Abstract
Background
Intensive insulin therapy has been associated with weight gain and increased hypoglycemia. In this pilot study, we determined the effect of optimized insulin therapy on weight gain and frequency of hypoglycemia in patients with long-standing type 1 diabetes mellitus.
Methods
Sixteen patients with long-standing type 1 diabetes participated in an interventional clinical trial. Before any pharmacologic intervention began, diabetes management was optimized by thorough review of carbohydrate counting and insulin dose adjustment.
Results
Optimizing insulin therapy and carbohydrate counting for 4–6 months decreased the enrollees' hemoglobin A1C (−0.7 ± 0.6%, P = 0.0003) without weight gain (−0.6 ± 2.9 kg, P = 0.44) or increased frequency of hypoglycemia (−0.5 ± 1.5 events per week, P = 0.22). The improved blood glucose control was achieved in most subjects by lowering their basal or long-acting insulin doses while making compensatory increases in meal-associated insulin doses.
Conclusions
“Fine tuning” of diabetes management with intensive insulin therapy was accomplished without inducing weight gain or worsening hypoglycemia. This was achieved by readjusting the ratio of basal to meal-associated insulin without increasing the total daily insulin dose.
Introduction
For almost 2 decades, the mainstay of treatment for patients with type 1 diabetes mellitus has been intensive insulin therapy,1–3 provided with multiple daily injections (MDI) or the use of continuous subcutaneous insulin infusion (CSII or insulin pump), and frequent blood glucose measurements with a home glucose monitoring system. Advances in the pharmacodynamics and pharmacokinetics of the insulin molecule in addition to new devices for the administration of insulin therapy have allowed treatment regimens to more closely mimic the physiologic insulin response of healthy individuals without type 1 diabetes mellitus.
Previous reports about intensifying insulin therapy have documented increased weight gain, increased insulin doses, and especially an increased frequency of hypoglycemia.1,4,5 We thus set out to test whether patients can intensify their insulin treatment without increasing weight and frequency of hypoglycemia while receiving close support and feedback from the study team.
Research Design and Methods
Patients interested in participating in a type 1 diabetes–related clinical trial contacted the National Institutes of Health's recruitment office. Details regarding the main study design and primary outcome have been published elsewhere.6 The protocol was approved by the institutional review board of the National Institute of Diabetes, Digestive and Kidney Diseases, and all patients provided written informed consent. Study inclusion criteria included the following: (1) age 18–60 years, (2) positive antibodies (glutamic acid decarboxylase 65, islet cell antibody 512) and/or a typical history of type 1 diabetes (childhood onset, immediate and persistent insulin dependence, positive family history), (3) body mass index (BMI) between 20 and 30 kg/m2, and (4) duration of disease ≥5 years.
Prior to beginning an experimental intervention, subjects entered an “optimization period” of 4–6 months duration with the goal of reaching the best achievable diabetes control. During a screening visit, subjects met with a dietician, who reviewed knowledge of carbohydrate counting, reading food labels, and portion sizes. No advice was given regarding weight management. For the remainder of the optimization period, subjects did not come to the clinic but engaged in frequent (in some cases daily) telephone and e-mail contact with the study team. Subjects performed self-assessment of blood glucose levels at least seven times daily (before meals and 2 h postprandially, at bedtime, and occasionally overnight) and entered this information into electronic spreadsheets that were shared with the diabetes team. Insulin dose adjustments were made per clinician judgment rather than a strict protocol, with a target blood glucose level of 80–140 mg/dL. Patients also recorded their carbohydrate intake on their spreadsheets but did not keep additional food records.
Capillary glucose measurements utilized the glucose oxidase method. Hemoglobin A1c (A1C) was determined by high-performance liquid chromatography (intra-assay coefficient of variation 0.58% for normal A1C and 0.42% for elevated A1C; inter-assay coefficient of variation 1.3% for normal A1C and 0.93% for elevated A1C).
Data were expressed as mean ± SD values. Statistical analyses were performed with GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA; www.graphpad.com). Differences before versus after the optimization period were tested by paired Student's t test. Correlations were performed using the Spearman method. A P value of <0.05 was considered statistically significant.
Results
Twenty individuals (nine men) were enrolled in the study. Four patients discontinued their participation after beginning intensified blood glucose management because of fear of hypoglycemia (n = 1) or psychosocial problems (e.g., divorce). Baseline characteristics of subjects are shown in Table 1. There were no clinically or statistically significant differences between subjects who did or did not complete the study.
Table 1.
Baseline Characteristics of Subjects
| Completers | Dropouts | |
|---|---|---|
| Number | 16 | 4 |
| Age (years) | 38.8 ± 10.3 (18.1–60.0) | 41.7 ± 15.2 (23–58) |
| Gender | 8 female, 8 male | 3 female, 1 male |
| Hemoglobin A1c (%) | 7.2 ± 0.9 (5.6–8.9) | 7.7 ± 1.9 (5.7–10.2) |
| Duration of diabetes (years) | 20.6 ± 11.1 (4.1–38.4) | 24.1 ± 9.7 (11.7–33.4) |
| BMI (kg/m2) | 24.4 ± 3.3 | 24.0 ± 3.6 |
| Insulin delivery | 10 CSII, 6 MDI* | 4 CSII |
Continuous variables are mean ± SD (range).
Two subjects changed from MDI to CSII use during the study.
BMI, body mass index; CSII, continuous subcutaneous insulin infusion (insulin pump); MDI, multiple daily injections (of insulin).
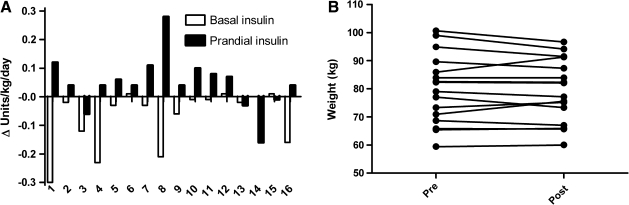
The period of optimized insulin administration led to the following observations: mean A1C decreased from 7.2 ± 0.9% to 6.5 ± 0.7% (P = 0.0003), with 14 of 16 subjects showing a decrease in A1C. In the two subjects whose A1C did not improve, A1C increased from 6.5% to 7% and from 6.2% to 6.4%, respectively. Subjects with the highest initial A1C showed the greatest improvements (R2 = 0.43, P = 0.0055). This improvement was achieved by keeping overall insulin doses the same (Δ = −0.03 ± 0.11 units/kg/day, P = 0.34) but reducing basal insulin doses from 0.34 ± 0.15 units/kg/day to 0.26 ± 0.10 units/kg/day (P = 0.01) and slightly increasing meal-related (prandial) insulin doses from 0.25 ± 0.1 to 0.30 ± 0.08 units/kg/day (P = 0.07) (Fig. 1A). The ratio of basal to prandial insulin changed from 1.9 ± 2.1 (two-thirds basal and one-third prandial) before the intervention to 1.0 ± 0.5 (half basal and half prandial) after the intervention. Dose changes for individual subjects are shown in Figure 1A. Blood glucose excursions (SD of the average self-monitored blood glucose levels during 1 week) were reduced from 68.3 ± 16.6 mg/dL to 58.4 ± 13.5 mg/dL (P = 0.03).
FIG. 1.
Changes in clinical parameters from time of study enrollment to end of optimization period for each subject. (A) Improved hemoglobin A1c was achieved via redistribution of basal insulin doses (significantly decreased, P = 0.01) and prandial doses (slightly increased, P = 0.07). (B) Body weight increased by more than 1 kg in only three subjects.
Subjects' weights remained stable (Fig. 1B) (79.9 ± 12.3 kg before and 79.3 ± 11.2 kg after optimization, P = 0.44, 95% confidence interval for weight change −0.97 to 2.1 kg). Three of the 16 subjects (patients 1, 11, and 12) gained more than 1 kg (5.4, 4.7, and 1.9 kg, respectively). Weight gain was associated with increases in prandial insulin dose (R2 = 0.35, P = 0.02) but not basal insulin dose (R2 = 0.005, P = 0.79). Greater lowering of A1C was associated with weight loss; however, this was not statistically significant (R2 = 0.20, P = 0.08).
Moderate to severe hypoglycemia (defined as symptomatic hypoglycemia or a measured blood glucose <54 mg/dL) did not occur more frequently (1.5 ± 1.5 episodes per week before and 1.0 ± 1.0 episodes per week after optimization, P = 0.22). Using a more liberal definition of hypoglycemia (blood glucose <70 mg/dL), frequency of hypoglycemia stayed stable at 4.4 ± 3.6 episodes per week before and 4.4 ± 3.3 episodes per week after optimization (P = 0.95).
Discussion
For patients with type 1 diabetes, the main goal of intensive insulin therapy is to improve blood glucose control (reflected by lower A1C values) because near-normal glycemia has been shown to positively impact morbidity and mortality. For many individuals, the “price” of such intensive therapy is weight gain and increased frequency of hypoglycemia.1,7 In this small cohort of individuals already receiving intensive insulin therapy, we found that individualized clinical management further improved blood glucose control while avoiding weight gain and more frequent hypoglycemia.
Although this was a small study, given the observed SD, there was 80% power to detect a mean weight change as small as 2.1 kg (only 2.6% of the subjects' initial body weight). In fact, only two of 16 subjects gained over 2.1 kg, and overall the cohort lost weight, supporting the idea that intensification of insulin as performed in this study does not cause weight gain. Similarly, the study had 80% power to detect an approximately twofold change in the incidence of hypoglycemia (for comparison, a threefold difference between conventional vs. intensive therapy was observed in the Diabetes Control and Complications Trial1).
A critical point to the success of this study may be that optimized therapy did not imply increased insulin doses; instead, basal insulin was reduced, carbohydrate counting was improved, and prandial insulin was increased. The ratio of basal to prandial insulin decreased from 2:1 to the recommended ratio of 1:1 after optimization, suggesting that some subjects may have been underdosing for meals and compensating with increased basal insulin. Improved carbohydrate counting, resulting in more accurate prandial insulin dosing, likely also contributed to the improved glycemia control seen in this cohort. Although most patients with type 1 diabetes are taught carbohydrate counting at the time of diagnosis, accuracy likely wanes over time, and many patients with long-standing diabetes may benefit from re-education in carbohydrate counting.
Although this study contrasts with findings of older, large-scale clinical trials such as the Diabetes Control and Complications Trial, similar observations have been reported. In a retrospective analysis of 19 patients with long-standing type 1 diabetes who were followed before and after transition from two to four daily injections of regular and NPH insulin to CSII, patients had improved blood glucose control, associated with decreased total daily insulin dose, decreased ratio of basal to prandial insulin, and no weight gain after 12 or more months.8 This pattern has frequently been observed when transitioning from MDI to CSII regimens. In another observational study, 65 patients transitioning from NPH to MDI with glargine did not experience weight gain or severe hypoglycemia (<40 mg/dL).9
Jacob et al.10 examined the reasons for weight gain with intensification of insulin treatment in 14 subjects with poorly controlled type 1 diabetes. After 6 months, A1c decreased by 1.7%, and weight increased nonsignificantly by 2.15 kg (five subjects lost weight, and 11 gained weight). Weight gain was not correlated with change in A1C, glycosuria, resting energy expenditure, activity, appetite, food intake, or hypoglycemia, and the authors speculated that insulin's direct effect on lipogenesis may have mediated weight gain. Unfortunately, insulin dose changes were not reported in this study. In our study, weight gain was correlated with increases in prandial insulin doses, supporting the idea of insulin-induced lipogenesis, but despite this the majority of subjects lost weight.
Although the paradigm of frequent blood glucose monitoring and almost daily contact with this study's research team will not be practical for most patients and their healthcare teams, the basic principles used in this pilot study apply to many motivated patients. Individuals with type 1 diabetes and their care providers should be aware that it is possible to improve blood glucose control while avoiding weight gain and worsening hypoglycemia. For many patients, optimizing insulin management may not involve increases in total daily insulin dose, but instead redistribution of insulin to the recommended 50% basal/50% prandial ratio, with careful attention to accurate insulin dosing for carbohydrate intake.
Acknowledgments
This research was supported by the intramural research program of the National Institute of Diabetes, Digestive and Kidney Diseases.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Giannini C. Mohn A. Chiarelli F. Technology and the issue of cost/benefit in diabetes. Diabetes Metab Res Rev. 2009;25(Suppl 1):S34–S44. doi: 10.1002/dmrr.986. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Nathan DM. Zinman B. Cleary PA. Backlund JY. Genuth S. Miller R. Orchard TJ. Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications experience (1983–2005) Arch Intern Med. 2009;169:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichard P. Berglund B. Britz A. Cars I. Nilsson BY. Rosenqvist U. Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. J Intern Med. 1991;230:101–108. doi: 10.1111/j.1365-2796.1991.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 5.Holl RW. Swift PG. Mortensen HB. Lynggaard H. Hougaard P. Aanstoot HJ. Chiarelli F. Daneman D. Danne T. Dorchy H. Garandeau P. Greene S. Hoey HM. Kaprio EA. Kocova M. Martul P. Matsuura N. Robertson KJ. Schoenle EJ. Sovik O. Tsou RM. Vanelli M. Aman J. Insulin injection regimens and metabolic control in an international survey of adolescents with type 1 diabetes over 3 years: results from the Hvidore study group. Eur J Pediatr. 2003;162:22–29. doi: 10.1007/s00431-002-1037-2. [DOI] [PubMed] [Google Scholar]
- 6.Rother KI. Spain LM. Wesley RA. Digon BJ., 3rd Baron A. Chen K. Nelson P. Dosch HM. Palmer JP. Brooks-Worrell B. Ring M. Harlan DM. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32:2251–2257. doi: 10.2337/dc09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care. 2001;24:1711–1721. doi: 10.2337/diacare.24.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford LM. Sinha RN. Odell RM. Comi RJ. Efficacy of insulin pump therapy: mealtime delivery is the key factor. Endocr Pract. 2000;6:239–243. doi: 10.4158/EP.6.3.239. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber SA. Russmann A. Long-term efficacy of insulin glargine therapy with an educational programme in type 1 diabetes patients in clinical practice. Curr Med Res Opin. 2007;23:3131–3136. doi: 10.1185/030079907X242827. [DOI] [PubMed] [Google Scholar]
- 10.Jacob AN. Salinas K. Adams-Huet B. Raskin P. Potential causes of weight gain in type 1 diabetes mellitus. Diabetes Obes Metab. 2006;8:404–411. doi: 10.1111/j.1463-1326.2005.00515.x. [DOI] [PubMed] [Google Scholar]