Abstract
Recent studies demonstrated a role of sphingosine-1-phosphate (S1P) in the protection against the stress of ischemia/reperfusion (I/R) injury. In experiments reported here, we have investigated the signaling through the S1P cascade by FTY720, a sphingolipid drug candidate displaying structural similarity to S1P, underlying the S1P cardioprotective effect. In ex vivo rat heart and isolated sinoatrial node models, FTY720 significantly prevented arrhythmic events associated with I/R injury including premature ventricular beats, VT, and sinus bradycardia as well as A–V conduction block. Real-time PCR and Western blot analysis demonstrated the expression of the S1P receptor transcript pools and corresponding proteins including S1P1, S1P2, and S1P3 in tissues dissected from sinoatrial node, atrium and ventricle. FTY720 (25 nM) significantly blunted the depression of the levels of phospho-Pak1 and phospho-Akt with ischemia and with reperfusion. There was a significant increase in phospho-Pak1 levels by 35%, 199%, and 205% after 5, 10, and 15 min of treatment with 25 nM FTY720 compared with control nontreated myocytes. However, there was no significant difference in the levels of total Pak1 expression between nontreated and FTY720 treated. Phospho-Akt levels were increased by 44%, 63%, and 61% after 5, 10, and 15 min of treatment with 25 nM FTY720, respectively. Our data provide the first evidence that FTY720 prevents I/R injury-associated arrhythmias and indicate its potential significance as an important and new agent protecting against I/R injury. Our data also indicate, for the first time, that the cardioprotective effect of FTY720 is likely to involve activation of signaling through the Pak1.
Keywords: S1P, FTY720, Pak1, Akt
1. Introduction
Disturbances of cardiac rhythm, including lethal ventricular arrhythmias, are a consequence of reperfusion following pathological and/or clinical instances of myocardial ischemia [1]. This includes arrhythmias arising from re-establishing flow after coronary spasm [2], cardiopulmonary bypass with ischemic cardiac arrest [3], and angioplastic/thrombolytic procedures [4]. The clinical occurrence and possible lethal consequences of ischemia/reperfusion arrhythmias have prompted considerable interest in determining the mechanisms responsible and in developing therapeutic approaches for their control. Effort has thus been made to minimize the adverse arrhythmic events related to myocardial ischemia/reperfusion (I/R) injury.
Several recent studies [5,6] have provided evidence for a role of sphingosine-1-phosphate (S1P) signaling in the protection against the stress of cardiac I/R injury. S1P, a product of sphingosine kinase (SphK) activation, is recognized as a vital lipid mediator activating a family of five G protein-coupled receptors (S1P1–5). These receptors regulate diverse cellular events, including cell survival, growth, motility, differentiation, cytoskeletal reorganization, and calcium mobilization [7]. S1P is a phosphorylated derivative of sphingosine, the structural backbone of all sphingolipids, which was initially described as an intermediate in the degradation of long-chain sphingoid bases [8]. FTY720 (fingolimod, 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diolhydrochloride), a sphingolipid drug candidate displaying structural similarity to S1P, is derived from myriocin, a component in the Chinese herb Iscaria sinclarii, which has been used as a treatment for asthma [9]. Moreover, FTY720 has demonstrated a protective effect in the prevention of liver I/R injury in an animal model [10]. Importantly, FTY720 is currently under evaluation in a long-term phase III clinical trial as an immunosuppressant agent for the treatment of autoimmune diseases and in organ transplantation [11].
The underlying key mechanism(s) and signaling pathway(s) for S1P cardioprotection and the possible effects of FTY720 remain unknown. A significant clue as to the mechanism came from experiments in a mammalian cell line, which demonstrated that p21-activated kinase (Pak1), a Ser/Thr kinase downstream of small G proteins, is activated by sphingosine and several related long-chain sphingoid bases in a time- and dose-dependent activation manner [12]. There is a large body of evidence that Pak1 activity is a key regulator of a number of cellular functions, including cytoskeletal dynamics, cell motility, growth and proliferation, cardiac ion channel activity, and contractility [13,14]. Pak1 also facilitates Akt stimulation and aids recruitment of Akt to the membrane. This reveals an important scaffolding function of Pak1 in the Akt pathway [15]. On the basis of these data, we speculated that Pak1 participates in the cardiac effect of S1P signaling.
We investigated the effect of FTY720 on ischemia/reperfusion-induced cardiac arrhythmias in an ex vivo rat heart and isolated sinoatrial node (SAN) models. Our results demonstrate a cardioprotection by FTY720 signaling through the S1P cascade to Pak1. Our data provide the first evidence that FTY720 prevents I/R injury-associated arrhythmias and can be a potentially important and new agent protecting against I/R injury including cardiac arrhythmias. We also determined, for the first time, that FTY720 activates Pak1.
2. Materials and methods
2.1. Ex vivo rat heart and isolated sinoatrial node ischemia/reperfusion models and electrophysiological recordings
Male adult Wistar rats (8–12 weeks old) were purchased from Charles River (UK). All animal procedures conformed to the United Kingdom Animals (Scientific Procedures) Act of 1986. Animals were killed by cervical dislocation. Hearts were then quickly excised and immediately immersed in cold buffer prior to mounting on the Langendorff perfusion system. In control groups, the hearts were first perfused with modified Krebs–Henseleit Tyrode solution at rate of ~8 ml/min for 30 min to achieve a steady-state condition as determined by observing a stable and regular sinus rhythm with one to one atrial–ventricular conduction via continual electrocardiogram (ECG) monitoring. Hearts were then perfused with ischemic solution (as described below) for 20 min and subsequently perfused with modified Krebs–Henseleit Tyrode solution (reperfusion phase) for 30 min. In the case of the FTY720-treated group, 25 nM FTY720 was added to the ischemic solution and Krebs–Henseleit buffer during the reperfusion phase. The perfusion sequence was the same as for the control groups. The modified Krebs–Henseleit Tyrode solution contained (in mM): 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.8 CaCl2, 11 glucose, 25 NaHCO3, and 1.2 KH2PO4 gassed with 95% O2–5% CO2 to give a pH of 7.4. The ischemic solution contained (in mM):130 NaCl, 10 KCl, 0.6 MgCl2, 1.8 CaCl2, 0.6 KH2PO4, 0 glucose, 1 NaHCO3, 10 HEPES, pH 6.6, and bubbled with 100% nitrogen gas for more than 30 min before the experiment was started. Temperature was set at 36 °C at the inner surface of the right ventricular apex. A pair of ECG electrodes was placed onto the epicardial surfaces of the atrium and left ventricle for ECG recording. ECG signal was amplified, filtered (0.5 Hz to 1 kHz band-pass filtered), and digitized at a sampling frequency of 5 kHz. Analysis of ECG and monophasic action potential (MAP) signals was performed using Spike 2 (Cambridge Electronic Design, Cambridge, UK).
In the case of the isolated SAN ischemia model, the tissues were prepared as previously described [16]. Extracellular potentials were recorded by two modified bipolar electrodes placed in the SAN and right atrium, as we previously described [16]. Electrical signals were amplified, filtered (0.5 Hz to 1 kHz band-pass filtered), digitized at a sampling frequency of 5 kHz, and stored for analysis.
2.2. Isolation and culture of rat ventricular cardiomyocytes
Neonatal rat ventricular cardiomyocytes were prepared from 2- to 3-day-old rats, as described previously by Mohamed et al. [17]. The dispersed cells were preplated for at least 30 min to minimize fibroblast contamination. Fibroblast contamination was <10%. The myocytes were cultured at 37 °C in 4:1 DMEM–M199 supplemented with 10% horse serum, 5% FCS, and 100 U/ml penicillin–streptomycin for the first 24 h. Thereafter, cells were maintained in an identical medium with a reduced serum concentration of 1% FCS for subsequent experiment.
2.3. Real-time PCR
Real-time PCR for S1P receptor was performed using cDNA generated from total RNA isolated from SAN, right atrium (RA), and left ventricle (LV) from seven rat hearts. qPCR was carried out as previously described [18]. For all transcripts and samples, at least three separate measurements were made with 1 μl aliquots of each cDNA sample. The abundance of a cDNA fragment is given relative to a housekeeping gene to correct for variations in input RNA. The abundance of housekeeping genes, namely 28S, 18S, and GAPDH, was constant in the tissues studied; 28S was chosen as the housekeeping gene for calculation, similar data were obtained using the two other housekeeping genes.
2.4. Western blot and antibodies
Tissue samples from SAN, RA, and LV were homogenized. The homogenate was filtered through two layers of cheesecloth to remove debris. Homogenates were centrifuged at 10,000×g for 5 min to generate a particulate and a cytosolic fraction. The particulate fraction was washed once with the isolation buffer. Western blot analysis was conducted using a specific antibody as described previously [17]. Various primary antibodies (Abs) (IgGs) were used for Western blot: rabbit anti-EDG01 (S1P1) against human EDG-1 (S1P1) residue 241–253 (1:100 dilutions, Cayman Chemical, Tyne & Wear, UK); rabbit anti-EDG-3 (S1P3) against human EDG-3 (S1P3) residue 12–25 (1:100 dilutions, Cayman Chemical); rabbit anti-EDG-5 (S1P2) against human EDG-5 (S1P2) 5 (EDG-5; S1P2) (1:100 dilutions, Exalpha Biologicals, Cambridge, UK); rabbit anti-PAK1 (1:1000 dilutions, Cell Signaling Technology, Hitchin, UK); rabbit anti-phospho-Pak1 (T423) (1:1000 dilutions, Cell Signaling Technology, Hitchin, UK); and rabbit anti-Akt and anti-phospho-Akt Thr308 residue (1:1000 dilutions, Cell Signaling Technology).
2.5. Chemicals
Sphingosine-1-phosphate (S1P), FTY720 (fingolimod, 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diolhydrochloride) and pertussis toxin (PTX) were purchased from Sigma-Aldrich (Dorset, UK).
2.6. Statistical analysis
All data are reported as means±S.E.M. Repeated-measure one-way ANOVA was used to compare values of measurements obtained from the same heart before and after treatment. When analysis of variance revealed the existence of a significant difference among values, Tukey’s test was applied to determine the significance of a difference between selected group means. A P value<0.05 was taken as an upper limit to indicate a significant difference.
3. Results
3.1. The effect of FTY720 on ischemia/reperfusion-induced cardiac arrhythmias in isolated heart and SAN preparations
In our first series of studies, we determined rhythm disturbances in hearts and SAN preparations perfused with normal Tyrode solution for 30 min, with the ischemic solution for 20 min, and then 30 min of reperfusion. FTY720 was added to the perfusion medium at the time of ischemia and reperfusion. With normal Tyrode solution, FTY720 (25 nM) caused a mild to moderate prolongation in RR interval by an average 4% in ex vivo hearts (control: 223±0.1 ms, FTY720: 232± 0.3 ms, n=12, P<0.05) and in cycle length (CL) by an average 8% in SAN preparations (control: 201.9±1.4 ms, FTY720: 218.6±0.7 ms, n=10, P<0.05). However, no significant alterations of P–R interval, QRS and QT duration, and arrhythmic events were observed in this condition.
Fig. 1 shows representative ECG recordings from hearts treated with 25 nM FTY720 in ischemic and reperfusion conditions. As summarized in Table 1, in the absence of FTY720, RR, P–R, QRS, and QT intervals were significantly increased during ischemia and reperfusion, but in the presence of FTY720, RR, P–R, QRS, and QT intervals were not significantly different than those during the control condition or during ischemia and reperfusion. Both tachycardia-related (ectopic beats, nonsustained, and sustained episodes of ventricular tachycardia (VT) and ventricular fibrillation (VF)) and bradycardia-related (sinus bradycardia, sinus pause, sinus arrest, and A-V conduction block) arrhythmic events were frequently observed after 5–10 min in the presence of ischemic conditions (Figs. 1A and B). As summarized in Table 1, frequent ectopic beats occurred in 9 of 14 and 12 of 14 hearts exposed to ischemic and reperfusion conditions respectively. Severe sinus bradycardia or A–V conduction block was observed in 14 of 14 hearts in ischemia and in 14 of 14 hearts in reperfusion conditions. Nonsustained and sustained VT was observed in 11 of 14 and 9 of 14 hearts in the ischemic and the reperfusion conditions respectively. In 15 hearts examined, FTY720 significantly prevented I/R injury-induced arrhythmic events. Treatment of 15 hearts with 25 nM FTY720 greatly reduced the occurrence of premature ventricular beats, VT, and sinus bradycardia as well as AV conduction block caused by I/R injury. Ectopic beats occurred in 1 of 15 hearts in ischemia and in 3 of 15 hearts in reperfusion conditions; nonsustained and sustained VT was not observed in any of the ischemia and reperfusion conditions. With FTY720 treatment, severe sinus bradycardia or A–V conduction block occurred in 3 of 15 hearts in ischemia and in 2 of 15 hearts in reperfusion conditions. Thus, FTY720 significantly prevented both bradycardiac and tachycardiac arrhythmic events induced by I/R injury in the Langendorff ex vivo heart model.
Fig. 1.
The effect of FTY720 on rhythm disturbance induced by ischemia/reperfusion in rat heart preparations. (A–C) ECG recordings from Langendorff perfused heart in the presence and absence of FTY720. (D–G) Bipolar extracellular potential recording from isolated SA node preparation in the presence and absence of FTY720. *P<0.05 for the CL comparison between the control vs. ischemia and reperfusion. ‡P<0.05 for the CL comparison between the ischemia vs. reperfusion.
Table 1.
Summary of ECG parameters and arrhythmias events in rat whole hearts during control, ischemia, and reperfusion in the absence and presence of 25 nM FTY720.
| N | RR interval (ms) | PR interval (ms) | QRS interval (ms) | QT (ms) | Ectopic beat (yes/no) | Sinus bradycardia (≥30% above baseline reduction of RR interval) (yes/no) | Sinus pause or arrest (yes/no) | A–V block (yes/no) | Nonsustained and sustained VT (yes/no) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 12 | 223±0.1 | 36.22±1.6 | 37.73±2.01 | 57.27±2.2 | 0:12 | 0:12 | 0:12 | 0:12 | 0:12 |
| 25 nM FTY720 | 12 | 232±0.3* | 38±1.6 | 35.5±1.2 | 55.7±1.7 | 2:10 | 0:12 | 0:12 | 0:12 | 0:12 |
| Ischemia | 14 | 342±5* | 57±3.5** | 56±0.8*** | 81±4.07**** | 9:5 | 14:0 | 9:5 | 11:3 | 11:3 |
| Reperfusion | 14 | 348±19* | 54±2.1** | 60±5.1*** | 92±8.55**** | 12:2 | 14:0 | 7:7 | 9:5 | 9:5 |
| Ischemia+25 nM FTY720 | 15 | 255±4* | 39±3.6 | 38±1.32 | 58±2.48 | 1:14 | 3:12 | 0:15 | 3:12 | 0:15 |
| Reperfusion+25 nM FTY720 | 15 | 261±1.6* | 41±2.5 | 36±2.12 | 54±3.42 | 3:12 | 3:12 | 0:15 | 2:13 | 0:15 |
| P value (one one-way ANOVA) | <0.05 | <0.05 | <0.05 | <0.05 |
Means±SE.
P<0.05 for the RR interval comparison between the control vs. 25 nM FTY720, ischemia, reperfusion, ischemia+25 nM FTY720 and reperfusion+25 nM FTY720.
P<0.05 for the PR interval comparison between the control vs. ischemia and reperfusion.
P<0.05 for the QRS interval comparison between the control vs. ischemia and reperfusion.
P<0.05 for the QT interval comparison between the control vs. ischemia and reperfusion.
We determined the effect of FTY720 on I/R injury-induced alteration of SAN pacemaker activity-isolated SAN preparations with the aid of a bipolar custom-made electrode for recording extracellular potentials (ECP) from the central SAN and surrounding atrial muscle. As shown in Figs. 1D–G, CL increased by 51% during ischemia and by 127% during reperfusion (control: 212.3±0.6 ms; ischemia: 320.6±2.8 ms; reperfusion: 483±5.2 ms, n=8, P<0.001). In the presence of FTY720, CL increased by 24% during ischemia and by 32% during reperfusion (control: 254±0.6 ms; ischemia: 316.6±0.3 ms; reperfusion: 336±0.4 ms, n=8, P<0.001). Thus, as in the heart studies, FTY720 attenuated both ischemia- and reperfusion-induced SAN rhythmic disturbance.
3.2. Pak1/Akt signaling as a potential intracellular pathway underlying FTY720 cardiac antiarrhythmic action
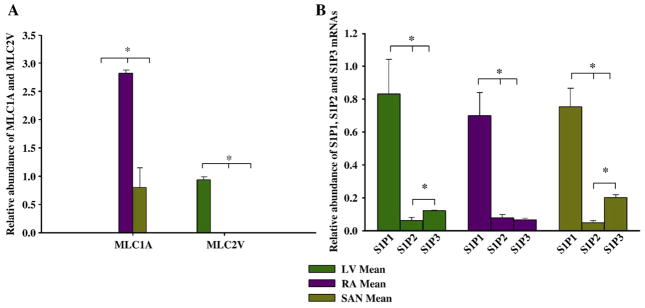
To clarify the key mechanism(s) and signaling pathway(s) for FTY720 cardioprotection under I/R injury conditions, we focused on Akt, and Pak1, a Ser/Thr kinase downstream of small G proteins, which is activated by sphingolipids [12]. We also employed real-time (RT) PCR to determine the expression of S1P1, S1P2, and S1P3 transcript pools in rat cardiac tissues [19,20]. The transcripts were detected and analysed in tissues dissected from SAN, RA, and LV (n=7 hearts). cDNA was generated from total RNA isolated from the tissues from these regions. The 28S reference gene was used for data normalization. This standardized method permits comparison of relative transcript expression level. The expression profile of each S1P receptor mRNA was compared between one compartment and the other compartments. To estimate cell populations in the SAN preparations, we used myosin light chain genes (MLC1A and MLC2V) as markers of atrial and ventricular cells. The S1P1 receptor isoform expression level was higher than S1P2 and S1P3 in these tissue types (Fig. 2), which indicates that the S1P1 receptor is the dominant isoform in rat heart tissues. As shown in Fig. 3, Western blot analysis also identified the expression of S1P1–3 receptors in rat heart tissues and myocytes.
Fig. 2.
Expression profiles of S1P receptor mRNAs in rat heart tissue. (A) Expression of regionally distributed MLCA1 and MLC2V in the atria, SAN, and ventricle tissues. (B) Expression profile of S1P receptors mRNAs in sinoatrial node tissue, in atrial tissue and in ventricular tissue. Means±SEM (n=7) shown. *P<0.05, one-way ANOVA. LV, left ventricle; RA, right atrium; SAN, sinoatrial node; MLC1A, atrial myosin light chain gene; MLC2V, ventricular myosin light chain gene.
Fig. 3.
Expression profiles of S1P receptor proteins in rat heart tissue. (A) Western blot analysis of S1P1 receptor protein in rat heart tissue. (B) Western blot analysis of S1P2 receptor protein in rat heart tissue. (C) Western blot analysis of S1P3 receptor protein in rat heart tissue. Brain (B), left ventricle (LV), right atrium (RA), and sinoatrial node (SAN), probed with anti-S1P1 (A), anti-S1P2 (B), and anti-S1P 3 (C) (purchased from Cayman Chemical, Exalpha Biologicals and Cayman Chemical, respectively). In all tissues, the antibody detected a primary band at 47 kDa.
To determine Pak1 activation, we employed Western blotting with an anti-phospho Thr 423 Ab and Akt activation with an anti-phospho-Thr 308 Ab [15] and we probed the same hearts used for arrhythmias studies (in the presence or absence of FTY720) for phosphorylation of Pak1/Akt. Compared to baseline levels under the control condition, there was a significant depression in the levels of phospho-Pak1 and phospho-Akt decreased by 62% and 64% in ischemic conditions and by 73% and 63.5% in reperfusion conditions (Fig. 4). However, in the presence of FTY720, phospho-Pak1 and Akt levels decreased by only 22% and 24% in ischemic conditions and by 30% and 29% in reperfusion conditions, respectively. In the presence of S1P, phospho-Pak1 and Akt levels increased by 10% and decreased only by 26%, respectively, in ischemic conditions and decreased by only 13% and 28.8%, respectively, in reperfusion conditions, compared to baseline level under the control condition.
Fig. 4.
Phospho-Pak1/Pak1 (A) and phospho-Akt/Akt (B) response to ischemia and follow-up reperfusion in the absence and presence of FTY720/S1P. Ex vivo hearts were either equilibrated for 20 min (lane 1), equilibrated followed by 20 min of ischemia (lane 2), equilibrated followed by 20 min of ischemia followed by 30 min of reperfusion (lane 3), equilibrated followed by 20 min of ischemia in the presence of 25 nM FTY720/S1P (lane 4), equilibrated followed by 20 min of ischemia followed by 30 min of reperfusion in the presence of 25 nM FTY720/S1P (lane 5). Hearts were collected, homogenized, and separated into cytosolic fraction and particulate fractions. (A) Western blot analysis for the cytosolic fraction using an antibody to phospho-Pak1 (Thr 423) and Pak1. (B) Western blot analysis for the cytosolic fraction using an antibody to phosphor-Akt (Thr 308). *P<0.05 for the phospho-Pak1 and phospho-Akt/Akt response comparison between the control vs. ischemia, reperfusion, ischemia+25 nM FTY720/S1P, and reperfusion+25 nM FTY720/S1P. ‡P<0.05 for the phospho-Pak1 and phospho-Akt/Akt response comparison between the ischemia vs. ischemia+25 nM FTY720/S1P. §P<0.05 for the phospho-Pak1 and phospho-Akt/Akt response comparison between the reperfusion vs. reperfusion+25 nM FTY720/S1P.
We addressed the important questions of whether FTY720-mediated Pak1 and Akt activation was through Gi by treating neonatal rat cardiac myocytes with 100 ng/ml PTX overnight and then stimulating with 25 nM FTY720 for 5 min. FTY720 induced a 1.6-fold increase in Pak1 phosphorylation, and a 1.45-fold increase in Akt phosphorylation relative to vehicle (Fig. 5). After PTX treatment, FTY720-mediated activation of Pak1 was reduced by 87%, and activation of Akt was reduced by 53%. These data demonstrate that a significant component of FTY720-mediated Akt and Pak1 activation in cardiomyocytes occurs through a Gi-coupled S1P receptor.
Fig. 5.
PTX sensitivity of FTY720-mediated activation of Pak1 and Akt in neonatal cardiac myocytes. Neonatal myocytes were stimulated with 25 nM FTY720 for 5 min and then assayed for phosphorylation of Pak1 and Akt by Western blotting. Representative blots are shown. Phosphorylation was quantitated by densitometry and normalized. Values are mean±S.E. (n=3 for each group). *P<0.01 versus control.
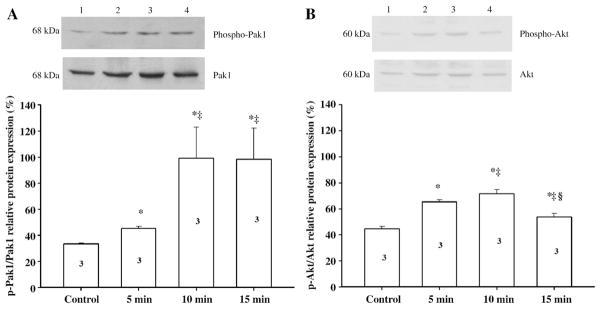
Fig. 6 illustrates the time course of activation of phospho-Pak1 and phospho-Akt in isolated nontreated adult cardiac myocytes (control) and myocytes treated with 25 nM FTY720. There was a significant increase in phospho-Pak1 levels by 35%, 199%, and 205% after 5, 10, and 15 min of treatment with 25 nM FTY720 compared with control nontreated myocytes. As detected using an antibody probing total Pak1, there was no significant difference in the levels Pak1 expression between nontreated and treated myocytes with FTY720. Consistent with the response to FTY720, we observed an increase in phospho-Pak1 level by 15% after treatment with 25 nM S1P compared with control cells.
Fig. 6.
Time-dependent activation of phospho-Pak1 and phospho-Akt in isolated adult rat ventricular myocytes treated with FTY720. Adult cardiac myocytes were exposed for 5 min, 10 min and 15 min. Subsequently, PAK1 and phospho-PAK1 (Thr 423) proteins were quantified by Western blot analysis in these cells. Results shown are representative of 3 independent experiments. In the corresponding bar graph, PAK1 and phospho-PAK1 (Thr 423) proteins expression are indicated as percentage of expression. *P<0.05 for the activation of phospho-Pak1 and phospho-Akt comparison between the control vs. 5-, 10-, and 15-min exposure of 25 nM FTY720. ‡P<0.05 for the activation of phospho-Pak1 and phospho-Akt comparison between the 5-min exposure of 25 nM FTY720 vs. 10- and 15-min exposure of 25 nM FTY720. §P<0.05 for the activation of phospho-Pak1 and phospho-Akt comparison between the 10-min exposure of 25 nM FTY720 vs. 15-min exposure of 25 nM FTY720.
We also compared the time course phospho-Akt levels in untreated control myocytes and in myocytes treated with 25 nM FTY720 (Fig. 6B). Compared to controls, phospho-Akt levels were increased by 44%, 63%, and 61% after 5, 10, and 15 min of treatment with 25 nM FTY720, respectively.
4. Discussion
4.1. The effect of FTY720 on ischemia/reperfusion injury and its associated arrhythmias
Our data demonstrate the important and novel finding that FTY720, an S1P analogue, effectively antagonizes both bradyarrhythmias and tachyarrhythmias induced by I/R injury, the treatment of which remains a major challenge. Our data also determined that FTY720 acts through Pak1/Akt signaling, which identifies this cascade as an important element in this cardiac protection effect. Our data also significantly extend earlier reports providing evidence for a role of S1P signaling in protection against I/R injury and its potential role in preconditioning and postconditioning [7,21]. Several studies [5–7,20] have provided evidence for a role of S1P signaling in protection against the stress of I/R injury, in particular its critical role in preconditioning and postconditioning mechanisms of rescue of hearts from I/R injury [7,21]. It is highly relevant that FTY720 is currently under evaluation in a long-term phase III clinical trial for the treatment of autoimmune disease and in organ transplantation [11,22]. FTY720 was found to be effective in prolonging allograft survival in preclinical models of cardiac, renal, and hepatic transplantation. Moreover, FTY720 has been shown to attenuate small-for-size liver graft injury by activation of cell survival Akt signaling and down-regulation of the MAPK pathway [23].
4.2. The action of S1P and FTY720 on S1P receptors
A key question is how S1P and FTY720 interact with S1P receptor (s). S1P stimulates all S1P receptors (S1P1–3) in the heart resulting in activation of (Gi, Gq, and G12/3). In contrast, FTY720, a prodrug that is phosphorylated in vivo by sphingosine kinase (SphK2) [21] to the biologically active FTY720-phosphate (FTY720-P), only activates the S1P1 and SIP3 receptors resulting in activation of Gi. There is also evidence that FTY720 may induce depletion of the S1P1 receptor [24] based on the finding from S1P1 receptor knockout mice that showed the same phenotype as FTY720-treated mice. Mullershausen et al. [25] reported that although FTY720 and S1P both led to a marked internalization of S1P1 receptors within 60 min of treatment, the effect was considerably more pronounced upon treatment with FTY720. This mechanism of attenuation of the FTY720 effect compared to its natural analogue S1P may not be prominent in our studies inasmuch as the kinetics of Pak1/Akt phosphorylation were very rapid (within minutes). We cannot exclude that the effect we saw with FTY720 in cardiomyocytes is due to rapid phosphorylation to phospho-FTY720 by sphingosine kinase. However, since phosphorylation of Pak1/Akt occurred within minutes, it appears possible that FTY720 may directly activate S1P1 receptor or intracellular targets.
An important issue in the interpretation of our data is the potential role of effects of FTY 720 on S1P receptors in fibroblasts, a predominant cell type in the heart. However, similar to our findings with perfused hearts, we demonstrated activation of signaling through the Pak1 and Akt cascade by FTY720 in cultured neonatal rat cardiac myocytes with fibroblasts removed. Moreover, this activation was significantly blunted by the treatment of PTX (Fig. 5).
4.3. Pak1/AKT signaling as a potential intracellular pathway underlying FTY720 cardiac protection effect
Our data are the first to demonstrate a down-regulation of phospho-Pak1 during I/R model. Moreover, we found a strong correlation of the activity of Pak1 and Akt with the incidence of I/R-induced arrhythmias with and without FTY 720. The detailed mechanisms underlying the cardioprotective effect of Pak1/Akt activation on I/R-induced arrhythmias is likely to be complex and may involve primary effects on ion channels/transporters and secondary effect to protect cardiac myocytes from hypoxia-induced stress and cell death. Earlier studies also indicated that sphingosine and several related long-chain sphingoid bases can directly activate Pak1 in vitro at a higher concentration than the FTY720 we used in this study [12,26]. Whether FTY720, which is structurally similar to S1P, can directly activate Pak1 in cardiac cells without prior conversion to FTY720 phosphate remains unclear. Recent studies also indicate that FTY720 activates PP2A and induces dephosphorylation of Erk-1/2 in immunocells, which also provides a mechanistic insight into understanding the cardiac effects of FTY720 demonstrated in this study [27].
Our hypothesis is that the upstream molecular mechanism by which FTY720 elicits cardioprotection involves signaling through the inhibitory G protein, Gi. Several lines of evidence support this hypothesis. Earlier studies indicated that FTY720 is converted to FTY720-phosphate in mammalian cells [28] and FTY720-phosphate binds to S1P receptors, which are coupled to Gi [28–30]. Moreover, these studies indicate that Pak1 and Akt are regulated by the large G proteins, sensitive to pertussis toxin. There is also evidence that response to some extracellular factors showing cardioprotective or preconditioning effects are abolished when the inhibitory G proteins are blocked by pertussis toxin treatment in the heart [31]. In cardiac cells, S1P stimulates the activity of acetylcholine-regulated potassium channel (KACh) and inhibits cardiac pacemaker activity [32]. Further evidence indicating that FTY720 functions through Gi-mediated signaling pathways in cardiac cells comes from studies on the phosphorylation of cardiac troponin I (cTnI). β-Adrenergic-induced phosphorylation of cTnI at PKA sites is reversed by Pak1 activation through up-regulation of PP2A phosphatase activity [13]. Dephosphorylation of cTnI is also responsive to pertussis toxin [33] and FTY720 is able to induce dephosphorylation of cTnI in ventricular myocytes (R.J. Solaro et al., unpublished observations).
Akt is a well-established regulator of myocardial growth and survival, contractile function, and coronary angiogenesis [34]. Studies showed that Pak1 is able to activate Akt [35], whereas Akt can phosphorylate Pak1 [36]. These data indicate a complex relationship between Pak1 and Akt. Mao et al. [37] recently showed that both Pak1 and Akt can be activated by multiple hypertrophic stimuli and growth factors in a PI3K-depenedent manner, suggesting that Pak1 and Akt may lie in the same signaling pathway in cardiomyocytes. Indeed, using both gain- and loss-of-function approaches in vitro and in vivo, the authors demonstrated that Pak1 is sufficient to activate Akt and is essential for growth factor-induced Akt activity in cardiomyocytes [37]. The functional significance of Pak1-Akt signaling is underscored by the observation that the prosurvival effect of Pak1 is diminished by Akt inhibition [34]. The Pak1-confered protection was blocked by the Akt inhibitor X, suggesting that the protective effect of Pak1 is mediated, at least in part, by Akt signaling [37]. These findings demonstrate an important role for Pak1–Akt signaling in cardiomyocyte survival.
Although the precise mechanisms underlying the activation of Pak1/Akt signaling by FTY720 in preventing arrhythmias induced by I/R injury require further investigation, our results suggest that activation of Pak1 and Akt signaling pathways play a role in the FTY720/S1P-mediated cardioprotection mechanism in the rat heart.
In conclusion, we have provided the first evidence that FTY720 prevents arrhythmias induced by I/R injury and can be a potentially important and novel agent protecting against I/R injury and its associated arrhythmias. We also determined, for the first time, that the cardioprotective effect of FTY720 is likely to involve activation of signaling through the Pak1.
Acknowledgments
We thank Dr. James Tellez and Dr. Mohamed Shaheen for their support. The project was supported by The Wellcome Trust (M.L.), The British Heart Foundation (M.L. and E.J.C.) and National Institute of Health grants RO1 HL 64035 and PO1 HL 62426 (Project 1) (R.J.S.).
References
- 1.Corr PB, Witkowski FX. Potential electrophysiologic mechanisms responsible for dysrhythmias associated with reperfusion of ischemic myocardium. Circulation. 1983;68:I16–24. [PubMed] [Google Scholar]
- 2.Tzivoni D, Keren A, Granot H, Gottlieb S, Benhorin J, Stern S. Ventricular fibrillation caused by myocardial reperfusion in Prinzmetal’s angina. Am Heart J. 1983;105:323–5. doi: 10.1016/0002-8703(83)90534-3. [DOI] [PubMed] [Google Scholar]
- 3.Robinson LA, Braimbridge MV, Hearse DJ. Creatine phosphate: an additive myocardial protective and antiarrhythmic agent in cardioplegia. J Thorac Cardiovasc Surg. 1984;87:190–200. [PubMed] [Google Scholar]
- 4.Goldberg S, Greenspon AJ, Urban PL, Muza B, Berger B, Walinsky P, et al. Reperfusion arrhythmia: a marker of restoration of antegrade flow during intracoronary thrombolysis for acute myocardial infarction. Am Heart J. 1983;105:26–32. doi: 10.1016/0002-8703(83)90274-0. [DOI] [PubMed] [Google Scholar]
- 5.Jin ZQ, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Vessey DA, Li L, Kelley M, Zhang J, Karliner JS. Sphingosine can pre- and postcondition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Mol Toxicol. 2008;22:113–8. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 7.Peters SL, Alewijnse AE. Sphingosine-1-phosphate signaling in the cardiovascular system. Curr Opin Pharmacol. 2007;7:186–92. doi: 10.1016/j.coph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Stoffel W, Assmann G, Binczek E. Metabolism of sphingosine bases: 13. Enzymatic synthesis of 1-phosphate esters of 4t-sphingenine (sphingosine), sphinganine (dihydrosphingosine), 4-hydroxysphinganine (phytosphingosine) and 3-dehydrosphinganine by erythrocytes. Hoppe-Seylers Z Physiol Chem. 1970;351:635–42. doi: 10.1515/bchm2.1970.351.1.635. [DOI] [PubMed] [Google Scholar]
- 9.Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, et al. Fungal metabolites: Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo) 1994;47:208–15. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 10.Kaudel CP, Frink M, van GM, Schmiddem U, Probst C, Bergmann S, et al. FTY720 application following isolated warm liver ischemia improves long-term survival and organ protection in a mouse model. Transplant Proc. 2007;39:493–8. doi: 10.1016/j.transproceed.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Budde K, Schutz M, Glander P, Peters H, Waiser J, Liefeldt L, et al. FTY720 (fingolimod) in renal transplantation. Clin Transplant. 2006;20:17–24. doi: 10.1111/j.1399-0012.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 12.Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, et al. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998;273:8137–44. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- 13.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004;94:194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 14.Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, et al. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res. 2007;100:1317–27. doi: 10.1161/01.RES.0000266742.51389.a4. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–64. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M. Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovasc Res. 2007;73:729–38. doi: 10.1016/j.cardiores.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed TMA, Oceandy D, Prehar S, Alatwi N, Hegab Z, Baudoin FM, et al. Specific role of neuronal nitric oxide synthase when tethered to the plasma membrane calcium pump in regulating the beta-adrenergic signal in the myocardium. J Biol Chem. 2008;284:12091–8. doi: 10.1074/jbc.M809112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tellez JO, Dobrzynski H, Greener ID, Graham GM, Laing E, Honjo H, et al. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ Res. 2006;99:1384–93. doi: 10.1161/01.RES.0000251717.98379.69. [DOI] [PubMed] [Google Scholar]
- 19.Alewijnse AE, Peters SL, Michel MC. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br J Pharmacol. 2004;143:666–84. doi: 10.1038/sj.bjp.0705934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alewijnse AE, Peters SL. Sphingolipid signalling in the cardiovascular system: good, bad or both? Eur J Pharmacol. 2008;585:292–302. doi: 10.1016/j.ejphar.2008.02.089. [DOI] [PubMed] [Google Scholar]
- 21.Karliner JS. Sphingosine kinase regulation and cardioprotection. Cardiovasc Res. 2009;82:184–92. doi: 10.1093/cvr/cvn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvadori M, Budde K, Charpentier B, Klempnauer J, Nashan B, Pallardo LM, et al. FTY720 versus MMF with cyclosporine in de novo renal transplantation: a 1-year, randomized controlled trial in Europe and Australasia. Am J Transplant. 2006;6:2912–21. doi: 10.1111/j.1600-6143.2006.01552.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Man K, Lo CM, Ng KT, Li XL, Sun CK, et al. Attenuation of small-for-size liver graft injury by FTY720: significance of cell-survival Akt signaling pathway. Am J Transplant. 2004;4:1399–407. doi: 10.1111/j.1600-6143.2004.00527.x. [DOI] [PubMed] [Google Scholar]
- 24.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 25.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–34. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 26.Ke Y, Solaro RJ. Use of a decoy peptide to purify p21 activated kinase-1 in cardiac muscle and identification of ceramide related activation. Biologics: Target Therapy. 2008;2:903–9. doi: 10.2147/btt.s3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–84. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 29.Goodemote KA, Mattie ME, Berger A, Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995;270:10272–7. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- 30.van KC, Meyer zu HM, Laser KT, Zhang C, Jakobs KH, Bunemann M, et al. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996;271:2082–7. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- 31.Schultz JE, Hsu AK, Barbieri JT, Li PL, Gross GJ. Pertussis toxin abolishes the cardioprotective effect of ischemic preconditioning in intact rat heart. Am J Physiol. 1998;275:H495–500. doi: 10.1152/ajpheart.1998.275.2.H495. [DOI] [PubMed] [Google Scholar]
- 32.Guo J, MacDonell KL, Giles WR. Effects of sphingosine 1-phosphate on pacemaker activity in rabbit sinoatrial node cells. Pfiugers Arch. 1999;438:642–8. doi: 10.1007/s004249900067. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Hofmann PA. Modulation of protein phosphatase 2a by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol. 2003;285:H97–103. doi: 10.1152/ajpheart.00956.2002. [DOI] [PubMed] [Google Scholar]
- 34.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–65. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 35.Gorlach A, BelAiba RS, Hess J, Kietzmann T. Thrombin activates the p21-activated kinase in pulmonary artery smooth muscle cells. Role in tissue factor expression. Thromb Haemost. 2005;93:1168–75. doi: 10.1160/TH05-01-0006. [DOI] [PubMed] [Google Scholar]
- 36.Bokoch GM. Biology of thep21-activated kinases. Annu Rev Biochem. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 37.Mao K, Kobayashi S, Jaffer ZM, Huang Y, Volden P, Chernoff J, et al. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J Mol Cell Cardiol. 2008;44:429–34. doi: 10.1016/j.yjmcc.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]