Abstract
Chloride influx through GABA-gated Cl− channels, the principal mechanism for inhibiting neural activity in the brain, requires a Cl− gradient established in part by K+–Cl− cotransporters (KCCs). We screened for Caenorhabditis elegans mutants defective for inhibitory neurotransmission and identified mutations in ABTS-1, a Na+-driven Cl−–HCO3− exchanger that extrudes chloride from cells, like KCC-2, but also alkalinizes them. While animals lacking ABTS-1 or the K+–Cl− cotransporter KCC-2 display only mild behavioural defects, animals lacking both Cl− extruders are paralyzed. This is apparently due to severe disruption of the cellular Cl− gradient such that Cl− flow through GABA-gated channels is reversed and excites rather than inhibits cells. Neuronal expression of both transporters is upregulated during synapse development, and ABTS-1 expression further increases in KCC-2 mutants, suggesting regulation of these transporters is coordinated to control the cellular Cl− gradient. Our results show that Na+-driven Cl−–HCO3− exchangers function with KCCs in generating the cellular chloride gradient and suggest a mechanism for the close tie between pH and excitability in the brain.
Keywords: C. elegans , chloride, GABA, transporter
Introduction
GABA is the primary inhibitory neurotransmitter in the mammalian brain (Mody et al, 1994). Cl− influx through GABAA receptors results in neuronal hyperpolarization and inhibition. This response depends on maintenance of a relatively low intracellular Cl− concentration, resulting in a Cl− reversal potential more negative than the cell's resting membrane potential (Farrant and Kaila, 2007). Many immature neurons maintain high intracellular Cl− levels, resulting in a Cl− reversal potential more positive than the cell's resting potential, so that GABA can elicit Cl− efflux and an excitatory response (Ben-Ari, 2002; Blaesse et al, 2009). Thus, neurons regulate intracellular Cl− levels to control the magnitude and polarity of the effects of GABA, and this in turn has been shown to powerfully affect neural development and activity (Fiumelli et al, 2005; Akerman and Cline, 2006). Understanding this type of ‘ionic plasticity’ (Rivera et al, 2005) requires understanding which transporters control intracellular Cl− levels.
Multiple transporters move Cl− across the plasma membrane. The most extensively studied are the Cl−-extruding K+–Cl− cotransporter KCC2 and the Cl−-accumulating Na+–K+–2Cl− cotransporter NKCC1 (Blaesse et al, 2009). The excitatory effect of GABA on immature hippocampal and cortical neurons is due in part to their low KCC2 expression and high NKCC1 expression. GABA becomes inhibitory as neurons upregulate expression of KCC2 and downregulate expression of NKCC1 (Rivera et al, 1999; Yamada et al, 2004; Akerman and Cline, 2006). However, KCC2 is apparently not the sole Cl− extruder (Rivera et al, 1999; Gulacsi et al, 2003). Na+-driven Cl−–HCO3− exchangers (also known as Na+-driven anion exchangers, or NDAEs) can extrude Cl− and could contribute to the cellular Cl− gradient and hyperpolarizing GABA action (Gulacsi et al, 2003; Kim and Trussell, 2009). However, to date no specific transporter of this type has been shown to have such a role.
The SLC4 family of ion transporters includes multiple Cl−–HCO3− exchangers (Romero et al, 2004). Among these, human and squid NDCBE and Drosophila NDAE1 have been shown to function as NDAEs that extrude Cl− (Romero et al, 2000; Grichtchenko et al, 2001; Virkki et al, 2003). Despite expression of NDCBE in mammalian neurons and its characterized role in regulating neuronal intracellular pH (Schwiening and Boron, 1994; Grichtchenko et al, 2001; Chen et al, 2008a, 2008b), its role in regulating neuronal intracellular Cl− homeostasis has not been fully investigated.
The nematode Caenorhabditis elegans has been developed as a model organism for genetic analysis of GABAergic neurotransmission (Jorgensen, 2005). C. elegans possesses 19 GABAergic motor neurons that synapse on the body wall muscles (BWMs) to promote hyperpolarization and lengthening of muscles contralateral to contracting muscles, producing sinusoidal body bends and coordinated locomotion (McIntire et al, 1993b). UNC-49 is the C. elegans GABAA receptor homologue (Bamber et al, 1999; Richmond and Jorgensen, 1999). UNC-49 function is required in the BWMs to generate coordinated body bends (McIntire et al, 1993a, 1993b; Bamber et al, 1999).
More recently, C. elegans has been established as a model system in which to identify Cl− transporters that control GABA signalling. C. elegans egg-laying behaviour can be blocked by inactivating the hermaphrodite-specific neurons (HSNs) with a mutation in the putative transmembrane receptor EGL-47 (Moresco and Koelle, 2004). Suppressor mutations that reactivate the HSNs to allow egg laying to occur identify genes required for inhibitory signalling by GABA, including the gene encoding the K+–Cl− cotransporter KCC-2 (Tanis et al, 2009). In this work, we have used this screening strategy to identify the Na+-driven Cl−–HCO3− exchanger ABTS-1 and show that it functions partially redundantly with KCC-2 to control GABAergic signalling in C. elegans neurons and muscles.
Results
Mutations in abts-1 suppress egl-47(dm) to reactivate egg laying
Wild-type animals retain 13.1±0.9 eggs in utero (Figure 1A), while egl-47(dm) mutants fail to lay eggs and thus retain a far larger number, 57.5±2.7 (Figure 1B). More strikingly, wild-type young adults lay many eggs through the first ∼12 h of adulthood (70.5±4.4 eggs per pool of five animals assayed), while we have never observed a young adult egl-47(dm) animal lay an egg (Figure 1D). Thus, egl-47(dm) animals provided an exquisitely clean background in which we were able to identify suppressor mutations that restore HSN activity to reactivate egg laying.
Figure 1.
Mutations in abts-1 suppress the egl-47(dm) egg-laying defect. (A–C) Representative images of wild-type, egl-47(dm), and abts-1(ok1566); egl-47(dm) worms. The average number of unlaid eggs is shown (±95% confidence interval). n⩾30 animals for each genotype. Eggs with eight or fewer cells (arrowheads) and eggs with more than eight cells (arrows). (D) Eggs laid by pools of five young adult worms, by 18 h after the late-L4 stage. Wild-type young adults lay many eggs, while egl-47(dm) young adults never lay any eggs. Mutations in abts-1 significantly suppress the egl-47(dm) egg-laying defect (P<0.005; comparing bars with white asterisks to the black asterisked control with zero eggs). For each genotype n=6 pools of five worms each. Error bars in this and subsequent figures represent standard errors except when otherwise noted. (E) Proportion of eggs laid at an early developmental stage. Wild-type (bar 1), egl-47(dm) (bar 2), abts-1(vs145) (bar 3), abts-1(ok1566) (bar 5), and kcc-2(vs132) (bar 7) animals all lay a low proportion of early-stage eggs that have not yet reached the eight-cell stage. abts-1(vs145); egl-47(dm) (bar 4), abts-1(ok1566); egl-47(dm) (bar 6), and kcc-2(vs132); egl-47(dm) (bar 8) animals all lay a high proportion of such early-stage eggs (asterisks indicate P<0.05 compared with the bar 2 control). n⩾100 eggs for each genotype, error bars show the 95% confidence interval. These results show that egl-47(dm) significantly stimulates egg laying in the absence of ABTS-1 or KCC-2.
We used the egl-47(dm) suppressor screen to identify the vs145 suppressor mutation, which strongly reactivated egg laying in an egl-47(dm) background (Figure 1C and D). We mapped and cloned vs145 and discovered a mutation in the anion bicarbonate transporter gene, abts-1, which encodes a protein similar to mammalian HCO3− transporters of the SLC4 family (Sherman et al, 2005). The vs145 mutation results in the substitution of an arginine for a glycine residue within a predicted transmembrane helix (Figure 3A). We obtained a deletion mutation ok1566 that is a putative null since it removes sequences encoding all of one and parts of two other predicted transmembrane helices. Because vs145 and ok1566 are phenotypically similar (Figure 1D and E; data not shown), vs145 appears to be a strong loss-of-function mutation. Both the vs145 and ok1566 alleles restored egg laying in egl-47(dm) young adults. The double mutants abts-1(vs145); egl-47(dm) and abts-1(ok1566); egl-47(dm) (Figure 1C) retained only 7.3±1.2 and 7.3±0.8 eggs in utero, respectively, compared with the 57.5±2.7 eggs retained by egl-47(dm) single mutants (Figure 1B). As young adults, the double mutants abts-1(vs145); egl-47(dm) and abts-1(ok1566); egl-47(dm) laid 20.3±4.0 and 30.2±4.5, respectively, compared with the 0.0±0.0 eggs laid by egl-47(dm) single mutants (Figure 1D). Thus, mutations in abts-1 suppress egl-47(dm) by reactivating egg laying in young adults.
Loss of abts-1 causes the egl-47(dm) mutation to stimulate rather than inhibit egg laying
abts-1 mutations are similar to kcc-2 mutations in that both show the same remarkable interaction with egl-47(dm). While egl-47(dm) strongly inhibits egg laying in a wild-type genetic background, it instead stimulates egg laying in a kcc-2 mutant background such that kcc-2; egl-47(dm) mutants lay eggs at a much higher rate than do wild-type animals (Tanis et al, 2009). The stimulatory effect of the egl-47(dm) mutation on function of HSNs lacking KCC-2 may be analogous to the excitatory action of GABA on immature mammalian neurons that do not express KCC2 (Rivera et al, 1999). To assay for an increased rate of egg laying, we measured the developmental stage of freshly laid eggs. C. elegans eggs are fertilized internally and undergo cell divisions in utero prior to being laid. In wild-type animals only ∼5% of freshly laid eggs are at an early developmental stage (defined as eight cells or fewer). Mutants that lay eggs at a higher rate than do wild-type animals retain eggs in utero for a shorter period of time and thus lay a higher percentage of their eggs at an early developmental stage (Chase and Koelle, 2004). Using this assay, we found that while wild-type animals, egl-47(dm) mutants, and abts-1 single mutants lay a low percentage of early-stage eggs, abts-1; egl-47(dm) double mutants lay over 60% of their eggs at an early developmental stage (Figure 1E). Thus, abts-1 mutations, like kcc-2 mutations, reverse the effect of egl-47(dm) on egg laying, but do not change egg-laying behaviour in the absence of egl-47(dm) or other challenges to the egg-laying system. abts-1 mutants lay fewer cumulative eggs than do wild-type animals (Figures 1C and 4), but this observation can be attributed to decreased egg production in abts-1 mutants (Supplementary Figure S1), an effect that is also observed in kcc-2 mutants (Tanis et al, 2009).
ABTS-1 is a Na+-driven chloride–bicarbonate exchanger
To analyse ABTS-1 function, we used Xenopus oocytes to express the ABTS-1 protein and monitored membrane ion transport using intracellular ion-selective microelectrodes (Figure 2). Previous experiments with the ABTS-1 ‘B’ isoform (isoforms are shown in Figure 3A) revealed that it functioned as an electroneutral Na+/HCO3− cotransporter (Romero and Boron, 1998). However, this early study did not address the potential role of Cl−, as the transport activity was quite low. Studies of the ABTS-1 ‘A’ isoform indicated that it functions as a Cl−–HCO3− exchanger, but did not address any potential role of Na+ (Sherman et al, 2005). In the present study, we sought to more fully characterize the transport properties of ABTS-1, specifically to determine whether it is an NDAE that acts to extrude Cl−.
Figure 2.
ABTS-1 is a sodium-driven chloride–bicarbonate exchanger. (A–C) Electrophysiology data from pH microelectrodes in Xenopus oocytes. In the presence of 1.5% CO2/10 mM HCO3− (pH 7.5), both ABTS-1 (A) and Drosophila NDAE1 (B) responded to Cl− replacement (0Cl−) by increasing pHi (HCO3− influx to oocyte), while water-injected oocytes (C) showed no response. By contrast, Na+ removal (0 Na+) resulted in dramatic pHi decreases (HCO3− efflux from oocyte) in oocytes expressing ABTS-1 (A) and NDAE1 (B), while water-injected oocytes (C) showed no response. Rates of alkalinization during Cl− and Na+ removal (# × 10−5 pH units/s) are indicated above the respective segments of the pHi traces. (D) Electrophysiology data from halide microelectrodes in Xenopus oocytes. In oocytes expressing ABTS-1 and water-injected controls, replacement of extracellular Cl− with gluconate produced no change in [Cl−]. When extracellular Cl− was replaced by I−, the electrode sensed I− influx in oocytes expressing ABTS-1, but not in water-injected controls. (E) Models illustrating the suggested transport activity of ABTS-1 and NDAE1 in response to removal of extracellular Cl− (a) and in response to removal of extracellular Na+ or addition of extracellular I− (b). The transport activity illustrated in panel (Ea) is also the predicted activity of ABTS-1 under normal conditions. The stoichiometry shown, with two HCO3− ions transported, is consistent with the observed electroneutrality of the transport.
Figure 3.
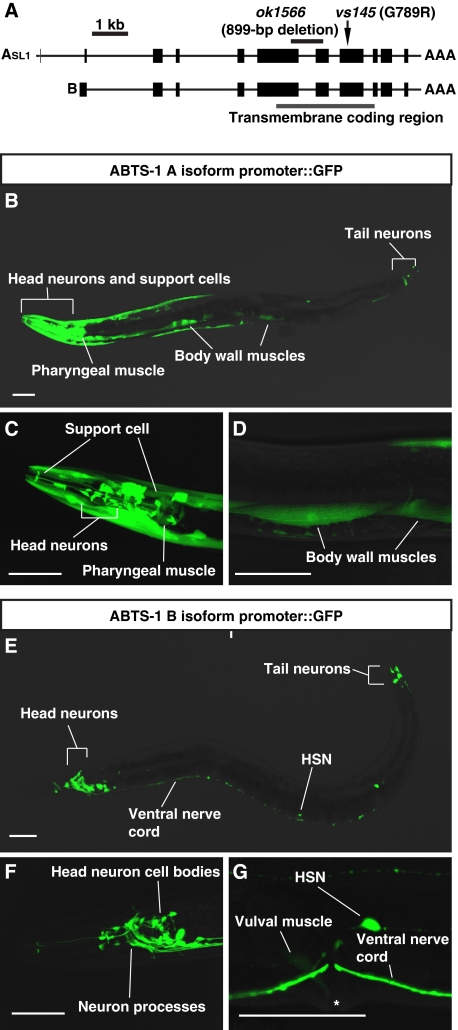
ABTS-1 is expressed predominantly in neurons and muscles. (A) The gene structure of abts-1. Two abts-1 transcripts, abts-1a and abts-1b, have different start sites that splice onto common exons. Black bars represent exons and connecting lines indicate introns. Indicated are the polyadenylation site (AAA) and the SL1 trans splice leader (SL1). Also displayed are the transmembrane domain coding region (grey bar) and the locations of the vs145 point mutation and the ok1566 deletion mutation. (B–D) GFP fluorescence in animals carrying an abts-1a promoter::gfp::abts-1 3′ UTR reporter transgene. This reporter transgene was expressed in many cells including the BWMs, pharyngeal muscles, and head and tail neurons. (E–G) GFP fluorescence in animals carrying an abts-1b promoter::gfp::abts-1 3′ UTR reporter transgene. This reporter transgene was expressed primarily in neurons, including the HSN, and in the vulval muscles. Scale bars in all images are 50 μm.
We found that the transport activity of ABTS-1 is consistent with Na+-driven Cl−–HCO3− exchange. The ion transport activity by ABTS-1 is somewhat lower than that of many other HCO3− transporters (note differences in the magnitude of pH change in Figure 2Ab and Bb), complicating some measures of its transport activity. We therefore tested the hypothesis that ABTS-1 is an NDAE by directly comparing ABTS-1 activity (Figure 2A) to that of a known Na+-driven Cl−–HCO3− exchanger, Drosophila NDAE1 (Figure 2B). Water-injected control oocytes had minimal background transport activity (Figure 2C). ABTS-1 (Figure 2A) and NDAE1 (Figure 2B) expression resulted in HCO3− transport (measured with intracellular pH microelectrodes) in response to removal of Cl− or Na+. With Cl− removal (0 Cl−) both ABTS-1 and NDAE1 produced an increase of intracellular pH (pHi), indicating HCO3− movement into the cell (model in Figure 2Ea). This response is characteristic of Cl−–HCO3− exchange (Romero et al, 2000; Chang et al, 2009). Next, we removed Na+ (0 Na+). Both ABTS-1 and NDAE1 responded by decreasing pHi, indicating HCO3− movement out of the cell (model in Figure 2Eb). This effect is consistent with Na+-driven Cl−–HCO3− exchange (Romero et al, 2000; Grichtchenko et al, 2001). Monitoring of Vm indicated that this transport activity was electroneutral (Supplementary Figures S2 and S3).
Many Cl− transport systems will also transport other halides (e.g. I− and Br−). The order of preference of halides (the Hoffmeister series) may be used to distinguish anion transport systems (Wright and Diamond, 1977). Figure 2D shows that ABTS-1 expression provided Xenopus oocytes with I− transport activity. By calibrating our anion-sensitive microelectrodes (see Materials and methods), we determined that for ABTS-1-expressing oocytes incubated in high extracellular [I−], intracellular [I−] reached ∼250 μM within 5 minutes. In contrast, intracellular [I−] did not increase in control oocytes injected with water rather than ABTS-1 copy RNA (cRNA) (Figure 2D). The model in Figure 2Eb represents the proposed transport mechanism that occurs during Na+ removal (Figure 2A) and during I− addition (Figure 2D): ABTS-1 transports Na+ and HCO3− out of the cell while transporting halides into the cell.
ABTS-1 is broadly expressed in C. elegans neurons and muscles
C. elegans KCC-2 is expressed in excitable cells such as neurons and muscles where it presumably extrudes Cl− to support inhibitory neurotransmission by ligand-gated Cl− channels (Tanis et al, 2009). We observed a similar pattern of ABTS-1 expression. To examine the expression pattern of ABTS-1, we constructed abts-1 promoter::gfp::abts-1 3′ UTR reporter transgenes in which GFP expression was driven by 5′ promoter and 3′ untranslated region sequences. Because the available ABTS-1 cDNAs showed the presence of two transcripts with different 5′ exons (Figure 3A), we generated two reporter constructs using the promoter regions for each of the two isoforms (Supplementary Figure S4). We found that the abts-1a promoter drove expression of GFP in head, tail, and lateral neurons as well as in BWMs, pharyngeal muscles, and neural support cells (Figure 3B–D). The abts-1b promoter drove expression of GFP in head neurons, tail neurons, and the HSNs, as well as weak expression in the vulval muscles (Figure 3E–G).
ABTS-1 is required for UNC-49-mediated inhibition of egg laying
Given the apparently similar mutant phenotypes, Cl− extrusion activities, and expression patterns of ABTS-1 and KCC-2, we hypothesized that ABTS-1 may have a similar role to KCC-2 in establishing the cellular Cl− gradient that supports the inhibitory action of GABA-gated Cl− channels. UNC-49 is the C. elegans GABAA receptor homologue, and its function is dependent on KCC-2 (Bamber et al, 1999; Tanis et al, 2009). The GABAA receptor agonist muscimol is a potent inhibitor of egg laying in wild-type animals, but not in animals that have compromised UNC-49 function. Thus, muscimol does not inhibit egg laying in animals lacking UNC-49 or in animals lacking KCC-2 (Tanis et al, 2009).
We found that muscimol also fails to inhibit egg laying in abts-1 mutants. In control wild-type animals, muscimol inhibited egg laying by 97%: over 2 h, pools of five animals laid 83.5±5.6 eggs in the absence but only 2.7±0.9 eggs in the presence of muscimol (Figure 4A). This effect requires UNC-49 and KCC-2, since the corresponding mutants showed only 11 and 12% inhibition of egg laying by muscimol, respectively (Figure 4A). Similarly, we found that the abts-1 mutant showed only 12% inhibition of egg laying by muscimol (Figure 4A). Thus, ABTS-1 may function similarly to KCC-2 in generating the Cl− gradient required for muscimol to inhibit egg laying via UNC-49.
Figure 4.
ABTS-1 is required for the inhibition of egg-laying behaviour by muscimol. The average number of eggs laid by pools of five animals over 2 h in the presence or absence of 0.5 mM muscimol. unc-49, kcc-2, and abts-1 mutants were all resistant to the inhibitory effects of muscimol on egg-laying behaviour. Brackets indicate the percent inhibition by muscimol. For each genotype n=6 pools of five worms each.
Inhibition of egg laying by muscimol requires KCC-2 function in the HSNs (Tanis et al, 2009). We found that ABTS-1 function is also required in the HSNs for normal sensitivity to muscimol. The inhibitory effect of muscimol on egg laying was partially restored by re-expression of ABTS-1 in the HSNs of abts-1 mutants as compared with abts-1 mutants expressing a GFP control protein (Supplementary Figure S5). The fact that re-expression of ABTS-1 only partially rescued muscimol sensitivity may indicate that ABTS-1 function is required in an additional cell type or that our transgene drives expression of ABTS-1 in the HSNs at a sub-wild-type level. We also observed that re-expression of ABTS-1 in the HSNs of the abts-1; egl-47(dm) mutant rescued the egl-47(dm) egg-laying defect (Supplementary Figure S6), providing additional evidence for ABTS-1 function in the HSNs.
UNC-49 activation excites rather than inhibits BWMs in the absence of ABTS-1
While GABA is normally an inhibitory neurotransmitter, neurons that do not have a mechanism to extrude Cl− may have relatively high intracellular Cl− levels that lead to an excitatory effect of GABAA receptor activation (Kaneko et al, 2004). Such an effect is observed in C. elegans BWMs, which receive inputs from GABAergic motor neurons that act to inhibit and lengthen the BWMs during the animal's locomotion (McIntire et al, 1993b). While wild-type animals exposed to muscimol undergo simultaneous relaxation of all BWMs and thus an increase in total body length, kcc-2 mutants experience muscle contraction and shortening of total body length (Tanis et al, 2009). The standard electrophysiological method for assaying changes in Cl− reversal potential (Blaesse et al, 2009) appears not to work on C. elegans muscle cells due to the failure of the ionophore gramicidin to perforate the membranes of these cells (data not shown). Nevertheless, the behavioural response to muscimol strongly suggest that there is a depolarizing shift in the Cl− reversal potential in the muscles of kcc-2 mutants, resulting in an excitatory effect of UNC-49 activation.
We found that abts-1 mutants exposed to muscimol undergo a decrease in body length similar to that observed in kcc-2 mutants. Wild-type animals exposed to muscimol experienced a significant increase in body length from 1207±6 μm before exposure to 1277±9 μm after exposure (Figure 5A, B, and E). In contrast, the abts-1 mutant underwent a significant decrease in body length when exposed to muscimol from 1021±7 μm before exposure to 984±8 μm following exposure (Figure 5C–E). This shortening effect was comparable to that observed in kcc-2 mutants (Figure 5E). Both the lengthening observed in wild-type animals and the shortening observed in abts-1 and kcc-2 mutants were dependent on the UNC-49, as loss of UNC-49 function in any genetic background resulted in no change in body length following muscimol exposure (Figure 5E).
Figure 5.
ABTS-1 is required for the inhibition of muscle contraction by muscimol. (A, B) Images of representative wild-type animals in the absence (A) or presence (B) of 1 mM muscimol. A line was drawn down the centre of each worm image as indicated to measure body length. Average lengths±standard errors are shown for each condition. n⩾30 animals for each genotype. Scale bars=100 μm. (C, D) Images of representative abts-1 animals in the absence (C) or presence (D) of 1 mM muscimol. (E) Average body length before and after 2 h of exposure to 1 mM muscimol. Wild-type animals displayed significant increases in body length following muscimol exposure, while abts-1 and kcc-2 mutants displayed significant decreases. Strains with unc-49 mutations showed no changes in body length. For each genotype, body-length measurements were normalized to body length prior to muscimol exposure. n⩾30 animals for each genotype. Asterisks indicate significant changes with P<0.005. (F) Average body length of transgenic animals before and after 2 h of exposure to 1 mM muscimol. A muscle-specific promoter was used to transgenically express cDNAs encoding the control protein GFP or ABTS-1. Re-expression of ABTS-1 in the BWMs of abts-1 mutants caused a significant increase in body length following muscimol exposure, in contrast to the muscimol-induced shortening observed in abts-1 mutants expressing GFP in the BWMs. n⩾50 animals for each genotype. Asterisks are as in panel (E). (G) Model depicting that Cl−-extruding transporters establish a gradient of Cl− ions across the membrane such that the Cl− reversal potential is more negative than the membrane resting potential. Thus, UNC-49 activation leads to Cl− influx and hyperpolarization. (H) Model depicting that cells not expressing Cl−-extruding transporters maintain relatively high intracellular Cl− levels such that the Cl− reversal potential is more positive than the resting potential. Thus, UNC-49 activation leads to Cl− efflux and depolarization.
KCC-2 and UNC-49 both function in the BWMs to mediate the effect of muscimol on body length (Tanis et al, 2009), and we found that ABTS-1 also functions in the muscles to control this effect of muscimol. Wild-type and abts-1 mutant animals expressing the control protein GFP in their BWMs showed lengthening and shortening in response to muscimol, respectively, comparable to that observed in non-transgenic wild-type and abts-1 mutant animals (Figure 5F). Re-expression of ABTS-1 in the muscles of abts-1 mutants converted their muscimol response from shortening back to a lengthening similar to that seen in the wild-type controls (Figure 5F).
These results are consistent with a model in which ABTS-1 acts as a Cl− extruder in the BWMs, along with KCC-2, to help set the cellular Cl− gradient required for UNC-49-mediated inhibition (Figure 5G) and muscle lengthening. In this model, loss of ABTS-1 or KCC-2 function results in accumulation of intracellular Cl− and thus a depolarizing shift in the Cl− reversal potential to produce UNC-49-mediated excitation and muscle contraction (Figure 5H).
ABTS-1 and KCC-2 function redundantly in the BWMs to mediate endogenous inhibitory GABA signalling
Mutants that lack UNC-49 display sluggish and uncoordinated locomotion (McIntire et al, 1993a; Bamber et al, 1999). Despite the fact that abts-1 and kcc-2 mutants show excitatory rather than inhibitory responses to the GABAA agonist muscimol, these mutants still show well-coordinated locomotion (Supplementary Videos S1, S2, S3, and S4). One model suggested by these observations is that modest changes in the cellular Cl− gradient produced by loss of one transporter do reverse the flow of Cl− through the UNC-49 Cl− channel, but the low levels of UNC-49 activation caused by endogenous, synaptically released GABA still result in ‘shunting’ inhibition of the muscles to allow coordinated movement (see the Discussion for further elaboration of this model).
The model that ABTS-1 and KCC-2 act in parallel predicts that a mutant lacking both ABTS-1 and KCC-2 would show phenotypic defects more severe than the relatively mild defects observed in abts-1 or kcc-2 single mutants. We found that the abts-1; kcc-2 double mutant indeed has severe defects in muscle contractility and locomotion not observed in either single mutant. We measured the body length of animals not exposed to any drug and found that wild-type body length was 1212±6 μm, and the body lengths of abts-1 and kcc-2 mutants were modestly shorter at 1064±8 μm and 1145±6 μm respectively (Figure 6A, B, C, and E). The unc-49 mutant was also modestly shorter than wild-type animals (Figure 6E). The modest shortening in all these mutants was presumably due to increased BWM contraction. However, the abts-1; kcc-2 double mutant exhibited a much more severe body-length defect, as these animals were only 510±10 μm long (Figure 6D and E).
Figure 6.
ABTS-1 and KCC-2 function redundantly in the BWMs to mediate muscle inhibition and relaxation. (A–D) Images of representative wild-type and mutant animals. Genotypes for each worm are indicated. A line was drawn down the centre of each worm image as indicated to measure body length. Average lengths±standard errors are shown for each condition. Scale bars=100 μm. (E) Average body length of wild-type and mutant animals. Mutants defective in muscimol responsiveness were slightly shorter than wild-type animals. The abts-1; kcc-2 double mutant was dramatically shorter than wild-type animals. n⩾15 animals for each genotype. (F) Average number of body bends executed by wild-type and mutant animals in a 1-min period. Body bends were moderately reduced in abts-1 mutants, more severely reduced in unc-49 mutants, and almost completely absent in abts-1; kcc-2 double mutants. n⩾30 animals for each genotype (see also Supplementary Movies S1, S2, S3, S4, S5, S6, S7). (G) Average body length of muscle-rescued transgenic animals. A muscle-specific promoter was used to transgenically express cDNAs encoding the control protein GFP, ABTS-1, or KCC-2. Re-expression of ABTS-1 or KCC-2 in the muscles partially rescued the body-length defect of the abts-1; kcc-2 double mutant. n⩾30 animals for each genotype.
The abts-1; kcc-2 double mutant also exhibited severe locomotion defects. We counted the number of body bends executed over a 1-min period and found that wild-type animals and kcc-2 mutants performed a similar number of coordinated body bends, while abts-1 mutants performed a lower number (Figure 6F; Supplementary Videos S1, S3, and S4) The unc-49 mutant executed even fewer body bends (Figure 6F), and these body bends were not coordinated (Supplementary Video S2), presumably due to lack of normal muscle relaxation. The abts-1; kcc-2 double mutant executed almost no body bends and was essentially incapable of locomotion (Figure 6F; Supplementary Video S5).
Both the double-mutant body-length and locomotion defects described above presumably result from BWM hypercontraction, and we performed cell-specific rescue experiments to demonstrate that re-expression of either ABTS-1 or KCC-2 in the BWMs is sufficient to at least partially rescue each of these defects. Double-mutant animals carrying a control transgene driving GFP expression in the BWMs exhibited similar body lengths to the non-transgenic animals described above (compare Figure 6E and G) and were never capable of coordinated locomotion (Supplementary Video S6). However, abts-1; kcc-2 mutant animals carrying transgenes driving expression of either ABTS-1 or KCC-2 in the BWMs displayed significant rescue of the abts-1; kcc-2 body-length defect (Figure 6G), and often showed well-coordinated movement (Supplementary Video S7). These results are consistent with a model in which ABTS-1 and KCC-2 act in parallel in the BWMs to extrude Cl− and in which loss of both transporters results in muscle hypercontraction that produces dramatic defects in body length and in locomotion.
Mutations that eliminate GABAergic transmission reduce the severity of the abts-1; kcc-2 double-mutant phenotype, consistent with a model in which excitatory GABA signalling contributes to this phenotype. The body lengths of abts-1; unc-49; kcc-2 and abts-1; unc-25; kcc-2 triple mutants (unc-25 encodes the GABA biosynthetic enzyme glutamic acid decarboxylase) are slightly, but significantly, longer than abts-1; kcc-2 double mutants (Supplementary Figure S7). The fact that mutations in unc-49 and unc-25 only partially ameliorate the abts-1; kcc-2 phenotype suggests that chloride conductances in addition to those of UNC-49 are disrupted in abts-1; kcc-2 double mutants.
Transcriptional regulation of ABTS-1 and KCC-2 expression are coordinately controlled
In mammalian brain, expression of Kcc2 is transcriptionally upregulated during the late stages of neuronal development, and this is associated with a negative shift in the Cl− reversal potential that converts the effect of GABA from excitatory to inhibitory (Rivera et al, 1999; Ben-Ari, 2002). In C. elegans, KCC-2 expression is transcriptionally upregulated in the HSNs during the transition from larva to adult (Tanis et al, 2009), coinciding with the timing of HSN synapse formation (Adler et al, 2006; Ding et al, 2007).
We examined expression of GFP from an abts-1 transcriptional reporter transgene during development. We first examined the HSNs, as the development of these neurons is especially well characterized (Desai et al, 1988; Adler et al, 2006; Ding et al, 2007). The abts-1b promoter::gfp::abts-1 3′ UTR reporter transgene drives GFP expression in the HSNs of transgenic animals and is the only abts-1 isoform-reporter that does so (Figure 3). Thus, we measured GFP fluorescence levels in the HSNs of L3- and L4-stage larval and adult transgenic animals carrying the abts-1b reporter. GFP fluorescence was generally not seen in the HSNs of L3 animals (Figure 7A and I). GFP was reliably expressed at a low level in L4 transgenic animals (Figure 7B and I), and there was an additional 3.7-fold increase in GFP fluorescence in the HSNs of adult animals as compared with L4 animals (Figure 7C and I). Although the upregulation of GFP expression is seen with both abts-1 and kcc-2 reporter transgenes (Tanis et al, 2009), it is not a property of all transgenes that drive GFP expression in the HSN: an unc-86 promoter::gfp transgene drives strong GFP expression in the HSNs in L3, L4, and adult stages (Figure 7D, E, F, and J). Additionally, the upregulation of abts-1 appears to be associated with neuronal maturation, not the transition from larva to adult, as similar levels of GFP expression in the BDU neuron, which matures early in embryonic development (Walthall and Chalfie, 1988), were observed at the larval and adult time points tested (Figure 7K).
Figure 7.
Expression of abts-1 in the HSNs is upregulated during neuronal maturation and synapse formation. (A–C) Images of representative HSNs at three stages of development expressing GFP from the abts-1b promoter::gfp::abts-1 3′ UTR transgene. (D–F) Images of representative HSNs at three stages of development expressing GFP from the unc-86 promoter::gfp transgene. (G, H) Images of representative HSNs of wild-type and kcc-2 mutant animals (as indicated by white text) expressing GFP from the abts-1b promoter::gfp::abts-1 3′ UTR transgene. Scale bars in panels (A–H)=5 μm. (I) Mean GFP fluorescence of the HSN cell body in animals at three stages of development carrying the abts-1b promoter::gfp::abts-1 3′ UTR reporter transgene. GFP fluorescence increased from barely detectable to strong between the L3 and adult stages. For each stage n⩾30 cell bodies. (J) Mean GFP fluorescence of the HSN cell body in animals at three stages of development carrying the unc-86 promoter::gfp reporter transgene. GFP was highly expressed at all developmental stages analysed. For each stage n⩾30 cell bodies. (K) Mean GFP fluorescence of the BDU cell body in larval and adult animals carrying the abts-1b promoter::gfp::abts-1 3′ UTR reporter transgene. GFP was highly expressed at all developmental stages analysed. For each stage n⩾30 cell bodies. (L) Mean GFP fluorescence of the HSN cell body in wild-type and kcc-2 mutant animals carrying the abts-1b promoter::gfp::abts-1 3′ UTR reporter transgene. Expression of the reporter is increased in the HSNs of kcc-2 mutants as compared with wild-type animals. For each stage n⩾30 cell bodies. In panels (I–L) different exposure settings were used to capture images for analysis in each panel. Thus, arbitrary fluorescence units cannot be compared across experiments.
We observed that abts-1::gfp expression was significantly increased in the HSNs of kcc-2 mutants (Figure 7G, H, and L). There was a 2.3-fold increase in GFP fluorescence in the HSNs of kcc-2 mutants as compared with wild-type animals. This result suggests that abts-1 expression may be upregulated to compensate for loss of KCC-2 function. In contrast, kcc-2 promoter::gfp expression in the HSNs of animals was not significantly altered in abts-1 mutant as compared with wild-type animals (data not shown), so such compensation was not seen in reverse.
Discussion
ABTS-1 and KCC-2 function partially redundantly to mediate inhibitory GABA signalling
Our results suggest that ABTS-1 and KCC-2 function partially redundantly. Each transporter is required in the HSNs for the inhibition of egg laying by EGL-47(dm), a putative transmembrane receptor whose ligand and precise mechanism of inhibiting neural activity remain unidentified. ABTS-1 and KCC-2 are also required in the HSN neurons for inhibition of egg-laying behaviour by the GABA receptor agonist muscimol. In addition to the HSNs, ABTS-1 and KCC-2 also function in the BWMs to mediate the inhibitory effects of muscimol on muscle contraction. Muscimol activates the GABAA receptor UNC-49 in wild-type animals to produce muscle lengthening and increased body length. However, the abts-1 and kcc-2 mutations reverse the effects of muscimol so that body shortening is observed. Analogously, abts-1 and kcc-2 mutations reverse the effect of the egl-47(dm) mutation so that it strongly stimulates rather than inhibits egg laying, strongly suggesting that the unknown mechanism of neural inhibition by EGL-47 involves the activation of a chloride conductance. While the abts-1 and kcc-2 single mutants display remarkably similar effects when challenged by the egl-47(dm) mutation or muscimol, neither transporter mutant shows strong behavioural defects in the absence of these challenges. However, we found that the abts-1; kcc-2 double mutant displays severe defects in body length and locomotion attributable at least in part to lack of inhibitory GABA signalling onto BWMs. Consistent with our hypothesis that ABTS-1 is required for proper inhibition of BWMs, Liao et al (2010) recently observed that abts-1 mutants are hypersensitive to an acetylcholine agonist that stimulates BWM contraction. Taken together, these observations strongly suggest that ABTS-1 and KCC-2 function partially redundantly to mediate inhibition of neurons and muscles.
Our work suggests that the redundant function shared by KCC-2 and ABTS-1 is Cl− extrusion, and that ABTS-1 is as important as KCC-2 in controlling the cellular Cl−gradient. We have characterized the transport activities of KCC-2 (Tanis et al, 2009) and ABTS-1, and their only shared ion transport activity is the ability to extrude intracellular Cl−. Past studies of neuronal Cl− extrusion have focused on the KCCs (Blaesse et al, 2009). NDAEs such as ABTS-1, which use Na+ influx to drive Cl−–HCO3− exchange resulting in Cl− extrusion and increased intracellular pH, have been previously cloned and characterized in invertebrate and mammalian systems (Romero et al, 2000; Grichtchenko et al, 2001; Virkki et al, 2003). Despite the Cl−-extrusion of these transporters, they have been studied and discussed mainly in the context of cellular pH regulation (Schwiening and Boron, 1994; Baxter and Church, 1996). ABTS-1 presumably regulates BWM pH in vivo by coupling Cl− extrusion to HCO3− import. The HCO3− content of the extracellular space surrounding the BWMs is unknown, but the BWMs do express the carbonic anhydrase, CAH-4, which may act a source of HCO3− for ABTS-1 transport (Hall et al, 2008). Thus, it is possible that ABTS-1 controls muscle contractility through its pH regulatory function. However, intracellular acidification, the expected result of loss of ABTS-1 function, is associated with loss of muscle contractility (Westerblad et al, 1998), which is the opposite of the abts-1 mutant phenotype. Our results suggest that mutants for KCC-2, the principal KCC of C. elegans neurons and muscles (Tanis et al, 2009), are only partially defective for cellular Cl− extrusion, that mutants for ABTS-1 are about as defective for this function as are mutants for KCC-2, and that profound defects in Cl− extrusion are only seen in double mutants lacking both transporters. These results imply that a principle physiological function of NDAEs is to control the cellular Cl− gradient and that further analysis of NDAEs will provide a more complete understanding of Cl− extrusion from excitable cells.
The relationship between KCC-2 and ABTS-1 function is revealed by single and double mutants for these transporters. High levels of Cl− extrusion in the wild type produce a steep cellular Cl− gradient such that opening of GABAA channels results in Cl− influx and hyperpolarization. Partial loss of Cl− extrusion, such as may occur in kcc-2 or abts-1 single mutants, could reduce the Cl− gradient such that the Cl− reversal potential is more positive than the cell's resting potential and opening of Cl− channels results instead in Cl− efflux. Given these results, it might appear puzzling that although the normal inhibitory signalling through UNC-49 is required for coordinated locomotion in C. elegans (McIntire et al, 1993a), kcc-2 and abts-1 single mutants still show coordinated locomotion. We propose that in these mutants, the relatively weak GABAA activation produced by endogenous synaptic GABA release results in shunting inhibition, a phenomenon in which the GABAA-mediated current effectively short-circuits excitation by tending to prevent depolarization beyond the Cl− reversal potential to the more positive levels needed to excite the cell (Farrant and Kaila, 2007). Consistent with this idea, previous work has shown that, at certain Cl− reversal potentials, weak GABAA activation can be inhibitory while massive and prolonged GABAA activation can become excitatory by setting the resting potential to a more positive level (Kaila, 1994). Finally, our model proposes that in absts-1; kcc-2 double mutants, lack of any Cl− extrusion function and the likely presence of an NKCC (Figure 8) could result in intracellular Cl− accumulation, and the reversed Cl− gradient produced would cause even relatively weak synaptic GABA release to result in strong depolarization and excitation. This would explain the hypercontracted muscles and uncoordinated locomotion observed in the double transporter mutants.
Figure 8.
Schematic of ion fluxes mediated by NKCCs, KCCs, NDAEs, and GABAA receptors. NKCCs mediate Cl− influx driven by the cellular Na+ gradient, while KCCs mediate Cl− efflux driven by the K+ gradient. NDAEs mediate Cl− efflux and acid extrusion (HCO3− influx driven by the cellular Na+ gradient). GABAA receptors are anion channels that allow Cl− and HCO3− ions across the membrane, with the direction of flow dictated principally by the ion concentration gradients produced by the three types of transporters diagrammed. Designations for both mammalian and C. elegans homologues of each protein are indicated.
Implications for understanding control of neural excitability in the mammalian brain
In the mammalian brain, NDAEs are likely to provide a Cl− extrusion function analogous to that of ABTS-1. Gulacsi et al (2003) observed that dopaminergic neurons in the rat substantia nigra do not express KCC2, but still exhibit inhibitory responses to GABA that are dependent upon the presence of extracellular HCO3−. More recently, glycinergic neurons of the brainstem have been shown to exhibit activity-dependent negative shifts in Cl− reversal potential that are dependent upon extracellular HCO3− and are blocked by inhibitors of SLC4 transporters (Kim and Trussell, 2009). A candidate for the transporter mediating these effects is the Na+-driven Cl−–HCO3− exchanger (NDCBE) encoded by slc4a8 (Kim and Trussell, 2009), which has well-characterized electroneutral Na+-driven Cl−–HCO3− exchange activity (likely Na+:2HCO3− exchange for Cl−) and is expressed in mammalian neurons (Grichtchenko et al, 2001; Chen et al, 2008a, 2008b). However, a role for NDCBE in controlling inhibitory neurotransmission has not been unambiguously demonstrated, as previous studies (Gulacsi et al, 2003; Kim and Trussell, 2009) have inhibited Na+-driven Cl−–HCO3− exchange with manipulations that would be expected to affect all brain-expressed SLC4 family members, only some of which are NDAEs. Thus, our study is the first to specifically inactivate an NDAE and demonstrate its role in controlling inhibitory GABA signalling.
A role for NDAEs in regulating GABA signalling may explain the relationship between pH and neural excitability in the mammalian brain. KCCs function primarily as Cl− extruders, whereas NDAEs both extrude Cl− and have an additional role, via HCO3− influx, in intracellular alkalinization (Figure 8). NDAEs are activated by intracellular acidification (Schwiening and Boron, 1994), suggesting that regulation of the Cl− gradient by NDAEs may be closely tied to regulation of cellular pH. Neuronal acidification may be induced by prolonged firing (reviewed by Chesler, 2003), by efflux of HCO3− through GABAA receptors (Kaila and Voipio, 1987; Pasternack et al, 1993), or during pathological states such as epileptic seizures (Somjen, 1984). In each case, activation of NDAEs by acidification may help to maintain a hyperpolarized Cl− reversal potential and thus promote the inhibitory action of GABA. Acidification of the brain is known to be associated with termination of seizure activity (Xiong et al, 2000). We propose that activation of NDAEs by acidosis may also contribute to seizure termination by promoting a more negative Cl− reversal potential and thus promoting the inhibitory effects of GABA. Consistent with the hypothesis that NDAEs use activity-dependent acidosis to control the Cl− reversal potential, Kim and Trussell (2009) have shown that prolonged spiking drives intracellular acidification that, in turn, leads to negative shifts in the Cl− reversal potential that require SLC4 transport activity. Additionally, mutations that disrupt the putative human NDAE gene slc4a10 are associated with epilepsy (Gurnett et al, 2008), a finding that is consistent with an antileptic activity of NDAEs.
Transcriptional regulation of Cl−-extruding transporter expression regulates inhibitory neurotransmission
Our demonstration that multiple Cl−-extruding transporters generate the cellular Cl− gradient suggests that a cell's response to inhibitory neurotransmitters is shaped by the combination of Cl− extruders that it expresses. Both ABTS-1 and KCC-2 (Tanis et al, 2009) are expressed in the HSNs and BWMs and both contribute to inhibitory neurotransmission in these cells. Some cells however express only ABTS-1 or KCC-2, such as the ventral cord motor neurons, which express only KCC-2. This observation may explain the fact that kcc-2 mutants are resistant to paralysis by exogenous serotonin (Tanis et al, 2009), while abts-1 mutants are not (data not shown), as the paralytic effects of serotonin are mediated in part by the serotonin-gated Cl− channel MOD-1, which is expressed in ventral cord motor neurons (Ranganathan et al, 2000).
The demonstration that expression of abts-1 is upregulated during the maturation of the HSNs coincident with upregulation of kcc-2 expression and synapse formation suggests that maturation of the cellular Cl− gradient and responses to inhibitory neurotransmitter involves the coordinated upregulation of these two transporters. Studies in mammals have focused on the upregulation of KCC2 expression and also the coincident downregulation of NKCC1 expression as the driving forces behind the maturation of the cellular Cl− gradient and inhibitory GABA signalling (Rivera et al, 1999; Yamada et al, 2004; Romero et al, 2009). No comprehensive analysis of SLC4 transporter expression in the nervous system across development has been completed, although NDCBE is detected in the choroid plexus of embryonic rats (Chen et al, 2008a) and throughout the brain of early postnatal mice (Chen et al, 2008b). Our work suggests that developmental regulation of these transporters may participate in fine-tuning the cellular Cl− gradient during development to produce mature neurons that exhibit proper inhibitory neurotransmission and electrical excitability.
We found that transcription from the abts-1 promoter is upregulated in the HSNs of adult kcc-2 mutants. This suggests that transcription of these transporters is regulated dynamically in the adult nervous system, potentially in response to changes in either the intracellular [Cl−] or electrical excitability. This extends the concept of ionic plasticity (Rivera et al, 2005) to one in which excitable cells have the ability to monitor and adjust levels of inhibitory neurotransmission through continuous adjustment Cl− transporter expression to alter of intracellular Cl− levels.
Materials and methods
C. elegans strains
All strains used in this work are detailed in the Supplementary data.
Transgenes
Transgenic strains were constructed by injection of plasmid DNA or PCR products into the germline (Mello et al, 1991). We constructed abts-1 promoter::gfp::abts-1 3′ UTR plasmids for the abts-1a (pAB9A) and abts-1b (pAB36A) promoters. PCR-amplified promoter::gfp::3′ UTR cassettes from these plasmids were injected (Etchberger and Hobert, 2008). For analysis of developmental changes in abts-1b expression (Figure 7), the extrachromosomal array containing abts-1b promoter::gfp::3′ UTR reporter transgene was chromosomally integrated using psoralen/UV mutagenesis. The resulting integrated strains were outcrossed at least four times. The unc-86 promoter::gfp transgene was as described previously (Adler et al, 2006).
The HSN cell-specific rescue plasmid (pAB11A) was obtained by inserting the abts-1a cDNA into an HSN expression vector (Tanis et al, 2008). A construct used to express GFP in the HSNs (pJM60A) and an empty HSN vector (pJM66A) were used as controls. The HSN cell-specific rescue construct was injected with pJM60A and the coinjection marker myo-2::gfp (pJKL449.1) (Huang and Stern, 2004) into LX1320 abts-1(ok1566). Wild-type animals and LX1320 were injected with pJM60A, pJM66A, and pJKL449.1 to generate transgenic control strains. The muscle cell-specific rescue plasmid (pAB23A) was generated by inserting the abts-1a cDNA into the pPD96.52 vector that drives muscle expression using the myo-3 promoter (Reynolds et al, 2005). A myo-3::gfp construct (pAB25A) was used as a control and a construct expressing nuclear-localized GFP in the muscles (pSAK2) was used as a coinjection marker (Fire et al, 1998). Wild-type and LX1320 animals were injected with pAB25A and pSAK2 to generate control strains. LX1320 animals were injected with pAB23A and pSAK2 to generate muscle-rescued strains.
Details of all transgenes and plasmids used in this work are described in the Supplementary data.
Behavioural assays
Animals for behavioural assays were picked at the late-fourth larval (L4) stage, a time point about 6 h prior to adulthood. Animals were assayed as adults after 24 h (Figures 4, 5, 6) or 30 h (Figure 1A, B, C, and E) of incubation at 20°C. Suppression of egl-47(dm) (Figure 1D) was assayed by measuring the number of eggs laid by pools of five worms over 18 h after staging as late-L4s. Unlaid eggs and early-stage eggs were measured as described by Chase and Koelle (2004). Muscimol inhibition of egg laying was assayed as previously described (Tanis et al, 2009). At least six plates carrying five worms each were assayed per genotype (Figure 4A). For cell-specific rescue experiments, at least two plates carrying five worms each from each of five transgenic lines were assayed (Supplementary Figure S5).
Muscimol-induced changes in body length were measured as described by Tanis et al (2009). For non-transgenic wild-type and mutant animals, at least 30 animals were analysed per genotype (Figure 5E). For experiments testing cell-specific rescue of the abts-1 muscimol sensitivity defect, at least 10 animals for each of five transgenic lines were analysed (Figure 5F). Body lengths of non-drug-exposed animals were also measured using this method. At least 15 animals were analysed per genotype (Figure 6E). In experiments testing cell-specific rescue of the abts-1; kcc-2 mutant phenotype, at least 30 animals from one transgenic line was analysed for each strain (Figure 6G).
Body bends (Figure 6F) were measured as described by Chase and Koelle (2004). At least 30 animals were assayed per genotype, for 1 min each.
Microscopy
Animals were immobilized for microscopy on agar pads with 10 mM levamisole. Transgenic animals containing abts-1 reporter transgenes imaged with a Zeiss LSM 510 confocal microscope and reconstructed using Volocity software (Improvision). For analysis of changes in abts-1 and unc-86 expression during development and in the kcc-2 mutant background, images were obtained using a Carl Zeiss Axioskop. Single images through a central focal plane of the left HSN cell body were acquired for each animal, and mean GFP intensity for the area of the entire cell body was calculated for each HSN using Openlab software (Improvision). Developmental age was scored using distal tip cell migration for L3 larvae and vulval morphology for L4 larvae. Adult animals were staged 12 h after the late-L4 stage. At least 30 animals were analysed per developmental stage and genotype.
Xenopus oocyte expression and electrophysiology
The coding sequence for ABTS-1a was subcloned into the Xenopus oocyte expression vector pGEMHE. ABTS-1 and NDAE1 cRNA was synthesized in vitro using the T7 mMessage mMachine (Ambion, Austin, TX). Stage V/VI oocytes were isolated by limited collagenase digestion (Romero et al, 1998). Oocytes were injected with 25 ng cRNA in 50 nl (0.5 μg/μl) or 50 nl RNAse-free water. Oocytes were studied 3–7 days after injection in a perfusion chamber and monitoring pHi, [Cl−]i, and Vm as previously (Romero et al, 2000; Kato et al, 2009). Oocytes were perfused with ND96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2 and 5 mM HEPES, pH 7.5, 195±5 mOsm). The 1.5% CO2/10 mM HCO3− solution replaced 10 mM NaCl with 10 mM NaHCO3 but maintained pH 7.5 (Romero et al, 2000). Recordings shown in Figure 2 are each representative of results from 4 to 6 oocytes.
Since changes in intracellular [Cl−] were not obvious, we monitored [I−] using a halide-sensitive microelectrode. This approach greatly increases the signal-to-noise ratio as free [I−] is typically <1 μM in most vertebrate cells. Iodide calibration was performed for each electrode by varying [I−] between 100 μM and 10 mM (NaI) in a background of 40 mM NaCl (resting [Cl−] in these ABTS-1 oocytes). This approach documented the offset for 100 μM I− in a constant [Cl−] background. The I−-specific electrode slope was then determined between 100 μM and 10 mM NaI.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center for strains, Keith Nehrke for the abts-1 cDNA, and Bob Horvitz for sharing unpublished information about abts-1. This work was supported by NIH grants NS36918 (to MRK), DK056218 and EY017732 (to MFR), DK83007Z-02S2 (to TH), and NS060432 (to AB).
Footnotes
The authors declare that they have no conflict of interest.
References
- Adler CE, Fetter RD, Bargmann CI (2006) UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci 9: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT (2006) Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci 26: 5117–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber BA, Beg AA, Twyman RE, Jorgensen EM (1999) The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci 19: 5348–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter KA, Church J (1996) Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. J Physiol 493: 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y (2002) Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3: 728–739 [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K (2009) Cation-chloride cotransporters and neuronal function. Neuron 61: 820–838 [DOI] [PubMed] [Google Scholar]
- Chang MH, Plata C, Zandi-Nejad K, Sindic A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF (2009) Slc26A9 - anion exchanger, channel and Na+ transporter. J Membr Biol 128: 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Koelle MR (2004) Genetic analysis of RGS protein function in Caenorhabditis elegans. Methods Enzymol 389: 305–320 [DOI] [PubMed] [Google Scholar]
- Chen LM, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA, Boron WF (2008a) Expression and localization of Na-driven Cl-HCO(3)(-) exchanger (SLC4A8) in rodent CNS. Neuroscience 153: 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Haddad GG, Boron WF (2008b) Effects of chronic continuous hypoxia on the expression of SLC4A8 (NDCBE) in neonatal versus adult mouse brain. Brain Res 1238: 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M (2003) Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221 [DOI] [PubMed] [Google Scholar]
- Desai C, Garriga G, McIntire SL, Horvitz HR (1988) A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 336: 638–646 [DOI] [PubMed] [Google Scholar]
- Ding M, Chao D, Wang G, Shen K (2007) Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science 317: 947–951 [DOI] [PubMed] [Google Scholar]
- Etchberger JF, Hobert O (2008) Vector-free DNA constructs improve transgene expression in C. elegans. Nat Methods 5: 3. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K (2007) The cellular, molecular, and ionic basis of GABAA receptor signaling. Prog Brain Res 160: 59–87 [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811 [DOI] [PubMed] [Google Scholar]
- Fiumelli H, Cancedda L, Poo MM (2005) Modulation of GABAergic transmission by activity via postsynaptic Ca2+-dependent regulation of KCC2 function. Neuron 48: 773–786 [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF (2001) Cloning, characterization, and chromosol mapping of a human electroneutral Na+-driven Cl-HCO3− exchanger. J Biol Chem 276: 8358–8363 [DOI] [PubMed] [Google Scholar]
- Gulacsi A, Lee CR, Sik A, Viitanen T, Kaila K, Tepper JM, Freund TF (2003) Cell type-specific differences in chloride-regulatory mechanisms and GABAA receptor-mediated inhibition in rat substantia nigra. J Neurosci 23: 8237–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, Bowcock A (2008) Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Arch Neurol 65: 550–553 [DOI] [PubMed] [Google Scholar]
- Hall RA, Vullo D, Innocenti A, Scozzafava A, Supuran CT, Klappa P, Mühlschlegel FA (2008) External pH influences the transcriptional profile of the carbonic anhydrase, CAH-4b in Caenorhabditis elegans. Mol Biochem Parasitol 161: 140–149 [DOI] [PubMed] [Google Scholar]
- Huang P, Stern MJ (2004) FGF signaling functions in the hypodermis to regulate fluid balance in C. elegans. Development 131: 2595–2604 [DOI] [PubMed] [Google Scholar]
- Jorgensen EM (2005) GABA. In WormBook, The C. elegans Research Community (ed) WormBook, doi/10.1895/wormbook.1.14.1, http://www.wormbook.org [Google Scholar]
- Kaila K, Voipio J (1987) Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature 330: 163–165 [DOI] [PubMed] [Google Scholar]
- Kaila K (1994) Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol 42: 489–537 [DOI] [PubMed] [Google Scholar]
- Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T (2004) Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci 24: 7931–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Kurita Y, Nakada T, Ogoshi M, Nakazato T, Doi H, Chang M-H, Hirose S, Romero MF (2009) Identification of renal transporters involved in sulfate excretion in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 297: R1647–R1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Trussell LO (2009) Negative shift in the glycine reversal potential mediated by a Ca2+- and pH-dependent mechanism in interneurons. J Neurosci 29: 11495–11510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao VH, Liu JT, Li WT, Yu CW, Hsieh YC (2010) Caenorhabditis elegans bicarbonate transporter ABTS-1 is involved in arsenite toxicity and cholinergic signaling. Chem Res Toxicol 23: 926–932 [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Horvitz HR (1993a) Genes required for GABA function in Caenorhabditis elegans. Nature 364: 334–337 [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Kaplan J, Horvitz HR (1993b) The GABAergic nervous system of Caenorhabditis elegans. Nature 364: 337–341 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in Celegans: extrachromosomol maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I (1994) Bridgeing the cleft at GABA synapses in the brain. Trends Neurosci 17: 517–525 [DOI] [PubMed] [Google Scholar]
- Moresco JJ, Koelle MR (2004) Activation of EGL-47, a Gαo-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J Neurosci 24: 8522–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack M, Voipio J, Kaila K (1993) Intracellular carbonic anhydrase activity and its role in GABA-induced acidosis in isolated rat hippocampal pyramidal neurones. Acta Physiol Scand 148: 229–231 [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Cannon SC, Horvitz HR (2000) MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408: 470–475 [DOI] [PubMed] [Google Scholar]
- Reynolds NK, Schade MA, Miller KG (2005) Convergent, RIC-8-dependent Gα signaling pathways in the Caenorhabditis elegans synaptic signaling network. Genetics 169: 651–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM (1999) One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2: 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K (1999) The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397: 251–255 [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K (2005) Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol 562: 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MF, Kanai Y, Gunshin H, Hediger MA (1998) Expression cloning using Xenopus laevis oocytes. Methods Enzymol 296: 17–52 [DOI] [PubMed] [Google Scholar]
- Romero MF, Boron WF (1998) Identification and expression of an electroneutral Na/HCO3 cotransporter from Caenorhabditis elegans (ceNBC). J Am Soc Nephrol 9: 11A [Google Scholar]
- Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM (2000) Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. J Biol Chem 275: 24552–24559 [DOI] [PubMed] [Google Scholar]
- Romero MF, Fulton CM, Boron WF (2004) The SLC4 family of HCO3− transporters. Pflugers Arch 447: 495–509 [DOI] [PubMed] [Google Scholar]
- Romero MF, Chang MH, Mount DB (2009) From cloning to structure, function, and regulation of chloride transporters. In Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases, Alvarez-Leefmans FJ, Delpire E (eds) pp 43–80. Salt Lake City, UT: Elsevier Academic Press [Google Scholar]
- Schwiening CJ, Boron WF (1994) Regulation of intracellular pH in pyramidal neurons from the rat hippocampus by Na+-dependent Cl—HCO3− exchange. J Physiol 475: 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman T, Chernova MV, Clark JS, Jiang L, Alper SL, Nehrke K (2005) The abts and sulp families of anion transporters from Caenorhabditis elegans. J Physiol Cell Physiol 289: 341–351 [DOI] [PubMed] [Google Scholar]
- Somjen GG (1984) Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res 331: 186–188 [DOI] [PubMed] [Google Scholar]
- Tanis JE, Moresco JJ, Lindquist RA, Koelle MR (2008) Regulation of serotonin biosynthesis by the G proteins Gαo and Gαq controls serotonin signaling in Caenorhabditis elegans. Genetics 178: 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis JE, Bellemer A, Moresco JJ, Forbush B, Koelle MR (2009) The potassium chloride cotransporter KCC-2 coordinates development of inhibitory neurotransmission and synapse structure in Caenorhabditis elegans. J Neurosci 29: 9943–9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkki LV, Choi I, Davis BA, Boron WF (2003) Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol 285: C771–C780 [DOI] [PubMed] [Google Scholar]
- Walthall WW, Chalfie M (1988) Cell-cell interactions in the guidance of late-developing neurons in Caenorhabditis elegans. Science 239: 643–645 [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Bruton JG, Allen DG, Andrade FH, Lännergren J (1998) Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue. Acta Physiol Scand 162: 253–260 [DOI] [PubMed] [Google Scholar]
- Wright EM, Diamond JM (1977) Anion selectivity in biological systems. Physiol Rev 57: 109–156 [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Saggau P, Stringer JL (2000) Activity-dependent intracellular acidification correlates with the duration of seizure activity. J Neurosci 20: 1290–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A (2004) Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurons is mediated by NKCC1. J Physiol 557: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.