Abstract
We examined the effect of subcutaneous fluoroquinolone antibiotic administration on persistence and density of vancomycin-resistant Enterococcus faecium stool colonization in mice. Levofloxacin and ciprofloxacin did not promote colonization in comparison to saline controls, whereas moxifloxacin and gatifloxacin promoted persistent overgrowth in a dose-dependent fashion.
In a mouse model and in colonized patients, we have demonstrated that antibiotics with potent in vitro activity against intestinal anaerobes promote persistent high-density colonization with vancomycin-resistant Enterococcus faecium (VRE), whereas antibiotics with minimal antianaerobe activity (including ciprofloxacin) do not (4, 5). Although some fluoroquinolone antibiotics have enhanced in vitro activity against anaerobes (e.g., moxifloxacin and gatifloxacin) (1, 13), studies with human volunteers have demonstrated that none of the currently available agents in this class cause a significant reduction in the density of intestinal anaerobes (2, 6-9, 14). Therefore, we examined the effect of fluoroquinolone antibiotics with various levels of antianaerobe activity on the persistence of VRE stool colonization in mice.
The mouse model that we have previously utilized to study the effect of antibiotics on persistence of VRE stool colonization was used (4). Male CF1 mice (Harlan Sprague-Dawley, Indianapolis, Ind.) weighing 25 to 30 g were housed in individual cages and fed rodent chow and water ad libitum. VRE colonization with Enterococcus faecium strain C68 was established in all mice by administering oral vancomcyin (250 μg/liter) in drinking water for 2 days before and 5 days after esophageal instillation of 108 CFU of VRE in 0.5 ml (4).
After discontinuation of oral vancomycin, mice were begun on twice-daily subcutaneous injections of saline (negative controls) or antibiotics for 15 days. Three different doses of the fluoroquinolone antibiotics were studied: a low dose equal to the usual human dose administered over a 24-h period (milligrams of antibiotic per gram of body weight); a medium dose of 5 times the low dose, and a human-equivalent dose of 12 times the usual human dose calculated by the technique of Freireich et al. (11). The dose of levofloxacin was based on the 750-mg-per-day human dose. Gatifloxacin and moxifloxacin were administered only at the lower two dosages because the highest dosage of these agents resulted in significant subcutaneous tissue irritation. The dosages included ciprofloxacin at 0.4, 2, and 4.8 mg/day; levofloxacin at 0.375, 1.875, and 4.7 mg/day; gatifloxacin at 0.2 and 1 mg/day; and moxifloxacin at 0.2 and 1 mg/day. Clindamycin at 1.4 mg/day promoted persistent high-density colonization in previous experiments and was included as a positive control (4).
Four mice were included in each experimental group, and the experiments were performed twice (eight mice per group). Stool samples were collected prior to starting subcutaneous antibiotics and then on days 5, 10, and 15 of antibiotic therapy. To measure the densities of VRE and total facultative and aerobic gram-negative bacilli, stool samples were weighed, diluted in sterile normal saline, homogenized with a pestle, serially diluted in sterile saline, and plated onto Enterococcosel agar (Becton Dickinson, Cockeysville, Md.) containing 6 μg of vancomycin/ml and MacConkey agar (Difco Laboratories, Detroit, Mich.), respectively (4).
Statistical analyses were performed using Stata, version 5.0 (Stata Corp., College Station, Tex.). To compare VRE densities among groups, mean densities were determined for individual mice with pooled data from days 5, 10, and 15. Means were then examined by one-way analysis of variance for the antibiotic treatment group. Overall differences and pairwise differences were examined, with P values adjusted for multiple comparisons by using the Scheffe correction. For the experiments in which fluoroquinolone dosages were equivalent to the usual human dosages, paired t tests (P < 0.05) were performed to compare densities of total facultative and aerobic gram-negative bacilli from experimental groups with those for the saline control group on day 5 of antibiotic treatment.
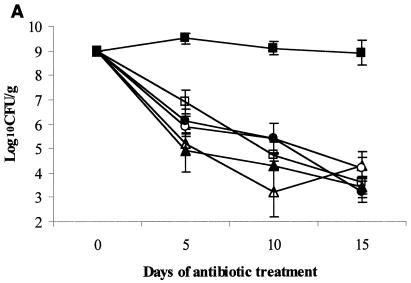
The results for the groups treated with the usual human-equivalent fluoroquinolone dosages are shown in Fig. 1A. Clindamycin promoted persistent high-density VRE stool colonization that was significantly higher than that for the saline controls or for the fluoroquinolone treatment groups (P < 0.001). The densities of VRE for each of the fluoroquinolone treatment groups on days 5 to 15 did not differ significantly from the densities for the saline control group (P > 0.12 for each comparison).
FIG. 1.
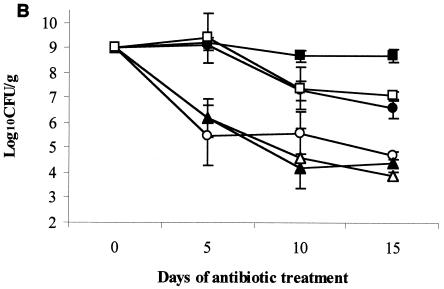
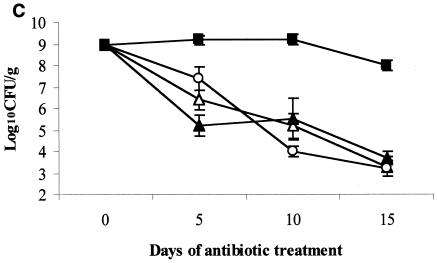
Effect of subcutaneous antibiotic administration on persistence and density of VRE in the stool of mice. All mice were colonized with VRE on day 0 (mean, 9 log10CFU/g of stool). Subcutaneous antibiotics (eight mice per group) were administered every 12 h from day 0 to day 15. Densities of VRE are shown for low-dose antibiotics (equal to human doses on a milligram-per-kilogram basis) (A), medium-dose antibiotics (five times the low dose) (B), and corrected human-equivalent doses of antibiotics (C). If VRE was not detected in stool, the lower limit of detection (∼2 log10CFU/g) was assigned. ▵, normal saline; ○, levofloxacin; •, gatifloxacin; □, moxifloxacin; ▴, ciprofloxacin; ▪, clindamycin.
On day 0 (Fig. 1.A.), the density of total facultative and aerobic gram-negative bacilli among all of the mice was 6.9 ± 1.52 log10CFU/g (mean ± standard deviation). On day 5 of antibiotic treatment, the densities of total and facultative gram-negative bacilli for each of the fluoroquinolone groups (range, 2.1 ± 0.05 to 2.3 ± 0.16 log10CFU/g) were significantly reduced in comparison to those for the saline control group (6.0 ± 0.58 log10CFU/g) (P < 0.05 for each comparison).
The results for the groups treated with five times the usual human dosages of fluoroquinolones are shown in Fig. 1B. Clindamycin (positive control), moxifloxacin, and gatifloxacin promoted persistent high-density VRE stool colonization that was significantly higher than that for the saline controls or for the levofloxacin and ciprofloxacin treatment groups at each time point (P < 0.001 for each comparison). The gatifloxacin and moxifloxacin groups did not differ significantly from the clindamycin group (P = 0.32 and 0.64, respectively).
The results for the groups treated with human-equivalent doses calculated based on the technique of Freireich et al. (11) are shown in Fig. 1C. Clindamycin promoted overgrowth of VRE in comparison to saline controls (P < 0.001), but levofloxacin and ciprofloxacin did not (P = 0.999 and 0.994, respectively).
The impact of antibiotics on the intestinal bacterial flora is determined by the concentrations achieved in the intestinal tract, the amount of inactivation that occurs, and the activity of the agent under the in vivo conditions present in the intestinal tract (4, 18). Fluoroquinolones are actively secreted into the human and rodent intestinal tract, resulting in intestinal concentrations that are typically 100 to 1,000 times higher than serum concentrations after oral or parenteral dosing (12, 16). These agents inhibit susceptible facultative gram-negative organisms in the intestinal tract but have much less effect on intestinal anaerobes than would be predicted based on the drug levels achieved (10, 17). For example, the MIC at which 90% of isolates are inhibited of ciprofloxacin for Bacteroides species is 1 to 32 μg/ml (18), but this agent achieves concentrations of 185 to 2,220 μg/g in stool (3, 14). The minimal impact of the fluoroquinolone antibiotics on intestinal anaerobes has been attributed to high degrees of reversible binding of these agents to fecal matter, reduced susceptibility of anaerobic bacteria to fluoroquinolones under strictly anaerobic conditions, and an inoculum effect for inhibition of anaerobes (10, 17).
Our data suggest that fluoroquinolone antibiotics with minimal antianaerobic activity (e.g., ciprofloxacin and levofloxacin) are unlikely to promote intestinal colonization with VRE in patients. Despite the fact that fluoroquinolone antibiotics with enhanced in vitro antianaerobic activity (e.g., moxifloxacin and gatifloxacin) have been shown to cause minimal disruption of the anaerobic microflora of humans (6, 8, 9), our data raise concern that these agents could promote overgrowth of VRE, particularly if administered at higher doses. Our findings are consistent with a previous study that found that low doses of BMS-284756, a novel des-F(6)-quinolone antibiotic, did not affect the concentration of intestinal anaerobes in healthy human volunteers, whereas higher doses resulted in significant inhibition of anaerobes (15).
Our conclusions are limited by several factors. The effect of the antibiotics studied on VRE colonization might differ for mice and patients. Our previous studies using this mouse model, however, demonstrated good correlation of findings in mice and in colonized patients (4, 5). The concentrations of the antibiotics in the intestinal tract were not assessed; however, significant inhibition of total facultative gram-negative bacilli was observed for all of the fluoroquinolones studied, suggesting that the antibiotics were present in the intestinal tract. The density of VRE within different sections of the colon may vary; however, the level of VRE in stool is likely to be most important in facilitating contamination of clinical sites or transmission to other patients.
Acknowledgments
This research was supported by an Advanced Research Career Development Award from the Department of Veterans Affairs to C.J.D. and by a grant from Ortho-McNeil Pharmaceuticals.
REFERENCES
- 1.Appelbaum, P. C. 1999. Quinolone activity against anaerobes. Drugs 58(Suppl. 2):60-64. [DOI] [PubMed] [Google Scholar]
- 2.Bergan, T., C. Delin, S. Johansen, I. M. Kolstad, C. E. Nord, and S. B. Thorsteinsson. 1986. Pharmacokinetics of ciprofloxacin and effect of repeated dosage on salivary and fecal microflora. Antimicrob. Agents Chemother. 29:298-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brumfitt, W., I. Franklin, D. Grady, J. M. T. Hamilton-Miller, and A. Iliffe. 1984. Changes in the pharmacokinetics of ciprofloxacin and fecal flora during administration of a 7-day course to human volunteers. Antimicrob. Agents Chemother. 26:757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, and L. B. Rice. 1999. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 180:384-390. [DOI] [PubMed] [Google Scholar]
- 5.Donskey, C. J., T. K. Chowdhry, M. T. Hecker, C. K. Hoyen, J. A. Hanrahan, A. M. Hujer, R. A. Hutton-Thomas, C. C. Whalen, R. A. Bonomo, and L. B. Rice. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343:1925-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edlund, C., and C. E. Nord. 1999. Effect of quinolones on intestinal ecology. Drugs 58(Suppl. 2):65-70. [DOI] [PubMed] [Google Scholar]
- 7.Edlund, C., S. Sjostedt, and C. E. Nord. 1997. Comparative effect of levofloxacin and ofloxacin on the normal oral and intestinal microflora. Scand. J. Infect. Dis. 29:383-386. [DOI] [PubMed] [Google Scholar]
- 8.Edlund, C., and C. E. Nord. 1999. Ecological effect of gatifloxacin on the normal human intestinal microflora. J. Chemother. 11:50-53. [DOI] [PubMed] [Google Scholar]
- 9.Edlund, C., G. Beyer, M. Hiemer-Bau, S. Ziege, H. Lode, and C. E. Nord. 2000. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal flora. Scand. J. Infect. Dis. 32:81-85. [DOI] [PubMed] [Google Scholar]
- 10.Edlund, C., L. Lindqvist, and C. E. Nord. 1988. Norfloxacin binds to human fecal material. Antimicrob. Agents Chemother. 32:1869-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freireich, E., E. Gehan, D. Rall, L. Schmidt, and H. Skipper. 1966. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother. Rep. 50:219-244. [PubMed] [Google Scholar]
- 12.Hooper D. C. 1995. Quinolones, p. 364-376. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 13.Horn, R., and H. G. Robson. 2001. Susceptibility of the Bacteroides fragilis group to newer quinolones and other standard anti-anaerobic agents. J. Antimicrob. Chemother. 48:127-130. [DOI] [PubMed] [Google Scholar]
- 14.Krueger, W. A., G. Ruckdeschel, and K. Unertl. 1997. Influence of intravenously administered ciprofloxacin on aerobic intestinal microflora and fecal drug levels when administered simultaneously with sucralfate. Antimicrob. Agents Chemother. 41:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nord, C. E., D. A. Gajjar, and D. M. Grasela. 2002. Ecological impact of the des-F(6)-quinolone, BMS-284756, on the normal intestinal microflora. Clin. Microbiol. Infect. 8:229-239. [DOI] [PubMed] [Google Scholar]
- 16.Ramon, J., M. Ben-Haim, M. Shabtai, and E. Rubenstein. 2001. Transepithelial intestinal excretion of ciprofloxacin in humans. Clin. Infect. Dis. 32:822-823. [DOI] [PubMed] [Google Scholar]
- 17.van Saene, J. J. M., H. K. F. van Saene, and C. F. Lerk. 1986. Inactivation of quinolone by feces. J. Infect. Dis. 153:999-1000. [DOI] [PubMed] [Google Scholar]
- 18.Vollaard, E. J., and H. A. L. Clasener. 1994. Colonization resistance. Antimicrob. Agents Chemother. 38:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]