Abstract
The in vitro and in vivo activities of a mixture of six oleane triterpene saponins, recovered from the methanolic extract of the leaves of the Vietnamese plant Maesa balansae (PX-6518), were evaluated against drug-sensitive visceral Leishmania strains. The in vitro 50% inhibitory concentration (IC50) against intracellular Leishmania infantum amastigotes was 0.04 μg/ml. The cytotoxic concentrations causing 50% cell death (CC50s) were about 1 μg/ml in murine macrophage host cells and >32 μg/ml in human fibroblasts (MRC-5 cell line). Evaluation in the Leishmania donovani BALB/c mouse model indicated that a single subcutaneous administration of 0.4 mg/kg at 1 day after infection reduced liver amastigote burdens by about 95% in all treated animals. If treatment was delayed until 14 days after infection, a dose of 1.6 mg/kg of body weight was required to maintain the same level of activity. Single 250-mg/kg doses of sodium stibogluconate (Pentostam) 1 and 14 days after infection produced comparable efficacies. A single dose of PX-6518 at 2.5 mg/kg administered 5 days before infection was still 100% effective in preventing liver infection, suggesting a particularly long residual action. Spleen and bone marrow could not be cleared by PX-6518 nor sodium stibogluconate. PX-6518 did not show activity after oral dosing at up to 200 mg/kg for 5 days. This study concludes that triterpenoid saponins from M. balansae show promising in vitro and in vivo antileishmanial potential and can be considered as new lead structures in the search for novel antileishmanial drugs.
Leishmaniasis is a growing health problem in many parts of the world, with about 350 million people living in areas of disease endemicity and about 2 million new cases each year (10). As for the other neglected diseases of poverty (28), treatment options for leishmaniases are limited and rely on pentavalent antimonials as first-line and amphotericin B and pentamidine as second-line drugs (8, 13). However, the excessively high cost of liposomal amphotericin B precludes any practical use (41). An important step forward was the recent approval of miltefosine as the first oral treatment for visceral leishmaniasis (37).
Given the prospect that antileishmanial vaccines may not become available in the near future (14), the search for better drugs should be continued, whereby natural products may offer an unlimited source of chemical diversity for identification of new drug templates (1, 5, 11).
During a random drug screening program, the methanolic extract of the leaves of the Vietnamese plant Maesa balansae Mez. (Myrsinaceae) was found to possess strong antileishmanial potential (23), and pentacyclic triterpene saponins were identified as the active constituents (12). Saponins have been demonstrated in a large number of plant species, and very different biological activities have been described (16). Antileishmanial activity has been reported for Hedera (9, 31), Dracaena (26), and Yucca (29) saponins, but their value as drug candidates cannot be fully assessed, since in vivo data from animal models were not generated.
The studies reported here give a first description of the in vitro and in vivo antileishmanial properties of the purified methanolic extract (PX-6518) of the leaves of M. balansae. A few comparative evaluations are included with extracts from other available Maesa species, since analogue saponins have been described to occur in the Maesa plant family (19, 20, 34).
MATERIALS AND METHODS
Plant material and extraction.
Leaves of the Maesa species M. balansae, M. sinensis, M. tomentella, and M. crassifolia were collected in the Thai Nguen province in Vietnam (voucher specimens deposited at the Institute of Ecology and Biological Resources at National Center for Natural Sciences and Technology, Hanoi, Vietnam). Leaves of M. lanceolata were collected in the Karura Forest, Nairobi, Kenya (voucher specimen deposited at the Department of Botany, UG, Ghent, Belgium). The leaves were dried, ground, and extracted in a percolator in methanol-water (9:1). The crude extracts were filtered, evaporated to dryness in a rotating evaporator, and stored at −20°C until further use. Successive plant collections (n = 5) and bio-guided fractionation and purification were performed for M. balansae only (Table 1).
TABLE 1.
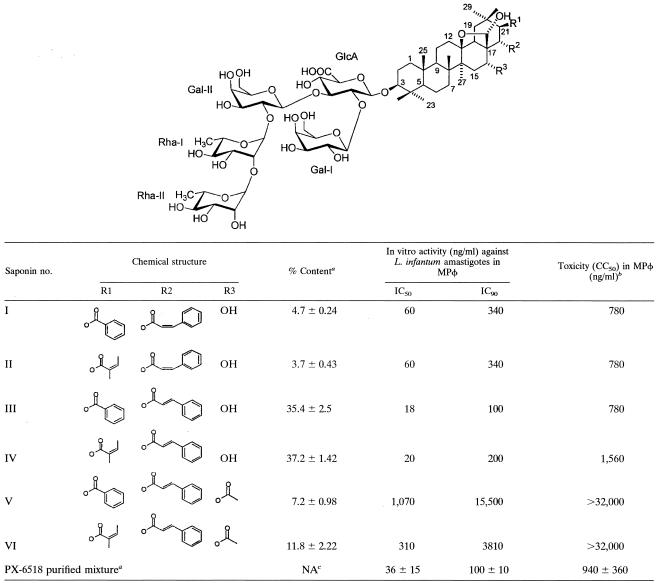
Chemical structure and antileishmanial activity of the 6 triterpene saponins in the PX-6518 mixture, isolated from the leaves of M. balansae
Results represent the mean ± standard deviation of five separate batches collected in the same geographical area and during the same season.
No cytotoxicity on MRC-5 cells (CC50, >32,000 ng/ml).
NA, not applicable.
Purification and identification of the active constituents.
The active constituents were purified and identified in a single large-scale production batch as described by Germonprez et al. (12). Briefly, powdered leaves (3 kg) were subsequently extracted with chloroform (10 × 5 liters) and methanol-water (9:1) (10 × 5 liters). The methanol extract was filtered, evaporated to dryness under reduced pressure at 40°C, and partitioned between n-BuOH (saturated with water) (1 liter) and water (1 liter). The aqueous fraction was extracted six times with n-BuOH (1 liter) (saturated with water). The combined n-BuOH soluble fractions were evaporated to dryness, and the residue was suspended in acetone (1 liter) and stirred for 4 h. The residue after filtration produced the purified PX-6518 mixture. All in vivo experiments were carried out with the latter. Identification of the individual saponins (referred to as maesabalides) involved preparative high-performance liquid chromatography on C18 BDS (Hypersil BDS, 8 μm, 200 g), using a column with axial compression (50-mm inside diameter, packed at 60 bars). 1H, 13C, and two-dimensional nuclear magnetic resonance (NMR) spectra were recorded with a Bruker AVANCE-400 spectrometer. The NMR data of the saponins were measured in pyridine-D. The negative-ion-mode fast atom bombardment mass spectrometry spectra were recorded on a Micromass VG70SEQ instrument with glycerol as a liquid matrix.
Compounds.
Plant extract solutions for biological testing and the reference drugs were prepared in 100% dimethyl sulfoxide (DMSO; Sigma) at 20 mg/ml. Reference drugs included sodium stibogluconate (Pentostam; GSK), amphotericin B (Fungizone; Squib), pentamidine isethionate (Sigma), allopurinol (Sigma), ketoconazole (Sigma), and trifluralin (Sigma). The latter compound was added because of its claimed antileishmanial potential (6, 15).
Parasites.
The visceral Leishmania species were reference strains known to be sensitive to the available antileishmania reference drugs. Leishmania donovani (MHOM/ET/67/L82) was used for the in vivo experiments, and Leishmania infantum [MHOM/MA(BE)/67] was used for the in vitro assays. Both strains were obtained as cryostabilates from the Institute of Tropical Medicine in Antwerp, Belgium, and further maintained in the laboratory by serial passage in Golden hamsters (Mesocrisetus auratus). Amastigotes for in vitro and in vivo infection were harvested from the spleens of donor hamsters with 6-week-established infections. The spleen was weighed, and a smear impression was made on a microscopic glass for Giemsa staining and microscopic counting. The organ was homogenized in 10 ml of cold Hanks balanced salt solution (Gibco) in a tissue grinder, and the amastigotes were separated by two successive centrifugation cycles. The total recovered number of amastigotes was determined according to the method of Stauber (35): Ntotal = [spleen weight (g)] × [n amastigotes/cell × 2.108].
Amastigote culture and drug sensitivity assay.
The in vitro sensitivity of amastigotes to the test compounds was determined in primary mouse peritoneal macrophages (MPφ) (24). Briefly, MPφ (NMRI mouse; Charles River, Sulzfeld, Germany) were induced by intraperitoneal (i.p.) administration of 2 ml of 2% potato starch in physiological saline and harvested about 24 to 48 h later in cold RPMI 1640 medium (Gibco) supplemented with penicillin-streptomycin (P/S) (Gibco). For the drug sensitivity assay, the 20-mg/ml compound stock solutions were fourfold serially diluted in DMSO, followed by an additional dilution in culture medium (RPMI 1640 supplemented with 20 mM l-glutamine, 16.5 mM NaHC03, 5% heat-inactivated fetal calf serum [FCSi], and 2% P/S solution). The highest in-test drug concentration was 32 μg/ml. Assays were performed in quadruplet in 96-well microtiter tissue culture plates (Nunc), with each well containing the test compound dilutions together with 3 × 104 MPφ and 3 × 105 amastigotes per well. After 5 days of incubation at 37°C in 5% CO2-air, intracellular amastigote burdens (and cytotoxicity) were microscopically assessed after Giemsa staining. The results are expressed as percent reduction of parasite burden compared to the level in untreated control wells, and the 50% inhibitory concentration (IC50) was determined from the concentration-response curves. The selectivity index (SI) was calculated according to the 50% cytotoxic concentration (CC50) on MPφ.
Cytotoxicity against mammalian cells.
Diploid human lung fibroblasts (MRC-5; BioWittaker) were cultured in minimum essential medium (MEM; Gibco), supplemented with 20 mM l-glutamine, 16.5 mM NaHC03, 5% FCSi, and 2% P/S solution. The assay was performed in 96-well tissue culture plates, with each well containing the test compound dilutions together with 5 × 103 MRC-5 cells per well. After 7 days of incubation at 37°C in 5% CO2-air, cell proliferation or viability was assessed after addition of Alamar Blue (25) (5 μl of a 1/10 solution per well), and fluorescence was measured (Fluostar Optima [550-nm excitation, 590-nm emission]) after 4 h of incubation at 37°C. The results are expressed as percent reduction in cell viability compared to untreated control wells, and a CC50 was determined.
Activity against other microorganisms.
In view of the wide range of biological activities that have been described for saponins in general (16), a more extended antimicrobial in vitro profiling was performed for PX-6518 and included a number of viruses, bacteria, yeasts, fungi, and parasites (Table 2). The highest in-test concentration was 32 μg/ml, and a test compound was considered inactive if the IC50 was >32 μg/ml. Published standard drug screening techniques were used. The antibacterial, antiyeast, and antifungal testing systems were broth based with a turbidity endpoint reading (30, 40). The activity against human immunodeficiency virus type 1 (HIV-1) was evaluated in exponentially growing HIV-infected MT4 cells with cell viability measurement after addition of MTT (27). The fluorimetric assay for neuraminidase activity was used for evaluation of activity against human influenza virus (3). The antimalarial activity was determined against chloroquine-sensitive Plasmodium falciparum (Ghana strain) in human erythrocyte cultures after 48 h of incubation and addition of the Malstat reagent (18). The test method for Trypanosoma cruzi was identical to the one described above for Leishmania. Bloodstream forms of T. brucei were cultivated in HMI-9 medium, and parasite multiplication was measured colorimetrically after addition of MTT (18). Toxoplasma gondii (RH strain) was grown in MRC-5 fibroblast cultures, and tachyzoite burdens were microscopically determined after Giemsa staining (33). For evaluation of activity against the sheep nematode Trichostrongylus colubriformis, fresh unembryonated worm eggs were incubated at room temperature in water and supplemented with MEM culture medium and Escherichia coli extract, and the number of viable L3 larvae was microscopically determined after 4 days of incubation (38).
TABLE 2.
In vitro antimicrobial profiling of PX-6518a
| Category | Microorganism(s) |
|---|---|
| Viruses | Influenza virus, HIV |
| Bacteria | Escherichia coli, Salmonella paratyphi, Streptococcus pyogenes, Staphylococcus aureus, Pseudomonas aeruginosa, Mycobacterium smegmatis |
| Yeasts | Candida albicans, Cryptococcus neoformans |
| Fungi | Aspergillus niger, Microsporum canis, Trichophyton rubrum |
| Protozoa | Leishmania promastigotes, Plasmodium falciparum, Trypanosoma brucei, Trypanosoma cruzi, Toxoplasma gondii |
| Nematodes | Trichostrongylus colubriformis (larvae) |
IC50, >32 μg/ml.
Promastigote transformation assay.
A promastigote transformation assay was used to trace viable amastigotes in target tissues (spleen and bone marrow) of treated animals. Small organ pieces (1 by 1 mm) were incubated for 7 days at 26°C before being scored microscopically for the presence of promastigotes. The growth medium consisted of MEM, supplemented with 7.5% sodium bicarbonate, 2% glutamine, 200 mM 0.3% [wt/vol] d-glucose, 1% HEPES, 4% adenine, 0.3% folic acid, 0.2% d-biotin, and 2% P/S. After adjustment to pH 7.5 with NaOH, the solution was filter sterilized (0.22-μm pore diameter), and 10% FCSi and 0.5% bovine hemin were added just before use.
In vivo evaluation of PX-6518 in the L. donovani mouse model.
Male BALB/c mice (Charles River) were infected with 107 amastigotes of L. donovani in the lateral tail vein on day 0 and randomly allocated into groups of six animals each. The amastigotes for infection were obtained from the spleen of a heavily infected donor hamster. PX-6518 was formulated in isotonic saline at 2 mg/ml and filter sterilized through a 0.22-μm-pore filter (Millipore). By further dilution in saline, formulations for injection were prepared at 1, 0.5, 0.25, and 0.125 mg/ml. Sodium stibogluconate was diluted in physiological saline to prepare a 50-mg/ml stock solution. Amphotericin B as a soluble powder was dissolved in saline to prepare a 2-mg/ml stock solution. Fresh solutions were made before each experiment. Depending on the specific experimental objective, the test compounds were administered as either five daily oral or i.p. doses (Table 3, primary in vivo screening) or as a single subcutaneous (s.c.) dose (Tables 4, 5, and 6, secondary in vivo profiling).
TABLE 3.
In vitro and in vivo primary evaluation against visceral Leishmania species
| Crude extract or reference drug | In vitro activity (μg/ml) against:
|
In vivo activity against L. donovani (mean % reduction of liver amastigote burden)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
L. infantum
|
MPφ (CC50) | MRC-5 (CC50) | Oral (200 mg/kg) | i.p.
|
|||||
| IC50 | IC90 | 40 mg/kg | 20 mg/kg | 10 mg/kg | 5 mg/kg | ||||
| M. balansae (PX-6518) | 0.4 | 1.2 | 12 | >32 | <50 | 100 | 100 | 100 | 100 |
| Stibogluconate | 5.6 | 25 | >32 | >32 | ND | 98 | ND | 95 | ND |
| Amphotericin B | 0.1 | 0.6 | 12 | >32 | ND | T | T | ND | |
| Pentamidine | >32 | >32 | >32 | >32 | ND | <50 | |||
| Ketoconazole | >32 | >32 | >32 | >32 | <50 | <50 | |||
| Trifluralin | >32 | >32 | >32 | >32 | ND | <50 | |||
| Allopurinol | 8.8 | 32 | >32 | >32 | ND | <50 | |||
| M. lanceolata | 0.2 | 0.4 | 1.5 | >32 | <50 | T | 100 | 100 | NA |
| M. sinensis | 0.1 | 0.5 | 5 | >32 | ND | 99 | NA | ||
| M. crassifolia | 1.2 | 3.5 | 16 | >32 | ND | NA | |||
| M. tomentella | >32 | >32 | >32 | >32 | ND | NA | |||
L. donovani BALB/c mouse model, three animals per group with daily dosing for 5 consecutive days. T, occurrence of severe toxicity (death or adverse clinical symptoms) in treated animals. NA, no compound available for testing; ND, not done.
TABLE 4.
Dose titration of PX-6518 against L. donovani in BALB/c mice
| Dosing group (1 × s.c.)a | % Body wt gain (day 0-7)b |
L. donovani parasite burden in target organc
|
|||
|---|---|---|---|---|---|
| Liver amastigote burden
|
No. of animals with positive:
|
||||
| Mean ± SD LDU | % Reduction | Spleen | Bone marrow | ||
| Placebo | 2.7 | 1,425 ± 131 | 6 | 6 | |
| PX-6518 | |||||
| 5 mg/kg | 3.2 | 0 ± 0 | 100 | 6 | 0 |
| 2.5 mg/kg | 3.5 | 0 ± 0 | 100 | 6 | 1 |
| 1.25 mg/kg | 4.5 | 2 ± 2 | 99.9 | 6 | 6 |
| 0.8 mg/kg | 0.4 | 2 ± 3 | 99.9 | 5 | 6 |
| 0.6 mg/kg | 7.0 | 3 ± 1 | 99.8 | 6 | 6 |
| 0.4 mg/kg | 0.0 | 69 ± 121 | 95.2 | 6 | 6 |
| 0.2 mg/kg | 1.3 | 421 ± 104 | 70.5 | 6 | 6 |
| 0.1 mg/kg | 2.2 | 1,102 ± 207 | 22.7 | 6 | 6 |
| Amphotericin (10 mg/kg) | 3.7 | 1,485 ± 152 | 0 | 6 | 6 |
| Stibogluconate (250 mg/kg) | 5.1 | 4 ± 4 | 99.7 | 6 | 6 |
One day postinfection.
Mean body weight at start, 22.3 ± 0.56 g (6 animals/experimental group).
Autopsy performed at 7 days posttreatment.
TABLE 5.
Five-day residual and curative activity of PX-6518 against L. donovani in BALB/c micea
| Dosing group (1 × s.c.) | Timing (day postinfection) |
L. donovani parasite burden in target organb
|
|||
|---|---|---|---|---|---|
| Liver amastigote burden
|
No. of animals with positive:
|
||||
| Mean ± SD LDU | % Reduction | Spleen | Bone marrow | ||
| Placebo | 1,170 ± 251 | 6 | 6 | ||
| PX-6518 (2.5 mg/kg) | −5 | 0.4 ± 0.6 | 100 | 5 | 4 |
| −3 | 0 ± 0 | 100 | 6 | 3 | |
| −1 | 0 ± 0 | 100 | 6 | 1 | |
| 1 | 0 ± 0 | 100 | 5 | 1 | |
| 3 | 0 ± 0 | 100 | 6 | 5 | |
| 5 | 22 ± 10 | 98.5 | 6 | 5 | |
The percent body weight gain was not determined.
Autopsy performed at 7 days postinfection.
TABLE 6.
Activity of PX-6518 against established (2 week) infections of L. donovani in BALB/c mice
| Dosing group (1 × s.c.)a | % Body wt gain (day 0-7)b |
L. donovani parasite burden in target organc
|
|||
|---|---|---|---|---|---|
| Liver amastigote burden
|
No. of animals with positive:
|
||||
| Mean ± SD LDU | % Reduction | Spleen | Bone marrow | ||
| Placebo | 4.3 | 1,339 ± 262 | 6 | 6 | |
| PX-6518 | |||||
| 1.6 mg/kg | 3.9 | 37 ± 35 | 97.2 | 6 | 5 |
| 0.8 mg/kg | 4.4 | 350 ± 149 | 73.9 | 6 | 6 |
| 0.4 mg/kg | 7.8 | 876 ± 191 | 34.6 | 6 | 6 |
| Stibogluconate (250 mg/kg) | 13.8 | 135 ± 86 | 89.9 | 6 | 6 |
Fourteen days postinfection.
Mean body weight at start, 22.0 ± 0.94 g (groups of six animals).
Autopsy performed at 7 days posttreatment.
Three separate profiling experiments with PX-6518 were performed with mice. In experiment 1, a dose titration trial was used to establish the lowest active dose (LAD) (Table 4). Experiment 2 involved evaluation of residual and early curative activity (Table 5). Finally, experiment 3 examined the curative potential in established visceral leishmania infections (Table 6).
Experimental details can be found in the respective tables. On day 0 of each experiment, animals were randomized into the various treatment groups and intravenously infected with L. donovani amastigotes. At the start and at termination of experiments 1 and 3, the mice were weighed to monitor general health status. The animals were not weighed individually but as a single group to minimize manipulation stress. Autopsy was performed at 7 days posttreatment for determination of amastigote burdens in the target organs liver, spleen, and bone marrow. The livers of individual animals were weighed, and impression smears were stained with 10% Giemsa solution for microscopic enumeration of the number of amastigotes per liver cell. The results are expressed as Leishman Donovan units (LDU [mean number of amastigotes per liver cell per milligram of liver]) (4), and a reduction of at least 80% was adopted as a criterion for efficacy. Because of a very low level of infection in the spleen and the bone marrow, making microscopic evaluation virtually impossible, the presence of viable amastigotes in these organs was assessed qualitatively by incubating a small piece of tissue into promastigote medium at 26°C for at least 1 week (promastigote transformation test).
RESULTS
Extraction and identification of the active constitutents.
The extraction yield for the purified PX-6518 mixture was about 3.3% (100 g from of 3 kg of dried leaves). Structural analysis (Table 1) revealed that PX-6518 consisted of six closely related oleane triterpene saponins, all with a hemiacetal moiety between C13 and C17. Other major components were not present. Maesabalides III and IV demonstrated the highest antileishmanial potency (IC50 of about 20 ng/ml) and contributed both for about 73% of the total saponin mass. Their respective cis-isomers (maesabalides I and II) were also highly active (IC50 = 40 ng/ml) and contributed to about 8% of the mixture. The acetoxy derivatives (maesabalides V and VI) were far less potent. Successive plant collections (n = 5) revealed good consistency with the regard to the relative content of the different maesabalides.
In vitro antimicrobial profiling.
PX-6518 was devoid of inhibitory effects (IC50, >32 μg/ml) on several viruses (influenza virus and HIV), bacteria (Staphylococcus, Streptococcus, Pseudomonas, Escherichia, Salmonella, and Mycobacterium), yeasts (Candida and Cryptococcus), fungi (Aspergillus, Microsporum, and Trichophyton), and parasites (Plasmodium, Trypanosoma, and Trichostrongylus), suggesting a highly specific primary pharmacological action. It is important to note the total lack of activity against Leishmania promastigotes (Table 2).
Primary in vitro and in vivo action against visceral leishmania.
The laboratory strain of L. infantum was used in the primary in vitro screening, and the IC50 and IC90s were determined for PX-6518, four other Maesa extracts, and six reference compounds (Table 3). PX-6518 was found to be highly active, with an IC50 of 0.4 μg/ml. Most of the reference drugs performed poorly (IC50, >32 μg/ml), except for amphotericin (IC50 = 0.1 μg/ml), sodium stibogluconate (IC50 = 5.5 μg/ml), and allopurinol (IC50 = 8.8 μg/ml). No obvious cytotoxicity occurred on MRC-5 cells (CC50, >32 μg/ml), and only marginal toxicity was noted on MPφ for PX-6518 and amphotericin B (CC50 = 12 μg/ml). After establishing the primary activity of PX-6518, a comparative in vitro profiling was added on extracts of a few other Maesa species to ascertain that M. balansae would be the best selection for potential further drug evaluation among the available alternatives. It was clearly shown that all species tested, except M. tomentella, contain constituents with strong antileishmanial potential (IC50, <2 μg/ml). To refine the evaluation and to include information on drug tolerance, a small-scale in vivo confirmation test was performed using the L. donovani BALB/c mouse model. To ensure maximal drug exposure, the test substances were administered as five daily oral or i.p. doses at up to 200 or 40 mg/kg of body weight, respectively. After oral dosing at the maximal attainable dose level of 5× 200 mg/kg, no antileishmanial activity could be demonstrated for M. balansae and M. lanceolata. The other Maesa extracts were therefore not evaluated orally, and all subsequent experiments included parenteral dosing (i.p. or s.c.). Within the series tested, the M. sinensis extract was the most potent extract in vitro (IC50 = 0.1 μg/ml, SI = 50), but it failed to induce complete cure in vivo after a single i.p. dose of 40 mg/kg (99% efficacy). The extract from M. lanceolata was also highly active (IC50 = 0.2 μg/ml and fully effective at 10 mg/kg), but was not selected as candidate because of the high level of cytotoxicity (SI = 8) and the fact that toxic symptoms were observed at 40 mg/kg. M. crassifolia was not retained because of its overall lower potency. Consequently, M. balansae remained the primary choice for further follow-up because of its better overall activity profile (IC50 = 0.4 μg/ml, SI = 30, absence of in vivo toxicity at 40 mg/kg, and full efficacy down to 5 × 5 mg/kg i.p.).
In vivo single-dose titration of PX-6518 against L. donovani.
Compared to the primary in vivo confirmation, PX-6518 was now administered as a single s.c. dose, with dose levels ranging between 0.1 and 5 mg/kg (Table 4). During the 7-day experimental period, no adverse effects on body weight (0 to 7% gain) were noted, endorsing a minimal impact of the infection and/or the treatment on the overall health status of the animals. In the placebo-treated infected controls and the amphotericin B-treated animals, comparably high levels of infection were present in the liver (LDU = about 1,450). At necropsy of the latter group, yellow s.c. deposits were present at the injection site, suggesting nonabsorption and explaining the lack of activity. In the PX-6518-treated animals, good control of infection was obtained at ≥0.4 mg/kg, with very few amastigotes being recovered from the liver (>95% reduction). Lowering the dose of PX-6518 resulted in a dose-dependent increase in liver amastigote burdens, with almost no efficacy left at 0.1 mg/kg (22.7% reduction). The spleen remained weakly positive at all doses, and the bone marrow could only be cleared at the highest dose levels (≥2.5 mg/kg). Accepting 80% reduction of liver amastigote burdens as the target for efficacy, the lowest active dose of PX-6518 becomes 0.4 mg/kg. Amphotericin B at 10 mg/kg was totally ineffective, and sodium stibogluconate at 250 mg/kg was almost fully effective (99.7% reduction).
Five-day residual and curative activities of PX-6518 against L. donovani.
In the placebo-treated infected controls, high burdens of amastigotes were present in the liver (LDU = 1,170 ± 251), spleen, and bone marrow. After a single s.c. administration of PX-6518 at 2.5 mg/kg, administered either on day −5 or day 3, no amastigotes could be recovered from the liver. When the treatment was delayed until day 5, very low levels of infection could be demonstrated (LDU in liver = 22 ± 10). The presence of infection in the spleen and bone marrow was erratic in all groups, with the spleen being almost consistently, but weakly positive. These results indicate that a single 2.5-mg/kg dose of PX-6518 has a residual activity period or a prophylactic potential of about 5 days (>98.5% reduction of liver amastigote burdens). It is important to note that this reduction is already obtained within 2 days after dosing (Table 5).
Activity of PX-6518 against established infections of L. donovani.
The experiment described above (Table 5) indicated a slight decrease in activity if PX-6518 treatment is delayed until day 5 after infection. This dose titration experiment evaluated the efficacy against fully (14 day) established infections with 0.4 mg/kg as the lowest dose level (Table 6). During the experiment, no marked effects on body weight gain were noted in any of the experimental groups, except in the sodium stibogluconate group, which had a slightly higher gain (14% versus 4 to 8% in the other groups). In the PX-6518-treated animals, almost no control of infection was obtained after dosing at 0.4 mg/kg (34.6% reduction). At higher doses, a dose-related inhibition was noted, and at 1.6 mg/kg, the inhibition was almost complete (97.2%), with low burdens of amastigotes in the liver, but with none of the animals totally cleared. The spleen and the bone marrow remained positive in all groups. PX-6518 at 0.8 mg/kg produced 73.9% reduction and could be considered roughly comparable to sodium stibogluconate at 250 mg/kg (89.9% reduction). These data suggest that 1.6 mg/kg or possibly 0.8 mg/kg could be regarded as the lowest active dose of PX-6518 for curative treatment 2 weeks after infection.
DISCUSSION
Efforts to find new chemotherapeutics for leishmaniasis have been ongoing for decades, and numerous potentially new drug candidates and/or putative drug targets have indeed been proposed (8). Except for the phospholipid miltefosine, recently approved in India for oral treatment of visceral leishmaniasis (37), no new drugs have become available to the patient. Therefore, continuation of drug screening efforts must remain a priority, partly also because resistance to first-line drugs is growing (36).
The potent in vitro activity of PX-6518 against intracellular L. infantum amastigotes (IC50 = 0.4 μg/ml) in the absence of obvious cytotoxicity on macrophage host cells (CC50 = 12 μg/ml) and MRC-5 cells (CC50 >32 μg/ml) formed the basis for advanced exploration of this promising lead activity. Chemical analytical work showed that its action is linked to six closely related oleane triterpene saponins (12). The most potent maesabalides III and IV (IC50 = 20 ng/ml) contributed to about 73% in the mixture, and acceptable consistency among successive batches was obtained (Table 1). However, potency should never be used as the sole criterion for lead selection, since selectivity is another, probably more relevant characteristic for defining lead candidates. Cytotoxicity on MPφ (SI = 30) was lower than for MRC-5 cells (SI > 80), suggesting that the action of PX-6518 may be influenced by selective intracellular accumulation in phagocytic cells. This phenomenon has already been demonstrated with antimonial preparations (32) and forms the basis for the enhanced activity of liposomal amphotericin B (21). The pharmacological selectivity evaluation against an extended set of bacteria, yeasts, fungi, viruses, and parasites (Table 2) demonstrated that PX-6518 is devoid of any relevant inhibitory activity (IC50, >32 μg/ml). This high selectivity may indicate that its action is not related to an aspecific cell or membrane toxic mechanism, believed to be responsible for most of the antimicrobial (antiviral, antibacterial, antifungal, piscicidal, and larvicidal) activities described (16). Very little is known about the antileishmanial potential of saponins. In vitro activity against promastigotes and/or intracellular amastigotes was demonstrated for Hedera (9, 31), Dracaena (26), and Yucca (29), but selectivity evaluations and in vivo confirmation studies with these extracts were unfortunately not performed. In the present study, extracts from different Maesa species were evaluated with the anticipation that they contain structural analogue saponins, as already documented for M. lanceolata (34) and Maesa japonica (19). All except one had potent antileishmanial activity: however, with quite variable selectivity (Table 3). Structural information is not yet available for M. sinensis, M. crassifolia, and M. tomentella, and further insight into the structure-activity relationship could be gained if, for example, M. tenera (20), M. ramentacea (39), M. japonica (19), and M. laxiflora (17) could be included in a comparative in vitro evaluation.
This report is the first to describe the antileishmania activity of Maesa saponins in the L. donovani BALB/c mouse model: extracts from M. balansae (PX-6518), M. lanceolata, and M. sinensis were all effective, but at different dose levels and with various degrees of toxicity (Table 3). PX-6518 was retained as the most promising candidate. Establishment of infection could be controlled after a single s.c. dose at 0.4 mg/kg if administered 1 day after infection (Table 4). If treatment was delayed until 14 days after infection, 1.6 mg/kg was required (Table 6). It is important to note that a single dose of PX-6518 at 2.5 mg/kg 5 days before infection was still 100% effective at preventing liver infection (Table 5), suggesting a slow clearance from the body, either by a low level of metabolic elimination or by a high degree of drug targeting in the macrophage host cell. Pilot in vitro metabolism data with human, dog, and rat microsomes (22) indeed suggest a high metabolic stability, but no experimental evidence is yet available to support the drug-targeting hypothesis. Another important observation was that both PX-6518 and sodium stibogluconate were not capable of preventing animals from becoming infected, as shown by the presence of low numbers of amastigotes in either spleen and/or bone marrow in all treated groups. This has already been documented for sodium stibogluconate, with which clearance of infection from spleen and bone marrow was much more difficult to achieve than from the liver (7). It is reasonable to assume that clearance of the infection from these two target organs may not be achieved within the short time frame of the present experiments (i.e., within 7 days after treatment). For example, studies with sodium stibogluconate indicated that clearance can be obtained within 50 days with a nonionic vesicular formulation but not with the free drug (2).
In conclusion, this study provides valuable data that PX-6518 exhibits interesting antileishmanial properties with minimal toxicity for mammalian cells and the laboratory rodent host. Its unique and potent activity profile represents an important new asset in the search for novel antileishmanial drugs, and the “proof-of-concept” that saponins possess strong in vivo antileishmania potential should be further explored. Before PX-6518 can be promoted into a formal drug development program, additional studies on cutaneous Leishmania species, pharmacological profiling of the individual maesabalide saponins, mechanism-of-action studies, and toxicity evaluations will be required. These factors are currently being investigated.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) and from the Belgian Directorate for Overseas Development (DGOS). Cos Paul is a postdoctoral researcher of the Fund for Scientific Research (FWO-Flanders, Belgium).
REFERENCES
- 1.Akendengue, B., E. Ngou-Milama, A. Laurens, and R. Hocquemiller. 1999. Recent advances in the fight against leishmaniasis with natural products. Parasite 6:3-8. [DOI] [PubMed] [Google Scholar]
- 2.Banduwardene, R., A. B. Mullen, and K. C. Carter. 1997. Immune responses of Leishmania donovani infected BALB/c mice following treatment with free and vesicular sodium stibogluconate formulations. Int. J. Immunopharmacol. 19:195-203. [DOI] [PubMed] [Google Scholar]
- 3.Bantia, S., C. D. Parker, S. L. Ananth, L. L. Horn, K. Andries, P. Chand, P. L. Kotian, A. Dehghani, Y. El-Kattan, T. Lin, T. L. Hutchison, J. A. Montgomery, D. L. Kellog, and Y. S. Babu. 2001. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley, D. J. 1977. Regulation of Leishmania populations within the host. II. Genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin. Exp. Immunol. 30:130-140. [PMC free article] [PubMed] [Google Scholar]
- 5.Camacho, M. R., S. L. Croft, and J. D. Phillipson. 2000. Natural products as sources of antiprotozoal drugs. Curr. Opin. Anti-Infect. Investig. Drugs 2:47-62. [Google Scholar]
- 6.Chan, M. M., and D. Fong. 1990. Inhibition of leishmanias but not host macrophages by the antitubulin herbicide trifluralin. Science 249:924-926. [DOI] [PubMed] [Google Scholar]
- 7.Collins, M., K. C. Carter, and A. J. Baillie. 1992. Visceral leishmaniasis in the BALB/c mouse: antimony tissue disposition and parasite suppression after the administration of free stibogluconate. Ann. Trop. Med. Parasitol. 86:35-40. [DOI] [PubMed] [Google Scholar]
- 8.Croft, S. L., and V. Yardley. 2002. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 8:319-342. [DOI] [PubMed] [Google Scholar]
- 9.Delmas, F., C. Di Giorgio, R. Elias, M. Gasquet, N. Azas, V. Mshvildadze, G. Dekanosidze, E. Kemertelidze, and P. Timon-David. 2000. Antileishmanial activity of three saponins isolated from ivy, alpha-hederin, beta-hederin and hederacolchiside A1, as compared to their action on mammalian cells cultured in vitro. Planta Med. 66:343-347. [DOI] [PubMed] [Google Scholar]
- 10.Desjeux, P. 2001. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 95:239-243. [DOI] [PubMed] [Google Scholar]
- 11.Fournet, A., and V. Munoz. 2002. Natural products as trypanocidal, antileishmanial and antimalarial drugs. Curr. Top. Med. Chem. 2:1215-1237. [DOI] [PubMed] [Google Scholar]
- 12.Germonprez, N., L. Van Puyvelde, L. Maes, M. Van Tri, and N. De Kimpe. New pentacyclic triterpene saponins with strong anti-leishmanial activity from the leaves of Maesa balansae. Tetrahedron, in press.
- 13.Guerin, P., J. P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 14.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havens, C. G., N. Bryant, L. Asher, L. Lamoreaux, S. Perfetto, J. J. Brendle, and K. A. Werbovetz. 2000. Cellular effects of leishmanial tubulin inhibitors on L. donovani. Mol. Biochem. Parasitol. 110:223-236. [DOI] [PubMed] [Google Scholar]
- 16.Hostettmann, K., and A. Marston. 1995. Chemistry and pharmacology of natural products. Saponins, p. 239-284. Cambridge University Press, Cambridge, United Kingdom.
- 17.Jiang, Z., J. F. Gallard, M. T. Adeline, V. Dumontet, M. V. Tri, T. Sevenet, and M. Pais. 1999. Six triterpenoid saponins from Maesa laxiflora. J. Nat. Prod. 62:873-876. [DOI] [PubMed] [Google Scholar]
- 18.Jonckers, T. H., S. van Miert, K. Cimanga, C. Bailly, P. Colson, M. C. De Pauw-Gillet, H. van den Heuvel, M. Claeys, F. Lemiere, E. L. Esmans, J. Rozenski, L. Quirijnen, L. Maes, R. Dommisse, G. L. Lemiere, A. Vlietinck, and L. Pieters. 2002. Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J. Med. Chem. 45:3497-3508. [DOI] [PubMed] [Google Scholar]
- 19.Koike, K., M. Kudo, Z. Jia, T. Nikaido, Y. Ide, and T. Sakura. 1999. New triterpenoid saponins from Maesa japonica. J. Nat. Prod. 62:228-232. [DOI] [PubMed] [Google Scholar]
- 20.Koike, K., Z. Jia, and T. Nikaido. 2001. New triterpenoid saponins from Maesa tenera. Chem. Pharm. Bull. 49:758-761. [DOI] [PubMed] [Google Scholar]
- 21.Legrand, P., A. Vertut-Doi, and J. Bolard. 1996. Comparative internalization and recycling of different amphotericin B formulations by a macrophage-like cell line. J. Antimicrob. Chemother. 37:519-533. [DOI] [PubMed] [Google Scholar]
- 22.Maes, L. 2003. Lead identification and development of a new anti-leishmanial saponin PX-6518, isolated from the Vietnamese plant Maesa balansae. Ph.D. thesis. Faculty of Pharmaceutical, Biomedical and Veterinary Sciences, Antwerp University, Antwerp, Belgium.
- 23.Maes, L., N. Germonprez, L. Van Puyvelde, M. Van Tri, T. Ngoc Ninh, and N. De Kimpe. July. 2000. Antiprotozoal saponins. International patent WO 00/38700.
- 24.Neal, R. A., and S. L. Croft. 1984. An in vitro system for determining the activity of compounds against the intracellular form of Leishmania donovani. J. Antimicrob. Chemother. 14:463-475. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien, J., I. Wilson, T. Orton, and F. Pognan. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267:5421-5426. [DOI] [PubMed] [Google Scholar]
- 26.Okunji, C. O., M. M. Iwu, J. E. Jackson, and J. D. Tally. 1996. Biological activity of saponins from two Dracaena species. Adv. Exp. Med. Biol. 404:415-428. [DOI] [PubMed] [Google Scholar]
- 27.Pauwels, R., K. Andries, Z. Debyser, P. Van Daele, D. Schols, P. Stoffels, K. De Vreese, R. Woestenborghs, A. M. Vandamme, C. G. Janssen et al. 1993. Potent and highly selective human immunodeficiency virus type 1 (HIV-1) inhibition by a series of alpha-anilinophenyl acetamide derivatives targeted at HIV-1 reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:1711-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pecoul, B., P. Chirac, P. Trouiller, and J. Pinel. 1999. Access to essential drugs in poor countries: a lost battle? JAMA 281:361-367. [DOI] [PubMed] [Google Scholar]
- 29.Plock, A., W. Sokolowska-Kohler, and W. Presber. 2001. Application of flow cytometry and microscopical methods to characterize the effect of herbal drugs on Leishmania spp. Exp. Parasitol. 97:141-153. [DOI] [PubMed] [Google Scholar]
- 30.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridoux, O., C. Di Giorgio, F. Delmas, R. Elias, V. Mshvildadze, G. Dekanosidze, E. Kemertelidze, G. Balansard, and P. Timon-David. 2001. In vitro antileishmanial activity of three saponins isolated from ivy, alpha-hederin, beta-hederin and hederacolchiside A(1), in association with pentamidine and amphotericin B. Phytother. Res. 15:298-301. [DOI] [PubMed] [Google Scholar]
- 32.Roberts, W. L., J. D. Berman, and P. M. Rainey. 1995. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob. Agents Chemother. 39:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romand, S., C. Della Bruna, R. Farinotti, and F. Derouin. 1996. In vitro and in vivo effects of rifabutin alone or combined with atovaquone against Toxoplasma gondii. Antimicrob. Agents Chemother. 40:2015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sindambiwe, J. B., A. M. Balde, T. De Bruyne, L. Pieters, H. Van den Heuvel, M. Claeys, D. A. Vanden Berghe, and A. J. Vlietinck. 1996. Triterpenoid saponins from Maesa lanceolata. Phytochemistry 41:269-277. [DOI] [PubMed] [Google Scholar]
- 35.Stauber, L. A. 1966. Characterisation of strains of Leishmania donovani. Exp. Parasitol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 36.Sundar, S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849-854. [DOI] [PubMed] [Google Scholar]
- 37.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, M. A. 1990. A larval development test for the detection of anthelminthic resistance in nematodes of sheep. Res. Vet. Sci. 49:198-202. [PubMed] [Google Scholar]
- 39.Tuntiwachwuttikul, P., O. Pancharoen, W. Mahabusarakam, P. Wiriyachitra, W. C. Taylor, W. A. Bubb, and G. H. Towers. 1997. A triterpenoid saponin from Maesa ramentacea. Phytochemistry 44:491-495. [DOI] [PubMed] [Google Scholar]
- 40.Vanden Berghe, D. A., and A. J. Vlietinck. 1991. Screening methods for antibacterial and antiviral agents from higher plants. Methods Plant Biochem. 6:47-69. [Google Scholar]
- 41.Yardley, V., and S. L. Croft. 2000. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents 13:243-248. [DOI] [PubMed] [Google Scholar]