Abstract
The need for daily parenteral administration represents one of the most serious limitations in the clinical use of pentavalent antimonials against leishmaniasis. In this work, we investigated the ability of β-cyclodextrin to enhance the oral absorption of antimony and to promote the oral efficacy of meglumine antimoniate against experimental cutaneous leishmaniasis. The occurrence of interactions between β-cyclodextrin and meglumine antimoniate was demonstrated through the changes induced in the spin lattice relaxation times of protons in both compounds. When free and complexed meglumine antimoniate were given orally to Swiss mice, plasma antimony levels were found to be about three times higher for the meglumine antimoniate-β-cyclodextrin complex than for the free drug. Antileishmanial efficacy was evaluated in BALB/c mice experimentally infected with Leishmania amazonensis. Animals treated daily with the complex (32 mg of Sb/kg of body weight) by the oral route developed significantly smaller lesions than those treated with meglumine antimoniate (120 mg of Sb/kg) and control animals (treated with saline). The effectiveness of the complex given orally was equivalent to that of meglumine antimoniate given intraperitoneally at a twofold-higher antimony dose. The antileishmanial efficacy of the complex was confirmed by the significantly lower parasite load in the lesions of treated animals than in saline-treated controls. This work reports for the first time the effectiveness of an oral formulation for pentavalent antimonials.
Pentavalent antimonials, including meglumine antimoniate, are the main drugs used in the treatment of all forms of leishmaniasis (1, 2, 9). These highly water-soluble compounds are considered inactive when given enterally and are subject to rapid renal clearance after parenteral administration, requiring a multiple-dosing regimen. Antimony (Sb) therapy is often accompanied by local pain during and just after intramuscular injections and by severe systemic side effects requiring very careful medical supervision. All these factors contribute to high cost and compliance difficulties that may ultimately lead to treatment failures. This also explains why so much effort is being devoted to the search for orally active antileishmanial drugs (4, 7, 13).
The association of drugs with carrier systems is a feasible strategy to improve oral absorption. Among drug carrier systems, cyclodextrins, which are cyclic oligosaccharides composed of glucose units joined through α-1,4 glucosidic bonds, have been one of the most successful drug absorption enhancers for oral delivery (5, 14).
We show here that β-cyclodextrin forms a complex with meglumine antimoniate and report the impact of this association on the oral absorption of antimony in mice and on the efficacy of meglumine antimoniate in an experimental model of cutaneous leishmaniasis.
MATERIALS AND METHODS
Materials.
N-methyl-d-glucamine and SbCl5 were obtained from Aldrich Chemical Co. (Milwaukee, Wis). β-Cyclodextrin and Dulbecco modified Eagle medium were obtained from Sigma Chemical Co. (St. Louis, Mo.). Heat-inactivated fetal calf serum was obtained from Cultilab (Campinas, Brazil).
Parasites.
Leishmania amazonensis (MHOM/BR/75/Josefa strain) promastigotes transfected with green fluorescent protein (L. amazonensis-GFP promastigotes) were used. Parasites were routinely isolated from mouse lesions and maintained as promastigotes in Dulbecco modified Eagle medium containing 10% heat-inactivated fetal calf serum and 150 μg of Geneticin/ml at 26°C.
Preparation and characterization of meglumine antimoniate-β-cyclodextrin complex.
Meglumine antimoniate was synthesized according to the method of Demicheli et al. (3) from an equimolar mixture in water of N-methyl-d-glucamine and freshly precipitated and hydrated antimony pentoxide, obtained from SbCl5 hydrolyzed in water. The resulting product contained 29% Sb by weight, as determined by inductively coupled plasma optical emission spectrometry.
The meglumine antimoniate-β-cyclodextrin complex was prepared by mixing β-cyclodextrin and meglumine antimoniate in distilled water at a 1:1 cyclodextrin/Sb molar ratio, heating the mixture for 48 h at 55°C, and finally freeze-drying the resulting solution. The resulting compound was characterized by C,H,N elemental analysis, inductively coupled plasma optical emission spectrometry (Sb), thermogravimetry, and nuclear magnetic resonance (NMR) spectroscopy. 1H NMR studies were performed in D2O on a Bruker DRX 400-Advance spectrometer operating at 400 MHz at 27°C using trimethylsilyl as an internal standard. Typical spectrometer conditions were 65,536 data points, 11.19-kHz spectral width, and 10.0-s recycle time. Proton resonances were assigned using data from the literature for β-cyclodextrin (11) and from previous work for meglumine antimoniate (3). 1H inversion recovery (T1) peak intensity data (I[t]) were fitted to a three-parameter exponential equation: I[t] = I[0] + P · exp(−t/T1), where I[t] is the signal intensity at t = τ, I[0] is the maximum measurable signal intensity, P is a constant whose value depends on the initial conditions (typically, P = 2I[0]), and T1 is the spin lattice relaxation time; 10 τ values, the time between the 180 and 90° pulses, of 10 to 15,000 ms were used.
Elemental and Sb analyses showed the following composition: C, 33.51%; H, 6.57%; N, 0.78%; and Sb, 7.94%. Thermogravimetry indicated the presence of nine water molecules. According to this data, the resulting product consisted of a 1:1 meglumine antimoniate-β-cyclodextrin complex (calculated: C, 33.89%; H, 6.57%; N, 0.81%; Sb, 7.02%).
Oral absorption of antimony from meglumine antimoniate and its complex with β-cyclodextrin.
Swiss mice (female; weighing 25 ± 3 g) received through oral inoculum, with the aid of a needle gauge, 0.3 ml of an aqueous solution of either meglumine antimoniate or its complex with β-cyclodextrin at 100 mg of Sb/kg of body weight. Three mice from each group were sacrificed at the following times: 0.5, 1, 2, 3, 4, 6, 12, and 24 h after inoculation. Blood samples were obtained, and the plasma was recovered and frozen.
The plasma was assayed for antimony by graphite furnace atomic absorption spectrometry without digestion of the sample, using zirconium (Zr) and rhodium (Rh) as permanent modifiers. Briefly, samples were diluted five times with 1% (vol/vol) nitric acid containing 0.02% tricetyl methyl ammonium. Pyrolytic-graphite-coated tubes were initially treated, as previously described (12), with 10 μl of a mixture containing 500 μg of Zr and 250 μg of Rh; 20 μl of the diluted samples was then added to the graphite tube. All measurements were carried out with a Hitachi (Mitorika, Ibaraki, Japan) Z-8200 atomic absorption spectrometer equipped with a graphite furnace and an autosampler (SSC-300; Hitachi) and with polarized Zeeman effect background correction. The hollow cathode lamp for Sb was operated at 12.5 mA with a slit of 0.4 nm. Pyrolysis and atomization temperatures of 900 and 1,900°C, respectively, which gave symmetric absorption peaks and Sb recuperation efficiencies close to 100% for Sb-contaminated samples, were selected.
Antileishmanial activity of meglumine antimoniate and its complex with β-cyclodextrin.
Five groups of BALB/c mice (five mice per group) were infected in the ear with 2 × 106 L. amazonensis-GFP promastigotes. The lesion sizes were periodically measured using a dial caliper and were expressed as the difference between the thicknesses of the infected and uninfected ears. Treatment was initiated 10 days after infection with the following preparations: meglumine antimoniate-β-cyclodextrin compound by the oral route (8 mg in 200 μl of saline, equivalent to 32 mg of Sb/kg, for each daily dose), meglumine antimoniate by the oral route (8 mg in 200 μl of saline, equivalent to 120 mg of Sb/kg), and meglumine antimoniate by the intraperitoneal (i.p.) route (4 mg in 100 μl of saline, equivalent to 60 mg of Sb/kg). The controls received saline (phosphate buffered) by the i.p. route. The animals were treated daily on days 10 through 16 and 31 through 36 of infection. On day 80 of infection, the animals were sacrificed, and the parasite loads in the lesions were quantitated by fluorimetry as previously described (10). Briefly, each infected ear was cut off and homogenized in 2 ml of phosphate-buffered saline with a tissue grinder. After the removal of tissue debris by gravity sedimentation for 10 min, 200 μl of twofold dilutions of the cell suspensions was transferred in triplicate to black microplates, and the fluorescence was read in a plate reader fluorimeter (Fluoroskan; LabSystems) at 435-nm excitation and 538-nm emission. The specific fluorescence was expressed as the difference between the infected and uninfected ears. The data were analyzed by one-way analysis of variance followed by a Tukey posttest.
RESULTS
Physicochemical characteristics of meglumine antimoniate-β-cyclodextrin complex.
The meglumine antimoniate-β-cyclodextrin complex was obtained following reaction of an equimolar mixture of meglumine antimoniate and β-cyclodextrin in water and freeze-drying of the resulting solution. Thermogravimetry analysis indicated the presence of nine water molecules in the association compound, suggesting that β-cyclodextrin did not release originally included water molecules (8) into the bulk water upon association with meglumine antimoniate.
The occurrence of interactions between meglumine antimoniate and β-cyclodextrin was investigated by 1H NMR in D2O. The proton NMR spectrum for the association compound was registered and compared to the spectra of individual solutions of meglumine antimoniate and β-cyclodextrin. In the case of the complex, the proton resonances for meglumine antimoniate and β-cyclodextrin were not significantly altered, showing changes of <0.02 parts per million (data not shown). Since the proton resonances of meglumine antimoniate differ markedly from those of free meglumine (3), one can infer that the antimonial drug did not suffer dissociation in the association compound.
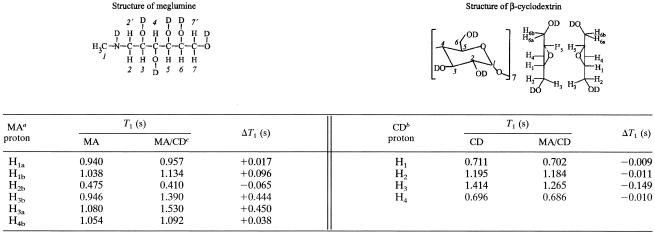
Evidence for the association of meglumine antimoniate with β-cyclodextrin was obtained through analysis of the spin lattice relaxation times of protons in both compounds: H1a, H1b, H2b, H3a, H3b, and H4b for meglumine antimoniate and H1, H2, H3, and H4 for β-cyclodextrin (Table 1). The other protons could not be analyzed because of the overlay between proton resonances of β-cyclodextrin and meglumine antimoniate. Marked alterations of the spin lattice relaxation times were observed for H3 in β-cyclodextrin and for H3a and H3b in meglumine antimoniate, indicating a change in the environment of these protons. These results, in light of the truncated cone-shaped structure of β-cyclodextrin, which exhibits a hydrophilic outer surface and a hydrophobic central cavity, suggest that meglumine antimoniate interacts with β-cyclodextrin through hydrogen bonds with the hydrophilic outer surface of the cyclodextrin molecule, presumably at the largest rim's torus, where OH-3 (hydroxyls at the 3 position) are located.
TABLE 1.
Spin lattice relaxation time T1-1H measurements for meglumine antimoniate, β-cyclodextrin, and their association compound
MA, meglumine antimoniate.
CD, β-cyclodextrin.
MA/CD, meglumine antimoniate-β-cyclodextrin.
Oral absorption of antimony from meglumine antimoniate and its complex with β-cyclodextrin.
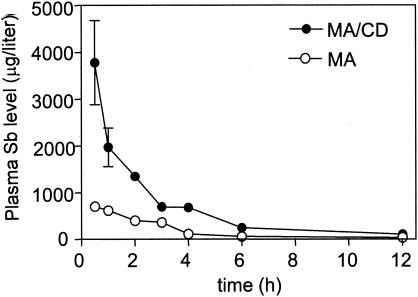
Figure 1 displays the levels of antimony in plasma when meglumine antimoniate or its complex with β-cyclodextrin were orally administered to mice at 100 mg of Sb/kg. The antimony concentrations were found to be about three times higher for the association compound than for meglumine antimoniate. These data clearly established that the association of meglumine antimoniate with β-cyclodextrin strongly improved the bioavailability of antimony by the oral route.
FIG. 1.
Levels of antimony in plasma following oral administration of meglumine antimoniate (MA) or its complex with β-cyclodextrin (MA/CD) in Swiss mice at 100 mg of Sb/kg. The results are expressed as means ± standard deviations (error bars) (n = 3).
Efficacy by oral route of meglumine antimoniate and its complex with β-cyclodextrin against experimental cutaneous leishmaniasis.
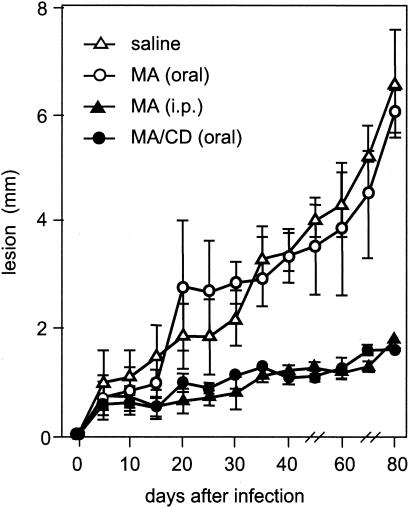
Figure 2 shows the course of infection in BALB/c mice upon treatment with meglumine antimoniate or its complex with β-cyclodextrin. As expected, the i.p. treatment with meglumine antimoniate effectively controlled lesion growth, whereas the oral treatment was ineffective, despite the double amount of antimony given orally. In agreement with the toxicological data obtained for β-cyclodextrin (6) showing that it is not absorbed across the gastrointestinal tract, no significant effect on the course of cutaneous leishmaniasis was found using β-cyclodextrin alone (data not shown). On the other hand, complexation of meglumine antimoniate with β-cyclodextrin rendered it highly effective by the oral route. The effectiveness of the complex by the oral route was equivalent to that of meglumine antimoniate given parenterally at a twofold-higher antimony dose.
FIG. 2.
Lesion growth in mice treated with meglumine antimoniate (MA) or its complex with β-cyclodextrin (MA/CD). The mice were infected in the ear with L. amazonensis-GFP and then treated daily with meglumine antimoniate-β-cyclodextrin compound (32 mg of Sb/kg) or meglumine antimoniate (120 mg of Sb/kg) on days 10 to 16 and 31 to 36 of infection. The controls received saline or meglumine antimoniate (60 mg of Sb/kg) by the i.p. route. The ear thickness was measured on the indicated days and expressed as means ± standard deviations (n = 5).
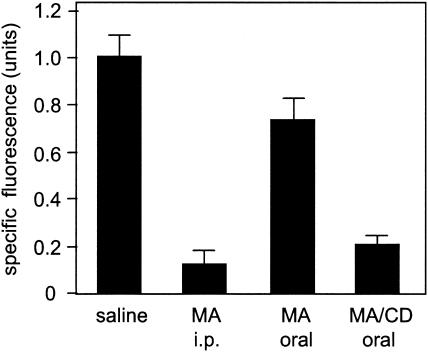
The effectiveness of the association compound by the oral route against cutaneous leishmaniasis was further evaluated by measuring the parasite load in the lesions on day 80 of infection, as expressed by the fluorescence in the ear tissues infected with L. amazonensis-GFP. The results displayed in Fig. 3 show that the complex, given orally as daily doses of 32 mg of Sb/kg, significantly reduced the number of parasites in the lesions compared to saline (P < 0.001). The parasite load was similar to that achieved with meglumine antimoniate given i.p. at a twofold-higher antimony dose (P > 0.05), confirming the great effectiveness of the association compound in killing the parasites when administered by the oral route. Although oral meglumine antimoniate partially reduced the parasite burden compared to saline controls (P < 0.001), the burden in animals treated with the complex was significantly lower than that after treatment with meglumine antimoniate (P < 0.001). These data clearly establish that the association of meglumine antimoniate with β-cyclodextrin renders the antimonial drug orally active, most probably through enhancement of its oral absorption.
FIG. 3.
Parasite loads in mice treated with meglumine antimoniate (MA) or its complex with β-cyclodextrin (MA/CD). The mice were infected and treated as described in the legend to Fig. 2. On day 80 of infection, the fluorescence in the ear homogenate was measured and expressed as the mean plus standard deviation (n = 5).
DISCUSSION
In the present study, we showed that β-cyclodextrin forms a complex with meglumine antimoniate and that this association enhanced the oral absorption of antimony in mice. The resulting formulation was found to be effective by the oral route in a mouse model of cutaneous leishmaniasis.
Cyclodextrins are well known in recognition chemistry as molecular hosts capable of including, with a degree of selectivity, water-insoluble guest molecules via noncovalent interactions within their hydrophobic cavities. Taking into account that meglumine antimoniate is highly soluble in water and most probably interacts with the external surface of β-cyclodextrin, the association compound obtained in the present study differs physicochemically from conventional inclusion compounds. The higher oral absorption of antimony observed with the association compound strongly suggests enhanced permeability of antimony across the intestinal barrier. This interpretation is consistent with previous findings that β-cyclodextrin increases the percutaneous absorption of antimony across isolated mouse skin (R. Ochoa, L. A. Ferreira, F. Frézard, J. B. da Silva, R. D. Sinisterra, and C. Demicheli, Proc. 30th Annu. Meet. Exposition Control. Release Soc., abstr. 864, 2003). In that study, a twofold increase in the flux of antimony across the skin was observed when meglumine antimoniate was applied onto the skin in the form of the association compound with β-cyclodextrin. Moreover, the partition of antimony into the skin was also found to be enhanced significantly with the association compound. Strikingly, the increase in antimony flux promoted by the association of meglumine antimoniate with β-cyclodextrin was even higher (∼8-fold) when the skin was used without the stratum corneum. These data are therefore consistent with the model in which β-cyclodextrin increases the lipophilicity and permeability of antimony across biological membranes, presumably through its free internal cavity. This hypothesis is further supported by the observation of Uekama et al. (14) that free β-cyclodextrin can remove components of the membrane surface, thereby modifying the transport properties of the membranes and facilitating drug absorption, especially for water-soluble drugs.
The fact that pentavalent antimonials have to be given parenterally represents one of the most serious limitations of the present chemotherapy for leishmaniasis. The recent introduction of the orally active drug miltefosine (13) illustrates well the great effort devoted to the search for orally active antileishmanial agents. However, this drug showed some adverse side effects, and its use will probably remain limited to visceral leishmaniasis. Allopurinol is another example of an orally active antileishmanial compound, but its efficacy is quite low in comparison to conventional antimonial therapy (2). In this context, the demonstration of the oral efficacy of meglumine antimoniate when given in association with β-cyclodextrin opens promising new perspectives for the treatment of leishmaniases. The importance of these results is reinforced by the fact that oral efficacy was achieved with a low dose of antimony.
Acknowledgments
This work was supported by grants from the Brazilian agencies CNPq (521010/97-7 and Brazilian Nanobiotechnology Network), CAPES, and FAPEMIG.
REFERENCES
- 1.Berman, J. D. 1988. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 10:560-586. [DOI] [PubMed] [Google Scholar]
- 2.Berman, J. D. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. [DOI] [PubMed] [Google Scholar]
- 3.Demicheli, C., R. Ochoa, I. S. Lula, F. C. Gozzo, M. N. Eberlin, and F. Frézard. 2003. Pentavalent organoantimonial derivatives: two simple and efficient synthetic methods for meglumine antimonate. Appl. Organomet. Chem. 17:226-231. [Google Scholar]
- 4.Dietze, R., S. F. Carvalho, L. C. Valli, J. Berman, T. Brewer, W. Milhous, J. Sanchez, B. Schuster, and M. Grogl. 2001. Phase 2 trial of WR6026, as an orally administered 8-aminoquinoline, in the treatment of visceral leishmaniasis caused by Leishmania chagasi. Am. J. Trop. Med. Hyg. 65:685-689. [DOI] [PubMed] [Google Scholar]
- 5.Hirayama, F., and K. Uekama. 1999. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 36:125-141. [DOI] [PubMed] [Google Scholar]
- 6.Irie, T., and K. Uekama. 1997. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 86:147-162. [DOI] [PubMed] [Google Scholar]
- 7.Kayser, O., C. Olbrich, V. Yardley, A. F. Kiderlen, and S. L. Croft. 2003. Formulation of amphotericin B as nanosuspension for oral administration. Int. J. Pharm. 254:73-75. [DOI] [PubMed] [Google Scholar]
- 8.Lindner, K., and W. Saenger. 1978. Beta-cyclodextrin dodecahydrate-crowding of water-molecules within a hydrophobic cavity. Angew. Chem. Int. Ed. Engl. 17:694-695. [Google Scholar]
- 9.Marsden, P. D. 1985. Pentavalent antimonials: old drug for new diseases. Rev. Soc. Bras. Med. Trop. 18:187-198. [Google Scholar]
- 10.Rossi-Bergmann, B., A. Lenglet, C. R. Bezerra-Santos, D. Costa-Pinto, and Y. M. Traub-Czeko. 1999. Use of fluorescent Leishmania for faster quantitation of parasite growth in vitro and in vivo. Mem. Inst. Oswaldo Cruz 94:74. [Google Scholar]
- 11.Schneider, H.-J., F. Hacket, and V. Rudiger. 1998. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 98:1755-1785. [DOI] [PubMed] [Google Scholar]
- 12.Silva, J. B. B., M. B. O. Giacomelli, and A. J. Curtius. 1998. Iridium and rhodium as permanent chemical modifiers for the determination of Ag, As, Bi, Cd and Sb by electrothermal atomic absorption spectrometry. Microchem. J. 60:249-254. [Google Scholar]
- 13.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fisher, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 14.Uekama, K., F. Hiramaya, and T. Irie. 1998. Cyclodextrin drug carrier system. Chem. Rev. 98:2045-2076. [DOI] [PubMed] [Google Scholar]