Abstract
Introduction
Little is known about how the development of a new chronic health condition affects management of existing chronic conditions over time. New conditions might worsen management of existing conditions because of competing demands or improve management of existing conditions because of increased engagement with heath care. We assessed the effect of incident stage 0, 1, 2 or 3 breast, colon or prostate cancer; incident depression; or an exacerbation of chronic pulmonary disease on control of type 2 diabetes (DM2).
Methods
We conducted a longitudinal, historical cohort study within an integrated, not-for-profit HMO. Of a cohort of persons with diagnoses of DM2 between 1998 and 2008, 582, 2,959 and 2,332 developed incident cancer, depression or pulmonary disease exacerbation, respectively. We assessed change in hemoglobin A1c (A1c) as a function of the occurrence of the incident comorbidity in each subcohort for a period of 1 to 5 years after the occurrence of the incident comorbidity. Secondary outcomes were systolic blood pressure (SBP) and low density lipoprotein (LDL) levels. Multivariate linear regression was adjusted for demographics, morbidity level, BMI, numbers of primary and specialty visits, and continuity of primary care. Latent class analyses assessed post-comorbidity outcome trajectories. All time-varying covariates were calculated for a 24-month pre-diagnosis period and 0 to 24- and 24 to 60-month post-diagnosis periods.
Results
For each condition, A1c did not change significantly from before to after the incident comorbidity. This was confirmed by latent class growth curve analyses that grouped patients by their A1c trajectories. SBP and LDL were also not significantly changed pre- and post-diagnosis of the incident comorbidities.
Discussion
Although incident comorbidities inevitably will affect patients’ and clinicians’ care priorities, we did not observe changes in these particular outcomes. Additional investigation of interactions between diseases will inform changes in care that benefit complex patient populations.
INTRODUCTION
Patients regularly report multiple medical concerns, and clinicians routinely assist patients in managing multiple co-occurring conditions.1–4 Therefore, it is important to understand the effect of comorbid conditions on health outcomes. In particular, little is known about how the development of a new health condition affects management of existing conditions over time. Assessing the effect of incident conditions on the outcomes of existing chronic conditions may help clarify approaches that patients and their clinicians can use to balance the competing demands of multiple comorbidities.
Most investigations into the effects of managing comorbid chronic conditions have been based on cross-sectional assessments of co-prevalent conditions. In some studies, the presence of more chronic conditions has been associated with under-treatment of other acute or chronic coexisting conditions, or under-provision of preventive services.5–8 In other assessments, higher levels of morbidity have been associated with greater attainment of disease-specific quality indicators such as timely measurement of hemoglobin A1c and LDL, and receipt of appropriate medications for asthma.9–11 In at least one study, attaining these quality measures was primarily a function of visit frequency.12 This inconsistency in the literature suggests that two contradictory hypotheses are plausible about the effect of a new comorbid disease on control of existing disorders. If patient and clinician are focused on a new competing demand, pre-existing conditions may assume a lower priority. Alternatively, increased frequency of contact with the medical system resulting from the diagnosis and treatment of a new disease may result in more rigorous attention to all coexisting conditions.
In this investigation we assess the acute and longitudinal effects of three specific new chronic comorbid conditions on process of care outcomes for an existing chronic condition. Within three separate cohorts of persons with pre-existing type 2 diabetes (DM), we examined the effect of the onset of treatable cancer, depression or exacerbations of chronic pulmonary disease on three measures of guideline-concordant diabetes care: glycemic control, systolic blood pressure and (LDL) cholesterol. We hypothesized that the occurrence of the incident condition would present competing demands for the care of pre-existing diabetes and would thereby decrease attainment of diabetes-specific care goals in the period following the new diagnosis. Furthermore, we postulated that these effects would vary over time after the new diagnosis.
METHODS
Study Design
We conducted a retrospective, longitudinal, cohort study of adults with type 2 diabetes who were members of an integrated, group model, not-for profit HMO and who developed new onset stage 0, 1, 2 or 3 breast, colon or prostate cancer; new onset depression; or an exacerbation of chronic pulmonary disease (chronic obstructive pulmonary disease or asthma).
Study Population and Definitions of Comorbidities
The cohort of participants with diabetes was based on membership in a validated diabetes registry. In addition, we required a minimum of 2 years of continuous enrollment and at least two diabetes diagnoses (ICD-9 codes of 250 with fifth digit of 0 or 2) at any point between January 1998 and September 2008 in order to maximize the chances that cohort members were under active treatment for DM.13,14 For each cohort member we established an index date as the date of their second diabetes diagnosis after at least 1 year of continuous enrollment.
Within this cohort, we identified three subcohorts of individuals who developed incident cases of cancer, depression, or exacerbations of COPD or asthma at least 60 days after the diabetes index date. Cancer patients were identified from the HMO’s cancer registry, which is validated through pathology reports, claims and diagnosis data and is compatible with, and linked to, the validated Colorado state cancer registry. If a member had multiple incident cancer diagnoses during their cohort eligibility, only the first was included for analyses. We chose to study both asthma and COPD rather than either disease alone in order to cover the spectrum of adults with chronic lung disease. COPD is rarely diagnosed before the age of 35; however, asthma is often diagnosed in childhood and continues into adulthood. Furthermore, it is often hard to separate symptoms of the two conditions in adults.
Cohort members with depression were identified if they met at least one of the following criteria: diagnosis of depression (ICD-9 codes: 296.2, 296.3, 300.4, 309.0, 309.1, 309.28, 311) from a mental health provider; diagnosis of depression from any clinician in conjunction with either contacting a mental health department or receiving a prescription of a non-tricyclic antidepressant within 30 days of diagnosis; or two diagnoses of depression from any clinician within a 3-month period.15–18 Although we required that cohort members had not received treatment for depression for at least 6 months after the DM index date, it is possible that some cohort members identified through this process may have experienced previous episodes of depression. Including such potentially recurrent ‘incident’ cases would still be consistent with the aims of the investigation.
The subgroup of cohort members with chronic asthma or COPD were identified by any one of the following criteria: an ICD9 code for an acute exacerbation of asthma or COPD (493.x1, 493.x2, 491.21, 493.2), an ICD9 code for asthma (493.x) or COPD (490, 491, 492, 496) in conjunction with a prescription for oral prednisone within 3 days of that diagnosis, or an emergency department (ED) visit or hospitalization with asthma or COPD as the primary diagnosis19–22. For members with multiple exacerbations, only the first exacerbation was assessed.
Outcome Measures
Our primary outcome variable was change in hemoglobin A1c from prior to the date of diagnosis of the incident comorbidity to up to 5 years after the diagnosis date. Secondary outcomes were pre- and post-diagnosis levels of (SBP) and (LDL). The independent variable of interest was the occurrence of the incident comorbidity. Data on all variables used in the study were available through the electronic medical record and housed in the HMO’s up-to-date virtual data warehouse.
Statistical Analysis
We used mixed-effects models (SAS Proc Mixed) to assess outcomes as a function of time before and after the occurrence of incident condition, adjusting for covariates. We used a step-function to model the effect of the comorbidity over time on the outcome variable. Time was measured in months relative to the time of the comorbidity (0 representing the diagnosis of the comorbidity). The time periods of interest were -24 to -6, -6 to 0, 0 to 6, 6 to 12, 12 to 24 and 24 to 60 months, with a step being modeled for each time period. Separate models were developed for each combination of the three subcohorts (defined by incident comorbidity—cancer, pulmonary disease and depression) with each of the three outcomes (A1c, SBP and LDL). Using mixed-effects models made optimal use of the patient data since such models allow for irregularly timed outcomes and covariates, which was the situation with our data. This analytic method enabled each cohort member to serve as his/her own control and minimized the potential confounding associated with assessing physiologic outcomes across heterogeneous study populations.
In addition, we wanted to explore the composition of the subcohorts based on the strata of our primary outcome variable. We initially conducted regression analyses stratified by initial level of A1c. However, results followed a typical pattern of ‘regression to the mean’ and did not elucidate outcome patterns as a function of the incident comorbidity. Therefore, we used adjusted latent class growth models to identify unique trajectories of members within each subcohort for hemoglobin A1c after diagnosis of each of the comorbid conditions.23–25 This approach, used in other longitudinal analyses of heterogeneous cohorts, improves on a simple stratified analysis because it takes into account time-varying outcome variables.26 These models are based on the assumption that the observed data are a mixture of subpopulations (latent classes) that have distinct trajectories, such as stable throughout, loss of control that is regained and loss of control that is not regained. Each individual is thus classified into one of the classes based on their posterior group membership probabilities. We tested the probability of agreement among certain latent class outcomes using a Kappa statistic. Agreement would be indicated by values close to 1, while values close to 0 indicate no agreement other than what would be expected by chance. Using this analysis we carefully examined the 18-month time period around the occurrence of the incident comorbidity.
Covariates in the adjusted models included demographics, morbidity level, body mass index (BMI) and patient-clinician contact (numbers of primary and specialty visits and continuity of primary care) as continuity of care and visit frequency may be associated with some chronic disease outcomes.12,27 In order to account for any changes in BMI over time that might be associated with the incident comorbidity and independently affect outcomes, we treated BMI as a time-varying covariate: We calculated the rolling median BMI over three consecutive measurements in order to smooth the variability of the covariate associated with any inaccurate data point. We estimated morbidity using the Quan variation of the Elixhauser morbidity index by assessing morbidity at baseline and adding relevant morbidities over time as recommended by Wang et al.28,29 We calculated continuity of care for ambulatory primary care visits using the methods of Gill and Mainous.30 We calculated continuity of care, number of specialty visits, number of primary care visits and morbidity scores for each cohort member for the following intervals: –24 to 0; 0 to 24 and 24–60 months. The composition of the analytic subcohorts based on outcome data available for analysis is listed in Table 1.
Table 1.
Cohort Data Available for Analysis
| Diagnosis | Diabetes | ||||||
|---|---|---|---|---|---|---|---|
| Cancer | Pulmonary disease | Depression | |||||
| Outcome | Subjects | Outcome observations | Subjects | Outcome observations | Subjects | Outcome observations | |
| A1c | Incident case | 579 | 5,377 | 2,310 | 20,089 | 2,938 | 27,548 |
| Missing covariate | (26) | (583) | (113) | (1,613) | (199) | (2,686) | |
| Analyzed | 553 | 4,794 | 2,197 | 18,476 | 2,739 | 24,862 | |
| SBP | Incident case | 582 | 20,065 | 2,329 | 72,141 | 2,959 | 91,525 |
| Missing covariate | (25) | (1,741) | (116) | (4,327) | (197) | (6,406) | |
| Analyzed | 557 | 18,324 | 2,213 | 67,814 | 2,762 | 85,119 | |
| LDL | Incident case | 579 | 4,376 | 2,290 | 16,747 | 2,916 | 22,259 |
| Missing covariate | (25) | (448) | (118) | (1,347) | (198) | (2,021) | |
| Analyzed | 554 | 3,928 | 2,172 | 15,400 | 2,718 | 20,238 | |
The study was approved by the Institutional Review Boards of the HMO (source of the data) and of the analytical site.
RESULTS
In the overall cohort of persons with diabetes, 582 individuals developed incident cancer, 2,332 developed an exacerbation of chronic pulmonary disease and 2,959 developed incident depression. The three cohorts were of similar age (median age 62 to 65 years), had slightly more females than males and had an average of four to five chronic medical conditions. Descriptions of members of the three subcohorts are listed in Table 2.
Table 2.
Characteristics of Study Cohorts
| Characteristic | Diabetes and cancer | Diabetes and pulmonary disease | Diabetes and depression |
|---|---|---|---|
| N = 582 | N = 2,332 | N = 2,959 | |
| n (%) | n (%) | n (%) | |
| Female | 318 (54.64) | 1244 (53.34) | 1670 (56.44) |
| Race and ethnicity | |||
| Black (any ethnicity) | 66 (11.34) | 107 (4.59) | 114 (3.85) |
| Hispanic (non-black) | 79 (13.57) | 265 (11.36) | 338 (11.42) |
| White (non-Hispanic) | 366 (62.89) | 1215 (52.10) | 1379 (46.60) |
| Other | 18 (3.09) | 104 (4.46) | 87 (2.94) |
| Unknown | 53 (9.11) | 641 (27.49) | 1041 (35.18) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age at index date (years) | 65.53 (9.10) | 63.20 (11.49) | 62.04 (12.53) |
| Study enrollment (years) | 4.69 (1.75) | 5.01 (1.66) | 5.06 (1.74) |
| Study enrollment after diagnosis of incident comorbidity (years) | 2.70 (1.75) | 3.01 (1.66) | 3.07 (1.74) |
| Body mass index* | 31.09 (6.30) | 32.45 (7.40) | 32.01 (7.10) |
| Number of primary care visits† | 8.21 (5.73) | 9.65 (6.74) | 9.47 (6.68) |
| Number of specialty care visits† | 10.53 (11.24) | 10.99 (11.36) | 10.78 (10.93) |
| Primary care continuity of care score†30 | 0.66 (0.22) | 0.63 (0.22) | 0.64 (0.22) |
| Number of comorbidities†29 | 4.58 (2.57) | 4.99 (3.04) | 5.35 (2.96) |
SD = standard deviation
*The median BMI was calculated for each cohort member using all measurements during their study enrollment
†Measured for the 24-month period prior to comorbidity diagnosis
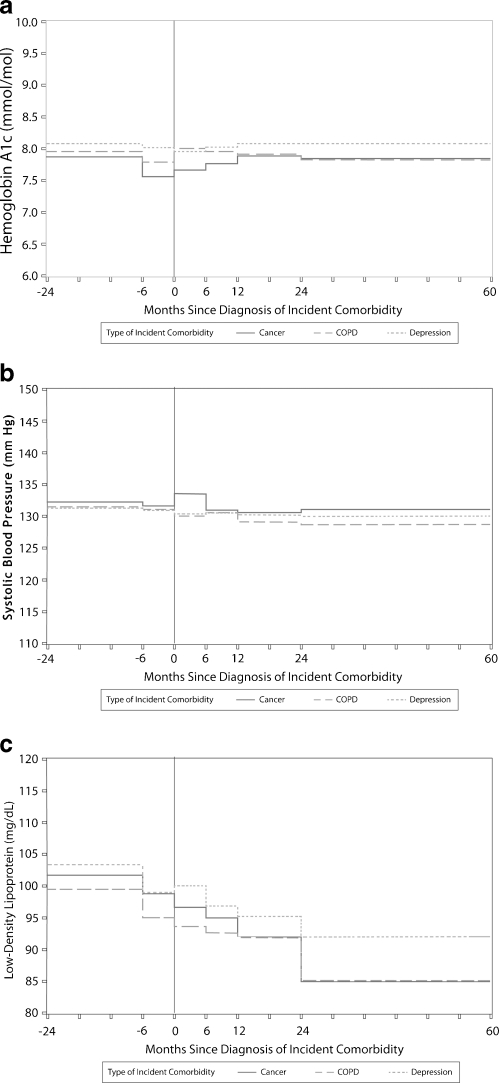
Figure 1a, b and c illustrates the adjusted outcomes of A1c, SPB and LDL as a function of the occurrence of each incident comorbidity over time. Mean level of hemoglobin A1c, SBP or LDL did not change significantly from pre-diagnosis to post-diagnosis of the incident comorbidity. The changes in A1c predicted in the adjusted models are listed in Appendix Table 3. Although several covariates including age, number of specialty visits, morbidity score, continuity of care and BMI were statistically significant in different adjusted models for the three outcomes, the changes in the values of the outcomes as a function of these covariates were not clinically significant (Appendix Table 4).
Figure 1.
a Adjusted model of hemoglobin A1c as a function of the occurrence of the incident comorbidity. b Adjusted model of systolic blood pressure as a function of the occurrence of the incident comorbidity. c Adjusted model of low density lipoprotein as a function of the occurrence of the incident comorbidity.
Table 3.
Adjusted Outcomes of A1c, SBP and LDL as a Function of Each Incident Comorbidity Over Time
| Outcome | Incident comorbidity | Time period (months relative to date of incident comorbidity: month 0) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -24—-6 | -6–0 | 0–6 | 6–12 | 12–24 | 24–60 | ||||||||
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | ||
| A1c | Cancer | 7.9 | (0.07) | 7.6 | (0.08) | 7.7 | (0.09) | 7.8 | (0.09) | 7.9 | (0.08) | 7.8 | (0.08) |
| Pulmonary | 7.9 | (0.03) | 7.8 | (0.04) | 8.0 | (0.04) | 7.9 | (0.04) | 7.9 | (0.04) | 7.8 | (0.04) | |
| Depression | 8.1 | (0.03) | 8.0 | (0.04) | 7.8 | (0.04) | 8.0 | (0.04) | 8.1 | (0.03) | 8.1 | (0.04) | |
| SBP | Cancer | 132 | (0.7) | 132 | (0.8) | 134 | (0.8) | 132 | (0.8) | 131 | (0.8) | 132 | (0.8) |
| Pulmonary | 132 | (0.3) | 131 | (0.3) | 130 | (0.3) | 131 | (0.3) | 129 | (0.3) | 129 | (0.3) | |
| Depression | 132 | (0.3) | 131 | (0.3) | 131 | (0.3) | 131 | (0.3) | 131 | (0.3) | 130 | (0.3) | |
| LDL | Cancer | 101 | (1.5) | 98 | (1.7) | 96 | (2.0) | 95 | (1.9) | 92 | (1.7) | 85 | (1.8) |
| Pulmonary | 99 | (0.7) | 95 | (0.8) | 94 | (0.8) | 92 | (0.9) | 91 | (0.8) | 86 | (0.8) | |
| Depression | 103 | (0.6) | 98 | (0.8) | 99 | (0.8) | 97 | (0.8) | 95 | (0.7) | 91 | (0.7) | |
Table 4.
Adjusted Models with Covariates: Outcomes as a Function of Incident Comorbidities
| Outcome | Incident comorbidity | Cancer N = 582 | Pulmonary disease N = 2,332 | Depression N = 2,959 | |||
|---|---|---|---|---|---|---|---|
| Covariate | Effect | 95% CI | Effect | 95% CI | Effect | 95% CI | |
| Hemoglobin A1c | Male | 0.16 | (-0.03, 0.35) | 0.02 | (-0.10, 0.13) | 0.02 | (-0.08, 0.13) |
| Black | 0.47 | (0.17, 0.76) | 0.18 | (-0.08, 0.44) | 0.34 | (0.07, 0.61) | |
| Hispanic | 0.12 | (-0.16, 0.40) | 0.20 | (0.02, 0.37) | 0.23 | (0.06, 0.40) | |
| Other race | 0.17 | (-0.37, 0.70) | 0.21 | (-0.05, 0.47) | -0.09 | (-0.39, 0.21) | |
| Unknown race | 0.16 | (-0.18, 0.50) | 0.28 | (0.15, 0.41) | 0.23 | (0.11, 0.35) | |
| Age (decade) | -0.33 | (-0.44, -0.22) | -0.33 | (-0.38, -0.27) | -0.28 | (-0.33, -0.24) | |
| BMI | 0.02 | (0.01, 0.03) | 0.00 | (-0.00, 0.01) | 0.01 | (0.0, 0.02) | |
| Number of conditions(29) | -0.02 | (-0.04, 0.00) | -0.04 | (-0.05, -0.03) | -0.05 | (-0.06, -0.04) | |
| Continuity of care(30) | -0.09 | (-0.29, 0.10) | -0.01 | (-0.13, 0.11) | -0.15 | (-0.25, -0.04) | |
| PC visits | 0.00 | (-0.00, 0.01) | 0.01 | (0.00, 0.01) | 0.00 | (0.00, 0.01) | |
| Specialist visits | -0.01 | (-0.01, -0.00) | -0.01 | (-0.01, -0.00) | -0.01 | (-0.01, -0.00) | |
| Systolic blood pressure | Male | -1.1 | (-3.1, 0.8) | -1.7 | (-2.7, -0.8) | -1.8 | (-2.6, -1.0) |
| Black | 3.7 | (0.6, 6.8) | 3.3 | (1.0, 5.5) | 5.2 | (3.1, 7.3) | |
| Hispanic | 0.4 | (-2.4, 3.3) | 0.3 | (-1.2, 1.8) | 1.0 | (-0.3, 2.3) | |
| Other race | -1.6 | (-4.8, 2.0) | 2.5 | (0.3, 4.8) | 0.1 | (-2.3, 2.4) | |
| Unknown race | -1.4 | (-4.8, 2.0) | 1.3 | (0.2, 2.4) | 0.7 | (-0.3, 1.6) | |
| Age (decade) | 2.8 | (1.7, 3.9) | 2.3 | (1.9, 2.8) | 3.0 | (2.6, 3.4) | |
| BMI | 0.6 | (0.5, 0.7) | 0.5 | (0.4, 0.5) | 0.4 | (0.4, 0.5) | |
| Number of conditions(29) | -0.4 | (-0.6, -0.2) | -0.4 | (-0.5, -0.3) | -0.5 | (-0.6, -0.4) | |
| Continuity of care(30) | -0.8 | (-2.4, 0.9) | 1.0 | (0.1, 2.0) | -1.2 | (-2.0, -0.3) | |
| PC visits | -0.1 | (-0.1, -0.0) | 0.0 | (-0.0, 0.0) | 0.0 | (-0.0, 0.0) | |
| Specialist visits | 0.0 | (-0.0, 0.0) | -0.0 | (-0.0, -0.0) | -0.0 | (-0.0, -0.0) | |
| Low-density lipoprotein | Male | -8.2 | (-12.1, -4.3) | -8.0 | (-10.2, -5.8) | -8.6 | (-10.6, -6.6) |
| Black | 1.5 | (-4.7, 7.7) | 8.4 | (3.2, 13.6) | 8.8 | (3.7, 13.9) | |
| Hispanic | -1.1 | (-6.9, 4.6) | 0.5 | (-3.0, 3.9) | -0.0 | (-3.2, 3.2) | |
| Other race | -0.9 | (-11.9, 10.1) | 1.4 | (-3.6, 6.5) | -3.4 | (-9.0, 2.2) | |
| Unknown race | 0.4 | (-6.7, 7.6) | 4.7 | (2.1, 7.3) | 4.7 | (2.5, 7.0) | |
| Age (decade) | 0.7 | (-1.6, 3.0) | -2.7 | (-3.7, -1.6) | -2.8 | (-3.7, -1.9) | |
| BMI | 0.0 | (-0.3, 0.3) | 0.1 | (-0.1, 0.2) | -0.0 | (-0.2, 0.1) | |
| Number of conditions (29) | -0.9 | (-1.4, -0.3) | -0.5 | (-0.7, -0.2) | -1.2 | (-1.4, -0.9) | |
| Continuity of care(30) | -3.5 | (-8.1, 1.2) | -1.4 | (-4.1, 1.3) | 1.5 | (-0.9, 3.9) | |
| PC visits | -0.2 | (-0.3, -0.0) | -0.1 | (-0.2, -0.1) | -0.0 | (-0.1, 0.1) | |
| Specialist visits | -0.0 | (-0.1, 0.1) | -0.1 | (-0.1, -0.0) | -0.0 | (-0.1, 0.0) | |
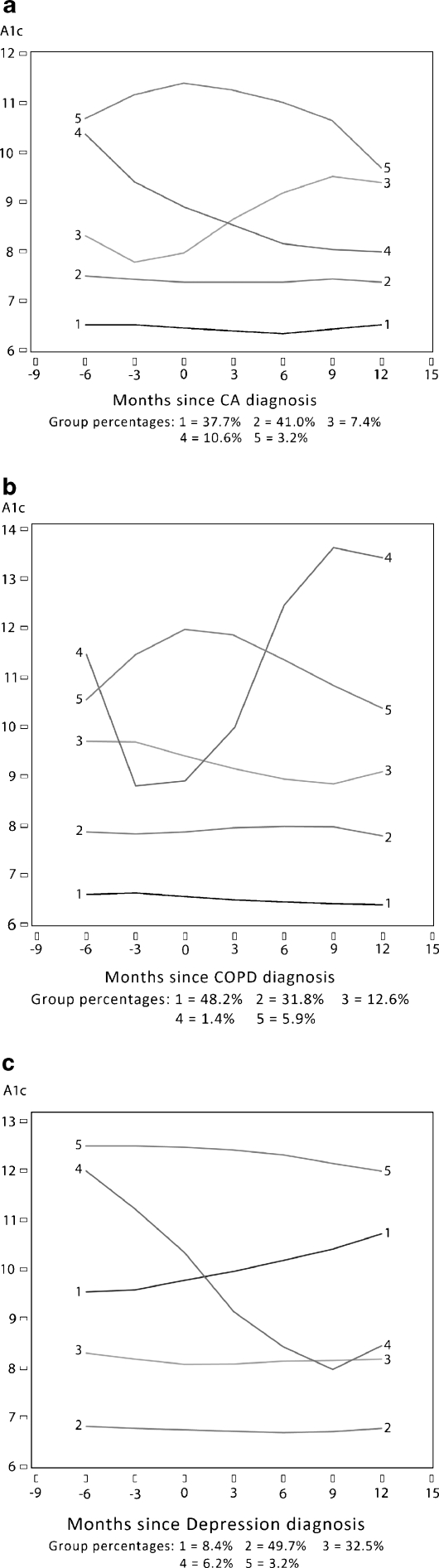
After determining that there was no change in mean A1c or other outcomes as a function of the incident comorbidity, we conducted latent class growth-curve analyses to explore whether there were different trajectories of outcomes within A1c strata. Results of this analysis confirmed that the majority of members of subcohorts have a flat trajectory of A1c pre- and post-diagnosis of the incident comorbidity. A minority show increasing or decreasing trends. Among the small numbers of members classified into one of these groups that change over time, there is no overlap over the three outcomes beyond what would be expected by random chance: The kappa statistics were all less than 0.050 (cancer -0.030 to -0.015; COPD 0.013 to 0.048; depression 0.003 to 0.032). Results of adjusted latent class growth curve analyses are illustrated in Figure 2a, b, and c.
Figure 2.
a Trajectory analysis of hemoglobin A1c as a function of incident cancer. b Trajectory analysis of hemoglobin A1c as a function of incident pulmonary disease. c Trajectory analysis of hemoglobin A1c as a function of incident depression
DISCUSSION
We hypothesized that a new diagnosis of cancer, an exacerbation of chronic pulmonary disease or depression would temporarily shift clinical priorities for both patients and clinicians, leading to changes in the intensity of blood glucose, systolic blood pressure and LDL cholesterol control among persons with underlying diabetes. Our results did not confirm this hypothesis. Based on our analysis, on a population level, none of these disease-specific outcomes was affected by the additional disease burden presented by the new comorbidities. This was suggested by the initial population-level analysis (Fig. 1a–c) and re-emphasized by the results of the latent class trajectory analysis (Fig. 2a–c). The latent class analyses suggest that over 80% of each subgroup had stable A1c levels over the time frame studied.
Previous investigations have described negative associations between these specific comorbidities and quality of care for DM. For example, increased depression severity has been associated with decreased adherence to DM care recommendations and DM control31,32, poor glycemic control has been associated with lower levels of lung function33,34, and cancer survivors may be less likely to receive certain aspects of recommended care for diabetes 6,35. Other studies suggest that treatment intensification in the face of competing demands may be partially a function of the extent of patients’ symptoms (e.g., chronic pain) or total number of comorbidities.36–38
In this longitudinal analysis of an historical cohort, we did not observe associations between the incident comorbidities and DM 2. There are several possible factors that may contribute to these findings: It is possible that these outcomes are largely a function of well-established self-care behaviors, including dietary habits, exercise patterns and medication adherence. It is also possible that other biopsychosocial factors such as duration of specific conditions, disease burden, depression, personal stress, financial constraints, physical functioning, self-efficacy and social support may influence these outcomes.39–43 Alternatively, clinicians (rather than patients) may perceive “competing demands” from a new diagnosis. For example, clinicians addressing increasing numbers of patient concerns during office visits are less likely to change medication management for diabetes, independent of A1c levels.44 Finally, incident comorbidities in themselves may simply have no bearing on the intensiveness of care that affects these specific process-of-care outcomes in these cohorts. Further investigation of the characteristics of subpopulations characterized by the different trajectories of glycemic control (and other outcomes) will be necessary to explain the processes underlying our population-level results.
In all three subcohorts, LDL and SBP declined overall throughout the time period and were not affected by the incident comorbidities. This may partially reflect secular trends that resulted from changes in clinical recommendations for these parameters for persons with DM and/or the effect of population management initiatives within the organization. (Although these effects would be somewhat attenuated by the varying time periods over which the follow-up measurements occurred.) To evaluate the possibility of underlying secular trends for these outcomes, we assessed average decline in SBP and LDL in the entire cohort of persons with DM (n = 27,432) and found that, on average, SBP decreased by 0.75 mmHg per year and LDL by 4.20 mg/dl per year.
We studied values for A1c, LDL and SBP rather than the frequency of measurement of these parameters. Previous evaluations of quality measures in complex patients have concluded that individuals with higher morbidity are more likely to have measurements of these intermediate outcomes and that this in turn is partly (but not completely) mediated by the increased number of visits to clinicians.12,45 Our results, in which none of three measures of patient-clinician contact (continuity of care, primary care visits or specialty care visits) meaningfully affected values of A1c, suggest that quality measures based on frequency of measurement may not reflect the actual values of these health outcomes in persons with multiple morbidities and calls into question measurement frequency as a relevant quality measure.
Our results should be interpreted in the context of several limitations. Administrative and electronic clinical data do not allow full exploration of the myriad biopsychosocial factors that may influence decision-making by both patients and clinicians. Therefore, we were unable to explore potential mechanisms that might explain our findings. For example, in communities of lower socioeconomic status (SES), both patients and clinicians experience increased demands within the clinical encounter, and we did not explore outcomes based on strata of SES.46 In addition, the first clinical diagnosis of an incident comorbidity may not truly represent the first occurrence of the condition, but merely the first time it is brought to clinical attention. In this case, the effect would be to bias the results towards the null (the effect of the ‘incidence’ would be reduced). We attempted to compensate for this limitation by creating a 6-month window of time after the index date during which each cohort member was enrolled, but did not meet criteria for diagnosis of the incident comorbidity. It is also possible that tools or criteria available for diagnosis of a comorbid condition may have changed over the time period of the longitudinal cohort. However, we are unaware of any specific changes in criteria for diagnoses of the comorbid conditions. Criteria for our primary outcome measure of A1c have always been standardized to the Diabetes Control and Complications Trial (DCCT), and internal assays have been stable over time.47
Finally, our results reflect the clinician and patient behavior of members of an integrated health care system that can readily track health outcomes, provide care reminders, and support chronic illness self-management independent of primary care or specialty visits. Given known trends in the organization, it is possible that cohort members may have received independent assistance with lipid management, but that blood pressure management and glycemic control were likely a function of primary care encounters.
CONCLUSION
Our investigation indicates that, in adults with type 2 diabetes, control of diabetes, systolic blood pressure or LDL cholesterol is neither worsened nor improved by the occurrence of any of the three incident comorbidities that we studied. Although incident comorbidities inevitably will affect patients’ and clinicians’ care priorities, these shifts in priorities were not necessarily manifested by changes in these particular outcomes in this cohort. Additional investigation into the effects of interactions between diseases—both for patients and for their clinicians—will better inform changes in care that benefit complex patient populations.
Acknowledgements
Supported by the Agency for Healthcare Research and Quality: 1 R21 HS017627-01 and K08 HS015476 (to E.A.B.). This material has not been presented prior to publication. Limited results were presented as a poster presentation to a small group conference at the AHRQ annual conference in September 2010 and at the annual conference of the North American Primary Care Research Group, Seattle, WA, November 2011.
Conflict of Interest None disclosed.
Appendix
References
- 1.Beasley J, Hankey T, Erickson R, et al. How many problems do family physicians manage at each encounter? A WReN study. Ann Fam Med. 2004;2(5):405–410. doi: 10.1370/afm.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starfield B, Lemke KW, Herbert R, Pavolvich WD, Anderson G. Comorbidity and the use of primary care and specialist care in the elderly. Ann Fam Med. 2005;3(3):215–222. doi: 10.1370/afm.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42(5):1871–1894. doi: 10.1111/j.1475-6773.2006.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parekh AK, Barton MB. The challenge of multiple comorbidity for the US health care system. JAMA. 2010;303(13):1303–1304. doi: 10.1001/jama.2010.381. [DOI] [PubMed] [Google Scholar]
- 5.Redelmeier D, Tan S, Booth G. The treatment of unrelated disorders in patients with chronic medical diseases. N Eng J Med. 1998;338(21):1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 6.Earle C, Neville B. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–1719. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 7.Chwastiak L, Rosenheck R, Leslie D. Impact of medical comorbidity on the quality of schizophrenia pharmacotherapy in a national VA sample. Med Care. 2006;44(1):55–61. doi: 10.1097/01.mlr.0000188993.25131.48. [DOI] [PubMed] [Google Scholar]
- 8.Kiefe C, Funkhouser E, Fouad M, May D. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med. 1998;13(6):357–365. doi: 10.1046/j.1525-1497.1998.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark RE, Weir S, Ouellette RA, Zhang J, Baxter JD. Beyond health plans: behavioral health disorders and quality of diabetes and asthma care for Medicaid beneficiaries. Med Care. 2009;47(5):545–552. doi: 10.1097/MLR.0b013e318190db45. [DOI] [PubMed] [Google Scholar]
- 10.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45(6):480–488. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- 11.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356(24):2496–2504. doi: 10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- 12.Bae SJ, Rosenthal MB. Patients with multiple chronic conditions do not receive lower quality of preventive care. J Gen Intern Med. 2008;23(12):1933–1939. doi: 10.1007/s11606-008-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selby J, Peng T, Karter A, et al. High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Am J Manag Care. 2004;10(2 part 2):70–163. [PubMed] [Google Scholar]
- 14.Zgibor JC, Orchard TJ, Saul M, et al. Developing and validating a diabetes database in a large health system. Diabetes Res Clin Pract. 2007;75(3):313–319. doi: 10.1016/j.diabres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Chermack ST, Zivin K, Valenstein M, et al. The prevalence and predictors of mental health treatment services in a national sample of depressed veterans. Med Care. 2008;46(8):813–820. doi: 10.1097/MLR.0b013e318178eb08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon GE, Korff M, Lin E. Clinical and functional outcomes of depression treatment in patients with and without chronic medical illness. Psychol Med. 2005;35(2):271–279. doi: 10.1017/S0033291704003071. [DOI] [PubMed] [Google Scholar]
- 17.Himelhoch S, Weller WE, Wu AW, Anderson GF, Cooper LA. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Med Care. 2004;42(6):512–521. doi: 10.1097/01.mlr.0000127998.89246.ef. [DOI] [PubMed] [Google Scholar]
- 18.Frayne SM, Miller DR, Sharkansky EJ, et al. Using administrative data to identify mental illness: what approach is best? Am J Med Qual. 2010;25(1):42–50. doi: 10.1177/1062860609346347. [DOI] [PubMed] [Google Scholar]
- 19.Akazawa M, Hayflinger DC, Stanford RH, Blanchette CM. Economic assessment of initial maintenance therapy for chronic obstructive pulmonary disease. Am J Manag Care. 2008;14(7):438–448. [PubMed] [Google Scholar]
- 20.McCrory DC, Brown C, Gray RN, et al. Management of acute exacerbations of chronic obstructive pulmonary disease. Evid Rep Technol Assess Summ. 2000;19:1–4. [PMC free article] [PubMed] [Google Scholar]
- 21.Naureckas ET, Dukic V, Bao X, Rathouz P. Short-acting beta-agonist prescription fills as a marker for asthma morbidity. Chest. 2005;128(2):602–608. doi: 10.1378/chest.128.2.602. [DOI] [PubMed] [Google Scholar]
- 22.Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132(2):403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 23.Nagin DS. Analyzing developmental trajectories: a semi-parametric group based approach. Psychol Methods. 1999;4(139):157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 24.Legler JM, Davis WW, Potosky AL, Ho RM. Latent variable modelling of recovery trajectories: sexual function following radical prostatectomy. Stat Med. 2004;23:2875–2893. doi: 10.1002/sim.1864. [DOI] [PubMed] [Google Scholar]
- 25.Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol. 2004;23(1):3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med. 2008;168(3):271–276. doi: 10.1001/archinternmed.2007.72. [DOI] [PubMed] [Google Scholar]
- 27.Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3(2):159–166. doi: 10.1370/afm.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Baldwin LM, Saver BG, et al. The contribution of longitudinal comorbidity measurements to survival analysis. Med Care. 2009;47(7):813–821. doi: 10.1097/MLR.0b013e318197929c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-Cm and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 30.Gill JM. Mainous AG3. The role of provider continuity in preventing hospitalizations. Arch Fam Med. 1998;7(4):352–357. doi: 10.1001/archfami.7.4.352. [DOI] [PubMed] [Google Scholar]
- 31.Richardson LK, Egede LE, Mueller M, Echols CL, Gebregziabher M. Longitudinal effects of depression on glycemic control in veterans with type 2 diabetes. Gen Hosp Psych. 2008;30(6):509–514. doi: 10.1016/j.genhosppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160(21):3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 33.Walter RE, Beiser A, Givelber RJ, O'Connor GT, Gottlieb DJ. Association between glycemic state and lung function: the Framingham Heart Study. Am J Respir Crit Care Med. 2003;167(6):911–916. doi: 10.1164/rccm.2203022. [DOI] [PubMed] [Google Scholar]
- 34.Davis WA, Knuiman M, Kendall P, Grange V, Davis TM. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27(3):752–757. doi: 10.2337/diacare.27.3.752. [DOI] [PubMed] [Google Scholar]
- 35.Keating NL, Zaslavsky AM, Herrinton LJ, Selby JV, Wolf RE, Ayanian JZ. Quality of diabetes care among cancer survivors with diabetes. Med Care. 2007;45(9):869–875. doi: 10.1097/MLR.0b013e31806728e9. [DOI] [PubMed] [Google Scholar]
- 36.Turner BJ, Hollenbeak CS, Weiner M, Have T, Tang SS. Effect of unrelated comorbid conditions on hypertension management. Ann Intern Med. 2008;148:578–586. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 37.Zulman DM, Kerr EA, Hofer TP, Heisler M, Zikmund-Fisher BJ. Patient-provider concordance in the prioritization of health conditions among hypertensive diabetes patients. J Gen Intern Med Feb 2, 2010; epub ahead of print. [DOI] [PMC free article] [PubMed]
- 38.Krein SL, Hofer TP, Holleman R, Piette JD, Klamerus ML, Kerr EA. More than a pain in the neck: how discussing chronic pain affects hypertension medication intensification. J Gen Intern Med. 2008;24(8):911–916. doi: 10.1007/s11606-009-1020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Fam Med. 2003;1(1):15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noel PH, Frueh C, Larme A, Pugh J. Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expect. 2005;8(1):54–63. doi: 10.1111/j.1369-7625.2004.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients' treatment priorities and self-management? J Gen Intern Med. 2007;22(12):1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST Study. Fam Med. 2001;33(5):354–360. [PubMed] [Google Scholar]
- 43.Sells D, Sledge WH, Wieland M, et al. Cascading crises, resilience and social support within the onset and development of multiple chronic conditions. Chronic Illn. 2009;5(2):92–102. doi: 10.1177/1742395309104166. [DOI] [PubMed] [Google Scholar]
- 44.Parchman M, Pugh J, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5(3):196–201. doi: 10.1370/afm.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner RM, Chang VW. The relationship between measured performance and satisfaction with care among clinically complex patients. J Gen Intern Med. 2008;23(11):1729–1735. doi: 10.1007/s11606-008-0734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercer SW, Watt GCM. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann Fam Med. 2007;5(6):503–510. doi: 10.1370/afm.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]