygous females and hemizygous males are maintained for breeding (Brinkhous and Graham 1950). The plasma level of F.VIII:Ag is <0.005 U/ml. As in humans, severe disease is associated with levels of F.VIII less than 1% of normal coagulant activity and the clinical severity is inversely proportional to the circulating F.VIII level in plasma. Likewise, spontaneous bleeding typically occurs in joints and soft tissues but can occur at any anatomic site.
Canine F.VIII cDNA and Identification of the Causative Molecular Defect
The isolation and characterization of canine F.VIII complementary DNA (cDNA1) by David Lillicrap’s team have advanced the field significantly (Cameron et al. 1998). The cDNA sequence identity is 77–92% similar to that of humans, mice, and pigs, and the domain structure is recapitulated with A1, A2, B, A3, C1, and C2 homologues present. Key functional motifs are preserved, including VWF binding sites, three thrombin cleavage sites, and the protein C cleavage site, and the six tyrosines known to be sulfated on human F.VIII are conserved. It is highly likely that the canine F.VIII expression is as complex as that of humans (Miao et al. 2004). Using the canine F.VIII cDNA, researchers found that both the Chapel Hill and Queen’s University (Ontario) strains of hemophilia A dogs have an intron 22 inversion (Hough et al. 2002; Lozier et al. 2002) and therefore appear to faithfully replicate a causative mutation present in about 40% of humans with severe hemophilia A (Antonarakis et al. 1995; Lakich et al. 1993; Naylor et al. 1993).
Development of Hemophilia A Dogs with Inhibitory Antibodies to F.VIII Activity
The development of inhibitory antibodies that neutralize the coagulant activity of F.VIII may be the most important adverse event during replacement therapy with F.VIII products (Tuddenham 2006). Such antibodies occur in approximately 20% of hemophilia A patients (Lozier 2004) and are more common in patients with severe hemophilia, especially those with complete deletion of the F.VIII gene (Oldenburg and Schwaab 2001). Most inhibitory anti-F.VIII antibodies form against the A1, A3, and C1/C2 regions (Astermark 2006), but treatment of inhibitors is difficult and controversial; thus there is a need for relevant animal models to study these issues (DiMichele 2007; Mannucci et al. 2006). Moreover, there appears to be a racial disparity, as African Americans and Latinos have a higher prevalence of inhibitors than Caucasians (Aledort and DiMichele 1998; Astermark 2006; Goudemand et al. 2006); in African-Americans the disparity may be due to the presence of rare polymorphic forms of F.VIII in this population (Howard et al. 2004).
Subsets of hemophilia A dogs with intron 22 inversion in both the Chapel Hill and Queen’s University strains develop inhibitors to canine F.VIII (Finn et al. 2008; Giles et al. 1984; Tinlin et al. 1993). This immunological phenotype makes these subsets of dogs more representative of the human hemophilia population, as inhibitors develop in some but not all humans with identical underlying mutations in the F.VIII gene (e.g., one of a pair of monozygotic twins, both of whom have hemophilia). Scientists do not know whether the A1, A3, and C1/C2 regions are the immunoepitopes for inhibitors in these hemophilia A dogs, which thus present a unique opportunity to define immunological mechanisms of their development, to explore other genetic modifiers of inhibitor development, and to test new preventive and therapeutic approaches including gene transfer (Finn et al. 2008).
Replacement Therapy
The use of plasma from hemophilia A dogs led to the development of several basic biochemical methods for preparing highly potent plasma-derived products for replacement therapy in hemophilia A (Brinkhous et al. 1952, 1956; Graham et al. 1949; Langdell et al. 1953). Testing in hemophilic and VWD dogs of the safety and hemostatic effectiveness of infusions of these different compounds during the developmental phase accurately predicted safe and successful translation to human medicine (Brinkhous et al. 1968; Wagner 1957; Wagner et al. 1968; Webster et al. 1965). Moreover, derivatives made from purified plasma F.VIII (Andersson et al. 1986; Brinkhous et al. 1985) or recombinant F.VIII without the B domain (making the F.VIII molecule considerably smaller) exhibited pharmacokinetics and pharmacodynamics similar to those of conventional plasma-derived F.VIII concentrates in hemophilia A dogs, including one with an inhibitor to F.VIII (Brinkhous et al. 2002). That a much smaller F.VIII molecule is fully functional has considerable significance for inclusion in gene therapy vectors that cannot accommodate large cDNAs and for the production of other variant molecules of F.VIII, such as the inactivation-resistant F.VIII (IR8) developed by Randal Kaufman and Steven Pipe (Nichols et al. 1999; Pipe and Kaufman 1997; Pipe et al. 1999). These findings indicate that development of smaller, fully functional, and nonimmunogenic versions of other large proteins such as dystrophin may be feasible.
Gene Therapy for Human Hemophilia A
There has been considerable progress in gene therapy for hemophilia A but several barriers remain (High 2005). Of the three gene therapy trials in human hemophilia A in the United States, the first used plasmid mediated transfection of autologous cells followed by reimplantation in 6 patients (Roth et al. 2001), the second a retroviral vector in 13 patients (Powell et al. 2003), and the third a “MiniAd” vector in one patient (White 2002). Both the nonviral transfection of autologous cells and the retroviral phase 1 trials have proven safe and a few patients had very low levels of F.VIII transiently detected. The MiniAd vector produced fever, thrombocytopenia, and elevation of liver enzymes. All three trials have been stopped due to low-level expression or vector toxicity. No inhibitor to F.VIII was detectable after gene transfer in these patients. Clearly, there is a need for additional preclinical and clinical studies to achieve successful gene therapy of hemophilia A in humans.
Gene Therapy for Canine Hemophilia A
In the following sections we review the history, limitations, and potential solutions for successful gene transfer strategies under study in hemophilia A dogs.
Organ Transplantation and Wild-Type Gene Therapy
“Wild-type” gene therapy entails the transfer of the gene of interest to a recipient host by transplanting the organ that normally expresses it. The possibility that hemophilia might be alleviated or even “cured” by wild-type gene therapy was first demonstrated more than 40 years ago in Chapel Hill and other strains of canine hemophilia A (Marchioro et al. 1969; Webster et al. 1967, 1971), when the transplantation of the spleen resulted in elevation of plasma F.VIII levels sufficient to change the phenotype from severe to mild (Webster et al. 1967). Liver transplantation was even more effective, normalizing (or nearly so) the F.VIII levels (Webster et al. 1971) and providing insights into F.VIII storage and release (Lamont and Ragni 2005). Although curative, this mode of gene therapy with the wild-type F.VIII gene is rarely used, because of the availability of effective plasma and recombinant products for replacement therapy, the limited number of liver donors, and, until relatively recently, the lack of effective antirejection therapy with acceptable levels of toxicity.
Retroviral Vectors
The Chapel Hill hemophilia A dogs were treated with B domain–deleted human F.VIII cDNA in a retroviral vector delivered via peripheral vein infusion in a dose escalation study (Greengard et al. 1997). All the dogs produced F.VIII activity, as evident in a shortening of the whole blood clotting time (WBCT1) from over an hour to between 12 and 16 minutes before development of antibodies to the human F. VIII. This study highlights the importance of using species-specific cDNA in gene therapy research in adult animals.
Katherine Ponder developed a gamma-retroviral vector expressing canine B domain–deleted F.VIII under the control of a hepatocyte-specific promoter (Xu et al. 2005). This vector was given to neonatal hemophilia A dogs within 72 hours of birth (6.71 to 8.71 × 109 transducing units (TU)/kg IV) which went on to express F.VIII activity over 100% for nearly 3 years. Quarterly measurements of liver enzymes revealed that they remained in the normal range during the study period. Interestingly,1-desamino-8-D-argininevasopressin(DDAVP1) increased von Willebrand factor but not F.VIII in these gene-treated dogs. These data suggest that the increase in plasma F. VIII in normal dogs after DDAVP is due to release of F.VIII synthesized by or taken up and stored in endothelial cells. These findings are consistent with recent data from hemophilia A liver transplant recipients that suggest that colocalization of F.VIII and VWF are necessary for F.VIII secretion after DDAVP (Lamont and Ragni 2005). Although these preclinical results are very encouraging, enthusiasm has been dampened by concerns of insertional mutagenesis with retroviral-based vectors (Hacein-Bey-Abina et al. 2003); several strategies have been proposed to address this issue (High 2005).
Adenoviral Vectors
The expression of high levels of a given transgene is a very attractive feature of adenoviral vectors that is offset by the toxicity associated with their use. To address this problem, there is now available a “MiniAdFVIII” construct that is a “gutless” adenovirus vector devoid of all viral genes and that carries the full-length human F.VIII (hF.VIII) cDNA under the control of the 12.5 kb human albumin promoter. In mouse models, the MiniAdFVIII vector directed expression of human F.VIII at therapeutic but gradually declining levels up to 1 year after a single intravenous injection (Balagué et al. 2000). For an assessment of the efficacy and safety of the MiniAdFVIII in larger animals, a hemophilia A dog received a single intravenous injection of the MiniAdFVIII at a dose of 3 × 1012 viral particles per kilogram; the WBCT decreased from a pre-treatment value of 24.5 minutes to a normal level of 11.5 minutes for several days, but human F.VIII activity was detected only transiently at levels 0.1 and 0.5%. Unfortunately, fever, thrombocytopenia, and elevation in liver transaminase levels were observed in this dog, as in nonhuman primates (Fang et al. 2000) and humans (White 2002). These undesirable side effects may be due to preexisting high antiadenoviral antibody titers, direct toxicity related to the innate immune response, and/or platelet-leukocyte-endothelial interactions mediated by VWF and P-selectin (Othman et al. 2007).
Researchers have pursued several strategies to reduce the toxicity associated with adenoviral vectors while retaining its capacity for high-level expression. Brendan Lee and Philip Ng have developed a helper-dependent (HD1) adenoviral vector that is devoid of all viral coding sequences and contains an expression cassette optimized for liver-restricted expression of canine B domain–deleted F.VIII (McCormack et al. 2006). Adenoviral vectors with reduced toxicity have also provided encouraging results in the hemophilia A dogs at Queen’s University (Brown et al. 2004; Chuah et al. 2003). Further study is required to determine whether successful reduction in the immune-mediated liver and hematological toxicities coupled with increasing expression of F.VIII is possible and whether it will allow exploitation of the persistent expression now documented with this vector (Arruda 2006).
Adeno-Associated Viral (AAV) Vectors
The approximately 8 kb cDNA of full-length F.VIII has presented a challenge because inserting sequences larger than 4.5 kb into adeno-associated viral (AAV1) vectors has been difficult. Researchers are addressing this issue with significantly smaller B domain–deleted canine F.VIII constructs that can be cloned into one AAV vector, as has been reported in mice (Chao et al. 2000; Ishiwata et al. 2006; Jiang et al. 2006a; Liu et al. 2004, 2008; Sarkar et al. 2004), as well as other single-chain constructs. An alternative approach originally described by Linda Couto (Burton et al. 1999; Scallan et al. 2003) has been to make two AAV vectors, one containing the F.VIII light chain and the other the F.VIII heavy chain, and to inject both concurrently. A potential practical limitation of this approach is the “chain imbalance” problem, the probable necessity of transducing a given cell with both vectors concurrently (Chen et al. 2007, 2009).
Recent reports have also shown that AAV8 and AAV9 may be more effective in male than female hemophilia A dogs at the same dose (1.52 × 1012 to 3 × 1013 GC/kg, portal vein) (Sabatino et al. 2007; Sarkar et al. 2006). The goal of ongoing studies of alternative AAV serotypes in hemophilia A dogs (Jiang et al. 2006a) is to determine the optimal AAV serotype, dosing, route of administration, and F.VIII cDNA construct as well as the effect of gender on gene transfer.
Lentiviral Vectors and Approaches
A key advance in gene therapy was the discovery that the use of lentiviral vectors can result in gene transfer to nondividing cells (Naldini et al. 1996). The development of unique packaging systems with self-inactivating vectors has facilitated use of this vector (Xu et al. 2001). Recently, the laboratories of David Wilcox, Mortimer Poncz, and Robert Montgomery used lentiviral transduction of hematopoietic cells to express F.VIII in platelets of hemophilia A mice with and without inhibitors; the researchers documented hemostatic efficacy (Gewirtz et al. 2008; Shi et al. 2003, 2006; Wilcox et al. 2003; Yarovoi et al. 2003) and novel insights into clotting dynamics (Neyman et al. 2008). Comparable studies are in progress in hemophilia A dogs.
Nonviral Methods and Combinations with Transposons
The use of plasmid DNA with various strategies including the Sleeping Beauty transposon system for stable integration (Ivics et al. 1997) has been successful in mice (Liu et al. 2006; Miao et al. 2006; Ohlfest et al. 2005; Ye et al. 2004). Comparable studies are planned with hemophilia A dogs.
Cell Therapy Using Blood Outgrowth Endothelial Cells
With the advent of techniques for isolating stem cells from a variety of sources, new avenues of therapy may become available and recent reports in mice are very encouraging (Matsui et al. 2007). The return of autologous cells to the hemophilic host after engineering them to produce antihemophilic factors remains an attractive approach (Follenzi et al. 2008). In addition, Robert Hebbel and colleagues have pioneered the technique of growing endothelium isolated from whole blood (Lin et al. 2000); these cultured cells are called blood outgrowth endothelial cells (BOECs1). This novel technique has opened the door for engineering BOECs with either nonviral or viral vectors, selecting clones that produce high levels of a transgene product, and reinfusing the transgene-producing cells into the original host. Monolayer cultures of BOECs are reproducible from human peripheral blood buffy coat mononuclear cells (Lin et al. 2000) and exhibit typical endothelial morphology and endothelial phenotypic markers including, among others, VWF, Weibel-Palade bodies, and adhesion cell molecules (Lin et al. 2000). These BOECs can be expanded from 20 starting cells to 1019 cells in 2 months. Thus, it is feasible to scale up to very large numbers of BOECs engineered to produce antihemophilic factors for infusion in hemophilic patients. When the Hebbel team gave NOD/SCID (non-obese diabetic/severe combined immunodeficiency) mice human BOECs engineered to produce human F.VIII, the animals expressed circulating levels from 10 to 200 ng/ml, or from 5% to over 100% of normal (Lin et al. 2002). The Hebbel team has successfully isolated BOECs from the Chapel Hill hemophilia A dogs for comparable experiments (Milbauer et al. 2008).
Canine Hemophilia B
Hemophilia B is an inherited X-linked disorder caused by a deficiency of F.IX. Characterization of the complete coding region for canine F.IX cDNA, completed in 1989 (Evans et al. 1989b), revealed that it is 86% conserved (compared to human F.IX) at the amino acid level. The leader peptide, Gla domain, epidermal growth factor (EGF) domains, and carboxy-terminal portion of the heavy chains have extensive sequence conservation between dogs and humans. All glutamic acid (Glu) residues undergoing gamma-carboxylation in humans are conserved in dogs. The 1989 study provided the necessary tools for identification of the molecular defects in several strains of hemophilia B dogs (Brooks et al. 1997, 2003; Evans et al. 1989a; Gu et al. 1999; Mauser et al. 1996). Two strains have been used extensively in gene therapy studies: one with a deletion mutation in Lhasa Apso dogs that are prone to develop inhibitory antibodies to infused canine F. IX, reported in 1996 from Auburn University in Alabama (recently moved to the University of Alabama at Birmingham, UAB; Mauser et al. 1996), and one with a missense mutation that does not develop inhibitory antibodies to infused canine F.IX, maintained in the Chapel Hill dogs since 1966 (Evans et al. 1989a). As in severe hemophilia B humans, the hemophilia B dogs from Auburn/UAB and Chapel Hill have a severe bleeder phenotype manifested by frequent spontaneous bleeds into joints and soft tissues. The well-described phenotype and genotype of these hemophilia B dogs make them very desirable for studying the pathophysiology of hemophilia B and for testing replacement therapies and gene therapy strategies.
Replacement Therapy with Recombinant F.IX
Advances in bioengineering have made possible the production in serum-free media of highly concentrated preparations of recombinant human F.IX (rF.IX1) (Brinkhous et al. 1996). Both rF.IX and plasma-derived human F.IX (pdF.IX) corrected the hemostatic defect in hemophilia B dogs in a dose-dependent fashion. The pharmacokinetic parameters and the immune response were the same for the two products. The percent recovery of recombinant F.IX (rF.IX) was somewhat lower than for pdF.IX, but the values of both products were less than half of the predicted levels. Lower recovery was subsequently seen in humans as well, especially in the pediatric population (Shapiro et al. 2005). In addition, a prophylactic regimen with daily administration of rF.IX resulted in a continuous therapeutic level of plasma F.IX that gradually rose for 5 days, with a twofold increase in recovery by day 5. The mechanism of the high recovery of the prophylactic regimen may be related to the degree of saturation of the vascular endothelial binding sites and to the altered dynamics of the balance of intravascular and extravascular compartments (Cheung et al. 1996; Schuettrumpf et al. 2005; Wolberg et al. 1997).
The pharmacodynamics and pharmacokinetics of various isoforms of F.IX have also been determined in these dogs. First, rF.IX deficient in gamma-carboxylated glutamic acid residues 36 and 40 was shown to have in vivo hemostatic efficacy and pharmacodynamics comparable to fully carboxylated F.IX. The discovery that gamma-carboxylation of Glu36 and Glu40 is not essential for in vitro clotting activity has important implications for therapy with recombinant products that may not be fully gamma-carboxylated (Gillis et al. 1997). Moreover, F.IX produced by gene therapy in extrahepatic sites may target cells with limited capacity for gamma-carboxylation (Wu et al. 1997). Second, high molecular weight (Mr) F.IX aggregates in pdF.IX products do not contribute substantially to F.IX activity in vivo and their absence from rF.IX does not alter its efficacy in vivo (Sigman et al. 1997). Third, newly constructed chimeric molecules of several coagulation factors have enhanced activity when compared to their wild-type form (Chang et al. 1997). In Chapel Hill, Jen-Yea Chang and colleagues made a chimeric F.IX molecule by replacing the first epidermal growth factor–like domain with that of F.VII. The resulting recombinant chimeric molecule, factor IXVIIEGF1, had at least a twofold increase in functional activity in the one-stage clotting assay when compared to recombinant wild-type F. IX, at least a threefold increase in clotting activity, and a biological half-life equivalent to recombinant wild-type F.IX when infused in hemophilia B dogs (Chang et al. 1997). The marked increased function of factor IXVIIEGF1 in vivo suggests that if this chimera or other F.IX variants (Pipe 2004; Schuettrumpf et al. 2005; Yan et al. 2006) were expressed even at low levels by existing gene therapy methods and compared to wild-type F.IX similarly expressed, they could produce a greater biological and perhaps “curative” effect. Whether these variant molecules might result in increased immunogenicity requires further study.
Extravascular Administration of F.IX
High-purity human plasma–derived and recombinant F.IX formulations have been exploited to investigate the disease mechanism and correction of hemophilia B. Standard clinical practice is to administer F.IX formulations intravenously with the attendant difficulties of establishing vascular access. Attempts to circumvent these limitations include extravascular administration of F.IX via intraperitoneal (IP), subcutaneous (SC), intramuscular (IM), and intratracheal (IT) routes of administration (Liles et al. 1997; McCarthy et al. 2002; Russell et al. 2001). The data from such efforts show that significant levels of F.IX may be obtained via extravascular injection in hemophilia B subjects, and indicate the importance of routes of administration in clinical uses of F.IX.
This work has direct bearing on gene therapy of hemophilia B with techniques that deliver F.IX extravascularly into the circulation, although a potential pitfall of such administration could be increased immunogenicity of the therapeutic protein. An adult male hemophilia B patient with a missense mutation in the signal peptide domain that substitutes a proline for leucine at position −24 received high-purity human plasma–derived F.IX subcutaneously under an approved protocol in the Clinical Research Unit at the University of North Carolina (Liles et al. 1997). Many months later, this same patient received IV treatment with F.IX for bleeding associated with a traumatic injury and recombinant F.IX in a clinical protocol that required monthly Bethesda assays. He subsequently developed a transient, low-titer inhibitor (1 to 2 Bethesda inhibitor units) but currently, even after many repeated exposures to rF.IX, has no detectable inhibitor. This patient had received extensive treatment in the past and had not shown evidence of developing an inhibitor, although he had not been as rigorously monitored as he was in this protocol.
This case raises the issue of whether extravascular administration of F.IX could result in an immune response with greater frequency than intravascular administration. Because host response can vary considerably, we offer several observations. First, none of the humans in the IM gene therapy trial developed an inhibitor (Manno et al. 2003). Second, in our canine model of hemophilia B we have detected functional canine F.IX that has traversed the extravascular space to reach the circulation in a series of gene therapy protocols that express canine F.IX from muscle; however, in some cases, inhibitory antibodies to canine F.IX formed and appear to be related to dosing and AAV serotype (Arruda et al. 2001, 2004a,b,c, 2005; Herzog et al. 2002, 1999). This information helped guide dosing in the human IM gene therapy trial in which none of eight patients developed an inhibitor (Manno et al. 2003). Third, many patients probably receive some F.IX in the extravascular space when IV lines infiltrate, a common occurrence. Whether hemophilic patients are more likely to develop an inhibitor with extravasation of F.IX is unknown. Fourth, Darrel Stafford, Ronald Heimark, and David Stern documented that F.IX binds to type IV collagen via lysine at position 5 or valine at position 10, identifying the mechanism(s) of extravascular and endothelial binding of F.IX (Cheung et al. 1996; Heimark and Schwartz 1983; Stern et al. 1987; Wolberg et al. 1997). Moreover, a recent study showed that factor IX variants that do not have these sites increase circulating levels of F.IX and thus improve gene therapy efficacy in mice (Schuettrumpf et al. 2005).
Considered together with our data on the extravascular administration of F.IX, it appears that F.IX can traverse not only from the extravascular space to the systemic circulation but also from the latter to the former (Zauber and Levin 1977). The exact function of F.IX in the extravascular space is unknown but likely prevents the bleeding into joints (Sun et al. 2008) and soft tissues characteristic of hemophilia B patients. The extravascular route of administration of F.IX warrants further investigation.
Induction of Tolerance to Recombinant Human F.IX (rF.IX) in Hemophilia B Dogs
The therapeutic potential and antigenicity of subcutaneous administration of F.IX were the focus of a study designed to induce tolerance to human F.IX in hemophilia B dogs (Russell et al. 2003). Administration (IV, IM, or SC) of rF.IX to adult hemophilia B dogs corrects the coagulopathy until inhibitory antibodies form to the xenoprotein. In our experience, the antibody response appears to be independent of the route of administration, thus allowing us to determine whether rF.IX, delivered subcutaneously, could induce immune tolerance under the correct experimental conditions.
We chose neonatal hemophilia B puppies to receive rF.IX daily (82.6 IU/kg or 1650 IU/kg) or thrice-weekly (82.6 IU/kg) SC injections from day 2 after birth (SC injections are tolerated well and circumvent the challenges associated with venipuncture) and achieved four goals in this study. First, five of eight hemophilia B dogs were tolerized to rF.IX. Second, the tolerized dogs thrived for over 4 years including interruptions in therapy, IV challenges, gestation and parturition, and surgical procedures. Third, the SC route and the dose shortened the WBCT and the activated partial thromboplastin time (aPTT1), and yielded F.IX antigen levels of approximately 1%. Fourth, and most importantly, our data show that sustained prophylactic administration of rF.IX to tolerized hemophilia B dogs significantly reduced the incidence of spontaneous bleeding events from an average of six bleeds per year to one per year (p < 0.05). This significant reduction in bleeding events is consistent with an improvement in phenotype. Moreover, these data underscore the advantages of prophylactic therapy for decreasing hemorrhages and associated complications in an animal model, and they support consideration of subcutaneous administration as an alternative to IV infusions if proven safe and efficacious in clinical trials in humans with hemophilia B.
Gene Therapy for Hemophilia B
Organ Transplantation and Wild-Type Gene Therapy
Following the “cure” of canine hemophilia A by liver and spleen transplantation discussed above, researchers demonstrated this same beneficial effect in the Chapel Hill strain of hemophilia B dogs (Webster et al. 1974). While this approach is feasible in humans, it is not the first choice of therapy given the quality of available recombinant F.IX for replacement therapy (Brinkhous et al. 1996). Nonetheless, the successful treatment of canine and human hemophilia B by liver transplantation makes this approach reasonable to consider in hemophilia B patients with severe liver damage from hepatitis or with nonmetastatic liver cancer.
Retroviral Vectors and Gene Therapy
Studies using the retroviral vectors created by Inder Verma at the Salk Institute, Savio Woo at Mt. Sinai Medical School, Mark Kay at Stanford University, and Kathy Ponder at Washington University in St. Louis illustrate both the advantages and the limitations of retroviral vectors in gene transfer. The advantages are that the vectors are replication-incompetent, have the ability to transduce a wide range of cells (including hepatocytes), and undergo permanent integration into the host genome, allowing for long-term expression of the transgene. Furthermore, it is possible to greatly increase the titer of the retroviral construct with the use of packaging cell lines that furnish essential DNA sequences deleted from the replication-deficient virus. The two practical limitations of early retroviral vectors are that they can infect only dividing cells and can accommodate an insert size of only about 7 kb.
In 1990, Inder Verma and Kenneth Brinkhous successfully accomplished gene therapy at the cellular level in the Chapel Hill hemophilia B dogs (Axelrod et al. 1990). Hemophilic and normal fibroblasts transduced with a retroviral construct produced functional F.IX and, with IP injection, resulted in the transient appearance of low levels of canine F.IX in the bloodstream (ex vivo gene therapy) (Lozier and Brinkhous 1994).
The first successful long-term somatic cell gene therapy in a large animal model was in the same strain of hemophilia B dogs in 1993 by Kay, Woo, and Brinkhous, resulting in a change in phenotype from severe hemophilia to a less severe form (Kay et al. 1993). The procedure consisted of conditioning hepatocytes by partial hepatectomy to establish a proliferative phase. With injection directly into the portal venous system, the vector that included the moloney retroviral long terminal repeat (LTR) as the promoter transduced an estimated 0.3–1.0% of the hepatocytes, which then expressed canine F.IX. Low but easily detectable levels of plasma F.IX (about 2–8 ng/ml) caused the whole blood clotting time to decrease from nearly 50 minutes to as low as 15 minutes, a distinct change in phenotype. The effect lasted more than 3 years. Two types of control hemophilia B animals were used in the protocol in addition to the experimental animals: (1) partial hepatectomy with prophylactic F.IX administration but without vector, and (2) partial hepatectomy with infusion of the retroviral vector without canine F.IX cDNA. In both cases, the WBCT, which shortened after the procedure due to administration of blood products, returned to that of the hemophilic state in 30 days. Most importantly, this study demonstrated the feasibility of in vivo retroviral-mediated gene transfer into the liver of a hemophilic animal, with improvement in the deficiency state (Kay et al. 1993).
Kathy Ponder has developed an alternative approach to retroviral gene therapy that circumvents the need for partial hepatectomy by exploiting the higher level of hepatocyte replication during the neonatal period (Xu et al. 2003). A murine leukemia virus (MLV)–based retroviral vector containing a strong liver-specific human alpha 1-antitrypsin promoter, the canine F.IX cDNA, and the Woodchuck hepatitis virus post-transcriptional regulatory element was generated and tested in very young mice with and without hepatocyte growth factor (HGF). The canine F.IX (cF.IX) antigen levels were over 100% with and without HGF. With these encouraging data, neonatal hemophilia B puppies were injected with comparable doses of this retroviral vector (7.84 × 109 to 1.27 × 1010 transducing units [TU]/kg IV), resulting in marked shortening of the puppies’ WBCT from over 60 minutes to between 8 and 12 minutes, shortening of the aPTT, and levels of F.IX antigen of about 10% (500 ng/ml). One puppy died of unrelated causes (pyelonephritis, sepsis, and acute renal failure). This was the first demonstration that high levels of F.IX activity are achievable with a retrovirus but without a partial hepatectomy or liver injury to induce hepatocyte replication. The Ponder laboratory has now extended this work to incorporate human F.IX cDNA into the retroviral vector cassette and has shown the feasibility of both long-term expression of therapeutic levels of human F.IX and tolerance to the xenoprotein (Xu et al. 2007). Remarkably, these tolerized hemophilia B dogs have been exposed to human F.IX intravenously several times and have not developed inhibitory antibodies.
This vector system is essentially the same as discussed above for hemophilia A (Xu et al. 2005). Concerns about the risk of insertional mutagenesis with retroviruses persist (Hacein-Bey-Abina et al. 2003), but these encouraging results support the pursuit of strategies to address these obstacles (High 2005) as well as consideration of alternative designs of clinical trials in the pediatric population (Monahan 2007).
Adenoviral Vectors
Investigators have reviewed many aspects of the use of replication-deficient recombinant adenoviruses for gene transfer (Arruda 2006; Connelly and Kaleko 1997; Eisensmith and Woo 1997; Lozier et al. 2002; Pasi 2001) and have extensively characterized the human adenoviral prototypes of subgroup C, Ad2 and Ad5, both biochemically and genetically. The first successful result with somatic cell gene therapy in canine hemophilia B used an Ad5 construct containing the canine F.IX cDNA (Kay et al. 1994). A prompt and vigorous response was observed with F.IX plasma levels reaching normal or supernormal levels for about a week, followed by a slow return to pretreatment or baseline levels in 70 to 100 days.
The success of adenoviral-mediated gene therapy for hemophilia B has been limited by the host immune response to the adenoviral proteins as it is quite complex and variable among species. The adenoviral vector activates specific cytotoxic T lymphocytes (CTLs), T helper cells (both Th1 and Th2 subsets), and B cells, leading to inflammation, the destruction of infected tissues, and the cessation of transgene expression (Arruda 2006). The hypothesis that suppression of the host immune response would prolong transgene expression was tested by two methods. First, hemophilia B dogs received the canine F.IX adenoviral vector and an immunosuppressive agent, cyclosporin A, concomitantly (Fang et al. 1995). Cyclosporin A significantly increased the persistence of transgene expression after adenovirus-mediated transfer in vivo, and discontinuation of the drug resulted in attenuation of transgene expression. Second, CTLA4-Ig was equally effective in prolonging transgene expression (unpublished data, Kay, Read, and Nichols). Next, the recombinant adenoviral vector was modified to decrease its immunogenicity. No significant differences in the duration of transgene expression were observed in this animal model when the temperature-sensitive 125 (ts125) mutation was compared to the same transgene without the ts125 mutation in hemophilia B dogs (Fang et al. 1996). Thus, the immune response to this series of adenoviral vectors is clearly a key factor that limits its use for gene therapy in the hemophilia B dogs.
Exciting and important recent developments have been reported from Kay, Ng, Lee, Art Beaudet, and researchers in other laboratories using the helper-dependent adenoviral constructs that encode few to no viral proteins (Hardy et al. 1997; Kochanek et al. 1996; Lieber et al. 1996a,b; Schiedner et al. 1998; also discussed above under gene therapy for hemophilia A). Mark Kay and Anja Ehrhardt have constructed an HD adenoviral vector that contains the complete human F.IX cDNA, some of the intronic sequences, and the complete 3′ untranslated region (Ehrhardt and Kay 2002). They directly compared this HD vector in mice to a first-generation adenoviral vector using the same expression cassettes and found that the level of F.IX expression was comparable between the two vectors. However, the HD adenoviral vector was less hepatotoxic and had an in vivo dose threshold effect about fourfold lower than the first-generation adenoviral vector. Kay and colleagues substituted canine for human F.IX cDNA in this vector and injected it into the Chapel Hill hemophilia B dogs by peripheral vein at 8.6 × 1011 viral particles (vp)/kg (Ehrhardt et al. 2003). In contrast to other studies using first- or second-generation adenoviral vectors, these dogs expressed therapeutic levels of F.IX for up to 2 months, exhibited no hepatotoxicity as judged by liver enzymes and liver histology, and had no drop in platelet counts. The level of F.IX declined over time but persisted for up to 4½ months with no detectable Bethesda inhibitor. Kay and Ehrhardt have very recently developed and are testing a combination of an HD adenoviral canine F.IX vector construct to support high-level transgene expression and a Sleeping Beauty transposase vector that integrates into the host genome (Ehrhardt et al. 2006; Ivics et al. 1997). Ng and Beaudet have been able to extend these observations with an HD adenoviral construct that induced transient mild liver enzyme elevations and a decrease in platelet count but exhibited sustained expression for over a year (1 × 1011, 3.57 × 1012, and 1.3 × 1013 vp/kg) (Brunetti-Pierri et al. 2005).
These studies demonstrate that injection of an HD adenoviral vector results in complete but transient phenotypic correction of F.IX deficiency in canine models with little or no detectable toxicity, a marked improvement over earlier adenoviral vectors. In addition, sustained expression with nonintegrating vectors is an important achievement that addresses safety concerns about integrating vectors (Donsante et al. 2007; Hacein-Bey-Abina et al. 2003).
Adeno-Associated Viral Vectors
The most significant advances in gene therapy for hemophilia B have arguably come from use of the adeno-associated virus (Samulski et al. 1991). Gene transfer studies in mice, dogs, nonhuman primates, and humans have identified the immune response to the expressed transgenic F.IX and the AAV vector as the most significant barriers to successful gene transfer for hemophilia B. With respect to the risk of inhibitor formation to the expressed transgenic F.IX, these studies have identified the following risk factors: the underlying F.IX gene mutation in the host, vector dose per kilogram of recipient, vector dose per site, and route of administration. The development of reagents that rigorously characterize the canine immune response have enabled the identification of operative mechanisms in the hemophilia B dogs as well as the development of methods for preventing inhibitor formation. With respect to the vector capsid, immune responses have been observed in humans after gene transfer to the liver but not in any other species (Manno et al. 2006).
AAV has a broad host range for infectivity (e.g., humans, monkeys, dogs, mice) when coinfected with the appropriate helper. A number of features of AAV make it a promising candidate for somatic gene delivery in patients: (1) it is ubiquitous in humans, although it causes no known disease; (2) recent improvements in vector manufacturing techniques provide more concentrated and purer preps than previously available (Matsushita et al. 1998; Xiao et al. 1998); (3) it is predominantly nonintegrating, reducing the risk of insertional mutagenesis (Nakai et al. 2001; Niemeyer et al. 2008), although debate continues about its use in mice (Donsante et al. 2001, 2007; Han et al. 2008; Schultz and Chamberlain 2008); (4) it efficiently transduces nonproliferating target cells; and (5) at least 12 serotypes of AAV have been characterized (Gao et al. 2002). The availability of different serotypes has provided an opportunity to re-treat or readminister AAV vectors to animals that exhibited a strong neutralizing antibody response after initial treatment with a given serotype, and it is reasonable to speculate that the different AAV serotypes will afford humans the same possibility of retreatment. The ability to infect nonproliferating cells is of particular importance for gene therapy of hemophilia because of the terminally differentiated properties of most desirable target cells. The availability of an infectious recombinant AAV (rAAV1) clone and a helper-free packaging system have provided a manipulatable gene transfer vehicle that has tremendous potential in the field of eukaryotic viral vectors. Indeed, there is growing enthusiasm in the gene transfer field for the role of AAV as a vector for genetic diseases.
Work in several laboratories has established muscle and liver as promising platforms for rAAV vector–mediated gene therapy of canine hemophilia B (Herzog et al. 1999; Snyder et al. 1997; Xiao et al. 1996). The use of rAAV vectors to express canine F.IX in hemophilia B dogs has been exciting and informative (Chao et al. 1999; Herzog et al. 1999; Monahan et al. 1998; Mount et al. 2002; Snyder et al. 1999; Wang et al. 2000). Intramuscular, portal vein, hepatic artery, and peripheral vein administration of various constructs have successfully induced expression of F.IX activity (Arruda et al. 2004a,b,c, 2005; Harding et al. 2004; Herzog et al. 2002; Mount et al. 2002; Wang et al. 2005). The dogs tolerated all the routes well, but there are important differences in transgene expression that are not yet fully understood. For example, portal vein infusion in mice appears to yield higher levels of F.IX than similar IM doses (Nathwani et al. 2001). The same may be true in dogs, but differences in constructs, species-dependent promoter efficiency, transit to the circulation, or other factors make direct comparisons difficult (High 2005). A second example was the subject of collaborations between the High laboratory and the Chapel Hill and Auburn/UAB hemophilia B colonies (Herzog et al. 2002; Mount et al. 2002). In the animals that received IM injections, expression of F.IX has been reproducible and dose-dependent; the range has been from 3 to over 200 ng/ml using doses from 1011 to 1013 vp/kg. Unexpectedly, inhibitory anticanine F.IX antibodies (IgG1 and IgG2) formed in the Chapel Hill strain that were low-titer, self-limited, and preventable by immunomodulation with cyclophosphamide (Herzog et al. 2001, 2002). The vector dose per injected site appeared to be the strongest predictor of anti-F.IX antibody formation. The associated proliferative responses and cytokine production were documented when peripheral blood mononuclear cells were restimulated in vitro with canine F.IX antigen. These data were consistent with T cell–dependent anticanine F.IX antibody formation. Importantly, these studies showed that the Chapel Hill strain can be used to evaluate gene transfer strategies for their potential to activate F.IX-specific B and T cell responses. The animals that received rAAV by portal vein injection exhibited a dose-dependent effect in a range similar to that seen with the IM approach but without anti-F.IX antibody formation. Considered together, these studies provide evidence that liver-based gene therapy may be less likely to generate an inhibitory antibody to F.IX, a feared complication of any form of hemophilia therapy.
These encouraging preclinical data in multiple animal models strongly supported muscle- and liver-based gene transfer trials with AAV vectors in humans. The intramuscular trial tested for safety, which was documented at the expense of dosing for efficacy (Manno et al. 2003). Important and useful dosing information was obtained in the Chapel Hill hemophilia B dogs as described above (Herzog et al. 2002), and the ultimate doses of rAAV used in the human trials were chosen after consideration of these data and the underlying mutations in the hemophilia B dogs. Based on studies in hemophilic dogs, the initial muscle trial was limited to subjects whose disease was due to a missense mutation (i.e., the trial excluded those with nonsense mutations or gene deletions). None of the eight patients who received IM gene therapy has shown evidence of an inhibitor to date (TCN personal communication with High, Kay, and Manno, December 2008) or any other significant side effects (Kay et al. 2000; Manno et al. 2003). Muscle biopsies of injection sites performed 2 to 42 months after vector administration confirmed gene transfer (as evidenced by Southern blot) and transgene expression (as evidenced by immunohistochemical staining) (Jiang et al. 2006b). Preexisting high-titer antibodies to AAV did not prevent gene transfer or expression. Plasma levels of F.IX were mostly less than 1–2% of normal. The vector was administered by separate needle injections and higher dosing seemed impractical; in addition, studies in the dogs suggested that higher doses might induce inhibitor formation (Herzog et al. 2002).
The liver-based trial was initially promising and exciting as it resulted in F.IX levels of 10–12% (Manno et al. 2006). Unfortunately, there was a gradual decline in F.IX accompanied by a transient asymptomatic elevation of liver transaminases that resolved without treatment, both of which were associated with expansion of AAV capsid-specific CD8+ T cells in the circulation. The increased presence of these cells suggested that the transduced hepatocytes were being destroyed by cell-mediated immunity-targeting antigens of the AAV capsid, an event that was not predicted by the preclinical studies in any other species. Further studies are in progress in the hemophilic dogs to develop a model of this immune response. Future liver-based studies in humans may require optimization of the expression cassette (Wu et al. 2008) or immunomodulation to achieve long-term expression. The US Food and Drug Administration (FDA) has recently approved an immunomodulation protocol.
There are two additional important areas of rAAV research in the hemophilia B dogs. The first uses an isolated limb perfusion approach that has been shown to dramatically increase the level of muscle transduction with rAAV (Greelish et al. 1999). Valder Arruda and Hansell Stedman have performed both antegrade infusion with drugs that are vasodilating (i.e., papaverine, FDA-approved for human use) and vascular-permabilizing (i.e., histamine, not FDA-approved for human use) (Arruda et al. 2005) and retrograde infusion without such drugs (Arruda et al. 2004). While transient immune suppression may still be necessary, the early results are very encouraging. The second area uses alternate serotypes or packaging of rAAV to augment gene expression. Results obtained by the High laboratory do indeed show that AAV-1 in the hemophilia B dogs resulted in approximately fiftyfold higher levels of expression but that these came at the cost of increased inhibitor formation in mice and dogs (maximum dose 1 × 1012 vp/kg IM) (Arruda et al. 2004b). The increase in transgene expression may be due to a higher gene copy number and a larger number of cells transduced at each injection site. Studies by the Wilson and Wang laboratory with hybrid AAV 2/7, 2/8, and 2/9 have also been encouraging (3.34 to 5.26 × 1012 genome copies (GC)/kg, portal vein) (Wang et al. 2005). Moreover, hemophilia B dogs that had received treatment with AAV2 vectors were successfully transduced with alternate serotypes with apparent additive effect on the level of transgene expression (Wang et al. 2005). The magnitude of difference, the effect of gender on transgene expression, and the potential for adverse outcomes (e.g., inhibitor formation, germline transmission) are actively being studied with these alternate serotypes and packaging systems.
Remarkably, stable expression has persisted since 1998 without detectable toxicity in the liver and muscle of the Chapel Hill strain (unpublished data, High, Kay, Verma, and Nichols) and for several years in the liver of the Auburn/UAB strain (Niemeyer et al. 2008). These observations are largely based on monitoring of the WBCT and aPTT and do not exclude a subtle decrease or increase in transgene expression that would be detected by specific antigen or coagulation factor assays. The titer of anti-AAV antibodies has not been systematically characterized. Persistent AAV-mediated gene expression in muscle was expected given the low rate of myocyte turnover. Since AAV is predominantly a nonintegrating vector, persistent expression is somewhat unexpected in liver given that hepatocytes have a higher rate of replication. In spite of these predictions, prolonged transgene expression over 10 years was unexpected but a highly desirable outcome that suggests long-term exposure to AAV gene therapy is safe in dogs. These observations are particularly important when considering the occurrence of hepatocellular carcinoma and angiosarcomas in mice with the lysosomal storage disease mucopolysaccharidosis type VII (MPS VII) after treatment with rAAV vectors (Donsante et al. 2001, 2007; for a detailed discussion of lysosomal storage diseases, see Haskins 2009). Insertional mutagenesis is probably the operative mechanism but other possibilities include overexpression of a human transgene in rodents and colony contamination by oncogenic viruses. To address this issue we are screening all AAV-treated Chapel Hill hemophilia B dogs for evidence of tumor formation or other pathology at the sites of gene transfer (unpublished findings, Nichols, High, and Arruda). The availability of relevant animal models that survive for 10 years after gene therapy with a species-specific transgene has proven invaluable to assess toxicity over time.
Finally, the potential for germline transmission through IM administration of rAAV serotype 2 vectors was assessed to be quite low in sperm (Arruda et al. 2001; Schuettrumpf et al. 2005). Among the four species tested in this study, mice, rats, and rabbits were positive whereas dogs were negative for vector in semen between 7 and 90 days after gene transfer. Vector is detectable in hemophilic dog serum and saliva, urine, tears, and feces for 1 or 2 days after gene transfer using a sensitive reverse transcriptase-polymerase chain reaction (RT-PCR) method (unpublished data, High, Arruda, and Nichols); we have not collected semen from these dogs in this early, 1- or 2-day time frame. Notably, six of seven human subjects treated in the liver-based trial have shed vector in semen briefly after gene transfer (Manno et al. 2006). In 2002, theRecombinant DNA Advisory Committee (2002) and the FDA determined that the transient shedding of rAAV vector was an acceptable risk.
Lentiviral Vectors Expressing Canine F.IX
Although the development of lentiviral vectors has made possible the transduction of nonproliferative or quiescent liver cells (Kafri et al. 1997), the Kay laboratory has shown that hepatocyte proliferation augments the effect of lentiviral gene therapy (Park et al. 2000). Their study used human immunodeficiency virus (HIV)–based lentiviral vectors containing an EF1alpha enhancer/promoter driving human factor IX (hF.IX) cDNA expression for portal vein injection into C57Bl/6 mice. A dose escalation study produced a sustained increase in serum hF.IX levels to approximately 50–60 ng/mL. Importantly, partial hepatectomy resulted in a significant increase in hF.IX, up to 350 ng/mL compared with the nonhepatectomized mice. Other vector cassettes are being tested as are methods of inducing hepatocyte division. The most efficient vector(s) will have canine F.IX cDNA inserted for studies in hemophilia B dogs.
Lentiviral vectors expressing F.IX from hepatocyte-specific promoters failed to achieve sustained F.IX expression in hemophilia B mice in part because of the induction of an anti-F.IX cellular immune response (Follenzi et al. 2004). Further analysis suggested that this may be a result of off-target transgene expression in hematopoietic-lineage cells of the spleen. In order to overcome this problem, the Naldini laboratory modified this vector to contain a target sequence for the hematopoietic-specific microRNA, termed mir-142-3p (Brown et al. 2006). Addition of this microRNA eliminated off-target expression in hematopoietic cells and enabled sustained gene transfer in hemophilia B mice for more than 280 days after injection. Treated mice had over 10% of normal F.IX activity, no detectable anti-F.IX antibodies, and shortened tail-clip bleeding times, and they were unresponsive to F.IX immunization (Brown et al. 2007). Plans are under way to extend these studies in the hemophilic dogs.
Nonviral Methods for Expressing F.IX
A variety of nonviral methods have been tried in cell lines from the hemophilia B dogs. First was the development of molecular conjugate vectors containing DNA sequences that do not have a size limitation, in contrast to the retroviral, adenoviral, and rAAV vectors (Cristiano et al. 1993; Lozier et al. 1994). Second, RNA/DNA oligonucleotides that are chemically modified to be nuclease resistant underwent testing by Betsy Kren and Clifford Steer at the University of Minnesota (Kren et al. 1998) and Carol Miao at the University of Washington (Miao et al. 1999, 2000). Both groups used RNA/DNA oligonucleotides containing a G insertion at nucleotide 1477 to transfect cultured canine hemophilia B hepatocytes. At present, results from these nonviral methods do not justify in vivo experiments in hemophilia B dogs (De Meyer et al. 2007; Zhang et al. 1998). Third, a study in mice showed that electroporation induces pores in cells that allow materials to enter until the pores seal; in vivo electroporation immediately after IM plasmid delivery leads to levels of transgene expression that are 100- to 1000-fold greater than those possible with direct plasmid injection alone using reporter genes (Fewell et al. 2001). Based on these encouraging results in mice, we injected our hemophilia B dogs with comparable doses of canine F.IX (cF.IX) plasmid with electroporation (Fewell et al. 2000). Correction of WBCT was evident in all treated animals 1 day after injection but was transient (lasting about 10 days) due to the development of Bethesda inhibitors (1 to 5 BU) that persisted for up to 3 months. Immunomodulation delayed but did not prevent the development of anti-cF.IX antibodies. These data show that plasmid gene delivery augmented by electroporation may temporarily improve gene transfer, but that the immune response to the transgene after electroporation is a critical barrier to surmount.
Cell Therapy: Ex Vivo Gene Therapy with Transduced Hemophilic Cells
The return of modified cells to the body is an important point to consider for ex vivo gene therapy, but it is more challenging than one might initially imagine. The chief difficulty lies in establishing a support matrix and blood supply to sustain the implanted cells and deliver the desired coagulant factor to the bloodstream (reimplantation is not a problem for transduced blood or marrow cells, which can be returned directly to the bloodstream). Instead of implantation of transduced cells directly into tissues, several researchers have proposed the installation of subcutaneous capsules or ports that have semipermeable membranes and contain cultures of transduced cells producing antihemophilic protein (Chang et al. 1993; Kingdon et al. 1993; Lozier et al. 1991). These methods (or possibly BOECs as discussed above under hemophilia A) may be effective with canine hemophilic cells transduced with canine F.IX when the systems are optimized.
Canine von Willebrand Factor (VWF) and von Willebrand Disease (VWD)
Von Willebrand factor (VWF) has several roles in hemostasis and thrombosis in humans, dogs, and pigs, associated in part with its compartmentalization or cellular source (Sadler 2005). It is synthesized not only in endothelial cells that store and secrete the protein into the subendothelium and plasma (Ruggeri and Ware 1993; Wu et al. 1987) but also in megakaryocytes, and is present in human and porcine platelet alpha-granules (Bowie et al. 1986; Cramer et al. 1986; Koutts et al. 1978; Sporn et al. 1985). Studies in both humans and swine have shown that bleeding time prolongation is associated with decreased platelet VWF with or without changes in plasma VWF (Bowie et al. 1986; Gralnick et al. 1986; Nichols et al. 1995; Parker et al. 1992).2 Dogs that have no platelet VWF but normal plasma and endothelial VWF have normal bleeding times (Nichols et al. 1993) and, in contrast to humans and pigs with von Willebrand disease (VWD), infusion of canine VWF into VWD dogs nearly normalizes bleeding time (Nichols et al. 1993). Thus, bleeding time, as a measure of hemostasis, appears to have species specificity related to its distribution in plasma, platelets, and endothelium.
VWF has no known enzymatic activity but serves as a carrier for F.VIII in plasma in humans, pigs, and dogs and may have cofactor activity accelerating thrombin-catalyzed cleavage of F.VIII light chain (Hill-Eubanks and Lollar 1990). The VWF carrier function protects F.VIII from degradation by activated protein C and may provide for the delivery of F.VIII to sites of arterial injury (Koedam et al. 1988). Such events could both localize F.VIII activity to sites of VWF attachment on exposed subendothelium and promote thrombosis. However, BB3-BD5, a neutralizing monoclonal antibody raised to purified porcine VWF, does not affect F. VIII activity but interrupts VWF-dependent arterial thrombosis in pigs (Bellinger et al. 1987). In addition, dogs with hemophilia A (i.e., no detectable F.VIII but normal VWF) form occlusive arterial thromboses at sites of arterial stenosis and injury as readily as normal dogs (Nichols et al. 1993). Likewise, human subjects with hemophilia A also have no detectable F.VIII and normal VWF, but it is not yet clear whether they are protected from myocardial infarction (i.e., thrombosis in atherosclerotic arteries) (Brinkhous et al. 1989b; Kiechl and Willeit 2003; Sramek et al. 2003). Notably, VWF level in humans also correlates directly with thrombosis risk and inversely with bleeding risk (Sadler 2005). These results support the hypothesis that VWF has an intrinsic property that supports arterial thrombosis and appears to be independent of its association with F.VIII in dogs, pigs, and humans.
Von Willebrand disease (VWD) is characterized by qualitative or quantitative abnormalities in VWF (Sadler et al. 2006). Both humans and dogs inherit the disease in an autosomal fashion (Nichols et al. 2008; Read et al. 1978; Sadler et al. 2006). There are several types of VWD and taken together they are the most prevalent of inherited bleeding disorders in both dogs and humans (Goodeve et al. 2007; Schneppenheim et al. 1995), so there is great interest in developing better diagnostic and treatment methods for the disease in both species.
Canine VWF: cDNA and Identification of the Causative Molecular Defect
The Chapel Hill canine VWD strain was derived from a show-dog strain of Scottish terriers, and the colony was established in 1978 from a single heterozygote from one of these terriers (Read et al. 1978). The normal canine VWF gene has been localized to chromosome 273 in a region that is syntenic with human chromosome 12p13, the location of the human gene (Sheldon-Inloes et al. 1986). Robert Montgomery’s team has reported the cDNA sequence for canine VWF (Haberichter et al. 2000) and found that the human and canine VWF are 87.1% and 86.2 % identical at the nucleotide and protein levels, respectively. This cDNA and reports from others (Rieger et al. 1998) enabled us, in collaboration with Montgomery, to identify the causative mutation in the Chapel Hill strain of canine VWD (Haberichter et al. 2005). A single nucleotide deletion is present in the VWD canine sequence at base pair 255 that causes a frame shift mutation resulting in a premature stop codon in exon 4. Canine VWD mimics type 3 human VWD, which is characterized by undetectable VWF and severe bleeding, especially at mucocutaneous sites. Type 3 VWD is the least common and has the most severe bleeding phenotype among the various types of VWD. The Chapel Hill VWD dogs have proven extremely useful for studying the basic biology of VWF and testing replacement products and novel therapeutics.
Replacement Therapy with Human Recombinant VWF and Plasma-Derived VWF
The pharmacodynamics and pharmacokinetics of human recombinant furin-processed VWF (rVWF, Baxter) were compared with a plasma VWF-F.VIII concentrate, Humate-P (100 IU VWF:RCo/kg IV), in the homozygous VWD dogs in Chapel Hill (Nichols et al. 2007). Blood samples were taken up to 29 days after infusion and measured for human VWF:Ag, VWF activity, VWF multimers, and canine FVIII activity. rVWF, but not Humate-P, reduced primary saline bleeding time from more than 15 minutes to about 5 minutes; recovery with the two treatments was 102% and 76%, respectively. Elimination of rVWF was slower than Humate-P but both were completely eliminated from circulation in 96 to 144 hours. Canine F.VIII activity increased about 150% relative to baseline after VWF infusion and returned to baseline within 48 hours. Humate-P contains substantial amounts of human F.VIII so the level of F.VIII was approximately 250% immediately after infusion but declined to baseline within 30 hours. Thus human rVWF appears to stabilize endogenous (i.e., canine) F.VIII in VWD dogs. These very preliminary results suggest that pharmacokinetic properties of rVWF are similar to or slightly better than for pdVWF. The correction of bleeding time by rVWF but not Humate-P in the VWD dogs may have important clinical implications. Further studies are in progress to confirm these results.
Treatment of Canine and Human VWD with Recombinant IL-11 and DDAVP
Recombinant human interleukin 11 (rIL-111; Neumega™), a glycoprotein 130 (gp130)–signaling cytokine that is approved for treatment of thrombocytopenia, has been shown to induce elevations in VWF and F.VIII in humans and mice (Denis et al. 2001; Kaye et al. 1994). Mice are not responsive to DDAVP, a standard therapy for increasing VWF levels in humans (Federici et al. 2006). On the other hand, dogs do respond to DDAVP, and therefore a comparative study of DDAVP and rIL-11 was possible in VWF-deficient dogs. To determine whether rIL-11 would offer an advantage over DDAVP in the treatment of VWF and F.VIII deficiencies, VWD heterozygous (VWF+/−) and normal (VWF+/+) dogs received rIL-11 (50 μg/kg/SC × 7 days) and DDAVP (5 μg/kg/IV × 7 days) (Olsen et al. 2003). rIL-11 produced a gradual and sustained elevation of VWF and F.VIII levels in both the normal and VWF-deficient (VWF+/−) dogs, whereas DDAVP caused a rapid and nonsustained increase. rIL-11 treatment produced a 2.5- to 11-fold increase in VWF mRNA in the heart, aorta, liver, and spleen of normal dogs but not in homozygous (VWF−/−) VWD dogs, thus identifying a mechanism for elevation of plasma VWF in vivo. Moreover, dogs pretreated with rIL-11 retain a DDAVP-releasable pool of VWF and F.VIII, suggesting that rIL-11 does not significantly alter trafficking of these proteins to or from storage pools. The half-life of infused VWF was unchanged by rIL-11. These results strongly suggest that rIL-11 and DDAVP raise plasma VWF by different mechanisms, and collectively our data suggest that, if shown to be safe in clinical trials, treatment with rIL-11 with or without DDAVP may provide an alternative to blood-derived products for some VWD or hemophilia A patients or for patients who are unresponsive to DDAVP. Successful rIL-11 therapy could also reduce the use of plasma products in some patients.
Margaret Ragni recently extended these studies in humans and administered rIL-11 subcutaneously to nine subjects (5F, 4M, 21–49 y.o.) with mild or type 1 VWD (Sadler and Rodeghiero 2005) in an FDA-approved Phase 2 open-label study (Ragni et al. 2008). Three each received rIL-11 at 10 mcg/kg, 25 mcg/kg, and 50 mcg/kg SC daily for 7 consecutive days, and all nine received DDAVP 0.3 mcg/kg IV on day 7. VWF ristocetin cofactor (VWF:RCo), VWF antigen (VWF:Ag), and F.VIII increased over baseline to 179 ± 55% (mean ± standard error of the mean [SEM]), 240 ± 120%, and 143%, respectively, by day 4. After the DDAVP treatment on day 7, VWF:RCo further increased to 359 ± 152% and VWF:Ag to 277 ± 21%. Very high molecular weight VWF multimers were detected in plasma by gel electrophoresis only after DDAVP. Quantitative PCR revealed a two- to eightfold increase in platelet VWF mRNA between baseline and day 7 in one patient. The drug was well tolerated with less than grade 1 hypertension, hypokalemia, and fluid retention (the latter was associated with dilutional decrease in hemoglobin).
These data confirm that rIL-11 increases VWF in mild or type 1 VWD by means other than the DDAVP releasable pool of VWF, and possibly by increasing VWF mRNA. These encouraging results have provided the foundation for requests to perform two Phase 2 efficacy studies in mild or type 1 VWD patients, the first in patients with menorrhagia (Jankowitz et al. 2008) and the second in those undergoing elective surgery. The studies will determine whether the results of rIL-11 therapy in the VWD dogs can be translated into a new treatment for humans with mild to moderate VWD and a hemostatic challenge.
Canine VWF and Weibel-Palade Body Formation
Weibel-Palade bodies are considered to be endothelial-specific markers that both require VWF for their formation and serve as the intracellular site of VWF storage (Sadler 2005). VWD dogs lack VWF and therefore their endothelial cells do not have Weibel-Palade bodies. For this reason, Robert Montgomery used endothelium cultured from such dogs not only to study intracellular VWF trafficking and the role of VWF multimerization in Weibel-Palade body formation (Haberichter et al. 2005) but also specifically to test the hypothesis that expression of VWF protein would support Weibel-Palade body formation in canine VWD endothelium. Antibody staining for the endothelial cell marker PECAM-1 and immunofluorescence confocal microscopy demonstrated a homogeneous population of cultured VWD dog aortic endothelial cells (K9-VWD-AEC). Immunostaining of the cells revealed that, in contrast to normal endothelial cells, no Weibel-Palade bodies could be identified by P-selectin, VWF propeptide (VWFpp), or VWF antibodies. In results similar to those reported for murine VWF knockout endothelial cells (Denis et al. 2001), P-selectin was identified only as diffuse staining or by the presence of very small granules. Montgomery then used nucleofection of the K9-VWD-AEC to test the hypothesis that formation of Weibel-Palade bodies would be supported by expression of either wild-type human or canine VWF. VWF from both species induced elongated granules consistent with Weibel-Palade bodies as revealed by VWF and VWFpp immunostaining and confocal microscopy. Moreover, these VWF granules recruited endogenous P-selectin and reestablished the apparent Weibel-Palade body distribution of P-selectin.
Further studies tested the hypothesis that formation of Weibel-Palade bodies was dependent on the multimerization of VWF in canine VWD. Previous studies from the Montgomery laboratory characterized a Y87S-VWF mutant that produces only dimeric VWF. Multimer analysis of the culture supernatants of transfected K9-VWD cells confirmed the lack of multimerization using this construct as well as a construct lacking the VWFpp, termed Δpro VWF. Immunostaining and confocal microscopy demonstrated that the Δpro construct did not restore apparent storage granules and P-selectin staining remained unchanged. However, the Y87S-VWF mutant demonstrated the presence of both VWF and VWFpp in storage granules that recruited endogenous P-selectin to the granule membrane. These results support the hypothesis that Weibel-Palade body formation can be induced by expression of wild-type fully multimerized VWF or the mutant dimeric VWF in endothelium from a spontaneously occurring animal model of type 3 VWD. Furthermore, establishing these storage granules is dependent on the expression of the VWFpp by the construct.
Lentiviral-based Cell Therapy of von Willebrand Disease
BOECs grown from VWD dogs were successfully transduced with lentiviral vectors encoding human VWF and produced functional human VWF (De Meyer et al. 2006). If comparable results are possible with canine VWF cDNA, reimplantation in the VWD donor dog would be an important next step.
Factor VII and F.VII-Deficient Dogs
F.VII deficiency was recognized in dogs in 1962 (Mustard et al. 1962). As in human F.VII deficiency, the bleeding phenotype is severe. Canine F.VII cDNA was recently cloned in the High laboratory; the gene resides on canine chromosome 22, which is syntenic with human chromosome 13, the location of the human F.VII gene (Callan et al. 2006). The protein sequence contains 406 amino acids and shares a 75% identity with mature human F.VII. The availability of the canine cDNA enabled the identification of a G to A missense mutation at nucleotide 6385 in exon 5 that substitutes glycine 96 for a glutamic acid as the causative molecular defect in dogs from research colonies in Switzerland, Canada, and the United States (Callan et al. 2006) and in Alaskan Klee Kai dogs (Kaae et al. 2007).
Activated Recombinant F.VII in Factor VII Deficiency and Hemophilia
Activated recombinant F.VII (rF.VIIa1) has proven to be safe and efficacious in F.VII-deficient patients (Klitgaard and Nielsen 2008; Mariani et al. 1999). Although this is a relatively rare disorder in humans, the addition of recombinant F.VIIa for use in patients with hemophilia, especially those with inhibitors, has been a critically important advance (Brinkhous et al. 1989a; Hedner 2003, 2004). During purification, rF.VIIa is formed and composed of two polypeptide chains (Mr approximately 20,000 and 30,000 each). Before its use in humans, rF.VIIa was administered to hemophilic dogs to test the hypothesis that the introduction of sufficient rF.VIIa into the circulation can correct the hemostatic defect without causing systemic coagulation. This hypothesis is based on several earlier findings: (1) No specific plasma inhibitor for factor VII has been identified; hence, rF.VIIa should not be cleared rapidly from the circulation. (2) Lacking tissue factor (TF), rF.VIIa will be inactive in the circulation. (3) At the site of an injury, such as a bleeding-time (BT) wound, TF is generated as a cell-membrane receptor for F.VIIa; the F.VIIa-TF complex forms promptly and initiates the extrinsic pathway of coagulation. (4) The onset of extrinsic coagulation with rapid formation of fibrin in BT wounds is normal in hemophilia. Recombinant F.VIIa was produced in baby hamster kidney cells transfected with human factor VII cDNA, and hemophilic testing was done in hemophilia A, hemophilia B, and VWD dogs that all had the severe form of the disorder. A sufficient dose of rF.VIIa normalized hemostasis in both types of hemophilia but not VWD.
These studies indicate that administration of rF.VIIa can “bypass” both F.VIII and F.IX deficiencies in hemophilic dogs and correct the hemostatic defect without generalized coagulation. These data are in accord with the hypothesis that the TF-rF.VIIa complex forms at the site of injury with formation of an effective hemostatic plug, and that supplemental rF.VIIa in hemophilia results in sufficient thrombin generation with fibrin formation and platelet activation to facilitate formation of a stable hemostatic plug. The data on the hemophilia B dogs extend the hypothesis, indicating that activation of factor X alone in the absence of factor IX can correct the hemophilia B defect. The data on the VWD dogs indicate that activation of factors IX and X is inadequate to bypass the hemostatic defect in type 3 VWD (i.e., no VWF) (Brinkhous et al. 1989a). Current studies are addressing the safety and efficacy of modified versions of F.VIIa (Ghosh et al. 2007; Viuff et al. 2007).
Expression of Recombinant Canine F.VIIa by Gene Transfer in Hemophilia A and Hemophilia B
The High laboratory developed a novel treatment strategy with a recombinant adeno-associated viral vector delivering a modified F.VII transgene that can be intracellularly processed and secreted as activated F.VII (F.VIIa) (Margaritis et al. 2004). They documented long-term expression as well as phenotypic correction of hemophilia A and B in mice after gene transfer of the murine F.VIIa homologue, with no evidence of thrombotic complications at these doses. These studies have also identified an important therapeutic window beyond which thrombotic complications have occurred in mice (Aljamali et al. 2008). Comparable studies with the canine F.VIIa construct, in the Chapel Hill hemophilia A and hemophilia B dogs (Margaritis et al 2008), have shown marked reduction in bleeding events in the four treated animals: three have had no bleeds for 1 to 2 years, and the fourth, which received the lowest vector dose, has had three bleeding events related to trauma but no spontaneous bleeding. There has been no evidence of thrombotic complications in these dogs. Recently, our laboratories have shown that thromboelastography (TEG) is an effective way to monitor rF.VIIa activity in whole blood (Viuff et al. 2007) and that TEG parameters are markedly improved in the hemophilic dogs that received canine F.VIIa by gene transfer. These data hold great promise for a potential treatment for hemophilia, especially in inhibitor patients.
Summary
Readily available animal models of hemophilia A, hemophilia B, von Willebrand disease (VWD), and factor VII (F.VII) deficiency have facilitated the study of the pathophysiology of blood coagulation and hemostasis, and the development of safe and efficacious new therapeutic agents. In particular, dogs with well-characterized inherited bleeding disorders that mirror the respective human conditions have provided the investigational basis for the development of sensitive and reproducible detection assays (Goldsmith et al. 1978; Langdell et al. 1953; Read et al. 1978), the safe and successful transfer of many experimental therapies into clinical practice (Brinkhous et al. 1968, 1985, 1989a, 1996, 2002; White et al. 1997), and the continued preclinical evaluation of promising new therapies (Arruda et al. 2004, 2005; Ehrhardt et al. 2003; Harding et al. 2004; Jankowitz et al. 2008; McCormack et al. 2006; Mount et al. 2002; Ragni et al. 2008; Sarkar et al. 2006; Wang et al. 2005; Xu et al. 2003, 2007).
A number of recent reviews present the results of gene therapy studies that have used retroviral, adenoviral, lentiviral, and adeno-associated viral vectors, hydrodynamic injection of plasmid DNA, and transfer of stem cells (Chuah et al. 2004; High 2005; Lillicrap et al. 2006; Lozier 2004; Monahan and White 2002; Nathwani et al. 2004; Ponder 2006). For initial preclinical testing of protein replacement and gene therapy strategies, hemophilic mice are often the most practical animal model; the more successful protein replacement and gene therapy approaches are then tried in hemophilic dogs (Casal and Haskins 2006; High 2005; Ponder 2006; Rawle and Lillicrap 2004). This progression from small to large animal models has established a paradigm that is often used before the initiation of human trials as it allows investigators to address issues of scaling up protein or vector production, to access larger amounts of target tissue (e.g., muscle or liver), and to determine the immune response to the procedure in a mixed genetic or outbred animal (High 2005).
But the progressive experimental approach is not without limitations (High 2005; Ponder 2006; Rawle and Lillicrap 2004). With regard to gene therapy, the immune response to AAV2 capsid detected in humans after liver-based gene transfer (Manno et al. 2006) was not seen in rodents, hemophilia B dogs, or nonhuman primates (Mount et al. 2002; Snyder et al. 1999). A new trial of gene therapy for hemophilia B is under consideration based on results of testing in nonhemophilic nonhuman primates (Nathwani et al. 2007) without testing in hemophilic dogs, and other human gene therapy trials also have not relied on direct testing in hemophilic dogs (Roth et al. 2001).
The primary benefits of preclinical testing of gene transfer strategies in the hemophilic dogs are as follows:
successful prediction of correct vector dosing in the human liver-based trial (Manno et al. 2006),
assessment of the functional status of the expressed coagulation protein,
determination of the duration of expression of the functional protein over several years,
assessment of the degree of correction of the bleeder phenotype, including monitoring for a clinically relevant endpoint of reduction in spontaneous bleeds, which is not possible in mice (Margaritis et al. 2008),
biochemical analyses of the expressed protein to determine the extent to which important post-translational modifications are executed (e.g., propeptide cleavage, gamma carboxylation, sulfation, glycosylation),
monitoring of the immune response to the vector or expressed protein,
assessment of issues related to scaling up vector production, and
access to large amounts of target tissue for assessment of vector persistence and integration by analytical methods such as Southern blot, RNA expression, immunohistochemistry for detection of transgenic protein expression, and screening for symptoms of toxicity such as inflammation and mutagenesis.
Moreover, in both preclinical and clinical protein replacement and gene therapy trials, unexpected results arise that often require refining scientific questions that can best be addressed in animal models. We do not know the exact positive and negative predictive values for all of the complex experiments done in these dogs, but many protein replacement products that have been successfully introduced into human clinical practice were first shown to be safe and efficacious in these dogs using this approach (Brinkhous et al. 1968, 1985, 1989a, 1996, 2002; White et al. 1997). These historical successes are the primary reason many advisory boards strongly encourage investigators contemplating new therapies for hemophilia A, hemophilia B, and VWD to demonstrate safety and efficacy in bleeder dogs before initiating studies in humans.4
Dogs are clearly recognized as valid animal models of human bleeding and other disorders, with a strong preclinical predictive value for successful translation to human medicine (e.g., Bauer et al. 2009; Haskins 2009; Sleeper et al. 2009; Wang et al. 2009). It is highly likely that studies in dogs and other species will continue to provide complementary information that will allow the safest and most efficacious design of clinical trials approved for humans.
Acknowledgments
The authors acknowledge the contributions of Kenneth M. Brinkhous, MD (1908–2000), for his pioneering work in identifying the hemophilia A, hemophilia B, and VWD dogs used in many of the studies cited in this manuscript as well as his mentorship of several of the authors of this paper. The authors gratefully acknowledge the National Institutes of Health’s National Heart, Lung and Blood Institute (NIH/NHLBI) for providing continuous support of the Chapel Hill strains of bleeder dogs since 1947. The authors’ work was supported by NIH/NHLBI HL063098 to TCN and NIH/NHLBI P01 HL074124 to KAH.
Footnotes
Abbreviations used in this article: AAV, adeno-associated virus; aPTT, activated partial thromboplastin time; BOECs, blood outgrowth endothelial cells; cDNA, complementary DNA; DDAVP, 1-desamino-8-D-arginine vasopressin; F.VII, coagulant factor VII; F.VIII, coagulant factor VIII; F.IX, coagulant factor IX; HD, helper dependent; rAAV, recombinant adeno-associated virus; rIL-11, recombinant interleukin 11; rF.VIIa, activated recombinant F.VII; rF.IX, recombinant F.IX; VWD, von Willebrand disease; VWF, von Willebrand factor; WBCT, whole blood clotting time
Interestingly, in porcine and human type 3 (the most severe form) von Willebrand disease (VWD), replacement of both plasma and platelet VWF is required to normalize the bleeding time, whereas plasma VWF alone is insufficient (Bowie et al. 1986; Castillo et al. 1991; Mannucci et al. 1987; Nichols et al. 1995; Smiley et al. 1989).
Information about this genome is available from the website of the National Institutes of Health’s National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/genome/guide/dog/).
See Recommendations #137 and #160 of the Medical and Scientific Advisory Council (MASAC), available online (http://www.hemophilia.org/research/masac/masac_all.htm).
References
- Aledort LM, DiMichele DM. Inhibitors occur more frequently in Africo-Americans and Latino hæmophiliacs. Hæmophilia. 1998;80:779–783. doi: 10.1046/j.1365-2516.1998.0146c.x. [DOI] [PubMed] [Google Scholar]
- Aljamali MN, Margaritis P, Schlachterman A, Tai SJ, Roy E, Bunte R, Camire RM, High KA. Long-term expression of murine activated factor VII is safe, but elevated levels cause premature mortality. J Clin Invest. 2008;118:1825–1834. doi: 10.1172/JCI32878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson LO, Forsman N, Huang K, Larsen K, Lundin A, Pavlu B, Sandberg H, Sewerin K, Smart J. Isolation and characterization of human factor VIII: Molecular forms in commercial factor VIII concentrate, cryoprecipitate, and plasma. Proc Natl Acad Sci U S A. 1986;83:2979–2783. doi: 10.1073/pnas.83.9.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis SE, Rossiter JP, Young M, Horst J, de Moerloose P, Sommer SS, Ketterling RP, Kazazian HH, Jr, Negrier C, Vinciguerra C, Gitschier J, Goossens M, Girodon E, Ghanem N, Plassa F, Lavergne JM, Vidaud M, Costa JM, Laurian Y, Lin S-W, Lin S-R, Shen M-C, Lillicrap D, Taylor SAM, Windsor S, Valleix SV, Nafa K, Sultan Y, Delpech M, Vnencak-Jones C, Phillips JA, III, Ljung RCR, Koumbarelis E, Gialeraki A, Mandalaki T, Jenkins PV, Collins PW, Pasi KJ, Goodeve A, Peake I, Preston FE, Schwartz M, Scheibel E, Ingerslev J, Cooper DN, Millar DS, Kakkar VV, Giannelli F, Naylor JA, Tizzano EF, Baiget M, Domenech M, Altisent C, Tusell J, Beneyto M, Lorenzo JI, Gaucher C, Mazurier C, Peerlinck GMK, Matthijs K, Cassiman JJ, Vermylen J, Mori PG, Acquila M, Caprino D, Inaba H. Factor VIII gene inversions in severe hemophilia A: Results of an international consortium study. Blood. 1995;86:2206–2212. [PubMed] [Google Scholar]
- Arruda VR. Toward gene therapy for hemophilia A with novel adenoviral vectors: Successes and limitations in canine models. J Thromb Hæmost. 2006;4:1215–1217. doi: 10.1111/j.1538-7836.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel P, Donavan PJ, Herzog RW, Nichols TC, Biegel JA, Razavi M, Dake M, Huff D, Flake AW, Couto L, Kay M, High KA. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vectors in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Scallan C, Jiang H, Couto L, Herzog RW, Nichols TC, Pierce G, High KA. Comparison of the efficacy on gene transfer by AAV vectors delivered by distinct routes of administration to the liver of hemophilia B dogs. Mol Ther. 2004a;9 (abstr suppl):S103. [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, Mingozzi F, Xiao W, Couto LB, High KA. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004b;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Schuettrumpf J, Jiang H, Pierce G, Nichols TC, High KA. A novel method of regional intravenous delivery of AAV vector to skeletal muscle results in correction of canine hemophilia B phenotype. Blood. 2004c;104 abstract 3179. [Google Scholar]
- Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, Couto LB, High KA. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astermark J. Basic aspects of inhibitors to factors VIII and IX and the influence of non-genetic risk factors. Hæmophilia. 2006;12(Suppl 6):8–13. doi: 10.1111/j.1365-2516.2006.01360.x. discussion 13–14. [DOI] [PubMed] [Google Scholar]
- Axelrod JH, Read MS, Brinkhous KM, Verma IM. Phenotypic correction of factor IX deficiency in skin fibroblasts of hemophilic dogs. Proc Natl Acad Sci U S A. 1990;87:5173–5177. doi: 10.1073/pnas.87.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagué C, Zhou JM, Dai YF, Alemany R, Josephs SF, Andreason G, Hariharan M, Sethi E, Prokopenko E, Jan HY, Lou YC, Hubert-Leslie D, Ruiz L, Zhang WW. Sustained high-level expression of full-length human factor VIII and restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood. 2000;95:820–828. [PubMed] [Google Scholar]
- Bauer TR, Jr, Adler RL, Hickstein DD. Potential large animal models for gene therapy of human genetic diseases of immune and blood cell systems. ILAR J. 2009;50:168–186. doi: 10.1093/ilar.50.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DA, Nichols TC, Read MS, Reddick RL, Lamb MA, Brinkhous KM, Evatt BL, Griggs TR. Prevention of occlusive coronary artery thrombosis by a murine monoclonal antibody to porcine von Willebrand factor. Proc Natl Acad Sci U S A. 1987;84:8100–8104. doi: 10.1073/pnas.84.22.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs R, Douglas AS, Macfarlane RG, Dacie JV, Pitney WR, Merskey Christmas disease: A condition previously mistaken for hæmophilia. Br Med J. 1952;2:1378–1382. doi: 10.1136/bmj.2.4799.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie EJW, Solberg LA, Fass DN, Johnson CM, Knutson GJ, Stewart ML, Zoecklein LJ. Transplantation of normal bone marrow into a pig with severe von Willebrand’s disease. J Clin Invest. 1986;78:26–30. doi: 10.1172/JCI112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous KM, Graham JB. Hemophilia in the female dog. Science. 1950;111:723. doi: 10.1126/science.111.2896.723. [DOI] [PubMed] [Google Scholar]
- Brinkhous KM, Morrison FCJ, Muhrer ME. Comparative study of clotting defects in human, canine, and porcine hemophilia. Fed Proc. 1952;11:409a. [Google Scholar]
- Brinkhous KM, Penick GD, Langdell RD, Wagner RH, Graham JB. Physiologic basis of transfusion therapy in hemophilia. Arch Pathol. 1956;61:6–10. [PubMed] [Google Scholar]
- Brinkhous KM, Shanbrom E, Roberts HR, Webster WP, Fekete L, Wagner RH. A new high-potency glycine-precipitated anti-hemophilic factor (AHF) concentrate: Treatment of classical hemophilia and hemophilia with inhibitors. JAMA. 1968;205:613–617. [PubMed] [Google Scholar]
- Brinkhous KM, Sandberg H, Garris JB, Mattsson C, Palm M, Griggs TR, Read MS. Purified human factor VIII procoagulant protein: Comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci U S A. 1985;82:8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous KM, Hedner U, Garris JB, Diness V, Read MS. Effect of recombinant factor VIIa on the hemostatic defect in dogs with hemophilia A, hemophilia B, and von Willebrand disease. Proc Natl Acad Sci U S A. 1989a;86:1382–1386. doi: 10.1073/pnas.86.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous KM, Reddick RL, Griggs TR. Arterial thrombosis, atherosclerosis, and the factor VIII/von Willebrand factor complex. In: Zimmerman TS, Ruggeri ZM, editors. Coagulation and Bleeding Disorders: The Role of Factor VIII and von Willebrand Factor. New York and Basel: Marcel Dekker; 1989b. pp. 283–303. [Google Scholar]
- Brinkhous KM, Sigman JL, Read MS, Stewart PF, McCarthy KP, Timony GA, Leppanen SD, Rup BJ, Keith JC, Jr, Schaub RG. Recombinant human factor IX: Replacement therapy, prophylaxis, pharmacokinetics, and immunogenicity in canine hemophilia B. Blood. 1996;88:2603–2610. [PubMed] [Google Scholar]
- Brinkhous KM, Sandberg H, Widlund L, Read MS, Nichols TC, Sigman J, Oswaldsson U, Schaub RG, Mikaelsson M. Preclinical pharmacology of albumin-free B-domain deleted recombinant factor VIII. Semin Thromb Hemost. 2002;28:269–272. doi: 10.1055/s-2002-32661. [DOI] [PubMed] [Google Scholar]
- Brooks MB, Gu W, Ray K. Complete deletion of factor IX gene and inhibition of factor IX activity in a labrador retriever with hemophilia B. JAVMA. 1997;211:1418–1421. [PubMed] [Google Scholar]
- Brooks MB, Gu W, Barnas JL, Ray J, Ray K. A Line 1 insertion in the factor IX gene segregates with mild hemophilia B in dogs. Mamm Genome. 2003;14:788–795. doi: 10.1007/s00335-003-2290-z. [DOI] [PubMed] [Google Scholar]
- Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, Lillicrap D. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–810. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P, D’Angelo A, Naldini L. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, Beaudet AL, Ng P. Sustained phenotypic correction of canine hemophilia B following systemic administration of helper dependent adenoviral vector. Hum Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- Burton M, Nakai H, Colosi P, Cunningham J, Mitchell R, Couto L. Coexpression of factor VIII heavy and light chain adeno-associated viral vectors produces biologically active protein. Proc Natl Acad Sci U S A. 1999;96:12725–12730. doi: 10.1073/pnas.96.22.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan MB, Aljamali MN, Margaritis P, Griot-Wenk ME, Pollak ES, Werner P, Giger U, High KA. A novel missense mutation responsible for factor VII deficiency in research Beagle colonies. J Thromb Hæmost. 2006;4:2616–2622. doi: 10.1111/j.1538-7836.2006.02203.x. [DOI] [PubMed] [Google Scholar]
- Cameron C, Notley C, Hoyle S, McGlynn L, Hough C, Kamisue S, Giles A, Lillicrap DA. The canine factor VIII cDNA and 5′ flanking sequence. Thromb Hæmost. 1998;79:317–322. [PubMed] [Google Scholar]
- Casal M, Haskins M. Large animal models and gene therapy. Eur J Hum Genet. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- Castillo R, Mateagudo J, Escolar G, Ordinas A, Magallon M, Villar JM. Hemostatic effect of normal platelet transfusion in severe von Willebrand disease patients. Blood. 1991;77:1901–1905. [PubMed] [Google Scholar]
- Chang PL, Shen N, Westcott AJ. Delivery of recombinant gene products with microencapsulated cells in vivo. Hum Gene Ther. 1993;4:433–440. doi: 10.1089/hum.1993.4.4-433. [DOI] [PubMed] [Google Scholar]
- Chang JY, Monroe DM, Stafford DW, Brinkhous KM, Roberts HR. Replacing the first epidermal growth factor-like domain of factor IX with that of factor VII enhances activity in vitro and in canine hemophilia B. J Clin Invest. 1997;100:886–892. doi: 10.1172/JCI119604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H, Samulski R, Bellinger D, Monahan P, Nichols T, Walsh C. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther. 1999;6:1695–1704. doi: 10.1038/sj.gt.3301024. [DOI] [PubMed] [Google Scholar]
- Chao H, Mao L, Bruce AT, Walsh CE. Sustained expression of human factor VIII in mice using a parvovirus-based vector. Blood. 2000;95:1594–1599. [PubMed] [Google Scholar]
- Chen L, Zhu F, Li J, Lu H, Jiang H, Sarkar R, Arruda VR, Wang J, Zhao J, Pierce GF, Ding Q, Wang X, Wang H, Pipe SW, Liu XQ, Xiao X, Camire RM, Xiao W. The enhancing effects of the light chain on heavy chain secretion in split delivery of factor VIII gene. Mol Ther. 2007;15:1856–1862. doi: 10.1038/sj.mt.6300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lu H, Wang J, Sarkar R, Yang X, Wang H, High KA, Xiao W. Enhanced factor VIII heavy chain for gene therapy of hemophilia. A Mol Ther. 2009 doi: 10.1038/mt.2008.292. Epub PMID 19127250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WF, van den Born J, Kuhn K, Kjellen L, Hudson BG, Stafford DW. Identification of the endothelial cell binding site for factor IX. Proc Natl Acad Sci U S A. 1996;93:11068–11073. doi: 10.1073/pnas.93.20.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah MK, Schiedner G, Thorrez L, Brown B, Johnston M, Gillijns V, Hertel S, Van Rooijen N, Lillicrap D, Collen D, VandenDriessche T, Kochanek S. Therapeutic factor VIII levels and negligible toxicity in mouse and dog models of hemophilia A following gene therapy with high-capacity adenoviral vectors. Blood. 2003;101:1734–1743. doi: 10.1182/blood-2002-03-0823. [DOI] [PubMed] [Google Scholar]
- Chuah MK, Collen D, Vandendriessche T. Preclinical and clinical gene therapy for hæmophilia. Hæmophilia. 2004;10(Suppl 4):119–125. doi: 10.1111/j.1365-2516.2004.00984.x. [DOI] [PubMed] [Google Scholar]
- Connelly S, Kaleko M. Gene therapy for hemophilia A. Thromb Hæmost. 1997;78:31–36. [PubMed] [Google Scholar]
- Cramer EM, Caen JP, Drouet L, Breton-Gorius J. Absence of tubular structures and immunolabeling for von Willebrand factor in the platelet α-granules from porcine von Willebrand disease. Blood. 1986;86:774–778. [PubMed] [Google Scholar]
- Cristiano RJ, Smith LC, Kay MA, Brinkley BR, Woo SLC. Hepatic gene therapy: Efficient gene delivery and expression in primary hepatocytes utilizing a conjugated adenovirus-DNA complex. Proc Natl Acad Sci U S A. 1993;90:11548–11552. doi: 10.1073/pnas.90.24.11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer SF, Vanhoorelbeke K, Chuah MK, Pareyn I, Gillijns V, Hebbel RP, Collen D, Deckmyn H, VandenDriessche T. Phenotypic correction of von Willebrand disease type 3 blood-derived endothelial cells with lentiviral vectors expressing von Willebrand factor. Blood. 2006;107:4728–4736. doi: 10.1182/blood-2005-09-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer SF, Pareyn I, Baert J, Deckmyn H, Vanhoorelbeke K. False positive results in chimeraplasty for von Willebrand Disease. Thromb Res. 2007;119:93–104. doi: 10.1016/j.thromres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Denis CV, Kwack K, Saffaripour S, Maganti S, André P, Schaub RG, Wagner DD. Interleukin 11 significantly increases plasma von Willebrand factor and factor VIII in wild type and von Willebrand disease mouse models. Blood. 2001;97:465–472. doi: 10.1182/blood.v97.2.465. [DOI] [PubMed] [Google Scholar]
- Denis CV, Andre P, Saffaripour S, Wagner DD. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMichele D. Immune tolerance therapy for factor VIII inhibitors: Moving from empiricism to an evidence-based approach. J Thromb Hæmost. 2007;5(Suppl 1):143–150. doi: 10.1111/j.1538-7836.2007.02474.x. [DOI] [PubMed] [Google Scholar]
- Donsante A, Vogler C, Muzyczka N, Crawford JM, Barker J, Flotte T, Campbell-Thompson M, Daly T, Sands MS. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther. 2001;8:1343–1346. doi: 10.1038/sj.gt.3301541. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Ehrhardt A, Kay MA. A new adenoviral helper-dependent vector results in long-term therapeutic levels of human coagulation factor IX at low doses in vivo. Blood. 2002;99:3923–3930. doi: 10.1182/blood.v99.11.3923. [DOI] [PubMed] [Google Scholar]
- Ehrhardt A, Xu H, Dillow AM, Bellinger DA, Nichols TC, Kay MA. A gene-deleted adenoviral vector results in phenotypic correction of canine hemophilia B without liver toxicity or thrombocytopenia. Blood. 2003;102:2403–2411. doi: 10.1182/blood-2003-01-0314. [DOI] [PubMed] [Google Scholar]
- Ehrhardt A, Xu H, Dillow AM, Yant SR, Nichols TC, Kay MA. Transposition from a gene-deleted adenoviral vector results in phenotypic correction in a canine model of hemophilia B. Mol Ther. 2006;13:S5. doi: 10.1182/blood-2003-01-0314. [DOI] [PubMed] [Google Scholar]
- Eisensmith RC, Woo SL. Viral vector-mediated gene therapy for hemophilia B. [Review] Thromb Hæmost. 1997;78:24–30. [PubMed] [Google Scholar]
- Evans JP, Brinkhous KM, Brayer GD, Reisner HM, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A. 1989a;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP, Watzke HH, Ware JL, Stafford DW, High KA. Molecular cloning of a cDNA encoding canine factor IX. Blood. 1989b;74:207–212. [PubMed] [Google Scholar]
- Fang B, Eisensmith RC, Wang H, Kay MA, Cross RE, Landen CN, Gordon G, Bellinger DA, Read MS, Hu PC, Brinkhous KM, Woo SLC. Gene therapy for hemophilia B: Host immunosuppression prolongs the therapeutic effect of adenovirus-mediated factor IX expression. Hum Gene Ther. 1995;6:1039–1044. doi: 10.1089/hum.1995.6.8-1039. [DOI] [PubMed] [Google Scholar]
- Fang B, Wang H, Gordon G, Bellinger DA, Read MS, Brinkhous KM, Woo SLC, Eisensmith RC. Lack of persistence of E1 recombinant adenoviral vectors containing a temperature-sensitive E2A mutation in immunocompetent mice and hemophilia B dogs. Gene Ther. 1996;3:217–222. [PubMed] [Google Scholar]
- Fang X, Zhang WW, Sobol RE, Gomperts E, Thompson AR, Wong WY, Kohn D, Rogers R, Fischer T, Nichols T, Murthy KK, Cobb EK, Fahs S, Montgomery RR, White G. Studies in nonhuman primate and hemophilic dog models of a “gutless” adenovirus vector for treatment of hemophilia A. Blood. 2000;96:428a. [Google Scholar]
- Federici AB, Castaman G, Thompson A, Berntorp E. Von Willebrand’s disease: Clinical management. Hæmophilia. 2006;12(Suppl 3):152–158. doi: 10.1111/j.1365-2516.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- Fewell JG, Bruce D, Florack V, Wilson E, Anscombe I, Nolan E, Read MS, Bellinger DA, Fischer TH, Nichols TC, Smith LC. Expression of Factor IX in mice and hemophilic B dogs following intramuscular injection of PINC™-formulated plasmid with electroporation. Blood. 2000;96:803a. [Google Scholar]
- Fewell JG, MacLaughlin F, Mehta V, Gondo M, Nicol F, Wilson E, Smith LC. Gene therapy for the treatment of hemophilia B using PINC-formulated plasmid delivered to muscle with electroporation. Mol Ther. 2001;3:574–583. doi: 10.1006/mthe.2001.0295. [DOI] [PubMed] [Google Scholar]
- Finn JD, Sabatino DE, Ozelo MC, Zhou S, Lillicrap D, Kazazian HH, Nichols TC, Arruda VR. Induction of immune tolerance to canine FVIII in hemophilia A dogs with inhibitors using AAV-mediated expression of canine FVIII. Blood. 2008;112 Abstract 243. [Google Scholar]
- Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG, Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Benten D, Novikoff P, Faulkner L, Raut S, Gupta S. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest. 2008;118:935–945. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscl Throm Vas. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz J, Thornton MA, Rauova L, Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J Thromb Hæmost. 2008;6:1160–1166. doi: 10.1111/j.1538-7836.2008.02992.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ezban M, Persson E, Pendurthi U, Hedner U, Rao LV. Activity and regulation of factor VIIa analogs with increased potency at the endothelial cell surface. J Thromb Hæmost. 2007;5:336–346. doi: 10.1111/j.1538-7836.2007.02308.x. [DOI] [PubMed] [Google Scholar]
- Giles AR, Tinlin S, Hoogendoorn H, Greenwood P, Greenwood R. Development of factor VIII:C antibodies in dogs with hemophilia A (factor VIII:C deficiency) Blood. 1984;63:451–456. [PubMed] [Google Scholar]
- Gillis S, Furie BC, Furie B, Patel H, Huberty MC, Switzer M, Foster WB, Scoble HA, Bond MD. gamma-Carboxyglutamic acids 36 and 40 do not contribute to human factor IX function. Protein Sci. 1997;6:185–196. doi: 10.1002/pro.5560060121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith JC, Chung KS, Roberts HR. A simple assay for human factor IX: Use of canine hemophilia B plasma as substrate. Thromb Res. 1978;12:497–502. doi: 10.1016/0049-3848(78)90320-1. [DOI] [PubMed] [Google Scholar]
- Goodeve A, Eikenboom J, Castaman G, Rodeghiero F, Federici AB, Batlle J, Meyer D, Mazurier C, Goudemand J, Schneppenheim R, Budde U, Ingerslev J, Habart D, Vorlova Z, Holmberg L, Lethagen S, Pasi J, Hill F, Hashemi Soteh M, Baronciani L, Hallden C, Guilliatt A, Lester W, Peake I. Phenotype and genotype of a cohort of families historically diagnosed with Type 1 von Willebrand disease in the European study: Molecular and clinical markers for the diagnosis and management of Type 1 von Willebrand Disease (MCMDM-1VWD) Blood. 2007;109:112–121. doi: 10.1182/blood-2006-05-020784. (erratum in: Blood 111:3299–3300) [DOI] [PubMed] [Google Scholar]
- Goudemand J, Rothschild C, Demiguel V, Vinciguerrat C, Lambert T, Chambost H, Borel-Derlon A, Claeyssens S, Laurian Y, Calvez T. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood. 2006;107:46–51. doi: 10.1182/blood-2005-04-1371. [DOI] [PubMed] [Google Scholar]
- Graham JB, Buckwalter JA, Hartley LJ, Brinkhous KM. Canine hemophilia: Observations on the course, the clotting anomaly, and the effects of blood transfusion. J Exp Med. 1949;90:97–111. doi: 10.1084/jem.90.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick HR, Rick ME, McKeown LP, Williams SB, Parker RI, Maison-neuve P, Jenneau C, Sultan Y. Platelet von Willebrand factor: An important determinant of the bleeding time in type I von Willebrand’s disease. Blood. 1986;68:58–61. [PubMed] [Google Scholar]
- Greelish JP, Su LT, Lankford EB, Burkman JM, Chen H, Konig SK, Mercier IM, Desjardins PR, Mitchell MA, Zheng XG, Leferovich J, Gao GP, Balice-Gordon RJ, Wilson JM, Stedman HH. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- Greengard JS, Bodner M, McCormack J, Edwards WR, Sensintaffar JL, Sheridan PL, Phuong T, Mittelstaedt D, Brumm D, Nichols TC, Read M, Brinkhous KM, Jolly DJ, Chang SMW. Sustained expression of human factor VIII from peripheral retroviral gene delivery in rabbits and dogs. Blood. 1997;90:240a. [Google Scholar]
- Gu W, Brooks M, Catalfamo J, Ray J, Ray K. Two distinct mutations cause severe hemophilia B in two unrelated canine pedigrees. Thromb Hæmost. 1999;82:1270–1275. [PubMed] [Google Scholar]
- Haberichter SL, Fahs SA, Montgomery RR. von Willebrand factor storage and multimerization: 2 independent intracellular processes. Blood. 2000;96:1808–1815. [PubMed] [Google Scholar]
- Haberichter SL, Merricks EP, Fahs SA, Christopherson PA, Nichols TC, Montgomery RR. Re-establishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells. Blood. 2005;105:145–152. doi: 10.1182/blood-2004-02-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Han Z, Zhong L, Maina N, Hu Z, Li X, Chouthai NS, Bischof D, Weigel-Van Aken KA, Slayton WB, Yoder MC, Srivastava A. Stable integration of recombinant adeno-associated virus vector genomes after transduction of murine hematopoietic stem cells. Hum Gene Ther. 2008;19:267–278. doi: 10.1089/hum.2007.161. [DOI] [PubMed] [Google Scholar]
- Harding TC, Koprivnikar KE, Tu GH, Zayek N, Lew S, Subramanian A, Sivakumaran A, Frey D, Ho K, VanRoey MJ, Nichols TC, Bellinger DA, Yendluri S, Waugh J, McArthur J, Veres G, Donahue BA. Intravenous administration of an AAV-2 vector for the expression of factor IX in mice and a dog model of hemophilia B. Gene Ther. 2004;11:204–213. doi: 10.1038/sj.gt.3302142. [DOI] [PubMed] [Google Scholar]
- Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through CRE-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbrouck NC, High KA. AAV-mediated gene transfer for the treatment of hemophilia B: Problems and prospects. Gene Ther. 2008;15:870–875. doi: 10.1038/gt.2008.71. [DOI] [PubMed] [Google Scholar]
- Haskins M. Gene therapy for lysosomal storage diseases (LSDs) in large animal models. ILAR J. 2009;50:112–121. doi: 10.1093/ilar.50.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner U. Recombinant factor VIIa (NovoSeven) as a hemostatic agent. Dis Mon. 2003;49:39–48. doi: 10.1053/shem.2001.29507b. [DOI] [PubMed] [Google Scholar]
- Hedner U. Dosing with recombinant factor VIIa based on current evidence. Semin Hematol. 2004;41:35–39. doi: 10.1053/j.seminhematol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Heimark RL, Schwartz SM. Binding of coagulation factors IX and X to the endothelial cell surface. Biochem Bioph Res Co. 1983;111:723–731. doi: 10.1016/0006-291x(83)90365-0. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, Burton M, Bellinger DA, Read MS, Brinkhous KM, Podsakoff GM, Nichols TC, Kurtzman GJ, High KA. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-asssocated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brewbaker J, Armstrong E, McClintock D, Bellinger DA, Couto LB, Nichols TC, High KA. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- High KA. Gene transfer for hemophilia: Can therapeutic efficacy in large animals be safely translated to patients? J Thromb Hæmost. 2005;3:1682–1691. doi: 10.1111/j.1538-7836.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- Hill-Eubanks D, Lollar P. von Willebrand factor is a cofactor for thrombin-catalyzed cleavage of the factor VIII light chain. J Biol Chem. 1990;265:17854–17858. [PubMed] [Google Scholar]
- Hough C, Kamisue S, Cameron C, Notley C, Tinlin S, Giles A, Lillicrap D. Aberrant splicing and premature termination of transcription of the FVIII gene as a cause of severe canine hemophilia A: Similarities with the intron 22 inversion mutation in human hemophilia. Thromb Hæmost. 2002;87:659–665. [PubMed] [Google Scholar]
- Howard T, Machiah DK, Tran TT, Viel KR, Channell C, Soria JM, Ameri A, Iyer RV, Brown C, Doering C, Almasy L, Watts R, Davis J, Abshire TC. African-Americans express multiple haplotypic forms of the wildtype factor VIII (FVIII) protein: A possible role for pharmacogenetics in FVIII inhibitor development? Blood. 2004;104 (suppl 1):113a. [Google Scholar]
- Hoyer LW. Hemophilia A. N Engl J Med. 1994;330:38–47. doi: 10.1056/NEJM199401063300108. [DOI] [PubMed] [Google Scholar]
- Ishiwata A, Mimuro J, Kashiwakura Y, Niimura M, Takano K, Ohmori T, Madoiwa S, Mizukami H, Okada T, Naka H, Yoshioka A, Ozawa K, Sakata Y. Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene. Thromb Res. 2006;118:627–635. doi: 10.1016/j.thromres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Hackett PB, Plasterk RH, Izsvak Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Jankowitz RG, Ragni MV, Chapman HL, Merricks EP, Kloos MT, Dillow AM, Franck HWG, Nichols TC. Recombinant interleukin-11 in women with refractory menorrhagia and von Willebrand disease. Blood. 2008;112:Abstract 1210. [Google Scholar]
- Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, Powell S, Keller T, McMurray M, Labelle A, Nagy D, Vargas JA, Zhou S, Couto LB, Pierce GF. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006a;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, Sommer J, Luk A, Manno CS, High KA, Arruda VR. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006b;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Jones TC. The value of animal models. Am J Path. 1980;101:S3–S9. [PMC free article] [PubMed] [Google Scholar]
- Kaae JA, Callan MB, Brooks M. Hereditary factor VII deficiency in the Alaskan Klee Kai dog. J Vet Intern Med. 2007;21:976–981. doi: 10.1892/0891-6640(2007)21[976:hfvdit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- Kay MA, Rothenberg S, Landen CN, Bellinger DA, Leland F, Toman C, Finegold M, Thompson AR, Read MS, Brinkhous KM, Woo SLC. In vivo gene therapy of hemophilia B: Sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Kay MA, Landen CN, Rothenberg SR, Taylor LA, Leland F, Wiehle S, Fang B, Bellinger D, Finegold M, Thompson AR, Read MS, Brinkhous KM, Woo SLC. In vivo hepatic gene therapy: Complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci U S A. 1994;91:2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in hæmophilia B patients treated with an AAV vector [see comments] Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Kaye J, Loewy J, Blume J, Clendaniel A, Egan A, Garnick MB, Mitchell J, Knowles C, Ault K. Recombinant human interleukin eleven (Neumega rhIL-ss growth factor) increases plasma von Willebrand factor and fibrinogen concentrations in normal human subjects. Blood. 1994;84:1088a. [Google Scholar]
- Kiechl S, Willeit J. Cardiovascular protection in hæmophilia. Lancet. 2003;362:2026. doi: 10.1016/S0140-6736(03)15038-6. [DOI] [PubMed] [Google Scholar]
- Kingdon HS, Johnson RC, Brauker JH, Johnson RC, Carr-Brendel VE, Lozier JN, High KA. An immunoisolation device which facilitates cell-based prophylactic therapy for hemophilia. Blood. 1993;82:1772. [Google Scholar]
- Klitgaard T, Nielsen TG. Overview of the human pharmacokinetics of recombinant activated factor VII. Br J Clin Pharmacol. 2008;65:3–11. doi: 10.1111/j.1365-2125.2007.03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci U S A. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedam JA, Meijers JCM, Sixma JJ, Bouma BN. Inactivation of human factor VIII by activated protein C: Cofactor activity of protein S and protective effect of von Willebrand factor. J Clin Invest. 1988;82:1236–1243. doi: 10.1172/JCI113721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutts J, Walsh PN, Plow EF, Fenton JW, Bouma BN, Zimmerman TS. Active release of human platelet factor VIII-related antigen by adenosine diphosphate, collagen, and thrombin. J Clin Invest. 1978;62:1255–1263. doi: 10.1172/JCI109246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren B, Bandyopadhyay P, Presnell S, Bellinger D, Nichols T, Steer C. Site-directed correction of the hemophilia B point mutation in genomic DNA of isolated dog hepatocytes by a chimeric RNA/DNA oligonucleotide. Am Soc Gene Ther. 1998 Abstracts:181a. [Google Scholar]
- Lakich D, Kazazian H, Jr, Antonarakis SE, Gitschier J. Inversions disrupting the factor VIII gene are a common cause of severe hæmophilia A. Nat Genet. 1993;5:236–241. doi: 10.1038/ng1193-236. [DOI] [PubMed] [Google Scholar]
- Lamont PA, Ragni MV. Lack of desmopressin (DDAVP) response in men with hemophilia A following liver transplantation. J Thromb Hæmost. 2005;3:2259–2263. doi: 10.1111/j.1538-7836.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- Langdell RD, Wagner RH, Brinkhous KM. Effect of antihemophilic factor on one-stage clotting tests. J Lab Clin Med. 1953;41:637–647. [PubMed] [Google Scholar]
- Lieber A, He C-Y, Kirillova I, Kay MA. Recombinant adenoviruses with large deletions generated by CRE-mediated excision exhibit different biological properties compared with first generation vectors in vitro and in vivo. J Virol. 1996a;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber A, He CY, Kay MA. Adenoviral preterminal protein stabilizes mini-adenoviral genomes in vitro and in vivo. Nat Biotechnol. 1996b;15:1383–1387. doi: 10.1038/nbt1297-1383. [DOI] [PubMed] [Google Scholar]
- Liles D, Landen CN, Monroe DM, Lindley CM, Read MS, Roberts HR, Brinkhous KM. Extravascular administration of factor IX: Potential for replacement therapy of canine and human hemophilia B. Thromb Hæmost. 1997;77:944–948. [PubMed] [Google Scholar]
- Lillicrap D, VandenDriessche T, High K. Cellular and genetic therapies for hæmophilia. Hæmophilia. 2006;12(Suppl 3):36–41. doi: 10.1111/j.1365-2516.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Chang L, Solovey A, Healey JF, Lollar P, Hebbel RP. Use of blood outgrowth endothelial cells for gene therapy for hemophilia A. Blood. 2002;99:457–462. doi: 10.1182/blood.v99.2.457. [DOI] [PubMed] [Google Scholar]
- Liu YL, Zhu H, Schlachterman A, Chang H, Camire RM, High KA. A novel deletion in the FVIII B-domain that reduces transgene size while preserving FVIII activity. Blood. 2004;104 Abstract 3182. [Google Scholar]
- Liu L, Mah C, Fletcher BS. Sustained FVIII expression and phenotypic correction of hemophilia A in neonatal mice using an endothelial-targeted sleeping beauty transposon. Mol Ther. 2006;13:1006–1015. doi: 10.1016/j.ymthe.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Lozier JN. Gene therapy of the hemophilias. Semin Hematol. 2004;41:287–296. doi: 10.1053/j.seminhematol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Lozier JN, Brinkhous KM. Gene therapy and the hemophilias. JAMA. 1994;271:47–51. [PubMed] [Google Scholar]
- Lozier JN, Palmer TD, Thompson AR, Miller AD, Brinkhous KM, High KA. Production of recombinant canine factor IX by hollow fiber tissue culture. Thromb Hæmost. 1991;65:1158. [Google Scholar]
- Lozier JN, Thompson AR, Hu PC, Read M, Brinkhous KM, High KA, Curiel DT. Efficient transfection of primary cells in a canine hemophilia B model using adenovirus-polylysine-DNA complexes. Hum Gene Ther. 1994;5:313–322. doi: 10.1089/hum.1994.5.3-313. [DOI] [PubMed] [Google Scholar]
- Lozier JN, Dutra A, Pak E, Zhou N, Zheng Z, Nichols TC, Bellinger DA, Read M, Morgan RA. The Chapel Hill hemophilia A dog colony exhibits a factor VIII gene inversion. Proc Natl Acad Sci U S A. 2002;99:12991–12996. doi: 10.1073/pnas.192219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Chen L, Wang J, Huack B, Sarkar R, Zhou S, Xu R, Ding Q, Wang X, Wang H, Xiao W. Complete correction of hemophilia A with adeno-associated viral vectors containing a full-size expression cassette. Hum Gene Ther. 2008;19:648–654. doi: 10.1089/hum.2007.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane RG. Hæmorrhagic diseases in surgery: The Hæmostatic defect in Hæmophilia and its temporary correction. Proc R Soc Med. 1965;58:251–255. [PMC free article] [PubMed] [Google Scholar]
- Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscl Throm Vas. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco-Johnson ML, Funk S, Jacobson L, Valentino LA, Hoots WK, Buchanan GR, DiMichele D, Recht M, Brown D, Leissinger C, Bleak S, Cohen A, Mathew P, Matsunaga A, Medeiros D, Nugent D, Thomas GA, Thompson AA, McRedmond K, Soucie JM, Austin H, Evatt BL. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mannucci PM, Tuddenham EG. The hemophilias: From royal genes to gene therapy. N Engl J Med. 2001;344:1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- Mannucci P, Moia M, Rebulla P, Altieri D, Monteagudo J, Castillo R. Correction of the bleeding time in treated patients with severe von Willebrand disease is not solely dependent on the normal multimeric structure of plasma von Willebrand factor. Am J Hematol. 1987;25:55–65. doi: 10.1002/ajh.2830250106. [DOI] [PubMed] [Google Scholar]
- Mannucci PM, Abshire T, DiMichele D, Santagostino E, Blanchette V. Inhibitor development, immune tolerance and prophylaxis in hæmophilia A: The need for an evidence-based approach. Hæmophilia. 2006;12:429–434. doi: 10.1111/j.1365-2516.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- Marchioro TL, Hougie C, Ragde H, Epstein RB, Thomas ED. Hemophilia: Role of organ homografts. Science. 1969;163:188–190. doi: 10.1126/science.163.3863.188. [DOI] [PubMed] [Google Scholar]
- Margaritis P, Arruda VR, Aljamali M, Camire RM, Schlachterman A, High KA. Novel therapeutic approach for hemophilia using gene delivery of an engineered secreted activated factor VII. J Clin Invest. 2004;113:1025–1031. doi: 10.1172/JCI20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritis P, Roy E, Aljamali MN, Downey HD, Giger U, Zhou S, Merricks E, Dillow A, Ezban M, Nichols TC, High KA. Successful treatment of canine hemophilia by continuous expression of canine FVIIa. Blood. 2008 doi: 10.1182/blood-2008-07-168377. prepublished online Dec 23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani G, Testa MG, Di Paolantonio T, Molskov Bech R, Hedner U. Use of recombinant, activated factor VII in the treatment of congenital factor VII deficiencies. Vox Sang. 1999;77:131–136. doi: 10.1159/000031091. [DOI] [PubMed] [Google Scholar]
- Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C, Hebbel RP, Galipeau J, Hough C, Lillicrap D. Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo FVIII expression from lentivirally-engineered endothelial progenitors. Stem Cell. 2007;25:2660–2669. doi: 10.1634/stemcells.2006-0699. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- Mauser AE, Whitlark J, Whitney KM, Lothrop CD., Jr A deletion mutation causes hemophilia B in Lhasa Apso dogs. Blood. 1996;88:3451–3455. [PubMed] [Google Scholar]
- McCarthy K, Stewart P, Sigman J, Read M, Keith JC, Brinkhous KM, Nichols TC, Schaub RG. Pharmacokinetics of recombinant factor IX after intravenous and subcutaneous administration in dogs and cynomolgus monkeys. J Thromb Hæmost. 2002;87:824–830. [PubMed] [Google Scholar]
- McCormack WM, Jr, Seiler MP, Bertin TK, Ubhayakar K, Palmer DJ, Ng P, Nichols TC, Lee B. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J Thromb Hæmost. 2006;4:1218–1225. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CH, Chou SH, Nichols TC, Thompson AR. Chimeric RNA/DNA hybrid dumbbells capped with compact trinucleotide loops more consistently corrects the factor IX point mutation in canine hemophilic fibroblasts. Blood. 1999;94 (Suppl 1):373a. [Google Scholar]
- Miao CH, Chou SH, Nichols TC, Thompson AR. Targeted gene repair in canine hemophilia fibroblasts by chimeric RNA/DNA hybrid dumbbells capped with compact trinucleotide loops. Mol Ther. 2000;1(abstr suppl):S291. [Google Scholar]
- Miao CH, Ye P, Thompson AR, Rawlings DJ, Ochs HD. Immuno-modulation of transgene responses following naked DNA transfer of human factor VIII into hemophilia A mice. Blood. 2006;108:19–27. doi: 10.1182/blood-2005-11-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao HZ, Sirachainan N, Palmer L, Kucab P, Cunningham MA, Kaufman RJ, Pipe SW. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103:3412–3419. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- Milbauer LC, Enenstein J, Roney M, Solovey A, Bodempudi V, Nichols TC, Hebbel RP. BOEC migration and trapping in vivo: A window into gene therapy. Transl Res. 2008 doi: 10.1016/j.trsl.2008.12.009. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Monahan PE. Neonatal immune tolerance for hemophilia: Can we ‘tolerate’ new paradigms for gene therapy trials? J Thromb Hæmost. 2007;5:1801–1804. doi: 10.1111/j.1538-7836.2007.02659.x. [DOI] [PubMed] [Google Scholar]
- Monahan PE, White GC., 2nd Hemophilia gene therapy: Update. Curr Opin Hematol. 2002;9:430–436. doi: 10.1097/00062752-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ, Tazelaar J, Xiao X, Nichols TC, Bellinger DA, Read MS, Walsh CE. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscl Throm Vas. 2002;22:1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- Monroe DM, Hoffman M, Roberts HR. Fathers of modern coagulation. Thromb Hæmost. 2007;98:3–5. [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, Jr, High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Mustard JF, Secord D, Hoeksema TD, Downie HG, Rowsell HC. Canine factor-VII deficiency. Br J Haematol. 1962;8:43–47. doi: 10.1111/j.1365-2141.1962.tb06493.x. [DOI] [PubMed] [Google Scholar]
- Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff A, Hanawa H, Zhou JF, Vanin EF, Nienhuis AW. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97:1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Tuddenham EG. Prospects for gene therapy of hæmophilia. Hæmophilia. 2004;10:309–318. doi: 10.1111/j.1365-2516.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EG, Davidoff AM. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J, Brinke A, Hassock S, Green PM, Giannelli F. Characteristic mRNA abnormality found in half the patients with severe Hæmophilia A is due to large DNA inversions. Hum Mol Genet. 1993;2:1773–1778. doi: 10.1093/hmg/2.11.1773. [DOI] [PubMed] [Google Scholar]
- Neyman M, Gewirtz J, Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood. 2008;112:1101–1108. doi: 10.1182/blood-2008-04-152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TC, Bellinger DA, Reddick RL, Smith SV, Koch GG, Davis K, Sigman J, Brinkhous KM, Griggs TR, Read MS. The roles of von Willebrand factor and factor VIII in arterial thrombosis: Studies in canine von Willebrand disease and hemophilia A. Blood. 1993;81:2644–2651. [PubMed] [Google Scholar]
- Nichols TC, Samama CM, Bellinger DA, Roussi J, Reddick RL, Bonneau M, Read MS, Bailliart O, Koch GG, Vaiman M, Sigman JL, Pignaud GA, Brinkhous KM, Griggs TR, Drouet L. Function of von Wille-brand factor after crossed bone marrow transplantation between normal and von Willebrand disease pigs: Effect on arterial thrombosis in chimeras. Proc Natl Acad Sci U S A. 1995;92:2455–2459. doi: 10.1073/pnas.92.7.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TC, Pipe SW, Kaufman RJ, McClintock DWD, Zollner R, Booth J, Schaub RG, Read MS. Inactivation-resistant human coagulation factor VIIIa (IR8) corrects canine hemophilia A coagulopathy. Thromb Hæmost. 1999;82 :488. abstr suppl XVII Congress of the ISTH. [Google Scholar]
- Nichols TC, Merricks E, Muchitsch EM, Baumgartner B, Turecek PL, Ahmad RU, Gritsch H, Schwarz HP. Pharmacokinetics (PK) of rVWF in dogs and mice with VWD. 2007;XXI ISTH Abstracts:P-W-197. [Google Scholar]
- Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, Rick ME, Sadler JE, Weinstein M, Yawn BP. von Wille-brand disease (VWD): Evidence-based diagnosis and management guidelines. National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Hæmophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD., Jr Long term correction of inhibitor prone hemophilia B dogs treated with liver-directed AAV2 mediated factor IX gene therapy. Blood. 2008 doi: 10.1182/blood-2008-10-181479. prepublished online Oct 28, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlfest JR, Frandsen JL, Fritz S, Lobitz PD, Perkinson SG, Clark KJ, Nelsestuen G, Key NS, McIvor RS, Hackett PB, Largaespada DA. Phenotypic correction and long-term expression of factor VIII in hemophilic mice by immunotolerization and nonviral gene transfer using the Sleeping Beauty transposon system. Blood. 2005;105:2691–2698. doi: 10.1182/blood-2004-09-3496. [DOI] [PubMed] [Google Scholar]
- Oldenburg J, Schwaab R. Molecular biology of blood coagulation. Semin Thromb Hemost. 2001;27:313–324. doi: 10.1055/s-2001-16885. [DOI] [PubMed] [Google Scholar]
- Olsen EHN, McCain AS, Merricks EP, Fischer TH, Dillon IM, Raymer RA, Bellinger DA, Fahs SA, Montgomery RR, Keith JC, Jr, Schaub RG, Nichols TC. Comparative response of plasma VWF in dogs to up-regulation of VWF mRNA by interleukin-11 versus Weibel-Palade body release by desmopressin (DDAVP) Blood. 2003;102:436–441. doi: 10.1182/blood-2003-01-0290. [DOI] [PubMed] [Google Scholar]
- Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: The role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832–2839. doi: 10.1182/blood-2006-06-032524. [DOI] [PubMed] [Google Scholar]
- Owen CA., Jr . In: A History of Blood Coagulation. Nichols WL, Bowie EJW, editors. Rochester MN: Mayo Foundation for Medical Education and Research; 2001. [Google Scholar]
- Park F, Ohashi K, Kay MA. Therapeutic levels of human factor VIII and IX using HIV-1-based lentiviral vectors in mouse liver. Blood. 2000;96:1173–1176. [PubMed] [Google Scholar]
- Parker RI, McKeown LP, Gallin JI, Gralnick HR. Absence of the largest platelet-von Willebrand multimers in a patient with lactoferrin deficiency and a bleeding tendency. Thromb Hæmost. 1992;67:320–324. [PubMed] [Google Scholar]
- Pasi KJ. Gene therapy for Hæmophilia. Br J Hæmatol. 2001;115:744–757. doi: 10.1046/j.1365-2141.2001.03225.x. [DOI] [PubMed] [Google Scholar]
- Patek AJ, Taylor FH. Hemophilia. II. Some properties of a substance obtained from normal human plasma effective in accelerating the coagulation of hemophilic blood. J Clin Invest. 1937;16:113–24. doi: 10.1172/JCI100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton S. Canine Technologies, Model Patients: The Historical Production of Hemophiliac Dogs in American Biomedicine. New York: Routledge; 2004. pp. 191–213. [Google Scholar]
- Pipe SW. Coagulation factors with improved properties for hemophilia gene therapy. Semin Thromb Hemost. 2004;30:227–237. doi: 10.1055/s-2004-825636. [DOI] [PubMed] [Google Scholar]
- Pipe SW, Kaufman RJ. Characterization of a genetically engineered inactivation-resistant coagulation factor VIIIa. Proc Natl Acad Sci U S A. 1997;94:11851–11856. doi: 10.1073/pnas.94.22.11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipe SW, Nichols TC, McClintock DWD, Zollner R, Thomas J, Schaub R, Read MS, Saenko EL, Kaufman RJ. The conformation of the factor VIII light chain can modulate von Willebrand factor binding affinity without affecting cofactor activity in vitro and in vivo. Blood. 1999;94 (Suppl 1):453a. [Google Scholar]
- Ponder KP. Gene therapy for hemophilia. Curr Opin Hematol. 2006;13:301–307. doi: 10.1097/01.moh.0000239700.94555.b1. [DOI] [PubMed] [Google Scholar]
- Powell JS, Ragni MV, White GC, 2nd, Lusher JM, Hillman-Wiseman C, Moon TE, Cole V, Ramanathan-Girish S, Roehl H, Sajjadi N, Jolly DJ, Hurst D. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102:2038–2045. doi: 10.1182/blood-2003-01-0167. [DOI] [PubMed] [Google Scholar]
- Ragni MV, Jankowitz RC, Chapman HL, Merricks EP, Kloos MT, Dillow AM, Nichols TC. A phase II prospective open-label escalating dose trial of recombinant interleukin-11 in mild von Willebrand disease. Hæmophilia. 2008;14:968–977. doi: 10.1111/j.1365-2516.2008.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawle FE, Lillicrap D. Preclinical animal models for hemophilia gene therapy: Predictive value and limitations. Semin Thromb Hemost. 2004;30:205–213. doi: 10.1055/s-2004-825634. [DOI] [PubMed] [Google Scholar]
- Read MS, Shermer RW, Brinkhous KM. Venom coagglutinin: An activator of platelet aggregation dependent on von Willebrand Factor. Proc Natl Acad Sci U S A. 1978;75:4514–4518. doi: 10.1073/pnas.75.9.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recombinant DNA Advisory Committee Meeting. Hum Gene Ther; Recombinant DNA Advisory Committee Meeting; March 7, 2002; 2002. pp. 989–1000. [Google Scholar]
- Rieger M, Schwarz HP, Turecek PL, Dorner F, van Mourik JA, Mannhalter C. Identification of mutations in the canine von Willebrand factor gene associated with type III von Willebrand disease. Thromb Hæmost. 1998;80:332–337. [PubMed] [Google Scholar]
- Roosendaal G, Lafeber F. Prophylactic treatment for prevention of joint disease in hemophilia: Cost versus benefit. N Engl J Med. 2007;357:603–605. doi: 10.1056/NEJMe078098. [DOI] [PubMed] [Google Scholar]
- Roth DA, Tawa NE, Jr, O’Brien JM, Treco DA, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–1742. doi: 10.1056/NEJM200106073442301. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM, Ware J. von Willebrand factor. FASEB J. 1993;7:308–316. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- Russell KE, Read MS, Bellinger DA, Leitermann K, Rup BJ, McCarthy KP, Keith JC, Schaub RG, Khor SP, Nichols TC. Intratracheal administration of recombinant human factor IX (BeneFixTM) achieves therapeutic levels in hemophilia B dogs. Thromb Hæmost. 2001;85:445–449. [PubMed] [Google Scholar]
- Russell KE, Olsen EHN, Raymer RA, Merricks EP, Bellinger DA, Read MS, Rup BJ, Keith JC, Jr, McCarthy KP, Schaub RG, Nichols TC. Reduced bleeding events with subcutaneous administration of recombinant human factor IX in immune tolerant hemophilia B dogs. Blood. 2003;102:4393–4398. doi: 10.1182/blood-2003-05-1498. [DOI] [PubMed] [Google Scholar]
- Sabatino D, Lange AM, Sarkar R, Dillow AM, Nichols TC, Arruda VR, Kazazian HH. Successful long term expression of F.VIII after gene delivery of novel adeno-associated viral vector serotypes in hemophilia A dogs. 2007 XXI ISTH Abstracts:P-w-231. [Google Scholar]
- Sadler JE. von Willebrand factor: Two sides of a coin. J Thromb Hæmost. 2005;3:1702–1709. doi: 10.1111/j.1538-7836.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- Sadler JE, Rodeghiero F. Provisional criteria for the diagnosis of VWD type 1. J Thromb Hæmost. 2005;3:775–777. doi: 10.1111/j.1538-7836.2005.01245.x. [DOI] [PubMed] [Google Scholar]
- Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB. Update on the pathophysiology and classification of von Willebrand disease: A report of the Subcommittee on von Willebrand Factor. J Thromb Hæmost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Zhu X, Xiao X, Brook JD, Bouseman DE, Epstein N, Hunter LA. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, Wilson JM, Kazazian HH., Jr Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, Kazazian HH. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia A dogs and mice. Hum Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- Scallan CD, Liu T, Parker AE, Patarroyo-White SL, Chen H, Jiang H, Vargas J, Nagy D, Powell SK, Wright JF, Sarkar R, Kazazian HH, McClelland A, Couto LB. Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII. Blood. 2003;102:3919–3926. doi: 10.1182/blood-2003-01-0222. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Morral N, Parks RJ, Wu Y, Koopmans SC, Langston C, Graham FL, Beaudet AL, Kochanek S. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet. 1998;18:180–184. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- Schneppenheim R, Thomas KB, Sutor AH. Von Willebrand disease in childhood. [Review] Semin Thromb Hemost. 1995;21:261–275. doi: 10.1055/s-2007-1000648. [DOI] [PubMed] [Google Scholar]
- Schuettrumpf J, Herzog RW, Schlachterman A, Kaufhold A, Stafford DW, Arruda VR. Factor IX variants improve gene therapy efficacy for hemophilia B. Blood. 2005;105:2316–2323. doi: 10.1182/blood-2004-08-2990. [DOI] [PubMed] [Google Scholar]
- Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AD, Di Paola J, Cohen A, Pasi KJ, Heisel MA, Blanchette VS, Abshire TC, Hoots WK, Lusher JM, Negrier C, Rothschild C, Roth DA. The safety and efficacy of recombinant human blood coagulation factor IX in previously untreated patients with severe or moderately severe hemophilia B. Blood. 2005;105:518–525. doi: 10.1182/blood-2004-06-2283. [DOI] [PubMed] [Google Scholar]
- Sheldon-Inloes BB, Titani K, Sadler JE. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms. Biochemistry. 1986;25:3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wilcox DA, Fahs SA, Kroner PA, Montgomery RR. Expression of human factor VIII under control of the platelet-specific alphaIIb promoter in megakaryocytic cell line as well as storage together with VWF. Mol Genet Metab. 2003;79:25–33. doi: 10.1016/s1096-7192(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, Desai D, Morateck PA, Gorski J, Montgomery RR. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116:1974–1982. doi: 10.1172/JCI28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman JL, Read MS, Stewart PF, Keith JC, Garzone P, McCarthy K, Schaub RG, Brinkhous KM. High molecular weight factor IX aggregate isolated from a highly purified plasma-derived concentrate does not correct hemostasis in the hemophilia B dog. Thromb Hæmost. 1997;77:220. (abstr suppl) [Google Scholar]
- Sleeper MM, Bish LT, Sweeney HL. Gene therapy in large animal models of human cardiovascular genetic disease. ILAR J. 2009;50:199–205. doi: 10.1093/ilar.50.2.199. [DOI] [PubMed] [Google Scholar]
- Smiley R, Titley P, Rock G. Studies on the prolonged bleeding time in von Willebrand’s disease. Thromb Res. 1989;53:417–426. doi: 10.1016/0049-3848(89)90196-5. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, Gown AM, Winther B, Meuse L, Cohen LK, Thompson AR, Kay MA. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Donahue BA, Lin H-F, Stafford DW, Patel S, Thompson A, Nichols T, Bellinger D, Read M, Brinkhous KM, Kay MA. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- Sporn LA, Chavin SI, Marder V, Wagner D. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest. 1985;76:1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sramek A, Kriek M, Rosendaal FR. Decreased mortality of ischæmic heart disease among carriers of hæmophilia. Lancet. 2003;362:351–354. doi: 10.1016/s0140-6736(03)14021-4. [DOI] [PubMed] [Google Scholar]
- Stern DM, Knitter G, Kisiel W, Nawroth PP. In vivo evidence of intravascular binding sites for coagulation factor IX. Br J Hæmatol. 1987;66:227–232. doi: 10.1111/j.1365-2141.1987.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Hakobyan N, Valentino LA, Feldman BL, Samulski RJ, Monahan PE. Intra-articular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood. 2008;112:4532–4541. doi: 10.1182/blood-2008-01-131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinlin S, Webster S, Giles AR. The development of homologous (canine/anti-canine) antibodies in dogs with hæmophilia A (factor VIII deficiency): A ten-year longitudinal study. Thromb Hæmost. 1993;69:21–24. [PubMed] [Google Scholar]
- Tuddenham EGD. Factor VIII: Purer is not necessarily safer. Blood. 2006;107:4–5. [Google Scholar]
- Van Cott KE, Monahan PE, Nichols TC, Velander WH. Hæmophilic factors produced by transgenic livestock: Abundance that can enable alternative therapies worldwide. Hæmophilia. 2004;10(Suppl 4):70–76. doi: 10.1111/j.1365-2516.2004.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg HM, De Groot PH, Fischer K. Phenotypic heterogeneity in severe hemophilia. J Thromb Hæmost. 2007;5(Suppl 1):151–156. doi: 10.1111/j.1538-7836.2007.02503.x. [DOI] [PubMed] [Google Scholar]
- Viuff D, Ezban M, Lind V, Dillow A, Raymer R, Merricks E, Nichols T. Effect of rFVIIa and NN1731 (rFVIIa analogue) on thromboelastography (TEG) in whole blood obtained from hemophilia A dogs. 2007 XXI ISTH Abstracts:P-W-113. [Google Scholar]
- Wagner RH, Langdell RD, Richardson BA, Farrell RA, Brinkhous KM. Antihemophilic factor (AHF): Plasma levels after administration of AHF preparations to hemophilic dogs. Proc Soc Exp Biol Med. 1957;96:152–155. doi: 10.3181/00379727-96-23417. [DOI] [PubMed] [Google Scholar]
- Wagner RH, Roberts HR, Webster WP, Shanbrom E, Brinkhous KM. Glycine-precipitated antihemophilic factor concentrates and their clinical use. Thromb Diath Hæmorrh Suppl. 1968;35:41–48. [PubMed] [Google Scholar]
- Wang L, Nichols TC, Read MS, Bellinger DA, Verma IM. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, Wilson JM. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chamberlain JS, Tapscott SJ, Storb R. Gene therapy in large animal models of muscular dystrophy. ILAR J. 2009;50:187–198. doi: 10.1093/ilar.50.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster WP, Roberts HR, Thelin GM, Wagner RH, Brinkhous KM. Clinical use of a new glycine-precipitated antihemophilic fraction. Amer J Med Sci. 1965;250:643–651. doi: 10.1097/00000441-196512000-00005. [DOI] [PubMed] [Google Scholar]
- Webster WP, Penick GD, Peacock EE, Brinkhous KM. Allotransplantation of spleen in hemophilia. NC Med J. 1967;28:505–507. [Google Scholar]
- Webster WP, Zukoski CF, Hutchin P, Reddick RL, Mandel SR, Penick GD. Plasma factor VIII synthesis and control as revealed by canine organ transplantation. Am J Physiol. 1971;220:1147–1154. doi: 10.1152/ajplegacy.1971.220.5.1147. [DOI] [PubMed] [Google Scholar]
- Webster WP, Mandel SR, Reddick RL, Wagner JL, Penick GD. Orthotopic liver transplantation in canine hemophilia B. Am J Physiol. 1974;226:496–500. doi: 10.1152/ajplegacy.1974.226.3.496. [DOI] [PubMed] [Google Scholar]
- White GC. Gutted Adenoviral Vector Delivering Factor VIII: Clinical Results. National Hemophilia Foundation Fifth Workshop on Gene Therapies for Hemophilia; Philadelphia. 2002. [Google Scholar]
- White GC, Beebe A, Nielson B. Recombinant Factor IX. Thromb Hæmost. 1997;78:261–265. [PubMed] [Google Scholar]
- Wilcox DA, Shi Q, Nurden P, Haberichter SL, Rosenberg JB, Johnson BD, Nurden AT, White GC, 2nd, Montgomery RR. Induction of mega-karyocytes to synthesize and store a releasable pool of human factor VIII. J Thromb Hæmost. 2003;1:2477–2489. doi: 10.1111/j.1538-7836.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- Wolberg AS, Stafford DW, Erie DA. Human factor IX binds to specific sites on the collagenous domain of collagen IV. J Biol Chem. 1997;272:16717–16720. doi: 10.1074/jbc.272.27.16717. [DOI] [PubMed] [Google Scholar]
- Wu QY, Drouet L, Carrier JL, Rothschild C, Berard M, Rouault C, Caen J, Meyer D. Differential distribution of von Willebrand factor in endothelial cells: Comparison between normal pigs and pigs with von Willebrand disease. Arteriosclerosis. 1987;7:47–54. doi: 10.1161/01.atv.7.1.47. [DOI] [PubMed] [Google Scholar]
- Wu SM, Stafford DW, Frazier LD, Fu YY, High KA, Chu K, Sanchez-Vega B, Solera J. Genomic sequence and transcription start site for the human gamma-glutamyl carboxylase. Blood. 1997;89:4058–4062. [PubMed] [Google Scholar]
- Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, Samulski RJ, Monahan PE. Optimization of self-complementary AAV vectors for liver- directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompentent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Ma H, McCown TJ, Verma IM, Kafri T. Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol Ther. 2001;3:97–104. doi: 10.1006/mthe.2000.0238. [DOI] [PubMed] [Google Scholar]
- Xu L, Gao C, Sands MS, Cai SR, Nichols TC, Bellinger DA, Raymer RA, McCorquodale S, Ponder KP. Neonatal or hepatocyte growth factor-potentiated adult gene therapy with a retroviral vector results in therapeutic levels of canine factor IX for hemophilia B. Blood. 2003;101:3924–3932. doi: 10.1182/blood-2002-10-3050. [DOI] [PubMed] [Google Scholar]
- Xu L, Nichols TC, Sarkar R, McCorquodale S, Bellinger DA, Ponder KP. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc Natl Acad Sci U S A. 2005;102:6080–6085. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Mei M, Haskins ME, Nichols TC, O’Donnell P, Cullen K, Dillow A, Bellinger D, Ponder KP. Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice. Thromb Res. 2007;120:269–280. doi: 10.1016/j.thromres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Yan JB, Wang S, Huang WY, Xiao YP, Ren ZR, Huang SZ, Zeng YT. Transgenic mice can express mutant human coagulation factor IX with higher level of clotting activity. Biochem Genet. 2006;44:349–360. doi: 10.1007/s10528-006-9034-1. [DOI] [PubMed] [Google Scholar]
- Yarovoi HV, Kufrin D, Eslin DE, Thornton MA, Haberichter SL, Shi Q, Zhu H, Camire R, Fakharzadeh SS, Kowalska MA, Wilcox DA, Sachais BS, Montgomery RR, Poncz M. Factor VIII ectopically expressed in platelets: Efficacy in hemophilia A treatment. Blood. 2003;102:4006–4013. doi: 10.1182/blood-2003-05-1519. [DOI] [PubMed] [Google Scholar]
- Ye P, Thompson AR, Sarkar R, Shen Z, Lillicrap DP, Kaufman RJ, Ochs HD, Rawlings DJ, Miao CH. Naked DNA transfer of factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice. Mol Ther. 2004;10:117–126. doi: 10.1016/j.ymthe.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Zauber NP, Levin J. Factor IX levels in patients with hemophilia B (Christmas disease) following transfusion with concentrates of factor IX or fresh frozen plasma (FFP) Medicine (Baltimore) 1977;56:213–224. doi: 10.1097/00005792-197705000-00003. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Eriksson M, Falk G, Graff C, Presnell SC, Read MS, Nichols TC, Blomback M, Anvret M. Failure to achieve gene conversion with chimeric circular oligonucleotides: Potentially misleading PCR artifacts observed. Antisense Nucleic A. 1998;8:531–536. doi: 10.1089/oli.1.1998.8.531. [DOI] [PubMed] [Google Scholar]