Abstract
Interferon-α, currently used for the treatment of hepatitis C, is associated with a substantially elevated risk of depression. However, not everyone who takes this drug becomes depressed, so it is important to understand what particular factors may make some individuals more ‘at risk’ of developing depression than others. Currently there is no consensus as to why interferon-induced depression occurs and the range of putative risk factors is wide and diverse. The identification of risk factors prior to treatment may allow identification of patients who will become depressed on interferon, allowing the possibility of improved treatment support and rates of treatment adherence. Here, we consolidate and review the literature on risk factors, and we discuss the potential confounds within the research examined in order to better isolate the risk factors that may be important in the development of depression in these patients and which might help predict patients likely to become depressed on treatment. We suggest that interactions between psychobehavioral, genetic, and biological risk factors are of particular importance in the occurrence of depression in patients with hepatitis C taking interferon-α.
Keywords: interferon-α, risk factors, depression, hepatitis C
Background
Hepatitis C virus (HCV) is a major cause of liver disease and death and affects ∼170 million people worldwide.1 Recent epidemiological studies suggest more than 90% of transmission in developed countries occurs through the sharing of nonsterilized needles and syringes in the intravenous drug-using population, ‘unknown’ sources, and ‘other’ sources such as dialysis, hemophilia, sexual transmission, and tattoos or piercing.2–5 Rates through blood transfusion are decreasing dramatically in the developed world due to improved screening for viruses in blood donations since the early 1990s, although progress is somewhat slower in developing countries.2 Progression from initial infection to liver cirrhosis and cancer can take 20–30 years;6,7 thus, complications from chronic HCV infection are, therefore, anticipated to continue to increase,8 with rates of cirrhosis doubling, liver-related deaths tripling, and HCV becoming the leading reason for liver transplantation.9
There is no vaccine for HCV; thus, the need for effective treatments for this infection is critical. Currently, the main Federal Drug Authority-approved treatment for HCV is the use of the proinflammatory cytokine interferon-α (IFN-α), a multifunctional pleiotropic protein with antiproliferative, antiviral, and immunoregulatory functions,10 which is usually used in combination with broad-spectrum antiviral ribavirin. Unfortunately, this treatment is only effective in ∼40%–80% of patients, with efficacy varying dramatically between genotypes.11,12 Treatment is also expensive and has a well-documented profile of physical, behavioral, and psychiatric side effects, including flu-like symptoms, fatigue, insomnia, depressed mood, and irritability,12–16 all of which could impact upon compliance. Depression is a particularly common side effect that may occur in up to 60% of patients,17,18 and in some rare cases, it may be associated with deliberate self-harm or suicide attempts.19,20 The neuropsychiatric side effects of IFN-α therapy are among the commonest causes of treatment discontinuation,21,22 and risk factors such as baseline psychiatric symptoms and interpersonal problems can predict poor compliance.23 Identification of the risk factors that lead to neuropsychiatric side effects and the associated low adherence rates may help identify patients at risk who may then benefit from additional psychological assessment and support.
Current treatment for IFN-α-induced depression involves the coadministration of selective serotonin reuptake inhibitors (SSRIs), which have been shown to improve treatment adherence in depressed patients.24 However, variations in prescribing practice exist. Some clinicians wait for the depression to occur before prescribing these drugs, meaning up to a month may pass before a reduction in depressive symptoms occurs.25 Other clinicians adopt a policy of prophylactically administering SSRIs to reduce the occurrence of depression and depressive symptomology, with dose modification where necessary,26–28 but this means a large number of patients who do not need an SSRI could be prescribed one. Where studies have identified ‘at-risk’ patients for prophylaxis of antidepressants and psychological support, these have both proven effective methods of reducing the occurrence of depression.29–31
In order to avoid depressive complications and associated treatment compliance risks, identification of risk factors for the subsequent development of depression could assist clinicians in deciding to coprescribe an antidepressant and psychological support at the start of antiviral treatment. Here, we provide an overview of data on HCV patients taking IFN-α in order to assess potential risk factors that affect the development of depression.
The main risk factors identified and investigated in this literature review can be divided into five main categories: biological, demographic, treatment related, genetic, and psychobehavioral. Here, we examine these categories and review evidence that these potential risk factors pose a sufficiently substantive risk of depression that pretreatment support should be considered.
Methods
A literature search was conducted in PubMed using the keywords ‘depression’, ‘interferon-α’, and ‘hepatitis C’. A total of 169 English, full-text, original research articles where rates of depression were assessed in the target population were then screened for their assessment of risk factors of depression. Those articles that used formal statistical analysis to assess possible risk factors were then broken down into five broad categories: biological, demographic, treatment related, genetic, and psychobehavioral. Once these categories were identified, further PubMed searches were conducted including category titles and subtitles as additional keywords. In total, 52 articles were assessed as part of the main review, and results from these articles are presented along with supporting evidence.
Review of risk factors
Biological risk factors
Biological mechanisms
Many humans or animals administered with a cytokine develop a behavioral syndrome and set of somatic effects termed ‘sickness behavior’; these behaviors result in an alteration of the motivational state of an organism so that it can preferentially respond to infections.32–34 In other words, the organism will have depressed functioning of mood, activity, and metabolism so that all of its energy is put into fighting illness. The observation that sickness behavior and depression share common features35 (see Table 1) has led many researchers to implicate raised levels of cytokines in the pathology of depression, giving rise to the cytokine theory of depression.
Table 1.
| Depression | Sickness behavior | |
|---|---|---|
| Somatic effects | Fatigue/loss of energy | Fatigue |
| Weight loss/weight gain | Weight loss | |
| Appetite loss/appetite increase | Anorexia | |
| Sleep disorders | ||
| Insomnia/hypersomnia | Increased body temperature | |
| Behavioral effects | Depressed mood | Depressed mood |
| Feelings of worthlessness/guilt | Behavioral despair | |
| Social withdrawal | ||
| Loss of interest in activities | Anhedonia | |
| Anhedonia | Cognitive impairment | |
| Inability to concentrate | ||
| Psychomotor agitation/retardation | Suppression motor behavior | |
| Recurrent thoughts of death |
Note: Similarities between a major depressive episode and sickness behavior. Copyright © 2009, Les Laboratories Servier, Neuilly-sur-Seine, France. Adapted from Lotrich FE. Major depression during interferon-alpha treatment: vulnerability and prevention. Dialogues Clin Neurosci. 2009;11(4):417–425.
The cytokine theory of depression suggests biological vulnerability in certain individuals that is linked, in part, to the immune system.33 This could be exposed by a compound that stimulates the immune system (such as IFN-α), and increases in cytokines such as interleukin-6 (IL-6) and IL-10 are positively correlated with depressive symptoms.40,41 The enhanced cytokine response seen in depressed patients resonates with data that shows that people who become depressed during treatment are more likely to have a sustained antiviral response.42,43
Several potential biological mechanisms for this vulnerability to developing an IFN-α-induced depression have been examined.17,38,42,44–47 Three main mechanisms that are supported by clinical data are introduced briefly before clinical data assessing their relationship with depression is discussed.
Serotonin
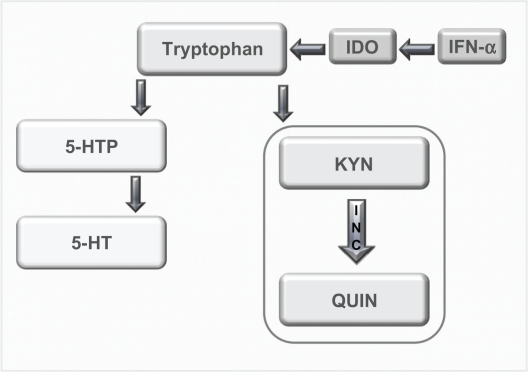
The neurotransmitter serotonin has long been believed to play a role in depression;48,49 however, more recent research has linked the observed decrease in serotonin with a cytokine-mediated pathway.50,51 When IFN-α is administered, it has been shown that there is an upregulation of the enzyme indoleamine 2,3-dioxygenase (IDO) that metabolizes tryptophan, the precursor to serotonin.34 Thus, when IDO is overstimulated, there is potentially a reduction in plasma tryptophan and serotonin in the brain (see Figure 1),42,45,52 which may lead to depression.51
Figure 1.
Tyrptophan–Kynurenine pathway. Diagram showing the alteration of tryptophan metabolism by IFN-α. Tryptophan is normally converted in 5-hydroxy tryptophan (5-HTP) and serotonin (5-HT). However, this metabolism is switched to the KYN pathway by IDO, which is induced by immune stimuli such as IFN-α, and it is this pathway that produced the neurotoxin quinolinic acid (QUIN). Copyright © 2002, Elsevier. Adapted with permission from Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25(3):154–159.
Serotonin as a risk factor
Clinical studies in HCV patients have provided mixed results regarding the involvement of serotonin in the IFN-α-induced depression; some studies support a correlation between lower tryptophan levels and scores on a depression scale53 and others do not find any correlation with tryptophan54 or whole blood serotonin.55 However, lower tryptophan has been correlated with other behavioral issues associated with depression such as increased irritability and aggression.56 Reasons for differences in results could be in part explained by the low number of participants in the studies and also the issue of correlating the centrally driven disorder of depression with peripheral biomarkers. This issue has been addressed by two recent studies conducted by Raison et al.57,58 The first study found that when IFN-α was administered peripherally, there was an increase in the amount of kynurenine (KYN) and quinolinic acid (QUIN) centrally, which correlated with Montgomery–Asberg Depression Rating Scale (MADRS) scores. The second study found that cerebrospinal fluid (CSF) levels of the serotonin metabolite 5-hydroxyindoleacetic acid were significant predictors of depressive symptoms.57
Further support for the involvement of this system is the observation that depressive symptoms in this cohort are effectively ameliorated by the use of SSRIs,25–28,59 implicating some role for serotonin in the relief of IFN-α-induced depression.
Hypothalamic–pituitary–adrenal axis
Interferons are acknowledged to activate the hypothalamic–pituitary–adrenal (HPA) axis,60 which could in part be linked to the development of depression.61–63 Recent research has identified a pathway where stress can increase glucocorticoids and corticotrophin-releasing hormones, which act in tandem with an increase in cytokines to disrupt levels of serotonin, norepinephrine, and dopamine.50,64,65 Vulnerability in this pathway could mean a stress-related response to cytokines is in part responsible for IFN-induced depression.
HPA axis as a risk factor
There are mixed results for the involvement of peripheral biomarkers of HPA axis functioning in IFN-α-induced depression. One study found that IFN-α does not induce a change in plasma cortisol levels and cannot be correlated with depression scores.55 Another found that although there was a significant increase in daily salivary cortisol following 8 weeks of treatment, this change was not significantly associated with depression scores.66
However, a recent study that compared HCV patients who were and were not taking IFN-α found that treatment was associated with a relative flattening of the diurnal slope for both salivary cortisol and adrenocorticotrophin-releasing hormone production and that flattening of the cortisol slope was associated with depressive symptoms, particularly somatic depressive symptoms.67 Issues leading to mixed results in these studies include the low number of participants and different measurements of cortisol (plasma cortisol and salivary cortisol). Another issue is the differing analyses employed by investigators with Raison et al67 analyzing the daily cortisol slope, whereas Wichers et al66 analyzed daily average cortisol levels.
Cytokine mechanisms
As IFN-α is a cytokine designed to affect immune system functioning, some researchers have chosen to look at baseline immune system functioning and the relative increase in cytokines induced by treatment to see if that had any effect on the subsequent development of depression.
One of the main questions regarding biological risk factors for development of an IFN-α-induced depression is how the immune system, which is a peripheral system, can exert effects on mood and behavior via the central nervous system (CNS). There are two main theories regarding this question; the first supposes that cytokines may enter the brain. There are a number of ways in which cytokines may enter the brain. The first is via circumventricular regions where the blood–brain barrier (BBB) is more permeable68 such as the organum vasculosum of the lamina terminalis, with uptake mechanisms for cytokines such as IL-1 being demonstrated at the surface of the BBB.69,70 Active transport mechanisms for transportation into the brain are also being identified for cytokines, including IL-171 and tumor necrosis factor-α (TNF-α).72 Another hypothesis concerns the induction of adhesion molecules such as vascular endothelial growth factor-1 in the brain endothelium, which increases the potential for circulating T-lymphocytes to cross the BBB.73
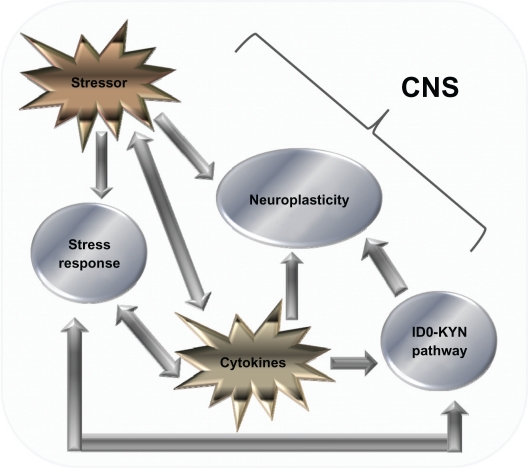
However, the second theory suggests that the immune system influences secondary factors that in turn interact with the CNS.32 In the second scenario, there is a complex interplay between stressors, the IDO–KYN pathway (see Figure 2), and factors that influence neuroplasticity in the development of cytokine-induced depression (see Figure 2).57,74,75
Figure 2.
Cytokine-mediated pathways that influence the CNS. Diagram showing the various factors that influence the CNS. There is a complex relationship, the relationship with monoamines and the IDO–Kyn pathway, growth factors, and stress. Stressors and cytokines both increase the stress response, which is reflected by an increase in the amount of CRH, both in the CNS and peripherally, which in turn activates ACTH and cortisol (CORT) levels. CRH also has a bidirectional relationship with serotonin (5-HT) levels, and gamma aminobutyric acid acts as a mediator for this process. 5-HT levels are also influenced by the production of IDO, which favors the production of the neurotoxin KYN over 5-HT. The stressor system and IDO–KYN pathway both lead to a reduction in 5-HT. Cytokines also influence oxidative and apoptotic mechanisms, leading to a reduction in growth factors such as brain-derived neurotrophic factor, which in turn leads to impaired neuroplastic processes and decreased neurogenesis, as well as cytokines having an indirect effect on growth factor levels; stress has also been shown to have a direct effect. The culmination of these three pathways can lead to the development of major depression. Copyright © 2005, Elsevier. Adapted with permission from Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135(3):659–678.
Abbreviation: CRH, corticotrophin-releasing hormone.
Cytokines as a risk factor
The role of cytokines in IFN-α-induced depression is a complex one, with circulating cytokines, but not central cytokines, appearing to have a relationship with depression scores. It was found that a higher IL-6 response at weeks 2–4 was predictive of a higher MADRS score 4–6 months later.40 Another study found that pretreatment levels of circulating IL-6 predicted the incidence of developing a major depressive disorder (MDD), and using regression analyses, the authors were able to predict the following month’s Beck Depression Inventory (BDI) score using the previous month’s IL-6 levels.41 Other studies have also found that a significantly increased baseline concentration of IL-6 can be a predictor for subsequent depression.76 In addition to IL-6, other peripheral cytokines shown to correlate with an increase in depressive symptoms include the soluble IL-2 receptor and TNF-α,66 with baseline levels of soluble IL-2 receptor and IL-10 being significantly increased in those patients who went on to develop depression.76 Another study that examined the relative importance of cytokines in depressive symptomology found that TNF-α and not IL-6 was correlated with mood scores.67
However, when central levels of cytokines were measured in CSF while IFN-α induced a significant increase in the cytokines IL-6 and monocyte chemoattractant protein-1 (MCP-1), only CSF concentrations of 5-hydroxyindoleacetic acid predicted depressive symptoms.57 These findings could mean that only peripheral cytokines can be correlated with depressive symptoms, possibly due to the influence of peripheral cytokines on the neurovegetative symptoms of depression.
Recent research is attempting to determine the specific neurobehavioral effect of cytokines by acknowledging the multidimensional nature of MDD as a disorder consisting of a number of specific subgroups of symptoms.47 There is a possibility that cytokines may be involved in certain aspects of the depressive syndrome, such as sleep and appetite changes, rather than being the central neurobiological cause of depression.35 There has been evidence that IL-6 may be linked in with poor sleep quality in patients taking IFN-α,41 with poor sleep quality being identified as a predicting factor for the development of a subsequent depression.41,77
There is substantive support for the role of biological risk factors in the development of the IFN-α-induced depression. However, as results are not always conclusive and replicable, there is a possibility that this risk factor acts in conjuntion with other factors in the development of a depression.
Genetic risk factors
Biological mechanisms responsible for IFN-α-induced depression will inevitably be influenced by underlying genetic vulnerability. Several genetic polymorphisms have been associated with an increased risk of developing depression. One of the most reproducible findings in this area is the observation that people who have the short allele in the functional 5′ promoter of the serotonin transporter gene (5-HTTLPR) are significantly more likely to be depressed in response to a major life event78,79 or in general80,81 than those people with the long version of this allele. Other genes implicated in depression are involved in the functioning of glucocorticoids,82 neuronal growth,83 dystonia,84 as well as the 5-HT1 A autoreceptor gene,85 and the IL-1β gene86 among others.87,88
These genes offer potential avenues for researchers looking to identify genetic risk factors to explore, and recent research conducted in HCV patients taking IFN has identified a number of genes that have been shown to correlate with depression (see Table 2). The 5-HT transporter gene has been shown to have a role in the development of depression in addition to polymorphisms in the interferon receptor-A1 gene, the apoloprotein (APOE) 4 allele, the cyclooxygenase 2 (COX2) and phospholipase A2 (PLA2) genes, and the IL-6 gene in patients taking IFN (see Table 2). A number of researchers have shown that the short version of the 5-HTTLPR increases the risk of developing a depressive episode when undertaking IFN-α therapy.92,93
Table 2.
Genetic risk factors
| Authors | No. of participants | Areas assessed | Results |
|---|---|---|---|
| Bull et al89 | 98 | Relationship between polymorphisms in the IL-6 and 5-HTT genes and symptoms of depression and fatigue in patients taking IFN-α | IL-6: A ‘low IL-6’ synthesizing genotype was associated with significantly fewer depressive symptoms during treatment 5-HTT: A ‘high transcription 5-HTT’ genotype was associated also with fewer depressive symptoms The authors propose an interaction in these genes offering a protective role |
| Gochee et al90 | 110 | Association between APOE genotypes and neuropsychiatric symptoms in patients taking IFN-α | APOEέ4 allele: More likely to be referred to a psychiatrist and had more neuropsychiatric symptoms (more likely to experience irritability, anger, and mood disturbances). In addition, more likely to experience a neuropsychiatric event sooner Depression carrier 10 (38.5%), noncarrier 29 (34.5%), irritability 15/17 (57.7%/20.2%), anxiety/other mood 15/18 (57.7%/21.4%) prior psychiatric 9/15 (34.6%/17.9%) However, this gene and a previous history of psychiatric illness was not significant |
| Kraus et al91 | 139 | Investigate the impact of genetic variations within the serotonin system on likelihood of depression development during treatment | 5-HT1A receptor gene HTR1A variation: Those patients homozygous for this gene were significantly more likely to develop depression during treatment and have significantly higher HADS scores when compared to patients who carried 5-HTT or TPH2 polymorphisms |
| Lotrich et al92 | 71 | Influence of the serotonin transporter gene in development of depression in patients taking IFN-α | 5-HTTLPR: Those patients with the LA -allele were less likely to develop depression, whereas those patients who displayed the S/S allele of this gene the most vulnerable to developing depression. This increased rate of depression in vulnerable patients was reported to be related to poor sleep quality in carriers of the S/S allele |
| Pierucci-Lagha et al93 | 1015 | Assess the influence of a polymorphism in the serotonin transporter gene on subsequent depression development | 5-HTTLPR: 5-HTLLPR genotype moderated the occurrence of depression in non-Hispanic Caucasians and Hispanic Caucasians but not African-Americans In non-Hispanic Caucasians, the L’ allele was associated with depression scores, whereas in Hispanic Caucasians, the S’-allele was associated with depression scores In both these populations, the 5-HTTLPR genotype interacted with psychiatric history and time to produce higher depression scale scores |
| Su et al94 | 132 | Ascertain the influence of polymorphisms in genes that regulate the metabolism of polyunsaturated fatty acids (PLA2 and COX2) | COX2 rs4648308 and PLA2 Banl GG genotypes: Those patients who had the COX2 rs4648308 allele were significantly more likely to develop a depression. The PLA2 Banl polymorphism was associated with a higher severity of the somatic symptoms of depression |
| Yoshida et al95 | 50 | Development of depression in HCV patients taking IFNα and how this relates to four promoter polymorphisms of the IFNAR1 gene | GT promoter repeat dinucleotide microsatellite polymorphism of the IFNAR1 gene: Patients with the 5/14 genotype of the GT repeat dinucleotide microsatellite polymorphism showed greater differences in the baseline to maximum difference in the SDS index score of neurovegetative/somatic symptoms (P = 0.0084) |
Notes: Studies that examine the impact of genetic risk factors on the development of depression during treatment with IFN-α.
One recent study demonstrates the difficulty in generalizing results across different ethnic groups, with non-Hispanic Caucasians and Hispanic Caucasians both showing opposite risk polymorphisms of the 5-HTTLPR gene for depression,93 meaning that results from different ethnic groups should be interpreted with caution. These studies are also limited by the different types of depression scales used such as the overall Hospital Anxiety and Depression Scale (HADS) score,91 BDI,92–94 Zung Self-Rating Depression Scale (SDS),95 and BDI or SDS.89
Some genes appear to be related to a specific subgroup of depressive symptoms rather than the depressive disorder as a whole. Both the interferon receptor-A1 and PLA2 genes were associated primarily with somatic and neurovegetative symptoms.94,95 The APOE 4 allele was shown to be more so associated with symptoms of anger and irritability,90 with a recent study showing that a TNF-α polymorphism is also associated with labile anger and fatigue but not depression.96 The 5-HTTLPR, IL-6, and 5-HT1A genes were associated with overall depression scores.89,91,92 The 5-HTTLPR gene was also associated with sleep quality,92 which is in itself a predictor of depression.41,77 Further work demonstrated that both the 5-HTTLPR and IL-6 genes are not associated with fatigue scores.92
These studies show that different genes are possibly involved in different aspects of the depressive disorder. There is also the possibility that the future may yield some useful genetic markers so that clinicians may identify ‘at-risk’ individuals and also identify what subgroups of symptoms patients may experience.
Demographic and social risk factors
Several demographic factors potentially impact on the development of depression,62 including age, gender, ethnicity, and education level. However, there is no consensus as to whether any of these factors impact on the development of depression in patients taking IFN-α (see Table 3).
Table 3.
Demographic risk factors
| Risk factor | Evidence for | Evidence against |
|---|---|---|
| Age | Castera et al97 Patients who developed mood disorders during treatment were significantly younger (mean age 43) than those who did not (mean age 48) Horikawa et al98 Only risk factor in their study where 23.2% of patients developed depression was advanced age Evon et al24 Patients who developed a depression were significantly younger than those who did not |
Hauser et al99 Of the 33% of their patients who became depressed, there was no difference in age to those who did not become depressed Miyaoka et al100 Martin-Santos et al101 Raison et al62 Su et al94 |
| Education level | Martin-Santos et al101 Those people with only a primary education level (ie, lower levels of education) were significantly more likely to develop a depressive or anxiety disorder |
Su et al94 |
| Gender | Gohier et al102 Sixty-four percent of women developed a depression on treatment Su et al94 Significantly more women (52.4%) than men (23.8%) developed a depression on treatment |
Bonaccorso et al103 Castera et al97 Hauser et al99 Lotrich et al104 Miyaoka et al100 Martin-Santos et al101 Raison et al62 |
| Ethnicity | Hauser et al99 The only demographic baseline characteristic that yielded a significant difference was that there were significantly more Caucasians (76.9%) who developed depression than African-Americans (23.1%) Martin-Santos et al101 Significantly more people who developed a depressive or anxiety disorder on treatment were immigrants (64%) compared with nonimmigrants (37%) Pierucci-Lagha et al93 At 12 weeks, non-Hispanic Caucasians had a significantly higher level of depression (39%) than African-Americans (29%) or Hispanic Caucasians (28%). However, this significant difference disappeared at 20 weeks |
Evon et al24 There was no significant difference in the development of depression between those patients who were Caucasian and those who were African-American |
| Social support | Evon et al24 Those patients who reported having lower levels of social support were significantly more likely to develop depression than those who had such support |
– |
Notes: Studies where impact of demographic risk factors was investigated with relation to depression occurrence during IFN-α treatment.
The problems with these studies are demonstrated by the work of Pierucci-Lagha et al93 who found that there was a significant difference between ethnic groups at week 12 but that this difference was no longer significant at week 20. This could mean that the observed significant difference was an artifact rather than a true reflection of ethnic differences in depression, and thus all other results regarding ethnicity must be interpreted with caution.
The only social risk factor that appears to influence levels of depression is that of social support, with lower levels leading to a significantly higher risk of developing a depression.24 Support for the involvement of this demographic factor also comes from research conducted in patients with malignant melanoma, where patients who employed effective coping strategies and had social support were less likely to become depressed.105 However, more work needs to be conducted in this area before any concrete conclusions can be drawn.
Treatment-related risk factors
Higher doses of IFN-α are associated with more numerous side effects.106,107 However, it is not clear whether this observed dose effect also holds for the development of depression (see Table 4). In general, rates of depression in patients taking IFN-α for HCV vary from 0%109,118 to up to 50%.21,62,97,99–101,104,108,113,116 Oncology patients taking up to five times the dose of IFN-α than those patients infected with HCV are prescribed have similar rates of depression from 5%119 up to 50%.120 Given that the doses of IFN-α given to the two populations are very different, there does not appear to be a convincing difference regarding the impact of dose on development of depression. However, a potential confounding factor is the retrospective design of most of these studies. This design can yield lower rates of depression than prospective depression-specific design, used by many contemporary researcher-led studies,62 perhaps also explaining the lower rates of depression seen in some of the studies in Table 4.107
Table 4.
Studies reviewed to assess whether treatment-related risk factors can be associated with development of depression in patients prescribed IFN-α
| Risk factor | Evidence for | Evidence against |
|---|---|---|
| Dose of interferon | Fried et al108 Of those patients taking 180 μg PEG–IFN, 20%–22% became depressed; however, 30% of those patients taking 3 MU of IFN-α b became depressed. (However, this was not reported to be significant). Mulder et al109 Ascribe the fact that they found no significant changes in depressive symptamology to lower doses of interferon used Renault et al110 Those patients taking a higher dose of IFN-α (10 MU every other day) were more likely to develop what the authors termed an ‘affective syndrome’ than those patients taking 5 MU every day Malaguarnera et al111 When different types of interferon were compared, mean scores on SDS were higher in those patients taking recombinant interferon-α-2a or lymphoblastoid interferon-α than recombinant interferon-α-2b or leukocyte interferon-α |
Horikawa et al98 No evidence that type and dose of interferon impacted on depression rates Iino112 In patients taking three regimens of high-dose IFN treatment (10 MU over varying periods up to 14 weeks to a total of 480 MU). Rates of reported depression ranged from 4.8% to 7.1% Kraus et al113 There was no significant difference in reported rates of depression between those patients taking 5 MU IFN-α (33.3%) and those taking 80–150 μg PEG–IFN-α (40%) Manns et al114 In a comparison of different dosing regimes of interferon, there was no significant difference in rates of depression between higher dose peginterefron plus ribavirin (31%), lower dose peginterefron + ribavirin (29%), and interferon plus ribavirin (34%) Okanoue et al107 In a clinical study where patients were taking 6–10 MU of interferon, only 3.5% became clinically depressed Zeuzem et al115 There was no significant difference in reported rates of depression between those patients taking 180 μg/week of PEG–IFN-α (16%) and those patients taking 3 MU IFN-α (23%). Clinically depressed |
| Length of treatment | – | Castera et al97 There was no significant difference in rates of depression between those on treatment for 24 weeks (53%) and those on treatment for 48 weeks (47%) Hauser et al99 Median time to depression in those patients who developed it was 12 weeks Horikawa et al98 73.9% of patients developed depression within first 8 weeks Robaeys et al116 Depression occurred in patients between 4 and 26 weeks, with the mean time to depression 10 weeks |
| Retreatment following nonresponse | – | Quarantini et al117 In nonresponder patients, rates of IFN-α-induced depression were comparable to studies in treatment naïve patients with 10% of 30 patients being diagnosed with depression during treatment |
| Ribavirin | Raison et al62 Patients who received weight-based dosing of ribavirin (800–1400 μg/day) were significantly more likely to develop moderate to severe depressive symptoms than those people who received a standard dose (800 μg/day) |
Davis et al12 No significant difference between development of depression in those patients taking IFN (11%) and those patients taking IFN + ribavirin (16%) Fried et al108 No difference in rates of depression between two groups both receiving PEG–IFN-α with either ribavirin (22%) or Placebo (20%) McHutchison et al21 There were no significant differences between the rates of depression in those people taking a standard dose of IFN (25%–37%) and those patients taking interferon and ribavirin (32%–36%) |
Other treatment-related factors such as coprescription of ribavirin and length of treatment do not seem to yield a general consensus on the impact of treatment-related risk factors on depression. This suggests that while treatment factors impact on the ‘sickness’ aspect of sickness behavior, that is, those somatic complaints that follow an infection or illness (see Table 1), there is little evidence suggesting that they impact hugely on the behavioral aspects of sickness behavior, one of which is depression.
Psychobehavioral risk factors
Risk factors implicating both psychological and behavioral vulnerabilities as being predictors of subsequent depression have been arguably the most researched in this field as simple reasoning would suggest that if a drug induces psychobehavioral side effects, a pre-existing vulnerability to developing a psychiatric disorder (be that history, current diagnosis, behavioral problems, or higher than normal rating on a psychiatric scale) should lead such patients to be more ‘at risk’ than others. Following an early observation that higher pretreatment scores on a depressive rating scale led to cancer patients having a higher risk of becoming depressed,121 several studies on HCV patients have explored this observation in more detail, with an emerging consensus that this risk factor results in patients being significantly more likely to develop depression when taking IFN-α (see Table 5). It could, however, also be argued that this ‘risk factor’ has the most support simply because it has been the most investigated. This risk factor goes hand in hand with findings which suggest that people with a past history of psychiatric illness, in particular MDD, are more vulnerable to becoming depressed on treatment.62,97 The research summarized in Table 5 suggests that some kind of ‘subsyndromal’ depression could leave people more susceptible to developing an IFN-α-induced depression.
Table 5.
Psychiatric risk factors
| Risk factor | Evidence for | Evidence against |
|---|---|---|
| Baseline personality/other mood scores | Castellvi et al122 In the 42% of patients who developed depression, low self-directedness was identified as a predictive factor Dell’Osso et al123 Patients who went on to develop depression had significantly higher state (but not trait) scores on the STAI-Y Of the 6 (12%) of patients who did develop depression, 33.3% presented with subthreshold manic-hypomanic symptoms compared with 7.5% of patients who did not develop depression Lotrich et al104 Baseline neuroticism and agreeableness predicted the occurrence of MDD Lotrich et al92 Regression analysis showed baseline neuroticism scores to be significantly associated with the occurrence of MDD |
– |
| Higher baseline depressive symptom score | Castellvi et al122 Higher baseline HADS scores were predictive of depression Castera et al97 There was a significant difference in baseline MADRS scores between those who did (baseline score 10.7) and those who did not (baseline score 4.2) become depressed Castera et al124 None of patients with normal MADRS baseline score (<3) developed depression. Forty-two percent of patients with higher MADRS baseline scores developed depressive symptoms Dieperink et al125 The only identified risk factor for the development of depressive symptoms was an elevated BDI score at baseline Lotrich et al104 A higher BDI at baseline was predictive of higher BDI scores at month 1 Martin-Santos et al101 A higher baseline HADS depression score led to a significantly increased risk of developing a depressive or anxiety disorder during treatment with IFN Miyaoka et al100 Those patients who subsequently became depressed had a significantly higher score at baseline on the HAM-D (3.5) than those who did not become depressed (2.0) Raison et al62 Elevated baseline SDS scores significantly predicted the severity of depressive symptoms. (However, the authors note that increased depressive symptoms were related to a history of depression). |
Dell’Osso et al12 Score’s on the depressive component of MOODS-SR at baseline did not differ at baseline between those who subsequently did and did not become depressed Robaeys et al116 Of the 19 (49%) of patients who became depressed, most of these had significantly higher scores on vegetative-depressive scales of the Zung self rating scale at week 4; however, there was no difference at baseline |
| Psychiatric history | Castellvi et al122 A history of mood disorders was predictive of depression Castera et al97 Patients in this study who became depressed were significantly more likely to have a history of psychiatric disorders (58%) than those who did not (30%) Ho et al126 Veterans with a prior psychiatric diagnosis had a greater incidence of neuropsychiatric adverse events (32% of whom 26% had depression) during interferon treatment than those who did not (14%); however, this result was not significant Raison et al62 When controlling for baseline SDS scores using logistic stepwise regression, it was found that a history of MDD was a significant predictor of moderate or severe depression |
Castera et al124 None of the 12% of patients who developed a depressive disorder had a psychiatric history Dieperink et al125 Of those patients receiving psychiatric care at baseline, 45% met criteria for MDD, which rose to 56% (11% incidence of new depression); however, of the patients not receiving psychiatric care at baseline, there was a 23% increase in new depression during treatment However, despite this, a history of two or more psychiatric diagnoses was identified as a risk factor for having to start antidepressant treatment during IFN, despite not being significant (P < 0.08), as well as a family history of mood disorders, which was significant. Yet none of these factors were identified as being important for the development of depressive symptoms Horikawa et al98 No evidence for prior history of psychiatric disorders was a risk factor Hauser et al99 No difference in past psychiatric history between those who did and did not become depressed Lotrich et al104 Of the 9 (39%) of patients who developed major depression, only 3 of these (33%) had a past history of a major depressive episode, whereas 36% of the group that did not become depressed also had a history of psychiatric illness Martin-Santos et al101 A past history of depressive or anxiety disorders did not impact significantly on the likelihood to develop a new depression. (Patients with depression at baseline were excluded from this analysis.) Mulder et al109 Despite the fact that having had a lifetime history of major depression meant higher mean depression scores throughout treatment, this did not predict the onset or worsening of these symptoms Pariante et al31 Patients with a pre-existing psychiatric disorder were not significantly more likely to develop psychiatric adverse events (24%) when compared to case controls (19%) |
| Psychiatric history | Renault et al110 Those patients with a past history of an affective disorder were not significantly more likely to develop an ‘affective syndrome’, as 57% of people who developed depression had this history, whereas 47% of people who did not develop any psychiatric side effects also had Schaefer et al30 More psychiatric patients had a higher baseline depression; however, there was no significant increase in depression in the psychiatric group (6%) compared with the methadone group (14%), former addiction group (29%), and the control group (12%) in the development of a new depression. In total, 16% of all patients developed a new depression However, those patients who were in the psychiatric group were more likely to develop severe depressive side effects |
|
| Sleep | Franzen et al77 Of the 19% of patients who developed MDD, when other factors were controlled for, poor sleep quality prior to treatment was a predictor of shorter time to development of depression Gohier et al102 Eighty-two percent of patients who presented with sleep disorders during treatment also showed anxiodepressive symptoms, with sleep disturbance also being a predictor of suicidal ideation Prather et al41 Poor sleep quality was a predictor of the next month’s BDI scores |
– |
| Substance abuse | Castera et al97 Forty-five percent of those patients that became depressed had a history of IV drug use, only 15% of the patients who did not become depressed were from this same population Schaefer et al30 Forty-three percent of patients who dropped out of study had former drug addiction (compared with 18% in psychiatric group, 14% methadone group, and 13% control group). These patients also tended to have more often, but milder episodes of depression |
Hauser et al99 Martin-Santos et al101 In this study, a past history of alcohol or opioid dependence did not make people more likely to develop depression Raison et al62 No evidence that past substance use impacts on rates of depression Renault et al110 None of the patients in the drug abuse group developed the ‘affective syndrome’, although 67% of them developed delirium |
Notes: Studies investigating the role of psychiatric risk factors on the development of depression during IFN-α treatment for HCV.
Abbreviations: STAI-Y, State-Trait Anxiety Inventory; MADRS, Montgomery–Asberg Depression Rating Scale; HAM-D, Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; HADS, Hospital Anxiety and Depression Scale; MOODS-SR, Mood Spectrum Self-Report.
It has been shown that those people who become depressed on treatment are more likely to have an increased baseline score on a depression scale (see Table 5). Raison et al127 propose that all people prescribed IFN-α develop a side effect profile, comprised of neurovegetative symptoms (those somatic aspects of sickness behavior) or depressive-specific symptoms (those symptoms specific to the behavioral parts of sickness behavior). Both of these side effect profiles are measured by depression rating scales. Thus, if a person scores highly at baseline on one of these scales, it would stand to reason that these scores would remain throughout the course of the treatment and that some additional treatment-related factors would also come into play. The additive effect would mean that this subset of patients would be more at risk of being diagnosed with a depressive disorder due to elevated scores on a depression rating scale. Should this be the case, then all patients would experience an increase in scores on a depression rating scale relative to time, which is something that much of the literature seems to support.23,25,104,108,111,113,114,125,128–134 In fact, some researchers have pinpointed a relative increase in somatic components of depressive rating scales as being primarily responsible for the increase in scores seen in many patients.46 However, many recent studies have become aware of the potential confounds associated with using only depression rating scales; the range cannot be deleted and so diagnose MDD using DSM-IV-TR criteria. In order to meet criteria for diagnosis of MDD, patients are required to show depressed mood for most of the days, nearly every day, and/or demonstrate a direct loss in interest in activities every day for 2 weeks. Neither of these factors are somatic in nature, although it could be said that they may result from a primarily somatic complaint.
Psychiatric morbidity at baseline is perhaps the most intriguing of all research in this area. Evidence, however, is varied with some studies finding no difference in development of depression in patients taking IFN-α, regardless of psychiatric history, and some indicating that patients are more likely to develop depression should they exhibit psychiatric vulnerability (see Table 5). This in part could be related to the fact that many of the studies that investigate a predefined ‘at-risk’ population such as those who have pre-existing psychiatric disorder are often coprescribed additional support at baseline with SSRIs and therapy.29–31
Another important issue with this population is whether researchers are investigating the incidence of total depression or ‘new’ depression only. Hauser et al99 reported an incidence of 6% of ‘new’ depression in the psychiatric group, which was not significant when compared against the incidence of ‘new’ depression in the other groups examined. However, if people depressed prior to treatment were included, then the total incidence of depression in this group was 44%, a much higher rate than in other groups. Some studies that have found evidence for a past psychiatric history impacting on IFN-α-induced depression include people who were diagnosed as being depressed prior to the start of treatment (see Table 5). This begs the question of which approach is better. If examining a ‘psychiatric group’, then a substantial proportion of this group may have depression, and removing this group from subsequent analyses will impact upon the level of depression observed. At the same time, however, these studies are supposedly examining IFN-α-induced depression, and if a person is depressed when commencing treatment, the depression observed while on treatment is not an IFN-α-induced phenomenon and should not be included.
The approach adopted by Hauser et al99 is perhaps the most sensible, given this dilemma. They report their results in terms of ‘new depression’ but also mention that those depressed prior to treatment were more likely to develop moderate or severe depression compared to patients in other groups, thus providing evidence that IFN-α may increase depressive symptoms relative to the baseline that people begin treatment at. Thus, people with a lower ‘baseline depression’ will have an increase in depressive symptoms, but this is not always necessarily enough of an increase to mean a diagnosis of depression. However, those people with higher depression scores when commencing treatment will have the same kind of increase in depressive symptoms leading, in their case, to a diagnosis of depression.
Another risk factor that has increasing support is personality, with those individuals who have low self-directedness122 and high neuroticism92,104 being more likely to become depressed on treatment. The likelihood is that these personality factors interact with other psychobehavioral risk factors; however, more research needs to be conducted in order to determine this.
A behavioral risk factor that is gaining increasing interest from researchers is the impact of sleep disorders on the subsequent development of depression. Studies have shown that those patients who exhibit higher scores on the Pittsburgh Sleep Quality Index at baseline are more likely to develop MDD in a shorter time span,77 with sleep disturbance on treatment being a predictive factor for subsequent depression scale scores41 and suicidal ideation.102 However, a recent literature review on sleep disturbance in HCV states that the presence of insomnia in this cohort is influenced by underlying psychiatric comorbidity such as depression.122 This could mean that sleep disturbance interacts with other risk factors in determining the possible risk of developing depression in individuals. This idea is supported by Lotrich et al92 who found that when assessed alone, baseline sleep quality and BDI could predict depression; however, when Pittsburgh Sleep Quality Index and BDI scores were combined with 5-HTTLPR genotype, the effect of sleep and depression scale scores were diminished and only genotype had a significant impact on the development of depression.
The area of psychobehavioral risk factors impacting on subsequent development of IFN-α-induced depression is perhaps the most convincing in terms of evidence gathered and rationale supplied by researchers. This risk factor is inevitably influenced by genetic and/or biological vulnerabilities.
Limitations
Limitations of cited studies
Many of the studies examined are retrospective and have only identified ‘risk factors’ following patients’ completion of treatment. It could, therefore, be argued that it is easy to retrospectively identify a risk factor using statistics to compare ‘depressed’ and ‘nondepressed’ groups. In fact, a prospective depression-specific design rather than the retrospective general design often employed in clinical trials generally yields much higher rates of depression.135 Despite this, a risk factor might only be identified using a prospective depression-specific design, and following identification of that risk factor, other studies could have the effect of that particular risk factor on the development of depression as their primary hypothesis. This has certainly been the case with many studies on psychiatric side effects, and this area of research has much stronger empirical evidence supporting it than others (eg, demographic risk factors, which when examined in a study, appears to be an afterthought examined only retrospectively). Paradoxically, many studies have focused from the outset on the side effect of depression and will often find higher rates of depression, as they are ‘on the look out’ for this specific side effect. Studies of general side effects rather than the specific side effect of depression have found lower rates of depression. It may be the same with other risk factors. Should researchers and clinicians be more aware that certain risk factors could lead to an increased likelihood of depression being developed, depression in these individuals may be diagnosed more quickly. Many of these studies employ scales to examine depressive symptoms, and these scales are often high in somatic items, which because of IFN-α being a drug that induces a ‘sickness behavior’, many patients taking this drug will score highly on these items simply because they are sick, rather than being depressed per se.46 It will be important in future studies to determine the subscale loads of those items that are somatic or affective.
When assessing the role of the various biological and genetic risk factors identified in this review, there are some key issues that limit the interpretation of results. Many of the biological risk factors identified were not readily identifiable at baseline and instead are correlated with depressive symptoms after the induction of therapy. Another issue is the problem of correlating mood scores with a biomarker; high scores on a depression scale are not neccesarily representative of a depressive disorder and could instead be linked in to higher somatic symptoms. Some studies have shown that their biomarker of interest is preferentially correlated with somatic symptoms,67,94,95 and future work should determine which cluster of symptoms each biomarker is associated with. The majority of studies examined in this review chose to conduct correlational and regression analysis, with no studies comparing between groups who did and did not develop a depression. In future, it would be interesting to conduct analyses that include all comparisons in order to gain a full appreciation of the role of biomarkers in IFN-α-induced depression.
Moreover, in issues relating to experimental design, there is the simple, yet often ignored issue that many patients who have HCV are more depressed than a healthy population; in fact, rates of depression in HCV patients can be as high as 40%.136 Yates and Gleason137 found that in a study of 78 patients who were HCV positive, the main reasons for being referred for psychiatric consultation were showing psychiatric symptoms, consultations either prior to listing for liver transplantation and/or therapy with IFN-α, and that the lowest number of patients that were referred for treatment in this group were those patients receiving IFN-α who had developed depressive symptoms. Furthermore, populations acquiring HCV tend to be from backgrounds that can lead them to have a higher level of depression regardless of treatment with IFN-α.138 This evidence demonstrates that the HCV population is a vulnerable group generally and that focusing on only treatment-associated problems may lead clinicians and researchers alike to underestimate those problems that are associated with HCV itself.
Limitations of review
The results reported in this review are also limited by a number of factors. The first one being that only accessible articles were included within the review, and those articles that could not be accessed due to language constraints or inability to access full-text content were not included, which could have an impact on the risk factors identified and discussion of these factors.
In addition, there are some risk factors shown to impact on rates of depression that do not necessarily fit within the pre-defined categories and are not readily identifiable at baseline. Robaeys et al116 found that vegetative-depressive symptoms at week 4 of treatment were predictive of a subsequent diagnosis of MDD; however, there were no differences between groups at baseline. Likewise, decreased motor speed was associated with increased symptoms of depression and fatigue.134
There are also some biological factors that influence IFN-α-induced depression that were not explored in the literature review due to the paucity of literature in that area. One such factor is brain-derived neurotrophic factor, which has been shown to correlate with BDI scores.139
Other studies have examined risk factors in different patient populations. IFN-α is also used as treatment for certain cancers such as malignant melanoma,52,140 and there are studies within this population that support various risk factors that have been examined within this review such as HPA axis biomarkers,140 lower levels of tryptophan,52 higher pretreatment depression scores,141 low social support, sleep disturbance, pessimistic thoughts, and sadness.105
Only those studies that looked at risk factors for depression were examined. In future, it will be important to determine what protective factors prevent people from developing a depression. There is some evidence presented that social support may be a protective factor against the development of depression,24 and further research could determine what other protective factors are important.
When interpreting the results from this review, clinicians also need to be aware of the individual nature of depression and the varying risk factors that will cause each individual to become depressed. Even though this review has found strong evidence for certain risk factors, this does not mean to say that any person who experiences these factors will become depressed; it means that they are at a higher risk of developing depression than others who do not have these risk factors and thus extra assessment and support should be administered prior to commencement of treatment in those individuals who have been ascertained to be at risk of developing depression.
Finally, all results should be interpreted with caution due to the differing methodologies and analyses undertaken in all the studies within this review. While care has been taken to narrow the scope of the review by concentrating only on HCV patients, there are still differences between all the studies included here. These include the number of patients in each study, the type of statistical analysis run, the types of depression analysis undertaken (clinician rated and self-rated, depression scale or clinical diagnosis), and how each researcher defined depression (clinical diagnosis or cutoff point on depression scale). These should be recognized to be limiting factors in interpreting the results of this review.
Conclusions
The current climate of psychiatric research is one where there is an acknowledgment of the multidisciplinary factors that play into any psychiatric disorder. Current research is, therefore, focused on finding interactions between genetic, biological, demographic, and psychobehavioral factors that lead some people to be more vulnerable to developing psychiatric disorders than others. It would be very difficult to identify a single risk factor that would lead people to be more susceptible to becoming depressed on IFN-α than others. However, the most convincing research conducted in this area is that which has examined biological,41,57,58 genetic,89,91–93 and psychobehavioral41,62,77,92,97 risk factors, and it is no surprise that these three risk factors interact with one another (for an example of the way these three risk factors could interact to produce depression, see Figure 3).
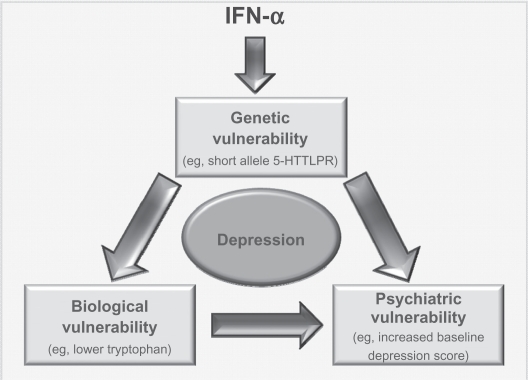
Figure 3.
Interaction of risk factors. Model by which various risk factors could interact to possibly produce depression in those patients taking IFN-α. It is possible that administration of IFN-α could lead to some underlying genetic vulnerability (eg, the short allele in 5-HTLLPR) impacting upon levels of a chemical such as tryptophan. This vulnerability, along with the biological response it could induce, would also interact with a psychiatric vulnerability. As these people have the genetic vulnerability, they are more likely to be depressed at baseline and/or have had a history of psychiatric disorders. All this would lead the person to be vulnerable to developing depression following administration of IFN.
Clinicians and researchers should be wary when trying to pinpoint one risk factor to concentrate on when attempting to identify ‘at-risk’ individuals. Instead, there should be an awareness of the complex interplay of biological, psychobehavioral, and genetic factors that lead some people to develop depression while taking IFN-α (see Figure 3). Two recent studies have examined interactions between genetic and psychobehavioral risk factors,92,93 demonstrating the need for more translational research in determining the relationship between different risk factors. Only when more research of this nature has been conducted, more concrete conclusions regarding the interaction and importance of individual risk factors can be determined.
Clinicians should attempt to assess people fully at baseline using as many tools available to them, such as depression rating scales, sleep disturbance questionnaires, psychiatric histories, and biological analysis of tryptophan and/or stress hormones. In future, it may also be possible to use genetic tools to identify individuals susceptible to development of IFN-α-induced depression. HCV-infected patients taking IFN-α are undoubtedly a vulnerable population. Effective pretreatment screening for baseline risk factors combined with more effective support during treatment will enable health care workers to better identify patients at risk and provide the additional treatment required should depression develop, thus improving adherence rates and improving clearance of HCV infection.
Acknowledgments
The authors thank all staff in the Hepatology Centre, in particular, Barbara Hynes, Clodagh Quinn, and Helena Irish, as well as the Psychological Services Department at St James’s Hospital, for their help in allowing us to better understand the patients’ perspective of taking IFN-α. Supported by the Health Research Board (Ireland).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362(9401):2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 3.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48(1):148–162. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Rantala M, van de Laar MJ. Surveillance and epidemiology of hepatitis B and C in Europe – a review. Euro Surveill. 2008;13(21) doi: 10.2807/ese.13.21.18880-en. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18880. Accessed 2010 Sep 18. [DOI] [PubMed] [Google Scholar]
- 5.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32(3):344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 7.Leone N, Rizzetto M. Natural history of hepatitis C virus infection: from chronic hepatitis to cirrhosis, to hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2005;51(1):31–46. [PubMed] [Google Scholar]
- 8.Patel K, Muir AJ, McHutchison JG. Diagnosis and treatment of chronic hepatitis C infection. BMJ. 2006;332(7548):1013–1017. doi: 10.1136/bmj.332.7548.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9(4):331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 10.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 11.Bacon BR. Managing hepatitis C. Am J Manag Care. 2004;10(Suppl 2):S30–S40. [PubMed] [Google Scholar]
- 12.Davis GL, Esteban-Mur R, Rustgi V, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339(21):1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 13.Cotler SJ, Wartelle CF, Larson AM, Gretch DR, Jensen DM, Carithers RL., Jr Pretreatment symptoms and dosing regimen predict side-effects of interferon therapy for hepatitis C. J Viral Hepat. 2000;7(3):211–217. doi: 10.1046/j.1365-2893.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- 14.Foster G, Mathurin P. Hepatitis C virus therapy to date. Antivir Ther. 2008;13(Suppl 1):S3–S8. [PubMed] [Google Scholar]
- 15.Kowdley KV. Hematologic side effects of interferon and ribavirin therapy. J Clin Gastroenterol. 2005;39(Suppl 1):S3–S8. doi: 10.1097/01.mcg.0000145494.76305.11. [DOI] [PubMed] [Google Scholar]
- 16.Ong JP, Younossi ZM. Managing the hematologic side effects of antiviral therapy for chronic hepatitis C: anemia, neutropenia, and thrombocytopenia. Cleve Clin J Med. 2004;71(Suppl 3):S17–S21. doi: 10.3949/ccjm.71.suppl_3.s17. [DOI] [PubMed] [Google Scholar]
- 17.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56(11):819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000;157(6):867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 19.Fukunishi K, Tanaka H, Maruyama J, et al. Burns in a suicide attempt related to psychiatric side effects of interferon. Burns. 1998;24(6):581–583. doi: 10.1016/s0305-4179(98)00073-4. [DOI] [PubMed] [Google Scholar]
- 20.Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21(2):241–243. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 21.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339(21):1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein D, Kleinman L, Barker CM, Revicki DA, Green J. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35(3):704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 23.Kraus MR, Schafer A, Csef H, Faller H, Mork H, Scheurlen M. Compliance with therapy in patients with chronic hepatitis C: associations with psychiatric symptoms, interpersonal problems, and mode of acquisition. Dig Dis Sci. 2001;46(10):2060–2065. doi: 10.1023/a:1011973823032. [DOI] [PubMed] [Google Scholar]
- 24.Evon DM, Ramcharran D, Belle SH, Terrault NA, Fontana RJ, Fried MW. Prospective analysis of depression during peginterferon and ribavirin therapy of chronic hepatitis C: results of the Virahep-C study. Am J Gastroenterol. 2009;104(12):2949–2958. doi: 10.1038/ajg.2009.528. [DOI] [PubMed] [Google Scholar]
- 25.Kraus MR, Schafer A, Schottker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531–536. doi: 10.1136/gut.2007.131607. [DOI] [PubMed] [Google Scholar]
- 26.Gleason OC, Fucci JC, Yates WR, Philipsen MA. Preventing relapse of major depression during interferon-alpha therapy for hepatitis C – a pilot study. Dig Dis Sci. 2007;52(10):2557–2563. doi: 10.1007/s10620-006-9729-5. [DOI] [PubMed] [Google Scholar]
- 27.Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 28.Morasco BJ, Rifai MA, Loftis JM, Indest DW, Moles JK, Hauser P. A randomized trial of paroxetine to prevent interferon-alpha-induced depression in patients with hepatitis C. J Affect Disord. 2007;103(1–3):83–90. doi: 10.1016/j.jad.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol. 2005;42(6):793–798. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37(2):443–451. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- 31.Pariante CM, Orru MG, Baita A, Farci MG, Carpiniello B. Treatment with interferon-alpha in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999;354(9173):131–132. doi: 10.1016/S0140-6736(98)04793-X. [DOI] [PubMed] [Google Scholar]
- 32.Dantzer R, Bluthe RM, Gheusi G, et al. Molecular basis of sickness behavior. Ann N Y Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 33.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25(3):154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 35.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29(4–5):891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 37.Charlton BG. The malaise theory of depression: major depressive disorder is sickness behavior and antidepressants are analgesic. Med Hypotheses. 2000;54(1):126–130. doi: 10.1054/mehy.1999.0986. [DOI] [PubMed] [Google Scholar]
- 38.Lotrich FE. Major depression during interferon-alpha treatment: vulnerability and prevention. Dialogues Clin Neurosci. 2009;11(4):417–425. doi: 10.31887/DCNS.2009.11.4/felotrich. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Bonaccorso S, Puzella A, Marino V, et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105(1–2):45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- 41.Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23(8):1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82(2):175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Maddock C, Landau S, Barry K, et al. Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry. 2005;10(4):332–333. doi: 10.1038/sj.mp.4001634. [DOI] [PubMed] [Google Scholar]
- 44.Capuron L, Dantzer R, Miller AH. Neuro-immune interactions in psychopathology with the example of interferon-alpha-induced depression. J Soc Biol. 2003;197(2):151–156. [PubMed] [Google Scholar]
- 45.Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (IFNalpha) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(4):731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 46.Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18(11):2316–2326. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 47.Loftis JM, Huckans M, Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol Dis. 2010;37(3):519–533. doi: 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40(2):288–295. [PubMed] [Google Scholar]
- 50.Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J. Neurobiology of depression: an integrated view of key findings. Int J Clin Pract. 2007;61(12):2030–2040. doi: 10.1111/j.1742-1241.2007.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynure-nine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11(3):198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- 52.Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 53.Bonaccorso S, Marino V, Puzella A, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Maes M, Bonaccorso S, Marino V, et al. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6(4):475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 55.Fontana RJ, Kronfol Z, Lindsay KL, et al. Changes in mood states and biomarkers during peginterferon and ribavirin treatment of chronic hepatitis C. Am J Gastroenterol. 2008;103(11):2766–2775. doi: 10.1111/j.1572-0241.2008.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo S, Kema IP, Haagsma EB, et al. Irritability rather than depression during interferon treatment is linked to increased tryptophan catabolism. Psychosom Med. 2005;67(5):773–777. doi: 10.1097/01.psy.0000171193.28044.d8. [DOI] [PubMed] [Google Scholar]
- 57.Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16(6):1091–1099. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- 60.Dafny N, Yang PB. Interferon and the central nervous system. Eur J Pharmacol. 2005;523(1–3):1–15. doi: 10.1016/j.ejphar.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 61.Asnis GM, de La Garza R., 2nd Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40(4):322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 62.Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl 1):S302–S306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 64.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34(1):4–20. [PMC free article] [PubMed] [Google Scholar]
- 65.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62(2):207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15(5):535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105(1):83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 69.Begley DJ. The interaction of some centrally active drugs with the blood-brain barrier and circumventricular organs. Prog Brain Res. 1992;91:163–169. doi: 10.1016/s0079-6123(08)62331-6. [DOI] [PubMed] [Google Scholar]
- 70.Ermisch A, Brust P, Kretzschmar R, Ruhle HJ. Peptides and blood-brain barrier transport. Physiol Rev. 1993;73(3):489–527. doi: 10.1152/physrev.1993.73.3.489. [DOI] [PubMed] [Google Scholar]
- 71.Dunn AJ. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exp Ther. 1992;261(3):964–969. [PubMed] [Google Scholar]
- 72.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47(2):169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 73.Brown MD, Wick TM, Eckman JR. Activation of vascular endothelial cell adhesion molecule expression by sickle blood cells. Pediatr Pathol Mol Med. 2001;20(1):47–72. [PubMed] [Google Scholar]
- 74.Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- 75.Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135(3):659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 76.Wichers MC, Kenis G, Leue C, Koek G, Robaeys G, Maes M. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry. 2006;60(1):77–79. doi: 10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 77.Franzen PL, Buysse DJ, Rabinovitz M, Pollock BG, Lotrich FE. Poor sleep quality predicts onset of either major depression or subsyndromal depression with irritability during interferon-alpha treatment. Psychiatry Res. 2010;177(1–2):240–245. doi: 10.1016/j.psychres.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 79.Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene-environment interaction. J Affect Disord. 2008;111(1):1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Hoefgen B, Schulze TG, Ohlraun S, et al. The power of sample size and homogenous sampling: association between the 5-HTTLPR serotonin transporter polymorphism and major depressive disorder. Biol Psychiatry. 2005;57(3):247–251. doi: 10.1016/j.biopsych.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 81.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 82.Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 83.Tadokoro K, Hashimoto R, Tatsumi M, Kosuga A, Kamijima K, Kunugi H. The gem interacting protein (GMIP) gene is associated with major depressive disorder. Neurogenetics. 2005;6(3):127–133. doi: 10.1007/s10048-005-0003-3. [DOI] [PubMed] [Google Scholar]
- 84.Heiman GA, Ottman R, Saunders-Pullman RJ, Ozelius LJ, Risch NJ, Bressman SB. Increased risk for recurrent major depression in DYT1 dystonia mutation carriers. Neurology. 2004;63(4):631–637. doi: 10.1212/01.wnl.0000137113.39225.fa. [DOI] [PubMed] [Google Scholar]
- 85.Lemonde S, Turecki G, Bakish D, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23(25):8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosa A, Peralta V, Papiol S, et al. Interleukin-1beta (IL-1beta) gene and increased risk for the depressive symptom-dimension in schizophrenia spectrum disorders. Am J Med Genet B Neuropsychiatr Genet. 2004;124B(1):10–14. doi: 10.1002/ajmg.b.20074. [DOI] [PubMed] [Google Scholar]
- 87.Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 88.Shyn SI, Hamilton SP. The genetics of major depression: moving beyond the monoamine hypothesis. Psychiatr Clin North Am. 2010;33(1):125–140. doi: 10.1016/j.psc.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bull SJ, Huezo-Diaz P, Binder EB, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2009;14(12):1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gochee PA, Powell EE, Purdie DM, et al. Association between apolipoprotein E epsilon4 and neuropsychiatric symptoms during interferon alpha treatment for chronic hepatitis C. Psychosomatics. 2004;45(1):49–57. doi: 10.1176/appi.psy.45.1.49. [DOI] [PubMed] [Google Scholar]
- 91.Kraus MR, Al-Taie O, Schafer A, Pfersdorff M, Lesch KP, Scheurlen M. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132(4):1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 92.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65(4):344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pierucci-Lagha A, Covault J, Bonkovsky HL, et al. A functional serotonin transporter gene polymorphism and depressive effects associated with interferon-alpha treatment. Psychosomatics. 2010;51(2):137–148. doi: 10.1176/appi.psy.51.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su KP, Huang SY, Peng CY, et al. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67(6):550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshida K, Alagbe O, Wang X, et al. Promoter polymorphisms of the interferon-alpha receptor gene and development of Interferon-induced depressive symptoms in patients with chronic hepatitis C: preliminary findings. Neuropsychobiology. 2005;52(2):55–61. doi: 10.1159/000086605. [DOI] [PubMed] [Google Scholar]
- 96.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon alfa treatment is associated with a polymorphism in tumor necrosis factor alpha. Clin Neuropharmacol. 2010;33(4):191–197. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Castera L, Constant A, Henry C, et al. Impact on adherence and sustained virological response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2006;24(8):1223–1230. doi: 10.1111/j.1365-2036.2006.03107.x. [DOI] [PubMed] [Google Scholar]
- 98.Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective study. Gen Hosp Psychiatry. 2003;25(1):34–38. doi: 10.1016/s0163-8343(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 99.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7(9):942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 100.Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156(7):1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- 101.Martin-Santos R, Diez-Quevedo C, Castellvi P, et al. De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. Aliment Pharmacol Ther. 2008;27(3):257–265. doi: 10.1111/j.1365-2036.2007.03568.x. [DOI] [PubMed] [Google Scholar]
- 102.Gohier B, Goeb JL, Rannou-Dubas K, Fouchard I, Cales P, Garre JB. Hepatitis C, alpha interferon, anxiety and depression disorders: a prospective study of 71 patients. World J Biol Psychiatry. 2003;4(3):115–118. doi: 10.1080/15622970310029904. [DOI] [PubMed] [Google Scholar]
- 103.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72(3):237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 104.Lotrich FE, Rabinovitz M, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18(3):205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Lindsay KL, Davis GL, Schiff ER, et al. Response to higher doses of interferon alfa-2b in patients with chronic hepatitis C: a randomized multicenter trial. Hepatitis Interventional Therapy Group. Hepatology. 1996;24(5):1034–1040. doi: 10.1002/hep.510240509. [DOI] [PubMed] [Google Scholar]
- 107.Okanoue T, Sakamoto S, Itoh Y, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25(3):283–291. doi: 10.1016/s0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 108.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 109.Mulder RT, Ang M, Chapman B, Ross A, Stevens IF, Edgar C. Interferon treatment is not associated with a worsening of psychiatric symptoms in patients with hepatitis C. J Gastroenterol Hepatol. 2002;15(3):300–303. doi: 10.1046/j.1440-1746.2000.02090.x. [DOI] [PubMed] [Google Scholar]
- 110.Renault PF, Hoofnagle JH, Park Y, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147(9):1577–1580. [PubMed] [Google Scholar]
- 111.Malaguarnera M, Di Fazio I, Restuccia S, Pistone G, Ferlito L, Rampello L. Interferon alpha-induced depression in chronic hepatitis C patients: comparison between different types of interferon alpha. Neuropsychobiology. 1998;37(2):93–97. doi: 10.1159/000026485. [DOI] [PubMed] [Google Scholar]
- 112.Iino S. High dose interferon treatment in chronic hepatitis C. Gut. 1993;34(Suppl 2):S114–S118. doi: 10.1136/gut.34.2_suppl.s114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kraus MR, Schafer A, Csef H, Scheurlen M. Psychiatric side effects of pegylated interferon alfa-2b as compared to conventional interferon alfa-2b in patients with chronic hepatitis C. World J Gastroenterol. 2005;11(12):1769–1774. doi: 10.3748/wjg.v11.i12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 115.Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343(23):1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 116.Robaeys G, de Bie J, Wichers MC, et al. Early prediction of major depression in chronic hepatitis C patients during peg-interferon alpha-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World J Gastroenterol. 2007;13(43):5736–5740. doi: 10.3748/wjg.v13.i43.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quarantini LC, Bressan RA, Galvao A, Batista-Neves S, Parana R, Miranda-Scippa A. Incidence of psychiatric side effects during pegylated interferon- alpha retreatment in nonresponder hepatitis C virus-infected patients. Liver Int. 2007;27(8):1098–1102. doi: 10.1111/j.1478-3231.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 118.Amodio P, de Toni EN, Cavalletto L, et al. Mood, cognition and EEG changes during interferon alpha (alpha-IFN) treatment for chronic hepatitis C. J Affect Disord. 2005;84(1):93–98. doi: 10.1016/j.jad.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Bannink M, Fekkes D, van Gool AR, et al. Interferon-alpha influences tryptophan metabolism without inducing psychiatric side effects. Neuropsychobiology. 2007;55(3–4):225–231. doi: 10.1159/000108382. [DOI] [PubMed] [Google Scholar]
- 120.Donnelly S. Patient management strategies for interferon alfa-2b as adjuvant therapy of high-risk melanoma. Oncol Nurs Forum. 1998;25(5):921–927. [PubMed] [Google Scholar]
- 121.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N Engl J Med. 1999;340(17):1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 122.Castellvi P, Navines R, Gutierrez F, et al. Pegylated interferon and ribavirin-induced depression in chronic hepatitis C: role of personality. J Clin Psychiatry. 2009;70(6):817–828. doi: 10.4088/jcp.08m04230. [DOI] [PubMed] [Google Scholar]
- 123.Dell’Osso L, Pini S, Maggi L, et al. Subthreshold mania as predictor of depression during interferon treatment in HCV+ patients without current or lifetime psychiatric disorders. J Psychosom Res. 2007;62(3):349–355. doi: 10.1016/j.jpsychores.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 124.Castera L, Zigante F, Bastie A, Buffet C, Dhumeaux D, Hardy P. Incidence of interferon alfa-induced depression in patients with chronic hepatitis C. Hepatology. 2002;35(4):978–979. doi: 10.1053/jhep.2002.32104. [DOI] [PubMed] [Google Scholar]
- 125.Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44(2):104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 126.Ho SB, Nguyen H, Tetrick LL, Opitz GA, Basara ML, Dieperink E. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96(1):157–164. doi: 10.1111/j.1572-0241.2001.03468.x. [DOI] [PubMed] [Google Scholar]
- 127.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dan AA, Martin LM, Crone C, et al. Depression, anemia and health-related quality of life in chronic hepatitis C. J Hepatol. 2006;44(3):491–498. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 129.Fontana RJ, Bieliauskas LA, Lindsay KL, et al. Cognitive function does not worsen during pegylated interferon and ribavirin retreatment of chronic hepatitis C. Hepatology. 2007;45(5):1154–1163. doi: 10.1002/hep.21633. [DOI] [PubMed] [Google Scholar]
- 130.Fontana RJ, Schwartz SM, Gebremariam A, Lok AS, Moyer CA. Emotional distress during interferon-alpha-2B and ribavirin treatment of chronic hepatitis C. Psychosomatics. 2002;43(5):378–385. doi: 10.1176/appi.psy.43.5.378. [DOI] [PubMed] [Google Scholar]
- 131.Hunt CM, Dominitz JA, Bute BP, Waters B, Blasi U, Williams DM. Effect of interferon-alpha treatment of chronic hepatitis C on health-related quality of life. Dig Dis Sci. 1997;42(12):2482–2486. doi: 10.1023/a:1018852309885. [DOI] [PubMed] [Google Scholar]
- 132.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64(6):708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 133.Maddock C, Baita A, Orru MG, et al. Psychopharmacological treatment of depression, anxiety, irritability and insomnia in patients receiving interferon-alpha: a prospective case series and a discussion of biological mechanisms. J Psychopharmacol. 2004;18(1):41–46. doi: 10.1177/0269881104040230. [DOI] [PubMed] [Google Scholar]
- 134.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22(6):870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schafer A, Wittchen HU, Seufert J, Kraus MR. Methodological approaches in the assessment of interferon-alfa-induced depression in patients with chronic hepatitis C – a critical review. Int J Methods Psychiatr Res. 2007;16(4):186–201. doi: 10.1002/mpr.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yovtcheva SP, Rifai MA, Moles JK, van der Linden BJ. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411–415. doi: 10.1176/appi.psy.42.5.411. [DOI] [PubMed] [Google Scholar]
- 137.Yates WR, Gleason O. Hepatitis C and depression. Depress Anxiety. 1998;7(4):188–193. doi: 10.1002/(sici)1520-6394(1998)7:4<188::aid-da7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 138.Johnson ME, Fisher DG, Fenaughty A, Theno SA. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93(5):785–789. doi: 10.1111/j.1572-0241.1998.225_a.x. [DOI] [PubMed] [Google Scholar]
- 139.Kenis G, Prickaerts J, van Os J, et al. Depressive symptoms following interferon-alpha therapy: mediated by immune-induced reductions in brain-derived neurotrophic factor? Int J Neuropsychopharmacol. 2010 Jul 29;:1–7. doi: 10.1017/S1461145710000830. Epub 2010 Sep 1. [DOI] [PubMed] [Google Scholar]
- 140.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160(7):1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 141.Heinze S, Egberts F, Rotzer S, et al. Depressive mood changes and psychiatric symptoms during 12-month low-dose interferon-alpha treatment in patients with malignant melanoma: results from the multicenter DeCOG trial. J Immunother. 2010;33(1):106–114. doi: 10.1097/CJI.0b013e3181b8bdb9. [DOI] [PubMed] [Google Scholar]
- 142.Sockalingam S, Abbey SE, Alosaimi F, Novak M. A review of sleep disturbance in hepatitis C. J Clin Gastroenterol. 2010;44(1):38–45. doi: 10.1097/MCG.0b013e3181b314ea. [DOI] [PubMed] [Google Scholar]