Abstract
The impact of behavioral functioning on medication adherence in children with perinatally acquired HIV infection is not well-explored, but has important implications for intervention. This report addresses the relationship between behavioral functioning and child self-report or caregiver report of medication adherence among children and adolescents enrolled in Pediatric AIDS Clinical Trials Group Protocol 219C (conducted 2000–2007). A total of 1134 participants, aged 3–17 years, received a behavioral evaluation and adherence assessment. Complete adherence was defined as taking 100% of prescribed antiretroviral medications during three days preceding the study visit. Multivariable logistic regression models were used to evaluate associations between adherence and behavioral functioning, adjusting for potential confounders, including demographic, psychosocial, and health factors. Children demonstrated higher than expected rates of behavioral impairment (≈7% expected with T > 65) in the areas of conduct problems (14%, z = 7.0, p < 0.001), learning problems (22%, z = 12.2, p < 0.001), somatic complaints (22%, z = 12.6, p < 0.001), impulsivity-hyperactivity (20%, z = 11.1, p < 0.001), and hyperactivity (19%, z = 10.6, p < 0.001). Children with behavioral impairment in one or more areas had significantly increased odds of nonadherence [adjusted odds ratio (aOR) = 1.49, p = 0.04]. The odds of nonadherence were significantly higher for those with conduct problems and general hyperactivity (aOR = 2.03, p = 0.005 and aOR = 1.68, p = 0.02, respectively). Psychosocial and health factors, such as recent stressful life events and higher HIV RNA levels, were also associated with nonadherence. Knowledge of behavioral, health, and social influences affecting the child and family should guide the development of appropriate, evidence-based interventions for medication adherence.
Introduction
Advances in medical treatment, through combination antiretroviral therapy (ART) and highly active antiretroviral therapy (HAART), have resulted in improved health and longer lives for children and adolescents with perinatally acquired human immunodeficiency virus (HIV) infection.1,2 Adherence to ART/HAART regimens is difficult, however, due to the demanding nature of ART and the challenges faced by youth with HIV and their caregivers. Antiretroviral medications share characteristics that amplify the inherent difficulties of medication adherence for children and adolescents: poor palatability, heavy pill burden, dietary restrictions, acute and long-term side effects, and restrictions on daily schedules.3,4 Additionally, children with HIV infection often face other life stressors that affect adherence, including poverty, parental HIV disease, stigmatization, and limited social support.5,6 Poor adherence is dangerous in HIV disease, potentially resulting in diminished treatment efficacy, genotypically resistant viral mutations, vulnerability to viral rebound, reduction in HIV treatment options, and higher risk of transmission of HIV to sexual partners.
Despite the importance of adherence in HIV disease, our understanding of factors predictive of adherence in children and adolescents remains incomplete. Multiple contextual factors have been associated with adherence in children with HIV infection, including disease characteristics, features of the medications, and caregiver/family characteristics such as education and problem-solving skills, familial relationship to the child, and stress.7–10 Youth characteristics, such as older age, cognitive status, knowledge of HIV diagnosis, psychological adjustment, and coping skills have been implicated in nonadherence, although findings remain inconsistent.11–16
Emotional and behavioral problems, including attention problems and hyperactivity, and psychiatric disorders, such as anxiety and attention deficit hyperactivity disorder (ADHD), have been observed among children with perinatal HIV exposure and HIV infection.17–21 Nozyce and colleagues,18 for example, identified significant behavioral problems among clinically and immunologically stable children with HIV infection (HIV+) compared to a general non-HIV–infected population. Mellins and colleagues19 observed similar rates of behavioral problems among children with HIV infection and a comparison group of HIV-exposed but uninfected children (HIV−), suggesting that problems may be related to shared demographic and psychosocial characteristics of children's high-risk families rather than to HIV disease itself. In recent investigations of the prevalence of psychiatric disorders, as defined by the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV), higher than expected rates of disorders were observed in both HIV+ and HIV-exposed or affected youth,20,21 although those with HIV infection had significantly higher rates of ADHD than those without HIV.21
Emotional and behavioral problems, including depression, conduct problems, and alcohol use have been associated with nonadherence in older adolescents and adults with HIV infection.13,22–24 Although there is evidence suggesting emotional and behavioral problems are related to adherence in children with other chronic illnesses,25 the relationship between such problems and adherence in children with HIV disease is not well-described. Clarifying this relationship is critical since emotional and behavioral problems in the context of chronic illness may reduce the ability to take medications as directed and affect a youth's assumption of responsibility for this important health maintenance behavior.
The purpose of this investigation was to examine the behavioral functioning of youth with HIV and to evaluate the relationship between behavioral functioning and medication adherence. We hypothesized that behavioral problems would occur at higher rates among children with HIV infection relative to the general population and behavioral problems would be associated with increased risk for nonadherence. We examined potential confounding factors, including demographic characteristics, biological markers of health, medication and adherence characteristics, and psychosocial factors presumed to influence behavior and adherence.
Methods
Participants
Our analyses used data from the Pediatric AIDS Clinical Trials Group (PACTG) Late Outcomes Protocol 219C, a prospective cohort study designed to assess long-term effects of HIV infection and in utero exposure to ART.26 Children were enrolled at over 80 sites in the United States and Puerto Rico. Eligibility criteria for this investigation included: perinatally acquired HIV, age 3–17 years, on ART during participation, and a behavioral evaluation and adherence assessment within 6 months of the protocol-required cognitive evaluation (see Malee et al.11). The sites' human subject research Institutional Review Boards (IRBs) approved the protocol. Written informed consent was obtained from children's parents or legal guardians or from older adolescents who could self-consent; written assent was obtained from children in accordance with IRB guidelines.
PACTG 219C opened to accrual in 2000 and closed to follow-up in 2007. As of April 2004, 2,384 children with perinatally acquired HIV infection were enrolled; 2191 were 3–17 years old at some point during study follow-up. Of these, 1682 (77%) had a cognitive test attempted and within that group 1134 (67%) completed an adherence assessment and behavioral evaluation. This sample (N = 1134) comprises the study cohort.
Measures
Medication adherence.
Medication adherence was assessed every 3 months with a validated 3-day self-report measure, using a scripted interview with the person who self-identified as responsible for medication administration, either parent/caregiver or child/adolescent. The adherence measure was based on a version of the ACTG instrument modified for use in pediatric studies.27 Respondents identified each medication in the ART regimen and reported the number of doses missed during each of the preceding 3 days. We defined separate indicators of adherence for each drug class: nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs). Earlier guidelines for ART use suggested 95% adherence for therapeutic effect.28 However, since each antiretroviral drug has different relationships between adherence, viral suppression and drug resistance and because recent patterns of adherence rather than average adherence over time may be important in determining the risk of viral rebound,29 we defined complete adherence as taking all prescribed ART medications (100%) during the 3 days preceding the study visit. The interviewer recorded the following data: medication burden (total number of expected doses over the past 3 days), the need to prompt respondents for medication names, and the types of aids utilized as reminders (e.g., pillboxes, buddy system).
Behavioral functioning.
The Conners' Parent Rating Scale (CPRS-48)30 is a screening instrument used in 219C to evaluate children's behavioral functioning at ages 3, 6, 9, 12, and 15 years. It was administered to the parent/caregiver in English or Spanish, depending upon the caregiver's primary language. Caregivers rated the child on 48 descriptors of behavior, as observed during the previous month, using a four-point Likert scale (not at all, just a little, pretty much, very much) that yielded a standardized T score [mean (M) ± standard deviation (SD) = 50 ± 10] in each of six behavioral domains: conduct problems, learning problems, psychosomatic symptoms, impulsivity–hyperactivity, anxiety, and general hyperactivity. The conduct problems index assesses rule-breaking behavior, problems with authority, and the extent to which individuals are quarrelsome or unhappy; the learning problems index assesses difficulties with learning, task completion, and sustained attention/effort; the psychosomatic symptom index assesses the degree of physical symptoms; the impulsivity–hyperactivity index assesses the extent of restlessness, difficulty sitting still, and impulsivity; the anxiety scale assesses tendencies toward worrying and fearfulness, and the general hyperactivity index provides an assessment of the extent to which behaviors are consistent with ADHD. On any scale, higher T scores reflect more problematic behavior. For this investigation, behavioral impairment was defined as T > 65 on any of the six scales. Based on population norms, approximately 7% of the general population would be expected to have impairment for a single Conners' scale. All comparisons and analyses were based on age-standardized scores provided in the Conners' manual.
Procedures
Data collection for PACTG 219C was described in Williams et al.13 Sociodemographic information, including age, gender, race/ethnicity, primary language, and caregiver characteristics was collected at study entry, as were detailed medical histories, including clinical diagnoses and ART medication history. Measures of virologic and immunologic health status, including HIV-1 RNA viral load and CD4+ T-lymphocyte count (CD4) and percentage (CD4%) were collected at each study visit, scheduled to occur every 3 months. Data obtained closest to and within 6 months of the adherence assessment were used. Centers for Disease Control and Prevention (CDC) classification of HIV disease31 was recorded at each study visit, as was information regarding the current use of ART/HAART medications and adherence. The latest adherence assessment and the behavioral evaluation conducted closest to and within 6 months of this adherence assessment were used in the present analyses.
Psychosocial information, such as occurrence of stressful life events and presence or absence of physical/social role limitations, was obtained from the General Health Assessment for Children (GHAC), a validated health-related quality-of-life measure, completed annually.32 Data obtained closest to and within 12 months prior to the adherence assessment were used. Caregivers reported negative life events during the prior 12 months, such as parental job loss, illness, or death in the family.33 Participants were dichotomized by whether they had experienced at least one stressor. Five different physical/social role functioning events were classified as having occurred or not for participants over 5 years of age: repeating a grade, receiving special help in school, participating in sports, and having limitations in physical activity or school attendance.
Data analytic strategy
We conducted a cross-sectional analysis of the association between medication adherence and behavioral impairment. One-sample tests based on Student's t distribution were used to evaluate whether the mean Conners' T scores were significantly different than the general population means and one-sample tests based on the normal approximation to the binomial distribution were used to evaluate whether impairment rates were significantly higher than in the general population (with expected rates of approximately 7% for each Conners' scale). Wilcoxon's signed rank tests for paired data were used to compare percentage adherence between different drug classes. Mantel-Haenszel χ2 tests for trend were used to assess age trends in rates of behavioral impairment. All conclusions were based on a p < 0.05 level of significance.
Fisher's exact tests were used to compare the proportions of children with complete adherence between those with behavioral impairment versus those without. Two-sample t tests were used to compare the distributions of Conners' T scores between adherent and nonadherent participants, with adjustments for unequal variance when applicable.
Separate multiple logistic regression models were used to evaluate associations between adherence and impairment based on each of the Conners' behavior scales, adjusting for covariates associated with medication adherence in previous investigations. These included demographic characteristics (gender, age, race/ethnicity, non-English primary language), biological markers of HIV disease (CD4, CD4%, HIV viral load greater than 400 copies per milliliter, and CDC class C classification), medication and adherence factors (ART and other medication use, duration on HAART, person responsible for medication administration, medication burden, need for prompting, types of reminders used), child and family psychosocial characteristics (child knowledge of HIV status, primary caregiver (biological parent or other adult), caregiver education level, occurrence of recent stressful life events, physical/social role functioning), and child psychiatric/neurologic profiles (medication and diagnosis history). Model selection was done using backward elimination with a 10% significance level. Final logistic regression models were determined for impairment in any behavioral domain and for each of the individual behavioral domains, adjusting for significant confounders. Only potential predictors with p ≤ 0.25 in either the univariate models or the full multiple regression model (including all potential predictors) were initially considered in the model selection process. All analyses were conducted using SAS 8.1 software (SAS Institute Inc., Cary, NC).
Results
Study population
Fifty-two percent of the participants were female and 66% were in the 6–12 year age range (Table 1). Eighty-five percent were African American (61%) or Latino (24%). Forty-four percent had HIV RNA less than 400 copies per milliliter, and 25% had CDC class C classification. Fifteen percent had a neurologic diagnosis (e.g., encephalopathy, cerebral palsy, seizure disorder) and 17% had a psychiatric diagnosis (e.g., ADHD, depression). Forty-four percent lived with a biological parent, and the remaining lived with a relative (26%) or nonrelative adult (29%). Thirty-eight percent of participants knew their HIV diagnosis and 12% reported on their own medication adherence. Fifty-four percent reported at least one recent significant life stressor.
Table 1.
Selected Characteristics of PACTG 219C Participants with Adherence and Behavioral Assessments (N = 1134)
| Characteristic | Overall n (%) | Adherent to whole regimen n (%) | Nonadherent to whole regimen n (%) | p Value |
|---|---|---|---|---|
| Female | 584 (52) | 492 (50) | 92 (58) | 0.09a |
| Age | 0.22b | |||
| 3–6 years | 192 (17) | 171 (18) | 21 (13) | |
| > 6–9 years | 342 (30) | 292 (30) | 50 (31) | |
| > 9–12 years | 404 (36) | 347 (36) | 57 (36) | |
| > 12–15 | 193 (17) | 163 (17) | 30 (19) | |
| > 15–17 | 3 (<1) | 2 (<1) | 1 (<1) | |
| Race/ethnicity | 0.03c | |||
| White | 153 (13) | 143 (15) | 10 (6) | |
| African American | 687 (61) | 584 (60) | 103 (65) | |
| Latino | 275 (24) | 231 (24) | 44 (28) | |
| Other | 19 (2) | 17 (2) | 2 (1) | |
| Child/adolescent knows HIV status | 434 (38) | 365 (37) | 69 (43) | 0.16a |
| Primary caregiver | 0.08c | |||
| Biological parent | 504 (44) | 419 (43) | 85 (53) | |
| Adult relative | 294 (26) | 258 (26) | 36 (23) | |
| Nonrelative adult | 332 (29) | 294 (30) | 38 (24) | |
| Other | 4 (<1) | 4 (<1) | 0 (0) | |
| Education level of primary caregiver | 0.02c | |||
| Grade 1–11 | 293 (26) | 247 (25) | 46 (29) | |
| High school graduate | 352 (31) | 312 (32) | 40 (25) | |
| Some college/technical school | 287 (25) | 235 (24) | 52 (33) | |
| College graduate or higher | 125 (11) | 116 (12) | 9 (6) | |
| Other/not reported | 77 (7) | 65 (7) | 12 (8) | |
| CD4 percent | 0.66b | |||
| 15% or less | 94 (8) | 82 (8) | 12 (7) | |
| 15–25% | 217 (19) | 181 (19) | 36 (23) | |
| 25% or more | 823 (73) | 712 (73) | 111 (70) | |
| HIV-1 viral load (copies/mL) | < 0.01b | |||
| 400 copies or less | 501 (44) | 450 (46) | 51 (32) | |
| 401–10,000 copies | 356 (31) | 300 (31) | 56 (35) | |
| 10,001–100,000 copies | 277 (24) | 225 (23) | 52 (33) | |
| CDC class Cd | 287 (25) | 255 (26) | 32 (20) | 0.12a |
| Complete adherence (prior 3 days) | n/a | |||
| To all ART drugs (N = 1134) | 975 (86) | n/a | n/a | |
| To NRTIs (N = 1127) | 986 (87) | n/a | n/a | |
| To NNRTIs (N = 407) | 375 (92) | n/a | n/a | |
| To PIs (N = 830) | 733 (88) | n/a | n/a | |
| On PI at adherence visit | 830 (73) | 723 (74) | 107 (67) | 0.08a |
| On boosted PI regimen | 221 (20) | 193 (20) | 28 (18) | 0.59a |
| Antiretroviral regimen | 0.07c | |||
| HAART with PI | 805 (71) | 700 (72) | 105 (66) | |
| HAART without PI | 129 (11) | 112 (11) | 17 (11) | |
| Single NRTI | 11 (1) | 10 (1) | 1 (<1) | |
| Dual NRTI | 115 (10) | 90 (9) | 25 (16) | |
| > 3 NRTIs | 42 (4) | 33 (3) | 9 (6) | |
| Other combination therapy | 32 (3) | 30 (3) | 2 (1) | |
| Duration of HAART (years) | 0.01b | |||
| < 2 | 370 (33) | 309 (32) | 61 (38) | |
| 2–4 | 500 (44) | 427 (44) | 73 (46) | |
| > 4–6 | 230 (20) | 208 (21) | 22 (14) | |
| > 6 | 34 (3) | 31 (3) | 3 (2) | |
| Memory prompt required | 312 (28) | 260 (27) | 52 (33) | 0.13a |
| Type of ART reminderse | ||||
| Activity of daily living | 291 (26) | 257 (26) | 34 (21) | 0.20a |
| Pillboxes | 166 (15) | 135 (14) | 31 (20) | 0.07a |
| Buddy system | 82 (7) | 75 (8) | 7 (4) | 0.18a |
| Labels | 154 (14) | 137 (14) | 17 (11) | 0.32a |
| Physical/social role functioningf | ||||
| Limited activities due to illness | 119 (10) | 106 (13) | 13 (9) | 0.33a |
| Limited school attendance | 126 (13) | 112 (13) | 14 (10) | 0.34a |
| Repeated a grade | 238 (24) | 205 (24) | 33 (24) | 1.00a |
| Participated in sports | 614 (63) | 523 (62) | 91 (65) | 0.51a |
| Attended special class/help | 348 (36) | 301 (36) | 47 (34) | 0.70a |
| Responsibility for medication (person reporting adherence) | < 0.01c | |||
| Biological parent | 460 (41) | 382 (39) | 78 (49) | |
| Adult relative | 247 (22) | 225 (23) | 22 (14) | |
| Nonrelative adult | 284 (25) | 256 (26) | 28 (18) | |
| Selfg | 143 (12) | 112 (11) | 31 (20) | |
| Had recent life stressh | 608 (55) | 506 (53) | 102 (66) | < 0.01a |
| At least one neurologic diagnosis | 171 (15) | 151 (15) | 20 (13) | 0.40a |
| At least one psychiatric diagnosis | 191 (17) | 164 (17) | 27 (17) | 1.00a |
Fisher's Exact test.
Mantel-Haenszel χ2 test.
χ2 test.
Three missing observations.
Participants could report using more than one type of reminder.
153–157 missing observations.
Includes four children who were assisted by caregiver in reporting adherence.
31 missing observations.
ART, antiretrovirals; CDC, Centers for Disease Control and Prevention; HAART, highly active antiretroviral therapy; NRTIs, nucleoside reverse transcriptase inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors.
Rates of adherence
Complete adherence to the total antiretroviral regimen was reported for 86% of participants. Within individual drug classes, complete adherence was identified among 87% of participants taking NRTIs, 92% of those taking NNRTIs, and 88% of those taking PIs. The percentage of all prescribed ARV medications taken over the last 3 days ranged from 0% to 100%, with a median of 100%. The median and range were the same for medications taken in each of the three classes. There were no significant differences in adherence percentages between any pair of drug classes.
Among the 14% of participants who did not report complete adherence to the whole regimen, the median percentage of medication taken ranged from 0% to 97%, with a median of 81%. The median percentage among those who were not adherent in each of the drug classes was: 42% for NNRTIs, 75% for NRTIs, and 67% for PIs.
Behavioral functioning
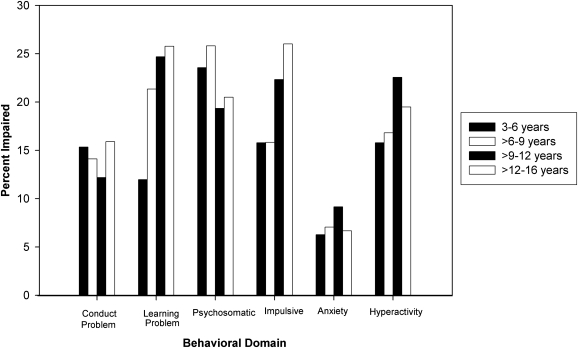
All of the Conners' mean scale scores, with the exception of anxiety (mean = 49.6) and conduct problems (mean = 50.7), were significantly higher for the total sample than the population mean of 50 (hyperactivity: mean = 53.8, t = 8.5, p < 0.001; impulsivity-hyperactivity: mean = 53.2, t = 8.0, p < 0.001; learning problems: mean = 55.3, t = 11.6, p < 0.001; psychosomatic: mean = 56.0, t = 13.3, p < 0.001). Based on caregiver reports, children demonstrated behavioral impairment at higher than expected rates (T > 65) in the areas of conduct problems (14%, z = 7.0, p < 0.001), learning problems (22%, z = 12.2, p < 0.001), psychosomatic complaints (22%, z = 12.6, p < 0.001), impulsivity–hyperactivity (20%, z = 11.1, p < 0.001), and hyperactivity (19%, z = 10.6, p < 0.001). The prevalence of behavioral impairment increased with age (Fig. 1) for impulsive–hyperactive behaviors (Mantel-Haenszel χ2 = 10.3, p = 0.001) and learning problems (Mantel-Haenszel χ2 = 12.0, p < 0.001).
FIG. 1.
Prevalence of impaired behavioral functioning (Connors' T score > 65) by age. Significantly increasing rates for impulsive-hyperactive (p = 0.001) and learning problems (p < 0.001).
Behavioral functioning and nonadherence
Nonadherence was identified among 17% of children with behavioral impairment in at least one Conners' domain and among 11% of children with no behavioral impairment (Fisher's Exact Test, p = 0.01). Rates of nonadherence (Table 2) were significantly greater in children with impairment on the scales labeled conduct problems (p = 0.004), psychosomatic complaints (p = 0.01), impulsive-hyperactive (p = 0.05), and hyperactivity (p = 0.05). Comparison of the distributions of Conners' mean T scores revealed that nonadherent participants had significantly higher Conners' scores than adherent participants for conduct problems (p = 0.01), impulsive–hyperactive (p = 0.01), and hyperactivity (p = 0.01).
Table 2.
Percentage of Nonadherent Participants by Behavioral Impairment
| |
Percent nonadherent (n/N) |
|
|
|---|---|---|---|
| Behavioral domain | Impaired (T > 65) | Unimpaired (T ≤ 65) | p Valuea |
| At least one Conners' scale | 17% (91/539) | 11% (65/584) | 0.01 |
| Anxiety | 16% (14/86) | 14% (144/1044) | 0.52 |
| Conduct problems | 22% (34/157) | 13% (123/969) | 0.004 |
| Hyperactivity index | 18% (39/215) | 13% (117/908) | 0.05 |
| Impulsive–hyperactive | 18% (41/225) | 13% (118/905) | 0.05 |
| Learning problem | 17% (42/245) | 13% (115/884) | 0.12 |
| Psychosomatic behavior | 20% (49/251) | 12% (109/879) | 0.005 |
Fisher's exact test.
Final logistic regression models for behavioral impairment in any domain and in each individual behavioral domain, adjusting for significant confounders, are shown in Table 3. Children with behavioral impairment in at least one domain were significantly more likely to be nonadherent than children without behavioral impairment, and the odds of nonadherence increased by 49% for those with any behavioral impairment. Among individual behavioral domains, impairment in conduct problems (adjusted odds ratio [aOR] = 2.03) and hyperactivity (aOR = 1.68) were significantly associated with nonadherence, after adjusting for significant covariates. Significantly increased odds of nonadherence were associated with African-American or Latino race/ethnicity (aORs = 3.47, p = 0.002 and 3.27, p = 0.005, respectively), recent life stress (aOR = 1.65, p = 0.01), HIV RNA detectability (aOR = 1.61, p = 0.02), and knowledge of HIV status (aOR = 1.61, p = 0.04). Factors associated with lower odds of nonadherence included more years on HAART (aOR = 0.85, p = 0.003), use of daily activities as an adherence support (aOR = 0.63, p = 0.05), limitations in school attendance (aOR = 0.54, p = 0.06), and adult relative or nonrelative responsible for medication administration (aORs = 0.43, p = 0.003 and 0.46, p = 0.005, respectively).
Table 3.
Adjusted Multivariate Logistic Regression Models for Predictors of Nonadherence
| Predictor | Adjusted ORa | 95% Confidence interval | p Value |
|---|---|---|---|
| Behavioral domainb | |||
| At least one Conners' scale with T >65 | 1.49 | 1.02–2.19 | 0.04 |
| Anxiety T >65 | 1.09 | 0.56–2.11 | 0.79 |
| Conduct problem T >65 | 2.03 | 1.25–3.31 | 0.005 |
| Hyperactivity index T >65 | 1.68 | 1.08–2.62 | 0.02 |
| Impulsive–hyperactive T >65 | 1.37 | 0.88–2.13 | 0.16 |
| Learning problem T >65 | 1.49 | 0.97–2.29 | 0.07 |
| Psychosomatic behavior T >65 | 1.51 | 0.98–2.33 | 0.06 |
| Covariatesc | |||
| Race/ethnicity | — | — | 0.01 |
| White | 1.00 | — | (ref ) |
| Black | 3.47 | 1.61–7.49 | 0.002 |
| Latino | 3.27 | 1.44–7.42 | 0.005 |
| Other race | 1.12 | 1.13–9.95 | 0.92 |
| Recent life stress | 1.65 | 1.11–2.46 | 0.01 |
| School attendance limited | 0.54 | 0.29–1.02 | 0.06 |
| Adherence assessor | — | — | < 0.001 |
| Biological parent | 1.00 | — | (ref ) |
| Self or other | 1.21 | 0.69–2.11 | 0.51 |
| Relative | 0.43 | 0.25–0.74 | 0.003 |
| Other adult | 0.46 | 0.26–0.79 | 0.005 |
| Child/adolescent knows HIV status | 1.56 | 1.02–2.39 | 0.04 |
| HIV-1 RNA >400 copies/mL | 1.61 | 1.07–2.41 | 0.02 |
| Duration of HAART | 0.85 | 0.77–0.95 | 0.003 |
| ART reminder: daily activities | 0.63 | 0.40–1.00 | 0.05 |
Adjusted for covariates shown in Table 3 (separate models were run for each domain).
Sample sizes ranged from 973–980 due to missing behavioral and covariate data.
Covariate odds ratios and p values based on the “at least one Conners' scale” model.
HAART, highly active antiretroviral therapy.
Discussion
In a large sample of children and adolescents with perinatal HIV infection, the prevalence of child behavioral problems was greater than expected compared to population norms, but consistent with previous reports of the association between childhood chronic illnesses and behavioral problems.34 Furthermore, our results are consistent with earlier investigations that identified higher than expected rates of behavioral problems and psychiatric disorders among children with HIV infection and HIV exposure.18–21 Parents and caregivers of youth in our sample reported higher than expected rates of conduct problems, learning difficulties, psychosomatic complaints, hyperactivity and impulsive–hyperactive behaviors. Whether these problems arise due to early or later neurotrophic effects of HIV infection or are related to other biological or psychosocial vulnerabilities, their identification and treatment are important considerations as youth prepare for the challenges of adolescence and young adulthood.
In our investigation, specific difficulties, such as learning problems and impulsive–hyperactive behaviors, were observed at higher rates with increasing age. Learning problems were reported more often in older school-age children, perhaps because difficulties and frustration with learning, including reduced attention, became more apparent to caregivers with the increasing performance and behavioral demands of the upper grades. Impulsive–hyperactive behaviors may be reported more frequently if children are unable to meet expectations for self-monitoring and well-regulated behavior within school and home environments. In addition, older children with HIV infection may have histories of suboptimal viral suppression at younger ages, prior to the widespread use of ART/HAART, extensive ARV drug resistance, and/or incomplete eradication of HIV even in the context of HAART, potentially increasing their vulnerability to impaired neurobehavioral functioning as they grow older.35,36
Consistent with our hypothesis, parent-reported behavioral problems were associated with an increased risk for nonadherence. This relationship was anticipated given the known challenges associated with adherence to ART/HAART and the powerful impact of child behavioral impairment upon any family system. Behavioral problems may interfere with children's ability to comply with directions and may affect parents' ability to foster positive adherent behaviors in their children. Hyperactive youth, for example, may require a structured and consistently reinforcing approach to medication administration to achieve success. Children with conduct problems similarly require establishment of routines, monitoring, and positive support for adherent behaviors. The ability of families affected by HIV to manage difficult child behaviors while also maintaining adherence routines likely varies, depending on multiple family factors related to caregiver physical and mental health and adequate financial, psychosocial, and educational resources. When behavioral problems persist, the risk for nonadherence is likely to increase as children enter adolescence and are faced with the typical and multiple challenges associated with that developmental stage.
Previous investigations have identified the important role of the caregiving environment for HIV medication adherence.7,9,11,13 Similarly, in this investigation, recent life stressors experienced by the family were associated with higher odds of nonadherence, suggesting that adherent behavior is sensitive to variations in family well-being. Nonadherence was also associated with minority group membership, which in itself may place families at increased risk for greater stress and poorer health. Factors detrimental to health, such as crime, violence, lack of access to services, poor housing, and proximity to environmental hazards are disproportionately found in low income, minority neighborhoods37 and may increase the risk for poor psychological adjustment.38,39
Several psychosocial and caregiving factors appeared to be protective, reducing the risk for nonadherence. Children whose medications were administered by a relative (e.g., aunts, grandparents) or nonrelative adult (e.g., foster or adoptive parents) demonstrated lower odds of nonadherence. It is possible that the health and quality of life of these caregivers is less compromised by the burden of HIV disease relative to biological parents. It is also possible that biological parents acknowledge adherence difficulties more readily than other types of caregivers. Regardless, this finding suggests that some biological parents may require increased support to ensure medication success for their children, particularly if their current or chronic life stress is high.40
Although these findings are important and relevant, as with any study, there are limitations. The cross-sectional design does not allow establishment of a causal link between behavioral problems and adherence or allow exploration of dynamic developmental processes, such as changes in adherence behaviors related to intermittently-experienced biomedical and psychosocial stressors in families affected by HIV infection. The use of the Conners' standardization sample as the control group may be problematic since children with HIV infection may differ in important ways (e.g., socioeconomic status, stressors) from this sample. These results may not adequately characterize adherence among all youth with HIV infection because the children and families willing to participate in PACTG 219C may represent an inherently more adherent subgroup. Finally, there are limitations associated with the measurement methods, including reliance on caregiver report; biomedical, adherence and behavioral data collection at study visits within a 6-month interval; the use of a 3-day measure of adherence, which may underestimate the true prevalence of nonadherence; that the same informant reported on both behavior and adherence.41
This investigation has important strengths as well. To date, this is the largest sample of children with HIV infection whose adherence and behavioral functioning were examined at different age levels, allowing investigation of variations in behavioral functioning as a function of age. We were also able to examine demographic, health, and psychosocial factors potentially associated with both adherence and nonadherence. As expected, we found that adherence is a complex process influenced by multiple factors, including child behavioral functioning, family and caregiver factors, and disease characteristics. These results suggest that targeted, multilevel, evidence-based interventions and support to children and families at highest risk for nonadherence, such as children with behavioral problems and families under stress, may be fruitful. Prospective longitudinal investigations are necessary to further identify factors associated with adherence success and to evaluate the efficacy of adherence interventions that specifically address behavioral problems associated with nonadherence.
Acknowledgments
The IMPAACT Network, which is now supervising publications from PACTG 219C, now requires the following acknowledgment for every publication using PACTG 219C data.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Protocol Team Members (versions 1 and 2 of PACTG-219)
Mary Culnane, M.S. C.R.N.P., Elizabeth Hawkins, Lynne Mofenson, M.D., Yvonne J Bryson, M.D., Edward M. Connor, M.D., Lawrence D'Angelo, M.D., M.P.H., Mark Mintz, M.D., Karen J. O'Donnell, Ph.D., Margaret Oxtoby, M.D., Andrea Rubin Hale, R.N., M.P.H., Richard D Gelber, Ph.D., Steven Gortmaker, Ph.D., William Lenderking Ph.D., Lynn Marrow, Christina Joy, R.N., M.S.M., Colleen Clark, M.P.H., Bethann Cunningham, M.S., Rhoda Sperling, M.D., Gwendolyn B. Scott, M.D., Courtney Fletcher, Pharm.D., Blake Caldwell, M.D., Dianne Donovan.
Protocol Team Members (versions 3 and 4 of PACTG-219C)
Elizabeth Smith, M.D., Anne Fresia, Gregory Ciupak, Carol Elgie, Michelle Eagle, P.A., Dorothy R. Smith, M.S., C.P.N.P., Paul Palumbo, M.D., John Sleasman, M.D., James Connor M.D., Michael Hughes, Ph.D., Rebecca Oyomopita, M.Sc., George Johnson, M.D., Andrew Wiznia, M.D., Nancy Hutton, M.D., Andrea Kovacs, M.D., Mary Sawyer, M.D., Martin Anderson, M.D., Audrey Rogers, Ph.D., M.P.H., William Borkowsky, M.D., Jane Lindsey, Sc.D., Jack Moye, M.D., Myron Levin, M.D., Marilyn Crain, M.D., M.P.H., Paul Britto, M.S., Ruth Toumala, M.D., Joseph Cervia, M.D., Eileen Monagham, Kenneth Dominguez, M.D., Melody Higgins, R.N., M.S., George Seage, D.Sc., M.P.H., Denise Gaughan, M.P.H., Phil Gona, Ph.D., William Shearer, M.D., Ph.D., Lois Howland, D.P.H., M.S., R.N., Deborah Storm, Ph.D., R.N., Kathleen Malee, Ph.D., Wendy Mitchell, M.D., Carol Gore, Eve Powell, Michelle McConnell, M.D., Newana Beatty, Susan Brogly, Ph.D., Jennifer Bryant, C.R.A., Miriam Chernoff, Ph.D., Barbara Heckman, B.S., Dawn English, Edward Handelsman, M.D., Patrick Jean-Philippe, M.D., Kathleen Kaiser, Joyce Kraimer, M.S., Linda Millar, Shirley Traite, M.S.W., Paige Williams, Ph.D., Elizabeth Woods, M.D., M.P.H., Carol Worrell, M.D.
The following institutions and clinical site investigators participated in PACTG Protocol 219C:
Baylor Texas Children's Hospital: F. Minglana, M.E. Paul, C.D. Jackson; University of Florida, Jacksonville: M.H. Rathore, A. Khayat, K. Champion, S. Cusic; Chicago Children's Memorial Hospital: R. Yogev, E. Chadwick; University of Puerto Rico, University Children's Hospital AIDS Program: I. Febo-Rodriguez, S. Nieves; Bronx Lebanon Hospital Center; M. Purswani, S. Baksi, E. Stuard, M. Dummit; San Juan Hospital: M. Acevedo, M. Gonzalez, L. Fabregas, M.E. Texidor; University of Miami: G.B. Scott, C.D. Mitchell, L. Taybo, S. Willumsen; University of Medicine & Dentistry of New Jersey: L. Bettica, J. Amour, B. Dashefsky, J. Oleske; Charity Hospital of New Orleans & Earl K. Long Early Intervention Clinic: M. Silio, T. Alchediak, C. Borne, M. Cowie; UCSD Mother, Child & Adolescent HIV Program: S.A. Spector, R. Viani, M. Caffery, L. Proctor; Howard University: S. Rana, D. Darbari, J.C. Roa, P.H. Yu; Jacobi Medical Center: M. Donovan, R. Serrano, M. Burey, R. Auguste; St. Christopher's Hospital for Children, Philadelphia: J. Chen, J. Foster; Baystate Medical Center Children's Hospital: B.W. Stechenberg, D.J. Fisher, A.M. Johnston, M. Toye; Los Angeles County Medical Center/USC: J. Homans, M. Neely, L.S. Spencer, A. Kovacs; Children's Hospital Boston: S. Burchett, N. Karthas; Children's Hospital of Michigan: E. Moore, C. Cromer; St. Jude Children's Research Hospital, Memphis: P.M. Flynn, N. Patel, M. Donohoe, S. Jones; New York University School of Medicine/Bellevue Hospital: W. Borkowsky, S. Chandwani, N. Deygoo, S. Akleh; The Children's Hospital at Downstate: E. Handelsman, H.J. Moallem DM Swindell, JM Kaye; The Columbia Presbyterian Medical Center & Cornell University New York Presbyterian Hospital: A. Higgins, M. Foca, P. LaRussa, A. Gershon; The Children's Hospital of Philadelphia: R.M. Rutstein, C.A. Vincent, S.D. Douglas, G.A. Koutsoubis; Children's Hospital of Oakland: A. Petru, T. Courville; UCSF, Moffitt Hospital: D. Wara, D. Trevithick; Children's Hospital, University of Colorado, Denver: E. McFarland, C. Salbenblatt; Johns Hopkins University Pediatrics: N. Hutton, B. Griffith, M. Joyner, C. Kiefner; Children's Hospital and Regional Medical Center, Washington: M. Acker, R. Croteau, C. McLellan, K. Mohan; Metropolitan Hospital Center: M. Bamji, I. Pathak, S. Manwani, E. Patel; Children's National Medical Center: H. Spiegel, V. Amos; University of Massachusetts Medical School: K. Luzuriaga; University of Alabama at Birmingham: R. Pass, M. Crain; University of Maryland Medical Center: J. Farley, K. Klipner; Schneider Children's Hospital: V.R. Bonagura, S.J. Schuval, C. Colter, L. Campbell; Boston Medical Center: S.I. Pelton, A.M. Reagan; University of Illinois: K.C. Rich, K. Hayani, M. Bicchinella; SUNY Stony Brook: S. Nachman, D. Ferraro, S. Madjar; North Broward Hospital District: A. Puga; Duke University: F. Wiley, K. Whitfield, O. Johnson, R. Dizney; Harlem Hospital: S. Champion, M. Frere, M. DiGrado, E.J. Abrams; Cook County Hospital: J. Martinez; University of South Alabama: M. Mancao; Connecticut Children's Medical Center: J. Salazar, G. Karas; University of North Carolina at Chapel Hill: T. Belho, B. Pitkin, J. Eddleman; Ruiz Arnau University Hospital: W. Figueroa, E. Reyes; SUNY Upstate Medical University: L.B. Weiner, K.A. Contello, W.A. Holz, M.J. Famiglietti; Children's Medical Center of Dallas; University of Florida at Gainesville: R. Lawrence, J. Lew, C. Delany, C. Duff; Children's Hospital at Albany Medical Center: A.D. Fernandez, P.A. Hughes, N. Wade, M.E. Adams; Lincoln Medical & Mental Health Center; Phoenix Children's Hospital: J.P. Piatt, J. Foti, L. Clarke-Steffen; Public Health Unit of Palm Beach County: J. Sleasman, C. Delaney; Medical College of Georgia: C.S. Mani; Yale University School of Medicine: W.A. Andiman, S. Romano, L. Hurst, J. de Jesus; Vanderbilt University Medical Center: G. Wilson; University of Rochester Medical Center: G.A. Weinberg, F. Gigliotti, B. Murante, S. Laverty; St. Josephs Hospital and Medical Center, New Jersey: N. Hutchcon, A. Townley; Emory University Hospital: S. Nesheim, R. Dennis; University of South Florida: P. Emmanuel, J. Lujan-Zilberman, C. Graisberry, S. Moore; Children's Hospital of the King's Daughters: R.G. Fisher, K.M. Cunnion, T.T. Rubio, D. Sandifer; Medical University of South Carolina: G.M. Johnson; University of Mississippi Medical Center: H. Gay, S. Sadler; Harbor-UCLA Medical Center: M. Keller, J. Hayes, A. Gagajena, C. Mink; Mount Sinai Medical Center: D. Johnson; Children's Hospital of Los Angeles: J. Church, T. Dunaway, C. Salata; Long Beach Memorial: A. Deveikis, L. Melton; Robert Wood Johnson Medical School: S. Gaur, P. Whitley-Williams, A. Malhotra, L. Cerracchio; Sinai Children's Hospital: M. Dolan, J. D'Agostino, R. Posada; The Medical Center, Pediatric Columbus, Georgia: C. Mani, S. Cobb; Medical College of Virginia: S.R. Lavoie, T.Y. Smith; Cooper Hospital-University Medical Center: A. Feingold, S. Burrows-Clark; University of Cincinnati: J. Mrus, R. Beiting; Columbus Children's Hospital: M. Brady, J. Hunkler, K. Koranyi; Sacred Heart Children's CMS of Florida: W. Albritton; St. Luke's/Roosevelt Hospital Center: R. Warford, S. Arpadi; Incarnation Children's Center, New York: A. Gershon, P. Miller; Montefiore Medical–AECOM: A. Rubinstein, G. Krienik; Children's Hospital of Los Angeles: A. Kovacs and E. Operskalski; San Francisco General Hospital: D. Wara, A. Kamrin, S. Farrales; Cornell University New York Presbyterian: R. Johan-Liang, K. O'Keefe; St. Louis Children's Hospital: K.A. McGann, L. Pickering, G.A. Storch; North Shore University Hospital: S. Pahwa, L. Rodriquez; Oregon Health and Science University: P. Lewis, R. Croteau.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Brady MT. Oleske JM. Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1–infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel K. Hernan MA. Williams PL, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: A 10-year follow-up study. Clin Infect Dis. 2008;46:507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 3.Byrne M. Honig J. Jurgrau A. Heffernan S. Donahue M. Achieving adherence with antiretroviral medications for pediatric HIV disease. AIDS Read. 2002;12:151–164. [PubMed] [Google Scholar]
- 4.Pontali E. Facilitating adherence to highly active antiretroviral therapy in children with HIV infection: What are the issues and what can be done? Paediatr Drugs. 2005;7:137–149. doi: 10.2165/00148581-200507030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Brown LK. Lourie KJ. Pao M. Children and adolescents living with HIV and AIDS: A review. J Child Psychol Psychiatry. 2000;41:81–96. [PubMed] [Google Scholar]
- 6.Steele RG. Nelson TD. Cole BP. Psychosocial functioning in children with AIDS and HIV infection: Review of the literature from a socioecological framework. J Dev Behav Pediatr. 2007;28:58–69. doi: 10.1097/DBP.0b013e31803084c6. [DOI] [PubMed] [Google Scholar]
- 7.Hammani N. Nostlinger C. Hoeree T. Lefevre P. Jonckheer T. Kolsteren P. Integrating adherence to highly active antiretroviral therapy into children's daily lives: A qualitative study. Pediatrics. 2004;114:591–597. doi: 10.1542/peds.2004-0085. [DOI] [PubMed] [Google Scholar]
- 8.Martin S. Elliot-DeSorbo DK. Wolters PL, et al. Patient, caregiver and regimen characteristics associated with adherence to highly active antiretroviral therapy among HIV-infected children and adolescents. Pediatr Infect Dis J. 2007;26:61–67. doi: 10.1097/01.inf.0000250625.80340.48. [DOI] [PubMed] [Google Scholar]
- 9.Mellins C. Brackis-Cott E. Dolezal C. Abrams E. The role of psychosocial and family factors in adherence to antiretroviral treatment in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2004;23:1035–1041. doi: 10.1097/01.inf.0000143646.15240.ac. [DOI] [PubMed] [Google Scholar]
- 10.Naar-King S. Arfken C. Frey M. Harris M. Secord E. Ellis D. Psychosocial factors and treatment adherence in paediatric HIV/AIDS. AIDS Care. 2006;18:621–628. doi: 10.1080/09540120500471895. [DOI] [PubMed] [Google Scholar]
- 11.Malee K. Williams PL. Montepiedra G, et al. The role of cognitive functioning in medication adherence of children and adolescents with HIV infection. J Pediatr Psychol. 2009;34:164–175. doi: 10.1093/jpepsy/jsn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simoni JM. Montgomery A. Martin E. New M. Demas PA. Rana S. Adherence to antiretroviral therapy for pediatric HIV infection: A qualitative systematic review with recommendations for research and clinical management. Pediatrics. 2007;119:e1371–e1384. doi: 10.1542/peds.2006-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams P. Storm D. Montepiedra G, et al. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics. 2006;118:e1745–e1757. doi: 10.1542/peds.2006-0493. [DOI] [PubMed] [Google Scholar]
- 14.Haberer J. Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6:194–200. doi: 10.1007/s11904-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merzel C. VanDevanter N. Irvine M. Adherence to antiretroviral therapy among older children and adolescents with HIV: A qualitative study of psychosocial contexts. AIDS Patient Care STDs. 2008;22:977–987. doi: 10.1089/apc.2008.0048. [DOI] [PubMed] [Google Scholar]
- 16.Michaud PA. Suris JC. Thomas R. Gnehm HE. Cheseaux JJ. The Swiss HIV Mother & Child Cohort Study. Coping with HIV infection. Swiss Med Wkly. 2010;140:247–253. doi: 10.4414/smw.2010.12834. [DOI] [PubMed] [Google Scholar]
- 17.Havens JF. Mellins CA. Psychiatric aspects of HIV/AIDS. In: Rutter M, editor; Bishop D, editor; Pine D, editor; Scott S, editor; Stevenson JS, editor; Taylor EA, editor; Thapar A, editor. Rutter's Child and Adolescent Psychiatry. 5th. Oxford: Blackwell Publishing; 2008. pp. 945–955. [Google Scholar]
- 18.Nozyce M. Lee S. Wiznia A, et al. A behavioral and cognitive profile of clinically stable HIV infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 19.Mellins CA. Smith R. O'Driscoll P, et al. High rates of behavioral problems in perinatally HIV-infected children are not linked to HIV disease. Pediatrics. 2003;111:384–393. doi: 10.1542/peds.111.2.384. [DOI] [PubMed] [Google Scholar]
- 20.Gadow KG. Chernoff M. Williams PL, et al. Co-occurring psychiatric symptoms in children perinatally infected with HIV and peer comparison sample. J Dev Behav Pediatr. 2010;31:116–128. doi: 10.1097/DBP.0b013e3181cdaa20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellins CA. Brackis-Cott E. Leu C, et al. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry. 2009;50:1131–1138. doi: 10.1111/j.1469-7610.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catz SL. Kelly JA. Bogart LM. Benotsch EG. McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19:124–133. [PubMed] [Google Scholar]
- 23.Murphy DA. Belzer M. Durako S. Sarr M. Wilson C. Muenz L. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 24.Walkup J. Akincigil A. Bilder S. Rosato NS. Crystal S. Psychiatric diagnosis and antiretroviral adherence among adolescent Medicaid beneficiaries diagnosed with human immunodeficiency virus/acquired immunodeficiency syndrome. J Nerv Ment Dis. 2009;197:354–361. doi: 10.1097/NMD.0b013e3181a208af. [DOI] [PubMed] [Google Scholar]
- 25.Rapoff MA. Adherence to Pediatric Medical Regimens. New York: Kluwer Academic/Plenum; 1999. [Google Scholar]
- 26.Gortmaker SL. Hughes M. Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 27.Van Dyke RB. Lee S. Johnson GM, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have Human Immunodeficiency Virus Infection. Pediatrics. 2002;109:e61. doi: 10.1542/peds.109.4.e61. [DOI] [PubMed] [Google Scholar]
- 28.Paterson DL. Swindells S. Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 29.Bangberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197:S272–278. doi: 10.1086/533415. [DOI] [PubMed] [Google Scholar]
- 30.Conners CK. Conners Rating Scales: Technical Manual, Revised. North Tonawanda, NY: MultiHealth Systems; 1989. [Google Scholar]
- 31.Center for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep. 1994;43:1–10. [Google Scholar]
- 32.Gortmaker SL. Lenderking WR. Clark C. Lee S. Fowler MG. Oleske J. Development and use of a pediatric quality of life questionnaire in AIDS clinical trials: Reliability and validity of the General Health Assessment for Children (GHAC) In: Drotar D, editor. Assessing Pediatric Health-Related Quality of Life and Functional Status: Implications for Research, Practice and Policy. Mahwah, NJ: Lawrence Feldbaum Associates; 1998. pp. 219–235. [Google Scholar]
- 33.Lee GM. Gortmaker SL. McIntosh K. Hughes MD. Oleske JM. Quality of life for children and adolescents: Impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117:273–283. doi: 10.1542/peds.2005-0323. [DOI] [PubMed] [Google Scholar]
- 34.Wallander JL. Varni JW. Effects of pediatric chronic physical disorders on child and family adjustment. J Child Psychol Psychiatry. 1998;39:29–46. [PubMed] [Google Scholar]
- 35.Van Rie A. Harrington PR. Dow A. Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: A global perspective. Eur J Paediatr Neurol. 2007;11:1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Valcour VG. Shiramizu BT. Shikuma CM. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol. 2010;87:1–6. doi: 10.1189/jlb.0809571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tashiro C. The meaning of race in healthcare and research-part 2. Current controversies and emerging research. Pediatr Nurs. 2005;31:305–308. [PubMed] [Google Scholar]
- 38.Wadsworth ME. Raviv T. Reinhard C. Wolff B. Santiago CD. Einhorn L. Association between poverty and child functioning: The role of children's poverty-related stress. J Loss Trauma. 2008;13:156–185. [Google Scholar]
- 39.Kishiyama MM. Boyce WT. Jimenez AM. Perry LM. Knight RT. Socioeconomic disparities affect prefrontal function in children. J Cogn Neurosci. 2008;21:1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- 40.Silver E. Heneghan A. Bauman L. Stein R. The relationship of depressive symptoms to parenting competence and social support in inner-city mothers of young children. Matern Child Health J. 2006;10:105–112. doi: 10.1007/s10995-005-0024-4. [DOI] [PubMed] [Google Scholar]
- 41.Rutter M. Environmentally mediated risks for psychopathology: Research strategies and findings. J Am Acad Child Adolesc Psychiatry. 2005;44:18. doi: 10.1097/01.chi.0000145374.45992.c9. [DOI] [PubMed] [Google Scholar]