Abstract
OBJECTIVE
To examine the utility of psychotherapy in managing treatment resistant depression.
DATA SOURCES
PubMed, PsycInfo, Embase, Cochrane Registry of Controlled Clinical Trials, article bibliographies.
REVIEW METHODS
Eligible articles had to be in English and include English-speaking adult outpatients from general medical or mental health clinics. Studies had to be randomized clinical trials (RCT) involving at least one of the following psychotherapy modalities: cognitive therapy, interpersonal therapy, or behavior therapy. Patients were considered treatment resistant if they reported partial or no remission following treatment with an adequate antidepressant dose for ≥6 weeks. Exclusion criteria included receiving psychotherapy at the time of recruitment, and/or comorbid psychiatric conditions unlikely to be treated outside of specialized mental health care (e.g., severe substance abuse). Due to heterogeneity in study designs, a summary estimate of effect was not calculated. Studies were critically analyzed and a qualitative synthesis was conducted.
RESULTS
Of 941 original titles, 13 articles evaluating 7 unique treatment comparisons were included. Psychotherapy was examined as an augmentation to antidepressants in five studies and as substitution treatment in two studies. A total of 592 patients were evaluated (Mean age ~40 y; Females = 50-85%; Caucasians ≥75%). The STAR*D trial used an equipoise stratified randomization design; the remaining studies were RCTs. Compared to active management, two good quality trials showed similar benefit from augmenting antidepressants with psychotherapy; one fair quality and one poor quality trial showed benefit from psychotherapy augmentation; and one good and one poor trial found similar benefit from substituting psychotherapy for antidepressants. One fair quality trial showed lithium augmentation to be more beneficial than psychotherapy.
CONCLUSIONS
Review demonstrates the utility of psychotherapy in managing treatment resistant depression. However, evidence is sparse and results are mixed. Given that quality trials are lacking, rigorous clinical trials are recommended to guide practice. In the interim, primary care providers should consider psychotherapy when treating patients with treatment resistant depression.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-010-1608-2) contains supplementary material, which is available to authorized users.
KEY WORDS: treatment resistant depression, depression treatment, primary care, psychotherapy
According to the World Health Organization, major depressive disorder (MDD) is the leading cause of disability in the developed world.1 It is associated with increases in health care utilization, functional impairment, and mortality.2 Of the estimated 13% that will experience MDD in their lifetimes, 20% will experience chronic depression and 60-85% will experience relapse or recurrence.2,3 Providers have access to several effective and efficacious treatment options, including a wide array of antidepressant medications4–8 and psychotherapeutic interventions.9–13 For chronic MDD, a combination of psychotherapy and medication has been found to be the most effective.14,15 Such diversity of treatment alternatives is necessary, as fewer than 50% of patients fully remit after an adequate trial of antidepressants or psychotherapy.16–19
MDD patients who do not fully remit after initial (“Step 1”) treatment are thought to have treatment resistant depression, defined as an inadequate response to at least one trial of an antidepressant, at an adequate dose, for 6 weeks or longer.20 According to guidelines, treatment resistant depression may be treated with four Step 2 strategies: augmenting current regimens with another antidepressant, substituting current regimens with another antidepressant, augmenting treatment through adding psychotherapy, or substituting psychotherapy for antidepressant medications.21–23 However, studies have shown that it is more common to consider medication changes than to consider psychotherapy.24,25 This may be in part related to insufficient empirical consensus regarding the use of psychotherapy in treatment resistant depression.
To address this issue, the current systematic review aimed to determine whether substituting or augmenting current antidepressant treatment with psychotherapy is effective for treating adults with treatment resistant depression.
METHODS
We searched published literature in PubMed, Embase, PsycInfo, Cochrane Database of Controlled Clinical Trials, and bibliographies of articles for relevant randomized clinical trials. Searches were conducted based on three concepts: a) depression or depressive disorder; b) treatment resistance/refractory depression; and c) psychotherapy. Sources were searched from database inception to 07 September 2010 and the study population was limited to adults 18 years and older.
To be eligible for inclusion, articles had to be published in English and include English-speaking adult outpatients from general medical or mental health clinics. Studies that recruited patients exclusively from specialty medical clinics (e.g., heart failure clinics) were excluded to yield samples reflective of a primary care patient population. To be eligible, trials had to involve at least one of the following psychotherapy modalities: cognitive therapy, interpersonal therapy, or behavior therapy. These were selected because they have the strongest empirical support in the treatment for depression.26
Patients were considered treatment resistant if they experienced either partial or no remission after being treated with adequate dose of an antidepressant for at least 6 weeks. Studies were excluded if patients were receiving psychotherapy at the time of recruitment, and/or if patients had comorbid psychiatric conditions that were unlikely to be treated by primary care clinicians (e.g., suicidal ideation, severe substance abuse). Two of the authors who were trained researchers (RBT, JAN) independently reviewed citations identified through the literature search. Articles clearly not meeting the inclusion criteria were excluded at the title and abstract level. The remaining articles were identified for full text review; those that did not meet inclusion criteria were excluded. Disagreements were resolved through discussion between the two reviewers and full consensus was achieved at each stage.
Study characteristics and results for included RCTs were synthesized along the following parameters: definition of treatment resistant depression; type of psychotherapy; comparator; setting; sample size; inclusion/exclusion criteria; age/sex/race of sample; duration of current depressive episode; number of prior episodes; number of hospitalizations; number of suicide attempts; interviewer-rated depression severity (if applicable); self-reported depression severity (if applicable); and duration of follow-up.
The overall quality of each study was rated based on the following characteristics: completeness of follow-up (<30% drop-out rate, <10% differential drop-out rate considered optimal); method to address incomplete data; adequacy of randomization; adequacy of allocation concealment; outcome assessment blind to intervention allocation; and whether there was stated or implied conflict of interest. Because of considerable heterogeneity in study designs, a summary estimate of effect was not calculated. Instead, studies were critically analyzed and a qualitative synthesis was conducted, which is summarized in narrative and evidence tables (Appendix A; available online).
RESULTS
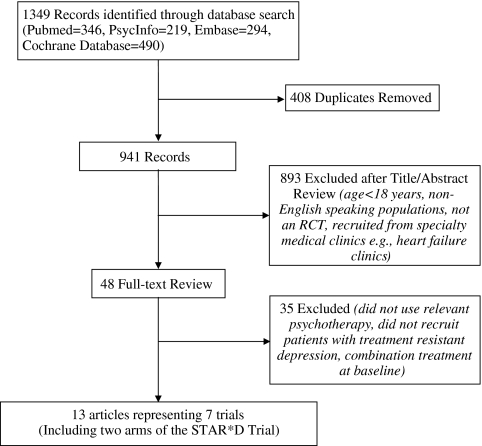
Our search identified 941 unique articles; 893 were excluded at the title and abstract level, and 35 were subsequently excluded following full-text review (Fig. 1). Thirteen articles, representing six unique studies, were included in the final review and are described in detail under Appendix B (available online).20,23,27–37 One of the studies—the STAR*D trial—examined psychotherapy in separate treatment arms as either an augmentation or substitution treatment. Because each employed a unique comparison group, these two arms were treated as separate studies in this review. Therefore, the narrative and evidence tables summarize seven studies, with the caveat that these are from six trials since two of the studies represent separate arms of the STAR*D trial.
Figure 1.
Literature flow.
Sample Characteristics
A total of 592 patients were evaluated. Participants were recruited from mental health and primary care clinics. Three studies (including both STAR*D arms) were conducted in the United States, three in the United Kingdom, and one in Canada. Sample sizes ranged from 24 to 304 participants; two studies contributed 78% of the subjects.23,33 Average age was approximately 40 years. Females comprised half to eighty percent of the studies’ participants, and Caucasians represented at least 75% of the racial makeup in the three studies that reported race. The average length of patients’ current depressive episodes ranged from 30 to 123 weeks, with the average number of lifetime depressive episodes ranging from 2.2 to 8.5. Depression severity was measured prior to initiating Step 2 treatment in all studies using self-report measures. These characteristics are summarized in Table 1 and show significant heterogeneity in depression severity and chronicity.
Table 1.
Sample Characteristics
| Study | Thase et al. 200723 (Augment) | Thase et al. 200723 (Substitute) | Scott et al. 200033 | Harley et al. 200828 | Kennedy et al. 200337 | Blackburn and Moore 1997a27 | Wiles et al. 200835 |
|---|---|---|---|---|---|---|---|
| Sample size, n | |||||||
| Psychotherapy | 65 | 36 | 80 | 13 | 23 | 17 | 14 |
| Comparator | 117 | 86 | 78 | 11 | 21 | 20 | 11 |
| Age, y (M ± SD) | |||||||
| Psychotherapy | 40.6 ± 11.5 | 43.4 ± 14.7 | 43.5 ± 9.8 | 41.8 | 40.7 ± 12.5 | 37.8 ± 13.1 | 45.5 ± 12.8 |
| Comparator | 39.7 ± 13.5 | 41.5 ± 13.3 | 43.2 ± 11.2 | 37.7 ± 11.3 | 40.1 ± 12.7 | 45.1 ± 11.1 | |
| Female, n (%) | |||||||
| Psychotherapy | 41 (63%) | 22 (61%) | 37 (46%) | 18 (75%) | 12 (52%) | 17 (77%)a | 12 (85.7%) |
| Comparator | 78 (67%) | 53 (62%) | 41 (53%) | 12 (57%) | 17 (65%)a | 9 (81.8%) | |
| Caucasian, n (%) | |||||||
| Psychotherapy | 52 (80%) | 28 (78%) | _ | 20 (83%) | _ | _ | _ |
| Comparator | 99 (85%) | 63 (73%) | |||||
| Duration of current episode, wks (M±SD) | |||||||
| Psychotherapy | 129 ± 214 | 76 ± 135 | 62.9 | 28.7 ± 18.8 | 126 ± 170 | 30.4 ± 6.1 | 86% > 1 yr |
| Comparator | 87 ± 206 | 115 ± 234 | 56.4 | 41.8 ± 53.6 | 120 ± 161 | 29.9 ± 5.6 | 82% > 1 yr |
| Number of prior MDD episodes (M ± SD) | |||||||
| Psychotherapy | 7.3 ± 14.1 | 8.7 ± 18.8 | 2 (median) | Not reported | 2.1 ± 1.5 | 4.1 ± 3.4 | Not reported |
| Comparator | 4.6 ± 5.4 | 8.4 ± 16.0 | 2 (median) | 2.3 ± 1.4 | 3.2 ± 2.2 | ||
| Baseline HAM-Db scores | |||||||
| Psychotherapy | 17.8 ± 5.7 | 16.4 ± 6.2 | 12.1 ± 2.7 | 16.2 ± 4.5 | 12.1 ± 2.2 | 11.8 ± 6.3 | Not administered |
| Comparator | 16.0 ± 6.7 | 16.0 ± 6.7 | 12.2 ± 2.9 | 18.6 ± 4.7 | 11.6 ± 1.9 | 10.6 ± 6.8 | |
| Baseline self-report scores | QIDS-SRd | QIDS-SR | BDIc | BDI | BDI | BDI | BDI |
| Psychotherapy | 11.9 ± 4.3 | 11.2 ± 4.3 | 21.7 ± 7.7 | 27.3 ± 8.8 | 22.7 ± 8.6 | 20.4 ± 11.1 | 31.1 ± 8.5 |
| Comparator | 12.0 ± 4.6 | 12.1 ± 4.6 | 22.3 ± 8.0 | 27.4 ± 11.7 | 22.4 ± 10.3 | 19.7 ± 14.2 | 26.8 ± 6.8 |
| Setting | MHC & PCf | MHC & PC | MHCe | MHC | MHC | MHC | PC |
| Location | U.S.A. | U.S.A. | England | U.S.A. | Canada | Scotland | England |
aHAM-D, BDI and QIDS-SR scores for this study based on smaller number that enrolled in phase 2 of the study following initial treatment with antidepressants; bHAM-D = Hamilton Depression Scale; cBDI = Beck Depression Inventory; dQIDS-SR = Quick Inventory of Depressive Symptoms-Self Report; eMHC = Mental Health Clinic; fPC = Primary Care Clinic
To determine treatment resistant depression, studies used different criteria (Table 2) but followed similar methodology. First, a baseline diagnosis of MDD was confirmed. Second, patients underwent antidepressant treatment at a pre-defined adequate dose. Third, patients were evaluated using a validated measure of depression severity to ensure residual symptoms of MDD. All studies except Harley et al. (2008) reported criteria used to determine initial depression diagnosis. Hamilton Depression Rating Scale (HAM-D) scores, either singly or in combination with Beck Depression Inventory (BDI) scores, were the most common measure of residual depression although Wiles et al. (2008) used the Revised Clinical Interview Schedule to determine whether patients continued to meet ICD-10 criteria for MDD.
Table 2.
Criteria to Determine Treatment Resistant Depression
| Study | Thase et al. 200723 | Scott et al. 200033 | Harley et al. 200828 | Kennedy et al. 200337 | Blackburn and Moore 199727 | Wiles et al. 200835 |
|---|---|---|---|---|---|---|
| Criteria for initial MDD diagnosis | Hx of MDD; HAM-D ≥ 14 | MDD episode in previous 18 months according to DSM-III-R; residual symptoms for ≥8 weeks | Not Reported | HAM-D = 17 ≥ 16 | Unipolar MDD using SADSa; HAM-D ≥ 16 | Step 1: Current AD use |
| Step 2: BDI ≥ 15 & adherent with AD treatment (Morisky Scale) | ||||||
| 1st step ADb treatment: Type and Dosage | Citalopram 20 mg/day titrated to 40 mg by week 4 if needed; Max. 60 mg/day by week 6 | Tricyclic AD, SSRI, atypical AD, or MAOI; Min. dose equivalent to 125 mg amitriptyline | As prescribed by non-study psychiatrist | Moclobemide (300-600 mg/day) OR Paroxetine (20-40 mg/day) OR Sertraline (50-200 mg/day) OR Venlafaxine (75-225 mg/day) | As prescribed by non-study practitioner; Equivalent to 100 mg amitriptyline OR 45 mg phenelzine OR 20 mg Sertraline | As prescribed by non-study general practitioner |
| 1st Step AD Treatment: Duration | 14 weeks | ≥8 weeks (at least 4 weeks of adequate dose) | ≥6 weeks | 8-14 weeks | 16 weeks | ≥6 weeks |
| Was 1st AD step treatment provided in the study? | Yes | No | No | Yes | No | No |
| Criteria to Determine Persistence Following 1st Step AD Treatment | HAM-D ≥ 14 | HAM-D ≥ 8 & BDI ≥ 9 | MDD on SCID-Ic | HAM-D = 8-15 | Moderate symptoms on BDI and HAM-D > 11 | ICD-10 diagnosis of MDD based on Revised Clinical Interview Schedule |
aSADS = Schedule for Affective Disorders and Schizophrenia; bAD = Antidepressant; cSCID-I = Structured Clinical Interview for DSM-III-R, I
Study Design & Interventions
The two STAR*D studies used an equipoise stratified randomization design, that is, patients were allowed to refuse randomization to non-preferred treatment arms and were randomized only within those arms which they deemed acceptable. The remainder used true randomization. Follow-up durations ranged between 8 and 104 weeks. Psychotherapy was examined as an augmentation strategy in five studies and as a substitution strategy in two studies. One study used dialectical behavior therapy,28 whereas all others used cognitive therapy as their psychotherapeutic intervention.
Patients in the comparison groups were taking antidepressant medications from a wide array of classes. Four of the comparison groups received medication in a maintenance condition, and three of the comparison groups received an active systematic alteration to their medication regimens (including both arms of the STAR*D trial). Retention rates ranged from 25% to 100%. All but one study35 used the HAM-D as their clinician-administered severity rating tool along with a self-report measure of depression. Three studies reported a sample size calculation. We rated three studies as good quality, two as fair quality, and two as poor quality. Study design and intervention details are provided in Table 3.
Table 3.
Study Design and Interventions
| Study | Thase et al. 2007 (Augment)23 | Thase et al. 2007 (Substitute)23 | Scott et al. 200033 | Harley et al. 200828 | Kennedy et al. 200337 | Blackburn and Moore 199727 | Wiles et al. 200835 |
|---|---|---|---|---|---|---|---|
| Duration of follow up, wks | 14 | 14 | 20 | 16 | 8 | 104 | ~16b |
| Study design | Equipoise Stratified Randomization | Equipoise Stratified Randomization | RCT | RCT | RCT | RCT | RCT |
| Augment or Substitute? | Augment | Substitute | Augment | Augment | Augment | Substitute | Augment |
| Psychotherapy Intervention | Cognitive therapy | Cognitive therapy | Cognitive therapy | Group DBTa | Cognitive therapy | Cognitive therapy | Cognitive Therapy |
| # of sessions | 24 | 24 | 16 | 16 | 12 | 27 | 9.5 |
| Comparator | AD Augment | AD Substitute | AD Continue | AD Continue | Lithium Augment | AD Continue | AD Continue |
| Power calculation | Yes | Yes | Yes | No | No | No | No |
| Quality rating | Good | Good | Good | Fair | Fair | Poor | Poor |
aDBT = Dialectical Behavior Therapy; b12-20 sessions for 4 months were planned; however, mean follow-up duration was not reported
Psychotherapy as Step 2 Treatment
Table 4 summarizes outcomes of all the studies. The good quality STAR*D trial20,23,31,36 was a multistage, multicenter trial that examined both cognitive therapy and medication as either augmentation or substitution treatments to initial treatment with citalopram. Treatment resistant depression was defined as having HAM-D ≥14 after 14 weeks of citalopram use. The unique equipoise-stratified randomization design allowed patients to refuse randomization to treatment strategies that they found unacceptable. Less than one third of participants agreed to true randomization, resulting in asymmetrical sample sizes in the treatment arms and inadequate power to detect small or moderate effects in the cognitive therapy arms. Although this may have adversely impacted internal validity, accounting for patient preferences improves applicability.
Table 4.
Results of the Psychotherapy Intervention
| Study | Thase et al. 2007 (Augment)23 | Thase et al. 2007 (Substitute)23 | Scott et al. 200033 | Harley et al. 200828 | Kennedy et al. 200337 | Blackburn and Moore 199727 | Wiles et al. 200835 |
|---|---|---|---|---|---|---|---|
| Retention rate, | |||||||
| Psychotherapy | 59 (91%) | 30 (83%) | 61 (76%) | 10 (77%) | 17 (74%) | 6 (35%) | 14 (100%) |
| Comparator | 95 (81%) | 63 (73%) | 66 (85%) | 9 (82%) | 15 (71%) | 5 (25%) | 9 (81.8%) |
| Post-treatment HAM-D (M±SD) | |||||||
| Remission: | Remission: | ||||||
| Psychotherapy | 23.1% | 25.0% | 8.7 ± 5.3 | 11.3 ± 5.3 | 14.8 ± 9.9 | 8.6 ± 5.6 | N/A |
| Comparator | 33.3% | 27.9% | 9.4 ± 5.3 | 17.1 ± 6.2 | 9.2 ± 6.7 | 9.3 ± 7.2 | |
| Effect Size | NS | NS | NSa | d = 1.45 | d = .32 | NS | |
| Post-treatment | Scores not reported; ITT analyses regression co-efficient = -8.4 (95%CI: -21.9, 5.1) | ||||||
| BDI Scores (M±SD) | |||||||
| Psychotherapy | 8.2 ± 5.1 | 9.1 ± 5.4 | 13.8 ± 9.6 | 15.1 ± 12.1 | 19.9 ± 10.3 | 14.2 ± 9.9 | |
| Comparator | 8.2 ± 4.8 | 9.1 ± 5.0 | 16.1 ± 10.0 | 25.9 ± 16.3 | 15.1 ± 11.4 | 18.1 ± 13.1 | |
| Effect Size | NSb | NS | NS | d = 1.31 | NS | NS | |
aNS = Not significant at p < 0.05; bResults are for QIDS-C
In the augmentation arm of STAR*D, 180 patients with inadequate response to initial citalopram treatment received augmentation through cognitive therapy (n = 65) or antidepressants (n = 117). Cognitive therapy patients received 16 sessions over 12 weeks and continued citalopram, while patients on antidepressants had citalopram augmented with bupropion or buspirone. While participants in both conditions improved, post treatment analyses found no significant differences between the two groups for percent remitted on the HAM-D, defined as HAM-D ≤7 (cognitive therapy = 23.1%, antidepressants = 33.3%; p > 0.05) or for mean scores on the QIDS-SR (cognitive therapy = 8.2, antidepressants = 8.2; p > 0.05). The retention rate was 91% in the cognitive therapy condition and 81% in the antidepressant condition.
In the substitution arm of STAR*D, patients discontinued citalopram and either switched to 16 therapy sessions over 12 weeks (n = 36) or switched to treatment with bupropion, sertraline, or venlafaxine (n = 86). Similar to the augmentation arm, patients in both treatment groups improved to a similar degree (percent remitted on the HAM-D, cognitive therapy = 25.0%, antidepressants = 27.9%. The retention rate was 83% in the cognitive therapy condition and 73% in the antidepressant condition.
Another comparison in the STAR*D trial evaluated augmentation with cognitive therapy.29,30,33,34 One hundred and fifty-eight patients were randomized to clinical management plus cognitive therapy (n = 80) or clinical management alone (n = 78). Cognitive therapy was delivered in 16 sessions over 20 weeks, with 2 booster sessions. Clinical management comprised of 30-minute appointments with psychiatrists every 4 weeks. The a priori sample size of 160 gave 80% power to detect a reduction in relapse rates from 40% in one group to 20% in the other at p = 0.05 at baseline. Treatment resistant depression was defined as having HAM-D ≥8 and BDI ≥9 after at least 8 weeks of adequate antidepressant treatment. Retention was measured as participating until relapse or until the end of the study at 68 weeks, resulting in a 76% retention rate in the cognitive therapy condition and an 85% retention rate in the clinical management condition. While participants in both conditions improved, post treatment analyses found no significant differences between the two groups for mean scores on the HAM-D (Cognitive therapy = 8.7, Clinical management = 9.4; p > 0.05) or BDI (Cognitive therapy = 13.8, Clinical management = 16.1; p > 0.05). A limitation of this study is that it allowed patients with partially remitted depressive symptoms and no diagnosis of MDD to participate. In summary, all of the three “good” quality studies supported the utility of psychotherapy in the treatment for treatment resistant depression.
A small, fair quality trial compared a dialectical behavior therapy group (DBT) (n = 13) to a waitlist control group (n = 11).28 Intervention patients received 16 weekly sessions of a 90-minute coping skills group; however, they remained on antidepressants and could continue individual psychotherapy provided it was not cognitive therapy. Waitlisted patients continued treatment as usual. Treatment resistant depression was defined as residual depression following stable adequate antidepressant treatment for at least 6 weeks. Post-treatment analyses showed greater clinical improvement in the DBT condition based on HAM-D (F = 4.63, p < 0.05) and BDI scores (F = 9.50, p < 0.01). The retention rate was 77% in the DBT group and 82% in the WL condition. Study quality was limited by the small sample size and confound of allowing patients to continue in individual therapy.
In another fair quality trial, 44 patients had their antidepressant augmented with either 12 sessions of cognitive therapy (n = 23) or lithium carbonate (n = 21).37 Patients in the lithium condition were seen every 2 weeks for medication check-up; those receiving cognitive therapy were seen every 4 weeks. Treatment resistant depression was defined as having HAM-D scores between 8 and 15 after 8-14 weeks of antidepressants treatment. Post-treatment analyses showed lower HAM-D scores in the lithium condition compared to the therapy condition (t = 2.02, p < 0.05). No significant difference was found for BDI scores. The retention rate was 74% in the therapy condition and 71% in the lithium condition. A limitation of this study is that it only included partial responders to initial antidepressant treatment (i.e., HAM-D between 8 and 15) and excluded non-responders (i.e., HAM-D ≥16). It also did not report a sample size calculation and likely lacked sufficient statistical power to detect clinically important differences.
One poor quality study examined the efficacy of cognitive therapy as a substitution strategy by randomizing 37 patients to cognitive therapy (n = 17) or continuing antidepressant (n = 20).27 Patients in the cognitive therapy condition received 27 sessions over 104 weeks. Patients in the antidepressant condition received a medication of their prescriber’s choice and were seen approximately every 3 weeks. Treatment resistant depression was not specifically defined in this study, but after 16 weeks of antidepressant treatment, patients continued to have depressive symptoms at a level comparable to that of the other patient populations included in this review (see Table 1). Post-treatment analyses found no significant differences between the two groups for mean scores on the HAM-D (Cognitive therapy = 8.6; Antidepressant = 9.3; p > 0.05) or BDI (Cognitive therapy = 14.2; Antidepressant = 18.1; p > 0.05). Significant study limitations included: poor retention rate (<35%), lack of statistical power, unorthodox length of cognitive therapy, and lack of operational definition for treatment resistance.
Finally, a poor quality feasibility study examined cognitive therapy as an augmentation strategy by randomizing 25 patients to cognitive therapy (n = 14) or usual care (n = 11). Patients in the cognitive therapy condition received a median of 9.5 sessions over 4 months. No restrictions were imposed on the usual care group. Treatment resistant depression was defined as adequate dose of antidepressant for at least 6 weeks (based on chart review and self-report), BDI ≥15, and ICD-10 criteria for MDD as determined by the Revised Clinical Interview Schedule (CIS-R). This study used the BDI as its primary outcome measure; no clinician based measure was administered at follow-up. In intent-to-treat analyses, the cognitive therapy group showed an 8-point reduction in BDI scores after controlling for multiple covariates; however, the results were not statistically significant (Regression coefficient = -8.4, 95% CI: -21.9, 5.1). No raw scores were provided. Significant study limitations included a small sample size, lack of a clinician administered depression measure, and lack of baseline data on history of depression.
In summary, two good quality, moderate sized trials showed benefit when treatment was augmented with 16 sessions of cognitive therapy, as compared to antidepressant treatment. Another moderate-sized, good quality study showed benefit when existing antidepressant treatment was substituted with cognitive therapy. Of the two fair quality trials, augmenting treatment with psychotherapy was found to be beneficial in one but not the other. The two small, poor quality studies showed similar benefit when existing antidepressant treatment was either augmented or substituted with cognitive therapy.
DISCUSSION
The key observation that emerges from a systematic review of the literature is that current evidence examining the effect of psychotherapy as augmentation or substitute therapy for treatment resistant depression is sparse and reveals mixed results. The three good quality studies, one fair quality study, and two poor quality studies all demonstrated that psychotherapy may be beneficial in managing treatment resistant depression whether used as a substitution or augmentation strategy. One fair quality study demonstrated that medications may be better than cognitive therapy. We conclude that although evidence is sparse, psychotherapy appears to be effective and is a reasonable treatment option for treatment resistant depression.
The utility of psychotherapy in treatment resistant depression is further supported by various theoretical and clinical reasons. First, maladaptive cognitions and behaviors endemic to MDD may lead to chronic depressive symptoms. Such cognitions and behaviors may be best modified with psychotherapy techniques such as cognitive restructuring, behavioral activation, or skills training. This is especially true when patients are experiencing acute stressors (e.g., divorce), where psychotherapy may improve patients’ long-term outcomes.13 Second, antidepressants have side effects which may increase in number or severity upon adding another antidepressant. Side effects are known to reduce quality of life and increase the chances of non-adherence, thereby interfering with the treatment of MDD.38 Using psychotherapy can help mitigate the issue of side effects. Third, patients may not respond to antidepressant treatment, may prefer not taking medications, or may become frustrated at the lack of response to treatment regimens. For all these reasons, psychotherapy may be an important treatment option for treatment resistant depression.
Despite these advantages, treatment via psychotherapy continues to face numerous barriers. First, access to psychotherapy can be limited if patients live in underserved areas. This issue is exacerbated by the greater time commitment required to receive traditional psychotherapy, which often requires weekly or biweekly face-to-face contact, typically for an hour each appointment. Second, the relative cost of delivering psychotherapy compared to antidepressants can be a barrier. The short-term costs of psychotherapy are typically higher, especially when delivered by a mental health professional such as a psychologist.39,40 However, psychotherapy may have more favorable cost profiles when mid to long-term outcomes are examined.39,41,42 In addition, psychotherapy may have unique economic advantages in the domains of work absenteeism,43 treatment of medical comorbidities,44 and relapse.32 A recent study examining the cost-effectiveness of psychotherapy in treatment resistant depression concluded that adjunctive cognitive therapy was more costly but also was more effective than antidepressants alone.32 Because treatment resistant depression is both common and costly,45 large, high quality, long-term randomized trials are needed to evaluate both the effectiveness and cost-effectiveness of different treatment strategies for patients with treatment resistant depression.
One strategy to increase access and cost-effectiveness of psychotherapy involves collaborative care. Recent research has shown that training non-mental health professionals (e.g., nurses) to provide brief psychotherapeutic interventions are effective in reducing depressive symptoms.46–48 Collaborative care models involving depression care managers have been shown to improve the quality of depression care, symptom severity, patient satisfaction, and functional impairment.8,40 A few of these trials utilized empirically based psychotherapy as a treatment option for treatment resistant depression.47,49 Unfortunately, psychotherapy in these trials was delivered as part of a package of collaborative care and its unique contribution to improved outcomes cannot be assessed. Nevertheless, evidence suggests that training non-mental health professionals to deliver brief psychotherapy may improve outcomes in primary care patients without excessively burdening limited resources.
Limitations
Several limitations of the current literature emerged upon review. First, few RCTs exist that adequately address the question of treatment resistant depression. Whereas each of the included studies addressed a portion of the research question, none of the studies provided a complete answer nor did an evidence synthesis across the studies provide an entirely satisfactory answer. Most studies appeared to be underpowered to detect moderately large treatment effects. Conclusions are tempered by the heterogeneity in study designs and patient populations, as well as the limited number of good quality trials.
Second, there was significant heterogeneity in the definition of treatment resistant depression as well as the measures used to determine MDD. Measures included clinician-administered scales (e.g., HAM-D), self-report scales (e.g., BDI), diagnostic criteria (DSM-III-R, DSM-IV, ICD-10), and clinical judgment. Third, the majority of trials used cognitive therapy. Traditionally, cognitive therapy requires a minimum of 12-16 sessions and is often delivered by trained experts. As a result, it is questionable whether the psychotherapies reviewed are suitable for use in primary care settings or will be accepted by primary care patients. Brief therapies such as problem-solving therapy have been adapted by non-mental health professionals as first step treatments in primary care settings, with demonstrated effectiveness.40 Newer interventions continue to be adapted for treatment within primary care settings. For example, a recent study examining the cognitive behavioral analysis system of psychotherapy (CBASP) found no effects of psychotherapy in treating treatment resistant depression.50 This study was excluded because of the limited empirical base of CBASP; nonetheless, well-designed trials such as this one are necessary to evaluate the efficacy of using these newer and/or briefer therapies in primary care settings. Fourth and finally, we were unable to comment on the fidelity to psychotherapy protocols from the published studies. Future reports should provide information regarding treatment fidelity to ensure that the real world applicability of the research is accurately represented.
Future Directions
There is a pressing need to examine psychotherapy as a second step treatment in patients who have not responded to initial antidepressants treatment. This may be addressed in two ways: 1) re-analysis of existing data from trials in which patients with treatment resistant depression are recruited, or 2) conducting studies designed to examine this question. As a field, it is important to develop a standardized, operational definition of treatment resistant depression to facilitate comparisons across studies.51 Finally, comparative effectiveness studies that compare the effects of augmentation or substitution of psychotherapy to acceptable treatment options will help elucidate the utility of psychotherapy in the treatment resistant population. Future investigations should also address the cost-effectiveness of different treatment options. Ideally, studies designed for this purpose would involve longer follow-up, as well as measures of direct costs, indirect costs, costs associated with comorbid non-psychiatric conditions, and societal costs. Collectively, these studies will help improve treatment of this important clinical population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 82 kb)
(DOC 52 kb)
Acknowledgments
We would like to thank the VHA Office of Quality Performance for their support in completing the original report on which this manuscript is based. We would like to thank the project staff at the Durham VA Evidence-based Practice Center for their administrative support. Finally, we would like to thank the peer reviewers of the original evidence synthesis and the initial manuscript submission. Their comments and suggestions greatly improved the report and have been incorporated into this manuscript.
Dr. Trivedi was partly supported by an AHRQ T-32 fellowship at Duke University Medical Center, and resources within the VA Puget Sound Health Care System. Dr. Nieuwsma was partly supported by a VA Mid-Atlantic MIRECC fellowship. The views and opinions in this manuscript are those of the authors and do not reflect those of the VA.
This project was funded by the VA Health Services Research and Development Evidence Synthesis Program.
Conflict of Interest None disclosed.
References
- 1.World Health Organization. The global burden of disease: 2004 update. 2008. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
- 2.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on alcoholism and related conditions. Arch Gen Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin. 2009;25(1):161–175. doi: 10.1185/03007990802622726. [DOI] [PubMed] [Google Scholar]
- 5.Barbui C, Furukawa TA, Cipriani A. Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re-examination of published and unpublished data from randomized trials. Can Med Assoc J. 2008;178(3):296–305. doi: 10.1503/cmaj.070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipriani A, Furukawa TA, Geddes JR, et al. Does randomized evidence support sertraline as first-line antidepressant for adults with acute major depression? A systematic review and meta-analysis. J Clin Psychiatry. 2008;69(11):1732–1742. doi: 10.4088/JCP.v69n1108. [DOI] [PubMed] [Google Scholar]
- 7.Mulrow CD, Williams JW, Jr, Chiquette E, et al. Efficacy of newer medications for treating depression in primary care patients. Am J Med. 2000;108(1):54–64. doi: 10.1016/S0002-9343(99)00316-2. [DOI] [PubMed] [Google Scholar]
- 8.Williams JM, Teasdale JD, Segal ZV, Soulsby J. Mindfulness-based cognitive therapy reduces overgeneral autobiographical memory in formerly depressed patients. J Abnorm Psychol. 2000;109(1):150–155. doi: 10.1037/0021-843X.109.1.150. [DOI] [PubMed] [Google Scholar]
- 9.Bortolotti B, Menchetti M, Bellini F, Montaguti MB, Berardi D. Psychological interventions for major depression in primary care: a meta-analytic review of randomized controlled trials. Gen Hosp Psych. 2008;30(4):293–302. doi: 10.1016/j.genhosppsych.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26(1):17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Craighead WE, Sheets ES, Brosse AL, Ilardi SS. Psychosocial treatments for major depressive disorder. In: Nathan PE, Gorman JM, editors. A guide to treatments that work. 3. New York: Oxford; 2007. pp. 289–293. [Google Scholar]
- 12.DeRubeis RJ, Gelfand LA, Tang TZ, Simons AD. Medications versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatry. 1999;156(7):1007–1013. doi: 10.1176/ajp.156.7.1007. [DOI] [PubMed] [Google Scholar]
- 13.DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 14.Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- 15.Michalak EE, Lam RW. Breaking the myths: new treatment approaches for chronic depression. Can J Psychiatry. 2002;47(7):635–643. doi: 10.1177/070674370204700705. [DOI] [PubMed] [Google Scholar]
- 16.Cornwall PL, Scott J. Partial remission in depressive disorders. Acta Psychiatr Scand. 1997;95(4):265–271. doi: 10.1111/j.1600-0447.1997.tb09630.x. [DOI] [PubMed] [Google Scholar]
- 17.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. doi: 10.1016/S0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 18.Stimpson N, Agrawal N, Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression. Systematic review. Br J Psychiatry. 2002;181:284–294. doi: 10.1192/bjp.181.4.284. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi MH. Major depressive disorder: remission of associated symptoms. J Clin Psychiatry. 2006;67(Suppl 6):27–32. [PubMed] [Google Scholar]
- 20.Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119–142. doi: 10.1016/S0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 21.Fochtmann LJ, Gelenberg AJ. Guideline Watch: Practice Guideline for the Treatment of Patients with Depressive Disorder. 2. Arlington, VA: American Psychiatric Association; 2005. [Google Scholar]
- 22.National Center for Health and Clinical Excellence. Depression: The treatment and management of depression in adults. Clinical Guideline 90. 2009; http://www.nice.org.uk/nicemedia/pdf/CG90NICEguideline.pdf. Accessed November 18, 2010.
- 23.Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: A STAR*D report. Am J Psychiatry. 2007;164(5):739–752. doi: 10.1176/appi.ajp.164.5.739. [DOI] [PubMed] [Google Scholar]
- 24.Markowitz JC. When should psychotherapy be the treatment of choice for major depressive disorder? Curr Psychiatry Rep. 2008;10(6):452–457. doi: 10.1007/s11920-008-0073-7. [DOI] [PubMed] [Google Scholar]
- 25.Simon GE, Ludman E, Unutzer J, Bauer MS. Design and implementation of a randomized trial evaluating systematic care for bipolar disorder. Bipolar Disorders. 2002;4(4):226–236. doi: 10.1034/j.1399-5618.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- 26.Chambless DL, Baker MJ, Baucom DH, Beutler LE, Calhoun KS, Crits-Christoph P, et al. Update on empirically validated therapies, II. Clin Psychol. 1998;51:3–16. [Google Scholar]
- 27.Blackburn IM, Moore RG. Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. Br J Psychiatry. 1997;171:328–334. doi: 10.1192/bjp.171.4.328. [DOI] [PubMed] [Google Scholar]
- 28.Harley R, Sprich S, Safren S, Jacobo M, Fava M. Adaptation of dialectical behavior therapy skills training group for treatment-resistant depression. J Nerv Ment Dis. 2008;196(2):136–143. doi: 10.1097/NMD.0b013e318162aa3f. [DOI] [PubMed] [Google Scholar]
- 29.Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35(1):59–68. doi: 10.1017/S003329170400282X. [DOI] [PubMed] [Google Scholar]
- 30.Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- 31.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/appi.ajp.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 32.Scott J, Palmer S, Paykel E, Teasdale J, Hayhurst H. Use of cognitive therapy for relapse prevention in chronic depression. Cost-effectiveness study. Br J Psychiatry. 2003;182:221–227. doi: 10.1192/bjp.182.3.221. [DOI] [PubMed] [Google Scholar]
- 33.Scott J, Teasdale JD, Paykel ES, et al. Effects of cognitive therapy on psychological symptoms and social functioning in residual depression. Br J Psychiatry. 2000;177:440–446. doi: 10.1192/bjp.177.5.440. [DOI] [PubMed] [Google Scholar]
- 34.Teasdale JD, Scott J, Moore RG, Hayhurst H, Pope M, Paykel ES. How does cognitive therapy prevent relapse in residual depression? Evidence from a controlled trial. J Consult Clin Psychol. 2001;69(3):347–357. doi: 10.1037/0022-006X.69.3.347. [DOI] [PubMed] [Google Scholar]
- 35.Wiles NJ, Hollinghurst S, Mason V, et al. A randomized controlled trial of cognitive behavioural therapy as an adjunct to pharmacotherapy in primary care based patients with treatment resistant depression: A pilot study. Behav Cogn Psychother. 2008;36(31):21–33. [Google Scholar]
- 36.Wisniewski SR, Fava M, Trivedi MH, et al. Acceptability of second-step treatments to depressed outpatients: A STAR*D report. Am J Psychiatry. 2007;164(5):753–760. doi: 10.1176/appi.ajp.164.5.753. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy SH, Segal ZV, Cohen NL, Levitan RD, Gemar M, Bagby RM. Lithium carbonate versus cognitive therapy as sequential combination treatment strategies in partial responders to antidepressant medication: An exploratory trial. J Clin Psychiatry. 2003;64(4):439–444. doi: 10.4088/JCP.v64n0414. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell AJ. Depressed patients and treatment adherence. Lancet. 2006;367(9528):2041–2043. doi: 10.1016/S0140-6736(06)68902-2. [DOI] [PubMed] [Google Scholar]
- 39.Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry. 2006;189:484–493. doi: 10.1192/bjp.bp.106.023655. [DOI] [PubMed] [Google Scholar]
- 40.Wolf NJ, Hopko DR. Psychosocial and pharmacological interventions for depressed adults in primary care: a critical review. Clin Psychol Rev. 2008;28(1):131–161. doi: 10.1016/j.cpr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Leff J, Vearnals S, Brewin CR, et al. The London Depression Intervention Trial. Randomised controlled trial of antidepressants v. couple therapy in the treatment and maintenance of people with depression living with a partner: clinical outcome and costs. Br J Psychiatry. 2000;177:95–100. doi: 10.1192/bjp.177.2.95. [DOI] [PubMed] [Google Scholar]
- 42.Simpson S, Corney R, Fitzgerald P, Beecham J. A randomized controlled trial to evaluate the effectiveness and cost-effectiveness of psychodynamic counselling for general practice patients with chronic depression. Psychol Med. 2003;33(2):229–239. doi: 10.1017/S0033291702006517. [DOI] [PubMed] [Google Scholar]
- 43.Mynors-Wallis L, Davies I, Gray A, Barbour F, Gath D. A randomised controlled trial and cost analysis of problem-solving treatment for emotional disorders given by community nurses in primary care. Br J Psychiatry. 1997;170:113–119. doi: 10.1192/bjp.170.2.113. [DOI] [PubMed] [Google Scholar]
- 44.Guthrie E, Moorey J, Margison F, et al. Cost-effectiveness of brief psychodynamic-interpersonal therapy in high utilizers of psychiatric services. Arch Gen Psychiatry. 1999;56(6):519–526. doi: 10.1001/archpsyc.56.6.519. [DOI] [PubMed] [Google Scholar]
- 45.Russell JM, Hawkins K, Ozminkowski RJ, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65(3):341–347. doi: 10.4088/JCP.v65n0309. [DOI] [PubMed] [Google Scholar]
- 46.Katon W, Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56(12):1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 47.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 48.Williams JW, Jr, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A. Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry. 2007;29(2):91–116. doi: 10.1016/j.genhosppsych.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Bruce ML, Have TR, Reynolds CF, 3rd, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 50.Kocsis JH, Gelenberg AJ, Rothbaum BO, et al. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009(11):1178-88. http://www.mrw.interscience.wiley.com/cochrane/clcentral/articles/454/CN-00722454/frame.html. [DOI] [PMC free article] [PubMed]
- 51.Souery D, Amsterdam J, Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1–2):83–91. doi: 10.1016/S0924-977X(98)00004-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 82 kb)
(DOC 52 kb)