Abstract
We examined the inhibitory effects of three heparins on the growth of Babesia parasites. The multiplication of Babesia bovis, B. bigemina, B. equi, and B. caballi in in vitro cultures and that of B. microti in vivo were significantly inhibited in the presence of heparins, as determined by light microscopy. Treatment with various concentrations of heparin showed complete clearance of the intracellular parasites. Interestingly, a higher percentage of abnormally multidividing B. bovis parasites was observed in the presence of low concentrations of heparin. Furthermore, fluorescein isothiocyanate-labeled heparin was preferably found on the surfaces of extracellular merozoites, as detected by confocal laser scanning microscopy. These findings indicate that the heparin covers the surfaces of babesial merozoites and inhibits their subsequent invasion of erythrocytes.
Babesia parasites are tick-transmitted intraerythrocytic protozoa of the phylum Apicomplexa. They affect a wide variety of wild and domestic animals and are responsible for enormous economic losses to the livestock industry worldwide (25). Moreover, some are major etiologic agents of human babesiosis (20). During the asexual growth cycle in a natural host, merozoites internalize the host erythrocytes (RBCs) via multiple adhesive interactions of several protozoan molecules with the host cell surface (45). Thus, the parasites destroy the infected RBCs, which results in severe clinical symptoms, such as high fever, anemia, hematuria, and hemoglobinuria, in the infected hosts. Therefore, understanding of the basic molecular mechanism(s) of the asexual growth cycle, particularly the process of merozoite invasion into the host RBC, may accelerate the development of effective therapeutic methods and methods for the prevention of babesiosis.
Heparin is a highly sulfated form of heparan sulfate (HS) (36) and is known to be an inhibitor of the blood coagulation system (19, 23) or as a coreceptor in the binding of fibroblast growth factor to its receptor (29). HS and heparin are complex entities composed of anionic, linear mucopolysaccharides with alternating uronic acid and hexosamine residues in which a limited set of monosaccharide units gives rise to a number of complex sequences by variable substitution with O-sulfate, N-sulfate, and N-acetyl groups (28), rendering the heparin polydisperse (26). While HS is produced in most cell types, heparin is a biosynthetically derived component of mast cells and basophils (16) and has a molecular weight (MW) of approximately 3,000 to 37,500, with an average MW of 13,000 (26). Due to the sulfate and carboxylate residues, heparin is highly negatively charged (9) and, moreover, is often used as a model glycosaminoglycan (GAG) to study the HS interaction with proteins (29).
Heparin has also been used for the treatment of patients affected by the cerebral form of Plasmodium falciparum infection (27, 30, 39). The therapeutic effect of heparin was demonstrated in rhesus monkeys experimentally infected with Plasmodium knowlesi (11). The growth-inhibitory effects of heparin were also described in in vitro studies with P. falciparum (7, 24, 38) and Theileria sergenti (14). However, the precise mechanisms of its inhibitory capacity against these hemoprotozoa are not fully understood.
In the present study, we examined the inhibitory effects of three kinds of heparin preparations on the in vitro growth of Babesia bovis, B. bigemina, B. equi, and B. caballi and characterized the inhibitory properties by light microscopy and confocal laser scanning microscopy. Additionally, the in vivo inhibitory effect of one heparin preparation against the rodent species B. microti was confirmed in mice, which has been successfully established as an experimental model for babesioses of many other animals (20, 32, 46). Finally, the mechanisms by which heparin interferes with the asexual growth cycle of Babesia parasites are discussed.
MATERIALS AND METHODS
Parasites, culture media, and mice.
The Texan strain of B. bovis (17), the Argentine strain of B. bigemina (21), and U.S. Department of Agriculture strains of B. equi and B. caballi (4, 18) were maintained in purified bovine or equine RBCs (4, 5, 31) with a microaerophilic stationary-phase culture system (2). Medium M199 (for bovine babesia isolates and B. equi) and RPMI 1640 (for B. caballi) (both of which were obtained from Sigma-Aldrich, Tokyo, Japan) supplemented with 40% normal bovine serum (for bovine babesia isolates) or normal equine serum (for equine babesia isolates), 60 U of penicillin G per ml, 60 μg of streptomycin per ml, and 0.15 μg of amphotericin B per ml (all three drugs were obtained from Sigma-Aldrich) were prepared and used as the culture media. Additionally, 13.6 μg of hypoxanthine (ICN Biomedicals Inc., Aurora, Ohio) per ml was added to the B. equi culture as a vital supplement (47), while 229 mg of N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid hemisodium salt (Sigma-Aldrich) per ml was added to the bovine babesia parasite cultures as a pH stabilizer (pH 7.2) (13).
The Munich strain of B. microti was maintained by passage in the blood of BALB/c mice (32, 46). Female BALB/c mice (age, 8 weeks) were purchased from CLEA Japan (Tokyo, Japan) and were used for the in vivo studies.
Chemicals.
Three unfractionated forms of heparin sodium salts were used in the in vitro growth-inhibitory studies and were designated heparin 1 (derived from porcine intestinal mucosa; Wako Pure Chemical Industries, Ltd., Osaka, Japan), heparin 2 (derived from porcine intestinal mucosa; MW, 17,000 to 19,000; Sigma-Aldrich), and heparin 3 (derived from bovine intestinal mucosa; Sigma-Aldrich). Only heparin 1 was used in the in vivo studies. Stock solutions of 100,000 U of the heparins per ml were prepared in double-distilled water and were kept frozen at −80°C until use. Fluorescein isothiocyanate (FITC)-labeled heparin (heparin-FITC) was obtained from Molecular Probes (Eugene, Oreg.).
In vitro growth-inhibitory assays.
The in vitro growth-inhibitory assays were conducted as described by Bork et al. (4, 5). One hundred microliters of infected bovine or equine RBCs was diluted with noninfected RBCs to obtain 1% parasitemia in a 0.1-μl volume, and the mixture was subsequently suspended in 0.9 ml of a suitable growth medium supplemented with the indicated concentrations of heparin. The suspension was added to 24-well culture plates (Nunc, Roskilde, Denmark), and the plates were incubated in a humidified multigas water-jacketed incubator at 37°C for 4 days. During the incubation period, the overlaid culture medium was replaced daily with 0.9 ml of fresh medium containing the different heparins at the concentrations indicated below. In parallel, heparin-free cultures were prepared as controls. All experiments were carried out in triplicate wells (for each heparin concentration) and in three separate trials to obtain conclusive results. Parasite growth in Giemsa-stained thin blood smears with approximately 1,000 RBCs was monitored daily and was determined on the basis of morphological appearance. After 4 days of treatment, 30 μl of each of the infected and heparin-treated (at the various concentrations indicated below) RBCs was mixed with 70 μl of parasite-free RBCs and suspended in fresh growth medium without heparin supplementation. The plates were incubated for the next 10 days. The culture medium was replaced daily, and parasite recrudescence was determined by light microscopy to evaluate the parasite viability (4, 5).
Effect of heparin on the course of B. microti infection in mice.
Sixty 8-week-old female BALB/c mice were divided into six groups (n = 10 mice in each group) and were intraperitoneally inoculated with 107 B. microti-infected RBCs. From days 1 to 10 postinfection, 500, 250, 100, 20, or 4 U of heparin 1 (dissolved in 100 μl of phosphate-buffered saline [PBS]) was administered to each mouse in groups one to five, respectively, and 100 μl of PBS was injected into the mice in the sixth group as a placebo control. All injections were via the subcutaneous route. The levels of parasitemia in all mice were monitored daily until 13 days postinfection by observing stained thin blood smears prepared from venous tail blood. All animal experiments were conducted in accordance with the Standard Relating to the Care and Management of Experimental Animals promulgated by the National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Hokkaido, Japan.
Confocal laser scanning microscopy.
Bovine and equine Babesia parasite-infected RBCs (approximately 5% parasitemia) and B. microti-infected RBCs (approximately 30% parasitemia) were collected from the cultures and infected mice, respectively, and washed three times with cold PBS. Twenty microliters of the infected RBCs was suspended in 500 μl of PBS, and then 50 μg of heparin-FITC was added. The suspension was incubated for 2 h at 4°C with gentle shaking. After the mixture was washed three times with cold PBS, thin smears were prepared on slides and fixed in 50% acetone-50% methanol for 10 min at −30°C. Subsequently, the slides were incubated with 25 μg of propidium iodide (PI; Molecular Probes) per ml containing 50 μg of DNase-free RNase (Roche Applied Science, Mannheim, Germany) per ml for 10 min at 37°C and then mounted in 50% glycerol in PBS. The slides were observed with a confocal laser scanning microscope (TCS NT; Leica, Heidelberg, Germany).
Statistical analyses.
The 50% inhibition concentrations (IC50s) were calculated on day 4 of in vitro culture or on the days when the highest level of parasitemia was detected in the in vivo experiment by interpolation after curve fitting by use of Cricket Graph (Cricket Software, Malvern, Pa.). Differences in percent parasitemias were analyzed statistically by the independent Student t test, with a P value <0.01 representing a significant difference.
RESULTS
Effects of heparins on in vitro growth of cultured parasites.
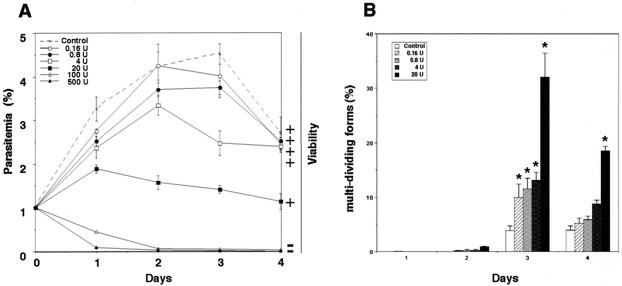
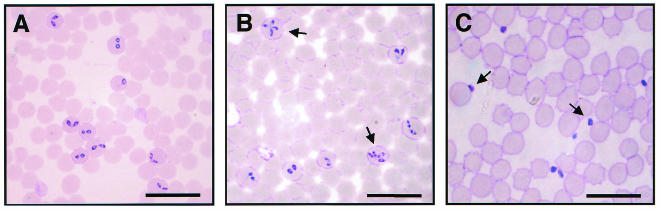
The in vitro growth of B. bovis was entirely arrested in the presence of 500 or 100 U of heparin 1 per ml, and a subsequent viability test revealed complete destruction of the parasite (Fig. 1A). Twenty units of heparin 1 could suppress growth but not clear the parasites from the host RBCs. The IC50 was determined to be 7 U/ml (35 μg/ml) (Table 1). The morphological phenomena observed by light microscopy showed that the parasites exposed to different concentrations of heparin showed a gradual increase in the number of abnormally multidividing forms (Fig. 1B and Fig. 2B). In particular, 20 U of heparin per ml was found to significantly increase the proportion of abnormally multidividing forms to 32% of the entire population within 3 days (Fig. 1B). On the other hand, the multidividing form was rarely observed in the control culture of B. bovis, in which a maximum of only about 4% multidividing forms was detectable. Additionally, concentrations of 20 U of heparin per ml and greater caused significant increases in the number of extracellular (free) merozoites (Fig. 2C) compared to the number in the untreated control groups (Fig. 2A). The apparent percentage of free merozoites reached a peak of 51.4% of the entire population on day 2 of culture in the presence of 20 U of heparin per ml, whereas the peak was 7.8% for the control group (data not shown).
FIG. 1.
(A) In vitro growth rate of B. bovis in the presence of different concentrations of heparin 1 (in units per milliliter). Parasitic viability was monitored in subcultures for 10 days without heparin. +, viable parasites; −, dead parasites. (B) Percentage of abnormally multidividing forms of heparin-treated B. bovis parasites in cultures. *, significant difference (P < 0.01) between the heparin-treated groups and the control group. Each value represents the mean ± standard deviation for three separate trials, carried out in triplicate.
TABLE 1.
IC50s of different heparin preparations
| Organism | IC50 (μg/ml [U/ml])a
|
||
|---|---|---|---|
| Heparin 1 | Heparin 2 | Heparin 3 | |
| B. bovis | 35 (7) | 410 (57) | 350 (52.5) |
| B. bigemina | 30 (6) | 210 (30) | 60 (9) |
| B. equi | 51 (10) | 50 (6.5) | 186 (28) |
| B. caballi | 148 (29) | 310 (44) | 353 (53) |
IC50s are per milliliter of growth medium and were determined on day 4 of in vitro culture by plotting the growth curves and subsequent interpolation.
FIG. 2.
Light micrographs of heparin 1-treated B. bovis in in vitro culture. Micrographs were taken on the first day of no treatment (A), treatment with 20 U of heparin per ml (B), and treatment with 100 U of heparin per ml (C). Note the increase in the numbers of abnormally multidividing forms (B) and free merozoites (C) (arrows) compared to those for the controls (A). Bars, 20 μm.
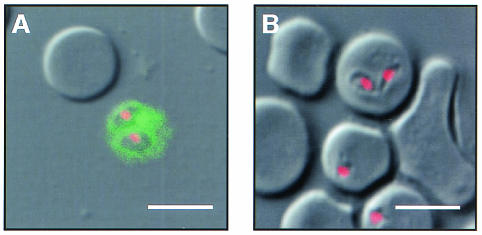
In order to locate the reaction site of heparin, we observed the preincubated parasites and RBCs in heparin-FITC by confocal laser scanning microscopy. Free merozoites exclusively showed strong fluorescence on their surfaces (Fig. 3A), while the infected RBCs, including the intracellular trophozoites and noninfected RBCs, did not show any reaction (Fig. 3B).
FIG. 3.
Demonstration of heparin binding on the surfaces of free merozoites. B. bovis-infected RBCs were preincubated with heparin-FITC, followed by fixation with acetone-methanol, and were then examined under a confocal laser scanning microscope. The heparin-antigen reaction (green) and nucleus (red) were visualized by staining with FITC and PI, respectively. A diffuse fluorescence reaction was detectable only around free merozoites (A) and not on the infected RBCs (B). Bars, 5 μm.
Similar results were obtained by applying heparin 1 to the B. bigemina (Fig. 4A), B. caballi (Fig. 4B), and B. equi (Fig. 4C) cultures. One hundred and 500 U of heparin 1 destroyed the parasites irretrievably, while 20 U/ml suppressed their growth but did not destroy them. The IC50s were calculated to be 6 U/ml (30 μg/ml) for B. bigemina, 10 U/ml (51 μg/ml) for B. equi, and 29 U/ml (148 μg/ml) for B. caballi (Table 1). Similar to the findings for B. bovis, heparin caused an increase in the number of free merozoites, and heparin-FITC reacted with the surfaces of free merozoites (data not shown). However, an abnormally multidividing form, like that observed with B. bovis, was not detectable with these Babesia parasites even if heparin was applied at a concentration of 20 U/ml (data not shown). For B. equi and B. microti, division of the trophozoite form leads to the characteristic Maltese cross form (which consists of four merozoites joined at their posterior ends) (46), similar to the multidividing form observed for B. bovis. However, the percentage of Maltese cross forms of B. equi decreased in the presence of 20 U of heparin per ml (a maximum of 1.4% on day 3 of culture, compared to 6.5% for the control culture) (data not shown).
FIG. 4.
In vitro growth rates of B. bigemina (A), B. caballi (B), and B. equi (C) in the presence of different concentrations of heparin 1 (in units per milliliter). Parasite viability was monitored in subcultures for 10 days without heparin. +, viable cells; −, dead cells. Each value represents the mean ± standard deviation for three separate trials, carried out in triplicate.
In order to exclude a potential incidental effect of one particular heparin preparation, we performed parallel in vitro experiments (described in Materials and Methods) using different sources of heparins (heparin 2 and heparin 3). The IC50s of heparin 2 and heparin 3 were subsequently compared to those of heparin 1, as shown in Table 1. It was found that all heparin preparations used in this study were able to inhibit parasite growth, although the effective concentrations varied among the parasites and heparins used. Except for B. equi, the IC50 of heparin 1 was lower than those of heparin 2 and heparin 3.
In vivo effect of heparin against B. microti infection.
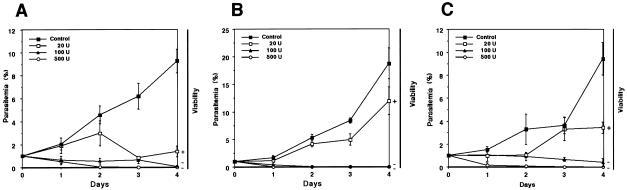
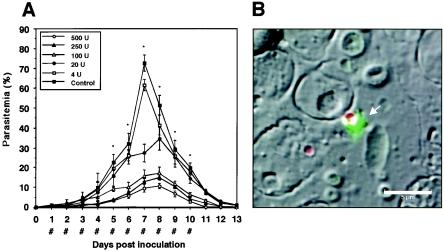
Having observed that heparin 1 had a strong inhibitory effect on the growth of the cultured parasites, we were encouraged to investigate its in vivo effect on B. microti infection in mice (Fig. 5A). When the infected mice showed about 1% parasitemia, they received subcutaneous injections of either 500, 250, 100, 20, or 4 U of heparin for 10 consecutive days. In the treated group, the levels of parasitemia increased significantly more slowly, achieving peak parasitemias of 10% (in the presence of 500 U), 15% (250 U), 17% (100 U), and 34% (20 U) on day 8 after inoculation. In contrast, in the control group, the peak parasitemia of more than 72% occurred on day 7 after inoculation. On the basis of these peak parasitemias, the IC50 for B. microti infection was calculated to be 16 U (81 μg) per mouse. In several mice treated with 250 and 500 U of heparin, hematomas occurred around the puncture site following the injections, and among the 10 mice in each of the groups treated with 500 and 250 U of heparin, three and one mice died, respectively, following the second or third injection. As for the other Babesia parasites, a greater percentage of free merozoites was seen in the heparin-treated groups, in which the peak proportion of free merozoites of 45% occurred on day 5 in the group treated with 100 U of heparin, while the highest value did not exceed 32% in the control group (data not shown). Additionally, binding of heparin-FITC was also observed on the surfaces of free merozoites (Fig. 5B). There were no significant differences in the percentages of the Maltese cross forms among the groups (data not shown).
FIG. 5.
(A) Inhibitory effects of 500, 250, 100, 20, or 4 U of heparin 1 per mouse on the course of B. microti infection in mice. Each value represents the mean ± standard deviation for observations for 10 mice in each group. *, statistically significant differences (P < 0.01) between the group treated with 100 U of heparin and the control group; #, times of subcutaneous inoculation of heparin or the control reagent (PBS). (B) Localization of heparin-recognizing locus. B. microti-infected blood was preincubated with heparin-FITC, followed by fixation with acetone-methanol, and was then examined under a confocal laser scanning microscope. The heparin-antigen reaction (green) and nucleus (red) were visualized by staining with FITC and PI, respectively. Distinct fluorescence was detectable around free merozoites (arrow) but not the infected RBC. Bar, 5 μm.
DISCUSSION
In the present study, different kinds of heparins were found to significantly inhibit the growth of Babesia parasites. The increase in the percentage of free merozoites of all parasites tested and the occurrence of an abnormally high percentage of the multidividing trophozoite form of B. bovis might be due to the blocking effects of heparin on the invasion of merozoites into RBCs and on the release of mature trophozoites from infected RBCs. In the presence of a low concentration of heparin, some B. bovis merozoites still retained their invasive capacity and subsequently divided in the infected RBCs. However, we conclude that the heparin-exposed parasites are not able to escape from the infected RBCs, probably because they have been carried over into the erythrocytic cytoplasm, resulting in an abnormal progression to further trophozoite division in the RBCs. Functionally, the multidividing form of B. bovis is not related to the Maltese cross form observed only in B. equi and B. microti because the proportion of the Maltese cross form of these parasites never increased, even in the presence of heparin.
The study with heparin-FITC indicated the presence of a molecule(s) with an affinity for heparin on the surfaces of free merozoites. Because heparin at a high concentration completely inhibited the invasion of merozoites, the interaction between the molecule with an affinity for heparin and exogenous heparin must play a critical role in disruption of the invasion process. Previous studies with heparin-FITC-labeled Toxoplasma gondii found that the fluorescence is localized near the subapical region of free tachyzoites (6). T. gondii surface antigen (SAG3) is known to show a heparin-binding property and mediates the attachment of the tachyzoite to the cellular HS proteoglycan of host cells (22). In P. falciparum, heparin interacts with the circumsporozoite protein expressed on the surface of the sporozoite (34). The binding of the circumsporozoite protein to cellular HS proteoglycans is required for sporozoite attachment to hepatocytes (33). The molecule(s) with an affinity for heparin observed in this study might also be essential for the process of invasion of RBCs by Babesia parasites. In several reports (40, 44), the HS-like GAG was described to be located on the surfaces of RBCs, suggesting the possibility that the molecule(s) of the free merozoite with an affinity for heparin might recognize the HS-like GAG as the binding partner, similar to the HS used for recognition by some other parasitic microbes (35).
In the erythrocytic stage of P. falciparum, heparin-FITC is detectable on the surfaces of infected RBCs (3). The infected RBCs, including the mature trophozoites, are bound deep in the microvasculature, primarily to endothelial cells (cytoadherence) and also to normal RBCs (rosetting) (8, 12, 15, 42). The sequestration is mediated by P. falciparum erythrocyte membrane protein 1 (PfEMP1), which is expressed as a parasite-derived polypeptide on the surfaces of infected RBCs (43) and which adheres to HS on endothelial cells and also to the HS-like GAG on normal RBCs (3). The resultant binding of infected RBCs in the inner organs prevents the parasite from being removed by splenic clearance (10, 37). Although it has also been suggested that some Babesia parasites sequester infected RBCs in the host microvasculature (1), the mechanism has not been fully determined.
In this study, the IC50 of heparin for the rodent babesia isolates was determined to be 16 U (81 μg) per mouse. However, some side effects (the occurrence of hematomas and the death of a total of four mice) were observed during treatment with either 500 or 250 U of heparin. These adverse reactions might be caused by the anticoagulation effect of heparin as a result of heparin binding to antithrombin AT-III and subsequent inhibition of the blood coagulation cascade (23, 41). Therefore, for babesiacidal therapy the application of heparin doses as high as those mentioned above is not recommended, even though these doses suppress parasite growth. Since we used unfractionated forms of heparin preparations in our study, it remains unknown which fraction of heparin exerts the highest degree of efficacy in the suppression of Babesia parasites without causing side effects. Further studies will be required to develop safety measures for heparin as therapy for babesiosis.
The present results suggest that the Babesia parasites do not have the PfEMP1-like molecule that confers a heparin-binding property to the surfaces of infected RBCs. However, the surfaces of extracellular merozoites of equine, bovine, and rodent Babesia parasites express the molecule(s) with an affinity for heparin. Furthermore, their covering with heparin was suggested to interfere with subsequent invasion of the RBCs. It might also be possible that the HS-like GAG on RBCs acts as a host receptor for merozoite attachment. There has been no report of the presence of a molecule with an affinity for heparin on the surfaces of free merozoites of Plasmodium parasites. Further investigation of the growth inhibition caused by heparin will be helpful in providing an understanding of the process of merozoite invasion. In particular, it will be very important to identify the molecule(s) with an affinity for heparin.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and by grants from the Special Coordination Funds for Science and Technology from the Science and Technology Agency and from The 21st Century COE Program (A-1), Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Allred, D. R. 2001. Antigenic variation in babesiosis: is there more than one ‘why'? Microbes Infect. 3:481-491. [DOI] [PubMed] [Google Scholar]
- 2.Avarzed, A., I. Igarashi, T. Kanemaru, K. Hirumi, Y. Omata, A. Saito, T. Oyamada, H. Nagasawa, Y. Toyoda, and N. Suzuki. 1997. Improved in vitro cultivation of Babesia caballi. J. Vet. Med. Sci. 59:479-481. [DOI] [PubMed] [Google Scholar]
- 3.Barragan, A., V. Fernandez, Q. Chen, A. von Euler, M. Wahlgren, and D. Spillmann. 2000. The duffy-binding-like domain 1 of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a heparan sulfate ligand that requires 12 mers for binding. Blood 95:3594-3599. [PubMed] [Google Scholar]
- 4.Bork, S., N. Yokoyama, T. Matsuo, F. G. Claveria, K. Fujisaki, and I. Igarashi. 2003. Growth inhibitory effect of triclosan on equine and bovine Babesia parasites. Am. J. Trop. Med. Hyg. 68:334-340. [PubMed] [Google Scholar]
- 5.Bork, S., N. Yokoyama, T. Matsuo, F. G. Claveria, K. Fujisaki, and I. Igarashi. 2003. Clotrimazole, ketoconazole, and clodinafop-propagyl as potent growth inhibitors of equine Babesia parasites in in vitro culture. J. Parasitol. 89:604-606. [DOI] [PubMed] [Google Scholar]
- 6.Botero-Kleiven, S., V. Fernandez, J. Lindh, A. Richter-Dahlfors, A. von Euler, and M. Wahlgren. 2001. Receptor-mediated endocytosis in an apicomplexan parasite (Toxoplasma gondii). Exp. Parasitol. 98:134-144. [DOI] [PubMed] [Google Scholar]
- 7.Butcher, G. A., C. R. Parish, and W. B. Cowden. 1988. Inhibition of in vitro growth of Plasmodium falciparum by complex polysaccharides. Trans. R. Soc. Trop. Med. Hyg. 82:558-559. [DOI] [PubMed] [Google Scholar]
- 8.Carlson, J., H. P. Ekre, H. Helmby, J. Gysin, B. M. Greenwood, and M. Wahlgren. 1992. Disruption of Plasmodium falciparum erythrocyte rosettes by standard heparin and heparan devoid of anticoagulatory activity. Am. J. Trop. Med. Hyg. 46:595-602. [DOI] [PubMed] [Google Scholar]
- 9.Casu, B. 1985. Structure and biological activity of heparin. Adv. Carbohydr. Chem. Biochem. 43:51-134. [DOI] [PubMed] [Google Scholar]
- 10.Craig, A., and A. Scherf. 2001. Molecules on the surface of the Plasmodium falciparum infected erythrocyte and their role in malaria pathogenesis and immune evasion. Mol. Biochem. Parasitol. 115:129-143. [DOI] [PubMed] [Google Scholar]
- 11.Dennis, L. H., J. W. Eichelberger, Jr., M. E. Conrad, and A. E. Von Doenhoff, Jr. 1966. A coagulation defect and its treatment with heparin in Plasmodium knowlesi malaria in rhesus monkeys. Mil. Med. 131(Suppl. 11):7-10. [PubMed] [Google Scholar]
- 12.De Souza, B. J., and E. M. Riley. 2002. Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect. 4:291-300. [DOI] [PubMed] [Google Scholar]
- 13.Erp, E. E., R. D. Smith, M. Ristic, and B. M. Osorno. 1980. Optimization of the suspension culture method for in vitro cultivation of Babesia bovis. Am. J. Vet. Res. 41:2059-2062. [PubMed] [Google Scholar]
- 14.Hagiwara, K., M. Takahashi, T. Ichikawa, M. Tsuji, K. Ikuta, and C. Ishihara. 1997. Inhibitory effect of heparin on red blood cell invasion by Theileria sergenti merozoites. Int. J. Parasitol. 27:535-539. [DOI] [PubMed] [Google Scholar]
- 15.Hainaut, P. 2000. Traitement de la maladie thrombo-embolique. Louv. Med. 119:S200-S203. (In French.) [Google Scholar]
- 16.Hileman, R. E., J. R. Fromm, J. M. Weiler, and R. J. Linhardt. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20:156-167. [DOI] [PubMed] [Google Scholar]
- 17.Hines, S. A., G. H. Palmer, D. P. Jasmer, T. C. McGuire, and T. F. McElwain. 1992. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 55:85-94. [DOI] [PubMed] [Google Scholar]
- 18.Hirata, H., H. Ikadai, N. Yokoyama, X. Xuan, K. Fujisaki, N. Suzuki, T. Mikami, and I. Igarashi. 2002. Cloning of a truncated Babesia equi gene encoding an 82-kilodalton protein and its potential use in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 40:1470-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsh, J., W. G. van Akren, A. S. Gallus, C. T. Dollery, J. F. Cade, and W. L. Yung. 1976. Heparin kinetics in venous thrombosis and pulmonary embolism. Circulation 53:691-695. [DOI] [PubMed] [Google Scholar]
- 20.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotzel, I., C. E. Suarez, T. F. McElwain, and G. H. Palmer. 1997. Genetic variation in the dimorphic regions of RAP-1 genes and rap-1 loci of Babesia bigemina. Mol. Biochem. Parasitol. 2:479-489. [DOI] [PubMed] [Google Scholar]
- 22.Jacquet, A., L. Coulon, J. De Neve, V. Daminet, M. Haumont, L. Garcia, A. Bollen, M. Jurado, and R. Biemans. 2001. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol. Biochem. Parasitol. 116:35-44. [DOI] [PubMed] [Google Scholar]
- 23.Jaques, L. B. 1979. Heparin: an old drug with a new paradigm. Nature 206:528-533. [DOI] [PubMed] [Google Scholar]
- 24.Kulane, A., H. P. Ekre, P. Perlmann, L. Rombo, M. Wahlgren, and B. Wahlin. 1992. Effect of different fractions of heparin on Plasmodium falciparum merozoite invasion of red blood cells in vitro. Am. J. Trop. Med. Hyg. 46:589-594. [DOI] [PubMed] [Google Scholar]
- 25.Kuttler, K. L. 1988. World-wide impact of babesiosis, p. 2-22. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Inc., Boca Raton, Fla.
- 26.Linhardt, R. J., K. G. Rice, Y. S. Kim, J. Engelken, and J. M. Weiler. 1988. Homogeneous, structurally defined heparin oligosaccharides with low anticoagulant activity inhibit the generation of the amplification pathway C3 convertase. J. Biol. Chem. 263:13090-13096. [PubMed] [Google Scholar]
- 27.Mitchell, A. D. 1974. Recent experiences with severe and cerebral malaria. S. Afr. Med. J. 48:1353-1354. [PubMed] [Google Scholar]
- 28.Mulloy, B., and M. J. Forster. 2000. Confirmation and dynamics of heparin and heparan sulfate. Glycobiology 10:1147-1156. [DOI] [PubMed] [Google Scholar]
- 29.Mulloy, B., and R. J. Linhardt. 2001. Order out of complexity—protein structures that interact with heparin. Curr. Opin. Struct. Biol. 11:623-628. [DOI] [PubMed] [Google Scholar]
- 30.Munir, M., H. Tjandra, T. H. Rampengan, I. Mustadjab, and F. H. Wulur. 1980. Heparin in the treatment of cerebral malaria. Paediatr. Indones. 20:47-50. [PubMed] [Google Scholar]
- 31.Nagai, A., N. Yokoyama, T. Matsuo, S. Bork, H. Hirata, X. Xuan, Y. Zhu, F. G. Claveria, K. Fujisaki, and I. Igarashi. 2003. Growth inhibitory effects of artesunate, pyrimethamine, and pamaquine against Babesia equi and Babesia caballi in in vitro cultures. Antimicrob. Agents Chemother. 47:800-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishisaka, M., N. Yokoyama, X. Xuan, N. Inoue, H. Nagasawa, K. Fujisaki, T. Mikami, and I. Igarashi. 2001. Characterization of the gene encoding a protective antigen from Babesia microti identified it as the η subunit of chaperonin-containing T-complex protein 1. Int. J. Parasitol. 31:1673-1679. [DOI] [PubMed] [Google Scholar]
- 33.Pinzon-Ortiz, C., J. Friedman, J. Esko, and P. Sinnis. 2001. The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for plasmodium sporozoite attachment to target cells. J. Biol. Chem. 276:26784-26791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathore, D., and T. F. McCutchan. 2000. Role of cysteines in Plasmodium falciparum circumsporozoite protein: interactions with heparin can rejuvenate inactive protein mutants. Proc. Natl. Acad. Sci. USA 97:8530-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rostand, K. S., and J. D. Esko. 1997. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasisekharan, R., and G. Venkataraman. 2000. Heparin and heparan sulfate: biosynthesis, structure, and function. Curr. Opin. Chem. Biol. 4:626-631. [DOI] [PubMed] [Google Scholar]
- 37.Shum, D. K., C. Baylis, and J. E. Scott. 1984. A micropuncture and renal clearance study in the rat of the urinary excretion of heparin, chondroitin sulphate and metabolic breakdown products of connective tissue proteoglycans. Clin. Sci. 67:205-212. [DOI] [PubMed] [Google Scholar]
- 38.Sivaraman, C. A., and A. N. Rai Chowdhuri. 1983. Effect of heparin sodium on in vitro development of Plasmodium falciparum. Indian J. Exp. Biol. 5:247-250. [PubMed] [Google Scholar]
- 39.Smitskamp, H., and F. H. Wolthuis. 1971. New concepts in the treatment of malignant tertian malaria with cerebral involvement. Br. Med. J. 1:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trybala, E., B. Svennerholm, T. Bergstrom, S. Olofsson, S. Jeansson, and J. L. Goodman. 1993. Herpes simplex virus type 1-induced hemagglutination: glycoprotein C mediates virus binding to erythrocyte surface heparan sulfate. J. Virol. 67:1278-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turnbull, J., A. Powell, and S. Guimond. 2001. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11:75-82. [DOI] [PubMed] [Google Scholar]
- 42.Udomsangpetch, R., B. Wahlin, J. Carlson, K. Berzins, M. Torii, M. Aikawa, P. Perlmann, and M. Wahlgren. 1989. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J. Exp. Med. 169:1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogt, A. M., A. Barragan, Q. Chen, F. Kironde, D. Spillmann, and M. Wahlgren. 2003. Heparan sulfate on endothelial cells mediates the binding of Plasmodium falciparum-infected erythrocytes via the DBL1α domain of PfEMP1. Blood 101:2405-2411. [DOI] [PubMed] [Google Scholar]
- 44.Xiao, L., C. Yang, P. S. Patterson, V. Udhayakumar, and A. A. Lal. 1996. Sulfated polyanions inhibit invasion of erythrocytes by plasmodial merozoites and cytoadherence of endothelial cells to parasitized erythrocytes. Infect. Immun. 4:1373-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama, N., B. Suthisak, H. Hirata, T. Matsuo, N. Inoue, C. Sugimoto, and I. Igarashi. 2002. Cellular localization of Babesia bovis merozoite rhoptry-associated protein 1 and its erythrocyte-binding activity. Infect. Immun. 70:5822-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoyama, N., S. Bork, M. Nishisaka, H. Hirata, T. Matsuo, N. Inoue, X. Xuan, H. Suzuki, C. Sugimoto, and I. Igarashi. 2003. Roles of the Maltese cross form in the development of parasitemia and protection against Babesia microti infection in mice. Infect. Immun. 71:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zweygarth, E., M. C. Just, and D. T. de Waal. 1995. Continuous in vitro cultivation of erythrocytic stages of Babesia equi. Parasitol. Res. 81:355-358. [DOI] [PubMed] [Google Scholar]