Abstract
Background:
The potential prognostic value of several commonly investigated immunohistochemical markers in resected pancreatic cancer is variably reported. The objective of this study was to conduct a systematic review of literature evaluating p53, p16, smad4, bcl-2, bax, vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) expression as prognostic factors in resected pancreatic adenocarcinoma and to conduct a subsequent meta-analysis to quantify the overall prognostic effect.
Methods:
Relevant literature was identified using Medline, EMBASE and ISI Web of Science. The primary end point was overall survival assessed on univariate analysis. Only studies analysing resected pancreatic adenocarcinoma were eligible for inclusion and the summary loge hazard ratio (logHR) and variance were pooled using an inverse variance approach. Evidence of heterogeneity was evaluated using the χ2 test for heterogeneity and its impact on the meta-analysis was assessed by the I2 statisic. Hazard ratios greater than one reflect adverse survival associated with positive immunostaining.
Results:
Vascular endothelial growth factor emerged as the most potentially informative prognostic marker (11 eligible studies, n=767, HR=1.51 (95% confidence interval, CI=1.18–1.92)) with no evidence of any significant publication bias (Egger's test, P=0.269). Bcl-2 (5 eligible studies, n=314, HR=0.51 (95% CI=0.38–0.68)), bax (5 studies, n=274, HR=0.63 (95% CI=0.48–0.83)) and p16 (3 studies, n=229, HR=0.63 (95% CI=0.43–0.92)) also returned significant overall survival differences, but in smaller patient series due to a lack of evaluable literature. Neither p53 (17 studies, n=925, HR=1.22 (95% CI=0.96–1.56)), smad4 (5 studies, n=540, HR=0.88 (95% CI=0.61–1.27)) nor EGFR (4 studies, n=250, HR=1.35 (95% CI=0.80–2.27)) was found to represent significant prognostic factors when analysing the pooled patient data. There was evidence of significant heterogeneity in four of the seven study groups.
Conclusion:
These results support the case for immunohistochemical expression of VEGF representing a significant and reproducible marker of adverse prognosis in resected pancreatic cancer.
Keywords: immunohistochemistry, molecular, pancreatic cancer, meta-analysis, prognosis
Pancreatic ductal adenocarcinoma is characterised by its singularly aggressive tumour biology and unfavourable patient outcomes. Despite overall 5-year survival rates of <5%, previous randomised trials have demonstrated that for patients presenting with localised disease, resection with administration of adjuvant chemotherapy is associated with 5-year survival rates of over 20% (Neoptolemos et al, 2004; Oettle et al, 2007).
Reliable identification of molecular prognostic markers is important in order to facilitate the rational selection of potential therapeutic targets in the development of novel cancer therapies and to allow meaningful and reproducible risk stratification as part of clinical trials. There is marked disparity in the literature between individual studies as to the relative prognostic impact of several immunohistochemical tissue markers in pancreatic cancer. This may, in part, be explained by heterogeneity in patient selection due to inclusion of resected and unresected patients in survival analyses or inclusion of mixed tumour types and laboratory methodology when comparing different studies. The objective of the present study was to conduct a systematic review and meta-analysis of published literature investigating the commonly reported immunohistochemical prognostic markers in resected primary tumour material from patients with pancreatic adenocarcinoma and to identify potential sources of heterogeneity when comparing the results of individual studies.
Materials and methods
Search strategy
Medline, EMBASE and ISI Web of Science were searched to identify potentially relevant published literature. No chronological search criteria were applied. Existing systematic reviews and reference lists were also checked for any potentially relevant additional studies. The most widely investigated and biologically relevant immunohistochemical tissue markers for pancreatic cancer were selected for meta-analysis. These comprised p53, smad4, p16, bcl-2, bax, vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR).
Selection criteria
The following criteria were used to search English language articles and abstracts: ‘(marker)’ AND (‘pancreas’ OR ‘pancreatic’) AND (‘survival’ OR ‘prognosis’ OR ‘prognostic’). Each search was repeated for individual markers by substituting the name of marker of interest along with relevant synonyms: ‘p53’ OR ‘TP53’ ‘p16’ OR ‘p16*’ OR ‘CDKN2A’ ‘smad4’ OR ‘smad-4’ OR ‘smad*’ ‘DPC4’ OR ‘DPC-4’ OR ‘DPC*’ ‘bcl-2’ OR ‘bcl2’ OR ‘bcl’ OR ‘bcl*’ ‘bax’ ‘vascular endothelial growth factor’ OR ‘VEGF’ OR ‘VEGF*’ ‘epidermal growth factor receptor’ OR ‘EGFR’ OR ‘c-erbB*’ OR ‘erbB*’ OR ‘HER*’. The search was performed in November 2009. Abstracts were initially checked for relevance and the full article was retrieved for all potentially eligible studies. Where part or all of the same patient series was included in more than one publication, only the more recent or most complete study was included in the analysis in order to avoid duplication of the same survival data.
The following inclusion criteria were used to select literature: only cases of resected pancreatic adenocarcinoma analysed, immunohistochemical expression assessed in resected primary tumour material, dichotomised univariate survival analysis reported (i.e. positive vs negative staining) and overall survival times used in analysis. For the analysis of VEGF, only studies investigating the prognostic value of VEGF-A expression were included. Authors were contacted for unpublished results in cases where insufficient survival data were reported to estimate the loge hazard ratio (logHR) and variance. Due to the minority of studies reporting multivariable analyses, no attempt was made to use any adjusted survival data as part of this meta-analysis (i.e. only univariate survival data were extracted).
End points
The primary outcome measure was overall survival (i.e. date of resection to date of death). Additional details were also collected in order to identify potential sources of heterogeneity. These included the specific primary antibody (and dilution) used for immunohistochemistry, the scoring criteria used to define positive staining and relevant clinico-pathological data. An assessment of study methodology was made according to previously defined criteria (Hayden et al, 2006; McShane et al, 2006). These principles were used to define 20 individual study characteristics, which were deemed to be key factors to report in an immunohistochemical prognostic study (Table 1). For any criterion not fulfilled according to the information outlined in the article, one point was deducted from a maximum of 20 and the final score was recorded as a percentage. The eligibility criteria and quality scoring were assessed by two independent investigators. Any disagreement was resolved by discussion.
Table 1. Methodological scoring criteria used.
| Study group | |
| Study population adequately described | |
| Gender/age | 1 Point |
| Histology | 1 Point |
| Period of recruitment | 1 Point |
| Inclusion/exclusion criteria used | 1 Point |
| Study attrition | |
| >90% of cases identified included in final analysis | 1 Point |
| Reasons for attrition/loss to follow-up given | 1 Point |
| Peri-operative mortality details | 1 Point |
| Scientific methodology | |
| IHC methodology outlined | |
| Details of 1°/2° Abs used | 1 Point |
| Concentration of 1° Abs used | 1 Point |
| Positive/negative controls outlined | 1 Point |
| Description of scoring technique | |
| >1 independent scorer | 1 Point |
| Scorers blinded to clinical data | 1 Point |
| Criteria for positivity clearly outlined | |
| Distribution (cytoplasm vs membranous vs nuclear) | 1 Point |
| % positive cells for immunostaining classification | 1 Point |
| Confounding factors considered | |
| Adjuvant therapy details provided | 1 Point |
| Histological breakdown according to IHC staining | 1 Point |
| Statistical analysis | |
| HR (confidence interval) provided | 1 Point |
| Exact P-value quoted | 1 Point |
| Numbers at risk for Kaplan–Meier curves | 1 Point |
| Number of censored cases recorded | 1 Point |
Abbreviations: HR=hazard ratio; IHC=immunohistochemical.
Statistical analysis
Previously reported indirect methods were utilised for extracting the logHR and variance due to the paucity of prognostic literature, which report these values directly (Parmar et al, 1998; Williamson et al, 2002; Tierney et al, 2007). These values were either calculated from the HR and 95% confidence interval (CI) where quoted, the log rank P-value, or from the Kaplan–Meier survival curves directly. The software used for these indirect calculations was designed by Matthew Sydes and Jayne Tierney of the Medical Research Council Clinical Trials Unit, London, UK (Tierney et al, 2007). The logHR and variance for individual studies were entered into RevMan 4.2 (Cochrane collaboration, Oxford, UK) and pooled using a random effects inverse variance approach. The overall prognostic effect of positive immunostaining was recorded as an HR and 95% CI (i.e. an HR>1 reflecting adverse survival associated with positive immunostaining). Heterogeneity was assessed using a χ2 test for heterogeneity with a P-value of <0.10 taken to reflect the presence of significant heterogeneity. The I2 statistic was calculated to quantify the degree of heterogeneity (Higgins and Thompson, 2002). A P-value of <0.050 was taken to reflect significance for all other analyses. Publication bias was assessed by inspection of the funnel plot with Egger's regression. Continuous data were compared using Spearman's rank correlation and two-sided Mann–Whitney testing for categorical data.
Results
VEGF
The initial search returned a total of 255 studies. Following review of these abstracts, 20 potentially relevant studies were identified as eligible of which nine were excluded for the following reasons: duplicated series of patients (Ikeda et al, 1999; Niedergethmann et al, 2000; Tang et al, 2001), only VEGF-C and/or VEGF-D analysed (Kurahara et al, 2004; Zhang et al, 2007), no dichotomised univariate survival analysis reported (Ellis et al, 1998; Fujioka et al, 2001), mix of resected and unresected cases included in survival analysis (Chung et al, 2006) and only VEGF receptor status analysed (Büchler et al, 2002).
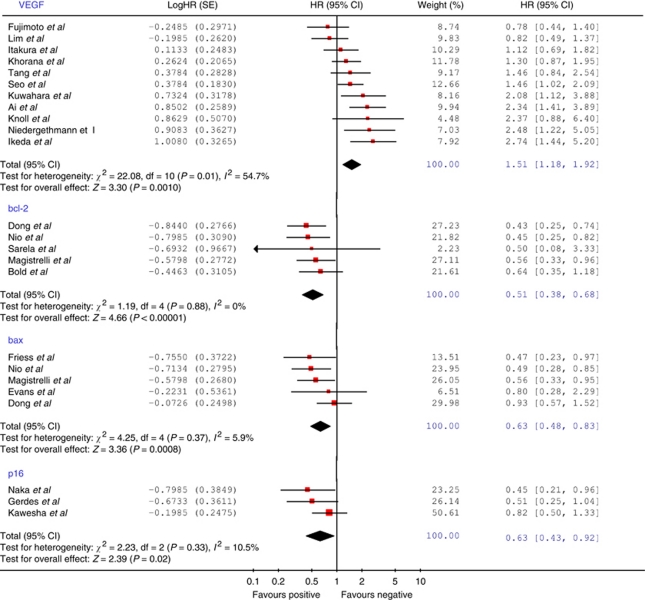
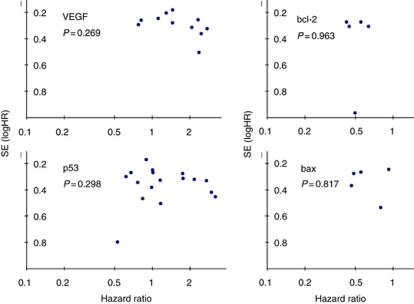
The 11 eligible studies (all retrospective) included a total of 767 patients with a median number of 62 patients per study (range=19–142). Table 2 outlines the demographic, clinico-pathological, methodological and outcome characteristics of these studies. The median quality score was recorded as 70% (range=60–95%). There was no significant difference in median quality scores between significant and non-significant studies (Mann–Whitney, P=0.516). Similarly, there was no significant correlation between study size and quality scores (Spearman's ρ=0.139, P=0.698). Figure 1 illustrates the Forrest plot for the survival data. Significant heterogeneity was demonstrated according to Cochran's χ2 test (χ2=22.08, P=0.01; I2=54.7%). The combined HR was recorded as 1.51 (95% CI=1.18–1.92), indicating that positive immunostaining for VEGF was significantly associated with adverse survival in the pooled patient group. When assessing the funnel plot for this analysis (Figure 2), the data points approximated a symmetrical distribution (Egger's test, P=0.269), indicating that publication bias is unlikely to be a significant confounding factor in describing this relationship.
Table 2. Methodological and clinico-pathological data for eligible prognostic studies evaluating VEGF, bcl-2, bax and p16.
| Reference | n | HR (95% CI) | Significant | 1° Ab (+dilution) | IHC +ve | IHC cutoff (%) | Male | Age | N1 | T3/T4 | Well | Mod. | Poor | Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VEGF | ||||||||||||||
| Itakura et al (1997) | 75 | 1.12 (0.69–1.82) | No | NC (30 μg ml–1) | 48 (64) | >10 | 46 (61) | 62 | 47 (63) | 43 (57) | 13 (17) | 44 (59) | 18 (24) | NS |
| Fujimoto et al (1998) | 50 | 0.78 (0.44–1.40) | No | Santa Cruz A20 (1 : 200) | 28 (40) | NS | 28 (56) | 62 | 29 (58) | 34 (68) | 9 (18) | 31 (62) | 10 (20) | NS |
| Seo et al (2000) | 142 | 1.46 (1.02–2.09) | Yes | Santa Cruz (NS) | 94 (66) | >30 | 79 (56) | 64 | 95 (67) | NS | NS | NS | NS | NS |
| Ikeda et al (2001) | 48 | 2.74 (1.44–5.20) | Yes | Santa Cruz (1 : 200) | 31 (65) | >10 | 37 (77) | 64 | 24 (50) | 40 (83) | 15 (31) | 28 (58) | 5 (11) | 48 (100) |
| Knoll et al (2001) | 19 | 2.37 (0.88–6.40) | No | R&D Ab293NA (1 : 200) | 13 (68) | >5 | 11 (58) | 58 | 18 (95) | 1 (5) | 1 (5) | 12 (63) | 6 (32) | 0 (0) |
| Niedergethmann et al (2002) | 70 | 2.48 (1.22–5.05) | Yes | Santa Cruz (1 : 200) | 28 (40) | >10 | 42 (60) | 63 | 41 (59) | NS | 25 (36) | 45 (64) | 22 (31) | |
| Kuwahara et al (2003) | 55 | 2.08 (1.12–3.88) | Yes | Santa Cruz sc152 (1 : 200) | 39 (71) | >50 | 34 (62) | 64 | 30 (55) | 40 (73) | 13 (24) | 33 (60) | 9 (16) | NS |
| Lim et al (2004) | 72 | 0.82 (0.49–1.37) | No | Santa Cruz (1 : 2000) | 23 (32) | >10 | 43 (60) | 60 | 38 (53) | 59 (82) | 14 (19) | 44 (61) | 14 (19) | 26 (36) |
| Khorana et al (2005) | 124 | 1.30 (0.87–1.95) | No | Zymed (1 : 50) | 70 (56) | >5 | 69 (56) | 67 | 56 (45) | 69 (58) | 23 (19) | 52 (43) | 45 (38) | 88 (79) |
| Tang et al (2006) | 50 | 1.46 (0.84–2.54) | No | NS (2 μg ml–1) | 25 (50) | >10 | 25 (50) | 63 | 39 (78) | 25 (50) | 15 (30) | 31 (62) | 4 (8) | NS |
| Ai et al (2008) | 62 | 2.34 (1.41–3.89) | Yes | Neomarkers (NS) | 37 (60) | >10 | 36 (58) | 65 | 49 (79) | 32 (52) | 17 (27) | 15 (24) | 30 (48) | 0 (0) |
| bcl-2 | ||||||||||||||
| Bold et al (1999) | 70 | 0.64 (0.35–1.18) | No | DAKO (NS) | 23 (33) | >25% | 36 (51) | 64 | 32 (46) | NS | 15 (22) | 37 (55) | 15 (22) | 19 (27) |
| Nio et al (2001b) | 66 | 0.45 (0.25–0.82) | Yes | DAKO M0887 (1 : 100) | 16 (24) | >5% | 31 (47) | 66 | 54 (82) | NS | 33 (50) | 29 (44) | 4 (6) | 36 (55) |
| Magistrelli et al (2006) | 67 | 0.56 (0.33–0.96) | Yes | DAKO c124 (1 : 40) | 45 (67) | >5% | 45 (67) | 63 | 34 (51) | 40 (62) | 14 (21) | 28 (42) | 15 (22) | 30 (45) |
| Sarela et al (2002) | 52 | 0.50 (0.08–3.33) | No | DAKO (1 : 40) | 6 (12) | >10% | 27 (52) | 64 | 40 (78) | 49 (94) | 11 (22) | 24 (47) | 16 (31) | NS |
| Dong et al (2005b) | 59 | 0.43 (0.25–0.74) | Yes | DAKO M124(1 : 100) | 21 (36) | >5% | 19 (32) | 55 | 54 (82) | NS | 19 (32) | 21 (36) | 19 (32) | NS |
| bax | ||||||||||||||
| Friess et al (1998) | 60 | 0.47 (0.23–0.97) | Yes | Santa Cruz (NS) | 50 (83) | NS | 32 (53) | 63 | 38 (63) | NS | NS | NS | NS | NS |
| Evans et al (2001) | 23 | 0.80 (0.28–2.29) | No | Santa Cruz (1 : 1600) | 6 (26) | >5% | 15 (65) | 59 | 38 (63) | NS | 5 (22) | 13 (54) | 5 (22) | 0 (0) |
| Nio et al (2001b) | 65 | 0.49 (0.28–0.85) | Yes | DAKO A3533 (1 : 100) | 42 (65) | >10% | 31 (47) | 66 | 54 (82) | NS | 33 (50) | 29 (44) | 4 (6) | 36 (55) |
| Magistrelli et al (2006) | 67 | 0.56 (0.33–0.95) | Yes | Zymed c2D2 (1 : 80) | 36 (54) | >10% | 45 (67) | 63 | 34 (51) | 40 (62) | 14 (21) | 28 (42) | 15 (22) | 30 (45) |
| Dong et al (2005b) | 59 | 0.93 (0.57–1.52) | No | DAKO A3533 (1 : 100) | 29 (49) | >10% | 19 (32) | 55 | 54 (82) | NS | 19 (32) | 21 (36) | 19 (32) | NS |
| p16 | ||||||||||||||
| Naka et al (1998) | 32 | 0.45 (0.21–0.96) | Yes | Santa Cruz C20 (1 : 500) | 19 (59) | NS | 20 (63) | 65 | 23 (72) | 13 (41) | NS | NS | NS | NS |
| Kawesha et al (2000) | 157 | 0.82 (0.50–1.33) | No | Santa Cruz (1 : 100) | 21 (13) | >5% | 100 (64) | 60 | 71 (46) | NS | 21 (13) | 77 (49) | 59 (38) | 13 (8) |
| Gerdes et al (2002) | 40 | 0.51 (0.25–1.04) | No | Pharmingen G175–405 (1 : 50) | 13 (33) | >5% | 22 (55) | NS | 16 (40) | NS | NS | NS | NS | 0 (0) |
Abbreviations: CI=confidence interval; HR=hazard ratio; IHC=immunohistochemical; NC=non-commercial; NS=not specified; VEGF=vascular endothelial growth factor.
% in parentheses unless otherwise stated. IHC and/or clinico-pathological data were incompletely reported in some studies. Well/Mod/Poor refers to tumour differentiation.
Figure 1.
Forrest plot to assess overall effect of VEGF, bcl-2, bax and p16 expression on survival.
Figure 2.
Forrest plot to assess overall effect of p53, smad4 and EGFR expression on survival.
The median proportion of patients classified as VEGF positive in the included studies was recorded as 60% (range=32–71%). The proportion of VEGF positive cases reported in each study failed to exhibit any correlation with the assessment of methodological quality (Spearman's P=0.491) or the % cutoff used to define positive immunostaining (Spearman's P=0.388). Only six studies reported the proportion of patients who received any form of adjuvant therapy (Table 2) and administered treatment modalities included a mix of both chemotherapy and chemoradiation. No studies reported use of any neoadjuvant therapy and only a single study reported use of intra-operative radiotherapy (Ikeda et al, 2001). Of the five studies that reported positive VEGF expression as a significant adverse prognostic variable, only three conducted some form of multivariate analysis. These three analyses included a variety of disparate covariates alongside VEGF. However, each reported that VEGF expression retained statistical significance.
bcl-2
The initial search returned a total of 232 abstracts of which 16 potentially eligible articles were retrieved. A total of 11 were excluded for the following reasons: duplicated series of patients (Nio et al, 2001a), mix of resected and unresected cases included (Gansauge et al, 1998; Mäkinen et al, 1998; Ohshio et al, 1998; Hu et al, 1999), inclusion of ampullary tumours (Sinicrope et al, 1996), no dichotomised univariate survival analysis conducted (Evans et al, 2001; Stipa et al, 2002; Sun et al, 2002) and insufficient survival data reported for indirect estimation of logHR and variance (Friess et al, 1998; Campani et al, 2001).
The five eligible studies included a total of 314 patients with a median number of 63 patients per study (range=52–70) (Table 2). The median quality score was recorded as 75% (range=65–85%) and the median proportion of bcl-2 positive cases was 33% (range=12–67%). Figure 2 illustrates the Forrest plot for the pooled survival data. There was no evidence of any significant heterogeneity (χ2=1.19, P=0.88). The combined HR was recorded as 0.51 (95% CI=0.38–0.68), indicating a significant association between positive bcl-2 immunostaining and more favourable survival in the pooled patient group. Despite the limited number of studies included, the funnel plot for this analysis failed to demonstrate any obvious asymmetry (Figure 3). Three studies reported use of either adjuvant chemotherapy or chemoradiation and a single study (Bold et al, 1999) also reported use of neoadjuvant chemoradiation in 43 out of the 70 patients analysed. Of the two studies rejected due to incomplete survival data (Friess et al, 1998; Campani et al, 2001), both failed to observe any significant prognostic effect associated with bcl-2 expression. Neither study reported the direction of the prognostic effect.
Figure 3.
Funnel plots to assess publication bias for VEGF, bcl-2, bax and p53 meta-analyses. Note: P-values for result of Egger's regression to assess publication bias.
bax
The initial search yielded 76 studies. Following review of the abstracts, a total of seven potentially eligible articles were identified. Two of these were excluded due to either a duplicated patient series (Hashimoto et al, 2005) or the inclusion of periampullary cancers of non-pancreatic origin in the survival analysis (Tomazic et al, 2004). Three of the five eligible studies investigated the prognostic effect of both bcl-2 and bax and were, therefore, included in both meta-analyses (Magistrelli et al, 2006; Nio et al, 2001b; Dong et al, 2005b).
The five eligible studies investigating bax included a total of 274 patients with a median number of 60 patients per study (range=23–67) (Table 2). The median quality score was 65% (range=55–85%) and the median proportion of bax positive cases was 54% (range=26–83%). Figure 2 illustrates the Forrest plot for the pooled survival data. There was no evidence of any significant heterogeneity (χ2=4.25, P=0.37; I2=5.9%). The combined HR was recorded as 0.63 (95% CI=0.48–0.83) and the funnel plot for this analysis is shown in Figure 3.
p16
The initial search returned 91 studies, seven of which were potentially relevant. Following review of these seven articles, three fulfilled all of the eligibility criteria. The remaining studies were rejected due to the inclusion of unresected cases (Hu et al, 1997; Biankin et al, 2002), no IHC used in tissue analysis (Ohtsubo et al, 2003) or only disease-free survival times reported (Jeong et al, 2005). A total of 229 patients were included in the pooled analysis. There was no evidence of any significant heterogeneity across the three included studies (χ2=2.23, P=0.33; I2=10.5%). A combined HR of 0.63 (95% CI=0.43–0.92) was obtained, indicating a significant association between p16 expression and more favourable survival.
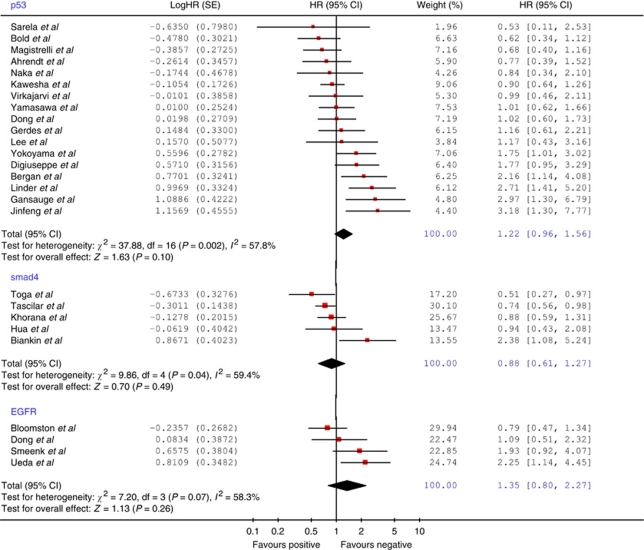
p53
The initial search returned a total of 337 studies. Following review of these abstracts, 58 potentially relevant studies were retrieved of which 17 fulfilled all of the inclusion criteria. The remaining studies were rejected for the following reasons: duplicated series of patients (Dergham et al, 1997a; Dong et al, 1998b; Nio et al, 1998; Nio et al, 1999; Dong et al, 2000; Linder et al, 2001; Nio et al, 2001a), no dichotomised univariate survival analysis conducted (Sessa et al, 1998; Karademir et al, 2000; Evans et al, 2001; Fujioka et al, 2001; Gazzaniga et al, 2001; Biankin et al, 2002; Dang et al, 2002; Hashimoto et al, 2005; Dong et al, 2007; Smeenk et al, 2007), no IHC used in tissue analysis (Weyrer et al, 1996; Li et al, 1999; Yamaguchi et al, 2000; Ohshio et al, 2002; Dong et al, 2003), unresected cases included in survival analysis (Zhang et al, 1994; Aizawa et al, 1996; Lundin et al, 1996; Coppola et al, 1998; Dergham et al, 1998; Mäkinen et al, 1998; Ohshio et al, 1998; Hu et al, 1999; Takikita et al, 2009), mix of different tumour types included (Sinicrope et al, 1996; Sato et al, 1997; Gansauge et al, 1998; Yu et al, 2004), only disease-free survival reported (Jeong et al, 2005) and insufficient survival data reported (Dergham et al, 1997b; Campani et al, 1999; Stipa et al, 2002; Hermanova et al, 2009).
The 17 eligible studies included a total of 925 patients with a median number of 48 patients per study (range=26–157) (Table 3). Nuclear staining of p53 was used for scoring in all cases. Five studies (29%) reported a significant adverse association between p53 expression and survival. The median quality score was recorded as 65% (range=45–90%) and the median proportion of patients exhibiting positive p53 immunostaining was 47% (range=25–68%). There was no significant association between the IHC cutoff score used and the proportion of cases classified as p53 positive (Spearman's ñ=0.389, P=0.206). Furthermore, there was no significant difference in median quality scores between significant and non-significant studies (Mann–Whitney, P=0.243).
Table 3. Methodological and clinico-pathological data for eligible prognostic studies evaluating p53, smad4 and EGFR.
| Reference | n | HR (95% CI) | Significant | 1° Ab (+ dilution) | IHC +ve | IHC cutoff (%) | Male | Age | N1 | T3/T4 | Well | Mod. | Poor | Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p53 | ||||||||||||||
| DiGiuseppe et al (1994) | 48 | 1.77 (0.95–3.29) | No | Novocastra CM-1 (1 : 1000) | 26 (54) | NS | 25 (52) | 61 | NS | NS | NS | NS | NS | NS |
| Yokoyama et al (1994) | 57 | 1.75 (1.01–3.02) | Yes | Novocastra DO7 (1 : 100) | 33 (58) | NS | NS | 64 | 25 (45) | 27 (47) | 37 (65) | 20 (35) | NS | |
| Lee et al (1995) | 26 | 1.17 (0.43–3.16) | No | Biogenex CM1 (NS) | 7 (27) | NS | 14 (54) | NS | NS | NS | 2 (8) | 20 (77) | 4 (15) | NS |
| Linder et al (1997) | 48 | 2.71 (1.41–5.20) | Yes | DAKO DO7 (1 : 50) | 22 (46) | >1 | 36 (68) | 66 | 18 (38) | 26 (49) | 5 (9) | 18 (34) | 30 (57) | NS |
| Virkajärvi et al (1997) | 36 | 0.99 (0.46–2.11) | No | Novocastra CM-1 (1 : 1000) | 15 (42) | >1 | 16 (44) | 64 | NS | NS | NS | NS | NS | NS |
| Naka et al (1998) | 32 | 0.84 (0.34–2.10) | No | Novocastra BP53-12 (1 : 50) | 19 (59) | NS | 20 (63) | 65 | 23 (72) | 13 (41) | NS | NS | NS | NS |
| Bold et al (1999) | 70 | 0.62 (0.34–1.12) | No | Oncogene DO1 (NS) | 33 (47) | >25 | 36 (51) | 64 | 38 (54) | NS | 15 (22) | 37 (56) | 15 (22) | 19 (27) |
| Gansauge et al (1999) | 26 | 2.97 (1.30–6.79) | Yes | Oncogene DO1 (1 : 500) | 11 (42) | NS | 12 (50) | 59 | 22 (85) | NS | NS | NS | NS | 26 (100) |
| Ahrendt et al (2000) | 43 | 0.77 (0.39–1.52) | No | DAKO DO7 (1 : 2000) | 26 (60) | >33 | 24 (55) | 63 | 23 (53) | 22 (51) | 11 (26) | 23 (55) | 8 (19) | 29 (66) |
| Bergan et al (2000) | 60 | 2.16 (1.14–4.08) | Yes | Novocastra DO7 (1 : 100) | 15 (25) | >5 | 41 (50) | 62 | 18 (30) | 21 (35) | 25 (42) | 23 (38) | 12 (20) | 0 (0) |
| Kawesha et al (2000) | 157 | 0.90 (0.64–1.26) | No | DAKO DO7 (1 : 300) | 64 (41) | >5 | 100 (64) | 60 | 71 (46) | NS | 21 (13) | 77 (49) | 59 (38) | 13 (8) |
| Gerdes et al (2002) | 40 | 1.16 (0.61–2.21) | No | DAKO DO7 (1 : 400) | 13 (33) | >10 | 22 (55) | NS | 16 (40) | NS | NS | NS | NS | 0 (0) |
| Sarela et al (2002) | 52 | 0.53 (0.11–2.53) | No | DAKO DO7 (1 : 100) | 28 (54) | >10 | 27 (52) | 64 | 40 (78) | 49 (94) | 11 (22) | 24 (47) | 16 (31) | NS |
| Yamasawa et al (2002) | 72 | 1.01 (0.62–1.66) | No | Oncogene DO1 (2 μg/ml) | 34 (47) | >20 | 34 (47) | 65 | 21 (29) | 42 (58) | 35 (49) | 32 (44) | 5 (7) | 41 (57) |
| Dong et al (2005a) | 59 | 1.02 (0.60–1.73) | No | DAKO DO7 (1 : 20) | 40 (68) | >10 | 38 (64) | NS | 47 (80) | NS | 19 (32) | 21 (36) | 19 (32) | NS |
| Magistrelli et al (2006) | 67 | 0.68 (0.40–1.16) | No | DAKO DO7 (1 : 50) | 32 (48) | >5 | 45 (67) | 63 | 34 (51) | 40 (62) | 14 (21) | 28 (42) | 15 (22) | 30 (45) |
| Jinfeng et al (2007) | 32 | 3.18 (1.30–7.77) | Yes | DAKO DO7 (1 : 50) | 13 (41) | >10 | 19 (59) | 63 | 18 (56) | 23 (72) | 11 (34) | 18 (56) | 3 (10) | NS |
| smad4 | ||||||||||||||
| Tascilar et al (2001) | 249 | 0.74 (0.56–0.98) | Yes | Santa Cruz B8 (1 : 100) | 111 (46) | NS | 139 (56) | 65 | NS | NS | NS | NS | NS | NS |
| Biankin et al (2002) | 45 | 2.38 (1.08–5.24) | Yes | Santa Cruz B8 (NS) | 10 (22) | >5 | 27 (60) | 61 | 21 (47) | NS | 5 (11) | 28 (62) | 12 (27) | 8 (16) |
| Hua et al (2003) | 34 | 0.94 (0.43–2.08) | No | Santa Cruz B8 (1 : 100) | 26 (76) | NS | 22 (65) | 55 | 14 (41) | NS | 27 (79) | 7 (21) | NS | |
| Toga et al (2004) | 88 | 0.51 (0.27–0.97) | Yes | Santa Cruz B8 (1 : 100) | 13 (15) | >10 | 43 (49) | 66 | 78 (89) | 33 (37) | 37 (42) | 45 (51) | 6 (7) | 58 (66) |
| Khorana et al (2005) | 124 | 0.88 (0.59–1.31) | No | Santa Cruz (1 : 400) | 59 (48) | >5 | 69 (56) | 67 | 56 (45) | 69 (58) | 23 (19) | 52 (43) | 45 (38) | 88 (79) |
| EGFR | ||||||||||||||
| Dong et al (1998a) | 57 | 1.09 (0.51–2.32) | No | Oncogene 985/996 (1 : 20) | 39 (68) | NS | 20 (35) | 55 | 46 (81) | NS | 18 (32) | 22 (39) | 17 (30) | 7 (12) |
| Ueda et al (2004) | 76 | 2.25 (1.14–4.45) | Yes | Zymed 31G7 (1 : 200) | 47 (62) | >10 | 57 (75) | 63 | 59 (78) | NS | 11 (14) | 32 (42) | 33 (43) | NS |
| Bloomston et al (2006) | 71 | 0.79 (0.47–1.34) | No | Dakocytomation 218C9 (NS) | 49 (69) | >1 | 40 (56) | 65 | 41 (58) | 57 (81) | 6 (9) | 45 (63) | 20 (28) | NS |
| Smeenk et al (2007) | 46 | 1.93 (0.92–4.07) | No | DAKO H11 (NS) | 11 (24) | >1 | 37 (66) | 63 | 29 (52) | 34 (61) | 6 (11) | 43 (77) | 7 (12) | 19 (34) |
Abbreviations: CI=confidence interval; EGFR=epidermal growth factor receptor; HR=hazard ratio; IHC=immunohistochemical; NC=non-commercial; NS=not specified.
% in parentheses unless otherwise stated. IHC and/or clinico-pathological data were incompletely reported in some studies. Well/Mod./Poor refers to tumour differentiation.
Figure 2 illustrates the Forrest plot for the survival data. There was no evidence of any significant publication bias (Egger's test, P=0.298). Significant heterogeneity was demonstrated according to Cochran's χ2 test (χ2=37.88, P=0.002; I2=57.8%). The combined HR was recorded as 1.22 (95% CI=0.96–1.56), indicating no significant overall association between p53 expression and survival. Of the four studies excluded due to incomplete reporting of survival data, only one reported a significant association between p53 expression and survival (Stipa et al, 2002).
smad4
The initial search returned 81 studies. Following review of these abstracts, five potentially relevant studies were identified, which were all found to be eligible for analysis. The combined number of patients was 540 with a median of 88 patients per study (range=34–249) (Table 3). The median quality score was 75% (range=60–95%) and the median proportion of patients exhibiting positive smad4 immunostaining was 45% (range=15–76%). Figure 2 illustrates the Forrest plot. There was evidence of significant heterogeneity across the included studies (χ2=9.86, P=0.04; I2=59.4%). A combined HR of 0.88 (95% CI=0.61–1.27) was recorded, indicating no significant overall association between smad4 expression and survival in the pooled patient group.
EGFR
The initial search identified 324 studies. Following review of these abstracts, 10 potentially relevant articles were retrieved. Six of these studies were rejected for the following reasons: duplicated series of patients (Uegaki et al, 1997; Ueda et al, 2006), no dichotomised univariate survival analysis conducted (Yamanaka et al, 1993; Zhang and Yuan, 2002) and unresected cases included in analysis (Gansauge et al, 1998; Takikita et al, 2009). The four eligible studies included a total of 250 patients (Table 3). Only a single study reported a significant relationship between EGFR expression and survival (Ueda et al, 2004). The median quality score was 70% (range=65–70%). Figure 2 illustrates the Forrest plot for the pooled data. Significant heterogeneity was demonstrated (χ2=7.20, P=0.07). The combined HR was recorded as 1.35 (95% CI=0.80–2.27), indicating no significant overall association between EGFR expression and survival.
Discussion
Previous meta-analyses of studies investigating the prognostic value of molecular markers have been published for different malignancies. These include VEGF (Delmotte et al, 2002; Kyzas et al, 2005a; Des Guetz et al, 2006), bcl-2 (Martin et al, 2003; Callagy et al, 2008) and p53 (Kyzas et al, 2005b; Malats et al, 2005). To date, no such meta-analysis has been undertaken for any studies evaluating immunohistochemical prognostic markers in resected pancreatic cancer.
Meta-analysis of prognostic literature is associated with a number of inherent limitations. One of these key limitations is the general prevalence of retrospective study design in this setting. None of the studies included in the current meta-analysis specified a prospective design and archived paraffin-embedded tumour material was utilised for IHC in all cases. This indicates that availability of tissue is invariably the main determinant of study size rather than any specific considerations relating to adequate statistical power in order to reliably detect a prognostic effect for the marker of interest. The availability and adequacy of corresponding clinico-pathological data is also a significant consideration in retrospective studies of this type and we identified several studies reporting incomplete datasets with regard to histopathological details. Alongside this, an additional hindrance to meta-analysis of prognostic literature is the general lack of multivariable survival data. This is usually attributable to the fact that the number of patients included in each study is typically small, precluding any meaningful attempt at analysing multiple covariates.
Additional challenges in the interpretation and comparison of immunohistochemical prognostic studies include variability in patient selection (i.e. resected and unresected cases, inclusion of non-pancreatic periampullary tumours), disparate immunohistochemical criteria used for prognostic classification, bias associated with the statistical approach to analysis of survival data (e.g. selection of data-driven cutoff values for continuous variables), incomplete reporting of survival data, duplicated patient series and publication bias arising as a result of selective reporting of ‘positive’ studies (Altman, 2001). In order to overcome some of these comparative difficulties, specific inclusion criteria were applied in order to select studies for meta-analysis. Only studies including resected pancreatic adenocarcinoma were included in order to avoid any confounding effects on survival associated with differing proportions of resected and unresected cases. Any studies including periampullary tumours of non-pancreatic origin were also excluded due to the disparity in survival outcomes characteristically associated with ampullary, duodenal and bile duct adenocarcinomas when compared with pancreatic adenocarcinoma (Riall et al, 2006). Furthermore, in cases where part or all of the same patient series was included in more than one publication, only the more recent or most complete study was included in the analysis in order to avoid duplicating the same patient data for the immunohistochemical marker of interest. For those studies where insufficient survival data was reported to generate indirect calculations for the logHR and variance, authors were contacted for additional survival data. However, in all cases the authors were either unable to provide any supplementary data or no response was received. The only supplementary raw data obtained was for two studies previously conducted at our own institution (Kawesha et al, 2000; Evans et al, 2001). Therefore, no subsequent attempt to request individual patient survival data for all eligible studies was undertaken, although this would have been potentially beneficial.
When analysing the overall relationships between individual study size, reported prognostic significance and methodological quality scores in the present study, there was a significant trend towards superior methodological quality in larger studies as one might reasonably expect, despite the fact that study size itself was not one of the criteria used for quality scoring. When considering the overall effect of potential publication bias in this analysis, only a minority of studies (21 out of 50) actually reported a statistically significant prognostic result. Furthermore, the funnel plots and Egger's tests for the individual analyses, although more difficult to interpret when fewer studies were included, were not generally indicative of any strong publication bias.
Vascular endothelial growth factor emerged as the most potentially informative immunohistochemical prognostic marker from the pooled data. Vascular endothelial growth factor comprises four ligands (VEGF-A, VEGF-B, VEGF-C and VEGF-D), which exhibit specific binding profiles with three transmembrane VEGF receptors (VEGF-I, -II and -III) and promote intracellular tyrosine kinase cascades when activated. The VEGF-A (usually referred to simply as VEGF) mediates the key pro-angiogenic properties of proliferation and migration of endothelial cells along with increasing vascular permeability (Yamazaki and Morita, 2006; Dallas et al, 2007). Alternate gene splicing results in a number of VEGF-A isoforms of differing amino-acid lengths, the smaller of which (e.g. 121 and 165) are secreted while the larger (e.g. 189 and 206) remains cell associated. VEGF-C and VEGF-D are implicated in the process of lymphangiogenesis (Achen and Stacker, 2008) while the function of VEGF-B is incompletely understood (Nash et al, 2006). Pancreatic cancer cells have been demonstrated to express both VEGF ligand and its receptors, implicating a potential VEGF-mediated autocrine loop in the proliferation of pancreatic malignancy (Büchler et al, 2002).
The results from the present study demonstrate that, despite variability between eligible studies as to the relative prognostic impact of VEGF expression in resected pancreatic adenocarcinoma, the observed survival trend is concordant with that reported for other malignancies in similar meta-analyses (Delmotte et al, 2002; Kyzas et al, 2005a, 2005b; Des Guetz et al, 2006). When comparing the value for the pooled HR identified in the present study (1.51 (95% CI=1.18–1.92)) with the above referenced studies, the order of magnitude for this effect is also broadly comparable for that quoted for both lung cancer (1.48 (95% CI=1.27–1.72)) and colorectal cancer (1.65 (95% CI=1.27–2.14)).
Significant heterogeneity was observed when analysing the logHR estimates from the eligible studies. Evaluation of the relevant methodological and clinico-pathological characteristics of each study revealed a number of potential sources of heterogeneity in study methodology. Nine studies reported use of commercially available anti-VEGF primary antibodies, all of which exhibit broadly comparable binding characteristics with the common splice variants of VEGF-A. When analysing the concentrations of primary antibody utilised, most studies reported comparable dilution ratios. However, the concentration was not specified in two studies. This issue is potentially relevant for the study reporting use of the lowest primary antibody dilution (Lim et al, 2004) as this was one of only two studies, which indicated a contradictory prognostic effect when compared with the overall group (i.e. a non-significant trend towards adverse survival with negative VEGF immunostaining).
When reviewing the immunohistochemical criteria used for VEGF scoring, the majority of studies reported a scoring system based on cytoplasmic staining of tumour cells. Where the distribution of immunostaining used for scoring was not explicitly stated in the text (i.e. cytoplasmic, membranous, nuclear, stromal, etc.), the figures of representative VEGF staining presented in the relevant studies were all strongly indicative of cytoplasmic staining being used to define positive VEGF expression in cancer cells. All studies with one exception utilised a system of dichotomising patients according to the percentage of positively stained cells present. Despite the range of values used to define VEGF positivity across the included studies, there was no evidence of any significant association between the % cutoff value used and the corresponding proportion of VEGF positive patients reported. Furthermore, if including only the six studies, which used a standardised cutoff value of >10% for meta-analysis, the significance of the association between VEGF staining and adverse survival was unchanged (HR=1.62 (95% CI=1.09–2.40)−random effects). These observations indicate that differences in the specific scoring criteria used for immunohistochemical classification appear unlikely to have a significant confounding effect in describing the underlying relationship between VEGF expression and survival observed for the overall group.
Broadly comparable demographic and histological tumour characteristics were observed across the eligible VEGF studies, indicating that similar patient populations were evaluated in the combined analysis. Data relating to adjuvant therapy was only reported in 6 out of 11 studies and the treatment modalities included a mix of both chemotherapy and chemoradiation. Importantly, no studies reported any policy of selection of patients for adjuvant therapy based on VEGF tumour expression as immunohistochemical evaluation was undertaken on a retrospective basis in all cases. This was equally true for studies evaluating the other markers of interest.
Both bcl-2 and bax emerged as potentially relevant immunohistochemical prognostic factors. These proteins belong to the bcl-2 family and regulate apoptosis by mediating cytosolic release of cytochrome C from mitochondria in response to cellular stress. Cytochrome C binds to APAF-1 and cleaves caspase-9 into its active form, thereby initiating the activation of executioner caspases resulting in cytoskeletal degradation and cell death (Hamacher et al, 2008). The bcl-2-associated X protein (bax) promotes release of cytochrome C and consequently exhibits pro-apoptotic properties. In contrast, bcl-2 inhibits mitochondrial release of cytochrome C and has anti-apoptotic effects as a result. The finding that bax expression is associated with more favourable survival in resected pancreatic cancer is, therefore, concordant with its physiological role. The observation that the same relationship is consistently seen for bcl-2 expression appears paradoxical. However, this finding is mirrored in other malignancies (Martin et al, 2003; Callagy et al, 2008) and it is believed that a complex interaction of competitive dimerisations between pro- and anti-apoptotic proteins governs the cell's fate in response to apoptotic stimuli (Westphal and Kalthoff, 2003). It is difficult to draw any reliable conclusions from the current meta-analysis of bcl-2 and bax for the pancreatic literature due to the limited number of evaluable studies. However, the overall trend towards both bax and bcl-2 expression being associated with more favourable survival outcomes is generally consistent with the findings seen in other malignancies.
The tumour suppressor gene p16 (CDKN2A) has a key role in pancreatic carcinogenesis (Schutte et al, 1997). p16 is a cell-cycle checkpoint protein, which binds to cyclin-dependent kinases resulting in cell-cycle arrest at the G1/S checkpoint. The observation that positive immunostaining for p16 appears to represent a favourable prognostic feature is, therefore, also consistent with its tumour suppressor function. However, the small number of eligible studies included in this analysis again precludes any meaningful conclusions regarding the reproducibility of p16 expression as a reliable marker of prognosis in resected pancreatic cancer.
Of the various factors evaluated in the present study, the tumour suppressor protein p53 was found to represent the most extensively investigated immunohistochemical prognostic marker. It also exhibited a significant degree of heterogeneity in the reported association between immunostaining and survival for individual studies. Although the overall trend was towards overexpression of p53 resulting in adverse survival for the pooled data, this did not reach significance and there is no obvious explanation for the contradictory results seen between the various studies. The majority of studies used either the monoclonal DO-7, DO-1 or polyclonal CM-1 primary antibodies, which all exhibit immunoreactivity with both wild-type and mutant forms of p53. Due to the increased stability of mutant p53, most of the nuclear immunostaining seen reflects the presence of the mutant rather than wild-type p53 protein. Despite the marked differences between studies in terms of the proportion of cases classified as p53 positive, reported primary antibody dilutions used and cutoff values selected for immunohistochemical scoring, there was no clear association between any of these factors and either the direction of the prognostic effect or the reported magnitude of the HR, which might potentially explain the disparity in survival trends. As a result of these findings, immunohistochemical overexpression of p53 cannot be recommended as a reliable or reproducible marker of prognosis in resected pancreatic cancer from the available evidence.
The smad4 (or DPC4) protein is a central component of the intracellular signalling pathway for transforming growth factor β (TGF-β), and loss of smad4 expression represents an important event in the progression of PanINs to invasive malignancy (Wilentz et al, 2000). The results from the analysis of the five studies evaluating smad4 expression again demonstrate unexplained heterogeneity in the reporting of the prognostic effect of this marker. Biankin et al reported an entirely contradictory survival trend to the other four studies with loss of smad4 expression being associated with significantly improved patient survival despite use of the same primary antibody and otherwise broadly comparable study methodology and patient groups. This survival trend appears at odds with the accepted tumour suppressor role of smad4 in mediating the inhibitory signalling associated with the TGF-β pathway. Despite the fact that the patient series reported by Biankin et al only accounts for 8% of all patients included in the combined analysis and 14% of the weighting allocated to the pooled survival data, the discrepancy in the results is such that sufficient heterogeneity is introduced to require a random effects approach resulting in a non-significant result for the overall analysis. These findings further underline the difficulties in making any reliable conclusions regarding the relative prognostic value of immunohistochemical markers when analysed in limited patient series.
Epidermal growth factor receptor is the cell surface receptor for a family of extracellular ligands, which include EGF and TGF-α and is coded for by the c-erbB1 proto-oncogene. Activation of EGFR stimulates intracellular tyrosine kinase phosphorylation with consequent activation of a number of signalling cascades including the MAPK (mitogen-activated protein kinase) and Akt (protein kinase) pathways, which promote cell proliferation (Ciardello and Tortora, 2008). The analysis of the four eligible studies included in the current meta-analysis again fails to make a strong case for tumoural overexpression of EGFR representing a reproducible prognostic marker. However, the laboratory methodologies reported in the four studies demonstrated more marked variability (e.g., use of four different EGFR primary antibodies) when compared with some of the other analyses.
Despite the inherent limitations of meta-analysing prognostic literature, the findings from the present study suggest that VEGF represents the most consistently reproducible molecular marker with prognostic value in resected pancreatic adenocarcinoma. This result is concordant with existing meta-analyses, which implicate a similar prognostic role for VEGF expression in other malignancies and lend further weight to the assertion that angiogenesis is a key determinant in driving pancreatic cancer progression. For several of the other markers evaluated in this study, directly contradictory prognostic effects were commonly observed with significant variability in the proportions of positive immunostaining reported, despite often broadly comparable patient groups and study methodologies. These results provide further evidence to suggest that in order to make reliable conclusions regarding immunohistochemical prognostic factors and to identify the relevance with which these factors can be translated into clinical use (e.g., individualised patient selection for adjuvant therapy modalities), large collaborative studies collecting tissue as part of prospective multicentre trials, with standardised approaches to both laboratory and statistical methodology, represent the optimal strategy to achieve these goals in the future (e.g. Farrell et al, 2009; Manuyakorn et al, 2010).
Acknowledgments
This study was supported by Cancer Research UK.
References
- Achen MG, Stacker SA (2008) Molecular control of lymphatic metastasis. Ann N Y Acad Sci 1131: 225–234 [DOI] [PubMed] [Google Scholar]
- Ahrendt SA, Brown HM, Komorowski RA, Zhu YR, Wilson SD, Erickson BA, Ritch PS, Pitt HA, Demeure MJ (2000) p21WAF1 expression is associated with improved survival after adjuvant chemoradiation for pancreatic cancer. Surgery 128: 520–530 [DOI] [PubMed] [Google Scholar]
- Ai KX, Lu LY, Huang XY, Chen W, Zhang HZ (2008) Prognostic significance of S100A4 and vascular endothelial growth factor expression in pancreatic cancer. World J Gastroenterol 14: 1931–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa S, Sasaki M, Wada R, Koyama M, Yagihashi S (1996) P53 protein expression in pancreatic tumors and its relationship to clinicopathological factors and prognosis. J Surg Oncol 62: 279–283 [DOI] [PubMed] [Google Scholar]
- Altman DG (2001) Systematic reviews of evaluations of prognostic variables. BMJ 323: 224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan A, Gladhaug IP, Schjolberg A, Bergan AB, Clausen OP (2000) p53 accumulation confers prognostic information in resectable adenocarcinomas with ductal but not with intestinal differentiation in the pancreatic head. Int J Oncol 17: 921–926 [DOI] [PubMed] [Google Scholar]
- Biankin AV, Morey AL, Lee CS, Kench JG, Biankin SA, Hook HC, Head DR, Hugh TB, Sutherland RL, Henshall SM (2002) DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol 20: 4531–4542 [DOI] [PubMed] [Google Scholar]
- Bloomston M, Bhardwaj A, Ellison EC, Frankel WL (2006) Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg 23: 74–79 [DOI] [PubMed] [Google Scholar]
- Bold RJ, Hess KR, Pearson AS, Grau AM, Sinicrope FA, Jennings M, McConkey DJ, Bucana CD, Cleary KR, Hallin PA, Chiao PJ, Abbruzzese JL, Evans DB (1999) Prognostic factors in resectable pancreatic cancer: p53 and bcl-2. J Gastrointest Surg 3: 263–277 [DOI] [PubMed] [Google Scholar]
- Büchler P, Reber HA, Büchler MW, Friess H, Hines OJ (2002) VEGF-RII influences the prognosis of pancreatic cancer. Ann Surg 236: 738–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callagy GM, Webber MJ, Pharoah PD, Caldas C (2008) Meta-analysis confirms bcl-2 is an independent prognostic marker in breast cancer. BMC Cancer 8: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campani D, Boggi U, Cecchetti D, Esposito I, Ceccarelli F, D’Antonio L, De Negri F, Mosca F, Bevilacqua G, Fornaciari G (1999) p53 overexpression in lymph node metastases predicts clinical outcome in ductal pancreatic cancer. Pancreas 19: 26–32 [DOI] [PubMed] [Google Scholar]
- Campani D, Esposito I, Boggi U, Cecchetti D, Menicagli M, De Negri F, Colizzi L, Del Chiaro M, Mosca F, Fornaciari G, Bevilacqua G (2001) Bcl-2 expression in pancreas development and pancreatic cancer progression. J Pathol 194: 444–450 [DOI] [PubMed] [Google Scholar]
- Chung GG, Yoon HH, Zerkowski MP, Ghosh S, Thomas L, Harigopal M, Charette LA, Salem RR, Camp RL, Rimm DL, Burtness BA (2006) Vascular endothelial growth factor, FLT-1, and FLK-1 analysis in a pancreatic cancer tissue microarray. Cancer 106: 1677–1684 [DOI] [PubMed] [Google Scholar]
- Ciardello F, Tortora G (2008) EGFR antagonists in cancer treatment. N Engl J Med 358: 1160–1174 [DOI] [PubMed] [Google Scholar]
- Coppola D, Lu L, Fruehauf JP, Kyshtoobayeva A, Karl RC, Nicosia SV, Yeatman TJ (1998) Analysis of p53, p21WAF1, and TGF-beta1 in human ductal adenocarcinoma of the pancreas: TGF-beta1 protein expression predicts longer survival. Am J Clin Pathol 110: 16–23 [DOI] [PubMed] [Google Scholar]
- Dallas NA, Fan F, Gray MJ, Van Buren II G, Lim SJ, Xia L, Ellis LM (2007) Functional significance of vascular endothelial growth factor receptors on gastrointestinal cancer cells. Cancer Metastasis Rev 26: 433–441 [DOI] [PubMed] [Google Scholar]
- Dang CX, Han Y, Qin ZY, Wang YJ (2002) Clinical significance of expression of p21 and p53 proteins and proliferating cell nuclear antigen in pancreatic cancer. Hepatobiliary Pancreat Dis Int 1: 302–305 [PubMed] [Google Scholar]
- Delmotte P, Martin B, Paesmans M, Berghmans T, Mascaux C, Meert AP, Steels E, Verdebout JM, Lafitte JJ, Sculier JP (2002) VEGF and survival of patients with lung cancer: a systematic literature review and meta-analysis. Rev Mal Respir 19: 577–584 [PubMed] [Google Scholar]
- Dergham ST, Dugan MC, Joshi US, Chen YC, Du W, Smith DW, Arlauskas P, Crissman JD, Vaitkevicius VK, Sarkar FH (1997a) The clinical significance of p21(WAF1/CIP-1) and p53 expression in pancreatic adenocarcinoma. Cancer 80: 372–381 [DOI] [PubMed] [Google Scholar]
- Dergham ST, Dugan MC, Kucway R, Du W, Kamarauskiene DS, Vaitkevicius VK, Crissman JD, Sarkar FH (1997b) Prevalence and clinical significance of combined K-ras mutation and p53 aberration in pancreatic adenocarcinoma. Int J Pancreatol 21: 127–143 [DOI] [PubMed] [Google Scholar]
- Dergham ST, Dugan MC, Sarkar FH, Vaitkevicius VK (1998) Molecular alterations associated with improved survival in pancreatic cancer patients treated with radiation or chemotherapy. J Hepatobiliary Pancreat Surg 5: 269–272 [DOI] [PubMed] [Google Scholar]
- Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY (2006) Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 94: 1823–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiuseppe JA, Hruban RH, Goodman SN, Polak M, van den Berg FM, Allison DC, Cameron JL, Offerhaus GJ (1994) Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol 101: 684–688 [DOI] [PubMed] [Google Scholar]
- Dong M, Dong Q, Zhang H, Zhou J, Tian Y, Dong Y (2007) Expression of Gadd45a and p53 proteins in human pancreatic cancer: potential effects on clinical outcomes. J Surg Oncol 95: 332–336 [DOI] [PubMed] [Google Scholar]
- Dong M, Ma G, Tu W, Guo KJ, Tian YL, Dong YT (2005a) Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J Gastroenterol 11: 2162–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT (1998a) Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res 18: 4613–4619 [PubMed] [Google Scholar]
- Dong M, Nio Y, Sato Y, Tamura K, Song MM, Tian YL, Dong YT (1998b) Comparative study of p53 expression in primary invasive ductal carcinoma of the pancreas between Chinese and Japanese. Pancreas 17: 229–237 [DOI] [PubMed] [Google Scholar]
- Dong M, Nio Y, Tamura K, Song MM, Guo KJ, Guo RX, Dong YT (2000) Ki-ras point mutation and p53 expression in human pancreatic cancer: a comparative study among Chinese, Japanese, and Western patients. Cancer Epidemiol Biomarkers Prev 9: 279–284 [PubMed] [Google Scholar]
- Dong M, Nio Y, Yamasawa K, Toga T, Yue L, Harada T (2003) p53 alteration is not an independent prognostic indicator, but affects the efficacy of adjuvant chemotherapy in human pancreatic cancer. J Surg Oncol 82: 111–120 [DOI] [PubMed] [Google Scholar]
- Dong M, Zhou JP, Zhang H, Guo KJ, Tian YL, Dong YT (2005b) Clinicopathological significance of Bcl-2 and Bax protein expression in human pancreatic cancer. World J Gastroenterol 11: 2744–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LM, Takahashi Y, Fenoglio CJ, Cleary KR, Bucana CD, Evans DB (1998) Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur J Cancer 34: 337–340 [DOI] [PubMed] [Google Scholar]
- Evans JD, Cornford PA, Dodson A, Greenhalf W, Foster CS, Neoptolemos JP (2001) Detailed tissue expression of bcl-2, bax, bak and bcl-x in the normal human pancreas and in chronic pancreatitis, ampullary and pancreatic ductal adenocarcinomas. Pancreatology 1: 254–262 [DOI] [PubMed] [Google Scholar]
- Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, Dicker AF, Mackey JR (2009) Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 136(1): 187–195 [DOI] [PubMed] [Google Scholar]
- Friess H, Lu Z, Graber HU, Zimmermann A, Adler G, Korc M, Schmid RM, Büchler MW (1998) bax, but not bcl-2, influences the prognosis of human pancreatic cancer. Gut 43: 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M (1998) Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer 34: 1439–1447 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Yoshida K, Yanagisawa S, Kawakami M, Aoki T, Yamazaki Y (2001) Angiogenesis in pancreatic carcinoma: thymidine phosphorylase expression in stromal cells and intratumoral microvessel density as independent predictors of overall and relapse-free survival. Cancer 92: 1788–1797 [DOI] [PubMed] [Google Scholar]
- Gansauge F, Gansauge S, Link KH, Beger HG (1999) p53 in relation to therapeutic outcome of locoregional chemotherapy in pancreatic cancer. Ann N Y Acad Sci 880: 281–287 [DOI] [PubMed] [Google Scholar]
- Gansauge F, Gansauge S, Schmidt E, Müller J, Beger HG (1998) Prognostic significance of molecular alterations in human pancreatic carcinoma – an immunohistological study. Langenbecks Arch Surg 383: 152–155 [DOI] [PubMed] [Google Scholar]
- Gazzaniga GM, Papadia FS, Dezzana M, Cappato S, Filauro M, Bandelloni R (2001) Role of p53 mutations on survival after pancreatoduodenectomy for ductal adenocarcinoma of the pancreatic head. Hepatogastroenterology 48: 1743–1745 [PubMed] [Google Scholar]
- Gerdes B, Ramaswamy A, Ziegler A, Lang SA, Kersting M, Baumann R, Wild A, Moll R, Rothmund M, Bartsch DK (2002) p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Ann Surg 235: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher R, Schmid RM, Saur D, Schneider G (2008) Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Nio Y, Koike M, Itakura M, Yano S, Higami T, Maruyama R (2005) Expression of retinoblastoma and p53 pathway-related proteins in resectable invasive ductal carcinoma of the pancreas: potential cooperative effects on clinical outcome. Anticancer Res 25: 1361–1368 [PubMed] [Google Scholar]
- Hayden JA, Côté P, Bombardier C (2006) Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 144: 427–437 [DOI] [PubMed] [Google Scholar]
- Hermanova M, Karasek P, Nenuti R, Kyr M, Tomasek J, Baltasova I, Dite P (2009) Clinicopathological correlations of cyclooxygenase-2, MDM2, and p53 expressions in surgically resectable pancreatic invasive ductal adenocarcinoma. Pancreas 38: 565–571 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558 [DOI] [PubMed] [Google Scholar]
- Hu YX, Watanabe H, Ohtsubo K, Yamaguchi Y, Ha A, Motoo Y, Okai T, Sawabu N (1999) Bcl-2 expression related to altered p53 protein and its impact on the progression of human pancreatic carcinoma. Br J Cancer 80: 1075–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YX, Watanabe H, Ohtsubo K, Yamaguchi Y, Ha A, Okai T, Sawabu N (1997) Frequent loss of p16 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. Clin Cancer Res 3: 1473–1477 [PubMed] [Google Scholar]
- Hua Z, Zhang YC, Hu XM, Jia ZG (2003) Loss of DPC4 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. World J Gastroenterol 9: 2764–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M (1999) Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer 79: 1553–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda N, Nakajima Y, Sho M, Adachi M, Huang CL, Iki K, Kanehiro H, Hisanaga M, Nakano H, Miyake M (2001) The association of K-ras gene mutation and vascular endothelial growth factor gene expression in pancreatic carcinoma. Cancer 92: 488–499 [DOI] [PubMed] [Google Scholar]
- Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Büchler MW, Korc M (1997) Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res 3: 1309–1316 [PubMed] [Google Scholar]
- Jeong J, Park YN, Park JS, Yoon DS, Chi HS, Kim BR (2005) Clinical significance of p16 protein expression loss and aberrant p53 protein expression in pancreatic cancer. Yonsei Med J 46: 519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinfeng M, Kimura W, Sakurai F, Moriya T, Mizutani M, Hirai I (2007) Prognostic role of angiogenesis and its correlations with thymidine phosphorylase and p53 expression in ductal adenocarcinoma of the pancreas. Hepatogastroenterology 54: 1635–1640 [PubMed] [Google Scholar]
- Karademir S, Sökmen S, Terzi C, Sağol O, Ozer E, Astarcioğlu H, Coker A, Astarcioğlu I (2000) Tumor angiogenesis as a prognostic predictor in pancreatic cancer. J Hepatobiliary Pancreat Surg 7: 489–495 [DOI] [PubMed] [Google Scholar]
- Kawesha A, Ghaneh P, Andrén-Sandberg A, Ograed D, Skar R, Dawiskiba S, Evans JD, Campbell F, Lemoine N, Neoptolemos JP (2000) K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16(INK4A), p21(WAF-1), cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer 89: 469–474 [DOI] [PubMed] [Google Scholar]
- Khorana AA, Hu YC, Ryan CK, Komorowski RA, Hostetter G, Ahrendt SA (2005) Vascular endothelial growth factor and DPC4 predict adjuvant therapy outcomes in resected pancreatic cancer. J Gastrointest Surg 9: 903–911 [DOI] [PubMed] [Google Scholar]
- Knoll MR, Rudnitzki D, Sturm J, Manegold BC, Post S, Jaeger TM (2001) Correlation of postoperative survival and angiogenic growth factors in pancreatic carcinoma. Hepatogastroenterology 48: 1162–1165 [PubMed] [Google Scholar]
- Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, Aikou T (2004) Impact of vascular endothelial growth factor-C and -D expression in human pancreatic cancer: its relationship to lymph node metastasis. Clin Cancer Res 10: 8413–8420 [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K (2003) Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas 26: 344–349 [DOI] [PubMed] [Google Scholar]
- Kyzas PA, Cunha IW, Ioannidis JP (2005a) Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res 11: 1434–1440 [DOI] [PubMed] [Google Scholar]
- Kyzas PA, Loizou KT, Ioannidis JP (2005b) Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst 97: 1043–1055 [DOI] [PubMed] [Google Scholar]
- Lee EU, Cibull ML, O’Daniel-Pierce E, Strodel WE, Jennings CD (1995) Expression of p53 protein in pancreatic adenocarcinoma. Relationship to cigaret smoking. Int J Pancreatol 17: 237–242 [DOI] [PubMed] [Google Scholar]
- Li Y, Bhuiyan M, Vaitkevicius VK, Sarkar FH (1999) Structural alteration of p53 protein correlated to survival in patients with pancreatic adenocarcinoma. Pancreas 18: 104–110 [DOI] [PubMed] [Google Scholar]
- Lim YJ, Lee JK, Park CK, Song SY, Jang WY, Ha HY, Park DI, Lee KT, Paik SW, Yoo BC, Rhee JC (2004) Prognostic value of VEGF in human pancreatic ductal adenocarcinoma. Korean J Intern Med 19: 10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Blåsjö M, von Rosen A, Parrado C, Falkmer UG, Falkmer S (2001) Pattern of distribution and prognostic value of angiogenesis in pancreatic duct carcinoma: a semiquantitative immunohistochemical study of 45 patients. Pancreas 22: 240–247 [DOI] [PubMed] [Google Scholar]
- Linder S, Parrado C, Falkmer UG, Blåsjö M, Sundelin P, von Rosen A (1997) Prognostic significance of Ki-67 antigen and p53 protein expression in pancreatic duct carcinoma: a study of the monoclonal antibodies MIB-1 and DO-7 in formalin-fixed paraffin-embedded tumour material. Br J Cancer 76: 54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin J, Nordling S, von Boguslawsky K, Roberts PJ, Haglund C (1996) Prognostic value of immunohistochemical expression of p53 in patients with pancreatic cancer. Oncology 53: 104–111 [DOI] [PubMed] [Google Scholar]
- Magistrelli P, Coppola R, Tonini G, Vincenzi B, Santini D, Borzomati D, Vecchio F, Valeri S, Castri F, Antinori A, Nuzzo G, Caraglia M, Picciocchi A (2006) Apoptotic index or a combination of Bax/Bcl-2 expression correlate with survival after resection of pancreatic adenocarcinoma. J Cell Biochem 97: 98–108 [DOI] [PubMed] [Google Scholar]
- Mäkinen K, Hakala T, Lipponen P, Alhava E, Eskelinen M (1998) Clinical contribution of bcl-2, p53 and Ki-67 proteins in pancreatic ductal adenocarcinoma. Anticancer Res 18: 615–658 [PubMed] [Google Scholar]
- Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, Kogevinas M, Real FX (2005) P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 6: 678–686 [DOI] [PubMed] [Google Scholar]
- Manuyakorn A, Paulus R, Farrell J, Dawson NA, Tze S, Cheung-Lau G, Hines OJ, Reber H, Seligson DB, Horvath S, Kurdistani SK, Dawson DW (2010) Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: results from RTOG 9704. J Clin Oncol 28: 1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Paesmans M, Berghmans T, Branle F, Ghisdal L, Mascaux C, Meert AP, Steels E, Vallot F, Verdebout JM, Lafitte JJ, Sculier JP (2003) Role of Bcl-2 as a prognostic factor for survival in lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 89: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) Reporting recommendations for tumor marker prognostic studies (REMARK). Exp Oncol 28: 99–105 [PubMed] [Google Scholar]
- Naka T, Kobayashi M, Ashida K, Toyota N, Kaneko T, Kaibara N (1998) Aberrant p16INK4 expression related to clinical stage and prognosis in patients with pancreatic cancer. Int J Oncol 12: 1111–1116 [DOI] [PubMed] [Google Scholar]
- Nash AD, Baca M, Wright C, Scotney PD (2006) The biology of vascular endothelial growth factor-B (VEGF-B). Pulm Pharmacol Ther 19: 61–69 [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW, European Study Group for Pancreatic Cancer (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350: 1200–1210 [DOI] [PubMed] [Google Scholar]
- Niedergethmann M, Hildenbrand R, Wolf G, Verbeke CS, Richter A, Post S (2000) Angiogenesis and cathepsin expression are prognostic factors in pancreatic adenocarcinoma after curative resection. Int J Pancreatol 28: 31–39 [DOI] [PubMed] [Google Scholar]
- Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S (2002) High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas 25: 122–129 [DOI] [PubMed] [Google Scholar]
- Nio Y, Dong M, Uegaki K, Hirahara N, Minari Y, Sasaki S, Takamura M, Iguchi C, Tamura K (1998) p53 expression affects the efficacy of adjuvant chemotherapy after resection of invasive ductal carcinoma of the pancreas. Anticancer Res 18: 3773–3779 [PubMed] [Google Scholar]
- Nio Y, Dong M, Uegaki K, Hirahara N, Minari Y, Sasaki S, Takamura M, Iguchi C, Tamura K (1999) Comparative significance of p53 and WAF/1-p21 expression on the efficacy of adjuvant chemotherapy for resectable invasive ductal carcinoma of the pancreas. Pancreas 18: 117–126 [DOI] [PubMed] [Google Scholar]
- Nio Y, Dong M, Iguchi C, Yamasawa K, Toga T, Itakura M, Tamura K (2001a) Expression of Bcl-2 and p53 protein in resectable invasive ductal carcinoma of the pancreas: effects on clinical outcome and efficacy of adjuvant chemotherapy. J Surg Oncol 76: 188–196 [DOI] [PubMed] [Google Scholar]
- Nio Y, Iguchi C, Yamasawa K, Sasaki S, Takamura M, Toga T, Dong M, Itakura M, Tamura K (2001b) Apoptosis and expression of Bcl-2 and Bax proteins in invasive ductal carcinoma of the pancreas. Pancreas 22: 230–239 [DOI] [PubMed] [Google Scholar]
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297: 267–277 [DOI] [PubMed] [Google Scholar]
- Ohshio G, Suwa H, Imamura M (2002) Clinical implication of anti-p53 antibodies and p53-protein in pancreatic disease. Int J Gastrointest Cancer 31: 129–135 [DOI] [PubMed] [Google Scholar]
- Ohshio G, Suwa H, Imamura T, Yamaki K, Tanaka T, Hashimoto Y, Imamura M (1998) An immunohistochemical study of bcl-2 and p53 protein expression in pancreatic carcinomas. Scand J Gastroenterol 33: 535–539 [DOI] [PubMed] [Google Scholar]
- Ohtsubo K, Watanabe H, Yamaguchi Y, Hu YX, Motoo Y, Okai T, Sawabu N (2003) Abnormalities of tumor suppressor gene p16 in pancreatic carcinoma: immunohistochemical and genetic findings compared with clinicopathological parameters. J Gastroenterol 38: 663–671 [DOI] [PubMed] [Google Scholar]
- Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival end-points. Stat Med 17: 2815–2834 [DOI] [PubMed] [Google Scholar]
- Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, Chang D, Yeo CJ (2006) Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery 140: 764–772 [DOI] [PubMed] [Google Scholar]
- Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ (2002) Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer 86: 886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Nio Y, Song MM, Sumi S, Hirahara N, Minari Y, Tamura K (1997) p53 protein expression as prognostic factor in human pancreatic cancer. Anticancer Res 17: 2779–2788 [PubMed] [Google Scholar]
- Schutte M, Hruban RH, Geradts J (1997) Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 57: 3126–3130 [PubMed] [Google Scholar]
- Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K (2000) High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 88: 2239–2245 [DOI] [PubMed] [Google Scholar]
- Sessa F, Bonato M, Bisoni D, Ranzani GN, Capella C (1998) Ki-ras and p53 gene mutations in pancreatic ductal carcinoma: a relationship with tumor phenotype and survival. Eur J Histochem 42: 67–76 [PubMed] [Google Scholar]
- Sinicrope FA, Evans DB, Leach SD, Cleary KR, Fenoglio CJ, Lee JJ, Abbruzzese JL (1996) bcl-2 and p53 expression in resectable pancreatic adenocarcinomas: association with clinical outcome. Clin Cancer Res 2: 2015–2022 [PubMed] [Google Scholar]
- Smeenk HG, Erdmann J, van Dekken H, van Marion R, Hop WC, Jeekel J, van Eijck CH (2007) Long-term survival after radical resection for pancreatic head and ampullary cancer: a potential role for the EGF-R. Dig Surg 24: 38–45 [DOI] [PubMed] [Google Scholar]
- Stipa F, Lucandri G, Limiti MR, Bartolucci P, Cavallini M, Di Carlo V, D’Amato A, Ribotta G, Stipa S (2002) Angiogenesis as a prognostic indicator in pancreatic ductal adenocarcinoma. Anticancer Res 22: 445–449 [PubMed] [Google Scholar]
- Sun CY, Wang BL, Hu CQ, Peng RY, Gao YB, Gu QY, Wang DW (2002) Expression of the bcl-2 gene and its significance in human pancreatic carcinoma. Hepatobiliary Pancreat Dis Int 1: 306–308 [PubMed] [Google Scholar]
- Takikita M, Altekruse S, Lynch CF, Goodman MT, Hermandez BY, Green M, Cozen W, Cockburn M, Siburg Saber M, Topor M, Zeruto C, Abedi-Ardekani B, Reichman ME, Hewitt SM (2009) Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res 69: 2950–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, Adsay V, Abrams RA, Cameron JL, Kern SE, Yeo CJ, Hruban RH, Goggins M (2001) The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res 7: 4115–4121 [PubMed] [Google Scholar]
- Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y (2001) Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas 22: 285–292 [DOI] [PubMed] [Google Scholar]
- Tang RF, Wang SX, Peng L, Wang SX, Zhang M, Li ZF, Zhang ZM, Xiao Y, Zhang FR (2006) Expression of vascular endothelial growth factors A and C in human pancreatic cancer. World J Gastroenterol 12: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga T, Nio Y, Hashimoto K, Higami T, Maruyama R (2004) The dissociated expression of protein and messenger RNA of DPC4 in human invasive ductal carcinoma of the pancreas and their implication for patient outcome. Anticancer Res 24: 1173–1178 [PubMed] [Google Scholar]
- Tomazic A, Pegan V, Ferlan-Marolt K, Pleskovic A, Luzar B (2004) Cyclin D1 and bax influence the prognosis after pancreatoduodenectomy for periampullary adenocarcinoma. Hepatogastroenterology 51: 1832–1837 [PubMed] [Google Scholar]
- Ueda S, Hatsuse K, Tsuda H, Ogata S, Kawarabayashi N, Takigawa T, Einama T, Morita D, Fukatsu K, Sugiura Y, Matsubara O, Mochizuki H (2006) Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol 19: 788–796 [DOI] [PubMed] [Google Scholar]
- Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H (2004) The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 29: 1–8 [DOI] [PubMed] [Google Scholar]
- Uegaki K, Nio Y, Inoue Y, Minari Y, Sato Y, Song MM, Dong M, Tamura K (1997) Clinicopathological significance of epidermal growth factor and its receptor in human pancreatic cancer. Anticancer Res 17: 3841–3847 [PubMed] [Google Scholar]
- Virkajärvi N, Pääkkö P, Soini Y (1997) Association between p53 overexpression, cell proliferation, tumor necrosis and extent of apoptosis in operated pancreatic adenocarcinoma. APMIS 105: 765–772 [DOI] [PubMed] [Google Scholar]
- Westphal S, Kalthoff H (2003) Apoptosis: targets in pancreatic cancer. Mol Cancer 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrer K, Feichtinger H, Haun M, Weiss G, Ofrer D, Weger AR, Umlauft F, Grunewald K (1996) p53, Ki-ras, and DNA ploidy in human pancreatic ductal adenocarcinoma. Lab Invest 74: 279–289 [PubMed] [Google Scholar]
- Wilentz RE, Iacobuzio-Donahue CA, Argani P (2000) Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 60: 2002–2006 [PubMed] [Google Scholar]
- Williamson PR, Smith CT, Hutton JL, Marson AG (2002) Aggregate data meta-analysis with time-to-event outcomes. Stat Med 21: 3337–3351 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Chijiiwa K, Torato N, Kinoshita M, Tanaka M (2000) Ki-ras codon 12 point and P53 mutations: a molecular examination of the main tumor, liver, portal vein, peripheral arterial blood and para-aortic lymph node in pancreatic cancer. Am J Gastroenterol 95: 1939–1945 [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M (1993) Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res 13: 565–569 [PubMed] [Google Scholar]
- Yamasawa K, Nio Y, Dong M, Yamaguchi K, Itakura M (2002) Clinicopathological significance of abnormalities in Gadd45 expression and its relationship to p53 in human pancreatic cancer. Clin Cancer Res 8: 2563–2569 [PubMed] [Google Scholar]
- Yamazaki Y, Morita T (2006) Molecular and functional diversity of vascular endothelial growth factors. Mol Divers 10: 515–527 [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Yamanaka Y, Friess H, Buchler M, Korc M (1994) p53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res 14: 2477–2483 [PubMed] [Google Scholar]
- Yu G, Zhu MH, Zhu Z, Ni CR, Zheng JM, Li FM (2004) Expression of ATM protein and its relationship with p53 in pancreatic carcinoma with tissue array. Pancreas 28: 421–426 [DOI] [PubMed] [Google Scholar]
- Zhang SY, Ruggeri B, Agarwal P, Sorling AF, Obara T, Ura H, Namiki M, Klein-Szanto AJ (1994) Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med 118: 150–154 [PubMed] [Google Scholar]
- Zhang B, Zhao WH, Zhou WY, Yu WS, Yu JM, Li S (2007) Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detect Prev 31: 436–442 [DOI] [PubMed] [Google Scholar]
- Zhang L, Yuan SZ (2002) Expression of c-erbB-2 oncogene protein, epidermal growth factor receptor, and TGF-beta1 in human pancreatic ductal adenocarcinoma. Hepatobiliary Pancreat Dis Int 1: 620–623 [PubMed] [Google Scholar]