Abstract
The repair of DNA double-strand breaks (DSBs) is the major mechanism to maintain genomic stability in response to irradiation. We hypothesized that genetic polymorphisms in DSB repair genes may affect clinical outcomes among non-small cell lung cancer (NSCLC) patients treated with definitive radio(chemo)therapy. We genotyped six potentially functional single nucleotide polymorphisms (SNPs) (i.e., RAD51 −135G>C/rs1801320 and −172G>T/rs1801321, XRCC2 4234G>C/rs3218384 and R188H/rs3218536 G>A, XRCC3 T241M/rs861539 and NBN E185Q/rs1805794) and estimated their associations with overall survival (OS) and radiation pneumonitis (RP) in 228 NSCLC patients. We found a predictive role of RAD51 −135G>C SNP in RP development (adjusted hazard ratio [HR] = 0.52, 95% confidence interval [CI], 0.31–0.86, P = 0.010 for CG/CC vs. GG). We also found that RAD51 −135G>C and XRCC2 R188H SNPs were independent prognostic factors for overall survival (adjusted HR = 1.70, 95% CI, 1.14–2.62, P = 0.009 for CG/CC vs. GG; and adjusted HR = 1.70; 95% CI, 1.02–2.85, P = 0.043 for AG vs. GG, respectively) and that the SNP-survival association was most pronounced in the presence of RP. Our study suggests that HR genetic polymorphisms, particularly RAD51 −135G>C, may influence overall survival and radiation pneumonitis in NSCLC patients treated with definitive radio(chemo)therapy. Large studies are needed to confirm our findings.
Introduction
Lung cancer leads all other cancers in both incidence and mortality worldwide. Non-small cell lung cancer (NSCLC) accounts for 89% of all lung cancer, and most patients have advanced stages at diagnosis, requiring radiotherapy alone or in combination with chemotherapy to improve the local control and overall survival. However, despite aggressive treatment in these patients, the prognosis is still unsatisfactory, with a 5-year survival rate of about 10% [1] and a median survival time (MST) of 16–18 months [2]. Meanwhile, radiation treatment-related pulmonary toxicity, such as pneumonitis and pulmonary fibrosis, may influence the prognosis of NSCLC patients, because these complications restrict the dose of radiation used and compromise pulmonary functions. Therefore, there has been a persistent interest in search for readily accessible molecular markers that may help assess therapeutic benefits by predicting clinical outcomes of those NSCLC patients who are treated with definitive radio(chemo)therapy.
The DNA double-strand breaks (DSBs) are the principle genotoxic lesions of ionizing radiation, which pose major threats to genomic integrity. It is estimated that a dose of ∼1 Gy of X-rays produces about 50–100 double-strand breaks in the DNA of a typical mammalian cell, leading to 50% cell death [3], [4]. There are two main pathways for DNA DSB repair—homologous recombination (HR) and non-homologous end-joining (NHEJ). In HR, the initial step involves recognition and signaling of DSB by a protein complex of NBN, MRE11 and RAD50; then RAD51 protein is recruited to catalyze the strand exchange reaction and some RAD51-related proteins, such as RAD51 B-D, XRCC2 and XRCC3, participate in the assembly of the RAD51 nucleoprotein filament and the selection and interaction with the appropriate recombination substrates [5].
There is a genetic basis of cellular responses to ionizing radiation in cancer treatment, because patients receiving similar treatment could have different response to radiotherapy. Previous studies demonstrated that elevated expression of some DSB repair proteins, such as RAD51, NBN and XRCC3, confers radioresistance [6], [7], [8], whereas loss of XRCC2 results in a severe delay in the early response of DSB [9]. Patients of a rare congenital disorder, Nijmegen breakage syndrome, are extremely sensitive to radiation because of a compromised DSB repair capacity due to NBN mutations [10]. Hence, it is reasonable to speculate that the inter-individual variability in DSB repair capacity may modulate phenotype of radiosensitivity and clinical outcomes of radiotherapy.
Since single nucleotide polymorphisms (SNPs) may modify gene function or can be used as genetic markers to detect nearby disease-causing variants through association or linkage studies, we sought to evaluate the association of six potentially functional SNPs (i.e., RAD51 −135G>C/rs1801320, −172G>T/rs1801321, XRCC2 4234G>C/rs3218384, R188H/rs3218536 G>A, XRCC3 T241M/rs861539, and NBN E185Q/rs1805794) of genes involved in HR pathway with radiation pneumonitis (RP) and overall survival (OS) of NSCLC patients treated with definitive radio(chemo)therapy in the present study.
Materials and Methods
Ethics statement
This study was approved by The University of Texas M. D. Anderson Cancer Center Institutional Review Board and informed consents were waived. We complied with HIPAA regulations.
Study populations
In the study, clinical data were derived from a dataset of 261 patients with histopathologically confirmed NSCLC, who were treated with definitive radiation at our institution between 1999 and 2005. Among these 261 patients, 231 patients had documented survival information. After we excluded those patients who had surgical resection or recurrence before radiotherapy, the final data pool consisted of 228 patients with stage IA to IV NSCLC and survival information and 196 patients with pneumonitis information and radiation dosimetric data. The details of the radiation treatment planning, follow-up schedule and tests, and dosimetric data analysis were described in our previous publication [11].
Selection of SNPs and genotyping
We selected six common (minor allele frequency >0.05 in Caucasians), well-studied functional variants of RAD51, XRCC2, XRCC3 and NBN genes involved in HR pathway: RAD51 −135G>C/rs1801320, −172G>T/rs1801321, XRCC2 4234G>C/rs3218384, R188H/rs3218536 G>A, XRCC3 T241M/rs861539 and NBN E185Q/rs1805794. This is because they are located in promoter region or cause nonsynonymous amino acid changes that have been reportedly associated with cancer risk or survival. Although MRE11 and RAD50 genes are also important in the initial stage of response to DSB, no functional SNPs have been reported so far and therefore, these two genes were not included in our study. Genomic DNA was extracted from the buffy coat fraction of each blood sample by using a Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. DNA purity and concentrations were determined by spectrophotometric measurement of absorbance at 260 and 280 nm by a UV spectrophotometer (Nano Drop Technologies, Inc., Wilmington, DE). Genotypes were generated by the polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) method (Figure S1). The primer sequences, restriction enzymes and PCR conditions used for the experiments were available upon request.
Selection of end points
We selected overall survival and occurrence of radiation pneumonitis as our study end points. There are five grades of radiation-treatment related pneumonitis according to the Common Terminology Criteria for Adverse Events version 3.0 [12]. Previous studies have used two different types of criterion in RP investigation: RP of any grade (grade≥1) [13], [14], [15] and RP requiring clinical intervention (grade≥2 or 3) [16], [17]. In this study, we used RP of any grade (grade≥1) to include more RP events. All times to the endpoints were calculated from the first day of radiation treatment until the date of event or the last known follow-up.
Statistical methods
The hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) were calculated by Cox proportional hazard analyses to evaluate the influence of various genotypes on overall survival and radiation pneumonitis. The HRs for OS were adjusted for age, sex, race, Karnofsky performance scores (KPS), smoking status, tumor histology, disease stage, application of chemotherapy and radiation dose; whereas the HRs for RP were adjusted for age, sex, race, KPS, smoking status, tumor histology, disease stage, application of chemotherapy and mean lung dose because mean lung dose is the traditional dosimetric factor to be associated with RP. Kaplan-Meier analysis was used to evaluate the effect of different genotypes on the cumulative probability of overall survival and radiation pneumonitis. All reported P values were two-sided, and P<0.05 indicates statistical significance. All analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC). The Bonferroni method was used to adjust for multiple comparisons.
Results
Population characteristics
The 228 patients used for the final analysis included 125 men and 103 women, with a median age of 63 years (range, 35 to 88 years). Among them, 74.6% were white, 82.5% had stage III/IV disease, 88.2% were treated with a combination of chemotherapy and radiotherapy, and 96.8% received radiation doses between 60 and 70 Gy. The median overall survival time was 20 months and the median time for RP development was 4.3 months. Patient-, disease-, and treatment-related characteristics and their influences on OS and RP (grade≥1) are shown in Table 1. In the univariate analysis, we found that male, tumor stage of III/IV, and histology of “not otherwise specified” were significantly associated with reduced OS, whereas tumor stage of I/II seemed to be associated with reduced RP. The most commonly used dosimetric factor of mean lung dose was only available for the 196 patients who were treated with 3-dimensional conformal radiation and who had pneumonitis information and was marginally associated with RP development in our analysis (Table 1).
Table 1. Patient demographics and association with clinical outcomes of overall survival (OS) and radiation pneumonitis (RP).
| OS (N = 228) | RP (grade≥1, N = 196) | |||||||
| Parameter | No. (%) | HR | 95% CI | P † | No. (%) | HR | 95% CI | P † |
| Sex | ||||||||
| Female | 103 (45.2) | 1.00 | 83 (42.4) | 1.00 | ||||
| Male | 125 (54.8) | 1.51 | 1.08–2.11 | 0.017 | 113 (57.6) | 1.09 | 0.78–1.54 | 0.608 |
| Age (years) | ||||||||
| <63 | 113 (49.6) | 1.00 | 97 (49.5) | 1.00 | ||||
| ≥63 | 115 (50.4) | 1.37 | 0.96–1.94 | 0.080 | 99 (50.5) | 1.02 | 0.73–1. 43 | 0.900 |
| Race | ||||||||
| White | 170 (74.6) | 1.00 | 141 (71.9) | 1.00 | ||||
| Black | 45 (19.7) | 0.96 | 0.60–1.54 | 0.875 | 42 (21.4) | 1.21 | 0.81–1.81 | 0.360 |
| Other | 13 (5.7) | 1.51 | 0.73–3.12 | 0.269 | 13 (6.7) | 1.11 | 0.54–2.28 | 0.776 |
| KPS | ||||||||
| <80 | 59 (25.9) | 1.00 | 40 (20.4) | 1.00 | ||||
| ≥80 | 169 (74.1) | 0.71 | 0.46–1.11 | 0.133 | 156 (79.6) | 1.02 | 0.61–1.71 | 0.934 |
| TNM stage§ | ||||||||
| III, IV | 188 (82.5) | 1.00 | 167 (85.2) | 1.00 | ||||
| I, II | 34 (14.9) | 0.55 | 0.32–0.94 | 0.029 | 29 (14.8) | 0.55 | 0.33–0.93 | 0.026 |
| Histology§ | ||||||||
| Adenocar | 77 (33.8) | 1.00 | 66 (33.7) | 1.00 | ||||
| NSCLC, NOS | 74 (32.5) | 1.56 | 1.04–2.33 | 0.032 | 67 (34.2) | 0.99 | 0.66–1.47 | 0.949 |
| Squamous cell | 70 (30.7) | 1.41 | 0.92–2.14 | 0.138 | 63 (32.1) | 0.73 | 0.48–1.12 | 0. 471 |
| Smoking status | ||||||||
| Ever | 200 (87.7) | 1.00 | 177 (90.8) | 1.00 | ||||
| Never | 28 (12.3) | 1.07 | 0.61–1.89 | 0.798 | 18 (9.2) | 1.36 | 0.77–2.40 | 0.292 |
| Chemotherapy§ | ||||||||
| No | 22 (9.6) | 1.00 | 20 (10.3) | 1.00 | ||||
| Yes | 201 (88.2) | 1.02 | 0.54–1.91 | 0.957 | 175 (89.7) | 1.61 | 0.84–3.07 | 0.151 |
| Mean lung dose (Gy) | ||||||||
| <17.67 | 57 (29.1) | 1.00 | ||||||
| ≥17.67 | 139 (70.9) | 1.40 | 0.95–2.04 | 0.087 | ||||
| Radiation dose (Gy) | ||||||||
| <63 | 46 (20.2) | 1.00 | 36 (18.4) | 1.00 | ||||
| ≥63 | 182 (79.8) | 0.79 | 0.50–1.21 | 0.272 | 160 (81.6) | 0.73 | 0.47–1.13 | 0.154 |
P values were calculated by univariate Cox proportional hazards model.
The numbers for certain variables may not add up to the total number because of the missing information.
NOS: Not otherwise specified; KPS: Karnofsky Performance Score.
Association between overall survival and polymorphisms
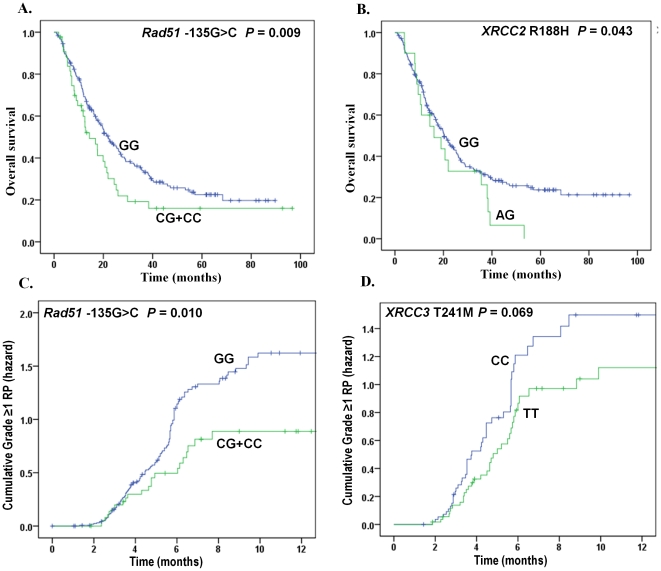
The genotype distributions of SNPs in genes involved in the HR pathway and the association with OS are summarized in Table 2. Compared with the homozygote RAD51 −135GG genotype, the CG/CC genotypes were marginally associated with a hazard of early death in the univariate analysis (HR = 1.46, 95% CI, 0.99–2.14, P = 0.056). After a multivariate adjustment for age, sex, smoking status, tumor histology, KPS, tumor stage, application of chemotherapy, and radiation dose, the HRs were statistically significant in both RAD51 −135G>C and XRCC2 R188H SNPs (CG/CC vs. GG: adjusted HR = 1.70; 95% CI, 1.14–2.62, P = 0.009; and AG vs. GG: adjusted HR = 1.70; 95% CI, 1.02–2.85, P = 0.043, respectively) (Table 2 and Fig. 1A, 1B).
Table 2. Univariate and multivariate analyses of different genotypes and OS for NSCLC (N = 228).
| Genotypes | Patient No. | Event | Crude | P * | Adjusted | P † | ||
| HR | 95% CI | HR | 95% CI | |||||
| RAD51 | ||||||||
| (rs1801320 −135G>C) | ||||||||
| GG | 183 | 124 | 1.00 | 1.00 | ||||
| CG | 39 | 29 | 1.53 | 1.02–2.30 | 0.040 | 1.71 | 1.11–2.64 | 0.014 |
| CC | 5 | 4 | 1.07 | 0.39–2.88 | 0.900 | 1.52 | 0.54–4.24 | 0.429 |
| CG+CC | 44 | 33 | 1.46 | 0.99–2.14 | 0.056 | 1.70 | 1.14–2.62 | 0.009 |
| RAD51 | ||||||||
| (rs1801321 −172G>T) | ||||||||
| TT | 144 | 100 | 1.00 | 1.00 | ||||
| TG | 84 | 58 | 1.14 | 0.82–1.58 | 0.429 | 1.03 | 0.73–1.45 | 0.879 |
| GG | 0 | 0 | N/A | N/A | ||||
| TG+GG | 84 | 58 | 1.14 | 0.82–1.58 | 0.429 | 1.03 | 0.73–1.45 | 0.879 |
| XRCC2 | ||||||||
| (rs3218384 4234G>C) | ||||||||
| GG | 137 | 97 | 1.00 | 1.00 | ||||
| GC | 78 | 53 | 0.77 | 0.55–1.08 | 0.125 | 0.81 | 0.56–1.17 | 0.260 |
| CC | 13 | 8 | 0.63 | 0.30–1.29 | 0.202 | 0.61 | 0.28–1.35 | 0.221 |
| GC+CC | 91 | 61 | 0.75 | 0.54–1.03 | 0.075 | 0.76 | 0.54–1.09 | 0.135 |
| XRCC2 | ||||||||
| (rs3218536 G>A R188H) | ||||||||
| GG | 205 | 138 | 1.00 | 1.00 | ||||
| AG | 20 | 18 | 1.44 | 0.88–2.36 | 0.144 | 1.70 | 1.02–2.85 | 0.043 |
| AA | 0 | 0 | N/A | N/A | ||||
| AG+AA | 20 | 18 | 1.44 | 0.88–2.36 | 0.144 | 1.70 | 1.02–2.85 | 0.043 |
| XRCC3 | ||||||||
| (rs861539 C>T T241M) | ||||||||
| CC | 71 | 46 | 1.00 | 1.00 | ||||
| CT | 98 | 73 | 1.22 | 0.84–1.77 | 0.297 | 1.17 | 0.79–1.72 | 0.428 |
| TT | 59 | 39 | 0.91 | 0.60–1.40 | 0.681 | 0.89 | 0.57–1.38 | 0.593 |
| CT+TT | 157 | 112 | 1.09 | 0.78–1.54 | 0.613 | 1.06 | 0.74–1.51 | 0.766 |
| NBN | ||||||||
| (rs1805794 E185Q) | ||||||||
| CC | 109 | 71 | 1.00 | 1.00 | ||||
| CG | 98 | 72 | 1.04 | 0.75–1.44 | 0.832 | 1.06 | 0.75–1.51 | 0.735 |
| GG | 20 | 14 | 1.06 | 0.60–1.87 | 0.850 | 0.89 | 0.48–1.65 | 0.707 |
| CG+GG | 118 | 86 | 1.08 | 0.79–1.47 | 0.653 | 1.06 | 0.76–1.49 | 0.720 |
*P values were calculated by Cox proportional model using univariate analysis.
P values were calculated with adjustment for age, sex, smoking status, tumor histology, KPS score, tumor stage, application of chemotherapy and radiation dose.
Figure 1. Overall survival and risk of radiation pneumonitis by selected polymorphisms of DNA double-strand break genes.
(A) and (B), overall survival by RAD51 −135G>C and XRCC2 R188H SNPs; (C) and (D), radiation pneumonitis by RAD51 −135G>C and XRCC3 T241M SNPs. The P values were obtained from the Cox hazards model with adjustment.
Association between radiation pneumonitis and polymorphisms
The distributions of genotypes and their association with RP (grade≥1) are summarized in Table 3. Similar to the data of OS, only RAD51 −135G>C SNP showed a significant association with RP in the univariate analysis (CG/CC vs. GG: HR = 0.56, 95% CI, 0.35–0.90, P = 0.017). After a multivariate adjustment, the significance still remained for RAD51 −135G>C SNP in the dominant model (CG/CC vs. GG: HR = 0.52, 95% CI, 0.31–0.86, P = 0.010). The TT genotype of XRCC3 T241M SNP showed a marginally protective effect against RP in the additive model (TT vs. CC: HR = 0.63, 95% CI, 0.38–1.04, P = 0.069) (Table 3 and Fig. 1C, 1D).
Table 3. Univariate and multivariate analyses of different genotypes and RP (grade≥1) for NSCLC (N = 196).
| Genotypes | Patient No. | Event | Crude | P * | Adjusted | P † | ||
| HR | 95% CI | HR | 95% CI | |||||
| RAD51 | ||||||||
| (rs1801320 −135G>C) | ||||||||
| GG | 156 | 115 | 1.00 | 1.00 | ||||
| CG | 34 | 18 | 0.55 | 0.34–0.91 | 0.020 | 0.50 | 0.29–0.84 | 0.009 |
| CC | 5 | 3 | 0.60 | 0.15–2.44 | 0.477 | 0.80 | 0.19–3.37 | 0.764 |
| CG+CC | 39 | 21 | 0.56 | 0.35–0.90 | 0.017 | 0.52 | 0.31–0.86 | 0.010 |
| RAD51 | ||||||||
| (rs1801321 −172G>T) | ||||||||
| TT | 125 | 89 | 1.00 | 1.00 | ||||
| TG | 71 | 48 | 0.82 | 0.58–1.17 | 0.279 | 0. 730 | 0.50–1.08 | 0.117 |
| GG | 0 | 0 | N/A | N/A | ||||
| TG+GG | 71 | 48 | 0.82 | 0.58–1.17 | 0.279 | 0. 730 | 0.50–1.08 | 0.117 |
| XRCC2 | ||||||||
| (rs3218384 4234G>C) | ||||||||
| GG | 120 | 85 | 1.00 | 1.00 | ||||
| GC | 66 | 47 | 1.03 | 0.72–1.47 | 0.870 | 1.21 | 0.81–1.80 | 0.361 |
| CC | 10 | 5 | 0.86 | 0.35–2.13 | 0.748 | 0.90 | 0.35–2.32 | 0. 830 |
| GC+CC | 76 | 52 | 1.01 | 0.72–1.43 | 0.949 | 1.17 | 0.79–1.73 | 0.437 |
| XRCC2 | ||||||||
| (rs3218536 G>A R188H) | ||||||||
| GG | 173 | 126 | 1.00 | 1.00 | ||||
| AG | 16 | 8 | 0.56 | 0.27–1.14 | 0.107 | 0.55 | 0.25–1.19 | 0.129 |
| AA | 0 | 0 | N/A | N/A | ||||
| AG+AA | 16 | 8 | 0.56 | 0.27–1.14 | 0.107 | 0.55 | 0.25–1.19 | 0.129 |
| XRCC3 | ||||||||
| (rs861539 C>T T241M) | ||||||||
| CC | 58 | 43 | 1.00 | 1.00 | ||||
| CT | 81 | 59 | 0.90 | 0.61–1.33 | 0.591 | 0.92 | 0.60–1.40 | 0.690 |
| TT | 57 | 35 | 0.70 | 0.45–1.09 | 0.119 | 0.63 | 0.38–1.04 | 0.069 |
| CT+TT | 138 | 94 | 0.81 | 0.57–1.17 | 0.263 | 0.80 | 0.54–1.19 | 0.271 |
| NBN | ||||||||
| (rs1805794 E185Q) | ||||||||
| CC | 93 | 68 | 1.00 | 1.00 | ||||
| CG | 85 | 57 | 0.98 | 0.69–1.39 | 0.909 | 1.09 | 0.75–1.60 | 0.651 |
| GG | 16 | 11 | 1.12 | 0.59–2.13 | 0.722 | 1.09 | 0.54–2.19 | 0.805 |
| CG+GG | 101 | 68 | 1.00 | 0.71–1.40 | 1.000 | 1.10 | 0.76–1.58 | 0.629 |
*P values were calculated by Cox proportional model using univariate analysis.
P values were calculated with adjustment for age, sex, smoking status, tumor histology, KPS score, tumor stage, application of chemotherapy and mean lung dose.
Modification effects of RP by SNPs of RAD51 −135G>C and XRCC2 R188H on overall survival
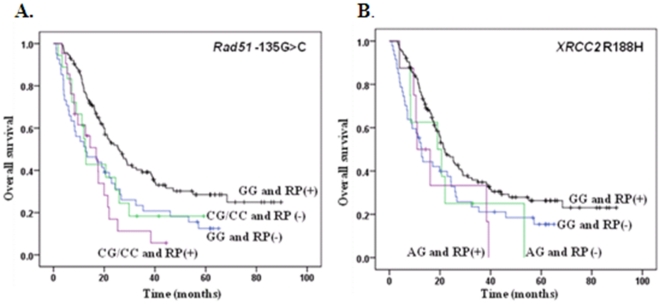
Since RAD51 −135G>C and XRCC2 R188H SNPs were also independent prognostic factors of overall survival, we then investigated the modification effect of RP by SNPs on OS. We found that both RAD51 −135G>C and XRCC2 R188H SNPs showed remarkable influence on OS only in the presence of RP (adjusted HR = 3.03. 95% CI, 1.69–5.45, P<0.001; and adjusted HR = 2.67. 95% CI, 1.13–6.29, P = 0.025, respectively) (Table 4 and Fig. 2). There was no difference in the death hazard between genotypes if RP was absent.
Table 4. RAD51 −135G>C and XRCC2 R188H genotypes and OS by RP (N = 196).
| Genotypes | Patient No. | Event | Crude | P * | Adjusted | P † | ||
| HR | 95% CI | HR | 95% CI | |||||
| RAD51 | ||||||||
| (rs1801320 −135G>C) | ||||||||
| Absence of RP | ||||||||
| GG | 41 | 35 | 1.00 | 1.00 | ||||
| CG+CC | 18 | 14 | 0.93 | 0.50–1.73 | 0.817 | 0.81 | 0.39–1.68 | 0.575 |
| Presence of RP | ||||||||
| GG | 115 | 73 | 1.00 | 1.00 | ||||
| CG+CC | 21 | 18 | 2.45 | 1.45–4.14 | 0.001 | 3.03 | 1.69–5.45 | <0.001 |
| XRCC2 | ||||||||
| (rs3218536 G>A R188H) | ||||||||
| Absence of RP | ||||||||
| GG | 47 | 38 | 1.00 | 1.00 | ||||
| AG | 8 | 7 | 1.02 | 0.45–2.29 | 0.889 | 1.22 | 0.51–2.92 | 0.653 |
| Presence of RP | ||||||||
| GG | 126 | 83 | 1.00 | 1.00 | ||||
| AG | 8 | 7 | 1.98 | 0.92–4.30 | 0.080 | 2.67 | 1.13–6.29 | 0.025 |
*P values were calculated by Cox proportional model using the univariate analysis.
P values were calculated with adjustment for age, sex, smoking status, tumor histology, KPS score, tumor stage, application of chemotherapy and radiation dose.
Figure 2. Overall survival by selected polymorphisms of DNA double-strand break genes according to the presence or absence of RP.
Bonferroni corrections
Because multiple testing may have been contributed to the findings in the evaluation of the statistical significance of our primary results, we also considered adjustment for the associations with the outcomes. After Bonferroni corrections, only RAD51 −135G>C SNP remained significant for its association with both OS and RP.
Discussion
To our knowledge, this is the first study to investigate the association between clinical outcomes and potentially functional polymorphisms of genes in the HR pathway in NSCLC patients treated with radiotherapy, with a reasonable sample size (a total of 228). We provided a strong evidence of the predictive value of RAD51 −135G>C SNP for RP development. RAD51 −135G>C and XRCC2 R188H SNPs were also independent prognostic factors of overall survival, and the SNP-survival association was most pronounced in the presence of RP.
Lung cancer is a disease resulting from interactions between genetic and environmental factors, and its progression and prognosis are also heavily influenced by patient host or treatment-related factors. Some well-known prognostic factors in inoperable NSCLC patients, such as being male, old age and advanced tumor stage [18], were present in the current study. Although the mean lung dose was marginally associated with RP development by the criterion of grade≥1, it was significantly associated with grade≥3 RP (data not shown), which was consistent with our previous report [19]. The most important finding from the current study, however, is the modification effect of RP by HR genetic polymorphisms on OS in NSCLC patients receiving radio(chemo)therapy.
Current models of HR-mediated DSB repair suggest that DSBs are first recognized and bound by the MRE11-RAD50-NBN (MRN) complex. MRE11 has both endonuclease and exonuclease activities that are important for DNA end processing, and RAD50 can bind to DNA that may be involved in tethering sister chromatids, whereas NBN forms the flexible adapter domain of MRN and provides the MRN complex with its signaling role through interactions and activation of its downstream proteins that ultimately activate the RAD51-dependent HR pathway [20].
The RAD51 gene family consists of several proteins that show DNA-stimulated ATPase activity and plays a central role in the HR activation. It interacts directly with several repair proteins, including XRCC2, XRCC3, BRCA1/2, to form a complex essential for repair of DNA cross-links (especially XRCC2 and XRCC3), which maintains genomic stability by using the sister chromatid as a template for precise repair. Previous studies found some potentially functional SNPs of HR genetic polymorphisms (e.g., RAD51-135 G>C, −172G>T, XRCC2 4234G>C, R188H, XRCC3 T241M and NBN E185Q) to be associated with various types of cancer risks, including cancers of the lung, breast, ovary, leukemia and head and neck [21]. Some SNPs also showed a significant effect on survival outcomes, such as RAD51-135 G>C in breast cancer [22], XRCC2 R188H in pancreatic cancer [23], and SNPs of RAD51-135 G>C and XRCC3 T241M in acute myeloid leukemia [24]. However, most studies performed in prostate or breast cancer failed to find any significant association with risk of radiation-induced complications [25], [26]. In the current study, there appeared a significant SNP-survival association (XRCC2 R188H) and a marginally significant SNP-RP association (XRCC3 T241M) in radiation-treated NSCLC patients. Most importantly, the SNP of the central HR gene, RAD51 −135G>C SNP, was robustly associated with both OS and RP after Bonferroni corrections. This result was confirmed by receiver operating characteristic (ROC) curves analyses, which showed that RAD51 −135G>C in particular was highly valuable to improve the predictive power of assessing clinical outcomes (e.g., OS and RP) of NSCLC patients treated with radiotherapy (data not shown). Taken together, our results demonstrated the importance of the HR pathway in response to thoracic radiation and possible tumor-specific influence from HR genetic polymorphisms.
To date, the functional changes of RAD51, XRCC2, XRCC3 and NBN caused by the SNPs included in the current study have not been well studied. Some studies found that the threonine-to-methionine substitution at codon 241 of the XRCC3 gene (T241M) was associated with radiosensitivity in non-cancer subjects but did not cause significant difference in DNA repair capacity between the variant and wild-type genotypes [27], [28], while the glutamine-to-glutamic acid substitution at codon 185 of NBN gene (E185Q) may influence the interaction between NBN and BRCA1 proteins responsible for recognition and repair of aberrant DNA [29], [30]. However, the role of arginine-to-histidine substitution at codon 188 of the XRCC2 gene (R188H) is largely unknown. The −135 G>C SNP, located in the promoter region of the RAD51 gene, results in the up-regulated gene expression through an increased promoter activity by substituting G for C allele [31]. RAD51 is essential for optimal repair of DSBs, and its expression level was a single important factor in modifying DSB repair capacity as reported in previous studies [32], [33]. Based on these findings, the association of the RAD51 −135C allele with a decreased hazard for RP and a reduced OS can be reasonably explained by an increased radioresistance due to the anticipated up-regulated RAD51 expression. Although the functional relevance of XRCC2 R188H with clinical outcomes of NSCLC patients has not been clarified, it may either result from its effect on the gene functions or its linkage with other functional SNPs, which need to be unraveled by additional mechanistic studies.
The relationship among RP, OS and SNPs of NSCLC patients was complex and could only be explored preliminarily in the current investigation. Inoue et al. reported severe RP (grade 3–4) to be an adverse prognostic factor [34], whereas our study showed RAD51 G>C to be associated with a reduced RP incidence but a decreased OS. This is not contradictory, because our RP group included a large proportion of patients with mild/moderate RP (grade 1 and 2), which was not likely to have an impact on patients' survival negatively. Furthermore, some other studies also did not find any association between RP grade and prognosis [35].
In this study, we were much interested in the finding that the influences of RAD51 −135G>C and XRCC2 R188H on overall survival were only evident in patients with RP. Several possibilities may explain these findings. First, irradiation of lung tissues induces immediate damage through intracellular protein denaturation, membrane disruption, and alterations of DNA. RP is largely a consequence of cellular DNA injury that appears in the second generation of cells [36]. The absence of RP may imply a low amount of DNA injury, which could result from an efficient DNA repair, whereas the presence of RP may indicate a high amount of DNA injury due to a suboptimal DNA repair. Therefore, it can be speculated that if the host cells have optimal DNA repair capacity, the activity of the entire HR machinery is less likely to be influenced by a single HR genetic polymorphism. On the contrary, if the DNA repair capacity is suboptimal, the subsequent changes in gene expression or activity resulting from functional SNPs may pose a substantial influence on the whole HR machinery. Second, RP per se is an inflammatory response to ionizing radiation, which triggers a large network of signaling events, including transient activation of pro-survival pathways such as the epidermal growth factor receptor (EGFR) pathway [37], and upregulation of a variety of cytokines, such as tumor necrosis factor alpha (TNF-α), interleukins, and transforming growth factor beta (TGF-β) [38], [39], [40]. Although many of these inflammatory responses are harmful to normal tissue, they confer a survival advantage of tumor cells and regulate cellular radiation response and DNA repair. Hence, the SNP-survival association could be more pronounced in the presence of RP. Additional mechanistic studies are needed to test these hypotheses.
Despite these positive findings, our study has some limitations. First, we were not able to explore the mechanism of how the HR genetic polymorphisms and RP influence survival outcomes of lung cancer patients. Secondly, we used a candidate polymorphism approach, which allowed us to focus on potentially functional SNPs reported in the literatures but did not comprehensively cover all SNPs in the entire gene. Some important SNPs may have been missed or the observed association may result from genetic linkages with other untyped SNPs. These warrant additional investigation of the tagging SNPs that may help find the underlying disease-causing variants in future studies. Thirdly, our sample size is still not large enough to perform stratified analyses for identifying the high-risk subgroups.
In summary, we found that HR genetic polymorphisms, particularly RAD51 −135G>C, may influence the overall survival and risk of radiation pneumonitis in NSCLC patients treated with definitive radio(chemo)therapy. Future prospective studies with large sample sizes and better study designs are required to confirm our findings.
Supporting Information
PCR-based restriction analysis. Genotypes of the RAD51, XRCC2, XRCC3 and NBN SNPs were shown on agarose electrophoresis.
(TIF)
Acknowledgments
We thank Kejing Xu, Jianzhong He and Min Zhao for laboratory assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was in part supported by National Institutes of Health grants R01 ES011740 and CA0131274 (to Q. W.) and CA 16672 (to M. D. Anderson Cancer Center). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 2.Cullen MH, Billingham LJ, Woodroffe CM, Chetiyawardana AD, Gower NH, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: effects on survival and quality of life. J Clin Oncol. 1999;17:3188–3194. doi: 10.1200/JCO.1999.17.10.3188. [DOI] [PubMed] [Google Scholar]
- 3.Nikjoo H, O'Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res. 2001;156:577–583. doi: 10.1667/0033-7587(2001)156[0577:cafdts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Ward JF. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: a review. Int J Radiat Biol. 1990;57:1141–1150. doi: 10.1080/09553009014551251. [DOI] [PubMed] [Google Scholar]
- 5.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 6.Qiao GB, Wu YL, Yang XN, Zhong WZ, Xie D, et al. High-level expression of Rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br J Cancer. 2005;93:137–143. doi: 10.1038/sj.bjc.6602665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohnishi K, Scuric Z, Schiestl RH, Okamoto N, Takahashi A, et al. siRNA targeting NBS1 or XIAP increases radiation sensitivity of human cancer cells independent of TP53 status. Radiat Res. 2006;166:454–462. doi: 10.1667/RR3606.1. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa T, Urade M, Yamamoto Y, Furuyama J. Increased expression of human DNA repair genes, XRCC1, XRCC3 and RAD51, in radioresistant human KB carcinoma cell line N10. Oral Oncol. 1998;34:524–528. doi: 10.1016/s1368-8375(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 9.Tambini CE, Spink KG, Ross CJ, Hill MA, Thacker J. The importance of XRCC2 in RAD51-related DNA damage repair. DNA Repair (Amst) 2010;9:517–525. doi: 10.1016/j.dnarep.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Tauchi H, Matsuura S, Kobayashi J, Sakamoto S, Komatsu K. Nijmegen breakage syndrome gene, NBS1, and molecular links to factors for genome stability. Oncogene. 2002;21:8967–8980. doi: 10.1038/sj.onc.1206136. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Liao Z, Wei X, Liu HH, Tucker SL, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, version 3. 2003 Available: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_glossary.pdf. Accessed 2011 April 25. [Google Scholar]
- 13.Armstrong J, Raben A, Zelefsky M, Burt M, Leibel S, et al. Promising survival with three-dimensional conformal radiation therapy for non-small cell lung cancer. Radiother Oncol. 1997;44:17–22. doi: 10.1016/s0167-8140(97)01907-5. [DOI] [PubMed] [Google Scholar]
- 14.Hernando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 15.Fu XL, Huang H, Bentel G, Clough R, Jirtle RL, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys. 2001;50:899–908. doi: 10.1016/s0360-3016(01)01524-3. [DOI] [PubMed] [Google Scholar]
- 16.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 17.Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. doi: 10.1016/s0360-3016(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 18.Maeda T, Ueoka H, Tabata M, Kiura K, Shibayama T, et al. Prognostic factors in advanced non-small cell lung cancer: elevated serum levels of neuron specific enolase indicate poor prognosis. Jpn J Clin Oncol. 2000;30:534–541. doi: 10.1093/jjco/hyd139. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Liao Z, Liu Z, Wang LE, Tucker SL, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27:3370–3378. doi: 10.1200/JCO.2008.20.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams GJ, Lees-Miller SP, Tainer JA. Mre11-Rad50-Nbs1 conformations and the control of sensing, signaling, and effector responses at DNA double-strand breaks. DNA Repair (Amst) 2010;9:1299–1306. doi: 10.1016/j.dnarep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chistiakov DA, Voronova NV, Chistiakov PA. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008;47:809–824. doi: 10.1080/02841860801885969. [DOI] [PubMed] [Google Scholar]
- 22.Costa S, Pinto D, Pereira D, Rodrigues H, Cameselle-Teijeiro J, et al. XRCC1 Arg399Gln and RAD51 5′UTR G135C polymorphisms and their outcome in tumor aggressiveness and survival of Portuguese breast cancer patients. Breast Cancer Res Treat. 2008;109:183–185. doi: 10.1007/s10549-007-9637-1. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Liu H, Jiao L, Chang DZ, Beinart G, et al. Significant effect of homologous recombination DNA repair gene polymorphisms on pancreatic cancer survival. Cancer Res. 2006;66:3323–3330. doi: 10.1158/0008-5472.CAN-05-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Yang L, Zhang Y, Xu ZF, Yu MH, et al. Polymorphisms of RAD51(G135C) and XRCC3(C241T) genes and correlations thereof with prognosis and clinical outcomes of acute myeloid leukemia. Zhonghua Yi Xue Za Zhi. 2008;88:378–382. [PubMed] [Google Scholar]
- 25.Damaraju S, Murray D, Dufour J, Carandang D, Myrehaug S, et al. Association of DNA repair and steroid metabolism gene polymorphisms with clinical late toxicity in patients treated with conformal radiotherapy for prostate cancer. Clin Cancer Res. 2006;12:2545–2554. doi: 10.1158/1078-0432.CCR-05-2703. [DOI] [PubMed] [Google Scholar]
- 26.Popanda O, Tan XL, Ambrosone CB, Kropp S, Helmbold I, et al. Genetic polymorphisms in the DNA double-strand break repair genes XRCC3, XRCC2, and NBS1 are not associated with acute side effects of radiotherapy in breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:1048–1050. doi: 10.1158/1055-9965.EPI-06-0046. [DOI] [PubMed] [Google Scholar]
- 27.Aka P, Mateuca R, Buchet JP, Thierens H, Kirsch-Volders M. Are genetic polymorphisms in OGG1, XRCC1 and XRCC3 genes predictive for the DNA strand break repair phenotype and genotoxicity in workers exposed to low dose ionising radiations? Mutat Res. 2004;556:169–181. doi: 10.1016/j.mrfmmm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Araujo FD, Pierce AJ, Stark JM, Jasin M. Variant XRCC3 implicated in cancer is functional in homology-directed repair of double-strand breaks. Oncogene. 2002;21:4176–4180. doi: 10.1038/sj.onc.1205539. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi J, Antoccia A, Tauchi H, Matsuura S, Komatsu K. NBS1 and its functional role in the DNA damage response. DNA Repair (Amst) 2004;3:855–861. doi: 10.1016/j.dnarep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, et al. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 31.Hasselbach L, Haase S, Fischer D, Kolberg HC, Sturzbecher HW. Characterisation of the promoter region of the human DNA-repair gene Rad51. Eur J Gynaecol Oncol. 2005;26:589–598. [PubMed] [Google Scholar]
- 32.Du LQ, Wang Y, Wang H, Cao J, Liu Q, et al. Knockdown of Rad51 expression induces radiation- and chemo-sensitivity in osteosarcoma cells. Med Oncol. 2010 doi: 10.1007/s12032-010-9605-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Brown ET, Holt JT. Rad51 overexpression rescues radiation resistance in BRCA2-defective cancer cells. Mol Carcinog. 2009;48:105–109. doi: 10.1002/mc.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, et al. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49:649–655. doi: 10.1016/s0360-3016(00)00783-5. [DOI] [PubMed] [Google Scholar]
- 35.Yamano M, Ogino H, Shibamoto Y, Horii N. Relationship between radiation pneumonitis and prognosis in patients with primary lung cancer treated by radiotherapy. Kurume Med J. 2007;54:57–63. doi: 10.2739/kurumemedj.54.57. [DOI] [PubMed] [Google Scholar]
- 36.Movsas B, Raffin TA, Epstein AH, Link CJ., Jr Pulmonary radiation injury. Chest. 1997;111:1061–1076. doi: 10.1378/chest.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 37.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83:781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 38.Meirovitz A, Kuten M, Billan S, Abdah-Bortnyak R, Sharon A, et al. Cytokines levels, severity of acute mucositis and the need of PEG tube installation during chemo-radiation for head and neck cancer–a prospective pilot study. Radiat Oncol. 2010;5:16. doi: 10.1186/1748-717X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legue F, Guitton N, Brouazin-Jousseaume V, Colleu-Durel S, Nourgalieva K, et al. IL-6 a key cytokine in in vitro and in vivo response of Sertoli cells to external gamma irradiation. Cytokine. 2001;16:232–238. doi: 10.1006/cyto.2001.0970. [DOI] [PubMed] [Google Scholar]
- 40.Xia DH, Xi L, Xv C, Mao WD, Shen WS, et al. The protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor beta1 and tumor necrosis factor alpha. Med Oncol. 2010;27:697–701. doi: 10.1007/s12032-009-9271-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR-based restriction analysis. Genotypes of the RAD51, XRCC2, XRCC3 and NBN SNPs were shown on agarose electrophoresis.
(TIF)