Abstract
Up-regulation of P2X4 receptors in spinal cord microglia is crucial for tactile allodynia, an untreatable pathological pain reaction occurring after peripheral nerve injury. How nerve injury in the periphery leads to this microglia reaction in the dorsal horn of the spinal cord is not yet understood. It is shown here that CCL21 was rapidly expressed in injured small-sized primary sensory neurons and transported to their central terminals in the dorsal horn. Intrathecal administration of a CCL21-blocking antibody diminished tactile allodynia development in wild-type animals. Mice deficient for CCL21 did not develop any signs of tactile allodynia and failed to up-regulate microglial P2X4 receptor expression. Microglia P2X4 expression was enhanced by CCL21 application in vitro and in vivo. A single intrathecal injection of CCL21 to nerve-injured CCL21-deficient mice induced long-lasting allodynia that was undistinguishable from the wild-type response. This effect of CCL21 injection was strictly dependent on P2X4 receptor function. Since neuronal CCL21 is the earliest yet identified factor in the cascade leading to tactile allodynia, these findings may lead to a preventive therapy in neuropathic pain.
Keywords: chemokines, neuron-microglia communication, peripheral nerve lesion, tactile allodynia
Introduction
Neuropathic pain is a pathological pain condition that often develops in response to peripheral nerve injury. Diabetes, cancer, infections with herpes zoster, nerve compression or trauma as well as autoimmune diseases are pathological conditions that may lead to the development of neuropathic pain (Campbell and Meyer, 2006). Tactile allodynia is the most distinctive symptom of neuropathic pain, whereby non-painful innoxiously stimuli, like light touch, become painful. Since neuropathic pain is an extremely intractable problem from the therapeutic point of view, new insights into the development of neuropathic pain may open new therapeutic windows.
Neuropathic pain is a result of abnormal neuronal activity, occurring throughout the neuraxis (Campbell and Meyer, 2006; White et al, 2007) and is underlined by an increased neuronal expression and/or activation of various ion channels and receptors that are responsible for the abnormal generation of action potentials and synaptic transmission in the pain pathways (White et al, 2007). Although originally thought to be a disease solely related to neurons, it is now increasingly evident that glial cells, in particular microglia, are actively involved in the development of neuropathic pain (Abbadie, 2005; Tsuda et al, 2005; Hains and Waxman, 2006; Scholz and Woolf, 2007; Saab and Haines, 2009). Rapidly after the first description that dorsal horn microglia activation correlates to the onset of tactile allodynia (Coyle, 1998) it was recognized that these cells are of crucial importance to the development of the disease (Watkins et al, 2001). An inhibition of microglia activity (i.e. with the antibiotic minocycline) attenuated predominately the development of tactile allodynia in animals (Raghavendra et al, 2003). More specifically, it is known now that up-regulation of ionotropic purinoceptors P2X4 in spinal cord microglia and subsequent release of brain-derived neurotrophic factor from these cells are crucial for the initiation and maintenance of tactile allodynia (Tsuda et al, 2003, 2005; Coull et al, 2005; Ulmann et al, 2008). However, it is not yet clear how nerve injury in the periphery leads to microglia activation in the dorsal horn of the spinal cord (Abbadie, 2005; Tsuda et al, 2005; Hains and Waxman, 2006; Scholz and Woolf, 2007; Saab and Haines, 2009). Since in peripheral nerve lesion, the activation of dorsal horn microglia depends on an intact dorsal root (Colburn et al, 1999), it was suggested that dorsal horn microglia activation is initiated by a factor derived from injured peripheral nerves (Tsuda et al, 2005). This factor, however, remained to be identified.
Neurons are important elements in the control of microglia activity under physiological circumstances and pathological conditions (Biber et al, 2007). Accordingly, microglia express numerous receptors for the various families of neuronal signalling molecules and respond to these stimuli with great specificity (Biber et al, 2007; Hanisch and Kettenmann, 2007; Ransohoff and Perry, 2009). Chemokines recently gained much attention in the field of neuron-microglia communication (Biber et al, 2008; Miller et al, 2008). The chemokine CCL21 functions in the CNS as microglia-activating factor that is exclusively expressed in endangered or mechanically injured neurons (Biber et al, 2001; de Jong et al, 2005). Since neuronal CCL21 is sorted into large-dense core vesicles and specifically transported into the axons of endangered neurons (de Jong et al, 2008), we here investigated the question whether CCL21 is involved in neuron-microglia signalling and tactile allodynia in response to peripheral nerve injury.
Results
Peripheral nerve injury induced CCL21 expression in DRG neurons and in the dorsal horn
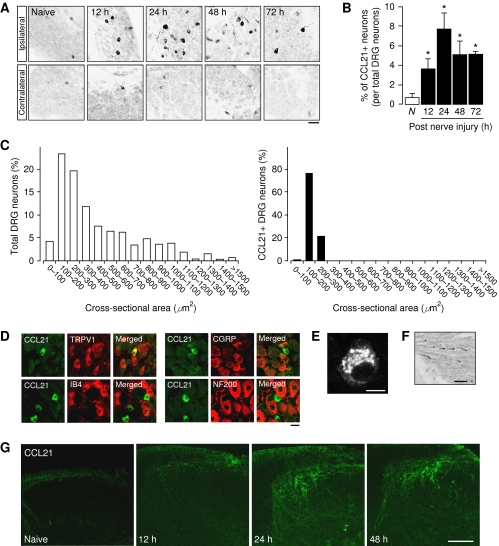
Spinal nerve injury induced by the transection of the L5 spinal nerve (Chung model) (Kim and Chung, 1992) induced rapid expression of CCL21 in ipsilateral L5 DRG cells (Figure 1A and B). No or very little CCL21 expression was found in contralateral L5 DRG neurons (Figure 1A) and L4 DRG neurons. Induction of CCL21 was prominent after 12 h, peaked at 24 h where almost 10% of the DRG neurons displayed CCL21 expression and slightly declined thereafter (Figure 1B). CCL21 was specifically found in small-sized C-fibres (determined by the cell size-frequency distribution of DRG neurons with CCL21 expression and double staining with TRPV1, IB4, CGRP or NF200) (Guseva and Chelyshev, 2006) (Figure 1C and D). Higher magnification revealed that neuronal CCL21 expression was found in vesicles located in the axonal hillock of C-fibres (Figure 1E) and within axons of the dorsal root (Figure 1F). The primary afferents of DRG neurons in the dorsal horn of the spinal cord showed no (naive) or little CCL21 (12 h after injury) expression, whereas 1 and 2 days after nerve injury expression of CCL21 was observed (Figure 1G). The staining for CCL21 in the dorsal horn of the spinal cord was only found in nerve terminals of the primary afferents and not in microglia (please refer to Supplementary Figure S1 for higher magnification) or in astrocytes.
Figure 1.
Expression of CCL21 in DRG neurons and spinal cord. (A) Rapid induction of CCL21 expression after spinal nerve injury in ipsilateral DRG. (B) Expression of CCL21 peaked at 24 h after spinal nerve injury with ∼8% positive neurons in the DRG. (C) Cell size-frequency histogram illustrating the distribution of the cross-sectional areas of total (left panel) and CCL21-positive (right panel) DRG neurons 24 h after spinal nerve injury in wild-type mice. (D) Double-staining experiments at 24 h after spinal nerve injury showed the exclusive expression of CCL21 (green fluorescence) in C-fibres positive to TRPV1 (red fluorescence) and negative to IB4, CGRP and NF200 (red fluorescence) (12). (E, F) CCL21 expression in vesicles at the axonal hillock (E) and in axons between the DRG and the spinal cord (F). (G) The primary afferents in the dorsal horn of the spinal cord showed CCL21 expression at 1 and 2 days after spinal nerve injury. The immunohistochemical staining showed similar results in at least four independent experiments. Data shown in (B) are derived from three animals. *Significantly different from control animals, P<0.05, Scale bars: (A) 50 μm; (D, F) 20 μm; (E) 10 μm; (G) 100 μm.
No development of tactile allodynia in plt animals after spinal nerve injury
To determine the functional relevance of CCL21, we compared wild-type animals with mice carrying the paucity of lymphoid T cells (plt) mutation, which lack the genes for CCL19 and CCL21-Ser (Nakano and Gunn, 2001), the CCL21 isoform that is expressed in lymphoid organs (Mori et al, 2001) and in neurons (Rappert et al, 2004). Immunohistochemistry confirmed the absence of CCL21 in lymph nodes and DRG neurons in plt animals (Supplementary Figure S2) and verified the specificity of the used antibody for CCL21. Since plt animals also lack the gene for CCL19, we investigated whether CCL19 is expressed in nervous structures. Neither RT–PCR experiments (unpublished data) nor immunohistochemical staining revealed expression of CCL19 in DRG neurons or spinal cord tissue (Supplementary Figure S2), confirming earlier findings that CCL19 is not expressed in neuronal tissue (Biber et al, 2001; Rappert et al, 2002).
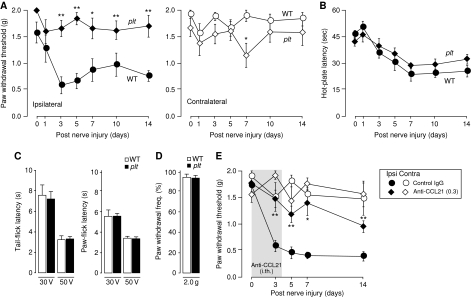
Spinal nerve injury induced a significant drop of the paw withdrawal threshold (PWT) in wild-type animals from 1.4±0.3 g (n=6 animals) before the injury to 0.4±0.09 g (n=6 animals) at day 3 (P=0.001) with no significant recovery of the PWT within 14 days after nerve injury (Figure 2A). In contrast, plt mice did not develop any sign of tactile allodynia in response to spinal nerve injury, PWT stayed at control levels 1.42±0.1 g throughout the experiment (Figure 2A). On the other hand, spinal nerve injury also enhanced the sensitivity to a heat stimulus as measured by the hot-plate test, but the thermal hypersensitivity of the operated hindpaw did not differ between wild-type and plt animals (Figure 2B). Moreover, acute pain responses were normal in plt mice as measured by tail-flick and paw-flick tests (Figure 2C), neither was the paw withdrawal frequency after mechanical stimulation (2.0 g) different between plt and wild-type animals (Figure 2D), indicating that plt animals had no general pain detection deficit. Intrathecally administration of 0.3 μg CCL21 neutralizing antibody to wild-type mice before and for the first 3 days after spinal nerve injury significantly attenuated tactile allodynia throughout the 14-day experiment (Figure 2E).
Figure 2.
Spinal nerve injury does not lead to tactile allodynia in plt mice. (A) The withdrawal threshold to tactile was examined at the ipsilateral (left panel, black symbols) and contralateral hindpaw (right panel, open symbols). Whereas spinal nerve injury induced within 2 days a significant drop in the paw withdrawal threshold (PWT) of the ipsilateral hindpaw in wild-type animals (circles), no such response was observed in plt animals (diamonds). No significant change in PWT after spinal nerve injury was observed at the contralateral hindpaw in both mouse strains. (B) Thermal sensitivity after spinal nerve injury was assessed by hot-plate test and revealed no difference of the ispilateral paw between wild-type (circles) and plt animals (diamonds). (C) Tail-flick and paw-flick tests revealed no differences between wild-type mice and plt mice in acute pain reception. (D) The paw withdrawal frequency after mechanical stimulation (2.0 g) did not differ between wild-type and plt animals. (E) Intrathecal injection of blocking antibodies (0.3 μg) for CCL21 twice a day from before and post-operative day 3 significantly attenuated the development of tactile allodynia. Data are presented in mean±s.e.m. from n=6 animals for (A, B), from five animals in (C, D) and from four to six animals for (E). *,**Statistically significant difference from control IgG-injected animals, P<0.05 and P<0.01, respectively.
CCL21 up-regulates P2X4 expression in microglia in vitro and in vivo
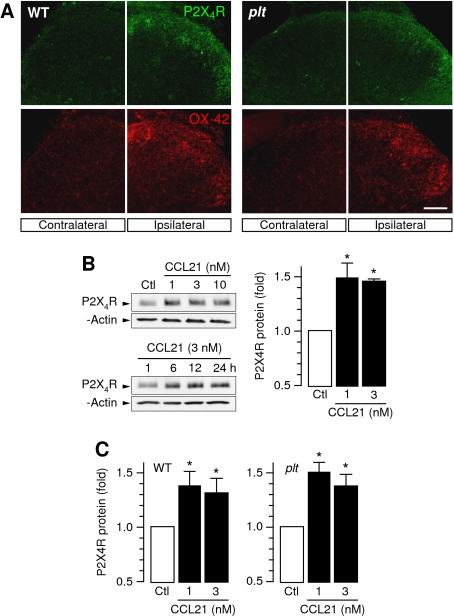
Since P2X4 receptor up-regulation in activated microglia is essential for the development of tactile allodynia after peripheral nerve lesion (Tsuda et al, 2003, 2009a; Ulmann et al, 2008), the possible effect of CCL21 on microglial P2X4 expression was assessed. Whereas in wild-type mice, spinal nerve injury induced a pronounced up-regulation of P2X4 receptor expression in dorsal horn microglia within 7 days (Figure 3A), this was attenuated in plt animals. Here, microglial P2X4 expression was barely detectable although OX-42 staining indicated a spinal nerve injury-dependent activation of microglia also in plt animals (Figure 3A). Moreover, direct stimulation of cultured rat microglia with CCL21 induced a rapid (<6 h) and significant up-regulation of P2X4 protein expression at concentrations as low as 1 nM (Figure 3B). Similar results have been obtained in microglia cultured from wild-type and plt mice (Figure 3C).
Figure 3.
CCL21 is required for microglial P2X4 receptor induction in vitro and in vivo, but not for morphological microglia activation in response to spinal nerve injury. (A) Immunohistochemical staining showed a spinal nerve injury-dependent induction of microglial P2X4 expression (green) and activation determined by OX-42 (red) expression at the ipsilateral side of the dorsal horn only. Whereas microglia OX-42 staining was unchanged in plt animals, the induction of P2X4 expression was strongly attenuated in plt mice. The immunohistochemical staining showed similar results in three independent experiments. (B) Western blot analysis showed a time and concentration-dependent induction of P2X4 receptor expression in cultured rat microglia by CCL21. *Significant difference of P2X4 receptor expression in CCL21 stimulated microglia compared with non-stimulated controls. (C) Western blot analysis showed a concentration-dependent induction of P2X4 receptor expression in cultured mouse wild-type and plt microglia by CCL21. *Significant difference of P2X4 receptor expression in CCL21 stimulated microglia. Data presented are mean±s.e.m. from five independent experiments. Scale bar, 100 μm.
Activation of spinal cord microglia
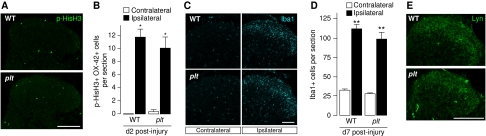
The OX-42 staining shown in Figure 3A revealed that in plt animals, the morphological activation of microglia did not differ from the microglia reaction in wild-type animals. We therefore compared several microglial activation markers in wild-type and plt animals at different time points (2, 7 and 14 days) after spinal nerve injury. Microglia proliferation (assessed by phosphorylated-histone H3 (p-HisH3) staining, shown for 2 days after spinal nerve injury, Figure 4A and B) was not prominent confirming earlier findings in rat (Tsuda et al, 2011) and did not differ between wild-type and plt animals. The proliferation of microglia was an early event, since p-HisH3-positive cells were not found at day 7 or 14 after spinal nerve injury (Supplementary Figure S3), whereas Iba1-positive microglia were observed at all time points investigated (Supplementary Figure S3). However, we did not observe differences in microglia morphology (determined by Iba1 staining, shown for 7 days after spinal nerve injury, Figure 4C and D) at any time point after spinal nerve injury between lesioned wild-type and plt animals. Moreover, we could not find a major difference in spinal nerve injury-dependent expression of microglial Lyn kinase. Although Lyn kinase was slightly less expressed in plt microglia 2 days after nerve injury (Supplementary Figure S3) this small difference between lesioned wild-type and plt animals vanished at 7 and 14 days after spinal nerve injury (Figure 4E; Supplementary Figure S3).
Figure 4.
Comparison of various aspects of microglia activation after spinal nerve injury between wild-type and plt animals. (A) Phosphorylated-histone H3 (p-HisH3) staining in the spinal cord 2 days after spinal nerve injury as a measure for microglia proliferation. (B) Quantification of p-HisH3-positive microglia did not reveal a difference between wild-type and plt animals. (C) Iba1 staining in the spinal cord 7 days after spinal nerve injury revealed the morphology of microglia. (D) Quantification of Iba1-positive cells did not show a difference between wild-type and plt animals. (E) Lyn kinase staining 7 days after spinal nerve injury showed no major difference between wild-type and plt animals. Data presented are mean±s.e.m. from four to five independent experiments. Scale bar, 100 μm. Statistically significant different from contralateral side, *P<0.05; **P<0.01.
Since plt animals have a changed peripheral immune system and a changed T-cell response (Mori et al, 2001), the possible role of peripheral T cells in the spinal cord was determined with the pan T-cell marker CD3. In general, very few T cells where found in the ipsilateral spinal cord at 7 days after spinal nerve injury with no differences between wild-type controls and plt animals (Supplementary Figure S4).
CCL21 requires P2X4 function to induce tactile allodynia
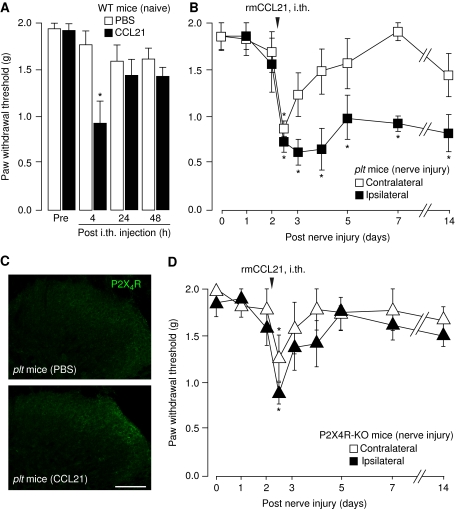
Next CCL21 was injected (0.06 μg/mouse) intrathecally into naive wild-type mice. After 4 h, a significant (P=0.036) but transient drop of the PWT to 0.93±0.25 g was observed, that returned to control values 1.44±0.10 g within 48 h (Figure 5A). Similar findings were observed in naive plt mice (unpublished data). CCL21 was furthermore injected into plt mice 2 days after spinal nerve injury (Figure 5B). The PWT of the ipsilateral hindpaw dropped rapidly to 0.721±0.11 g (P=0.003) and did not show any recovery throughout the experiment (Figure 5B). The PWT of the contralateral hindpaw showed a rapid (4 h) but transient decrease (0.865±0.08 g/P=0.014) (Figure 5B) similar to the response seen in naive animals after CCL21 injection.
Figure 5.
Intrathecal injection of CCL21 rescues normal tactile allodynia development after spinal nerve injury in plt animals but not in P2X4 receptor-deficient mice. (A) Intrathecal injection of CCL21 (0.06 μg) in naive wild-type animals resulted in a temporary (<24 h) drop of the PWT. (B) Intrathecal injection of CCL21 (0.06 μg) into plt animals 2 days after L5 spinal nerve lesion induced a long-lasting formation of tactile allodynia of the ipsilateral hindpaw (▪), whereas the PWT of the contralateral hindpaw was temporary (□). (C) Intrathecal injection of CCL21 (0.06 μg) enhanced the expression of P2X4 receptor in plt animals whereas control injections with PBS had no effect. (D) Intrathecal injection of CCL21 (0.06 μg) in P2X4 receptor-knockout mice caused a transient (<24 h) drop of the PWT of both hindpaws. Data are given as mean±s.e.m. from five to eight animals. Statistically significant difference from control values, *P<0.05.
The intrathecally injection of CCL21 (0.3 μg) caused up-regulation of microglial P2X4 receptor expression (Figure 5C), confirming our findings in cultured microglia (see Figure 3)
In order to further investigate the function of P2X4 for the allodynia-inducing effects of CCL21, P2X4 receptor-deficient animals were used. Intrathecal injection of CCL21 2 days after spinal nerve injury caused a transient drop (ipsi: 0.878±0.11 g/P<0.001; contra: 1.258±0.24 g/P=0.024) in PWT of both hindpaws (Figure 5D). However, unlike plt mice (Figure 5B), the CCL21-induced decreased PWT in the ipsilateral side of P2X4-deficient mice was not maintained, and the PWT recovered to control values within 48 h after the injection (Figure 5D).
Neither CCR7 nor CXCR3 are solely involved in CCL21-dependent induction of neuropathic pain
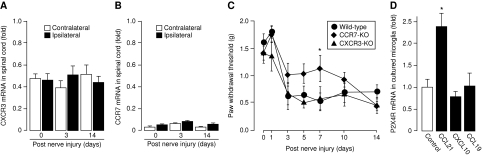
Two different receptors for CCL21 (CXCR3 and CCR7) have been described in mice, both of which can be found in microglia (Biber et al, 2001; Rappert et al, 2002; Dijkstra et al, 2006). Whereas CXCR3 is constitutively expressed in these cells, microglial CCR7 expression can be induced by activation (Dijkstra et al, 2006). To determine which of those receptors is mediating the neuropathic pain inducing effect of CCL21, we first determined the mRNA expression levels of these chemokine receptors in spinal cord tissue of mice. As shown in Figure 6, CXCR3 mRNA was detected in non-injured spinal cord tissue of the mouse (albeit at lower levels than in lymph node control tissue) (Figure 6A), whereas the signal for CCR7 mRNA was close to detection limit (Figure 6B), thereby confirming earlier findings that CCR7 mRNA is not expressed in healthy nervous tissue (Biber et al, 2001; Rappert et al, 2002, Dijkstra et al, 2006). Spinal nerve injury did not change the mRNA expression levels for CXCR3 or CCR7 at 3 and 14 days after the nerve injury (Figure 6A and B). Furthermore, chemokine receptor-deficient animals were subjected to spinal nerve injury and the PWT was analysed. As depicted in Figure 6C, there was no difference in the PWT of the ipsilateral paw between CXCR3 −/− and wild-type animals. The development of neuropathic pain in CCR7-deficient mice in the first week appeared somewhat less severe than in wild-type mice (statistically significant from wild-type controls at the 7-day time point only) but reached wild-type levels thereafter (Figure 6C). Cultured microglia were furthermore stimulated with different chemokine ligands (10 nM for 6 h) and P2X4 mRNA expression was assessed. Only CCL21 caused a significant up-regulation of P2X4 receptor mRNA expression, whereas neither the specific CXCR3 ligand CXCL10, nor the specific CCR7 ligand CCL19 had such an effect (Figure 6D).
Figure 6.
Chemokine receptor expression in mouse spinal cord tissue and the potential role of a yet unidentified chemokine receptor in neuropathic pain development. (A) Real-time PCR analysis of CXCR3 expression in mouse spinal cord. Compared with the mRNA expression levels in lymph nodes (reference point 1.0) less CXCR3 mRNA was detected in mouse spinal cord tissue, which was not affected by spinal nerve injury. (B) Real-time PCR analysis of CCR7 expression in mouse spinal cord. Compared with the mRNA expression levels in lymph nodes (reference point 1.0) CCR7 mRNA was close to detection limit in mouse spinal cord tissue and was not affected by spinal nerve injury. Data in (A, B) are given as mean±s.e.m. from three to five animals. (C) Spinal nerve injury induced development of neuropathic pain in CXCR3- and CCR7-deficient mice. There was no difference in the PWT of the ipsilateral hindpaw between wild-type (circles) and CXCR3 −/− animals (triangles) in response to spinal nerve injury. The development of neuropathic pain was significantly attenuated in CCR7 −/− animals (diamonds) at day 7 after spinal nerve injury. At earlier or later time points, no difference between CCR7 −/− and wild-type mice were observed. Data are given as mean±s.e.m. from five to eight animals. *Statistically significant from wild-type values. Only data from the ipsilateral paw are shown. (D) Real-time PCR analysis of P2X4 receptor mRNA expression in cultured microglia after 6 h stimulation with 100 nM of CCL21, CXCL10 (CXCR3 ligand) and CCL19 (CCR7 ligand). Data are given as mean±s.e.m. from six to nine experiments. Statistically significant difference from control values, *P<0.05.
Discussion
It was recognized in the last couple of years that microglia activity in the dorsal horn is crucial for the initiation and maintenance of tactile allodynia (Tsuda et al, 2005). It is therefore of interest to discover the signal(s) that control microglia activation in response to spinal nerve injury.
Neuronal CCL21 is crucial for the development of tactile allodynia
We here provide evidence for the neuronal chemokine CCL21 as such a signal. CCL21 is a microglia-activating chemokine that in nervous tissue is exclusively found in damaged neurons (Biber et al, 2001; de Jong et al, 2005, 2008). Using live cell imaging we showed that neuronal CCL21 is sorted into large-dense core vesicles and transported anterogradely along the axons to presynaptic terminals, indicating its function in directed neuron-microglia signalling (de Jong et al, 2005, 2008). In injured DRG neurons, CCL21 expression was detected within 12 h, specifically in vesicles of small-sized C-fibres. Since CCL21 expression was at later time points (24 h after injury) furthermore observed in axons of the dorsal root and in the primary afferents in the dorsal horn of the spinal cord, a vesicle-based, anterograde transport of CCL21 is suggested in small-sized C-fibres.
The importance of CCL21 for the development of tactile allodynia became evident in plt animals that lack neuronal CCL21. Spinal nerve injury in these animals did not induce any signs of tactile allodynia. However, the development of tactile allodynia was initiated in plt animals when spinal nerve injury was followed by an intrathecally injection of CCL21. Although CCL21 was injected only once, it induced a pain reaction that lasted until the end of the experiment (day 14) and was undistinguishable from the pain reaction in wild-type animals. An inhibition of CCL21 function by antibody treatment before and after spinal nerve injury prominently reduced the development of tactile allodynia in wild-type animals throughout the 14-day experiment. Furthermore, whereas wild-type mice up-regulated P2X4 receptor expression in microglia after spinal nerve injury (Tsuda et al, 2003; Ulmann et al, 2008), this response was reduced in plt animals. Stimulation with recombinant CCL21 induced a rapid and concentration-dependent increase in P2X4 protein expression in cultured microglia, indicating a direct CCL21 effect in these cells. This was further corroborated by intrathecally injections of CCL21 in plt animals, which resulted in up-regulation of P2X4 expression. It is therefore suggested that CCL21 in vivo is crucial for the induction of microglial P2X4 expression. This assumption is corroborated by the findings that intrathecal injection of CCL21 in P2X4-deficient animals did not induce long-lasting tactile allodynia, showing that CCL21 function is upstream of P2X4 up-regulation in the cascade leading to tactile allodynia.
CCL21 was not required for the development of thermal hypersensitivity after spinal nerve lesion as plt animals did not show a difference compared with wild-type animals. Since it is known that tactile allodynia and thermal hypersensitivity in the spinal nerve lesion model show differences in morphine response and disease course (Kim and Chung, 1992; Wegert et al, 1997; Lashbrook et al, 1999), our data about the selective involvement of CCL21 add up to the concept that tactile allodynia and thermal hypersensitivity are due to different mechanisms.
Taken together, these data show that the expression of neuronal CCL21 is an early event after spinal nerve injury, which is required and sufficient for the development of tactile allodynia specifically.
Temporary versus prolonged pain reaction
An intrathecal injection of inflammatory factors (cytokines and chemokines) often causes a temporary drop in PWT in rodents (Watkins et al, 2001; Abbadie, 2005), suggesting that this reflects a temporary hypersensitivity caused by local inflammation. In line with this it is shown here that in naive wild-type, plt and P2X4-deficient mice, injection of CCL21 induced a temporary drop of the PWT. This indicates that the general responsiveness to CCL21 and the overall inflammatory reaction in the spinal cord induced by CCL21 was comparable in all animal strains used in this study. Interestingly, injection of CCL21 only in combination with spinal nerve injury induced the development of long-lasting tactile allodynia in plt mice. This indicates not only that temporary hypersensitivity and development are most likely due to different processes but furthermore shows that more than one signal is needed for the development of tactile allodynia.
Microglia morphology is not a sufficient readout of their function
Based on morphological changes has microglia activation long been considered a stereotypic response; this view has been challenged recently. New notions concerning microglia activity suggest that these cells respond in quite distinct ways to different pathological situations, thereby integrating various inputs and responding appropriately with a variety of different reactions (Hanisch and Kettenmann, 2007; Ransohoff and Perry, 2009). Although the arrival of CCL21 in the primary afferents coincides timely with the first morphological signs of microglia activation, our data show that this chemokine is not required for the morphological microglia activation or proliferation. Numerous factors, such as cytokines, chemokines or ATP can induce morphological microglia activity (Davalos et al, 2005; Cardona et al, 2006; Haynes et al, 2006; Koizumi et al, 2007), are expressed and most likely released in the spinal cord after spinal nerve injury and may thus account for the morphological activation of microglia (Coull et al, 2005; Tsuda et al, 2005; White et al, 2007). Although spinal nerve injury leads to an accumulation of activated microglia in lamina I and II of the dorsal horn at day 7 after the nerve injury, at earlier time points there is a more equal distribution of activated cells. It is at the moment neither clear which factors are responsible for the activation of microglia nor is it understood whether the accumulation of activated microglia in lamina I and II is due to migration of microglia into these areas or a deactivation of microglia in other layers at day 7 after nerve injury. These questions are at the moment under investigation in our laboratory. However, our findings clearly show that morphologically activated microglia did not cause the development of tactile allodynia in the absence of CCL21 and subsequent P2X4 receptor up-regulation. The data presented here thus add up to that concept that microglia are able to integrate various inputs and that the lack of one factor significantly affects their reaction.
Potential role of a yet unknown CCL21 receptor in the induction of neuropathic pain
There are two known receptors for CCL21 in mice: CCR7 and CXCR3, both of which can be detected in microglia (Biber et al, 2001; Rappert et al, 2002; Dijkstra et al, 2006). Cultured microglia from CXCR3-deficient animals lose their chemotactic response to CCL21 stimulation (Rappert et al, 2002) and CXCR3-deficient animals display markedly reduced microglia activation after neuronal injury in the entorhinal cortex lesion model (Rappert et al, 2004), indicating a prominent role of CXCR3 in detecting CCL21 in the nervous system. However, the development of neuropathic pain was not affected in CXCR3-deficient animals, showing the involvement of a different receptor here. CCR7-deficient animals displayed a somewhat milder disease course, especially in the first days after spinal nerve injury. This delay in allodynia development might point to an induction of CCR7 expression in activated dorsal horn microglia, similar to what was found in a mouse model of multiple sclerosis (Dijkstra et al, 2006). However, in agreement with earlier studies we were not able to detect any CCR7 mRNA in the spinal cord, neither was CCR7 mRNA induced by the nerve lesion. Given this lack of CCR7 in spinal cord tissue, the slightly milder disease development after spinal nerve injury in CCR7-deficient animals is most likely due to a yet not understood effect in the periphery. The fact that only CCL21, but not the specific CXCR3 ligand CXCL10 or the specific CCR7 ligand CCL19 were able to induce P2X4 mRNA expression in cultured mouse microglia might point to another CCL21 receptor in these cells. Indeed, we have recently provided functional evidence for a third, yet not identified, CCL21 receptor in mouse astrocytes (van Weering et al, 2010), indicating that the question of CCL21 receptors in glia cells is more complex than originally anticipated. Taken together, the responsible receptor for the CCL21-dependent development of neuropathic pain after spinal nerve injury remains to be established.
CCL21 as potential drug target to prevent neuropathic pain
Whether or not blocking of CCL21 would be of therapeutic relevance to stop already ongoing neuropathic pain is unknown. Since CCL21 expression peaked very early after nerve injury and diminished thereafter and since the stop of CCL21-blocking antibody treatment did not lead to increased pain states for >10 subsequent days, our data might not argue for a role of CCL21 in the maintenance of neuropathic pain. The data presented in this study strongly indicate that inhibition of CCL21 expression in DRG neurons or attenuation of its function in the dorsal horn would prevent the development of neuropathic pain, which would be a welcomed therapeutic intervention for patients with acute nerve injury. Anatomical considerations might support the possibility that similar processes are also occurring in humans. In rodents it is known that microglia activity and subsequent development of neuropathic pain depends on an intact dorsal root, indicating the transport of activating molecules (Colburn et al, 1999; Obata et al, 2006a, 2006b). In humans cutting of dorsal roots (e.g. to treat spasticity or for tumour removal) does not lead to neuropathic pain, in contrast to lesions distal from the DRG (Campbell and Meyer, 2006), which might suggest a transport of microglia-activating molecules through the dorsal root also in humans. Whether or not CCL21 is also expressed in human DRG neurons in response to nerve injury, however, remains to be established.
Taken together, we provide evidence that neuronal CCL21 in mice is a rapid signal that up-regulates P2X4 receptor expression in spinal cord microglia, identifying CCL21 as the neuronal signal that is required and sufficient for the induction of tactile allodynia after spinal nerve injury.
Materials and methods
Animals
plt (Mori et al, 2001; Nakano and Gunn, 2001) and wild-type C57BL/6 mice (Clea Japan) were used. Animals were housed in groups of two to three per cage at a temperature of 22±1 °C with a 12-h light–dark cycle (light on from 08:30 to 20:30 hours), and fed food and water ad libitum. All animal experiments were conducted according to relevant national and international guidelines ‘Act on Welfare and Management of Animals’ (Ministry of Environment of Japan) and ‘Regulation of Laboratory Animals’ (Kyushu University), and under the protocols approved by the Institutional Animal Care and Use committee review panels at Kyushu University.
Spinal nerve injury
We used the spinal nerve injury model (Kim and Chung, 1992) with some modifications (Tozaki-Saitoh et al, 2008; Tsuda et al, 2009b) in 9- to 12-week-old mice. Under isoflurane (2%) anaesthesia, a small left-side incision at ∼L4–S2 was made. Paraspinal muscle and fat were removed from the L6 traverse process, and the part of this traverse process was removed to expose the parallel-lying L4 and L5 spinal nerves. The L5 nerve was isolated and cut. The wound was sutured with 3-0 silk. The surrounding skin was pulled together and sutured with 3-0 silk.
Behavioural studies
To assess the tactile allodynia, mice were placed individually in an opaque plastic cylinder, which was placed on a wire mesh and habituated for 1 h to allow acclimatization to the new environment. After that, calibrated von Frey filaments (0.02–2.0 g, Stoelting) were applied to the plantar surface of the hindpaw from below the mesh floor, and the 50% PWT was determined using the up–down method (Chaplan et al, 1994). The PWT was measured before and after the injury of the L5 spinal nerve (an animal model of neuropathic pain) or intrathecal administration of recombinant CCL21 (0.06 μg/mouse, R&D Systems). Intrathecal administration to mice was performed according to a procedure described previously (Tsuda et al, 2008a) using a 25-μl Hamilton syringe with 30-gauge needle. A neutralizing antibody for CCL21 (0.3 μg/mouse, Peprotech) was administered intrathecally twice a day from day 0 (15 min before nerve injury) to day 3. Nerve injury-induced thermal hyperalgesia was assessed using hot-plate test. Mice were placed on a metal surface maintained at either 49 °C within a 25-cm-high Plexiglas box (25 cm2). The latency to either lick or shake the hindpaw was measured as a nocifensive end point. Noxious heat-evoked hindpaw withdrawal responses were detected by the application of radiant heat (Ugo Basile, Italy) to the tail and the plantar surface of hindpaw, respectively (Tsuda et al, 2007). The intensity of the heat stimulus was adjusted to 30 or 50 V, and the latency of the paw withdrawal response (seconds) was measured (Tsuda et al, 2007). The sensitivity to noxious mechanical stimulus was assessed by measuring the frequency of withdrawal responses to application of a von Frey filament (2.0 g) 10 times. Motor coordination was assessed using the rotarod performance test (Tsuda et al, 2007).
Immunohistochemistry
According to a procedure described previously (Nakajima et al, 1992), anaesthetized mice were perfused transcardially with PBS, followed by 4% paraformaldehyde. The fifth lumbar (L5) segments of the DRG, dorsal root and spinal cord were removed, post-fixed in the same fixative for 3 h at 4 °C, and placed in 30% sucrose solution for 24 h at 4 °C. Sections (30 μm) were cut and processed for immunohistochemistry with antibodies for CCL21 (1:500, Peprotech), TRPV1 (1:1000, Santa Cruz), isolectin B4 (1:200, Sigma), CGRP (1:20 000, Peninsula Laboratories), NF200 (1:400, Sigma), OX-42 (1:1000, Serotec), Iba1 (1:2000, Wako), Lyn (1:200, Wako), phosphorylated-histone H3 (Ser10) (p-HisH3, 1:1000, Upstate/Millipore), CD3 (1:100, eBioscience) and P2X4 (1:1000, kindly provided by Dr Francois Rassendren) (Ulmann et al, 2008). Following incubation, the sections were incubated with secondary antibodies conjugated to Alexa Fluor™ 488 or 546 (1:1000, Molecular Probes). The sections were analysed using an LSM510 Imaging System (Zeiss). For the cell size-frequency analyses, the cross-sectional areas of total and CCL21-positive DRG neurons with clearly visible nuclei were measured.
Microglial culture
Primary cultured microglia were prepared in accordance with a method described previously (Nakajima et al, 1992). In brief, a mixed glial culture was prepared from neonatal Wistar rats, C57BL/6 and plt mice and maintained for 10–16 days in DMEM with 10% fetal bovine serum. Immediately before experiments, microglia were collected by a gentle shake as the floating cells over the mixed glial culture. The microglia were transferred to coverslips for subsequent experiments. The cultures were of >99% purity, determining by immunostaining for OX-42 and Iba1.
Western blotting
Whole-cell lysates of cultured microglial cells were prepared in accordance with the methods described previously (Tsuda et al, 2008b). Aliquots (4 μg) were subjected to a 10% polyacrylamide gel electrophoresis (Bio-Rad), and proteins were transferred electrophoretically to nitrocellulose membranes. After blocking, the membranes were incubated with anti-P2X4R rabbit polyclonal antibody (1:1000, Alomone) or anti-β-actin (1:2000, Sigma) then incubated with HRP-conjugated secondary antibody (1:1000). The blots were detected using ECL western blotting detection system (GE Healthcare) and an LAS-3000 imaging system (Fujifilm). Bands were quantified using the software NIH Image J 1.36.
Real-time PCR
Real-time amplification, using an iCycler (Bio-Rad) and iQ SYBR Green supermix (Bio-Rad), was performed on 4 ng cDNA derived from microglia culture or spinal cord tissue. RNA preparation and cDNA synthesis was performed as described recently (Dijkstra et al, 2006). The primers used for real-time PCR were designed using Primer Designer (Scientific and Educational Software, Version 3.0) and have been published earlier (Dijkstra et al, 2006). PCR amplification with CCR7 and CXCR3 primers was run in parallel with primers for the housekeeping gene hydroxymethylbilane synthase and quantified as described recently (Dijkstra et al, 2006). Primers used for P2X4 were (F): 5′-TGGCGGACTATGTGATTCCA-3′, primer (B): 5′-GGTTCACGGTGACGATCATG-3′. Lymph node tissue served as positive control and reference for CCR7 and CXCR3 expression.
Statistics
Statistical analyses of the results were made with Student's t-test, Mann–Whitney U-test or two-way repeated measures ANOVA (post hoc test using Tukey).
Supplementary Material
Acknowledgments
This work was supported by Dutch NWO-Vidi grant, Deutsche Forschungsgemeinschaft (DFG, FOR 1336) and a stipend from Kyushu University (KB), and by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MT, HT-S, KI) and in part from the Mochida Memorial Foundation for Medical and Pharmaceutical Research and the Astellas Foundation for Research on Metabolic Disorders (MT). We thank Drs Terutaka Kakiuchi, Fumio Ishikawa and Taku Kuwabara (Toho University School of Medicine, Tokyo, Japan) for providing some plt mice. We also thank Dr Francois Rassendren (Institut de Génomique Fonctionnelle, Montpellier, France) for providing the antibody for P2X4. KB, MT, HS-T, KT, TM and ET performed the experimental work. KB, MT and KI planned the project and designed the experiments. KB, MT, HB and KI wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abbadie C (2005) Chemokines, chemokine receptors and pain. Trends Immunol 26: 529–534 [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HWGM (2007) Neuronal on and off signals control microglia. Trends Neurosci 30: 598–602 [DOI] [PubMed] [Google Scholar]
- Biber K, Sauter A, Brouwer N, Copray SCVM, Boddeke HWGM (2001) Ischemia-induced neuronal expression of the microglia attracting chemokine secondary lymphoid-tissue chemokine (SLC). Glia 34: 121–133 [DOI] [PubMed] [Google Scholar]
- Biber K, Vinet J, Boddeke HWGM (2008) Neuron-microglia signaling: chemokines as versatile messengers. J Neuroimmunol 198: 69–74 [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA (2006) Mechanisms of neuropathic pain. Neuron 52: 77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9: 917–924 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63 [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA (1999) The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol 157: 289–304 [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438: 1017–1021 [DOI] [PubMed] [Google Scholar]
- Coyle DE (1998) Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia 23: 75–83 [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8: 752–758 [DOI] [PubMed] [Google Scholar]
- de Jong EK, Dijkstra IM, Hensens M, Brouwer N, Amerongen M, Liem RSB, Boddeke HWMG, Biber K (2005) Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci 25: 7548–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong EK, Vinet J, Stanulovic V, Wesseling EM, Meijer M, Schollema K, Boddeke HWGM, Biber K (2008) Expression, transport and axonal sorting of neuronal CCL21 in large dense core vesicles. FASEB J 22: 4136–4145 [DOI] [PubMed] [Google Scholar]
- Dijkstra IM, De Haas A, Brouwer N, Boddeke HWGM, Biber K (2006) Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo. Glia 54: 861–872 [DOI] [PubMed] [Google Scholar]
- Guseva D, Chelyshev Y (2006) The plasticity of the DRG neurons belonging to different subpopulations after dorsal rhizotomy. Cell Mol Neurobiol 26: 1226–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG (2006) Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26: 4308–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathological brain. Nat Neurosci 10: 1387–1394 [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9: 1512–1519 [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50: 355–363 [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446: 1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook JM, Ossipov MH, Hunter JC, Raffa RB, Tallarida RJ, Porreca F (1999) Synergistic antiallodynic effects of spinal morphine with ketorolac and selective COX1- and COX2-inhibitors in nerve-injured rats. Pain 82: 65–72 [DOI] [PubMed] [Google Scholar]
- Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, White FA, Zhang J (2008) Chemokine action in the nervous system. J Neurosci 28: 11792–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T (2001) Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J Exp Med 193: 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Shimojo M, Hamanoue M, Ishiura S, Sugita H, Kohsaka S (1992) Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem 58: 1401–1408 [DOI] [PubMed] [Google Scholar]
- Nakano H, Gunn MD (2001) Gene duplications at the chemokine locus on the mouse chromosome 4: multiple strain-specific haplitypes and the deletion of secondary lymphpoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol 166: 361–369 [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Noguchi K (2006a) Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J Neurosci 26: 11974–11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K (2006b) The effect of site and type of nerve injury on the expression of brain-derived neurotrophic factor in the dorsal root ganglion and on neuropathic pain behavior. Neuroscience 137: 961–970 [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA (2003) Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther 306: 624–630 [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH (2009) Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27: 119–145 [DOI] [PubMed] [Google Scholar]
- Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, Kovac A, Gerard C, Boddeke HWGM, Nitsch R, Kettenmann H (2004) CXCR3-dependent microglial recruitment is essential for dendritic loss after brain lesion. J Neurosci 24: 8500–8509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, Gerard NP, Gerard C, Boddeke HWMG, Kettenmann H (2002) Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl− current and chemotaxis in murine microglia. J Immunol 169: 3221–3226 [DOI] [PubMed] [Google Scholar]
- Saab CY, Haines BC (2009) Remote neuroimmune signaling: a long-range mechanism of nociceptive network plasticity. Trends Neurosci 32: 110–117 [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10: 1361–1368 [DOI] [PubMed] [Google Scholar]
- Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K (2008) P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci 28: 4949–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW (2005) Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 28: 101–107 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Ishii S, Masuda T, Hasegawa S, Nakamura K, Nagata K, Yamashita T, Furue H, Tozaki-Saitoh H, Yoshimura M, Koizumi S, Shimizu T, Inoue K (2007) Reduced pain behaviors and extracellular signal-related protein kinase activation in primary sensory neurons by peripheral tissue injury in mice lacking platelet-activating factor receptor. J Neurochem 102: 1658–1668 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K (2011) JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009a) Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K (2009b) IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci USA 106: 8032–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424: 778–783 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K (2008a) Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia 56: 579–585 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Tozaki-Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T, Inoue K (2008b) Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia 56: 50–58 [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28: 11263–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF (2001) Glia cells: a driving force for pathological pain. Trends Neurosci 24: 450–455 [DOI] [PubMed] [Google Scholar]
- Wegert S, Ossipov MH, Nichols ML, Bian D, Vanderah TW, Malan TP Jr, Porreca F (1997) Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats. Pain 71: 57–64 [DOI] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ (2007) Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci 104: 20151–20158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Weering HR, Jong AP, Haas AH, Biber K, Boddeke HW (2010) CCL21-induced calcium transients and proliferation in primary mouse astrocytes: CXCR3-dependent and independent responses. Brain Behav Immun 24: 768–775 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.