Abstract
Background
Good syndrome is a rare cause of combined B- and T-cell immunodeficiency that occurs in association with a thymoma. Patients affected with Good syndrome have increased susceptibility to bacterial, fungal, viral, and opportunistic infections.
Objective
To describe 2 unusual cases of infections in patients with Good syndrome and review the literature.
Methods
Case 1 describes a 51-year-old woman with Good syndrome who presented with a 10-day history of diarrhea, nausea, and fevers. During her hospitalization she became pancytopenic and underwent a bone marrow biopsy and evaluation of her peripheral blood smear. Case 2 describes an 89-year-old man with Good syndrome who presented with a nonhealing leg ulcer, which underwent biopsy. A literature search through MEDLINE was performed. Keywords included Good syndrome, thymoma, hypogammaglobulinemia, immunodeficiency, and infection.
Results
The peripheral blood smear in patient 1 showed ring-formed parasites in red blood cells suggestive of babesiosis. She began treatment with azithromycin, atovaquone, and doxycycline and recovered completely. Patient 2 underwent a biopsy of the foot. Immunohistochemical staining was positive for human herpesvirus 8 consistent with Kaposi sarcoma.
Conclusions
The concomitant occurrence of immunodeficiency and thymoma is known as Good syndrome. In contrast to other humoral immune defects, patients with this syndrome can develop opportunistic infections, and the prognosis appears less favorable compared with X-linked agammaglobulinemia or common variable immunodeficiency. Immunological investigations, including T-cell subsets, B cells, and quantitative immunoglobulins, should be considered part of the routine diagnostic evaluation in patients with a thymoma and recurrent infections.
INTRODUCTION
Good syndrome is a rare association of thymoma and immunodeficiency first described more than 50 years ago. Patients are most commonly between the ages of 40 and 70 years and have a thymoma, low to absent B cells in the peripheral blood, hypogammaglobulinemia, and defects in cell-mediated immunity. As reviewed by Tarr et al,1 patients often present with recurrent infections due to encapsulated bacteria, fungi, and viruses. Herein, we describe 2 patients with Good syndrome and their presentation of unusual infections and review the literature.
METHODS
We searched the literature using the MEDLINE (National Library of Medicine, Bethesda, MD) database. Keywords used in the search included Good syndrome, thymoma, hypogammaglobulinemia, immunodeficiency, and infection. Additional cases were identified using references of publications found. Only articles and abstracts published in English were included.
CASE REPORTS
Case 1
A 51-year-old white woman who was previously healthy presented with a persistent cough, several bouts of pneumonia, and oral ulcerations. A chest x-ray examination in May 2000 revealed a large thymoma, which was resected. Despite the resection, she continued to have oral ulcerations and a cough with shortness of breath. In October 2000, a lung biopsy specimen demonstrated fragments of alveolar parenchyma with large intra-alveolar myxoid tissue plugs consistent with cryptogenic organizing pneumonia, and she was given prednisone, 60 mg/d. There was transient improvement of the oral ulcerations with the prednisone. Her serologic test results for paraneoplastic pemphigus (indirect immunofluorescence for pemphigus antibodies, indirect immunofluorescence on rat bladder, and antigen-specific immunoprecipitation for any relevant antibodies) were negative, and the diagnosis of oral lichen planus was made after biopsy.
In June 2002, she was found to be hypogammaglobulinemic, with an IgG level of 447 mg/dL (reference range, 639–1,329 mg/dL), an IgM level of 56 mg/dL (reference range, 70–312 mg/dL), and an IgA level of 20 mg/dL (reference range, 56–352 mg/dL). She had a reduced CD4 T-cell count of 298/μL (reference range, 588–1,202/μL) and a decreased CD4:CD8 ratio of 0.7. The diagnosis of Good syndrome was made. Immunopathology of peripheral blood demonstrated few B cells that were polyclonal according to surface immunoglobulin expression and T cells without aberrant antigen expression. She lacked significant levels of protective antibodies to varicella zoster and denied a history of chicken pox but had protective levels of antibodies to tetanus (0.60 IU/mL; reference range, >0.15 IU/mL) and diphtheria (0.44 IU/mL; reference range, >0.01 IU/mL). She had received pneumococcal vaccination and produced 12 of 12 pneumococcal serotypes, indicating preserved antibody function.
Her cough and shortness of breath persisted, and in September 2003 a high-resolution computed tomography showed diffusely increased interstitial markings, reticular changes with areas of ground glass density, and fine nodularity. Pulmonary function tests demonstrated diffusion of 47% of predicted with restrictive ventilatory defect on spirometry. Anti-inflammatory therapy with azathioprine was recommended. She continued to have oral ulcerations for which she began taking daily cyclosporine, 3 mg/kg, but continued this only intermittently with 5 mg/d of prednisone.
She had been stable until July 2005, when she was admitted to the hospital with a 10-day history of nausea, vomiting, watery diarrhea, and fevers. On admission she was hypotensive, anemic, and hyperglycemic. Her medication regimen consisted of 5 mg/d of prednisone, 400 mg/kg per month of intravenous immunoglobulin (IVIG), 1,200 mg/d of calcium carbonate, and 70 mg/wk of alendronate. Given her hypotension and history of long-term steroid use, adrenal insufficiency was suggested and she was given high-dose steroids.
Her stool culture was positive for Clostridium difficile, which failed to respond to metronidazole, and she was prescribed oral vancomycin. Throughout the following days, she became pancytopenic with an inappropriately low reticulocyte response (reticulocyte count, 1.6). A disseminated intra-vascular coagulation panel suggested hemolysis. A bone marrow biopsy specimen revealed patchy hypercellularity. Her hemolysis continued, and on hospital day 15 a peripheral blood smear was significant for ring-formed parasites in red blood cells suggestive of babesiosis. Further history elicited included a weekend at Montauk, NY, where tickborne diseases are prevalent, and she was subsequently prescribed azithromycin, atovaquone, and doxycycline. She was discharged on hospital day 25 and completed a 14-day course of antibiotics with complete recovery.
Case 2
An 89-year-old white man was found to have a thymoma in 2002. He had a chronic cough with large amounts of white to green sputum and was being treated with nebulizers of albuterol and ipratropium bromide. His medical history included multiple pneumonias, bronchitis, deep venous thrombosis in the lower extremity, peripheral vascular disease, type 2 diabetes mellitus, multiple skin infections, and a blistering lesion on his leg for a number of years. He had been colonized by Pseudomonas aeruginosa and was intermittently prescribed antibiotics. In November 2002, laboratory tests revealed the following: IgG, 198 mg/dL; IgA, 466 mg/dL; and IgM, less than 4 mg/dL. Flow cytometry revealed virtually undetectable levels of peripheral B cells, normal levels of T cells and CD8 cells, but slightly reduced CD4 cells. Good syndrome was diagnosed, and he was given monthly IVIG at 400 mg/kg. His thymoma appeared to be stable on computed tomography.
In July 2005, he underwent a biopsy of the left foot lesion, which was thought to be chronic stasis dermatitis. He had multiple purplish raised nodules on both feet and along the medial anterior thigh in a linear pattern. Pathologic tests demonstrated a focus of superficial atypical vascular proliferation, which was positive for immunohistochemical staining by human herpesvirus 8 (HHV-8), consistent with Kaposi sarcoma.
DISCUSSION
Definition
The association between the presence of a thymoma and adult-onset hypogammaglobulinemia was first described by Dr Robert Good in 1955.2 There are a number of definitions for Good syndrome. Practice parameters in 20053 define it as a subset of common variable immunodeficiency; however, the reduced numbers of peripheral B cells noted in Good syndrome are not a feature of common variable immunodeficiency, which typically shows impaired B-cell maturation. Others choose to define it as hypogammaglobulinemia with thymoma consistent with Dr Good’s case. Our rationale for choosing to define Good syndrome as immunodeficiency with thymoma, a broader classification, is because the pathogenesis of the disease remains unknown and patients have several other immunological impairments in addition to hypogammaglobulinemia (Table 1).
Table 1.
Important Features of Good Syndrome
| Definition | Immunodeficiency with thymoma |
|---|---|
| Clinical manifestations and complications | Increased susceptibility to bacterial infections, especially encapsulated organisms and opportunistic viral and fungal infections; autoimmune diseases, such as myasthenia gravis, neutropenia, pure red blood cell aplasia, and anemia |
| Laboratory findings | Hypogammaglobulinemia, low to absent levels of B cells, CD4+ T lymphopenia, reduced T-cell mitogen proliferation response |
| Treatment options | Resection of thymoma if malignant, invasive, or obstructive; immunoglobulin replacement; antibiotics if indicated; immunosuppressive agents if associated with autoimmune disease |
Presentation of Thymoma
The initial patient described by Good and Varco2 was a 58-year-old man who presented with a 4-year history of recurrent pulmonary infections. He had weak antibody response to poliomyelitis vaccine but no response to several other vaccines. His initial chest x-ray examination revealed a thymoma, and the infections continued despite resection of the thymoma. The incidence of hypogammaglobulinemia in patients with a thymoma is estimated to be 6% to 11%.4,5 Patients with recurrent sinopulmonary infections are often referred for chest x-ray examinations at which time an anterior mediastinal mass is discovered indicative of a thymoma (Figs 1 and 2). However, thymomas are missed on standard chest x-ray examinations in approximately 20% to 24% of cases,6 and although most appear as an anterior mediastinal mass, an occasional thymoma may occur within the lung parenchyma.7 Chest computed tomography may be more sensitive for the detection of a thymoma and for evaluation of pulmonary complications.8 Most resected thymomas in Good syndrome are found to be benign, well-encapsulated tumors, and 75% are of the spindle type (Fig 3).9 Treatment of the thymoma may be surgical removal or debulking if either malignant or locally obstructive. However, the immunological abnormalities are apparently not reversed by removal of the thymoma,4 suggesting that the hypogammaglobulinemia is not directly caused by the thymoma but rather secondary to an autoimmune or other immunoregulatory process. Furthermore, thymomas may appear on chest x-ray films years before the diagnosis of hypogammaglobulinemia. In a case described by Ide et al,10 a 61-year-old woman had undergone a thymectomy 17 years before having any infectious symptoms. When she presented with pulmonary symptoms, bronchoalveolar lavage fluid cells contained cytomegalic inclusion bodies and cytomegalovirus DNA was demonstrated. Her immunological findings included hypogammaglobulinemia, and she was diagnosed as having Good syndrome presenting with cytomegalovirus pneumonia.
Figure 1.
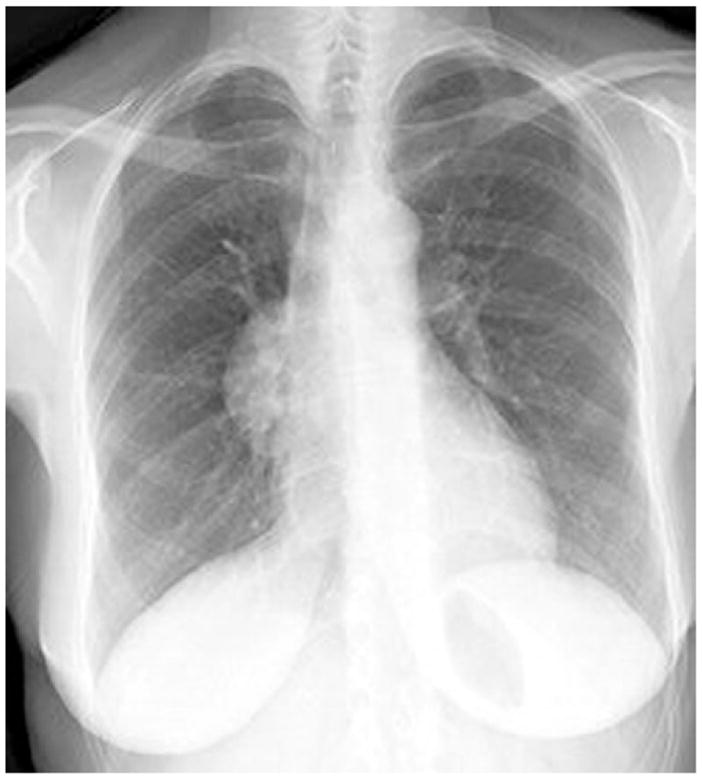
Chest x-ray film showing right mediastinal mass. Reprinted with permission from Alan Pestronk, MD, Department of Neurology, Washington University School of Medicine, St Louis, MO.
Figure 2.
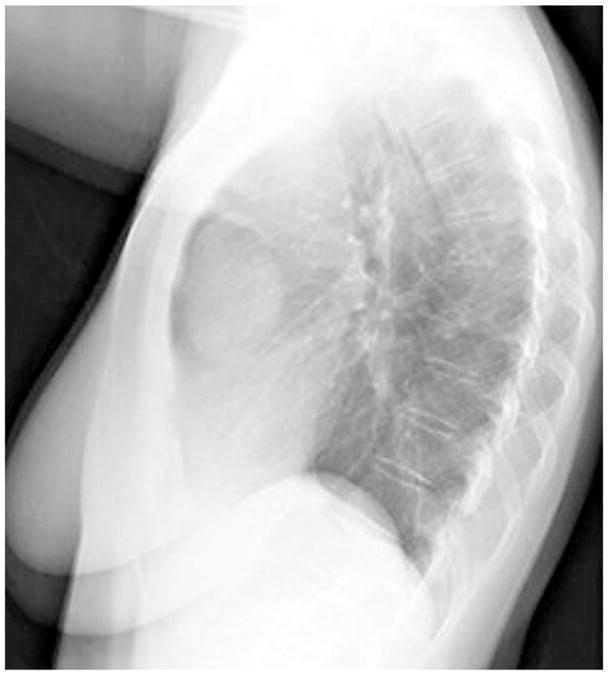
Chest x-ray film showing right anterior mediastinal mass. Reprinted with permission from Alan Pestronk, MD, Department of Neurology, Washington University School of Medicine, St Louis, MO.
Figure 3.
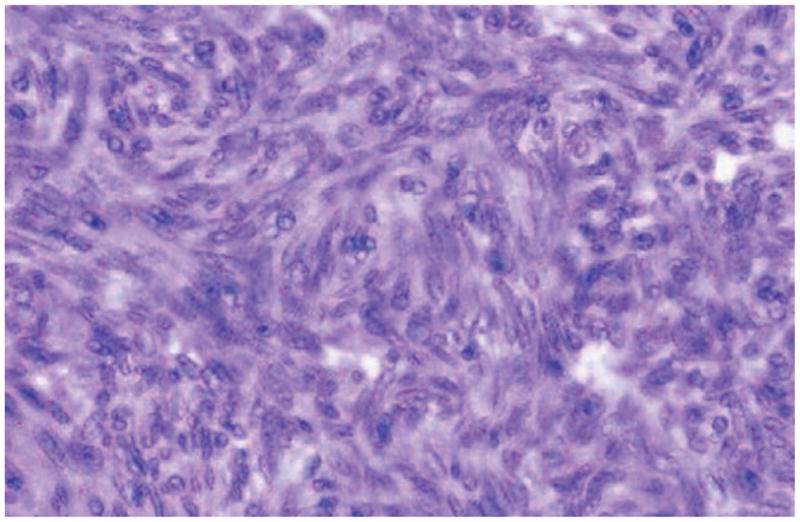
Spindle cell thymoma. Reprinted with permission of the American Society of Clinical Pathology from Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms: impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol. 2000;114: 760 –766.
Pathogenesis
The pathogenesis of Good syndrome is unknown; however, there are at least 3 suggested hypotheses. The first explanation derived from murine models demonstrates that cytokines such as limitin, an interferon-like cytokine produced by a bone marrow stromal cell line, influence B-cell precursor growth and differentiation, causing either cell arrest or impaired maturation.11 Second, many patients with Good syndrome experience opportunistic infections associated with defects in cell-mediated immunity, suggesting loss in either the naïve or memory CD4+ T-cell population.12 Third, studies of paraneoplastic phenomena in thymoma, such as pure red cell aplasia, show that T cells or autoantibodies can directly or indirectly inhibit erythropoiesis, suggesting that loss of B-cell function could be due to autoimmune destruction.13
Immunological Investigation
The immunologic hallmarks include few to absent levels of B peripheral cells, hypogammaglobulinemia, CD4+ T lymphopenia, and impaired T-cell response. Immunophenotyping is essential in detecting and monitoring immunodeficiency in patients with a thymoma, since treatment with IVIG may potentially replace the defective antibody production.14 IgG antibodies to toxoplasmosis and cytomegalovirus should be determined to evaluate whether a patient is at risk of reactivated infections. If immunologic test results are normal, testing should be performed periodically if the clinical suspicion of Good syndrome persists, because there can be an interval between the diagnosis of immunodeficiency and/or thymoma and development of infection.15
Infectious Complications
The initial clinical features of Good syndrome are varied. As reviewed by Tarr et al,1 in 51 patients, Good syndrome affects male and female patients equally. Most patients experienced infectious complications such as recurrent sinopulmonary infections secondary to encapsulated organisms (Haemophilus influenzae, Streptococcus pneumoniae), skin infections, bacterial diarrheas (Giardia lamblia, Salmonella spp, Campylobacter jejuni), and urinary tract infections. Herein, we described 2 unusual infections in patients with Good syndrome. Both illustrate typical features of Good syndrome, including the initial presentation of a mediastinal mass with a history of multiple sinopulmonary infections. Similar to other cases described in the literature,8,16 thymectomy in patient 1 did not lead to return of appropriate immune function.
In case 1, the patient with Good syndrome was found to have babesiosis. Babesiosis is a tickborne disease that is caused by a protozoa that infects red blood cells, leading to hemolysis. There are several species of Babesia; however, human disease is most commonly due to Babesia microti in the United States or Babesia divergens in Europe. Babesia have animal reservoirs such as rodents or cattle, but the disease is transmitted to humans via the ixodid tick. Definitive diagnosis is made by examination of the peripheral blood smear, which demonstrates intraerythrocytic parasites. Most cases in the United States occur in the Northeast region, specifically, the coastal areas off Massachusetts and islands near New York City, New Jersey, Rhode Island, and Connecticut.
Patient 1 had the first reported case of Good syndrome associated with babesiosis. We speculate that her manifestation of babesiosis occurred because of her underlying immunodeficiency, since 95% of patients infected are immuno-competent with normal spleens, and most of these are usually asymptomatic. Risk factors for more severe clinical disease include age older than 40 years, asplenia, and immunocompromised patients such as those with human immunodeficiency virus (HIV), corticosteroid administration, or other immunosuppressive therapy.17,18
Host defense mechanisms against the organism are mainly through cellular immunity. Mouse models have shown that spleen cells transferred from mice that cleared infection were protective if not depleted of CD4 cells. Furthermore, treatment with monoclonal antibodies to interferon-γ reduced the protective effect, and those mice deficient in interferon-γ were not protected from infection.19 Humoral immunity plays less of a role in host defense, and it is unclear whether protective immunity occurs in humans. Preventive measures include wearing long-sleeve garments and pants in endemic areas and examining the skin for ticks after exposure.
Opportunistic infections, such as Pneumocystis carinii and cytomegalovirus, which are not characteristic of other humoral immune defects (X-linked agammaglobulinemia or common variable immunodeficiency), develop more frequently in Good syndrome, suggesting still unclear defects in cell-mediated immunity. Kelleher and Misbah12 reviewed several studies of cell-mediated immunity that emphasized T-cell defects, manifested by cutaneous anergy to 2 or more test antigens or delayed rejection of skin allografts, as a common feature of Good syndrome. Furthermore, in vitro studies also show defects in T-lymphocyte proliferation and/or interleukin-2 production.9 Analysis of cellular immunity in patients with Good syndrome has shown that opportunistic infections can occur in the presence of greater CD4 T-cell numbers compared with that seen in patients with HIV.1
In case 2, we describe a patient who was found to have Kaposi sarcoma, an unusual skin tumor usually localized to the lower extremities. HHV-8 has been implicated in its development. Kaposi sarcoma was relatively rare until the early 1980s, when it was seen in homosexual men in association with HIV. In 1994, a new herpesvirus, now named HHV-8, was isolated and sequenced from more than 90% of Kaposi sarcoma tissues obtained from patients with acquired immunodeficiency syndrome (AIDS).20 Despite opportunistic infections occurring more commonly in patients with Good syndrome, there are few reported cases of associated Kaposi sarcoma, as described in case 2, without HIV infection or transplantation.21,22 Currently, 4 epidemiologic forms of Kaposi sarcoma exist: the classic form seen in Mediterranean or Eastern European males, usually affecting the lower extremities; an endemic or African form, which is not typically associated with immunodeficiency found in all parts of equatorial Africa, especially sub-Saharan Africa; a transplant-related form, which occurs after solid organ transplantation; and the AIDS-related or epidemic form, which occurs in HIV-infected individuals. Our patient is of Eastern European origin and thus may have been predisposed to the classic form of the disease; however, it is important to recognize that his underlying immunodeficiency and subsequently poor immune defense mechanisms may have played a role in manifestation of disease as well.
Autoimmune Association
Autoimmune diseases, such as myasthenia gravis, diabetes mellitus, polymyositis, pure red cell aplasia, aplastic anemia, and hemolytic anemia, may occur in association with Good syndrome.4,23–27 This association suggests a potential role of autoantibodies or autoreactive cell-mediated immunity in the pathogenesis of hypogammaglobulinemia. Ito et al28 reported a case in a patient with pure red cell aplasia accompanied by Good syndrome. The patient was a 57-year-old man with a mediastinal mass, hypogammaglobulinemia, and anemia. The bone marrow aspirate revealed markedly decreased hemopoiesis in erythroid cells. The patient underwent an extensive thymectomy with lymph node dissection and began treatment with prednisolone and cyclophosphamide. Although he received prophylactic treatment for infections, he developed herpes zoster, recurrent pneumonia, and cytomegalovirus retinitis. Thus, hypogammaglobulinemia and Good syndrome should also be suspected in patients with hematological and autoimmune diseases who develop unusual infections.
CONCLUSIONS
In patients with a thymoma, recognition of hypogammaglobulinemia is an important clinical consideration, since the prognosis for patients with Good syndrome appears worse when compared with common variable immunodeficiency or X-linked agammaglobulinemia.29 The major causes of death in patients with Good syndrome include infections, autoimmune diseases, and hematologic complications.1,12 In addition, the clinical course of disease may be more severe for those patients who require immunosuppressive agents for associated autoimmune processes. Therapy with IVIG should be given if humoral immunodeficiency is demonstrated, since IVIG can reduce the risk of infections, excess antibiotic administration, hospitalizations, and the development of pulmonary damage.
References
- 1.Tarr PE, Sneller MC, Mechanic LJ, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome): report of 5 cases and review of the literature. Medicine. 2001;80:123–133. doi: 10.1097/00005792-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Good RA, Varco RL. A clinical and experimental study of agammaglobulinemia. J Lancet. 1955;75:245–271. [PubMed] [Google Scholar]
- 3.Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 suppl 1):S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 4.Souadjian JV, Enriquez P, Silverstein MN, et al. The spectrum of diseases associated with thymoma: coincidence or syndrome. Arch Intern Med. 1974;134:374–379. [PubMed] [Google Scholar]
- 5.Rosenow EC, Hurley BT. Disorders of the thymus: a review. Arch Intern Med. 1984;144:763–772. [PubMed] [Google Scholar]
- 6.Brown LR, Muhm JR, Gray JE. Radiographic detection of thymoma. AJR Am J Roentgenol. 1980;134:1181–1188. doi: 10.2214/ajr.134.6.1181. [DOI] [PubMed] [Google Scholar]
- 7.Ryman NG, Burrow L, Bowen C, et al. Good’s syndrome with primary intrapulmonary thymoma. J R Soc Med. 2005;98:119–120. doi: 10.1258/jrsm.98.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arend SM, Dik H, van Dissel JT. Good’s syndrome: the association of thymoma and hypogammaglobulinemia. Clin Infect Dis. 2001;32:323–325. doi: 10.1086/318460. [DOI] [PubMed] [Google Scholar]
- 9.Raschal S, Sigel JN, Huml J, Richmond GW. Hypogammaglobulinemia and anemia 18 years after thymoma resection. J Allergy Clin Immunol. 1997;100:846–848. doi: 10.1016/s0091-6749(97)70283-5. [DOI] [PubMed] [Google Scholar]
- 10.Ide S, Koga T, Rikimaru T, et al. Good’s syndrome presenting with cytomegalovirus pneumonia. Intern Med. 2000;39:1094–1096. doi: 10.2169/internalmedicine.39.1094. [DOI] [PubMed] [Google Scholar]
- 11.Oritani K, Medina KL, Tomiyama Y, et al. Limitin: an interferon-like cytokine that preferentially influences B-lymphocyte precursors. Nat Med Immunol. 2000;3:659–666. doi: 10.1038/76233. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher P, Misbah SA. What is Good’s syndrome? immunological abnormalities in patients with thymoma. J Clin Pathol. 2003;56:12–16. doi: 10.1136/jcp.56.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles RJ, Sabo KM, Kidd PG, et al. The pathophysiology of pure red cell aplasia: implications for therapy. Blood. 1996;87:4381–4388. [PubMed] [Google Scholar]
- 14.Montella L, Masci AM, Merkabaoui G, et al. B-cell lymphopenia and hypogammaglobulinemia in thymoma patients. Ann Hematol. 2003;82:343–347. doi: 10.1007/s00277-003-0635-z. [DOI] [PubMed] [Google Scholar]
- 15.Tarr PE, Lucey DR. Good’s syndrome: the association of thymoma with immunodeficiency. Clin Infect Dis. 2001;33:585–586. doi: 10.1086/322708. [DOI] [PubMed] [Google Scholar]
- 16.Oshikiri T, Morikawa T, Sugiura H, Katoh H. Thymoma associated with hypogammaglobulinemia (Good’s syndrome): report of a case. Surg Today. 2002;32:264–266. doi: 10.1007/s005950200032. [DOI] [PubMed] [Google Scholar]
- 17.Meldrum SC, Birkhead GS, White DJ, et al. Human babesiosis in New York State: an epidemiological description of 136 cases. Clin Infect Dis. 1992;15:1019. doi: 10.1093/clind/15.6.1019. [DOI] [PubMed] [Google Scholar]
- 18.Pruthi RK, Marshall WF, Wiltsie JC, Persing DH. Human babesiosis. Mayo Clin Proc. 1995;70:853. doi: 10.1016/S0025-6196(11)63943-8. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi I, Suzuki R, Waki S, et al. Roles of CD4(+) T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect Immun. 1999;37:2887. doi: 10.1128/iai.67.8.4143-4148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 21.Moysset I, Lloreta J, Miguel A, et al. Thymoma associated with CD4+ lymphopenia, cytomegalovirus infection, and Kaposi’s sarcoma. Hum Pathol. 1997;28:1211–1213. doi: 10.1016/s0046-8177(97)90261-6. [DOI] [PubMed] [Google Scholar]
- 22.Sawai T, Tuchikawa K. Kaposi’s sarcoma developed in a patient with a thymoma in the setting of excess numbers of CD8-positive cells in the peripheral blood. Arch Pathol Lab Med. 1990;114:611–613. [PubMed] [Google Scholar]
- 23.International Union of Immunological Societies. Primary immunodeficiency diseases: report of an IUIS scientific committee. Clin Exp Immunol. 1999;118(suppl 1):1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asherson GL, Webster ADB. Diagnosis and Treatment of Immunodeficiency Diseases. Malden, MA: Blackwell Scientific Publications; 1980. [Google Scholar]
- 25.Murray WD, Webb JN. Thymoma associated with hypogammaglobulinaemia and pure red cell aplasia. Am J Med. 1966;41:974–980. doi: 10.1016/0002-9343(66)90054-4. [DOI] [PubMed] [Google Scholar]
- 26.Barnes RDS, O’Gorman P. Two cases of aplastic anaemia associated with tumors of the thymus. J Clin Pathol. 1962;15:264–268. doi: 10.1136/jcp.15.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mongan ES, Kern WA, Jr, Terry R. Hypogammaglobulinaemia with thymoma, haemolytic anemia, and disseminated infection with cytomegalovirus. Ann Intern Med. 1966;65:548–554. doi: 10.7326/0003-4819-65-3-548. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Kuriyama Y, Tauchi T, et al. A patient with pure red cell aplasia and Good’s syndrome. Haematologica. 1999;84:1048–1049. [PubMed] [Google Scholar]
- 29.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993;86:31–42. [PubMed] [Google Scholar]


