Abstract
The aim of this study was to assess whether patients with neurodegenerative disease, namely Alzheimer’s disease (AD) and Parkinson’s disease (PD), differed from age-matched, neurologically normal comparison participants in their ability to detect impending collisions. Six AD patients and 8 PD patients, together comprising the neurodegenerative disease group, and 18 comparison participants completed a collision detection simulation task where they must judge whether approaching objects would collide with them or pass by them. The neurodegenerative disease group was less sensitive in detecting collisions than the comparison group, and sensitivity worsened with increasing number of objects in the display and increasing time to contact of those objects. Poor performance on tests of cognition and visual attention were associated with poor collision detection sensitivity. The results of this study indicate that neurodegenerative disease impairs the ability to accurately detect impending collisions and that these decrements are likely the combined result of visual and cognitive disturbances related to disease status.
Keywords: collision detection, vision, driving, Alzheimer’s disease, Parkinson’s disease, cognitive impairment
1. Introduction
Considerable evidence indicates that crash risk increases with age and the presence of neurological disease (Harris, 1999; Lundberg et al., 1997; McKnight and McKnight, 1999; Rizzo et al., 1997, 2001; Uc et al., 2004, 2006, 2007). Individuals with neurological diseases are at a greater risk for both on-road and simulated crashes (Dubinsky et al., 2000; Rizzo et al., 1997, 2001; Uc et al., 2004, 2006). One important aspect of crash risk is the ability to detect impending collisions. Successful detection of these events must occur before other actions can be executed, such as collision avoidance (Andersen et al., 1999, 2000; DeLucia et al., 2003). This study examined collision detection abilities of Alzheimer’s disease (AD) and Parkinson’s disease (PD) patients compared to neurologically normal older adults.
Driving is a complex task that involves cognitive, visual, and motor processes that may be impaired by aging and neurological disease. AD, the most common form of dementia worldwide (Alzheimer’s Association, 2008), is a neurodegenerative disease characterized by progressive memory loss and accompanied by varying levels of impairment in vision, attention, executive function, and language (Jackson and Owsley, 2003; Rizzo et al., 2000; Silverman et al., 1994; Rizzo and Nawrot, 1998, O’Brien et al., 2001). PD is a less common, disabling progressive neurodegenerative disorder whose prevalence increases with aging (Lang et al., 1998). PD produces hallmark motor dysfunction, together with variable impairments of cognition, vision, sleep, autonomic function, and behavior (Lang et al, 1998; Uc et al., 2005).
Research has indicated that patients with AD and PD commit more driving errors, perform worse on various driving tasks, and are at greater risk for safety errors in standardized experimental tests of driving performance on the road and in driving simulators, when compared to neurologically normal older adults (Dubinsky et al., 2000; Lundberg et al., 1997; Rizzo et al., 1997, 2001, 2004, 2005; Uc et al., 2004, 2006, 2007). Drivers with AD were more likely than comparison drivers to crash or demonstrate risky avoidance behavior when approaching a potential crash scenario in a driving simulator (Rizzo et al., 2001; Uc et al., 2006). Drivers with cognitive and visual deficits due to PD were more likely to make incorrect turns, get lost, and commit at-fault safety errors than comparison drivers on a route following task in an instrumented vehicle (Uc et al., 2007).
Aging and neurological disease are associated with declines in several aspects of vision that may affect the ability to accurately detect collisions, such as the integration of visual cues, ability to perceive motion, and judgment of distances. Advancing age has been associated with impairments in several types of motion perception (Andersen and Atchley, 1995; Atchley and Andersen, 1998; Gilmore et al., 1992; Norman et al., 2003, 2004). The ability to accurately judge the time to contact (TTC) of an approaching object also decreases with age (DeLucia et al., 2003; Schiff et al., 1992). AD is reported to impair static spatial contrast sensitivity, visual attention, shape-from-motion, color, visuospatial construction and visual memory, in association with cognitive decline (Rizzo et al, 2000). Motion processing deficits in AD include problems with conscious motion perception (while subconscious motion detection remains intact) (Silverman et al., 1994), structure from motion (Rizzo and Nawrot, 1998), and optic flow (O’Brien et al., 2001). Similarly, PD is associated with deficits in visual attention, contrast sensitivity, motion perception, color vision, and cognition, in association with loss of dopaminergic neurons (Jackson and Owsley, 2003; Uc et al., 2005).
Decrements in the ability to detect collisions could occur at many levels, from basic vision to higher-order visual and cognitive abilities. Previous research has determined the visual information used by drivers to detect collisions during deceleration (Andersen et al., 1999) and has shown how visual information is used to regulate speed during braking to avoid a collision (Andersen and Sauer, 2004). Studies have also shown the visual information used to detect collisions at constant speeds and demonstrated the role of attention in detecting collisions when multiple moving objects are present (Andersen and Kim, 2001). In addition, studies have shown age related decrements in the use of visual information for detecting collisions during deceleration (Andersen et al., 2000) and at constant speeds (Andersen and Enriquez, 2006). Yet, this ability has been largely unexplored in clinical populations.
The present study sought to explore the difference in collision detection abilities between a group of individuals with neurodegenerative disease, specifically AD and PD, and a group of neurologically normal older adults. To measure collision detection sensitivity in neurodegenerative disease, this study employed a task developed by Andersen and Enriquez (2006) to study older adults. In this task participants were presented with driving simulation displays that depicted a roadway scene with the vehicle travelling at a fixed speed and objects that were approaching the driver on linear trajectories. On some trials the objects were on trajectories that would pass by the participant (a non-collision event). On other trials one of the objects was on a trajectory that would collide with the participant (a collision event). The participant’s task was to identify at the end of each trial whether any object was on a collision path with the observer. The total time before the object would either collide with the observer or pass by the participant was 9 sec. During the experiment participants were shown a limited presentation of the motion path. Detection performance was measured using the sensitivity measure d′.
Given that the ability to detect impending collisions seems to decline with age, and the heightened severity of visual and cognitive disturbances associated with neurodegenerative disease, researchers predicted that the AD and PD patients would be worse at detecting collisions than comparison participants. The experiment examined two hypotheses regarding possible performance differences between the clinical and comparison groups. The first hypothesis concerns the time needed to detect a collision. On each trial participants were shown either 6 seconds or 8 seconds of the motion path. The duration of the motion path was greater than that used by Andersen and Enriquez (2006) because of concerns that the participants in the clinical group may have had greater difficulty in detecting collision events at shorter durations. We refer to these conditions as the 3 sec and 1 sec time to contact (TTC) conditions (time on the last frame before the object would collide or pass by the participant). With greater difficulty in detecting an impending collision, the neurodegenerative group should require more time to detect collision events. Demonstration of an interaction between participant group and TTC of the display would support the hypothesized of decline.
The second hypothesis concerns the role of attention in detecting collision events. In the task of Andersen and Enriquez (2006) observers viewed a single moving object and had to judge whether or not the object would collide with them. In this study we examined conditions in which several moving objects were present in the driving scene. Andersen and Kim (2001) reported that sensitivity of collision detection declines and the time needed to for collision detection increases, with increasing numbers of moving objects in a driving scene. These findings indicate that attention is required to detect a collision in a scene with multiple moving objects, with participants needing to serially scan the driving scene to detect a collision object. If the clinical group, as compared to neurologically normal older adults, has greater difficulty in scanning and processing individual objects in a field of moving objects then we expect poorer performance for the clinical group as the number of objects is increased. To examine this hypothesis we presented participants with displays that contained either a single object or 6 objects.
Lastly, research has indicated that tests of cognitive ability, possibly more so than measures of vision, can be good predictors of driving performance (Amick et al., 2007; Rizzo et al., 2001, 2004; Uc et al., 2006, 2007). Therefore, the present study also explored possible relationships between cognitive abilities, as measured by a battery of neuropsychological tasks, collision detection sensitivity, and vision. Given that the collision detection task requires both basic and higher-order perceptual processing, researchers expected to find relationships between cognition, vision, and collision detection performance.
2. Methods
2.1 Participants
The neurodegenerative disease group comprised patients with cognitive impairment due PD and AD. This group had 27% women, with a mean age of 68.5, and included 8 PD patients (mean age = 61.86) and 6 AD patients (mean age = 77.5). The comparison group consisted of 18 age-matched, neurologically normal drivers (mean age = 69.67, 38% women). The two groups did not differ significantly in age, near vision, or far vision (p > .05). However, the neurodegenerative disease group had worse contrast sensitivity than the comparison group (p = .002). Due to the high contrast of both color and shade in the display scenes and the minimal distance that the observer sat from the stimuli, the experimenters did not find it necessary to control for differing contrast sensitivity. In addition, deficits in contrast sensitivity are a symptom of both PD and AD and to control for this is to diminish some of the effects that disease status has on participants ability in the collision detection task.
Participants with neurologic disorders other than AD and PD were excluded. No participant had acute, confounding medical or psychiatric conditions. Participants with AD were recruited from a registry in the Department of Neurology. The diagnosis of probable AD relied on the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984). Accordingly, all AD patients had symptoms of memory impairment and related cognitive complaints that interfered with their social or occupational life. Participants with PD were recruited from the Movement Disorders Clinics at the Department of Neurology, University of Iowa and Veterans Affairs Medical Center, Iowa City, Iowa, and met diagnostic criteria for PD (Gelb et al., 1999). All participants were community dwelling, independently living and licensed active drivers. The PD participants were not demented and ambulated independently. Comparison participants were recruited from the Johnson County community.
The neurodegenerative disease group had a mean Mini-Mental State Examination (MMSE) score 26.79 (24.5 for AD, 28.5 for PD), compared to 28.78 for the group of normal older adults. The disease group did not differ significantly from the comparison group on the MMSE measure, indicative of their mild to moderate disease status. However, performance on a battery of standardized neuropsychological tests suggested early cognitive decline in the AD/PD group (Salmon et al., 2002).
Results from a self-report measure of driving behavior indicated that, as expected, the neurodegenerative disease group drove less often and for fewer miles than comparison participants. (Table 1) The neurodegenerative disease group was also slightly more likely to have been in a car crash and to have been pulled over by police within the last two years. However, these differences were not significant. Independent analyses of both the AD and PD groups revealed that AD participants drove less often and were involved in fewer accidents than comparison group of older adults, while PD participants drove more often and were involved in more crashes than the comparison group. Yet, self-report of driving behavior is often inaccurate and can be misleading, particularly when the reporter is cognitively impaired. Also, on-road incidents (car crashes and traffic citations) were so infrequent, that they do not provide a reliable basis for comparing actual on-road performance of these drivers.
Table 1.
Mean values for several self-reported driving behaviors from the Driving Habits Questionnaire (DHQ; Sloane et al., 1990).
| AD (n = 6) |
PD (n = 8) |
Neurodegenerative Disease Group (AD+PD) (n = 14) |
Comparison Group (n = 18) |
|
|---|---|---|---|---|
| Number of days driven per week | 4.33 | 6.75 | 5.71 | 6.11 |
| Number of miles driven per week | 41.67* | 178.13 | 119.64 | 145 |
| Number of accidents in past 2 years | 0.0 | .25 | .15 | .11 |
| Number of times pulled over in last 2 years | 0.0 | .38 | .23 | .17 |
Denotes the only significant difference between comparison and disease groups.
2.2 Stimuli
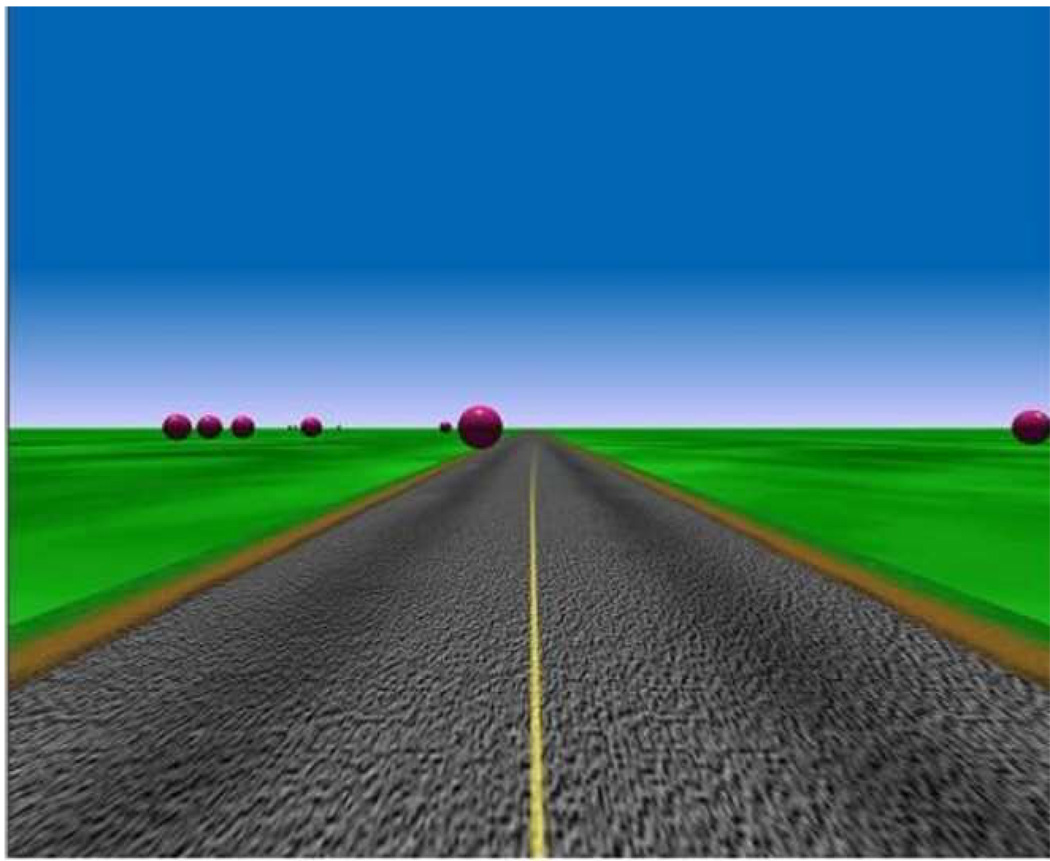
The stimuli used in this experiment were textured 3-D roadway scenes presented on a 39.8 by 32 deg visual angle display. The task was administered on a desktop computer with observers seated in front of a computer screen (viewing distance of 61cm). The scenes simulated a roadway with objects (bright red spheres of 2.0-unit diameter, with a unit representing 1 eyeheight in the scene or 1.7 m) translating at a constant speed on linear trajectories toward the observer. (Figure 1) The display simulated both driver vehicle motion and motion of the objects towards the driver. The speed of the driver's vehicle was 43.2 kmph. The speed of the approaching objects was also 43.2 kmph. The total dimension of the simulated space was 2000 by 1000 units. The roadway was 4 units horizontally and extended the entire length of the simulated space. A solid double-yellow line was projected down the length of the roadway. An irregular green texture pattern extended in all directions and surrounded the roadway. The projection point of the scene was 1.0 unit above the ground plane. Each object in the scene was shaded (using a Gouraud shading model) to enhance the spherical shape. The maximum luminance of the sphere was 28.7 cd/m2. The stimuli were high-contrast displays, with a Michelson contrast (for the object relative to the darkest region of the surrounding scene) of 0.88. The display resolution was 1280 × 1024. The refresh rate of the display was 30 Hz. Consistent with perspective, the size of the object varied as a function of distance.
Figure 1.
Static image of the driving scene used in the experiment.
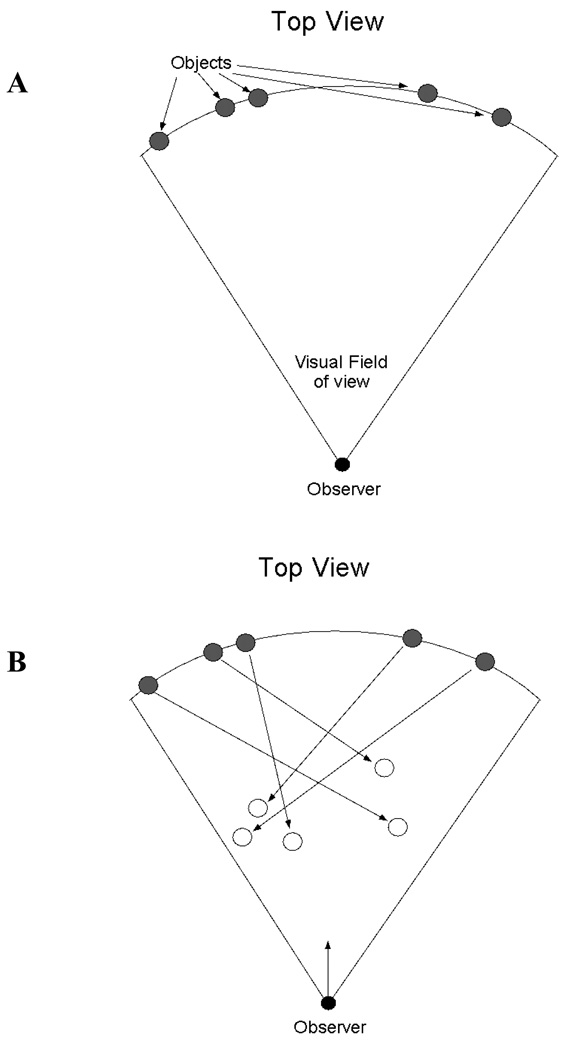
Collision and non-collision objects were located along an arc of fixed radial distance from the observer. (Figure 2) The motion of the objects was on a linear path towards the viewpoint of the driver. The trajectories of the translating objects were determined in the following manner. For non-collision objects an initial random trajectory was assigned that resulted in motion towards the simulated viewpoint. Next, the final position of each object, given the speed and trajectory of the object and of the driver’s vehicle, was derived. If the final position of the object projected outside the field of view, a new random trajectory was selected. This constraint was used to ensure that all objects remained in the field of view on each trial. A second constraint for non-collision objects was that the final position must project outside a horizontal region of 4 object widths---a value used in previous research on collision detection (Andersen and Kim, 2001).
Figure 2.
Schematic illustration of the aerial display geometry for collision events. All objects were positioned at a constant radial distance from the observer at the beginning of the trial (Figure 2A). During the trial the objects moved toward the viewpoint of the observer, each on its own linear path (Figure 2B). For collision trials one of the objects approached on a linear path that would eventually collide with the observer. The number of objects in the scene was either a single object or 6 objects.
Collision objects (targets) were defined in a similar manner to non-collision objects. Collision objects were assigned an initial random position along the fixed radial-distance arc. A trajectory was derived for the collision object such that it would intersect the simulated viewpoint given the speed of the object and driver’s vehicle.
The displays differed on two independent variables: number of objects and time to contact (TTC). On half of the trials a single red sphere approached the observer, while on the remaining trials 6 red spheres approached the observer. TTC was manipulated in the following manner. At the beginning of each trial all objects were located at an initial distance from the driver such that the TTC (given the speed of the driver’s vehicle and approaching objects) was 9 sec. For the 3 sec TTC condition the display depicted 6 sec of motion whereas for the 1 sec TTC the display depicted 8 sec of motion.
2.3 Cognitive and Visual Tests
In addition to completing the Collision Detection task, participants completed a battery of neuropsychological and vision tests. Judgment of Line Orientation (JLO) assessed visuospatial perception. Visuoconstructional ability was measured using the Rey-Osterreith Complex Figure Test Copy version (CFT-COPY) and Block Design subtest (BLOCKS) from the WAIS-R. The CFT-RECALL version and the Benton Visual Retention Test (BVRT) were used to test visual memory. The Rey Auditory Verbal Learning Test (AVLT) provided an index of anterograde verbal memory. The Trail Making Test, Subtest B time, was used as an index of set-shifting (an executive function). Controlled Oral Word Association (COWA) also tested executive functioning (maintaining task). These tasks are described in detail in Lezak, 1995. We calculated a composite measure of cognitive impairment (COGSTAT) by summing T scores (mean = 50, SD = 10) derived from raw (uncorrected) data from each of the eight tests from the neuropsychological assessment battery, as in our previous work (e.g., Rizzo et al., 2001; Uc et al., 2004).
The Useful Field of View (UFOV) task (Visual Attention Analyzer Model 3000, Visual Awareness Inc.), depends on speed of visual processing, divided attention, and selective attention, and UFOV scores are reported to correlate with crashes and performance impairments in drivers with older and cognitively impaired drivers (Owsley et al., 1991; Ball et al., 1993; Rizzo et al., 2001; Uc et al., 2006, 2007),. We used the sum of the subtests 1–4 (in msec) of the UFOV task (UFOVTOT) in our analyses, as well as conducting analyses on specific subtests. Contrast sensitivity was assessed using the standard Pelli-Robson chart (1988). The best corrected visual acuity was measured using the ETDRS chart (Ferris et al., 1982) for far visual acuity (FVA) and the reduced Snellen chart for near visual acuity (NVA). Both measures of acuity were expressed as LogMAR scores (logarithm of the minimum angle of resolution), with 0 representing 20/20 vision.
2.4 Procedure
Participants were told that they would be presented with roadway scenes where one or more spheres would be moving towards them. In each of these scenes, one of the spheres would either be on a collision path with the observer or all of the spheres would be on paths passing to the right or the left of the observer. The participants’ job was to judge whether or not any of the spheres would collide with them. Participants were then shown examples of collision and non-collision events. They were shown the entire collision or non-collision event during the practice scenes. For collision events the object filled the entire screen. For non-collision events the object moved off the screen beyond the simulated field of view.
After observers understood the two types of events, they were presented with eight practice trials and were asked to indicate whether a collision would have occurred in each display. These trials used a 0.5 sec TTC and half of the trials simulated a collision. A 0.5 sec TTC was used because it allowed for the approaching object to increase in size without filling the entire display (as would occur if the TTC was 0.0 sec). It was necessary for observers to correctly identify seven of the eight practice trials before proceeding to the experimental trials. This was done to ensure that the participants understood the task and response options. Comprehension of the task was also continually monitored by a trained experimenter. Observers completed two separate blocks of the task. Each block displayed scenes with approaching objects that looked as though they may or may not collide with the observer. Participants were asked to indicate whether or not a collision would have occurred on each trial. Observers were presented with 20 replications (10 collision and 10 non-collision events) for each combination of number of objects and TTC for a total of 80 trials.
3. Results
3.1 Collision Detection Performance
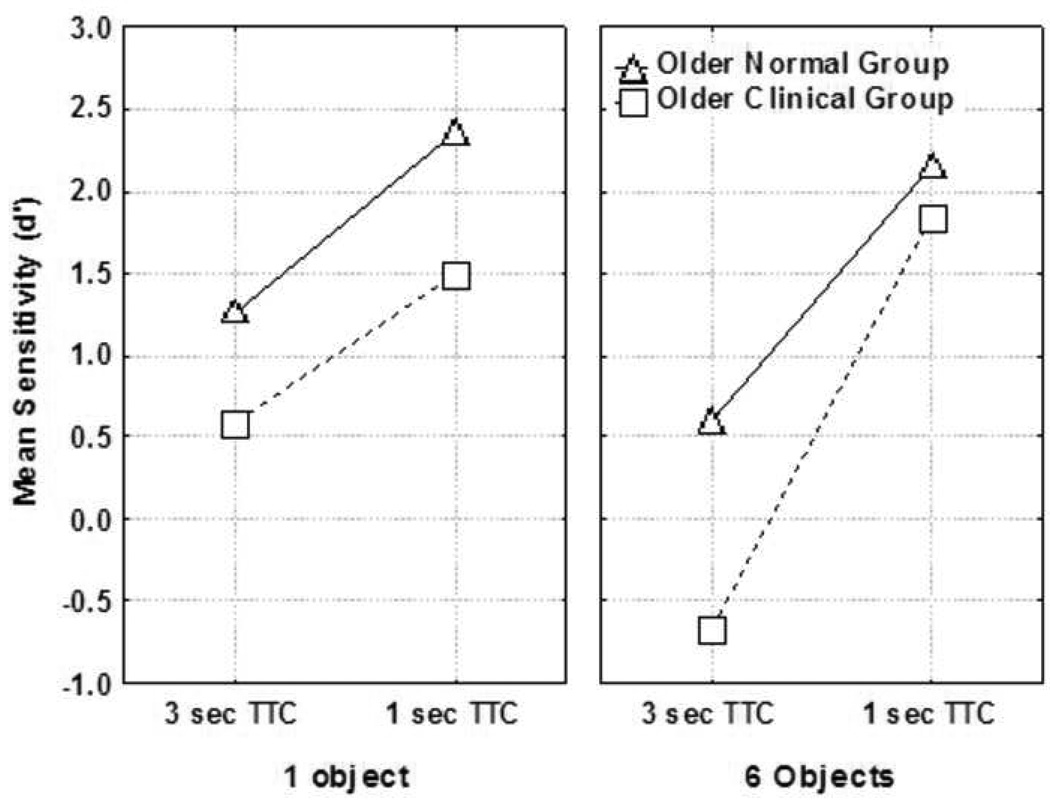
The average proportion of hits (collision responses for trials that simulated a collision) and the average proportion of false alarms (collision responses for trials that did not simulate a collision) were calculated for each observer in each condition and used to derive a sensitivity (d′) statistic (d′ = Z(hit rate) – Z(false alarm rate); Green and Swets, 1966) and analyzed in a 2 (group) by 2 (TTC) by 2 (number of objects) analysis of variance. Group was a between subjects variable and TTC and number of objects were repeated measures variables. The AD and PD participant sample was worse at detecting collisions than the age-matched comparisons across all four conditions. (Figure 3) The main effect of neurodegenerative disease was significant, F(1,30) = 12.3, p < .01, with mean d′ scores of 0.81 and 1.59 for the AD/PD group and comparison group, respectively. The main effects of number of objects, F(1,30) = 12.2, p < .01, and TTC, F(1,30) = 93.9, p < .01, were significant. Additionally, there was an interaction between TTC and number of objects, F(1,30) = 31.2, p < .01, indicating decreasing ability to detect a collision with increasing number of objects and increasing TTC. Collision detection sensitivity was therefore lowest for both groups in the 6 object, 3 second TTC condition. This was also the condition that resulted in the largest discrepancy in performance between the two groups. (Figure 3) Finally, the three-way interaction of group, TTC, and number of objects was significant, F(1,30) = 10.1, p < .01.
Figure 3.
Mean sensitivity (d’) as a function of TTC, number of objects, and observer group.
In addition, AD participants (n = 6) and PD participants (n = 8) were each separately compared to the group of neurologically normal, age-matched participants. Assessment of average d′ scores indicated that both AD (M d′ = .73, p = .007) and PD (M d′ = .87, p = .022) participants performed significantly worse than normal comparisons (M d′ = 1.59) on the collision detection task, with the AD participants performing slightly worse than PD overall. There was no significant difference in the collision detection performance between AD and PD participants.
3.2 Cognitive and Vision Measures
The neurodegenerative disease group had significantly worse scores on COGSTAT, a measure of composite neuropsychological exam performance, than the normal comparisons (p < .01). (Table 2) On the UFOV task the neurodegenerative disease group scored significantly worse on subtest 3 of Selective Attention (p < .05). Despite a significant difference in contrast sensitivity between the groups (p < .01), they did not differ on any other vision measures. When looking at the AD and PD participants individually, AD participants performed worse than the comparison group of older adults on all vision measures (far vision, near vision, contrast sensitivity, UFOV subtests 2–4, and UFOVtotal, p < .05). They also performed worse on the cognition measures of COGSTAT and MMSE (p < .05, p < .10, respectively). PD patients on the other hand, did not differ significantly from normal comparisons on any of the vision or cognition measures (p > .05), with the exception of contrast sensitivity (p = .04).
Table 2.
Group comparison of participants’ performance on cognitive and vision measures.
| Measure | AD (n = 6) M [SD] |
PD (n = 8) M [SD] |
Neurodegenerative Disease Group (AD+PD) (n = 14) M [SD] |
Comparison Group (n = 18) M [SD] |
|---|---|---|---|---|
| Far Vision (LogMAR) |
.14 [.08] p = .001* |
−.03 [.09] p = .96 |
.04 [.12] p = .06 |
−.03 [.10] |
| Near Vision (LogMAR) |
.15 [.09] p = .02* |
.03 [.04] p = .28 |
.08 [.09] p = .38 |
.05 [.07] |
| Contrast Sensitivity | 1.45 [.23] p = .0003* |
1.67 [.10] p = .04* |
1.58 [.19] p = .002* |
1.78 [.14] |
| UFOV 2 | 178.60 [102.20] p =.004* |
41.63 [40.79] p = .77 |
94.31 [96.24] p = .15 |
49.83 [72.49] |
| UFOV 3 | 363.20 [128.28] p = .02* |
209.00 [134.03] p = .23 |
268.31 [148.53] p = .03* |
161.61 [65.89] |
| UFOV 4 | 476.60 [39.02] p = .0002* |
321.13 [129.04] p = .81 |
380.92 [128.13] p = .27 |
333 [107.85] |
| UFOV Total | 1074.60 [215.32] p = .0001* |
590.38 [283.98] p = .82 |
776.61 [350.17] p = .07 |
567.89 [209.54] |
| COGSTAT | 298.00 [78.43] p = .02* |
368.21 [55.99] p = .09 |
338.13 [73.17] p = .004* |
405.38 [47.22] |
| MMSE | 24.50 [4.42] p = .06 |
28.5 [1.60] p = .60 |
26.79 [3.62] p = .07 |
28.78 [1.06] |
Denotes significant differences between comparisons and each of the disease groups.
3.3 Relationships Between Collision Detection and Cognition and Vision
Correlational analyses were conducted in order to assess the relationship between collision detection performance and measures of vision and cognition. Between participants there was a moderate correlation between average d′ scores and COGSTAT (Spearman r = .453, p = .009). This indicates that as collision detection sensitivity declines so does overall cognition, as measured by COGSTAT. Nonparametric correlations indicated moderate negative relationships between average d′ and both UFOV3 (r = −.396, p = .027) and UFOVTOT (r = −.367, p = .042). High scores on the UFOV task indicate poor performance while high d′ measures indicate greater sensitivity and therefore better performance on the collision detection task. These results indicate that poorer UFOV performance, a reported predictor of vehicle crashes (Ball et al., 1993), is associated with poorer sensitivity for detecting collisions.
4. Discussion
The current results indicate that neurodegenerative disorders, represented by AD and PD, impair the ability to detect impending collisions. Individuals with neurological disease performed worse than neurologically normal, age-matched comparisons at each of the four testing conditions. Collision detection sensitivity was worse for all participants during conditions that involved greater number of objects and longer TTC. The condition with the longest TTC and highest number of objects resulted in the largest decline in performance for the neurodegenerative group, indicative of declines in both perceptual (i.e. greater TTC conditions) and attentional processing (i.e., increased number of objects).
Drivers with neurodegenerative diseases, such as AD or PD, are at particular risk for impairment and error when confronted with several moving objects. When a single object is present in the driving scene both groups performed with some degree of sensitivity at each of the TTC conditions (i.e. the d′ values for both groups were greater than zero). However, examination of the results (Figure 3) for the 3 sec TTC/6 object condition indicates that the comparison group has some degree of sensitivity whereas the neurodegenerative group has no sensitivity to detect a collision (the d′ values were less than zero). A decrease in the TTC to 1 sec for the 6 object condition resulted in high sensitivity to detect a collision for both groups. From these results we infer that the amount of time to respond to an impending collision when 6 objects are present will be different for the two groups. The comparison group has some degree of sensitivity at the 3 sec TTC and thus will have up to 3 sec to react (steer or brake to avoid a collision). However, the neurodegenerative disease group has no sensitivity to detect collisions at the 3 sec TTC and thus will have less than 3 seconds to react to avoid a collision. These findings indicate that drivers with AD and PD need more time to detect impending collisions, which likely impairs their ability to avoid collision events, measured by the current simulation task.
We did not find a significant difference in the collision detection performance between AD and PD participants. The lack of group difference between AD and PD in this study is partially consistent with findings in another study, where we compared route following performance and driving safety errors of AD and PD drivers (Uc et al., 2004). The route following study showed that AD participants performed worse than PD on cognitive tests and had more incorrect turns, but there was no significant difference in the number of safety errors or times lost. Although it is possible that AD and PD participants may be performing similarly on collision detection, despite worse cognition in AD, our individual AD and PD group sample sizes are too small to draw definitive conclusions.
Several measures of vision and cognition were significantly impaired in participants with AD and PD. The neurodegenerative disease group as a whole had greater cognitive dysfunction than comparison participants, as measured by COGSTAT. They were also found to have diminished visual selective attention abilities on subtest 3 of the UFOV test. These findings are consistent with previous research indicating reduced cognitive and visual abilities in both AD and PD (Jackson and Owsley, 2003; Rizzo et al., 2000; Uc et al., 2005). Separate analysis of AD and PD participants revealed a greater level of impairment for AD participants. They were significantly worse than normal comparisons on all vision and cognition measures, while PD patients did not differ significantly from normal comparisons on any of these measures. The lack of observable differences between PD patients and comparisons may reflect the small sample size given that larger studies, including our own studies with PD and comparison cohorts from which the sample of this study was derived (Uc et al., 2005, 2006, 2007), have found patients with PD to be impaired on measures of vision and cognition when compared to neurologically normal, age-matched adults (Amick et al., 2007; Uc et al., 2005, 2006, 2007).
Various measures of vision and cognition have been related to driving ability and crash risk in patients with neurodegenerative disease (Ball et al, 2003; Fernandez et al., 2007; Kavcic et al., 2006; Rizzo et al., 2001; Uc et al., 2004, 2006). The current study found a significant correlation between the ability to detect collisions and visual attention, as measured by UFOV total and the UFOV selective attention subtest. There was also a correlation between collision detection sensitivity and cognition measured by a composite score of neuropsychological test performance, COGSTAT. Impairments on the collision detection task in patients with neurodegenerative impairment in this study probably reflect a variety of combined disturbances of visual-sensory processing, motion processing, attention, visuospatial skills, and executive functions.
This study successfully addressed mechanisms of collision detection using carefully controlled psychophysiological techniques, implemented in the context of a virtual environment and scenario that focused on a key aspect of driving. The present study employed the use of a low-fidelity simulation task that uses abstract (rather than photorealistic) representations of the virtual environment, and scenario design guided by cognitive neuroscience to localize performance errors in specific cognitive domains that are crucial to the real-world task being simulated, in this case, collision-detection. One benefit of this type of simulation is that it does not involve the expense and complex technical operation that high-fidelity simulators often require, while still being capable of providing useful insight into particular aspects of the driving experience.
The findings obtained using our collision detection scenario showed clear differences between drivers with neurodegenerative impairment and comparison drivers. While there is ample theory to suggest that motion cues and perception of moving objects are important to navigation and driving behavior, more data are needed to disclose relationships between performance on the collision detection task and real world evidence of driver behavior, such as performance profiles and errors on road tests, in instrumented vehicles, on test tracks, and in the epidemiologic records of moving violations and crashes.
Acknowledgements
This research was supported by NIH grant AG13419-06, NIH EY18334-01, NIH NS044930, and NIA grant AG 17177. We would especially like to thank Mi Jin Jang and Jamie Emerson for their assistance with the statistical analysis of the research findings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lindsay M. Vaux, Email: lindsay-vaux@uiowa.edu.
Rui Ni, Email: rui.ni@wichita.edu.
Ergun Y. Uc, Email: ergun-uc@uiowa.edu.
George J. Andersen, Email: john.andersen@ucr.edu.
References
- Alzheimer’s Association. Chicago: Report published by the Alzheimer’s Association; 2008. Alzheimer’s disease facts and figures. [DOI] [PubMed] [Google Scholar]
- Amick MM, Grace J, Ott BR. Visual and cognitive predictors of driving safety in Parkinson's disease patients. Arch Clin Neuropsychol. 2007;22(8):957–967. doi: 10.1016/j.acn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen GJ, Atchley P. Age-related differences in the detection of three dimensional surfaces from optic flow. Psychol Aging. 1995;10(4):650–658. doi: 10.1037//0882-7974.10.4.650. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Cisneros J, Saidpour A, Atchley P. Speed, size and edge rate information for the detection of collision events. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:256–279. doi: 10.1037//0096-1523.25.1.256. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Cisneros J, Saidpour A, Atchley P. Age-related differences in collision detection during deceleration. Psychol Aging. 2000;15(2):241–252. doi: 10.1037//0882-7974.15.2.241. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Kim RD. Perceptual Information and attentional constraints in visual search of collision events. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:1039–1056. doi: 10.1037//0096-1523.27.5.1039. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Enriquez A. Aging and the detection of observer and moving object collisions. Psychol Aging. 2006;21:74–85. doi: 10.1037/0882-7974.21.1.74. [DOI] [PubMed] [Google Scholar]
- Andersen GJ, Sauer CW. Optical information for collision detection during deceleration. In: Hecht H, Kaiser M, editors. Theories of time to contact, Advances in Psychology Series, North Holland. Amsterdam Netherlands: Elsevier; 2004. pp. 93–108. [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychol Aging. 1998;13(2):297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- DeLucia PR, Bleckley MK, Meyer LE, Bush JM. Judgments about collisions in younger and older drivers. Transportation Research Part F: Traffic Psychology & Behaviour. 2003;6:63–80. [Google Scholar]
- Dubinsky RM, Stein AC, Lyons K. Practice parameter: risk of driving and Alzheimer's disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;54(12):2205–2211. doi: 10.1212/wnl.54.12.2205. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Kavcic V, Duffy C. Neurophysiologic analyses of low- and high level visual processing in Alzheimer disease. Neurology. 2007;68:2066–2076. doi: 10.1212/01.wnl.0000264873.62313.81. [DOI] [PubMed] [Google Scholar]
- Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Wenk HE, Naylor LA, Stuve TA. Motion perception and aging. Psychol Aging. 1992;7(4):654–660. doi: 10.1037//0882-7974.7.4.654. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- Harris P. Vision and driving in the elderly. Journal of Optometric Vision Development. 1999;30(4):188–197. [Google Scholar]
- Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. In: Neurologic Clinics of North America, Vision and the Brain, Part II. 2003;21(3):709–728. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- Kavcic V, Fernandez R, Logan D, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer’s disease. Brain. 2006;129:736–746. doi: 10.1093/brain/awh727. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Lundberg C, Johansson K, Ball K, Bjerre B, Blomqvist C, Braekhus A, et al. Dementia and driving: an attempt at consensus. Alzheimer Dis Assoc Disord. 1997;11(1):28–37. doi: 10.1097/00002093-199703000-00006. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKnight AJ, McKnight AS. Multivariate analysis of age-related driver ability and performance deficits. Accid. Anal. Prev. 1999;31(5):445–454. doi: 10.1016/s0001-4575(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Norman JF, Ross HE, Hawkes LM, Long JR. Aging and the perception of speed. Perception. 2003;32(1):85–96. doi: 10.1068/p3478. [DOI] [PubMed] [Google Scholar]
- Norman JF, Clayton AM, Shular CF, Thompson SR. Aging and the perception of depth and 3-D shape from motion parallax. Psychol Aging. 2004;19(3):506–514. doi: 10.1037/0882-7974.19.3.506. [DOI] [PubMed] [Google Scholar]
- O'Brien HL, Tetewsky SJ, Avery LM, Cushman LA, Makous W, Duffy CJ. Visual mechanisms of spatial disorientation in Alzheimer's disease. Cereb Cortex. 2001;11(11):1083–1092. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, Sloane ME, Roenker DL, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychology and Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- Owsley C. Vision and driving in the elderly. Optometry and Vision Science. 1994;71:727–735. doi: 10.1097/00006324-199412000-00002. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical vision Sci. 1988;2:187–199. [Google Scholar]
- Rizzo M, Reinach S, McGehee D, Dawson J. Simulated car crashes and crash predictors in drivers with Alzheimer disease. Arch. Neurol. 1997;54(5):545–551. doi: 10.1001/archneur.1997.00550170027011. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Nawrot M. Perception of movement and shape in Alzheimer's disease. Brain. 1998;121(Pt 12):2259–2270. doi: 10.1093/brain/121.12.2259. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Anderson SW, Dawson J, Myers R, Ball K. Visual attention impairments in Alzheimer's disease. Neurology. 2000;54(10):1954–1959. doi: 10.1212/wnl.54.10.1954. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Anderson SW, Dawson J, Nawrot M. Vision and cognition in Alzheimer's disease. Neuropsychologia. 2000;38(8):1157–1169. doi: 10.1016/s0028-3932(00)00023-3. [DOI] [PubMed] [Google Scholar]
- Rizzo M, McGehee DV, Dawson JD, Anderson SN. Simulated car crashes at intersections in drivers with Alzheimer disease. Alzheimer Disease & Associated Disorders. 2001;15(1):10–20. doi: 10.1097/00002093-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Stierman L, Skaar N, Dawson JD, Anderson SW, Vecera SP. Effects of a controlled auditory-verbal distraction task on older driver vehicle control. Transportation Research Record: Journal of the Transportation Research Board No. 1865. 2004:1–6. [Google Scholar]
- Rizzo M, Shi Q, Dawson J, Anderson SW, Kellison I, Pietras TA. Stops for cops: Impaired response implementation in older drivers with cognitive decline. Transportation Research Record: Journal of the Transportation Research Board No. 1922. 2005:1–8. [Google Scholar]
- Salmon DP, Thomas RG, Pay MM, Booth A, Hofstetter CR, Thal LJ, Katzman R. Alzheimer's disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59(7):1022–1028. doi: 10.1212/wnl.59.7.1022. [DOI] [PubMed] [Google Scholar]
- Schiff W, Oldak R, Shah V. Aging persons' estimates of vehicular motion. Psychol Aging. 1992;7(4):518–525. doi: 10.1037//0882-7974.7.4.518. [DOI] [PubMed] [Google Scholar]
- Silverman SE, Tran DB, Zimmerman KM, Feldon SE. Dissociation between the detection and perception of motion in Alzheimer's disease. Neurology. 1994;44(10):1814–1818. doi: 10.1212/wnl.44.10.1814. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Shi Q, Anderson SW, Rodnitzky RL, Dawson JD. Route following in drivers with Parkinson's disease vs. Alzheimer's disease. Annals of Neurology. 2004:S50. (abstract) [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver route following and safety errors in early Alzheimer disease. Neurology. 2004;63(5):832–837. doi: 10.1212/01.wnl.0000139301.01177.35. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65:1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Unsafe rear-end collision avoidance in Alzheimer’s disease. Journal of the Neurological Sciences. 2006;251:35–43. doi: 10.1016/j.jns.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Impaired navigation in drivers with Parkinson’s disease. Brain. 2007;130(9):2433–2440. doi: 10.1093/brain/awm178. [DOI] [PubMed] [Google Scholar]