Abstract
Background
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a lipoprotein-associated enzyme that cleaves oxidized phosphatidylcholines, generating pro-atherosclerotic lysophosphatidylcholine and oxidized free fatty acids. Lp-PLA2 is independently associated with cardiovascular disease (CVD) in a variety of populations. Coronary calcium is a measure of subclinical CVD, and progression of coronary calcification predicts future CVD events. In type 1 diabetes there is an increase in coronary calcium and CVD despite a favorable lipid profile. Levels of Lp-PLA2 in type 1 diabetes are not known, nor is the relationship between Lp-PLA2 and progression of coronary calcification.
Methods
The Coronary Artery Calcification in Type 1 Diabetes study measured coronary calcium by electron-beam computed tomography twice over a 2.6 ± 0.3-year interval. Lp-PLA2 mass and activity were measured at baseline (n = 1,097 subjects, 506 with and 591 without type 1 diabetes).
Results
In type 1 diabetes Lp-PLA2 mass was marginally higher (285 ± 79 vs. 278 ± 78 ng/mL, P = 0.1), and Lp-PLA2 activity was significantly lower (137 ± 30 vs. 146 ± 36 nmol/min/mL, P < 0.0001) than in those without diabetes. There was a greater proportion of those with progression of coronary calcification in type 1 diabetes compared with those without diabetes (24% vs. 10%, P < 0.0001). Lp-PLA2 activity was independently associated with progression of coronary calcification in multivariate analysis (4th quartile verses bottom three quartiles, odds ratio = 1.77 [1.08–2.91], P = 0.02). LpPLA2 mass was not significantly associated with progression of coronary calcification in this cohort (P = 0.09).
Conclusions
Lp-PLA2 activity predicts progression of subclinical atherosclerosis in individuals with and without type 1 diabetes.
Introduction
In type 1 diabetes, coronary heart disease (CHD), the leading cause of death, occurs earlier in life, affects women as often as men, and has dramatically higher mortality.1 Type 1 diabetes can be characterized as a pro-inflammatory state. Pro-inflammatory cytokines are expressed in type 1 diabetes and in animal models of type 1 diabetes.2 As has been shown in those without diabetes, there is a strong relationship between inflammatory markers (e.g., interleukin-6, tumor necrosis factor α, and C-reactive protein [CRP]) and cardiovascular disease in type 1 diabetes.3 However, much of the excess risk of CHD in type 1 diabetes remains unexplained.
Coronary artery calcium (CAC), measured by electron-beam computed tomography (CT), is associated with atherosclerosis4 and independently predicts CHD events.5–7 Several groups, including our own, have found an increase in coronary calcium prevalence and magnitude in patients with diabetes.8–10 Factors related to coronary calcium in type 1 diabetes include inflammatory markers such as CRP11 and high white blood cell count.12 Progression of coronary calcification has been shown to predict clinical coronary disease events.13–15
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a macrophage-derived enzyme that may play an important role in the link among inflammation, oxidation, and atherosclerosis.16,17 Lp-PLA2 secreted from the macrophage is bound to low-density lipoprotein (LDL) through protein-to-protein interaction with apolipoprotein B.18 Upon oxidation of LDL, Lp-PLA2 cleaves oxidized phosphatidylcholine into lysophosphatidylcholine and oxidized nonesterified free fatty acids.16 This stimulates the secretion of cytokines from macrophages and promotes plaque development.
Lp-PLA2 has been shown to be an independent predictor of CHD events in most studies;19 however, the relationship between Lp-PLA2 and coronary calcium has not been consistent.20,21 The association between LpPLA2 and progression of coronary calcification has not been reported. Given the importance of inflammation in atherosclerosis, it is likely that inflammatory biomarkers will be associated with the progression of subclinical atherosclerosis in both type 1 diabetes and in those without diabetes. We hypothesize that the vascular-specific inflammatory marker, Lp-PLA2, will predict progressive of subclinical atherosclerosis.
Subjects and Methods
Study participants
The data presented in this report are baseline measurements of those participants completing a Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study follow-up visit. At baseline they were 19–56 years of age and were asymptomatic for coronary artery disease (CAD) (no history of coronary artery bypass graft, coronary angioplasty, or unstable angina). Patients with type 1 diabetes generally had been diagnosed when younger than 30 years of age, and among those who were 30 years or older at diagnosis, positive antibodies or a clinical course consistent with type 1 diabetes was present. Of the 1,416 persons enrolled at baseline, 1,215 (86%) were seen for a follow-up visit, and progression of subclinical coronary artery atherosclerosis was obtained for 1184 (97%). Of that group, 1,158 (98%) had available stored samples, and Lp-PLA2 mass and activity were measurable on 1,097 (95%) samples; of these, 1,021 (93%) had complete covariate. All subjects provided informed consent, and the study was approved by the Colorado Combined Institutional Review Board.
Laboratory measurements
After an overnight fast, blood was collected and centrifuged, and separated plasma was stored at −70°C until assayed. Lp-PLA2 mass was measured using the diaDexus PLAC test (diaDexus Inc., South San Francisco, CA), based on the principle of a sandwich enzyme immunoassay using two specific monoclonal antibodies.22 Monoclonal anti-Lp-PLA2 antibody (2C10) is immobilized to microwells, and plasma is added and incubated for 10 min at 20–26°C. A second monoclonal anti-Lp-PLA2 antibody (4B4) labeled with the enzyme horseradish peroxidase is then added and incubated at 20–26°C for 180 min. The wells are washed, and the substrate, tetramethylbenzidine, is then added and incubated at 20–26°C for 20 min. The absorbance at 450 nm is directly proportional to the concentration of Lp-PLA2 present. A set of Lp-PLA2 calibrators is used to plot a standard curve of absorbance versus Lp-PLA2 concentration from which the Lp-PLA2 concentration in the test sample can be determined in ng/mL.
Lp-PLA2 activity was measured with a colorimetric activity method provided by diaDexus Inc. Samples, standards, or controls are added to wells of a nonbinding 96-well microplate, followed by addition of the reaction buffer containing substrate. This reaction takes place in the presence of a mild detergent. In the presence of Lp-PLA2, the substrate is converted upon hydrolysis by the phospholipase enzyme. The change in absorbance is immediately measured at 405 nm over a 60–180-s interval. The level of Lp-PLA2 activity (in nmol/min/mL) is calculated from the slope (optical density at 405 nm/min), based on a standard conversion factor from a p-nitrophenol calibration curve.
It is important to note that the measurements of Lp-PLA2 mass and activity represent, in part, potentially different physiologically relevant species of Lp-PLA2. The mass assay represents Lp-PLA2 in which the epitopes are exposed to the antibody in the presence of intact lipoproteins, whereas the activity assay represents Lp-PLA2 after disruption of lipoproteins by detergent. Thus Lp-PLA2 mass represents “exposed” Lp-PLA2, and Lp-PLA2 activity represents total Lp-PLA2 in plasma. Studies have shown that Lp-PLA2 mass is distributed on specific subtypes of LDL and that Lp-PLA2 activity is different than that predicted by mass.23
Covariate measurements
Total plasma cholesterol and triglyceride levels were measured using standard enzymatic methods. High-density lipoprotein (HDL) cholesterol was separated using dextran sulfate, and LDL cholesterol was calculated using the Friedewald formula. High-performance liquid chromatography (Variant, Bio-Rad, Hercules, CA) was used to measure hemoglobin A1c. Plasma glucose was measured using the standard hexokinase method. Homocysteine was determined by the Abbott (Abbott Park, IL) IMX automated procedure. CRP was measured in the laboratory of Dr. Russell Tracy at the University of Vermont, Burlington, VT using the BNII nephelometer from Dade Behring (Deerfield, IL) utilizing a particle-enhanced immunonephelometric assay. Cystatin C was measured on stored serum samples in the clinical laboratory at the University of Colorado Hospital in Denver, CO, using a commercially available particle-enhanced immunonephelometric assay (Dade-Behring). Results are reported in mg/L with a sensitivity cutoff of 0.23 mg/L. Urine albumin was measured by radioimmunoassay, and the albumin excretion rate was determined by radioimmunoassay; the results of two timed overnight urine collections were averaged.
Anthropometric measurements
We measured height and weight and calculated body mass index. Minimum waist and maximum hip measurements were obtained in duplicate, and the results were averaged. Intra-abdominal fat and subcutaneous fat were assessed using abdominal CT scan at the L4–L5 levels. The total intra-abdominal fat volume and subcutaneous fat volume (in cm3) were measured using AccuAnalyzer software from Accu-Image (Sunnyvale, CA). Resting systolic blood pressure and fifth-phase diastolic blood pressure were measured three times while the subjects were seated, and the second and the third measurements were averaged.9 Hypertension was defined as current antihypertensive therapy or untreated hypertension (blood pressure ≥140/90 mm Hg) at the time of the study visit.
Interview measurements
Current and former smoking status was obtained by questionnaire. Participants completed a standardized questionnaire including medical history and medication inventory as previously reported.
Imaging
All patients underwent two electron beam CT scans within 5 min without contrast at baseline and two scans at follow-up. Images were obtained of the entire epicardial system using a CT scanner (C-150 Ultrafast, Imatron, South San Francisco) with a 100-ms exposure. The standard acquisition protocol was used.24 Scanning started from near the lower margin of the bifurcation of the main pulmonary artery. Images were electrocardiographically triggered at 80% of the R-R interval, and 30–40 contiguous 3-mm slices were acquired. The volume scores were calculated using the volumetric method, which is based on isotropic interpolation.25
Definition of progression of coronary calcification
We defined significant progression of coronary calcification as a difference in the square root transformed calcium volume score between baseline and follow-up of >2.5.26 This accounts for the measurement variability, is valid at any baseline calcium score, and is less than 1% likely to be due to interscan variability per se.
Statistical methods
All analyses were performed using SAS system version 9.1 (SAS Institute, Cary, NC). Data are presented as arithmetic means and SDs for continuous variables that are normally distributed and geometric means and ranges for log-transformed variables. Categorical variables are presented as percentages.
Correlation coefficients between Lp-PLA2 mass and activity and other measurements of interest were calculated and compared using Pearson correlation coefficients.
To assess the relationship between Lp-PLA2 mass and activity and coronary calcification progression, we chose an a priori model of progression of subclinical atherosclerosis constructed using known predictors; baseline coronary calcium, diabetes, age, sex, hypertension, waist circumference, apolipoprotein B, cystatin C (a measure of kidney function), statin use, and smoking. LDL cholesterol was substituted for apolipoprotein B in this model and did not alter the association between Lp-PLA2 and coronary calcification progression. Other potentially important variables were assessed using forward, backward, and stepwise logistic regression and included in the model where appropriate. Lp-PLA2 mass and activity were separately added to these models as quartiles. A secondary analysis was performed by adding participants with a clinical coronary disease event over the course of the follow-up. A CAD event was defined as myocardial infarction, coronary artery bypass graft, angioplasty with stent, or death attributed to CAD as adjudicated by a three physician committee.
Results
Participants with type 1 diabetes were more likely to be younger and to have a more favorable lipid profile with lower LDL-cholesterol and triglyceride and higher HDL-cholesterol (Table 1). Although body mass index and waist circumference were not different, those with type 1 diabetes had less visceral fat. There was significantly more hypertension and statin use among individuals with type 1 diabetes.
Table 1.
Characteristics of Study Population by Type 1 Diabetes Status
| Variable | DM (n = 506) | Non-DM (n = 591) | P value |
|---|---|---|---|
| Lp-PLA2 | |||
| Mass (ng/mL) | 285.3 ± 79.2 | 277.6 ± 77.7 | 0.1 |
| Activity (nmol/min/mL) | 137.2 ± 29.5 | 145.9 ± 35.6 | <0.0001 |
| Age (years) | 36.8 ± 8.9 | 39.8 ± 8.8 | <0.0001 |
| Male (%) | 46% | 51% | 0.38 |
| Total cholesterol (mg/dL) | 173.6 ± 33.4 | 191.5 ± 36.6 | <0.0001 |
| LDL cholesterol (mg/dL) | 99.4 ± 28.2 | 115.7 ± 32.8 | <0.0001 |
| HDL cholesterol (mg/dL) | 56.3 ± 16.3 | 51.4 ± 14.5 | <0.0001 |
| Triglycerides (mg/dL) | 89.8 (25–398) | 121.6 (27–400) | <0.0001 |
| BMI (kg/m2) | 26.1 ± 4.3 | 25.9 ± 4.8 | 0.61 |
| Average waist (cm) | 84.8 ± 12.2 | 85.6 ± 14.1 | 0.33 |
| Visceral fat at L4–L5 (cm2) | 10.3 (8–12) | 10.6 (8–12) | <0.0001 |
| Subcutaneous fat at L4–L5 (cm2) | 11.7 (9–13) | 11.7 (10–13) | 0.64 |
| CRP (μg/mL) | 2.0 ± 2.5 | 1.7 ± 1.8 | 0.06 |
| Progression | 23.5% | 10.0% | <0.0001 |
| Baseline CAC | 48.6 ± 216.0 | 12.8 ± 67.8 | <0.0001 |
| Smoking | |||
| Current | 10.2% | 9.2% | 0.56 |
| Ever | 30.4% | 30.9% | 0.87 |
| Statin use | 14.2% | 4.0% | <0.0001 |
| Hypertension | 40.2% | 14.6% | <0.0001 |
BMI, body mass index; CAC, coronary artery calcium; CRP, C-reactive protein; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2.
Lp-PLA2 mass was marginally higher in type 1 diabetes (285 ± 79 vs. 277 ± 78 ng/mL, P = 0.1) than those without diabetes (Table 1). Lp-PLA2 activity was significantly different in those with type 1 diabetes compared with individuals without diabetes (137 ± 30 vs. 145 ± 36 nmol/min/mL, P < 0.0001). Individuals with type 1 diabetes had more coronary calcium at baseline, and a significantly greater proportion of those with type 1 diabetes had progression of coronary calcification (23.5% vs. 10.0%, P < 0.0001) over an average of 2.6 years.
The 19% of individuals who demonstrated significant progression of coronary calcification were older and more likely to be male (Table 2). They had lower HDL, higher cholesterol and triglyceride, and more obesity and hypertension. Those who had progression of coronary calcification had significantly more baseline coronary calcium. CRP was marginally higher in participants with type 1 diabetes with progression of coronary calcification but not in those without diabetes. Lp-PLA2 mass was not different in those with progression of coronary calcification (type 1 diabetes, 294 ± 82 vs. 283 ± 78 ng/ml, P = 0.19; without diabetes, 292 ± 85 vs. 276 ± 77 ng/mL, P = 0.13); however, Lp-PLA2 activity was higher in those with progression of coronary calcification (type 1 diabetes, 141 ± 29 vs. 136 ± 29 nmol/min/mL, P = 0.09; without diabetes, 168 ± 34 vs. 144 ± 35 nmol/min/mL, P < 0.0001).
Table 2.
Characteristics of Study Population by Progression of Coronary Calcification and Type 1 Diabetes Status
| |
Progression of coronary calcification |
No progression of coronary calcification |
P value for difference between progression and no progression |
|||
|---|---|---|---|---|---|---|
| Variable | DM (n = 119) | Non-DM (n = 59) | DM (n = 387) | Non-DM (n = 532) | P DM | P non-DM |
| Lp-PLA2 | ||||||
| Mass (ng/mL) | 293.5 ± 82.4 | 291.9 ± 85.0 | 282.8 ± 78.1 | 276.0 ± 76.7 | 0.19 | 0.13 |
| Activity (nmol/min/mL) | 141.2 ± 2.9 | 167.8 ± 34.0 | 136.0 ± 28.8 | 143.5 ± 34.9 | 0.09 | <0.0001 |
| Age (years) | 42.4 ± 7.9 | 46.6 ± 7.3 | 35.0 ± 8.4 | 39.0 ± 8.6 | <0.0001 | <0.0001 |
| Male (%) | 57.90% | 83.10% | 42.60% | 45.10% | 0.003 | <0.0001 |
| Total cholesterol (mg/dL) | 176.1 ± 32.2 | 202.3 ± 40.9 | 172.9 ± 33.8 | 190.2 ± 35.9 | 0.36 | 0.02 |
| LDL cholesterol (mg/dL) | 101.1 ± 26.5 | 128.0 ± 34.1 | 98.9 ± 28.7 | 114.3 ± 32.4 | 0.45 | 0.002 |
| HDL cholesterol (mg/dL) | 55.7 ± 17.1 | 43.0 ± 8.7 | 56.5 ± 16.0 | 52.4 ± 14.7 | 0.67 | <0.0001 |
| Triglycerides (mg/dL) | 96.3 (32–368) | 156.5 (50–400) | 87.7 (25–357) | 117.7 (27–396) | 0.03 | <0.0001 |
| BMI (kg/m2) | 26.8 ± 4.8 | 29.6 ± 5.6 | 25.9 ± 4.1 | 25.6 ± 4.6 | 0.06 | <0.0001 |
| Average waist (cm) | 88.8 ± 13.0 | 99.3 ± 13.4 | 83.6 ± 11.7 | 84.1 ± 13.3 | <0.0001 | <0.0001 |
| Visceral fat at L4–L5 (cm2) | 10.5 (8–12) | 11.0 (10–12) | 10.3 (9–12) | 10.5 (8–12) | 0.0002 | <0.0001 |
| Subcutaneous fat at L4–L5 (cm2) | 11.7 (9–13) | 12.0 (8–13) | 11.7 (10–13) | 11.7 (9.7–13) | 0.94 | 0.0005 |
| CRP (μg/mL) | 2.2 (0.4–29) | 1.7 (0.6–5.8) | 1.9 (0.3–21) | 1.8 (0.2–17.3) | 0.23 | 0.85 |
| Baseline CAC | 164.7 ± 411.4 | 92.2 ± 189.9 | 12.9 ± 62.4 | 4.0 ± 19.9 | <0.0001 | <0.0001 |
| Smoking (%) | ||||||
| Current | 11.9% | 8.5% | 9.7% | 9.2% | 0.49 | 0.04 |
| Past | 36.4% | 28.8% | 28.5% | 31.1% | 0.1 | 0.13 |
| Statin use (% current) | 26.9% | 3.4% | 10.3% | 4.1% | <0.0001 | 0.07 |
| Hypertension | 64.7% | 27.1% | 32.6% | 13.2% | <0.0001 | 0.004 |
BMI, body mass index; CAC, coronary artery calcium; CRP, C-reactive protein; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2.
There was more statin use among those with diabetes compared with those without diabetes (14.2% vs. 4.0%, P < 0.0001). Among those with diabetes on statin therapy, there was no significant relationship between Lp-PLA2 mass (P = 0.23) or activity (P = 0.34) and progression of coronary calcification, whereas among those not taking statins there was a significant relationship between Lp-PLA2 activity and progression (144.1 ± 30.7 vs. 135.4 ± 28.8 nmol/min/mL, P = 0.014, progression vs. no progression, respectively).
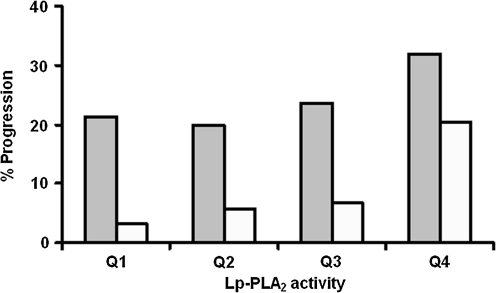
There was a trend toward an increased proportion of individuals with progression of coronary calcification by quartiles of Lp-PLA2 activity (Fig. 1).
FIG. 1.
Percentage of participants showing significant progression of subclinical atherosclerosis over the 2.6 years of follow-up by quartiles (Q1–Q4) of lipoprotein-associated phospholipase A2 (Lp-PLA2) activity (diabetes specific). Participants with type 1 diabetes are shown in the dark columns, and those without diabetes are shown in open columns. The Jonckheere–Terpstra Test for trend is marginally significant (P = 0.07) in type 1 diabetes and significant in those without diabetes (P < 0.0001).
Lp-PLA2 mass and activity are significantly positively correlated (Table 3), similar to other studies.27 Both Lp-PLA2 mass and activity show a strong positive correlation with total and LDL cholesterol as well as apolipoprotein B in both type 1 diabetes and in those without diabetes. Lp-PLA2 activity is negatively correlated with HDL cholesterol. Correlations between Lp-PLA2 and measures of obesity were modest with the strongest relationship being between Lp-PLA2 activity in those without diabetes (ρ ranging from 0.15 to 0.17, P < 0.001).
Table 3.
Correlations Between Lipoprotein-Associated Phospholipase A2 Mass and Activity and Other Important Cardiovascular Risk Factors
| |
Lp-PLA2 |
|||
|---|---|---|---|---|
| |
Mass |
Activity |
||
| Variable | DM | Non-DM | DM | Non-DM |
| Lp-PLA2 activity (nmol/min/mL) | 0.35*** | 0.43*** | 1 | 1 |
| Age (years) | 0.14** | −0.001 | 0.05 | 0.13** |
| Total cholesterol (mg/dL) | 0.19*** | 0.14** | 0.38*** | 0.36*** |
| LDL cholesterol (mg/dL) | 0.21*** | 0.21*** | 0.54*** | 0.48*** |
| HDL cholesterol (mg/dL) | 0.06 | −0.07 | −0.26*** | −0.36*** |
| Triglycerides (mg/dL) | 0.02 | −0.04 | 0.19*** | 0.23*** |
| APOB (mg/dL) | 0.11* | 0.13** | 0.51*** | 0.48*** |
| BMI (kg/m2) | −0.06 | 0.04 | 0.01 | 0.15** |
| Average waist (cm) | −0.05 | 0.03 | 0.04 | 0.17*** |
| Visceral fat at L4–L5 (cm2) | −0.09* | 0.03 | 0.07 | 0.17*** |
| Subcutaneous fat at L4–L5 (cm2) | −0.05 | 0.03 | 0.07 | 0.12** |
| CRP (μg/mL) | −0.05 | 0.01 | −0.15** | −0.08 |
| HbA1c (%) | −0.07 | −0.09* | 0.02 | −0.02 |
| Insulin dose (units/kg/day) (DM) | −0.11* | NA | −0.02 | NA |
| Duration of DM | 0.15** | NA | 0.01 | NA |
All correlations are controlled for gender.
P<0.05, **P<0.01, ***P<0.0001.
APOB, apolipoprotein B; BMI, body mass index; CRP, C-reactive protein; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; NA, not applicable.
Lp-PLA2 mass and activity were added separately to the a priori model. Lp-PLA2 mass was not significantly independently related to progression of coronary calcification (P = 0.09). LpPLA2 activity was positively related to progression of coronary calcification in the multivariate model (odds ratio = 1.77, 95% confidence interval 1.08–2.91, P = 0.02) (Table 4). CRP was not related to progression of coronary calcification.
Table 4.
Predictors of Progression of Coronary Calcification, a Priori Model in Multiple Logistic Regression Analysis (n = 1,021)
| Variable | OR (95% CI)* | P value |
|---|---|---|
| Baseline CVS | 2.15 (1.69–2.71) | <0.0001 |
| Diabetes | 2.89 (1.74–4.79) | <0.0001 |
| Age (10 years) | 2.19 (1.63–2.94) | <0.0001 |
| Sex (male) | 1.44 (0.88–2.36) | 0.15 |
| Hypertension | 1.62 (1.03–2.56) | 0.04 |
| Waist circumference | 1.76 (1.39–2.23) | <0.0001 |
| APOB | 0.77 (0.59–0.99) | 0.04 |
| Cystatin C | 1.23 (1.01–1.50) | 0.04 |
| Statin medication | 1.29 (0.73–2.31) | 0.37 |
| Smoker | ||
| Current | 1.12 (0.57–2.18) | 0.74 |
| Former | 1.09 (0.65–1.83) | 0.76 |
| Lp-PLA2 activity | 1.77 (1.08–2.91) | 0.02 |
Odds ratio (OR) and 95% confidence interval (CI) are per SD for each variable. The 1 SD value is as follows: calcium volume score (CVS), 4.6; waist circumference, 13.2 cm; apolipoprotein B (APOB), 25.9 mg/dL; cystatin C, 0.19 mg/L; lipoprotein-associated phospholipase A2 (Lp-PLA2), 4th quartile versus bottom three quartiles.
Discussion
This study shows that Lp-PLA2 activity is associated with progression of subclinical atherosclerosis as measured by a significant change in coronary calcification over a short follow-up period of 2.6 years. To our knowledge this is the first report to demonstrate a relationship between Lp-PLA2 activity and progression of subclinical vascular disease. This association is independent of other known risk factors as well as the baseline level of coronary calcium. In this cohort, Lp-PLA2 mass was not independently related to the progression of coronary calcification.
Lp-PLA2 mass or activity has been shown to be associated with long-term risk of cardiac events,28 and this study extends that work to a subclinical outcome. Lp-PLA2 activity has also been shown to be associated with mechanisms leading to atherosclerotic plaque vulnerability through macrophage cell death and monocyte attraction.29,30 Progression of coronary calcification is a consequence of these pathophysiologic mechanisms. This suggests that Lp-PLA2 activity is associated with plaque development and more advanced lesions that are calcified and ultimately lead to rupture and clinical events.
The CARDIA study found an association between Lp-PLA2 mass and cross-sectional coronary calcium but not with Lp-PLA2 activity.20 Cross-sectional coronary calcium as measured in the CARDIA study31 may not be as sensitive a measure of plaque evolution as progression of coronary calcification, although differences in the populations studied may also be an explanation for the differences observed. The Rotterdam Coronary Calcification Study found an association between Lp-PLA2 activity and coronary calcium21 that was removed by adding cholesterol to a multivariate model. The study also found an association between Lp-PLA2 activity and very high calcium (>1,000 vs. ≤100 Agatston units) cross-sectionally, in men and not in women in their older cohort (≥55 years old). These population-based studies suggest a positive relationship between Lp-PLA2 and the presence of coronary calcium. The current study expands this finding to the dynamic process of progression of coronary calcification.
Lp-PLA2 activity was significantly lower and Lp-PLA2 mass was marginally higher in individuals with type 1 diabetes compared with those without diabetes. This suggests differences in the distribution of Lp-PLA2 between those with and without type 1 diabetes. There is less total plasma Lp-PLA2 (as measured by Lp-PLA2 activity in the presence of detergent that disrupts lipoproteins) in type 1 diabetes. In comparison, “exposed” Lp-PLA2 (i.e., Lp-PLA2 that is either on the surface of lipoproteins or circulating free in plasma as measured by Lp-PLA2 mass) is marginally higher in type 1 diabetes. This may be related to differences in concentrations or in the composition of lipoproteins in type 1 diabetes compared with those without diabetes. This may be due to the differences in statin use in those with versus those without type 1 diabetes. Further studies on the lipoprotein distribution of Lp-PLA2 and the potential clinical significance of these differences are warranted.
There are several potential limitations to this study. The CACTI study has longitudinal measurements of coronary calcium and various covariates, but longitudinal measurements of Lp-PLA2 mass or activity have not yet been analyzed. Second, we use a surrogate, subclinical marker of CAD instead of hard events such as myocardial infarction or death. The CACTI cohort is relatively young and was asymptomatic for CAD at enrollment and has had few CAD events as of this writing (n = 15); data on patient outcomes are being collected prospectively. A secondary multivariate analysis that included these clinical events showed a very similar result (odds ratio = 2.11, 95% confidence interval = 1.23–3.64, P = 0.03, for the 4th quartile of Lp-PLA2 activity). Third, although in previous publications our group developed a valid method to define coronary calcification progression in the CACTI cohort,26 it must be acknowledged that measuring coronary calcium or its progression does not allow us to directly assess plaque stability. Plaque vulnerability and instability could be very important characteristics in the pathway between Lp-PLA2 and CAD events. In addition, we do not have a measure of oxidized LDL, oxidized phospholipids, lysophosphatidylcholine, or oxidized nonesterified free fatty acids that could aid in understanding the physiologic pathways linking Lp-PLA2 activity and progression of coronary calcification in our population of type 1 diabetes patients and those without diabetes. Finally, we do not have relevant genetic analysis related to Lp-PLA2 activity, stability, or association with apolipoprotein B, which could be important modifiers of the observed relationship of Lp-PLA2 activity with coronary calcification progression.
In conclusion, high Lp-PLA2 activity, but not Lp-PLA2 mass or CRP, is an independent predictor for significant progression of coronary calcification. These results suggest an association of Lp-PLA2 with the evolution of advanced, calcified plaques that lead to coronary disease events. This observation is seen over a short period of time (2.6 years) in a young cohort of individuals both with and without type 1 diabetes. Future studies will be required to determine whether Lp-PLA2 is merely a marker of progression of subclinical atherosclerosis or a potential target of treatment.
Acknowledgments
Support for the CACTI study was provided by grants RO1 HL61753 and RO1 HL079611 from the National Heart, Lung and Blood Institute, National Institutes of Health, postdoctoral fellowship 7-09-CVD-06 from the American Diabetes Association, and Clinical Investigation Core grant P30 DK57516 from the Diabetes Endocrinology Research Center. The study was performed at the Adult General Clinical Research Center at the University of Colorado Denver Anschutz Medical Center supported by grant MO1 RR000051 from the National Institutes of Health, at the Barbara Davis Center for Childhood Diabetes and at the Colorado Heart Imaging Center in Denver, CO. The authors would also like to thank diaDexus of South San Francisco, CA for their generous support in measuring Lp-PLA2.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Krolewski AS. Kosinski EJ. Warram JH. Leland OS. Busick EJ. Asmal AC. Rand LI. Christlieb AR. Bradley RF. Kahn CR. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 2.Bergholdt R. Heding P. Nielsen K. Nolsøe R. Sparre T. Størling J. Nerup J. Pociot F. Mandrup-Poulsen T. Type 1 database mellitus: an inflammatory disease of the islet. Adv Exp Med Biol. 2004;552:129–153. [PubMed] [Google Scholar]
- 3.Schram MT. Chaturvedi N. Schalkwijk CG. Fuller JH. Stehouwer CD. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes—the EURODIAB Prospective Complications Study. Diabetologia. 2005;48:370–378. doi: 10.1007/s00125-004-1628-8. [DOI] [PubMed] [Google Scholar]
- 4.O'Rourke RA. Brundage BH. Froelicher VF. Greenland P. Grundy SM. Hachamovitch R. Pohost GM. Shaw LJ. Weintraub WS. Winters WL., Jr Forrester JS. Douglas PS. Faxon DP. Fisher JD. Gregoratos G. Hochman JS. Hutter AM., Jr Kaul S. Wolk MJ. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000;102:126–140. doi: 10.1161/01.cir.102.1.126. [DOI] [PubMed] [Google Scholar]
- 5.Arad Y. Spadaro LA. Goodman K. Newstein D. Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253–1260. doi: 10.1016/s0735-1097(00)00872-x. [DOI] [PubMed] [Google Scholar]
- 6.Wong ND. Hsu JC. Detrano RC. Diamond G. Eisenberg H. Gardin JM. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–498. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 7.Raggi P. Callister TQ. Cooil B. He ZX. Lippolis NJ. Russo DJ. Zelinger A. Mahmarian JJ. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101:850–855. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 8.Colhoun HM. Rubens MB. Underwood SR. Fuller JH. The effect of type 1 diabetes mellitus on the gender difference in coronary artery calcification. J Am Coll Cardiol. 2000;36:2160–2167. doi: 10.1016/s0735-1097(00)00986-4. [DOI] [PubMed] [Google Scholar]
- 9.Dabelea D. Kinney G. Snell-Bergeon JK. Hokanson JE. Eckel RH. Ehrlich J. Garg S. Hamman RF. Rewers M. Coronary Artery Calcification in Type 1 Diabetes Study: Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. Erratum in Diabetes 2004;53:2177. [DOI] [PubMed] [Google Scholar]
- 10.Schurgin S. Rich S. Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24:335–338. doi: 10.2337/diacare.24.2.335. [DOI] [PubMed] [Google Scholar]
- 11.Colhoun HM. Schalkwijk C. Rubens MB. Stehouwer CD. C-reactive protein in type 1 diabetes and its relationship to coronary artery calcification. Diabetes Care. 2002;25:1813–1817. doi: 10.2337/diacare.25.10.1813. [DOI] [PubMed] [Google Scholar]
- 12.Forrest KY. Becker DJ. Kuller LH. Wolfson SK. Orchard TJ. Are predictors of coronary heart disease and lower-extremity arterial disease in type 1 diabetes the same? A prospective study. Atherosclerosis. 2000;148:159–169. doi: 10.1016/s0021-9150(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 13.Raggi P. Callister TQ. Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–1277. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 14.Raggi P. Cooil B. Shaw LJ. Aboulhson J. Takasu J. Budoff M. Callister TQ. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol. 2003;92:827–829. doi: 10.1016/s0002-9149(03)00892-0. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ. Raggi P. Coronary artery disease progression assessed by electron-beam computed tomography. Am J Cardiol. 2001;88:46E–50E. doi: 10.1016/s0002-9149(01)01767-2. [DOI] [PubMed] [Google Scholar]
- 16.Macphee CH. Nelson J. Zalewski A. Role of lipoprotein-associated phospholipase A2 in atherosclerosis and its potential as a therapeutic target. Curr Opin Pharmacol. 2006;6:154–161. doi: 10.1016/j.coph.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Zalewski A. Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923–931. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 18.Stafforini DM. Tjoelker LW. McCormick SP. Vaitkus D. McIntyre TM. Gray PW. Young SG. Prescott SM. Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J Biol Chem. 1999;274:7018–7024. doi: 10.1074/jbc.274.11.7018. [DOI] [PubMed] [Google Scholar]
- 19.Garza CA. Montori VM. McConnell JP. Somers VK. Kullo IJ. Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82:159–165. doi: 10.4065/82.2.159. [DOI] [PubMed] [Google Scholar]
- 20.Iribarren C. Gross MD. Darbinian JA. Jacobs DR., Jr Sidney S. Loria CM. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults: the CARDIA study. Arterioscler Thromb Vasc Biol. 2005;25:216–221. doi: 10.1161/01.ATV.0000148322.89911.44. [DOI] [PubMed] [Google Scholar]
- 21.Kardys I. Oei HH. Hofman A. Oudkerk M. Witteman JC. Lipoprotein-associated phospholipase A2, coronary calcification. The Rotterdam Coronary Calcification Study. Atherosclerosis. 2007;191:377–383. doi: 10.1016/j.atherosclerosis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Caslake MJ. Packard CJ. Suckling KE. Holmes SD. Chamberlain P. Macphee CH. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150:413–419. doi: 10.1016/s0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 23.Gazi I. Lourida ES. Filippatos T. Tsimihodimos V. Elisaf M. Tselepis AD. Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. Clin Chem. 2005;51:2264–2273. doi: 10.1373/clinchem.2005.058404. [DOI] [PubMed] [Google Scholar]
- 24.Snell-Bergeon JK. Hokanson JE. Jensen L. MacKenzie T. Kinney G. Dabelea D. Eckel RH. Ehrlich J. Garg S. Rewers M. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care. 2003;26:2923–2928. doi: 10.2337/diacare.26.10.2923. [DOI] [PubMed] [Google Scholar]
- 25.Agatston AS. Janowitz WR. Hildner FJ. Zusmer NR. Viamonte M., Jr Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 26.Hokanson JE. MacKenzie T. Kinney G. Snell-Bergeon JK. Dabelea D. Ehrlich J. Eckel RH. Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 27.Lp-PLA2 Studies Collaboration. Thompson A. Gao P. Orfei L. Watson S. Di Angelantonio E. Kaptoge S. Ballantyne C. Cannon CP. Criqui M. Cushman M. Hofman A. Packard C. Thompson SG. Collins R. Danesh J. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenig W. Khuseyinova N. Lowel H. Trischler G. Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–1908. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter KL. Dennis IF. Challis IR. Osborn DP. Macphee CH. Leake DS. Arends MJ. Mitchinson MJ. Inhibition of lipoprotein-associated phospholipase A2 diminishes the death-inducing effects of oxidised LDL on human monocyte-macrophages. FEBS Lett. 2001;505:357–363. doi: 10.1016/s0014-5793(01)02840-x. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y. Zhang P. Zhang L. Osman H. Mohler ER., 3rd Macphee C. Zalewski A. Postle A. Wilensky RL. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis. 2007;191:54–62. doi: 10.1016/j.atherosclerosis.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Reilly MP. Wolfe ML. Localio AR. Rader DJ. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis. 2004;173:69–78. doi: 10.1016/j.atherosclerosis.2003.10.010. [DOI] [PubMed] [Google Scholar]