Abstract
Autoparasitoids with the capacity of consuming primary parasitoids that share the same hosts to produce males are analogous to intraguild predators. The use of autoparasitoids in biological control programs is a controversial matter because there is little evidence to support the view that autoparasitoids do not disrupt and at times may promote suppression of insect pests in combination with primary parasitoids. We found that Encarsia sophia, a facultative autoparasitoid, preferred to use heterospecific hosts as secondary hosts for producing males. The autoparasitoids mated with males originated from heterospecifics may parasitize more hosts than those mated with males from conspecifics. Provided with an adequate number of males, the autoparasitoids killed more hosts than En. formosa, a commonly used parasitoid for biological control of whiteflies. This study supports the view that autoparasitoids in combination with primary parasitoids do not disrupt pest management and may enhance such programs. The demonstrated preference of an autoparasitoid for heterospecifics and improved performance of males from heterospecifics observed in this study suggests these criteria should be considered in strategies that endeavor to mass-produce and utilize autoparasitoids in the future.
Introduction
Aphelinid parasitoids have an outstanding record of success in programs of classical biological control against whiteflies and scale insects [1]. Some exhibit unusual host relationships with males developing on or in different hosts than do the females. Walter called them heteronomous parasitoids [2], which has been universally accepted [3]. Autoparasitoids, the main kind of heteronomous aphelinids, differ from the typical pattern of parasitoid development in which females always develop as primary parasitoids on homopteran or hemipteran hosts (primary hosts), but males develop as hyperparasitoids on their own species or on other primary parasitoids (secondary hosts) [1], [2], [4].
Autoparasitoids occur primarily in the genera Coccophagus, Coccobius, Coccophagoides and Encarsia [3]. Some autoparasitoids have been successfully used in biological control in several projects [4], [5]. Their use of immatures of heterospecific parasitoids to produce males results in effects analogous to those observed with intraguild predators [6], [7]. Like intraguild predators, autoparasitoids can consume and kill other primary parasitoids that share the same hosts with them. Because autoparasitoids interact with other conventional parasitoid species with a potential to disrupt pest suppression and eliminate conventional parasitoids, they have at times been considered to be questionable choices in introduction programs [8]–[10]. Several studies on secondary host selection indicate that autoparasitoids prefer heterospecific hosts to conspecific hosts most of the time [11]–[14], which could contraindicate their utility in biological control programs. However, the effects of the autoparasitoid in the context of the population dynamics of the target pest must also be considered.
A series of studies have been conducted worldwide that examine whether autoparasitoids disrupt pest suppression provided by primary parasitoids,. Mills & Gutierrez [8] and Briggs & Collier [15] predicted that autoparasitoids could disrupt pest suppression based on the models they designed. However, Ehler [5] and Heinz & Nelson [16] found that an autoparasitoid and a primary parasitoid together could suppress more pests than could either species used alone. These results appear to be in conflict. Additional studies have also demonstrated that pests can be suppressed with a complex of parasitoids that include at least one species of autoparasitoid and a primary parasitoid under field or greenhouse conditions [5], [7], [16]–[18]. The evidence from the model does not appear to be supported by empirical evidence from the field.
Encarsia sophia (Girault & Dodd) ( = En. transvena), an autoparasitoid, oviposits female eggs in whitefly nymphs and male eggs externally on female immatures of their own or of other Encarsia and Eretmocerus species [4]. Recent research demonstrates that the efficiency of En. sophia in biological control of whiteflies can be readily manipulated by controlling the duration of food deprivation and effects on mating status [19]–[21]. These parasitoids suppress more whiteflies through parasitism and host feeding than do other commonly used species [22].
In this study, we investigated secondary host selection of the autoparasitoid females on conspecific and heterospecific hosts; compared the suitability of different secondary host species for development of male En. sophia, and then explored the performance of males originated from different secondary host species on parasitism of En. sophia females. Lastly, we determined the whitefly suppression by En. sophia paired with different numbers of males under greenhouse conditions. The goal was to explore the mechanisms affecting heteronomous hyperparasitism in autoparasitoids and the implications this has for development of biological control strategies.
Materials and Methods
Parasitoids, whitefly hosts, and host plants
Three species of parasitoids were used in this study: Encarsia formosa Gahan, Eretmocerus melanoscutus Zolnerowich & Rose and Encarsia sophia (Girault & Dodd). En. formosa is one of the most commonly used parasitoids for biological control of greenhouse whiteflies. It is a thelytokous endoparasitoid, with females producing no male offspring and laying all its eggs in the whitefly hosts. Er. melanoscutus oviposits externally under the nymphal host. This species is a bi-parental primary parasitoid, with both males and females developing in whitefly nymphs. En. sophia is an autoparasitoid, and its females are primary parasitoids of whiteflies and males are hyperparasitic, developing on conspecific or heterospecific immatures which are parasitoids of whiteflies [23]. In our previous experiments, we found that the immatures of both En. formosa and Er. melanoscutus were secondary hosts parasitized by En. sophia females. Laboratory colonies of these parasitoids were established using Bemisia tabaci biotype B, one of the most serious of global pests [24], on cabbage plants in the Vegetable IPM Laboratory, Texas AgriLife Research and Extension Center in Weslaco, Texas, USA.
Cabbage (Brassica oleracea L. var. capitata, ‘Golden Acre’) was used as the host plant for B. tabaci. Plants were grown in 15-cm plastic pots filled with Metro-Mix 360 growing medium (Sun Gro, Horticulture Distribution Inc., Bellevue, WA, USA) and enclosed in whitefly-proof screen cages (110×80×80 cm). Plants with 3 fully extended true leaves were used in the experiments.
Secondary host selection
The preference for oviposition of male eggs by En. sophia on each stage of their own species and another species, Er. eremicus, has been determined by Hunter & Kelly [4]. Their results indicated that En. sophia prefers to lay male eggs on late larvae-prepupae of the parasitoids. In this experiment, larvae (3rd instar) of the conspecific En. sophia and the heterospecific En. formosa and Er. melanoscutus were used for examining secondary host selection by En. sophia females. In order to obtain the desired stage of secondary hosts, the following procedures were conducted. Thirty female and male adults of B. tabaci were introduced onto the lower leaf surface of a cabbage leaf on a potted plant with a leaf clip-on cage (4.0 cm in diameter) for oviposition for 12 h. The development of the eggs was monitored daily, and the nymphs were then monitored daily until they developed to early fourth instars. Then, six mated female parasitoids of En. sophia, Er. melanoscutus, or six En. formosa females were introduced into each clip-cage for oviposition for 12 h, respectively. After parasitoid removal, 20 whitefly adults were introduced into each leaf clip-on cage for oviposition for 6 h (these were the source of healthy whitefly nymphs to be used for host feeding by En. sophia females tested in the secondary host selection experiment). The development of parasitoid larvae was monitored daily until they developed to third instar larvae. Cabbage leaf discs prepared with the desired parasitoid stage and healthy whitefly hosts were then used for the following no-choice and paired-choice tests as the secondary hosts for En. sophia. All experiments were conducted in an air-conditioned insectary (28±2°C, 70±5% r.h., and a photoperiod of L14∶D10 h).
No-choice test
Only one species of late larval parasitoid (En. sophia, En. formosa or Er. melanoscutus) was exposed to an En. sophia female at a time. The leaf discs with late larval parasitoids and healthy whitefly nymphs as described above were cut out around the leaf clip-on cage's bottom rim. Twenty whitefly nymphs containing the desired stage of larval parasitoids and 20 healthy whitefly nymphs were used on each disk, and all other whitefly nymphs were carefully removed under a stereoscopic microscope using an insect pin. Then, the leaf discs were individually placed on the bottom of a Petri dish (5 cm in diameter and 2.0 cm in depth) covered with a thin layer (0.3–0.5 cm in thickness) of 1.5% agar gel. Two newly-emerged En. sophia virgin females (<3 h old) were introduced into each Petri dish. The dishes were inverted to simulate the upside-down natural conditions. After a 48-h exposure time, the survival of parasitoids in each treatment was recorded, and then they were removed. The number of secondary hosts that was parasitized, or fed on by En. sophia females were separately counted under a stereoscopic microscope 6 days after parasitoid removal. Parasitoid larvae parasitized by a male larva (“C”-shaped) were easily identified by inspecting the consumed remains of the secondary host [25]. If the secondary hosts were fed on by En. sophia, the whitefly nymph's body became flat, and the primary parasitoid larva was visible through the clear cuticle of the host. Each treatment was replicated 25 times.
Paired-choice test
The late larval instar of two parasitoid species (En. sophia vs En. formosa, En. sophia vs Er. melanoscutus or En. formosa vs Er. melanoscutus) were exposed to En. sophia female simultaneously. Half leaf discs (2.5 cm in diameter) had 20 late larval parasitoids of one parasitoid species and 10 healthy whitefly nymphs, and another half leaf disc had same numbers of larvae of another parasitoid species and healthy hosts. Then, two half leaf discs with different secondary parasitoid hosts were placed on the bottom of a Petri dish (5 cm in diameter and 2.0 cm in depth) covered with a thin layer (0.3–0.5 cm in thickness) of 1.5% agar gel. Two newly-emerged En. sophia virgin females (<3 h old) were introduced into each Petri dish for 48 h, and numbers of secondary hosts killed by parasitism or host feeding on each half leaf disc in each of the arenas were assessed as described in the no-choice test above. Each treatment was replicated 25 times.
Development and size of male En. sophia originated from different secondary hosts
The leaf discs with 20 late larval parasitoids and 20 healthy whitefly nymphs as described above were individually placed on the bottom of a Petri dish (5 cm in diameter and 2.0 cm in depth) covered with a thin layer of 1.5% agar gel. Five unmated En. sophia females (24 h old) were introduced into each Petri dish for hyperparasitism. The dishes were inverted to simulate the upside-down natural conditions. After a 6-h exposure time, the parasitoids in each arena were removed. Each treatment was replicated 20 times. Total numbers of secondary hosts by parasitism or by host-feeding were separately counted under a stereoscopic microscope 5 days after parasitoid removal. Unparasitized parasitoids and healthy whiteflies were removed using an insect pin. The development of male En. sophia on different secondary hosts was monitored daily until no parasitoids emerged, and the head width and body length was measured for 30–40 newly-emerged males originated from different secondary hosts.
Parasitism of En. sophia females mated with the males originated from different secondary hosts
One pair of newly-emerged En. sophia female and male originated from En. sophia, En. formosa or Er. melanoscutus was introduced onto a cabbage leaf (on a potted plant) with approximately 60 third-instar whitefly nymphs covered by a leaf clip-on cage (4.0 cm in diameter). Every 24 h, the live female and male parasitoids were transferred to a new cabbage leaf bearing a similar number of third-instar whitefly nymphs. During the experiment, only a single male was used to mate with each female. This process lasted for 12 days (preliminary experiments showed parasitism completion for En. sophia females in this duration). Host mortality by parasitism or by host feeding of the parasitoid adults on each cabbage leaf was examined under a stereoscopic microscope 6 days after parasitoids were transferred. Twenty pair of En. sophia for each treatment was initially used, and the data from 17 to 19 replicates were analyzed because a few females were lost during the experiment.
Whitefly suppression by En. sophia and En. formosa under greenhouse conditions
This experiment was conducted in an air-conditioned greenhouse (25–35°C, and 60–90% r.h.). Four potted cabbage plants having 3 fully expanded leaves were placed in each cage (80×45×55 cm) with a glass top and four screened sides. Thirty pairs of newly-emerged B. tabaci adults (1∶1 sex ratio) were released into each cage. The development of whitefly immatures was monitored daily. When fourth instar nymphs were first found on the leaves, all introduced whitefly adults were removed using an aspirator, and the plants were re-caged. On the same day, six treatments with different ratios of male to female for En. sophia released were conducted in this experiment: (1) 30 females +5 males (females∶males = 6∶1); (2) 30 females +10 males (females∶males = 3∶1); (3) 30 females +15 males (females∶males = 2∶1); (4) 30 females +20 males (females∶males = 3∶2); (5) 30 females +30 males (females∶males = 1∶1); and (6) 30 females of En. formosa without En. sophia. In this experiment, newly-emerged parasitoids (<6 h old) were used, and all males of En. sophia were originated from secondary host En. formosa. Each treatment had five replicates. Ten days after parasitoid releases, all leaves with whitefly nymphs from each cage were detached. Total numbers of whitefly nymphs killed by parasitism and host feeding and healthy whitefly nymphs were counted, respectively, as described by Zang & Liu [22].
Statistical analysis
Parasitism or host feeding by the autoparasitoid En. sophia on conspecific and heterospecific hosts under no-choice conditions was analyzed using one-way analysis of variance (ANOVA), and means were separated using Tukey's honestly significantly difference (HSD) test at P<0.05 [26]. Numbers of secondary hosts parasitized or fed on by En. sophia were transformed to square root to stabilize variance before being subjected to ANOVA. Similarly, one-way ANOVA was used in analyzing the development and size of male En. sophia originated from different secondary hosts, parasitism of En. sophia females mated with different males originated and whitefly suppression by En. sophia at various release ratios of male to female, and means were separated using Tukey's HSD test at P<0.05, respectively. Paired t-test was used in the analyses of parasitism or host feeding on late larvae of two parasitoid species by En. sophia females.
Results
Secondary host selection by En. sophia
No-choice test
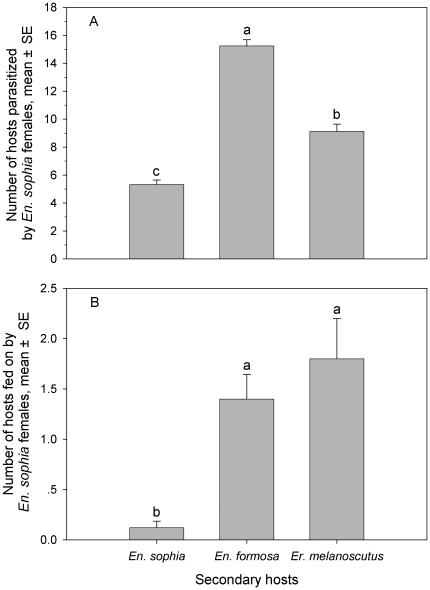
Parasitism or host feeding by En. sophia females on conspecific and heterospecific hosts varied (Fig. 1). Encarsia sophia females most preferred to use En. formosa as its secondary host, followed by Er. melanoscutus and En. sophia under no-choice conditions (F 2, 72 = 127.87; P<0.0001) (Fig. 1A). The number of En. formosa immatures parasitized by one En. sophia female in 48 h averaged 7.1, 2.9 and 1.6 fold more than that of En. sophia (2.7) and Er. melanoscutus (4.6), respectively. Encarsia sophia females rarely fed on their own offspring (Fig. 1B). The number of conspecific hosts fed on by En. sophia females was significantly fewer than those of heterospecific hosts (F 2, 72 = 10.30; P = 0.0001), Er. melanoscutus and En. formosa with no difference between the latter.
Figure 1. Number of secondary hosts (En. sophia, En. formosa, Er. melanoscutus) parasitized (A) and fed on (B) by two En. sophia female adults during 48-h exposure under no-choice conditions.
The same letters above bars in each figure indicate that means do not differ significantly (P>0.05, Tukey's HSD test).
Paired choice test
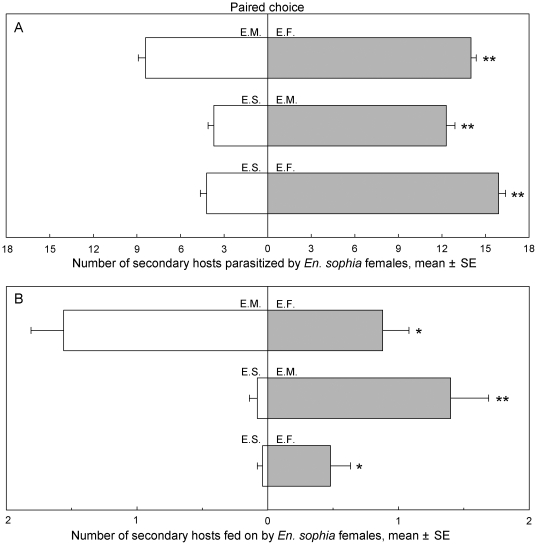
Encarsia sophia exhibited different host selection on different secondary host species under paired choice conditions (Fig. 2). When En. sophia was offered in combination with heterospecific species, En. sophia females significantly preferred parasitizing En. formosa or Er. melanoscutus compared to its own species (15.9 En. formosa vs 4.2 En. sophia; 12.3 Er. melanoscutus vs 3.7 En. sophia. t = 11.89−18.07, P<0.0001). When En. formosa and Er. melanoscutus were offered simultaneously, En. sophia females significantly preferred to parasitize En. formosa (14.0 vs 8.4, t = 9.10, P<0.0001) (Fig. 2A). When En. sophia was presented in combination with heterospecific species, En. sophia females significantly fed on fewer conspecific hosts than heterospecific hosts, En. formosa and Er. melanoscutus (t = 2.76−4.41, P = 0.0101−0.0002). However, when two heterspecific hosts were presented simultaneously, En. sophia females significantly fed on more Er. melanoscutus than En. formosa (t = 2.14, P = 0.0378) (Fig. 2B).
Figure 2. Number of secondary hosts parasitized (A) and fed on (B) by two En. sophia female adults during 48-h exposure under paired choice conditions.
The paired bars with an ‘*’ or ‘**’ indicate that the means differ significantly at P<0.05 or P<0.01 (paired t-test), respectively. Secondary hosts∶ E.S. = En. sophia, E.F. = En. formosa, E.M. = Er. melanoscutus.
Development and size of male En. sophia originated from different secondary hosts
Male development time was significantly different in different secondary host species (Table 1). En. sophia males developed fastest on En. formosa, followed by En. sophia and Er. melanoscutus. En. sophia males had greatest proportion of emergence on their own species. There was no difference with En. formosa, but significantly higher than on Er. melanoscutus. Males originated from different secondary host species differed significantly in size. The largest males were obtained from En. formosa (Table 1).
Table 1. Comparisons of development time, proportion of emergence and size of males originated from different secondary hosts.
| Secondary host species | Male development time (days) ± SE | Proportion of male emergence ± SE | Size of male | |
| Head width (mm) ± SE | Body length (mm) ± SE | |||
| En. sophia | 12.1±0.1 b | 0.92±0.03 a | 0.225±0.038 ab | 0.537±0.091 b |
| En. formosa | 11.5±0.1 c | 0.84±0.03 ab | 0.231±0.039 a | 0.565±0.096 a |
| Er. melanoscutus | 12.8±0.1 a | 0.75±0.03 b | 0.221±0.037 b | 0.517±0.087 b |
| F 2, 55 = 28.07P<0.0001 | F 2, 55 = 7.79P = 0.0011 | F 2, 102 = 6.55P = 0.0021 | F 2, 102 = 14.35P<0.0001 | |
Parasitism of En. sophia females mated with males originated from different secondary host species
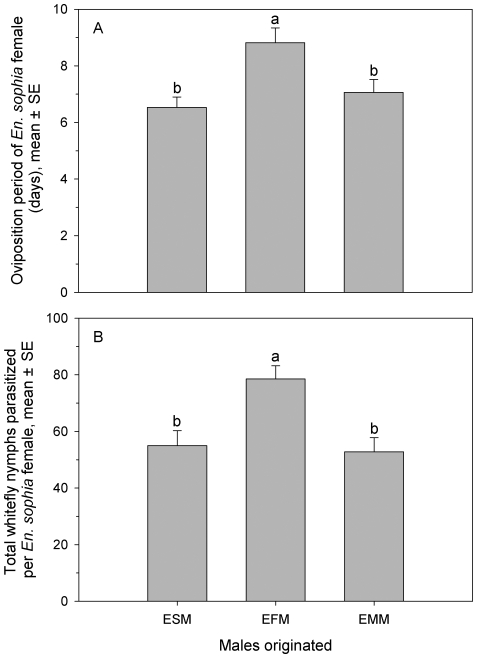
Encarsia sophia females had different oviposition periods and parasitized different numbers of whitefly nymphs throughout their lifespan when they were mated with males originated from different secondary host species (Fig. 3). Females mated with males originated from En. formosa had longer oviposition periods (F 2, 50 = 7.22; P = 0.0018) and parasitized more whitefly nymphs (F 2, 50 = 7.87; P = 0.0011) than those mated with males from En. sophia and Er. melanoscutus with no difference between them.
Figure 3. Oviposition period (A) and total whitefly nymphs parasitized (B) by En. sophia female mated with male originated from different secondary hosts.
The same letters above the bars in each figure indicate that means do not differ significantly (P>0.05, Tukey's HSD test). ESM, EFM and EMM indicated that males from En. sophia, En. formosa and Er. melanoscutus, respectively.
Whitefly suppression by En. sophia and En. formosa under greenhouse conditions
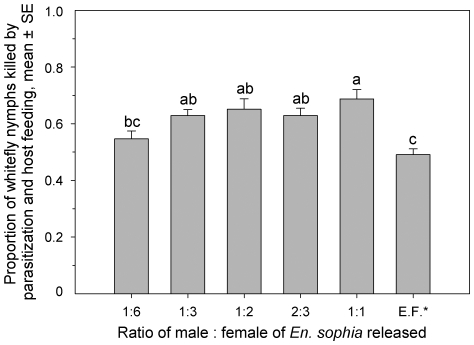
The availability of males significantly affected the efficiency of whitefly suppression by En. sophia (F 4, 20 = 3.07; P = 0.0402) (Fig. 4). Encarsia sophia released at a male to female ratio of 1∶1 caused the largest proportion of whitefly nymph mortality by parasitism and host feeding among all treatments of parasitoid releases. Generally, En. formosa caused significantly lower proportion of whitefly nymph mortality than En. sophia released at all male to female ratios except at 1∶6, with no difference between them.
Figure 4. Proportion of total whitefly nymphs killed due to parasitism and host feeding by En. sophia with different released ratio of male: female and En. formosa.
The same letters above bars in each figure indicate that means do not differ significantly (P>0.05, Tukey's HSD test). E.F. - En. formosa.
Discussion
The attack on heterospecific competitors through parasitism and host feeding by autoparasitoids represents a mechanism of interference similar to intraguild predation [9]. Similar to intraguild predators, the autoparasitoid females consume and kill both their competitors and a shared host [3]. As for threatening the establishment and survival of other parasitoid species, the relaxed predation induced by autoparasitoids possibly results in temporal outbreaks of some pests [10], and they are also predicted to have the potential to disrupt pest suppression based on the models established [8], [15]. However, numerous reports show that autoparasitoids have been successfully used for biological control of whiteflies, blackflies, scales and midges [5], [7], [16], [27].
Several studies on hyperparasitism behavior of autoparasitoids on secondary hosts have been conducted. In some Encarsia species, such as En. pergandiella Howard and En. transvena ( = En. sophia), no parasitism preference has been found between conspecific and heterospecific secondary hosts [4], [13]. However, En. tricolor Forster and En. smithi (Silvestri) exhibit parasitism preference for heterospecific hosts more than conspecific hosts [11], [12], [14], [28]. The present study indicates that En. sophia females prefer to use heterospecifics as secondary hosts, and particularly prefer to parasitize En. formosa under no-choice or choice conditions (Figs. 1, 2). The inconsistent results with Hunter & Kelly [4] possibly result from different host insects (B. tabaci vs Trialeurodes vaporariorum), host plants (cabbage vs green bean) and heterospecific secondary hosts (En. formosa, Er. melanoscutus vs Er. eremicus) in the two experimental systems. Certainly, another possible reason for differences is connected with virgin En. sophia females our study and mated females used in theirs. Encarsia sophia, a destructive host feeder, causes significant whitefly mortality by host feeding, equivalent to that caused by parasitism [22]. The results of secondary hosts killed by host feeding indicated an En. sophia female could feed on about 1–2 heterospecific hosts in 24 h, and hardly fed at all on conspecific hosts. The conspecific hosts are rarely fed on by En. sophia females; this may be related to the narrow window of vulnerability [4] and perhaps self-discriminatory behavior. Besides hyperparasitism, autoparasitoids also can feed on secondary hosts with a significant difference occurring between heterospecifics and conspecifics.
The autoparasitoid females might attack developing conspecific hosts and produce their males in future. Most studies demonstrated that there was host stage suitability for development of autoparasitic males [4], [29]–[31]. Generally, the host stages of late larvae to prepupae were suitable for development. Facultative autoparasitoids hyperparasitize conspecifics and heterospecifics [2], thus their males may have different secondary host origins. Avilla & Copland [30] investigated secondary host suitability of En. tricolor and En. formosa for development of En. tricolor males. Their results showed that En. tricolor males developed faster on En. formosa than on their own species when the suitable host stages were offered. Similarly, our study indicated that En. formosa was the most suitable host for male development of En. sophia with the largest individuals and highest proportion of emergence in the shortest period of time (Table 1). The high suitability of heterospecific hosts hints that facultative autoparasitism is not only a trait that self-regulates population densities and confers stability on host and parasitoid population but is a means of enabling coexistence with competitors in natural conditions [30]. Preference for parasitizing heterospecific hosts by an autoparasitoid may allow them to produce more males that contribute to their own population expansion. Further, the possibility for males to develop on females of their own species can be envisaged as a strategy for maintaining population persistence when there are no competing species in the same habitat. These are expected to be factors that affected autoparasitoid evolution.
As for facultative autoparasitoids, males may have different origins, conspecific or heterospecific hosts. In this study we found that the males originated from different host species affected parasitism of autoparasitoid females. Our results demonstrated that there was a significant difference in parasitism of En. sophia females on whiteflies mated with males from different secondary hosts (Fig. 3). Generally, En. sophia females had a longer oviposition period and parasitized more whiteflies when they were mated with males originated from En. formosa. In addition, En. sophia females mated with males from Er. melanoscutus and its own species expressed similar parasitism of whiteflies. The females of some parasitoid species such as Cotesia glomerata (L.), mated with haploid males, could produce more daughters than females mated with diploid males [32]. The present study indicated that males originated from different hosts also might influence the reproduction of autoparasitoid females. West & Rivero [33] estimated the factors that limit reproduction in parasitoids from autoparasitoid sex ratio data. Our results suggest that secondary host species may influence their reproduction as well. The special reproductive biology in autoparasitoids should be considered when developing theoretical models that predict the optimal oviposition strategy. Many authors suggest that autoparasitoids may be dominant more often than primary parasitoids in parasitoid communities [1]. The autoparasitoids may produce more males when they coexist with other parasitoid species. Consequently, the mated autoparasitoids using primary hosts may produce more females, which further contributes to the expansion of their own population. Our results present evidence to support the notion that autoparasitoids are the dominant species in the majority of natural parasitoid complexes [1].
The literature addressing the efficiency of most autoparasitoids as biological control agents to suppress primary hosts is documented in Table 2. In most cases, the addition of a facultative autoparasitoid such as Coccophagus lycimnia (Walker), En. smithi (Silv.) and En. tricolor, exhibit the potential to disrupt primary hosts. However, in several cases, primary hosts can be successfully suppressed by an autoparasitoid alone or with a parasitoid complex that includes at least one autoparasitoid and a primary parasitoid that is attacked by it in biological control programs (Table 2). Several autoparasitoids such as Zatropis capitis Burks, En. pergandiella and En. sophia, may at times suppress more pests in combination with primary parasitoids than either species used alone [5], [7], [16]. Our results indicate that males played a principal role in suppressing pests by autoparasitoids. When the ratio of male to female of En. sophia was not less than 1∶3, the autoparasitoid exhibited superior bio-control efficacy on whiteflies than did the primary parasitoid, En. formosa (Fig. 4). The preference of autoparasitoids to parasitize heterospecific hosts results in producing more males when they coexist with other primary parasitoids. On the other hand, the autoparasitoid females may parasitize more or similar number of pests when they mate with the males from heterospecific hosts. All of these may offset the adverse effects from autoparasitoids that consume primary parasitoids. The present study provides evidence to explain why an autoparasitoid in combination with a primary parasitoid that is attacked by it may suppress more insect pests. Our results could alleviate some fears that releasing autoparasitoids may disrupt parasitoid diversity and pest suppression. Presently many conventional primary parasitoids (e.g. En. formosa and Er. erimicus) have been commercially produced and widely applied in pest management. However, due to the biological and behavioral features, mass-production of autoparasitoids has been constrained for seasonal inoculative or inundative releases [34]. The apparent preference for heterospecific hosts and satisfactory performance of males from heterospecifics reveals a direction to mass-produce autoparasitoids in future.
Table 2. The efficiency on suppression of primary hosts by autoparasitoids alone or in combination with primary parasitoids in biological control programs.
| Parasitoid species | Male developmenta | Primary hosts | Evaluation as biological control agentb | Reference |
| Coccophagoides utilis Doutt | C | Parlatoria oleae (Colvee) | + + | 36 |
| Coccophagus gurneyiCompere | H-C | Pseudococcus calceolariae (Mask.) | 8, 37 | |
| C. lycimnia (Walker) | H-C | Coccus pseudomagnoliarum (Kuwana) | + − | 38 |
| Toumeyella pini (King) | + + | 39 | ||
| C. sp. nr gurneyi | H | Lantana montevidensis (Spreng) | + | 40 |
| C. cowperi Girault | H-C | Pulvinariella mesembryanthemis (Vallot) | + | 29 |
| Encarsia sp. | H-C | B. tabaci (Gennadius) | + − | 8 |
| Encarsia sp. | C | B. tabaci | + + | 8 |
| En. bimaculata(Heraty & Polaszek) | H-C | B. tabaci | + | 41 |
| En. lahorensis (Howard) | H-C | Dialeurodes citri (Ashmead) | + + | 42 |
| En. opulenta (Silv.) | C | Aleurocanthus woglumi Ashby | + + | 17, 35 |
| En. pergandiella Howard | H-C | B. argentifolii Bellows&Perring | + + + | 16 |
| En. perniciosi (Tower) | H-C | Quadraspidiotus perniciosus (Comstock) | + | 8 |
| Aonidiella aurantii (Mask.) | + + | 43 | ||
| En. smithi (Silv.) | H-C | A. spiniferus (Quaintance) | + | 8 |
| A. woglumi Ashby | + − | 35 | ||
| En. sophia (Girault&Dodd) | H-C | B. argentifolii | + + + | 7 |
| Parabemisia myricae (Kuwana) | + + | 8 | ||
| En. sublutea Silv. | H-C | B. tabaci | + + | 44 |
| En. tricolor Forster | H-C | Aleyrodes proletella L. | + − | 45 |
| Physcus seminotus Silv. | H-C | Aulacaspis tegalensis (Zhnt.) | + + | 46 |
| P. subflavus Annecke&Insley | H-C | A. tegalensis (Zhnt.) | + | 47 |
| Zatropis capitis Burks | H-C | Rhopalomyia californica Felt | + + + | 5 |
C: Males develop on conspecifics only; H: Males develop on heterospecifics only; H–C: Males may develop on heterospecifics or on conspecifics.
+: A potential biological control agent; + +: No disruption on the suppression of primary hosts; + −: Disrupt suppression of primary hosts in combination with primary parasitoids; + + +: Suppress more primary hosts in combination with primary parasitoids.
Acknowledgments
We thank Y.-M. Zhang and Texas AgriLife Research, Texas A&M University System for technical assistance during this study. We also greatly appreciate Prof. Marvin Harris (Department of Entomology, Texas A&M University, College Station, Texas) for review the revised version.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The research was partially supported by the National Natural Science Foundation of China (30930062 and 31071735) (provided partial support to the first author's research), the National Basic Research and Development Program of China (2009CB119200) (provided partial support the salary for LSZ and FHW) and Northwest A&F University (provided partial salary to TXL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Williams T. Invasion and displacement of experimental populations of a conventional parasitoid by a heteronomous hyperparasitoid. Biocontrol Sci Tech. 1996;6:603–618. [Google Scholar]
- 2.Walter GH. ‘Divergent male ontogenies’ in Aphelinidae (Hymenoptera: Chalcidoidea): a simplified classification and a suggested evolutionary sequence. Biol J Linn Soc. 1983;19:63–82. [Google Scholar]
- 3.Hunter MS, Woolley JB. Evolution and behavioral ecology of heteronomous aphelinid parasitoids. Annu Rev Entomol. 2001;46:251–290. doi: 10.1146/annurev.ento.46.1.251. [DOI] [PubMed] [Google Scholar]
- 4.Hunter MS, Kelly SE. Hyperparasitism by an exotic autoparasitoid: secondary host selection and the window of vulnerability of conspecific and native heterospecific hosts. Entomol Exp Appl. 1998;89:249–259. [Google Scholar]
- 5.Ehler LE. Utility of facultative secondary parasites in biological control. Environ Entomol. 1979;8:829–832. [Google Scholar]
- 6.Polis GA, Holt RD. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol Evol. 1992;5:151–154. doi: 10.1016/0169-5347(92)90208-S. [DOI] [PubMed] [Google Scholar]
- 7.Hunter MS, Collier TR, Kelly SE. Does an autoparasitoid disrupt host suppression provided by a primary parasitoid? Ecology. 2002;83:1459–1469. [Google Scholar]
- 8.Mills NJ, Gutierrez PA. Prospective modeling in biological control: an analysis of the dynamics of heteronomous hyperparasitism in a cotton-whitefly-parasitoid system. J Appl Ecol. 1996;33:1379–1394. [Google Scholar]
- 9.Reitz SR, Trumble JT. Competitive displacement among insects and arachnids. Annu Rev Entomol. 2002;47:435–465. doi: 10.1146/annurev.ento.47.091201.145227. [DOI] [PubMed] [Google Scholar]
- 10.Van Lenteren JC, Bale J, Bigler F, Hokkanen HMT, Loomans AJM. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu Rev Entomol. 2006;51:609–634. doi: 10.1146/annurev.ento.51.110104.151129. [DOI] [PubMed] [Google Scholar]
- 11.Williams T. Host selection and sex ratio in a heteronomous hyperparasitoid. Ecol Entomol. 1991;16:377–386. [Google Scholar]
- 12.Avilla J, Anadón J, Sarasúa MJ, Albajes R. Egg allocation of the autoparasitoid Encarsia tricolor at different relative densities of the primary host (Trialeurodes vaporariorum) and two secondary hosts (Encarsia formosa and E. tricolor). Entomol Exp Appl. 1991;59:219–227. [Google Scholar]
- 13.Pedata PA, Hunter MS. Secondary host choice by the autoparasitoid Encarsia pergandiella Howard (Hymenoptera: Aphelinidae). Entomol Exp Appl. 1996;81:207–214. [Google Scholar]
- 14.Huang Y, Loomans AJM, van Lenteren JC, Xu RM. Hyperparasitism behaviour of the autoparasitoid Encarsia tricolor on two secondary host species. BioControl. 2009;54:411–424. [Google Scholar]
- 15.Briggs CJ, Collier TR. Autoparasitism, interference, and parasitoid-pest population dynamics. Theor Pop Biol. 2001;60:33–57. doi: 10.1006/tpbi.2001.1529. [DOI] [PubMed] [Google Scholar]
- 16.Heinz KM, Nelson JM. Interspecific interactions among natural enemies of Bemisia in an inundative biological control program. Biol Contr. 1996;6:384–393. [Google Scholar]
- 17.Thompson CR, Cornell JA, Sailer RI. Interactions of parasites and hyperparasites in biological control of citrus blackfly, Aleurocanthus woglumi (Homoptera: Aleyrodidae), in Florida. Environ Entomol. 1987;16:140–144. [Google Scholar]
- 18.Bogran CE, Heinz KM, Ciomperlik MA. Interspecific competition among insect parasitoids: field experiments with whiteflies as hosts in cotton. Ecology. 2002;83:653–668. [Google Scholar]
- 19.Zang LS, Liu TX. Food-deprived host-feeding parasitoids kill more pest insects. Biocontrol Sci Tech. 2009;19:573–583. [Google Scholar]
- 20.Zang LS, Liu TX. Effects of food deprivation on host feeding and parasitism of whitefly parasitoids. Environ Entomol. 2010;39:912–918. doi: 10.1603/EN09266. [DOI] [PubMed] [Google Scholar]
- 21.Zang LS, Liu TX, Zhang F, Shi SS, Wan FH. Insect Sci. in press; 2011. Effects of mating status on host feeding and parasitism in two whitefly parasitoids. [Google Scholar]
- 22.Zang LS, Liu TX. Host feeding of three whitefly parasitoid species on Bemisia tabaci B biotype, with implication for whitefly biological control. Entomol Exp Appl. 2008;127:55–63. [Google Scholar]
- 23.Chiel E, Zchori-Fein E, Inbar M, Gottlieb Y, Adachi-Hagimori T, et al. Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS ONE. 2009;4:e4767. doi: 10.1371/journal.pone.0004767. doi: 10.1371/journal.pone.0004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, De Barro P, Zhao H, Wang J, Nardi F, et al. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PloS ONE. 2011;6:e16061. doi: 10.1371/journal.pone.0016061. doi: 10.1371/journal.pone.0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antony B, Palaniswami MS, Henneberry TJ. Encarsia transvena (Hymenoptera: Aphelinidae) development on different Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) instars. Environ Entomol. 2003;32:584–591. [Google Scholar]
- 26.SAS Institute. Cary, NC: SAS Institute; 2006. SAS/STAT version 9.1. [Google Scholar]
- 27.Dowell RV, Fitzpatrick GE, Reinert JA. Biological control of citrus blackfly in Southern Florida. Environ Entomol. 1979;8:595–597. [Google Scholar]
- 28.Nguyen R, Sailer RI. Facultative hyperparasitism and sex determination of Encarsia smithi (Silvestri) (Hymenoptera: Aphelinidae). Ann Entomol Soc Am. 1987;80:713–719. [Google Scholar]
- 29.Wilk BM, Kitayama CY. Host stage preference for deposition of male eggs by Coccophagus cowperi (Hymenoptera: Aphelinidae). Entomophaga. 1981;26:313–318. [Google Scholar]
- 30.Avilla J, Copland MJW. Effects of host stage on the development of the facultative autoparasitoid Encarsia tricolor (Hymenoptera: Aphelinidae). Ann Appl Biol. 1987;110:381–389. [Google Scholar]
- 31.Hunter MS. Suitability of stages of female Encarsia pergandiella (Hymenoptera: Aphelinidae) for development of conspecific male hyperparasites. Entomophaga. 1989;34:265–274. [Google Scholar]
- 32.Elias J, Mazzi D, Dorn S. No need to discriminate? Diploid males in a parasitoid with complementary sex determination. PloS ONE. 2009;4:e6024. doi: 10.1371/journal.pone.0006024. doi: 10.1371/journal.pone.0006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West SA, Rivero A. Using sex ratios to estimate what limits reproduction in parasitoids. Ecol Lett. 2000;3:294–299. [Google Scholar]
- 34.Loomans AJM, Huang Y, Bukovinszkiné-Kiss G, van Lenteren JC. Heteronomous hyperparasitoids for biological control of whiteflies: balancing benefits and risks. IOBC/WPRS Bull. 2002;25:143–146. [Google Scholar]
- 35.Nguyen R, Brazzel JR, Poucher C. Population density of the citrus blackfly, Aleurocanthus woglumi Ashby (Homoptera: Aleyrodidae), and its parasites in urban Florida in 1979-1981. Environ Entomol. 1983;12:878–884. [Google Scholar]
- 36.Kennett CE, Huffaker CB, Finney GL. The role of an autoparasitic aphelinid, Coccophagoides utilis Doutt, in the control of Parlatoria oleae (Colvee). Hilgardia. 1966;37:255–282. [Google Scholar]
- 37.Parkes GT, Walter GH. Mating behaviour and alternative oviposition sites for male eggs in the heteronomous hyperparasitoid Coccophagus gurneyi Compere (Hymenoptera: Aphelinidae). Aust J Enomol. 2001;40:74–78. [Google Scholar]
- 38.Bernal JS, Luck RF, Morse JG, Drury MS. Seasonal and scale size relationships between citricola scale (Homoptera: Coccidae) and its parasitoid complex (Hymenoptera: Chalcidoidea) on San Joaquin Valley Citrus. Biol Contr. 2001;20:210–221. [Google Scholar]
- 39.Clarke SR, Debarr GL, Berisford CW. The life history of Toumeyella pini (King) (Homoptera: Coccidae) in loblolly pine seed orchards in Georgia. Can Entomol. 1989;121:853–860. [Google Scholar]
- 40.Walter GH, Abeeluck D. Confirmation of the existence of alloparasitoids in nature – host relationships of an Australian Coccophagus species that parasitizes mealy bugs. Entomol Exp Appl. 2006;118:97–103. [Google Scholar]
- 41.Antony B, Palaniswami MS, Kirk AA, Henneberry TJ. Development of Encarsia bimaculata (Heraty and Polaszek) (Hymenoptera: Aphelinidae) in Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) nymphs. Biol Contr. 2004;30:546–555. [Google Scholar]
- 42.Hudson WG, Williams ML. Release of the parasitic wasp, Encarsia lahorensis (Hymenoptera: Aphelinidae), for control of citrus whitefly (Homoptera: Aleyrodidae) on gardenia in Alabama. Environ Entomol. 1986;15:585–589. [Google Scholar]
- 43.Yu DS, Luck RF, Murdoch WW. Competition, resource partitioning and coexistence of an endoparasitoid Encarsia perniciosi and an ectoparasitoid Aphytis melinus of the California red scale. Ecol Entomol. 1990;15:469–480. [Google Scholar]
- 44.Gerling D. Parasitoids attacking Bemisia tabaci (Hom.: Aleyrodidae). Entomophaga. 1985;30:163–165. [Google Scholar]
- 45.Williams T. The biology of Encarsia tricolor: an autoparasitoid of whtiefly. Biol Contr . 1995;5:209–217. [Google Scholar]
- 46.Williams JR. Some features of sex-linked hyperparasitism in Aphelinidae [Hymenoptera]. Entomophaga. 1977;22:345–350. [Google Scholar]
- 47.Williams JR. The biology of Physcus seminotus Silv. and P. subflavus Annecke & Insley (Aphelinidae), parasites of the sugar-cane scale insect Aulacaspis tegalensis (Zhnt.) (Diaspididae). Bull Entomol Res. 1972;61:463–484. [Google Scholar]