Abstract
Background
Corals worldwide are in decline due to climate change effects (e.g., rising seawater temperatures), pollution, and exploitation. The ability of corals to cope with these stressors in the long run depends on the evolvability of the underlying genetic networks and proteins, which remain largely unknown. A genome-wide scan for positively selected genes between related coral species can help to narrow down the search space considerably.
Methodology/Principal Findings
We screened a set of 2,604 putative orthologs from EST-based sequence datasets of the coral species Acropora millepora and Acropora palmata to determine the fraction and identity of proteins that may experience adaptive evolution. 7% of the orthologs show elevated rates of evolution. Taxonomically-restricted (i.e. lineage-specific) genes show a positive selection signature more frequently than genes that are found across many animal phyla. The class of proteins that displayed elevated evolutionary rates was significantly enriched for proteins involved in immunity and defense, reproduction, and sensory perception. We also found elevated rates of evolution in several other functional groups such as management of membrane vesicles, transmembrane transport of ions and organic molecules, cell adhesion, and oxidative stress response. Proteins in these processes might be related to the endosymbiotic relationship corals maintain with dinoflagellates in the genus Symbiodinium.
Conclusion/Relevance
This study provides a birds-eye view of the processes potentially underlying coral adaptation, which will serve as a foundation for future work to elucidate the rates, patterns, and mechanisms of corals' evolutionary response to global climate change.
Introduction
Reef-building corals (Cnidaria: Hexacorallia: Scleractinia) are of fundamental ecological significance in tropical and sub-tropical shallow marine environments as they form the most important components of coral reefs. These organisms are sensitive to the current rising global seawater temperatures [1] resulting in increased frequencies of mass coral bleaching events, which in turn have caused severe declines in live coral cover [2]. To this end, much effort has been committed to assessing factors affecting overall vulnerability and resilience of reef corals [3], [4], [5], [6]. Additional work has been devoted to the identification of stress-responsive genes [7], [8], [9], [10], [11], [12]. However, few studies have looked into the genetic makeup of corals that might help determining to what extent corals are able to respond to increasing disturbances and stress by means of evolutionary adaptation [13]. Thompson and van Woesik [14] found that corals at sites with a high-frequency of thermal stress displayed less bleaching than at other sites, despite being exposed to a greater level of stress. The authors suggest that bleaching resistance is most likely a consequence of rapid directional selection following an extreme thermal event, i.e. corals are able to respond adaptively from the pool of standing genetic variation. Other studies have shown that multicolored fluorescent proteins display a considerable amount of adaptive, convergent, and parallel evolution in corals [15], [16]. Schwarz et al. [17] characterized a ferritin in Acropora millepora and Acropora palmata that displays signs of positive selection. Hayes et al. detected adaptive evolution in tachylectins [18].
Adaptive evolution, at the molecular level, is characterized by an excess of nonsynonymous nucleotide substitutions (dN) in comparison to synonymous ones (dS) [19], [20], [21], [22], [23]. If this is the case, the so-called dN/dS ratio becomes >1, and the gene of interest may be under positive selection. Note that a single important amino acid change may be sufficient to demonstrate positive selection. However, methods for site-specific adaptive evolution analyses require multiple pair-wise comparisons, thus inclusion of sequence data from multiple species. Evolutionary screens are designed in a way that orthologs in a designated group of genes are ‘scanned’ for elevated dN/dS ratios. These screens provide a powerful way to identify, in a single effort, many candidate genes that are potentially subject to positive selection. Circumstantially, there is no a priori requirement to know the function of the protein, a factor that is particularly beneficial in non-model organisms such as corals. However, lack of annotation cannot be considered a difficulty exclusively associated with non-model species as the number of genes without any significant sequence similarity to genes of other species in any eukaryotic genome surveyed so far seems to be about 10–20% [24], [25], [26]. It is assumed that these genes represent lineage-specific adaptations of the species under study as they not only lack sequence similarity to genes or proteins in other organisms, but also display a narrow phylogenetic distribution. There is no general agreement or rule, but usually, proteins which do not show any sequence similarity in BLASTp searches with cut-off values of E<10−5 or E<10−10 have been denoted as so-called taxonomically-restricted genes (TRGs) [27], and have been hypothesized to provide one of the sources of phenotypic diversity [28], [29], [30]. TRGs are synonymously referred to as lineage-specific genes [29]. A recent screen by Sunagawa et al. [31] identified a family of small, cysteine-rich proteins (SCRiPs) that appear to be restricted to Hexacorallia. A study in Hydra identified Periculin-1, a peptide that has strong bactericidal activity and at present no identifiable orthologs in sequence databases [32].
The amount of positive Darwinian selection has not yet been systematically surveyed in any coral. We set out to conduct an evolutionary screen of orthologs in two congeneric acroporid coral species: Acropora millepora from the Indo-Pacific and Acropora palmata from the Caribbean. We identified a set of 2,604 orthologous cDNA sequences for which we calculated pair-wise dN/dS ratios in order to (i) identify the extent of adaptive evolution in scleractinian corals, and (ii) assess the nature of proteins that are potentially subject to positive selection. Our results indicate that a considerable fraction of coral proteins might be under positive selection, and that TRGs display on average significantly higher evolutionary rates. As such, they might represent important mediators of microevolution and lineage-specific adaptations that warrant further examination for assessing the future response of corals to a changing environment.
Results
Evolutionary Screen
Out of 99,091 assembled unique sequences for A. millepora and 14,647 unique sequences for A. palmata, we gathered 3,295 putative ortholog pairs. We applied several criteria to identify the correct open reading frame (see Materials and Methods, Figure S1), and calculated the ratio of nonsynonymous to synonymous divergence (dN/dS ratio) between both coral species for each putative ortholog pair using PAML [33] as implemented in Pal2Nal [34]. After filtering, we obtained 2,604 putative ortholog pairs. 2,281 ortholog pairs could be annotated according to BLASTx homology searches, while 323 sequences had no significant hit. From 2,604 orthologs, 190 genes showed dN/dS ratios larger than 1. Of those, 68 genes were among the presumably coral-specific (i.e. non-annotated) orthologs (Table 1). This led us to conclude two things: (i) a considerable portion of the orthologs analyzed here show dN/dS values exceeding 1, which is a strong indicator (although not a proof) of positive selection (7% of all orthologs) [23], and (ii) taxonomically-restricted genes had significantly higher dN/dS values (median dN/dS = 0.5040) compared with the annotated set (median dN/dS = 0.2260; PMWU<0.001). Although the elevated dN/dS ratios of lineage-specific proteins could result from positive selection, they could also result from relaxed selective constraints. Hence, this alone does not constitute evidence for positive selection. The non-annotated set had a significantly higher rate of amino acid substitution (non-annotated median dN = 0.0206) compared with the annotated set (annotated median dN = 0.0091; PMWU<0.001), and this elevated rate cannot be attributed to a difference in overall mutation rate as values of synonymous substitutions were similar (non-annotated median dS = 0.0416; annotated median ds = 0.0426; PMWU = 0.379). This result confirms that non-annotated proteins evolve faster on average than annotated ones.
Table 1. Number of annotated (conserved) and non-annotated (presumably lineage-specific) orthologs.
| orthologs | northologs | %all | annotated | %all | %annot | non-annotated | %all | %non-annot |
| all | 3295 | |||||||
| filtered | 2604 | 100 | 2281 | 88 | 323 | 12 | ||
| dN/dS<1 | 2414 | 93 | 2159 | 95 | 255 | 79 | ||
| dN/dS>1 | 190 | 7 | 122 | 5 | 68 | 21 |
Evolutionary rate distribution
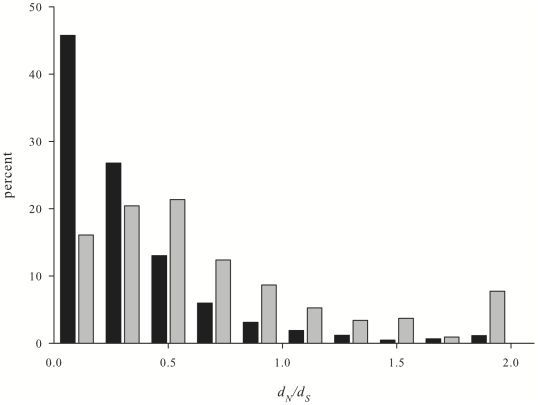
Although lineage-specific genes seem to evolve on average significantly faster than annotated genes, there is nonetheless a broad distribution of different rates for both classes (Figure 1). Annotated orthologs were most common at dn/ds<0.5 and successively diminished with increasing dn/ds. In contrast, lineage-specific orthologs were more evenly distributed across dn/ds values between 0 and 0.5, and were in particular present at values >1. We also found potential TRGs with very low divergence rates (dn/ds<0.01), indicative of high conservation. Those genes are particularly interesting as they might have arisen as a result of lineage-specific evolution until they reached an adaptive peak from which further evolution slowed [35]. As a result they are highly conserved between species of the same lineage but cannot be found outside of those lineages. Note that our BLAST-based annotation approach included the cnidarian Nematostella vectensis, so the TRGs are actually restricted even within cnidarians.
Figure 1. Discrete distribution of dN/dS ratios.
The percentages of conserved (black bars) and lineage-specific (grey bars) genes falling into the respective dN/dS classes. Note that dn/ds ratios over 1.8 were pooled for clarity.
Expression of dN/dS orthologs
The nature of TRGs does not allow for assigning functions based on homologies. Hence, it is not possible to compare ad hoc the functional distribution of conserved (i.e. annotated) and lineage-specific (i.e. non-annotated) orthologs. However, expression can be used as a first proxy to the function of a gene [36]. To this date, several studies used microarray expression profiling and whole mount in situ hybridization in corals to identify conserved and lineage-specific genes that play a role in development, bleaching, symbiosis, and heat stress [7], [8], [9], [11], [37], [38], [39]. Grasso et al. [38] conducted a microarray analysis of coral development in which they analyzed four major stages of coral development in A. millepora (prawnchip, planula, polyp, and adult stage). They were able to identify six major synexpression clusters that mapped onto the four stages of coral development. In order to compare expression between our set of conserved and lineage-specific orthologs, we mapped orthologs from A. millepora to the sequences that were contained in the expression clusters from the Grasso dataset via BLASTn. In each life stage, we identified the majority of the genes that were originally assigned to a cluster among our set of A. millepora orthologs (although our A. millepora EST dataset was derived from planulae larvae) (Table 2). First, we found no significant overrepresentation of lineage-specific genes in any of the clusters ((χ2-test, P = 0.224). Next, we conducted a Two Way ANOVA on log10-transformed dN/dS ratios with cluster and annotation as the dependent variables. As expected, there was a significant difference in the mean dN/dS values between the annotated and non-annotated orthologs (P<0.001). There was, however, no significant difference between clusters (P = 0.295), and also no significant interaction between clusters and conserved or lineage-specific orthologs (P = 0.666).
Table 2. Expression of dN/dS orthologs across developmental stages of Acropora millepora.
| Cluster sensu Grasso et al. | expression | n | northologs | % | nconserved | ncoral-specific | AV dN/dS conserved orthologs | AV dN/dS lineage-specific orthologs |
| I | prawnchip | 567 | 417 | 73.5 | 372 | 45 | 0.32 | 0.65 |
| II | planula | 110 | 86 | 78.2 | 76 | 10 | 0.38 | 0.76 |
| III | planula/polyp | 159 | 121 | 76.1 | 115 | 6 | 0.36 | 0.71 |
| IV | polyp | 77 | 65 | 84.4 | 61 | 4 | 0.44 | 0.67 |
| V | adult | 43 | 32 | 74.4 | 29 | 3 | 0.35 | 0.37 |
| VI | planula/polyp/adult | 205 | 155 | 75.6 | 146 | 9 | 0.32 | 0.82 |
| AV | 0.36 | 0.66 |
Functional distribution of orthologs with elevated dS, dN, and dN/dS
We applied Mann-Whitney U (MWU) test to see whether the indices of evolutionary rates were distributed unevenly across functional categories, based on annotations established using Gene Ontology (GO) terms for “biological process” and “molecular function”. Plotting the MWU test P-values across GO categories (Figures 2 and 3, Figures S2 and S3) indicated that the observed d N/d S variation is predominantly driven by variation in dN rates (as expected under varying selection pressures). We visualized a number of functional clusters showing a tendency to rank higher than the rest of the dataset with respect to dN/dS. Several of the highlighted GO categories passed the 10% false discovery rate cutoff [40] (Table 3).
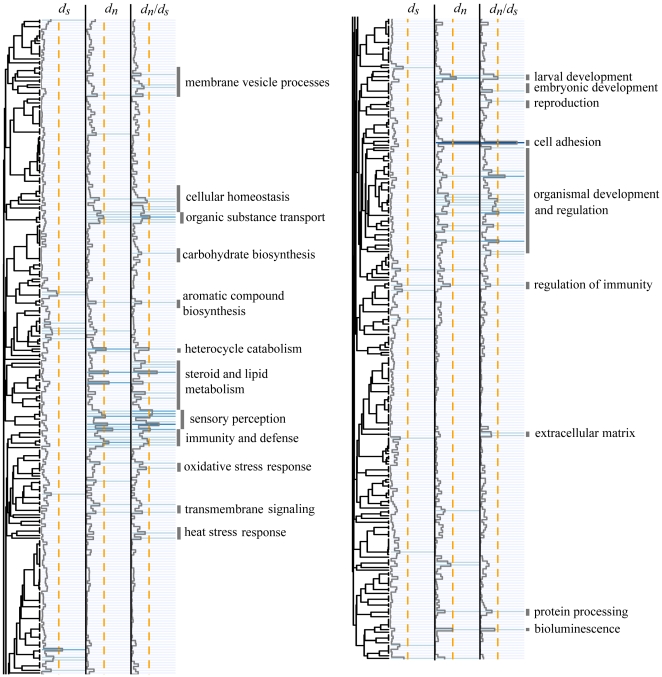
Figure 2. Detection of biological processes experiencing accelerated protein sequence evolution.
The dendrogram reflects the proportion of orthologs shared between different categories in our dataset (see Material and Methods). The colors of the corresponding cells and the overlying trace line represent P-values of Mann-Whitney U test for elevated dS, dN, and dN/dS values. The first transition to the darker color signifies P<0.05 in an individual comparison. The dashed orange line indicates the 10% false discovery rate cutoff.
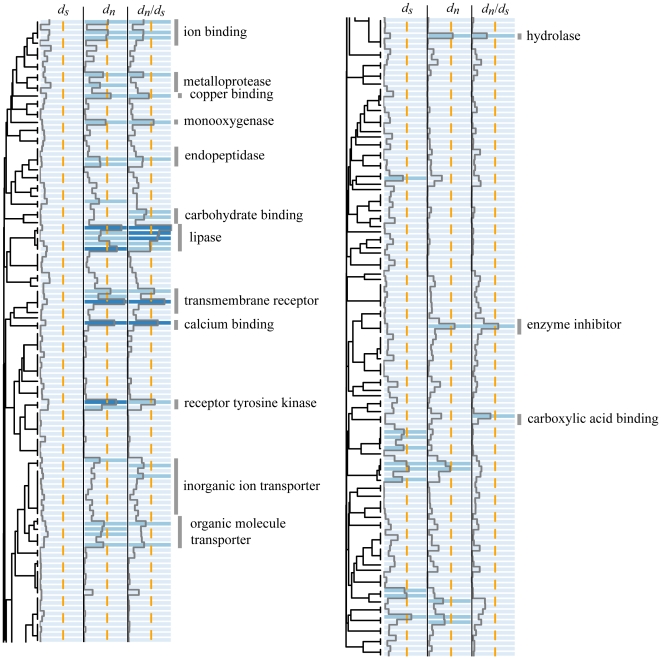
Figure 3. Detection of molecular functions experiencing accelerated protein sequence evolution.
The dendrogram reflects the proportion of orthologs shared between different categories in our dataset (see Material and Methods). The colors of the corresponding cells and the overlying trace line represent P-values of Mann-Whitney U test for elevated dS, dN, and dN/dS values. The first transition to the darker color signifies P<0.05 in an individual comparison. The dashed orange line indicates the 10% false discovery rate cutoff.
Table 3. List of GO categories most strongly enriched for orthologs with elevated dN/dS.
| Adjusted P MWU * | GO term | Description |
| Biological process: | ||
| 0.002 | GO:0007155 | cell adhesion |
| 0.021 | GO:0003008 | system process |
| 0.027 | GO:0006629 | lipid metabolic process |
| 0.027 | GO:0032501 | multicellular organismal process |
| 0.070 | GO:0007600 | sensory perception |
| 0.070 | GO:0007601 | visual perception |
| 0.087 | GO:0002009 | morphogenesis of an epithelium |
| 0.087 | GO:0002376 | immune system process |
| 0.087 | GO:0050877 | neurological system process |
| 0.087 | GO:0071702 | organic substance transport |
| 0.087 | GO:2000026 | regulation of multicellular organismal development |
| 0.099 | GO:0002164 | larval development |
| 0.102 | GO:0046700 | heterocycle catabolic process |
| 0.102 | GO:0051239 | regulation of multicellular organismal process |
| Molecular function: | ||
| 0.010 | GO:0016298 | lipase activity |
| 0.026 | GO:0004888 | transmembrane receptor activity |
| 0.053 | GO:0004620 | phospholipase activity |
| 0.053 | GO:0005509 | calcium ion binding |
| 0.069 | GO:0004714 | transmembrane receptor protein tyrosine kinase activity |
| 0.069 | GO:0004857 | enzyme inhibitor activity |
| 0.069 | GO:0004872 | receptor activity |
| 0.070 | GO:0004497 | monooxygenase activity |
*P-value of Mann-Whitney U test, adjusted according to the false discovery rate method (cutoff 10%) [40].
Candidate genes with potential relevance to cnidarian-dinoflagellate symbioses that display elevated rates of evolution
Many biological systems rely on symbiotic interactions between different organisms. Coral reef ecosystems, in particular, depend on the mutualistic relationship between corals and their endosymbiotic, dinoflagellate algae. Here, we generated a candidate gene list through literature perusal (and GO categories therein) containing homologs that are likely to be of relevance to cnidarian-dinoflagellate symbioses and that displayed elevated rates of evolution (Table 4). Among these, we found many members from the cellular stress-, heat stress-, and antioxidant-response system. Genes related to the innate immune system and sugar-binding proteins gave rise to a partial gene inventory (Table 4). Other genes that are likely to play a role in the cellular events surrounding the breakdown of symbiosis (exocytosis, apoptosis and/or autophagy [41], [42], [43], [44], [45], [46], [47]) were also identified.
Table 4. Potential symbiosis-related genes displaying elevated rates of evolution identified from the transcriptomes of A. millepora and A. palmate.
| Name | A. millepora SymBioSys ID | SwissProt Accession | E-value BLASTx | dN/dS | Notes |
| stress-/heat stress-/oxidative stress-/antioxidant activity-related | |||||
| Glutaredoxin-1 | SEQINDEX4598_C_c | GLRX | 6.00E-15 | 1.90 | cell redox homeostasis |
| Glutathione S-transferase omega-1 | SEQINDEX4951_C_c | GSTO1 | 2.00E-09 | 1.73 | oxidative stress response |
| Peroxidasin homolog | SEQINDEX7200_C_c | Pxdn | 3.00E-81 | 1.65 | oxidative stress response |
| Protein LSM14 homolog A | SEQINDEX12122_C_c | LSM14A | 2.00E-32 | 1.07 | |
| Endoplasmic reticulum resident protein 44 | SEQINDEX14162_C_c | Txndc4 | 1.00E-100 | 1.01 | |
| Heat shock protein Hsp-16.2 | SEQINDEX2354_C_c | hsp-16.2 | 5.00E-13 | 0.97 | stress response |
| Apoptosis-/Autophagy-related | |||||
| Apoptosis regulator BAX | SEQINDEX5561_C_c | Bax | 2.00E-18 | 1.09 | |
| Endo-, Exo-, Phagocytosis-related | |||||
| CD63 antigen | SEQINDEX6578_C_c | CD63 | 1.00E-29 | 2.31 | growth regulation |
| MIT domain-containing protein 1 | SEQINDEX12663_C_c | MITD1 | 9.00E-45 | 1.25 | endosomal protein transport |
| Gamma-glutamyl hydrolase | SEQINDEX13089_C_c | GGH | 1.00E-31 | 1.21 | |
| Synaptosomal-associated protein 29 | SEQINDEX2151_C_c | SNAP29 | 3.00E-21 | 1.20 | cellular membrane fusion |
| Immunity-related | |||||
| Gamma-IFN-inducible lysosomal thiol reductase | SEQINDEX2720_C_c | IFI30 | 1.00E-30 | 1.42 | innate immune response |
| Ectonucleoside triphosphate diphosphohydrolase 1 | SEQINDEX4337_C_c | ENTPD1 | 2.00E-40 | 1.37 | regulates homotypic adhesion |
| Lipopolysaccharide-binding protein | SEQINDEX3027_C_c | LBP | 5.00E-55 | 1.03 | binds to bacterial lipopolysaccharides (LPS), Toll signaling pathway |
| Toll-like receptor 2 | SEQINDEX6872_C_c | TLR2 | 4.00E-13 | 0.98 | mediates innate immune response to bacterial lipoproteins |
Discussion
Evolutionary Screen
A major factor that comes into play when assessing dN/dS ratios is that with higher evolutionary divergence, dS becomes saturated with multiple substitutions per site on long branches. Hence, neutral evolution is underestimated and, as a consequence, comparisons between different species are only valid within a given divergence range. The genus Acropora (Scleractinia: Acroporidae) is one of the most widespread genera of corals as it spans the Indian and Pacific Oceans and the Caribbean Sea. It is also the largest extant reef-building coral genus with numbers of species estimates ranging from 113 to 180 [48]. In this study, A. millepora is representing a member of the Indo-Pacific Acropora species and A. palmata is representing a member from the Caribbean. Molecular analyses suggest that A. millepora and A. palmata had their latest contact around 12 Myr ago, while Indo-Pacific Acropora species have radiated over the last 10 Myr [48]. If we assume a generation time of 1 to 10 years [49], [50] and a mutation rate of 10−8 per nucleotide site per generation for both species [51], we come up with the following proxy for dS: 107 generations (divergence time/generation time) * 10−8 (mutation rate) = 0.1. Hence, we expect an average divergence at neutrally evolving sites of approximately 10% (given that both species have the same mutation rate). This estimate is the same order of magnitude as the median dS of our set of orthologs (median dS = 0.043), and consequently our approach does not seem to inflate measures of dS. Even for genes that evolve fast, this divergence time frame allows one to identify the respective ortholog in both species.
We found that a considerable portion of the orthologs showed dN/dS values exceeding 1 (7% of all orthologs), and that TRGs had significantly higher dN/dS values. This finding might indicate that the group of TRGs plays a vital role in adaptive evolution. These genes did not show homology anywhere, including the sea anemone Nematostella vectensis, which belongs to the same subclass but a different order (subclass Hexacorallia, order Actiniaria). Although many of these genes may be coral-specific (i.e., restricted to stony corals, order Scleractinia), we cannot rule out that they are present in other, currently unsampled, orders of Hexacorallia (e.g. Corallimorpharia and Zoanthidea), or even have a broader pan-Anthozoan distribution but happen to be missing in the sea anemone. Studies in Drosophila have shown that TRGs represent a group of genes that on average display higher dN/dS ratios and are likely to play an important role in lineage-specific adaptations [35], [52]. Furthermore, a recent study on orthologs from coral symbionts, Symbiodinium spp., identified the highest dN/dS ratio in a Symbiodinium-specific gene, and the authors speculated accordingly that the portion of genes with elevated dN/dS values might be higher in the group of lineage-specific genes in comparison to conserved genes [53]. The authors further hypothesized that a symbiotic lifestyle might affect sequence evolution, as genes might need to coevolve with their symbiotic partners.
The ability to differentiate between self and non-self plays a particular role for reef-building corals in light of their mutualistic, intracellular symbioses with dinoflagellate algae as these need to be distinguished from other eukaryotic protists (dinoflagellates are alveolates and a sister group to the apicomplexans – obligate intracellular parasites – that may use the same receptors and signaling pathways to gain access to the host cell). In addition, competition between different symbiont strains might facilitate the evolution of genes involved in recognizing different clades of Symbiodinium, which often can associate with the same coral species [54]. A recent study suggested that transcriptomic states of the Caribbean coral Montastraea faveolata (a coral that can host multiple Symbiodinium genotypes) were correlated with differences in the Symbiodinium genotype hosted [55]. It will be interesting to test whether the percentage of genes under adaptive evolution is higher in corals that are able to host multiple versus only one genotype of Symbiodinium. Given that the generation time of Symbiodinium spp. is orders of magnitude smaller than those of corals, selection in corals might act on being less discriminating between different algal types that in turn evolve to cope with a changing environment.
Expession of dN/dS ortholgs
By definition, functional inference by homology to known genes is not available for TRGs. However, expression of those genes might indicate and serve as a proxy for functional significance. Given that some of the TRGs are likely a result of lineage-specific adaptations, it seems plausible to assume that they are expressed specifically with regard to life stage as 1) they presumably have specialized functions, and 2) restricted expression tends to minimize pleiotropic interference. Here, we wanted to test whether specific life-stages show an enrichment or depletion of high dN/dS genes in the group of annotated and non-annotated genes. While we were not able to show statistically significant differences, our data indicates that the adult life stage of corals has a similar dN/dS distribution for conserved (i.e. annotated) and coral-specific (i.e. non-annotated) genes (Table 2). By contrast, all other life stages show a higher mean dN/dS in supposedly coral-specific genes in comparison to conserved genes (Table 2). This might indicate that in order to better understand and investigate adaptive evolution of corals, particular attention has to be paid to non-annotated genes, and that expression of these genes are more easily found in life stages other than adult.
It is interesting to note that we found lineage-specific genes with low dN/dS values that showed stage-specific expression (e.g., lineage-specific orthologs in the adult stage). Those genes either arose de novo [56], through gene duplication and subsequent diversification [35], or were retained from a common ancestor but lost elsewhere [57]. They likely represent genes that support coral-specific adaptations, as they are conserved among corals but not found outside this lineage. A promising approach that arises from these considerations is that slowly evolving TRGs are enriched for “coral-specific” processes and are expressed stage-specifically. A combination of in silico and in situ approaches that couples evolutionary analyses with signatures of expression might prove a useful strategy to target such genes for further functional studies.
Functional analysis of the variation in protein evolution rates
Analysis of all the functional categories represented within our set of orthologs suggested a number of processes that experience accelerated rates of protein evolution (Figures 2 and 3, Table 3). Although many of these signatures may be due to relaxed purifying selection rather than positive selection, we detected anticipated targets of the latter. For example, proteins involved in immunity and defense, reproduction, and sensory perception (including transmembrane receptors and associated signaling pathways) are under positive selection in a wide variety of animals, from primates [22] and other mammals [58] to fruit flies [52]. “Bioluminescence” category in our case contained GFP-like fluorescent proteins, which have been shown to experience strong positive selection in corals [16]. In addition to these “usual suspects”, we saw elevated rates of evolution in several other functional groups that are not highlighted in studies of other animals and may therefore reflect the specifics of coral evolution. Some of these are ostensibly related to the corals' endosymbiotic relationship with Symbiodinium dinoflagellates, such as management of membrane vesicles, transmembrane transport of ions and organic molecules, and cellular homeostasis (which includes the category “maintenance of cellular location”). The category that was the most enriched with rapidly evolving proteins – cell adhesion – may also be related to symbiosis, as its members are linked to a number of cell surface molecules that may mediate host-symbiont recognition (Table S1). These proteins are expected to evolve under positive selection due to the need for frequent specificity readjustments and potentially due to “arms race” between the coral and cheater (i.e. non-compatible) strains of Symbiodinium.
Some of the functions highlighted by our analysis were rather unexpected. Most notably, proteins involved in metabolism of lipids and steroids feature prominently in both biological process and especially molecular function analyses (Table 3, Table S1, Table S2), for which we are not yet ready to offer a biological explanation. Some other functional groups may appear as rapidly evolving due to sharing of orthologs with other GO categories (reflected by the dendrogram in Figures 2 and 3). For example, “multicellular organismal development” may have become highlighted in our analysis due to substantial sharing of orthologs with the “cell adhesion” category. More generally, the inherent redundancy of the GO database leads to partial overlaps in the outcomes of different categories, so that any result on functional analyses based on GO annotations (irrespective of the methodology) must be viewed as the union of all possible interpretations of the data. Since only one of these interpretations is correct, some false positives are unavoidable. Selecting the correct interpretation would be possible based on additional systems biology data, which is still lacking for corals but may become available in the near future from whole-transcriptome expression profiling studies.
Candidate genes with potential relevance to cnidarian-dinoflagellate symbioses that display elevated rates of evolution
Since the global GO analysis may not adequately reflect the mechanisms specific for coral biology, we looked at a set of candidate genes that were either directly implicated in cnidarian-dinoflagellate symbiosis by empirical evidence, or functionally interconnected with them in molecular pathways. Within this gene set, proteins that play a role in corals' response to stress and genes related to immunity were the most prominently represented ones.
Stress-induced photoinhibition and damage to the algal photosystem II are thought to be responsible for an increased production of reactive oxygen species [59], [60], and consequently, diffusion of hydrogen peroxide (H2O2) through the membranes into the host cell(s) [61]. H2O2 then activates a cellular cascade, which results in expulsion of symbionts and bleaching [62]. The molecular pathways in the coral host to prevent bleaching (i.e. heat stress and oxidative stress) might therefore be under positive selection in order to mitigate the effects of stress on the coral-algae symbiosis. Consequently, many of the stress genes we identified (Table 4) were identified as differentially expressed in recent microarray studies on heat stress and bleaching in corals [7], [8], [10], [11]. Among the stress-related genes, we detected elevated rates of evolution in Hsp-16.2, Glutaredoxin-1, Glutathione S-transferase omega-1, and a Peroxidasin homolog (Table 4).
Genes related to innate immunity gave rise to another partial inventory of rapidly evolving genes. From the gene expression regulation standpoint, coral-algae specificity seems to arise not from the fact that a coral responds to an appropriate symbiont strain, but from active exclusion of other strains through immunity and apoptosis [37], [63]. Evolution of association with novel algal strains could therefore be enabled by mutations in recognition receptors typically responsible for their exclusion, such as immunity genes. Several genes thus far implicated in the establishment of coral-algae partnerships may indeed be broadly responsible for allorecognition and immune response regulation, such as glycans and lectins [64], [65], fasciclin [66], and MAPK-kinase and NF-kappa-B [67]. The latter two genes regulate antimicrobial response in invertebrates [68], which is somewhat different from their function in mammals. In this study, Gamma-IFN-inducible lysosomal thiol reductase, lipopolysaccharide-binding protein, and Toll-like receptor 2, all implicated in the innate immune response to bacterial pathogens, displayed elevated rates of evolution.
While our evolutionary screen between two coral species allowed for the delineation of fast-evolving functional categories, ultimately one is interested in identifying the specific genes and amino acid sites that are under adaptive evolution. Conducting similar analyses in a multi-species framework will make it possible to investigate this question in a robust statistical framework, allowing for amino acid site- and species-specific identification and characterization of positively selected genes. However, at the moment we have next to no information about the evolutionary mechanisms that brought about morphological, ecological, and physiological diversity of corals. This study provides an initial birds-eye view of genome-wide evolutionary patterns in corals and will serve as a guide for subsequent studies focusing on finer details of adaptation. Some of the genes that we highlighted in this initial screen may be responsible for thermal adaptation and therefore be targets of natural selection driven by increasing seawater temperatures as a consequence of climate change. They therefore represent a meaningful set of genes providing working hypotheses to look for genetic markers of climate change-driven evolution.
Materials and Methods
EST libraries, sequencing, assembly, and annotation
The datasets used in this study include an expanded version (see below) of a Sanger EST dataset from Acropora palmata [17] and a normalized 454 EST dataset from Acropora millepora [69]. Acropora millepora sequencing reads have been deposited in NCBI's SRA database, along with the assembly output (accession: SRA003728). Acropora palmata sequences are deposited in GenBank with the accession numbers DR982333–DR988505, EY021828–EY031784, FE038910–FE040597, DR982333-DR986349, EY021857-EY031784, and GW189124-GW218328. All sequences are available from the SymBioSys database at http://sequoia.ucmerced.edu/SymBioSys/index.php. For A. palmata, 29,205 additional ESTs were generated from a pooled EST library, which included RNA from unfertilized eggs, various larval stages, heat- and light-stressed larvae, and heat-stressed adult fragments. This library was normalized and sequenced at the DOE-Joint Genome Institute. Both datasets were processed as described in [70] using our EST pipeline and database SymBioSys (http://sequoia.ucmerced.edu/SymBioSys/index.php). Briefly, all unique sequences were annotated by a BLASTx homology search (E<10−5) against the UniProt, Swissprot, and TrEMBL databases. We denoted a gene as lineage-specific or taxonomically-restricted if the sequence did not yield a BLASTx hit to the TrEMBL database with an e-value smaller than 10−5. All raw, assembled, and annotated sequences are accessible as ‘Amil_v2’ and ‘Apal_EST’ through the SymBioSys database.
Identification of putative orthologs by Best Reciprocal BLAST Hit approach
Putative orthologs in A. millepora and A. palmata were identified by a Best Reciprocal BLAST Hit approach using the two assembled EST datasets described above (Figure S1). Initially, the method was developed as a shortcut to identify orthologs between genomes [71], [72], but is assumed to work equally well for EST sequences [73]. Briefly, all-against-all BLASTs of both datasets were conducted to obtain a library-specific best hit. We applied this method by identifying Best Reciprocal tBLASTx Hits with a bitscore cutoff of 300 for any given alignment (Table S3). Since tBLASTx hit alignments of translated nucleotide sequences may not necessarily correspond to the correct reading frame of the nucleotide sequences, we applied a series of tests to reduce the number of falsely calculated dN/dS ratios (see below). First, the correct orientation (5′ to 3′) of all A. palmata sequences was known, since all cDNAs were directionally cloned [17]. Accordingly, all A. millepora sequences were also oriented in the forward direction as determined from a BLASTn against the corresponding A. palmata sequence. Second, all A. millepora sequences were annotated according to Uniprot TrEMBL protein database entries using BLASTx (E<10−5). For those sequences that had BLASTx hits, we parsed the longest and second longest stop codon-free protein sequence (LSCFPS and 2nd LSCFPS) from the tBLASTx alignments and searched for homologs of both sequences in the Uniprot TrEMBL database using BLASTp (E<10−5). If either the LSCFPS or the 2nd LSCFPS BLASTp hit was identical to the BLASTx hit of the nucleotide sequence, we were highly confident that we identified the correct reading frame. If neither the LSCFPS nor the 2nd LSCFPS matched the BLASTx hit, we excluded the putative ortholog pair from further analysis. For non-annotated sequences, the alignment of the LSCFPS or the 2nd LSCFPS was called correct, if both nucleotide sequences for the LSCFPS or the 2nd LSCFPS were oriented in the forward direction, respectively. Again, the putative ortholog pair was excluded from further analysis if none of these rules applied to the tBLASTx alignments (Figure S1). Finally, sequences with homology search hits to mitochondrial sequences were removed. This was done by conducting BLASTn searches of all orthologs against the mitochondrial genome of Acropora tenuis (GenBank NC_003522).
Evolutionary Screen and Data analysis
We tested for evidence of positive selection by comparing the nonsynonymous substitution rate (dN) to the synonymous substitution rate (dS). Briefly, protein sequences of each ortholog pair were parsed from the tBLASTx results and aligned using ClustalW [74] with default parameters. The aligned protein sequences and corresponding nucleotide sequences were used as input files for Pal2Nal [34], which generates codon alignments and calculates gene-wide dN/dS values based on the codeml program implemented in PAML (Model M0) [75], [76] (Table S3). As dN/dS values for multiple mutations at a single site are not reliable, we chose to exclude those ortholog pairs that had dN or dS estimates greater than one. In addition, all dN/dS ratio estimates of 99 were removed from further analyses. To assess statistical significance of the difference in evolutionary rates between conserved and lineage-specific sets, we conducted Mann-Whitney U tests on dS, dN, and dN/dS distributions.
Functional analysis of accelerated protein evolution
Gene ontology (GO) [77] annotations were assigned to the ortholog pairs based on their best match to the UniProt database [78] and expanded based on the current GO hierarchy (as of March 18, 2011, http://www.geneontology.org/ontology/obo_format_1_2/gene_ontology.1_2.obo) to include all parents of the initially assigned terms. All subsequent calculations and plotting were performed in R [79]. The categories that were either represented by less than 5 orthologs or contained more than 25% of all orthologs were discarded; redundant categories (containing identical sets of orthologs) were removed to leave a single category with the most specific GO level. For “biological process” division, this filtering resulted in 1,426 orthologs in 502 GO categories, for “molecular function” division, the result was 1,433 orthologs in 239 GO categories. We then performed hierarchical clustering of GO categories following Kosiol et al. [58]. Specifically, the similarity of each pair of categories was calculated as the number of the shared orthologs divided by the size of the smaller of the two compared categories. The clustering was performed using a matrix of pairwise dissimilarities (1-similarity) using hclust function, method “average”. Such clustering simplifies the extensive hierarchy of the GO database, resulting in easily interpretable GO grouping tailored to the particular sequence dataset.
To avoid imposing arbitrary thresholds when selecting orthologs for GO analysis, we analyzed the distribution of dS, dN, and dN/dS values across all categories that passed our filters using one-sided Mann-Whitney U test, following the approach proposed earlier [58], [80]. The test identified categories containing orthologs that ranked significantly higher than the rest with respect to dS, dN, or dN/dS. The resulting P-values were plotted (after -log10 transformation) as a heat map (function heatmap.2) using the result of hierarchical clustering of GO categories as the grouping dendrogram. The custom color palettes for the heat maps were generated using function brewer.pal, such that the first transition to the darker color would correspond to P<0.05 in an individual test for a particular GO category. Thresholds at which the result would pass the 10% false discovery rate [40] cutoff were determined using function p.adjust. The GO terms in the resulting heat maps were manually summarized to indicate functional modules showing signatures of accelerated evolution. The heat maps listing descriptions for all represented GO categories, as well as full listing of annotated orthologs comprising the GO categories passing the 10% false discovery rate threshold, are available in supplementary data (Figures S2, Figure S3, Table S1, Table S2).
Supporting Information
Identification of putative orthologs between A. millepora and A. palmata by Best Reciprocal BLAST Hit and subsequent filtering approach.
(TIFF)
Detection of biological processes experiencing accelerated protein sequence evolution. The dendrogram reflects the proportion of orthologs shared between different categories in our dataset (see methods). The colors of the corresponding cells and the overlying trace line represent P-values of Mann-Whitney U test for elevated dS, dN, and dN/dS values. The first transition to the darker color signifies P<0.05 in an individual comparison. The dashed orange line indicates the 10% false discovery rate cutoff. The number preceding the definition of a GO category indicates the number of orthologs assigned to this category in our dataset.
(TIF)
Detection of molecular functions experiencing accelerated protein sequence evolution. The dendrogram reflects the proportion of orthologs shared between different categories in our dataset (see methods). The colors of the corresponding cells and the overlying trace line represent P-values of Mann-Whitney U test for elevated dS, dN, and dN/dS values. The first transition to the darker color signifies P<0.05 in an individual comparison. The dashed orange line indicates the 10% false discovery rate cutoff. The number preceding the definition of a GO category indicates the number of orthologs assigned to this category in our dataset.
(TIF)
List of biological process GO categories most strongly enriched with orthologs (false discovery rate 10%) displaying elevated dN/dS and their assigned orthologs.
(XLS)
List of molecular function GO categories most strongly enriched with orthologs (false discovery rate 10%) displaying elevated dN/dS and their assigned orthologs.
(XLS)
Orthologs of A. millepora and A. palmata with associated pairwise dN/dS estimates.
(XLSX)
Acknowledgments
We want to thank all the colleagues, divers and volunteers that helped over the years during the arduous spawning seasons in the Florida Keys, Panama, and Puerto Rico from 2003 to 2006. In particular, we want to thank Margaret Miller, Ernesto Weil, Nancy Knowlton, Don Levitan, Ben Mason and Cindy Lewis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by DEB-1054766 to M.V.M. and National Science Foundation grants IOS-0644438 and OCE-0313708 to M.M., and by a Collaborative Travel Fund to C.R.V. made by King Abdullah University of Science and Technology (KAUST). The work conducted by the U.S. Department of Energy Joint Genome Institute is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Marine and Freshwater Research. 1999;50:839–866. [Google Scholar]
- 2.Wilkinson C. Status of coral reefs of the world: 2004; 2004. Network GCRM, editor.
- 3.Weeks R, Russ GR, Alcala AC, White AT. Effectiveness of Marine Protected Areas in the Philippines for Biodiversity Conservation. Conserv Biol. 2009. [DOI] [PubMed]
- 4.Game ET, Bode M, McDonald-Madden E, Grantham HS, Possingham HP. Ecol Lett; 2009. Dynamic marine protected areas can improve the resilience of coral reef systems. [DOI] [PubMed] [Google Scholar]
- 5.Raymundo LJ, Halford AR, Maypa AP, Kerr AM. Functionally diverse reef-fish communities ameliorate coral disease. Proc Natl Acad Sci U S A. 2009;106:17067–17070. doi: 10.1073/pnas.0900365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett CY, Manua C, Cinner J, Sutton S, Jimmy R, et al. Conserv Biol; 2009. Comparison of Outcomes of Permanently Closed and Periodically Harvested Coral Reef Reserves. [DOI] [PubMed] [Google Scholar]
- 7.DeSalvo MK, Voolstra CR, Sunagawa S, Schwarz JA, Stillman JH, et al. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol Ecol. 2008;17:3952–3971. doi: 10.1111/j.1365-294X.2008.03879.x. [DOI] [PubMed] [Google Scholar]
- 8.Voolstra CR, Schnetzer J, Peshkin L, Randall CJ, Szmant AM, et al. Effects of temperature on gene expression in embryos of the coral Montastraea faveolata. BMC Genomics. 2009;10:627. doi: 10.1186/1471-2164-10-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Portune KJ, Voolstra CR, Medina M, Szmant AM. Development and heat stress-induced transcriptomic changes during embryogenesis of the scleractinian coral Acropora palmata. Marine Genomics. 2010;3:51–62. doi: 10.1016/j.margen.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Lanetty M, Harii S, Hoegh-Guldberg O. Early molecular responses of coral larvae to hyperthermal stress. Mol Ecol. 2009;18:5101–5114. doi: 10.1111/j.1365-294X.2009.04419.x. [DOI] [PubMed] [Google Scholar]
- 11.DeSalvo MK, Sunagawa S, Voolstra CR, Medina M. Transcriptomic responses to heat stress and bleaching in the elkhorn coral Acropora palmata. Marine Ecology Progress Series. 2010;402:97–113. [Google Scholar]
- 12.Downs CA, Mueller E, Phillips S, Fauth JE, Woodley CM. A molecular biomarker system for assessing the health of coral (Montastraea faveolata) during heat stress. Mar Biotechnol (NY) 2000;2:533–544. doi: 10.1007/s101260000038. [DOI] [PubMed] [Google Scholar]
- 13.Meyer E, Davies S, Wang S, Willis B, Abrego D, et al. Genetic variation in responses to a settlement cue and elevated temperature in the reef-building coral Acropora millepora. Marine Ecology Progress Series. 2009;392:81–92. [Google Scholar]
- 14.Thompson DM, van Woesik R. Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc Biol Sci. 2009;276:2893–2901. doi: 10.1098/rspb.2009.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alieva NO, Konzen KA, Field SF, Meleshkevitch EA, Hunt ME, et al. Diversity and evolution of coral fluorescent proteins. PLoS One. 2008;3:e2680. doi: 10.1371/journal.pone.0002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field SF, Bulina MY, Kelmanson IV, Bielawski JP, Matz MV. Adaptive evolution of multicolored fluorescent proteins in reef-building corals. J Mol Evol. 2006;62:332–339. doi: 10.1007/s00239-005-0129-9. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz JA, Brokstein PB, Voolstra C, Terry AY, Manohar CF, et al. Coral life history and symbiosis: functional genomic resources for two reef building Caribbean corals, Acropora palmata and Montastraea faveolata. BMC Genomics. 2008;9:97. doi: 10.1186/1471-2164-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes ML, Eytan RI, Hellberg ME. High amino acid diversity and positive selection at a putative coral immunity gene (tachylectin-2). BMC Evol Biol. 2010;10:150. doi: 10.1186/1471-2148-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AL, Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988;335:167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Bielawski JP. Statistical methods for detecting molecular adaptation. Trends Ecol Evol. 2000;15:496–503. doi: 10.1016/S0169-5347(00)01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen R. Molecular signatures of natural selection. Annu Rev Genet. 2005;39:197–218. doi: 10.1146/annurev.genet.39.073003.112420. [DOI] [PubMed] [Google Scholar]
- 23.Jensen JD, Wong A, Aquadro CF. Approaches for identifying targets of positive selection. Trends Genet. 2007;23:568–577. doi: 10.1016/j.tig.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Fischer D, Eisenberg D. Finding families for genomic ORFans. Bioinformatics. 1999;15:759–762. doi: 10.1093/bioinformatics/15.9.759. [DOI] [PubMed] [Google Scholar]
- 25.Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, et al. Comparative Genomics of the Eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson GA, Feil EJ, Lilley AK, Field D. Large-scale comparative genomic ranking of taxonomically restricted genes (TRGs) in bacterial and archaeal genomes. PLoS One. 2007;2:e324. doi: 10.1371/journal.pone.0000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson GA, Bertrand N, Patel Y, Hughes JB, Feil EJ, et al. Orphans as taxonomically restricted and ecologically important genes. Microbiology. 2005;151:2499–2501. doi: 10.1099/mic.0.28146-0. [DOI] [PubMed] [Google Scholar]
- 28.Kunin V, Cases I, Enright AJ, de Lorenzo V, Ouzounis CA. Myriads of protein families, and still counting. Genome Biol. 2003;4:401. doi: 10.1186/gb-2003-4-2-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalturin K, Hemmrich G, Fraune S, Augustin R, Bosch TC. More than just orphans: are taxonomically-restricted genes important in evolution? Trends Genet. 2009;25:404–413. doi: 10.1016/j.tig.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Tautz D, Schmid KJ. From genes to individuals: developmental genes and the generation of the phenotype. Philos Trans R Soc Lond B Biol Sci. 1998;353:231–240. doi: 10.1098/rstb.1998.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunagawa S, DeSalvo MK, Voolstra CR, Reyes-Bermudez A, Medina M. Identification and gene expression analysis of a taxonomically restricted cysteine-rich protein family in reef-building corals. PLoS One. 2009;4:e4865. doi: 10.1371/journal.pone.0004865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosch TC, Augustin R, Anton-Erxleben F, Fraune S, Hemmrich G, et al. Uncovering the evolutionary history of innate immunity: the simple metazoan Hydra uses epithelial cells for host defence. Dev Comp Immunol. 2009;33:559–569. doi: 10.1016/j.dci.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 34.Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609–612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domazet-Loso T, Tautz D. An evolutionary analysis of orphan genes in Drosophila. Genome Res. 2003;13:2213–2219. doi: 10.1101/gr.1311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allocco D, Kohane I, Butte A. Quantifying the relationship between co-expression, co-regulation and gene function. BMC Bioinformatics. 2004;5:18. doi: 10.1186/1471-2105-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voolstra CR, Schwarz JA, Schnetzer J, Sunagawa S, Desalvo MK, et al. The host transcriptome remains unaltered during the establishment of coral-algal symbioses. Mol Ecol. 2009;18:1823–1833. doi: 10.1111/j.1365-294X.2009.04167.x. [DOI] [PubMed] [Google Scholar]
- 38.Grasso LC, Maindonald J, Rudd S, Hayward DC, Saint R, et al. Microarray analysis identifies candidate genes for key roles in coral development. BMC Genomics. 2008;9:540. doi: 10.1186/1471-2164-9-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes-Bermudez A, DeSalvo MK, Voolstra CR, Sunagawa S, Szmant AM, et al. Gene expression microarray analysis encompassing metamorphosis and the onset of calcification in the scleractinian coral Montastraea faveolata. Marine Genomics. 2009;2:149–159. doi: 10.1016/j.margen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 41.Chen MC, Cheng YM, Sung PJ, Kuo CE, Fang LS. Molecular identification of Rab7 (ApRab7) in Aiptasia pulchella and its exclusion from phagosomes harboring zooxanthellae. Biochem Biophys Res Commun. 2003;308:586–595. doi: 10.1016/s0006-291x(03)01428-1. [DOI] [PubMed] [Google Scholar]
- 42.Downs CA, Kramarsky-Winter E, Martinez J, Kushmaro A, Woodley CM, et al. Symbiophagy as a cellular mechanism for coral bleaching. Autophagy. 2009;5:211–216. doi: 10.4161/auto.5.2.7405. [DOI] [PubMed] [Google Scholar]
- 43.Dunn SR, Schnitzler CE, Weis VM. Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: every which way you lose. Proc Biol Sci. 2007;274:3079–3085. doi: 10.1098/rspb.2007.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gates RD, Baghdasarian G, Muscatine L. Temperature stress causes host cell detachment in symbiotic cnidarians: implications for coral bleaching. Biol Bull. 1992;182:324–332. doi: 10.2307/1542252. [DOI] [PubMed] [Google Scholar]
- 45.Lesser M, Stochaj W, Tapley D, Shick J. Bleaching in coral reef anthozoans: Effects of irradiance, ultraviolet radiation and temperature, on the activities of protective enzymes against active oxygen. Coral Reefs. 1990;8:225–232. [Google Scholar]
- 46.Merle PL, Sabourault C, Richier S, Allemand D, Furla P. Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic Biol Med. 2007;42:236–246. doi: 10.1016/j.freeradbiomed.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 47.Sunagawa S, Choi J, Forman HJ, Medina M. Hyperthermic stress-induced increase in the expression of glutamate-cysteine ligase and glutathione levels in the symbiotic sea anemone Aiptasia pallida. Comp Biochem Physiol B: Biochem Mol Biol. 2008;151:133–138. doi: 10.1016/j.cbpb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Oppen MJ, McDonald BJ, Willis B, Miller DJ. The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol. 2001;18:1315–1329. doi: 10.1093/oxfordjournals.molbev.a003916. [DOI] [PubMed] [Google Scholar]
- 49.Hughes TP, Ayre D, Connell JH. The evolutionary ecology of corals. Trends in Ecology & Evolution. 1992;7:292–295. doi: 10.1016/0169-5347(92)90225-Z. [DOI] [PubMed] [Google Scholar]
- 50.Babcock RC. Comparative Demography of Three Species of Scleractinian Corals Using Age- and Size-Dependent Classifications. Ecological Monographs. 1991;61:225–244. [Google Scholar]
- 51.Baer CF, Miyamoto MM, Denver DR. Mutation rate variation in multicellular eukaryotes: causes and consequences. Nature Reviews Genetics. 2007;8:619–631. doi: 10.1038/nrg2158. [DOI] [PubMed] [Google Scholar]
- 52.Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- 53.Voolstra CR, Sunagawa S, Schwarz JA, Coffroth MA, Yellowlees D, et al. Comp Biochem Physiol Part D Genomics Proteomics; 2008. Evolutionary analysis of orthologous cDNA sequences from cultured and symbiotic dinoflagellate symbionts of reef-building corals (Dinophyceae: Symbiodinium). [DOI] [PubMed] [Google Scholar]
- 54.Frank SA. Host-symbiont conflict over the mixing of symbiotic lineages. Proceedings of the Royal Society of London Series B-Biological Sciences. 1996;263:339–344. doi: 10.1098/rspb.1996.0052. [DOI] [PubMed] [Google Scholar]
- 55.DeSalvo MK, Sunagawa S, Fisher PL, Voolstra CR, Iglesias-Prieto R, et al. Coral host transcriptomic states are correlated with Symbiodinium genotypes. Mol Ecol. 2010;19:1174–1186. doi: 10.1111/j.1365-294X.2010.04534.x. [DOI] [PubMed] [Google Scholar]
- 56.Heinen TJ, Staubach F, Haming D, Tautz D. Emergence of a new gene from an intergenic region. Curr Biol. 2009;19:1527–1531. doi: 10.1016/j.cub.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 57.Foret S, Knack B, Houliston E, Momose T, Manuel M, et al. New tricks with old genes: the genetic bases of novel cnidarian traits. Trends Genet. 2010;26:154–158. doi: 10.1016/j.tig.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Kosiol C, Vinar T, da Fonseca RR, Hubisz MJ, Bustamante CD, et al. Patterns of positive selection in six Mammalian genomes. PLoS genetics. 2008;4:e1000144. doi: 10.1371/journal.pgen.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 1998;21:1219. [Google Scholar]
- 60.Warner ME, Fitt WK, Schmidt GW. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Natl Acad Sci U S A. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Amsterdam, : Elsevier; 1987. pp. 227–287. and. Topics in photosynthesis, Vol 9: Photoinhibition. [Google Scholar]
- 62.Baird AH, Bhagooli R, Ralph PJ, Takahashi S. Coral bleaching: the role of the host. Trends Ecol Evol. 2009;24:16–20. doi: 10.1016/j.tree.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Dunn SR, Weis VM. Apoptosis as a post-phagocytic winnowing mechanism in a coral-dinoflagellate mutualism. Environmental microbiology. 2009;11:268–276. doi: 10.1111/j.1462-2920.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 64.Jimbo M, Yanohara T, Koike K, Sakai R, Muramoto K, et al. The D-galactose-binding lectin of the octocoral Sinularia lochmodes: characterization and possible relationship to the symbiotic dinoflagellates. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 2000;125:227–236. doi: 10.1016/s0305-0491(99)00173-x. [DOI] [PubMed] [Google Scholar]
- 65.Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell Microbiol. 2006;8:1985–1993. doi: 10.1111/j.1462-5822.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- 66.Reynolds WS, Schwarz JA, Weis VM. Symbiosis-enhanced gene expression in cnidarian-algal associations: cloning and characterization of a cDNA, sym32, encoding a possible cell adhesion protein. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2000;126:33–44. doi: 10.1016/s0742-8413(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Lanetty M, Phillips WS, Weis VM. Transcriptome analysis of a cnidarian-dinoflagellate mutualism reveals complex modulation of host gene expression. BMC Genomics. 2006;7:23. doi: 10.1186/1471-2164-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwanaga S, Lee BL. Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol. 2005;38:128–150. doi: 10.5483/bmbrep.2005.38.2.128. [DOI] [PubMed] [Google Scholar]
- 69.Meyer E, Aglyamova GV, Wang S, Buchanan-Carter J, Abrego D, et al. Sequencing and de novo analysis of a coral larval transcriptome using 454 GSFlx. BMC Genomics. 2009;10:219. doi: 10.1186/1471-2164-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sunagawa S, Wilson EC, Thaler M, Smith ML, Caruso C, et al. Generation and analysis of transcriptomic resources for a model system on the rise: the sea anemone Aiptasia pallida and its dinoflagellate endosymbiont. BMC Genomics. 2009;10:258. doi: 10.1186/1471-2164-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 73.Telford MJ. Phylogenomics. Curr Biol. 2007;17:R945–946. doi: 10.1016/j.cub.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 75.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol. 1994;11:725–736. doi: 10.1093/oxfordjournals.molbev.a040153. [DOI] [PubMed] [Google Scholar]
- 76.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 77.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Consortium UniProt. Ongoing and future developments at the Universal Protein Resource. Nucleic acids research. 2011;39:D214–219. doi: 10.1093/nar/gkq1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Team RDC. R: A language and environment for statistical computing: Vienna, Austria: R Foundation for Statistical Computing 2008.
- 80.Nielsen R, Bustamante C, Clark AG, Glanowski S, Sackton TB, et al. A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS biology. 2005;3:e170. doi: 10.1371/journal.pbio.0030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of putative orthologs between A. millepora and A. palmata by Best Reciprocal BLAST Hit and subsequent filtering approach.
(TIFF)
Detection of biological processes experiencing accelerated protein sequence evolution. The dendrogram reflects the proportion of orthologs shared between different categories in our dataset (see methods). The colors of the corresponding cells and the overlying trace line represent P-values of Mann-Whitney U test for elevated dS, dN, and dN/dS values. The first transition to the darker color signifies P<0.05 in an individual comparison. The dashed orange line indicates the 10% false discovery rate cutoff. The number preceding the definition of a GO category indicates the number of orthologs assigned to this category in our dataset.
(TIF)
Detection of molecular functions experiencing accelerated protein sequence evolution. The dendrogram reflects the proportion of orthologs shared between different categories in our dataset (see methods). The colors of the corresponding cells and the overlying trace line represent P-values of Mann-Whitney U test for elevated dS, dN, and dN/dS values. The first transition to the darker color signifies P<0.05 in an individual comparison. The dashed orange line indicates the 10% false discovery rate cutoff. The number preceding the definition of a GO category indicates the number of orthologs assigned to this category in our dataset.
(TIF)
List of biological process GO categories most strongly enriched with orthologs (false discovery rate 10%) displaying elevated dN/dS and their assigned orthologs.
(XLS)
List of molecular function GO categories most strongly enriched with orthologs (false discovery rate 10%) displaying elevated dN/dS and their assigned orthologs.
(XLS)
Orthologs of A. millepora and A. palmata with associated pairwise dN/dS estimates.
(XLSX)