Abstract
Major advances in screening have lowered the death rate from cervical cancer in the United States. One of the first major advances in cervical cancer screening was the Papanicolaou (Pap) test. The second major advance was liquid-based cytology (LBC). This review presents a wide range of data, discusses the strengths and weaknesses of the available information regarding Pap technologies, and reviews the meta-analyses, which have examined the differences in clinical performance. The review concludes with information on new and future developments to further decrease cervical cancer deaths.
Key words: Cervical cancer detection, Papanicolaou test, Liquid-based cytology
Major advances in screening have lowered the death rate from cervical cancer in the United States. One of the first major advances in cervical cancer screening was the Papanicolaou (Pap) test. Implementation of Pap testing was responsible for reducing the incidence of cervical cancer between 1955 and the mid-1980s.1 The second major advance in cervical cancer screening was liquid-based cytology (LBC). Today, LBC accounts for more than 90% of the Pap tests performed in the United States. This shift from conventional cytology to LBC has occurred due to improvements in sample quality, reproducibility, sensitivity, and specificity, as well as the ability to perform reflex molecular testing.
Since the introduction of LBC, there has been occasional debate regarding whether LBC provides clinical benefit over the use of the conventional Pap testing for detecting precancerous lesions.2,3 This review presents a wide range of data, discusses the strengths and weaknesses of the available information regarding Pap technologies, and reviews the meta-analyses, which have examined the differences in clinical performance. The review concludes with information on new and future developments to further decrease cervical cancer deaths.
Evolution of Cervical Cancer Screening: Cervical Cytology Approaches
In the 1930s and 1940s, when cervical cancer was a leading cause of death among women, Dr. George Papanicolaou and others discovered that precancerous and cancerous cells could be identified in cytologic samples from vaginal aspirates. This discovery led to the publication of the first manuscript on the Pap test in 1941.4 Subsequently, collection of the cervical sample (via common sampling devices such as the Ayre spatula), improvements in overall sample quality, and homogeneity of the sample became important as the work of these early pioneers began to receive attention in the medical community. It was not until 1957 that the American Cancer Society endorsed the use of Pap tests in cervical cancer screening programs.5
Retrospective analyses of 1960s Scandinavia provided initial insight into the potential success in decreasing cervical cancer incidence.6 The programs initiated in Finland, Sweden, and Iceland screened > 80% of the population; a significantly lower proportion of women participated in Norway, where screening was performed in only a single county. In 1960, cervical cancer incidence was similar in all four countries. Over the next two decades, cervical cancer incidence was reduced by approximately 50% in Finland, Sweden, and Iceland, yet remained unchanged in Norway.
A separate population-based cervical screening program was implemented in British Columbia, Canada.7 The province-wide program was introduced in 1949 and the impact was dramatic. Between 1955 and 1988, this program was credited with an 85% reduction in cervical cancer incidence and mortality.8 Upon more widespread global cervical cancer screening adoption in the 1950s, population studies began to further demonstrate the effectiveness of conventional Pap testing, the mainstay of successful cytologic screening programs at this time.
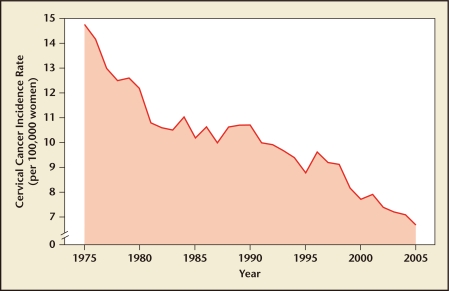
According to US data from the 1970s and 1980s, cervical cancer screening had emerged as the most common cancer screening test. Currently, among women aged 18 to 65 years, approximately 80% have received a Pap test within the previous 3 years.9 Screening with the Pap test was responsible for a reduction in cervical cancer incidence in the United States by nearly 70% between 1955 and the mid-1980s10 (Figure 1). However, an estimated 12,200 new cases and 4210 deaths from cervical cancer were predicted to occur in 2010, suggesting that there is room for improvement and/or need for continued vigilance and adherence to screening protocols.10
Figure 1.
Decrease in cervical cancer incidence rates in the United States over the past quarter century. Data from SEER Cancer Statistics Review, 1975–2005.1
Although the conventional Pap test was responsible for the initial success in reducing the incidence of cervical cancer, the clinical performance of the technology is not without limitations. A broad range of sensitivity (30%–87%) has been reported for the detection of high-grade lesions by the conventional Pap test.11 The conventional Pap test was also found to have a false-negative rate of about 14% to 33%, approximately two-thirds of which is due to limitations of sampling or slide preparation.12 Only a small portion of the sample taken from the patient is transferred to the conventional Pap slide; most of it is discarded along with the sampling device. These limitations may lead to inaccuracies and equivocal diagnoses when using this methodology.
To address these shortcomings, new technologies were introduced. In 1996, the ThinPrep® Pap test (Hologic, Inc, Marlborough, MA) became the first LBC approved by the US Food and Drug Administration (FDA). Implementation of this test, which uses LBC for improved detection of abnormal cervical cells, may be partially responsible for a further decline in cervical cancer following the plateau seen with use of the conventional Pap test (Figure 1). In 1999, a second LBC test was developed and approved by the FDA, the SurePath™ Pap test (Becton, Dickinson and Company, Franklin Lakes, NJ).
In general, LBC begins with the clinician-collected gynecologic sample. Then, using a FDA-approved cervical sampling device, the sample is added to a vial of transport medium preservative rather than being spread on a microscope slide as part of the conventional Pap testing protocol. Samples are then processed and deposited in a cell spot onto a microscope slide. This processing results in a monolayer preparation that may be analyzed by a computer-based imager and reviewed by a cytotechnologist and/or pathologist. As a result of these improvements in sample quality, the clinical sensitivity of LBC over the conventional Pap test in the detection of high-grade lesions has been reported to have increased from 88% to 93% for a single LBC test.13,14 Thus, the benefit of LBC compared with the conventional Pap test is evident not only in improvements in technology, but also in the clinical utility for detecting precancerous lesions or cervical cancer.
LBC Technologies
ThinPrep Pap Test
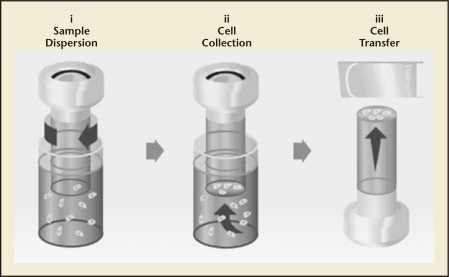
The ThinPrep Pap test represented the first significant advancement in cervical cytology screening in more than 50 years. The test is intended as a replacement for the conventional Pap test for use in screening for the presence of atypical cells, cervical cancer, or its precursor lesions (low-grade squamous intraepithelial lesions [LSIL] and high-grade squamous intraepithelial lesions [HSIL]), in addition to other cytologic categories15 as defined by The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses.16 The foundation of the ThinPrep system is the ThinPrep Processor, an automated slide preparation unit that produces uniform, thin-layer slides, and minimizes obscuring artifacts such as red blood cells and mucus. Specimens are first collected by the clinician with a cervical sampling device. The device is rinsed into a ThinPrep vial containing PreservCyt® transport medium (Hologic, Inc), rather than smearing the cells on a slide. This vial is labeled and sent to the laboratory, where the vial is placed into the ThinPrep Processor. The testing procedure uses a liquid-based filtration process for slide preparation whereby the sample is dispersed, randomized, filtered, and a representative sample is transferred to the slide (Figure 2).15 Slides are reviewed in conjunction with the patient’s clinical history and previous diagnostic cervical procedures.
Figure 2.
ThinPrep® System (Hologic, Inc, Marlborough, MA) sample processing and analysis. Processing a Thin-Prep Pap test sample for analysis involves three essential steps: (i) Rotation of the filter within the sample vial creates currents in the fluid that separate debris and disperse mucus yet preserve cell morphology; (ii) A gentle vacuum (controlled by software for the ThinPrep Processor) created within the filter collects cells on the exterior surface of the membrane; (iii) After the cells are collected on the membrane, the filter is inverted and gently pressed against the microscope slide. Natural attraction and slight positive air pressure cause the cells to adhere to the slide, resulting in an even distribution of cells in a defined circular area.
Pivotal Studies: ThinPrep Pap Test
The pivotal trial for the ThinPrep Pap test demonstrated that the test provides a 65% increase (P < .001) in the diagnosis of LSIL or greater cytology and improvement in specimen quality compared with the conventional Pap test (P < .001).17 The authors concluded that the ThinPrep Pap test is a statistically significant improvement over the use of the conventional Pap test for cervical cancer screening. Subsequent studies substantiated this finding. Díaz-Rosario and Kabawat18 found that the detection of LSIL (71.7%; P < .001) and HSIL (102.5%; P < .001) by the ThinPrep Pap test was significantly better compared with conventional Pap testing. Hatch and colleagues19 determined that the detection of HSIL or greater cytology was 59.7% greater than the conventional Pap test (P < .001). The detection of LSIL cytology was also significantly better than the conventional Pap test (P < .01).
Later studies demonstrated that the ThinPrep Pap test improved the sensitivity of detecting glandular lesions/adenocarcinoma,20–25 leading to FDA approval for detection of such lesions. In addition to cytologic assessment, the ThinPrep Pap test is approved by the FDA for use in testing for high-risk (HR) human papillomavirus (HPV), Chlamydia trachomatis, and Neisseria gonorrhoeae.15 The PreservCyt preservation medium is approved for use with molecular-based tests, including Cervista® HPV HR (Hologic, Inc), Cervista® HPV 16/18 genotyping (Hologic, Inc), Hybrid Capture® 2 (hc2; QIAGEN, Inc, Valencia, CA), Gen-Probe APTIMA COMBO 2® CT/NG (Gen-Probe, Inc, San Diego, CA), Roche COBAS AMPLICOR™ CT/NG (Roche Molecular Diagnostics, Pleasanton, CA), and BD ProbeTec™ CT/GC QX amplified DNA assay (Becton, Dickinson and Company). The FDA also approved a reprocessing step for unsatisfactory specimens originally sampled with the ThinPrep Pap test.26 This reprocessing procedure includes a wash step with 10% glacial acetic acid in CytoLyt for unsatisfactory ThinPrep specimens (Table 1).
Table 1.
FDA Approvals for the ThinPrep Pap and SurePath Pap Tests
| ThinPrep Pap Test | SurePath Pap Test | |
| FDA approval | 1996 | 1999 |
| Compared with conventional Pap test | Significantly more effective | Similar |
| Improved specimen adequacy | Yes | Yes |
| Improved HSIL detection | Yes | Yes |
| Glandular disease detection | Yes | No |
| Adjunctive HPV testing | ||
| Cervista HPV HR | Yes | No |
| Cervista HPV 16/18 genotyping | Yes | No |
| hc2 | Yes | No |
| Adjunctive CT/GN testing | ||
| BD ProbeTec CT/GC QX amplified DNA assay | Yes | Yes |
| Gen-Probe APTIMA COMBO 2 CT/NG | Yes | No |
| Roche COBAS AMPLICOR CT/NG | Yes | No |
| Reprocessing procedure for unsatisfactory specimens | Yes | No |
APTIMA COMBO 2® CT/NG is manufactured by Gen-Probe, Inc, San Diego, CA.
BD ProbeTec™ CT/GC QX amplified DNA assay and SurePath™ Pap test are manufactured by Becton, Dickinson and Company, Franklin Lakes, NJ.
Cervista® HPV HR, Cervista® HPV 16/18 genotyping, and ThinPrep® Pap test are manufactured by Hologic, Inc, Marlborough, MA.
COBAS AMPLICOR™ CT/NG is manufactured by Roche Molecular Diagnostics, Pleasanton, CA.
CT, Chlamydia trachomatis; FDA, US Food and Drug Administration; hc2, Hybrid Capture 2; HPV, human papillomavirus; HR, high risk; HSIL, high-grade squamous intraepithelial lesion; NG, Neisseria gonorrhoeae; Pap, Papanicolaou.
SurePath Pap Test
The second LBC system approved by the FDA, the SurePath Pap test, is intended as a replacement for the conventional Pap test for use in screening for the presence of atypical cells, cervical cancer, or its precursor lesions (LSIL and HSIL), in addition to other cytologic categories27,28 as defined by The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses.16 For the SurePath Pap test, cervical cytology samples are collected with a broom-like device or combination brush/spatula with detachable heads and then placed in a vial with collection medium (SurePath Preservative fluid), thus capturing the entire sample. The test uses a sedimentation process whereby samples are enriched to remove debris, followed by centrifugation to generate a pellet, a portion/subset of which is then applied to the slide for analysis. The automatic slide preparation is carried out by the BD PrepStain™ Slide Processor (Becton, Dickinson and Company) to generate a thin-layer cell sample that is then reviewed by the cytotechnologist.
Pivotal Studies: SurePath Pap Test
The SurePath Pap test increases the detection rate of LSIL and HSIL cytologies by 47% (P < .0011) and 116% (P < .0002), respectively, compared with the conventional Pap test.29 A subsequent study reported similar results, with the SurePath Pap test increasing the detection rate of atypical squamous cells of undetermined significance (ASC-US), LSIL, and HSIL cytologies by 75.1%, 107.2%, and 64.4% (P < .00001), respectively.30 This study also demonstrated a statistically significant reduction (−58.4%; P < .00001) in unsatisfactory analyses for the SurePath Pap test when compared with the conventional Pap test. Additional FDA-approved claims for the SurePath Pap test include use in testing for C trachomatis and N gonorrhoeae using the BD ProbeTec™ CT/GC QX amplified DNA assay.27,31 The SurePath Pap test is not approved by the FDA for HPV testing (Table 1).
Published Data and Evidence Supporting Clinical Utility of LBC Over the Conventional Pap Test
Additional LBC Evidence Supported by Meta-Analyses
Since FDA approval of the ThinPrep Pap test, a number of large, independent meta-analyses have been conducted comparing the clinical performance of LBC and the conventional Pap test. The studies assessed similar performance measures for comparison of the technologies, including the proportion of unsatisfactory slides and the rate of detection of ASC-US, LSIL, and HSIL cytologies. Some of these analyses included studies that only compared the ThinPrep Pap test to the conventional Pap test,32,33 whereas others included multiple technologies (including the ThinPrep Pap test, SurePath Pap test, and/or other technologies not approved by the FDA).34–36 As LBC technologies have different methodologies and different indications by the FDA, analysis of a singular LBC technology compared with conventional cytology may permit cleaner interpretation of the data. It is also important to note that the inclusion/exclusion criteria for each analysis were not uniform.
The analyses carried out by Bernstein and colleagues33 and Abulafia and coworkers32 assessed the performance of the ThinPrep Pap test alone, and demonstrated significant improvement in specimen adequacy and the detection rates of LSIL and HSIL cytologies compared with the conventional Pap test. Klinkhamer and colleagues36 stated that whereas additional analysis on clinical performance was warranted for the SurePath Pap test, the ThinPrep Pap test provided consistent improvements compared with the conventional Pap test in the detection of LSIL and HSIL cytologies. Additional metaanalyses assessed the performance of multiple LBC technologies compared with conventional cytology.34,35 In studies that included LBC technologies as a whole, it was determined that LBC did not provide an improvement in performance over the use of the conventional Pap test. A detailed summary of these studies is presented in Table 2.
Table 2.
Table 2 Meta-Analyses Comparing LBC and CP for Detection of Cervical Abnormalities
| Study | Number of Studies Included in Analysis | LBC Methodologies Included | Study Outcomes (LBC vs CP) |
| Bernstein SJ et al33 | 25 (1990–2000) | ThinPrep Pap test | ThinPrep Pap test similar to CP for diagnosis of ASC-US: OR, 1.03 (95% CI, 0.99–1.06) |
| ThinPrep Processor β1 | ThinPrep Pap test better than CP for diagnosis of: LSIL: OR, 2.15 (95% CI, 2.05–2.26) HSIL: OR, 2.26 (95% CI, 2.06–2.47) | ||
| Abulafia O et al32 | 24 (1990–2002) | ThinPrep Pap test | Overall sensitivity for ThinPrep Pap test was 76% vs 68% for CP |
| Overall specificity for ThinPrep Pap test was 86% vs 79% for CP | |||
| Klinkhamer PJ et al36 | 10 (1995–2000) | ThinPrep Pap test (n = 6) | Sensitivity for detecting ASC-US or greater cytology was lower than CP in studies that included the AutoCyte PREP/SurePath Pap test |
| AutoCyte PREP/SurePath Pap test (n = 4) | Results for detection of LSIL or HSIL cytologies were inconclusive | ||
| Sensitivity for detecting ASC-US or greater, LSIL, or HSIL cytologies trended higher than CP in studies that included the ThinPrep Pap test | |||
| Specificity for detecting ASC-US or greater cytology was lower for the ThinPrep Pap test, but remained unchanged for detection of LSIL and HSIL cytologies compared with CP | |||
| Davey E et al35 | 56; 58 data sets2 (1991–2004) | ThinPrep Pap test (n = 39) CytoRich (n = 9) | No statistically significant difference in UNSAT slides with LBC compared with CP |
| AutoCyte PREP/SurePath (n = 8) CytoScreen (n = 1) | Differences in detection rates between LBC and CP did not vary between paired and independent studies (P = .17; 0.44–0.08) | ||
| PapSpin (n = 1) | LBC classified more slides as HSIL compared with CP in both study designs; greatest difference in independent studies (P = .03) | ||
| Arbyn M et al34 | 9 (1991–2007) | ThinPrep Pap test, ThinPrep Processor β1 (n = 6) | Pooled sensitivities for ASC-US, LSIL, and HSIL cytologies were not statistically different between LBC and CP |
| • AutoCytePrep/SurePath Pap test (n = 1) | Pooled specificities for LSIL and HSIL cytologies were in the same range for LBC compared with CP; the pooled specificity for ASC-US was lower for LBC compared with CP | ||
| • CellSlide (n = 1) | |||
| • DNA-Citoliq (n = 1) |
AutoCyte PREP™ System, CYTORICH™, and SurePath™ Pap test are manufactured by Becton, Dickinson and Company, Franklin Lakes, NJ.
CellSlide™ is manufactured by Alphagenics International, Lausanne, Switzerland.
CytoScreen® is manufactured by BJ Medic, Sarajevo, Bosnia.
DNA-Citoliq is manufactured by Digene Brazil, São Paulo, SP, Brazil.
Shandon PapSpin™ is manufactured by ThermoShandon, Pittsburgh, PA.
ThinPrep® Pap test and ThinPrep Processor are manufactured by Hologic, Inc, Marlborough, MA.
The ThinPrep Processor β is a prototype instrument and not available for commercial use.
Data sets, representing distinct populations, were extracted from two separate studies.
ASC-US, atypical squamous cells of undetermined significance; CI, confidence interval; CP, conventional Papanicolaou test; HSIL, high-grade squamous intraepithelial lesions; LBC, liquid-based cytology; LSIL, low-grade squamous intraepithelial lesions; OR, odds ratio; UNSAT, unsatisfactory.
Independent Analysis From the College of American Pathologists Laboratory Survey
The effectiveness of LBC compared with the conventional Pap test has been analyzed in real-world settings beyond the limitations presented in clinical trials. The College of American Pathologists (CAP) recently conducted a survey to assess practice patterns in cervical cytology programs. These surveys form the basis for benchmarks in the CAP Laboratory Accreditation Program. This independently pooled analysis represents 679 of the 1621 laboratories surveyed across the United States. The objective of this survey was to quantify changes in cytology laboratory practices, detail the implementation of new technologies, and quantify the LBC reporting rates. It is currently estimated that approximately 90% of all cervical cytology in the United States is processed with LBC.37 Most laboratories in the United States use one of the two FDA-approved LBC methods for most, if not all, of their cytology screening. For the most recently published survey,37 questionnaires were mailed to 1621 laboratories and the benchmark data were based on 679 responding laboratories. The laboratories were asked to report 2006 data for The Bethesda System 2001 categories for conventional smears and the two LBC methods. Overall, between 2003 and 2006, there was a decrease in the number of laboratories offering only conventional Pap testing (24.4% vs 13.7%) and an increase in laboratories offering only LBC (9.3% vs 25.5%).37 Of the laboratories who reported using LBC (n = 587), approximately 70% (411/587) used the ThinPrep Pap test compared with 30% (176/587) of those who reported using the SurePath Pap test.37
A summary of the performancebased analysis, assessed via laboratory reporting rates, is found in Table 3. The median LSIL detection rates for SurePath Pap test (2.5%) and Thin-Prep Pap test (3.0%) were significantly greater than for the conventional Pap test (1.3%; P < .001). The median HSIL detection rates were significantly higher for the ThinPrep Pap test (0.6%) than either the SurePath Pap test or the conventional Pap test (both 0.3%; P < .05). Median unsatisfactory rates were significantly better for the SurePath Pap test (0.3%) compared with ThinPrep Pap test preparations and the conventional Pap test (1.1% and 1.0%, respectively; P < .05). The ASC-US detection rates for both the SurePath Pap test (4.1%) and the ThinPrep Pap test (4.9%) were significantly higher than the conventional Pap test (2.4%; P < .001). It is clear from this analysis that each of the LBC methods provides specific benefits compared with the conventional Pap test, yet the two LBC technologies differ in their clinical performance.
Table 3.
The CAP Laboratory Survey: Median Reporting Rates (50th Percentile) for Conventional Papanicolaou (Pap), SurePath Pap, and ThinPrep Pap Cytologic Preparations Across the Major Clinical Cytologic Categories37
| Conventional | SurePath Pap | ThinPrep | |
| Category | Pap Test | Test | Pap Test |
| ASC-US | 2.4 | 4.1* | 4.9* |
| LSIL | 1.3 | 2.5* | 3.0* |
| HSIL | 0.3 | 0.3 | 0.6† |
| UNSATS | 1.0 | 0.3‡ | 1.1 |
SurePath™ Pap test is manufactured by Becton, Dickinson and Company, Franklin Lakes, NJ.
ThinPrep® Pap test is manufactured by Hologic, Inc, Marlborough, MA.
P < .001 compared with CP.
P = .05 compared with conventional Pap and SurePath Pap tests.
P = .05 compared with conventional Pap and ThinPrep Pap tests.
ASC-US, atypical squamous cells of undetermined significance; CAP, College of American Pathologists; HSIL, high-grade squamous intraepithelial lesion; LBC, liquid-based cytology; LSIL, low-grade squamous intraepithelial lesion; UNSATS, unsatisfactory for evaluation.
Imaging Evidence
The latest development in the evolution of cervical cytology screening is the introduction of computer-guided imaging for slide interpretation. Each of the manufacturers of approved LBC technologies has a marketed proprietary imaging system. Hologic offers the ThinPrep Imaging System (TIS; Hologic, Inc). The TIS is a fully integrated, interactive computer system that assists cytotechnologists and pathologists in the primary screening and diagnosis of ThinPrep Pap test slides.38 This system combines imaging technology with human interpretive expertise to improve cervical cancer screening efficiency and performance. The TIS consists of an image processor and one or more review scopes. The system makes use of computer imaging and algorithms to select 22 fields of view for presentation to a cytotechnologist, who reviews and marks the slide. Becton, Dickinson and Company, the manufacturer of the SurePath Pap test, offers two systems: the BD FocalPoint Slide Profiler (formerly AutoPap Primary Screening System) and the BD FocalPoint GS Imaging System. The former uses multiple algorithms to rank slides based on probability of abnormality.39 Slides can be classified on a scale as well as a portion for no further review, which would not be reviewed by cytotechnologists. The BD FocalPoint GS Imaging System includes screening capabilities of the Slide Profiler with field location capability to assist in screening of BD SurePath Pap test slides. Clinical trial data for the ThinPrep Imaging System and the BD FocalPoint GS Imaging System showed increased disease detection when compared with manual screening methods. Studies from the real-world setting evaluating the ThinPrep Imager demonstrate significantly improved detection of cervical abnormalities (ASC-US, LSIL, and HSIL) as well as improvements in productivity compared with manually read preparations.40,41 These TIS studies have been conducted in North America, Europe, and Australia. To date, only the clinical trial data for the BD FocalPoint GS Imaging System have been published.42 No commercial use reports have appeared in the literature.
Further Improvements in Diagnostic Capabilities of Cervical Cancer Prevention
Although the Pap test has been effective, deaths from cervical cancer still occur. As discussed, Pap testing has demonstrated false-negative rates, many of which are caused by sampling or detection errors.43,44 By contrast, molecular-based HPV DNA testing identifies women at risk for developing preinvasive cervical lesions and subsequent invasive cancer while decreasing the subjectivity of cervical cytologic assessment.45 HPV DNA testing has also shown greater sensitivity compared with Pap testing in detection of preinvasive high-grade cervical lesions.46
There are approximately 40 HPV genotypes that infect the genital mucosa, 13 of which are considered HR and are precursors for the development of cervical cancer.47 Both persistence of HR HPV infection and genotype of the infecting HPV contribute to the development of cervical intraepithelial neoplasia (CIN),48–50 as well as progression from CIN to cervical cancer.51 HPV 16 and 18 are the most common HR types associated with cervical cancer, accounting for 76% of cases in the United States.52 Therefore, HPV molecular-based detection and genotyping technologies may offer additional clinical benefits when screening for cervical cancer. Advancements in this area include the following HR HPV tests approved by the FDA: hc2,53 CERVISTA HPV HR,54 and CERVISTA HPV 16/18 genotyping tests.54
Although co-collection is an option for using these tests in conjunction with the conventional Pap test, the ThinPrep Pap test is approved and allows for adjunctive and triage testing of women with ASC-US cytology results. The ThinPrep Pap test has been widely studied in the ASCUS/LSIL Triage Study (ALTS)53 for use with hc2 and also in the more recent clinical trial for the CERVISTA HPV HR and 16/18 genotyping tests.54 The incorporation of HPV genotyping identifies women infected with specific HR HPV types (ie, HPV 16 and/or 18). This approach may help to stratify women at greater risk for developing precancerous lesions and subsequent invasive cervical cancer. In addition, genotyping women ≥ 30 years of age with negative cytology and a positive HR HPV55 result provides another opportunity to identify women at risk for developing disease. 56 The combination of cervical cytology and HPV DNA testing may represent the potential to further improve the detection of preinvasive disease and thereby reduce the incidence of high-grade lesions compared with cytology testing alone. Improving molecular-based strategies will aid in the increased detection of precancerous lesions and cervical cancer.
Conclusions
There have been a number of technological advances in cervical cancer screening since the introduction of Pap testing more than 60 years ago. Among them, the implementation of routine screening programs is recognized as a public health success, whereas LBC represents a significant shift in testing methodology. For the ThinPrep Pap and SurePath Pap tests, the pivotal trials and subsequent FDA indications demonstrate the individual benefits of these tests. In addition, multiple meta-analyses provide evidence that the LBC tests perform differently from each other, and also from the conventional Pap test. The independent analysis presented by the CAP survey further demonstrates that LBC is the preferred methodology for cytologic testing across laboratories in the United States. However, we are very likely to see additional advances in cervical screening as computer imaging evolves and additional molecular markers are identified. As new technologies emerge, efforts must be vigilant to ensure that the unparalleled success of the past is carried forward into the future.
Main Points.
Although the conventional Papanicolaou (Pap) test was responsible for the initial success in reducing the incidence of cervical cancer, the clinical performance of the technology is not without limitations: a broad range of sensitivity (30%–87%) has been reported; a false-negative rate of about 14% to 33%, approximately two-thirds of which is due to limitations of sampling or slide preparation. These limitations may lead to inaccuracies and equivocal diagnoses when using this methodology.
The pivotal trial for the ThinPrep Pap test demonstrated that the test provides a 65% increase (P < .001) in the diagnosis of low-grade squamous intraepithelial lesions (LSIL) or greater cytology and improvement in specimen quality compared with the conventional Pap test (P < .001). Subsequent studies substantiated this finding.
The SurePath Pap test increases the detection rate of LSIL and high-grade squamous intraepithelial lesions cytologies by 47% (P < .0011) and 116% (P < .0002), respectively, compared with the conventional Pap test.
Overall, between 2003 and 2006, there was a decrease in the number of laboratories offering only conventional Pap testing (24.4% vs 13.7%) and an increase in laboratories offering only liquid-based cytology (9.3% vs 25.5%).
Human papillomavirus molecular-based detection and genotyping technologies may offer additional clinical benefits when screening for cervical cancer.
Footnotes
Editorial support was sponsored by Hologic, Inc, and provided by David E. Kaminsky, PhD, at AlphaBioCom. The authors had full control in the initiation and development of the content and assume such responsibility. The authors did not receive compensation for doing this work.
References
- 1.National Cancer Institute, authors. SEER Cancer Statistics Review, 1975–2005. [Accessed February 10, 2011]. http://seer.cancer.gov/csr/1975_2005/results_merged/sect_05_cervix_uteri.pdf.
- 2.Melnikow J, Kulasingam S, Slee C, et al. Surveillance after treatment for cervical intraepithelial neoplasia: outcomes, costs, and cost-effectiveness. Obstet Gynecol. 2010;116:1158–1170. doi: 10.1097/AOG.0b013e3181f88e72. [DOI] [PubMed] [Google Scholar]
- 3.Sawaya GF. Rightsizing cervical cancer screening: comment on “Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone. Arch Intern Med. 2010;170:985–986. doi: 10.1001/archinternmed.2010.133. [DOI] [PubMed] [Google Scholar]
- 4.Papanicolaou G, Traut H. Diagnostic value of vaginal smears in carcinoma of uterus. Am J Obstet Gynecol. 1941;42:193–206. [PubMed] [Google Scholar]
- 5.Breslow L, Wilner D, Agran L, et al. A History of Cancer Control in the US, With Emphasis on the Period 1946–1971. Bethesda, MD: National Institutes of Health; 1977. [Google Scholar]
- 6.Johannesson G, Geirsson G, Day N. The effect of mass screening in Iceland, 1965–74, on the incidence and mortality of cervical carcinoma. Int J Cancer. 1978;21:418–425. doi: 10.1002/ijc.2910210404. [DOI] [PubMed] [Google Scholar]
- 7.Cannistra SA, Niloff JM. Cancer of the uterine cervix. N Engl J Med. 1996;334:1030–1038. doi: 10.1056/NEJM199604183341606. [DOI] [PubMed] [Google Scholar]
- 8.Benedet JL, Anderson GH, Matisic JP. A comprehensive program for cervical cancer detection and management. Am J Obstet Gynecol. 1992;166:1254–1259. doi: 10.1016/s0002-9378(11)90618-8. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics, authors. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: US Department of Health and Human Services; 2010. [PubMed] [Google Scholar]
- 10.American Cancer Society Web site, authors. What are the key statistics about cervical cancer? [Accessed February 10, 2011]. http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-key-statistics. Revised December 23, 2010.
- 11.American College of Obstetricians and Gynecologists, authors. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol. 2008;112:1419–1444. doi: 10.1097/AOG.0b013e318192497c. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann K, Hall SA, Nanda K, et al. Systematic Evidence Review Number 25: Screening for Cervical Cancer. Rockville, MD: US Department of Health and Human Services; [Accessed February 10, 2011]. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. [Google Scholar]
- 13.Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7932 women. Br J Cancer. 2001;84:1616–1623. doi: 10.1054/bjoc.2001.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson ML, Zahniser DJ, Sherman ME, et al. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer. 1999;87:48–55. doi: 10.1002/(sici)1097-0142(19990425)87:2<48::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.The ThinPrep 2000 System [package insert], authors Marlborough, MA: Hologic, Inc;; 2010. [Google Scholar]
- 16.Solomon D, Davey D, Kurman R, et al. Forum Group Members; Bethesda 2001 Workshop, authors. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 17.Lee KR, Ashfaq R, Birdson GG, et al. Comparison of conventional Papanicolaou smears and a fluid-based, thin-layer system for cervical cancer screening. Obstet Gynecol. 1997;90:278–284. doi: 10.1016/S0029-7844(97)00228-7. [DOI] [PubMed] [Google Scholar]
- 18.Díaz-Rosario LA, Kabawat SE. Performance of a fluid-based, thin-layer Papanicolaou smear method in the clinical setting of an independent laboratory and an outpatient screening population in New England. Arch Pathol Lab Med. 1999;123:817–821. doi: 10.5858/1999-123-0817-POAFBT. [DOI] [PubMed] [Google Scholar]
- 19.Hatch KD, Sheets E, Kennedy A, et al. Multicenter direct to vial evaluation of a liquidbased pap test. J Low Genit Tract Dis. 2004;8:308–312. doi: 10.1097/00128360-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Ashfaq R, Gibbons D, Vela C, et al. ThinPrep Pap test. Accuracy for glandular disease. Acta Cytol. 1999;43:81–85. doi: 10.1159/000330872. [DOI] [PubMed] [Google Scholar]
- 21.Bai H, Sung CJ, Steinhoff MM. ThinPrep Pap test promotes detection of glandular lesions of the endocervix. Diagn Cytopathol. 2000;23:19–22. doi: 10.1002/1097-0339(200007)23:1<19::AID-DC4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter AB, Davey DD. ThinPrep Pap test: performance and biopsy follow-up in a university hospital. Cancer. 1999;87:105–112. doi: 10.1002/(sici)1097-0142(19990625)87:3<105::aid-cncr2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Guidos BJ, Selvaggi SM. Detection of endometrial adenocarcinoma with the ThinPrep Pap test. Diagn Cytopathol. 2000;23:260–265. doi: 10.1002/1097-0339(200010)23:4<260::aid-dc9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Schorge JO, Hossein Saboorian M, Hynan L, Ashfaq R. ThinPrep detection of cervical and endometrial adenocarcinoma: a retrospective cohort study. Cancer. 2002;96:338–343. doi: 10.1002/cncr.10761. [DOI] [PubMed] [Google Scholar]
- 25.Wang N, Emancipator SN, Rose P, et al. Histologic follow-up of atypical endocervical cells. Liquid-based, thin-layer preparation vs. conventional Pap smear. Acta Cytol. 2002;46:453–457. doi: 10.1159/000326860. [DOI] [PubMed] [Google Scholar]
- 26.FDA approval for reprocessing unsatisfactory ThinPrep samples with glacial acetic acid wash [press release] Marlborough, MA: Hologic, Inc; 2006. Jan 23, [Google Scholar]
- 27.SurePath? Collection [product insert] Franklin Lakes, NJ: Becton, Dickinson and Company; 2009. [Google Scholar]
- 28.US Food and Drug Administration, authors. [Accessed February 10, 2011];AutoCyte PREP? System Summary of Safety and Effectiveness Data. P970018. 1999 Jun 17; http://www.accessdata.fda.gov/cdrh_docs/pdf/P970018b.pdf. [Google Scholar]
- 29.Marino JF, Fremont-Smith M. Direct-to-vial experience with AutoCyte PREP in a small New England regional cytology practice. J Reprod Med. 2001;46:353–358. [PubMed] [Google Scholar]
- 30.Fremont-Smith M, Marino J, Griffin B, et al. Comparison of the SurePath liquid-based Papanicolaou smear with the conventional Papanicolaou smear in a multisite direct-to-vial study. Cancer. 2004;102:269–279. doi: 10.1002/cncr.20599. [DOI] [PubMed] [Google Scholar]
- 31.BD SurePath? Pap Test, authors. Becton, Dickinson and Company Web Site. [Accessed February 10, 2011]. http://www.bd.com/tripath/products/surepath/ctgc.asp.
- 32.Abulafia O, Pezzullo JC, Sherer DM. Performance of ThinPrep liquid-based cervical cytology in comparison with conventionally prepared Papanicolaou smears: a quantitative survey. Gynecol Oncol. 2003;90:137–144. doi: 10.1016/s0090-8258(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein SJ, Sanchez-Ramos L, Ndubisi B. Liquid-based cervical cytologic smear study and conventional Papanicolaou smears: a metaanalysis of prospective studies comparing cytologic diagnosis and sample adequacy. Am J Obstet Gynecol. 2001;185:308–317. doi: 10.1067/mob.2001.116736. [DOI] [PubMed] [Google Scholar]
- 34.Arbyn M, Bergeron C, Klinkhamer P, et al. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111:167–177. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 35.Davey E, Barratt A, Irwig L, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquidbased versus conventional cervical cytology: a systematic review. Lancet. 2006;367:122–132. doi: 10.1016/S0140-6736(06)67961-0. [DOI] [PubMed] [Google Scholar]
- 36.Klinkhamer PJ, Meerding WJ, Rosier PF, Hanselaar AG. Liquid-based cervical cytology. Cancer. 2003;99:263–271. doi: 10.1002/cncr.11673. [DOI] [PubMed] [Google Scholar]
- 37.Eversole GM, Moriarty AT, Schwartz MR, et al. Practices of participants in the College of American Pathologists interlaboratory comparison program in cervicovaginal cytology, 2006. Arch Pathol Lab Med. 2010;134:331–335. doi: 10.5858/134.3.331. [DOI] [PubMed] [Google Scholar]
- 38.Miller FS, Nagel LE, Kenny-Moynihan MB. Implementation of the ThinPrep imaging system in a high-volume metropolitan laboratory. Diagn Cytopathol. 2007;35:213–217. doi: 10.1002/dc.20627. [DOI] [PubMed] [Google Scholar]
- 39.Becton, Dickinson and Company Web site., authors Cervical Cytology: BD FocalPoint? GS Imaging System. [Accessed February 10, 2011]. http://www.bd.com/tripath/labs/fp_gs_system_process.asp.
- 40.Davey E, d’Assuncao J, Irwig L, et al. Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study. BMJ. 2007;335:31. doi: 10.1136/bmj.39219.645475.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papillo JL, St John TL, Leiman G. Effectiveness of the ThinPrep Imaging System: clinical experience in a low risk screening population. Diagn Cytopathol. 2008;36:155–160. doi: 10.1002/dc.20779. [DOI] [PubMed] [Google Scholar]
- 42.Wilbur DC, Black-Schaffer WS, Luff RD, et al. The Becton Dickinson FocalPoint GS Imaging System: clinical trials demonstrate significantly improved sensitivity for the detection of important cervical lesions. Am J Clin Pathol. 2009;132:767–775. doi: 10.1309/AJCP8VE7AWBZCVQT. [DOI] [PubMed] [Google Scholar]
- 43.Mandelblatt JS, Lawrence WF, Womack SM, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA. 2002;287:2372–2381. doi: 10.1001/jama.287.18.2372. [DOI] [PubMed] [Google Scholar]
- 44.Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and followup of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 45.Boulet GA, Horvath CA, Berghmans S, Bogers J. Human papillomavirus in cervical cancer screening: important role as biomarker. Cancer Epidemiol Biomarkers Prev. 2008;17:810–817. doi: 10.1158/1055-9965.EPI-07-2865. [DOI] [PubMed] [Google Scholar]
- 46.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 47.Munoz N, Bosch FX, Castellsagué X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 48.Castle PE, Rodriguez AC, Burk RD, et al. Proyecto Epidemiológico Guanacaste (PEG)^Group, authors. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez AC, Schiffman M, Herreo R, et al. Proyecto Epidemiológico Guanacaste Group, authors. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100:513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wheeler CM, Hunt WC, Schiffman M, Castle PE Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study Group, authors. Human papillomavirus genotypes and the cumulative 2-year risk of cervical precancer. J Infect Dis. 2006;194:1291–1299. doi: 10.1086/507909. [DOI] [PubMed] [Google Scholar]
- 51.Jit M, Gay N, Soldan K, et al. Estimating progression rates for human papillomavirus infection from epidemiological data. Med Decis Making. 2010;30:84–98. doi: 10.1177/0272989X09336140. [DOI] [PubMed] [Google Scholar]
- 52.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 53.Solomon D, Schiffman M, Tarone R ALTS Study group, authors. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293–299. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 54.Einstein MH, Martens MG, Garcia FA, et al. Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol. 2010;118:116–122. doi: 10.1016/j.ygyno.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 55.American Society for Colposcopy and Cervical Pathology (ASCCP), authors HPV Genotyping Clinical Update. Hagerstown, MD: ASCCP; 2009. [Accessed February 10, 2011]. http://www.asccp.org/pdfs/consensus/clinical_update_20090408.pdf. [Google Scholar]
- 56.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of typespecific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]