Abstract
Objective
Enteral nutrition is provided to mechanically ventilated patients who cannot eat normally, yet the amount of support needed is unknown. We conducted this randomized, open-label study to test the hypothesis that initial low-volume (i.e. trophic) enteral nutrition would decrease episodes of gastrointestinal intolerance/complications and improve outcomes as compared to initial full-energy enteral nutrition in patients with acute respiratory failure.
Design
Randomized, open-label study
Patients
200 Patients with acute respiratory failure expected to require mechanical ventilation for at least 72 hours
Interventions
Patients were randomized to receive either initial trophic (10 ml/hr) or full-energy enteral nutrition for the initial 6 days of ventilation.
Measurements and Main Results
The primary outcome measure was ventilator-free days to day 28. Baseline characteristics were similar between the 98 patients randomized to trophic and the 102 patients randomized to full-energy nutrition. At enrollment, patients had a mean APACHE II score of 26.9, PaO2/FiO2 of 182 and 38% were in shock. Both groups received similar duration of enteral nutrition (5.5 vs. 5.1 days; P=0.51). The trophic group received an average of 15 ± 11% of goal calories daily through day 6 compared to 74.8 ± 38.5% (P<0.001) for the full-energy group. Both groups had a median of 23.0 ventilator-free (P=0.90) and 21.0 ICU-free days (P=0.64). Mortality to hospital discharge was 22.4% for trophic vs. 19.6% for full-energy (P=0.62). In the first 6 days, the trophic group had trends for less diarrhea (19 vs. 24% of feeding days; P=0.08) and significantly fewer episodes of elevated gastric residual volumes (2 vs. 8% of feeding days; P<0.001).
Conclusions
Initial trophic enteral nutrition resulted in similar clinical outcomes in mechanically ventilated patients with acute respiratory failure as early full-energy enteral nutrition but with fewer episodes of gastrointestinal intolerance.
Keywords: enteral nutrition, acute respiratory failure, trophic, permissive underfeeding
Introduction
Acute respiratory failure (ARF) requiring mechanical ventilation afflicts more than 3 million patients in the United States annually and represents the single most common reason ICU patients cannot eat (1,2). With a median 7.7 days of ventilation, ARF results in more than 23 million ICU days annually for potential nutritional support (3,4). Because malnourishment is associated with poor outcomes, many physicians assume that providing artificial enteral nutrition to replace full energy needs is beneficial. Additionally, studies suggest enteral nutrition supports intestinal structure and function, helping to prevent the increased permeability, bacterial translocation, and consequent systemic inflammation seen with gut disuse (5-7). Although not consistent among all studies, enteral nutrition has been shown to attenuate the hypermetabolism of critical illness, decrease infectious complications, and shorten ICU and hospital lengths of stay compared to parenteral nutrition (8-11). Furthermore, a recent meta-analysis demonstrated reduced mortality when enteral nutrition was initiated early in critically ill patients (12).
Due to these reported benefits, clinical practice guidelines recommend enteral nutrition as the preferred route for caloric support with early initiation when possible (13-15). However, the necessary volume of enteral nutrition required to maintain intestinal integrity remains unknown and many patients receiving enteral nutrition experience feeding complications (4,16,17). Full enteral feedings remain one of the biggest risk factors for aspiration, which represents the leading cause of pneumonia in the ICU and significantly increases morbidity and mortality (18). Hence, some clinicians use low dose “trickle” or trophic feeds (10-30cc/hr) early in the course of critical illness to maintain gut integrity and function while decreasing complications (14). We conducted a randomized, controlled trial to test the hypothesis that initial trophic enteral nutrition would decrease gastrointestinal complications and improve outcomes compared to early goal-directed full-energy enteral nutrition in mechanically ventilated patients.
Materials and Methods
Enrollment, Randomization, and Study Initiation
This randomized, open-label study enrolled patients from August 20, 2003 through July 8, 2009 from two ICUs at a single academic center after approval from the IRB. Patients expected to require mechanical ventilation for at least 72 hours and whose primary team intended to initiate or continue enteral nutrition (full exclusion criteria in the online supplement) were randomized in a 1:1 ratio (see online supplement) to either initial full-energy enteral nutrition or initial trophic enteral nutrition for 6 days followed by advancement to full-energy enteral nutrition. All baseline assessments were obtained after obtaining informed consent from each participant or their legally authorized representative, but prior to randomization or initiation of study procedures (additional data collection detailed in online supplement).
Patients were assigned to either initial full-energy enteral nutrition or initial trophic enteral nutrition for 6 days followed by advancement to full-energy enteral nutrition in a 1:1 ratio according to a permuted block scheme with random block sizes of 2, 4 or 6 patients. Assignments were placed in consecutive-numbered, opaque envelopes and sealed prior to the start of the study by personnel not associated with the trial. All baseline assessments were obtained after obtaining informed consent from the participant or their legally authorized representative, but prior to initiation of study procedures. Then, the envelope corresponding to the number of the patient was opened and the assignment determined.
Enteral Nutrition Procedures
The designated feeding strategy was initiated within 12 hours of randomization and enteral nutrition was continued until death, extubation, or 28 days. In patients who were extubated then required re-intubation, enteral nutrition was restarted and managed according to the study protocol through study day 28.
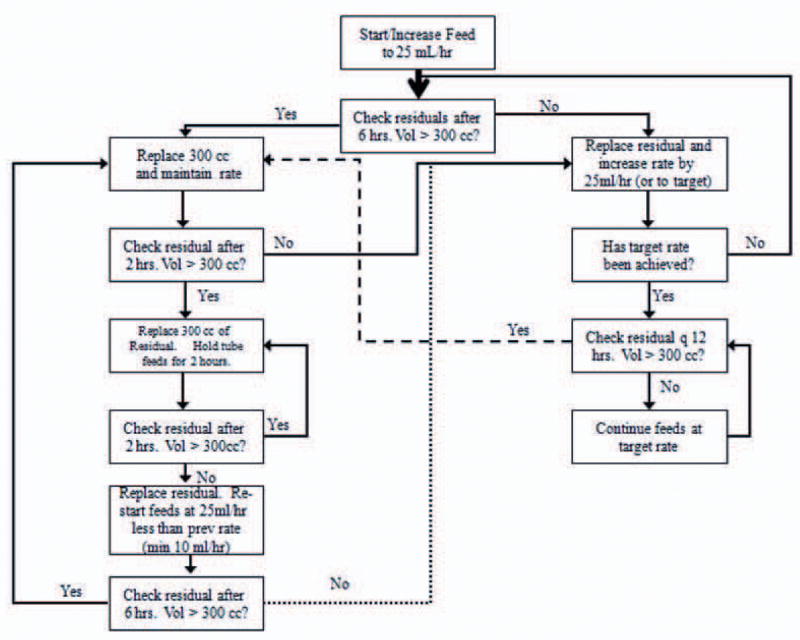
Patients randomized to the full-energy feeding group had enteral nutrition initiated at 25 cc/hr. Gastric residuals were checked every 6 hours while feeding rates were being advanced, with the feeding rate increased by 25 cc/hr every 6 hours until the full-energy feeding rate was achieved (Fig 1). Once full-energy rates were achieved, gastric residuals were checked every 12 hours. Dietary specialists determined full-energy feeding rates targeting 25-30 kcal/kg predicted body weight/day of non-protein energy and 1.2-1.6 g/kg predicted body weight/day of protein (details in online supplement). Patients randomized to the trophic group had enteral nutrition initiated at 10 cc/hr. GRV were checked every 12 hours. In patients still ventilated at 144 hours, enteral nutrition was advanced to full-energy target feeding rates using the same protocol as for the full-energy feeding group (Fig 1). Both feeding strategies directed when and for how long to hold enteral nutrition for elevated GRV and other GI intolerances (details in online supplement). In both groups, the primary team selected the enteral formula to be used. Most patients received a commercially available standard formula containing 1-1.2 kcal/cc, except patients with renal failure where the primary team could elect to prescribe an alternative commercially available formula consisting of 2 kcal/cc with restricted electrolytes.
Figure 1. Full-energy Feeding Protocol.
The instructions in the boxes on the right are intended to advance enteral nutrition rapidly while the boxes on the left are to ensure feeds are advanced safely.
Other Patient Care Decisions
Serum blood sugar levels were managed using the ICU's insulin drip protocol with the target blood sugar range determined by the primary medical team. Likewise, other aspects of care not associated with enteral nutrition, including fluid management, antibiotics, steroid administration, vasopressors, transfusions, acid suppression, and dialysis were at the sole discretion of the primary team. Per ICU protocol, patients were maintained in the semi-recumbent position whenever possible. Management of mechanical ventilation and weaning was standardized (details in online supplement).
Endpoints
The number of ventilator-free days (VFDs) to study day 28 represented the primary efficacy measure (detailed in online supplement). Secondary endpoints included 28-day and hospital all-cause mortality, organ-failure free days, ICU-free days, and hospital-free days to study day 28. Incidence of GI intolerances and development of new infections represented additional secondary endpoints. Patients were deemed to have developed a new presumed infection while on the study if the primary team started or changed antibiotics to treat a presumed or confirmed infectious process after study day 2.
Statistical Analysis
Prior to this trial, the mean VFDs to day 28 after initiation of mechanical ventilation in 60 consecutive mechanically ventilated patients in the ICU was 17.6 ± 7.3 days with a median of 22 (IQR 16-25) VFDs. An independent sample t-test, designed to demonstrate a 15% relative increase of 3.0 VFDs with 80% power and a two-sided p-value of 0.05, determined that 94 patients would be required in each arm. The study enrolled 200 to allow for a 5% withdrawal rate and compensate for the single interim analysis.
Variables were assessed by intention-to-treat analyses. Organ failure-free days and VFDs are reported as medians with IQR with a Mann-Whitney U test used to compare differences between groups. Mortality data were compared between groups using chi-square tests and Kaplan-Meier curves with log rank testing for 28-day survival analysis. Episodes of GI intolerance were reported as percent of days fed with intolerance and analyzed using Student's t-test (detailed in online supplement). Patients with ICU admitting diagnoses of acute lung injury, sepsis, or pneumonia were considered the most severely ill patients, defined as a subgroup a priori, and analyzed for differences in VFD, mortality, and ICU-free days. Patients with BMI ≥ 35 were similarly analyzed, but as a posthoc subgroup.
An independent Data and Safety Monitoring Board (DSMB) monitored the study for safety and efficacy and performed one interim analysis after 100 patients had completed study procedures. Data analysis and descriptive statistics were performed using PASW Statistics version 18.0 (SPSS, Inc. Chicago, IL, USA) with two-sided P-values ≤ 0.05 considered significant.
Results
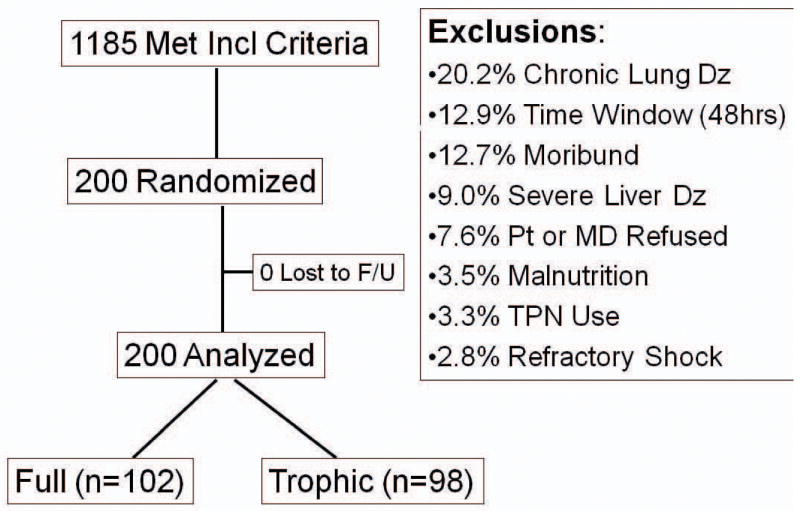
Of the 1187 mechanically ventilated patients who met the inclusion criteria, 290 lacked exclusion criteria but the patient's family refused surrogate consent for 51 and the primary doctor refused to allow patients' families to be approached for consent in an additional 39. The remaining 200 were consented and enrolled with 102 randomized to full-energy and 98 to initial trophic enteral nutrition (Figure 2). All 200 had complete follow-up to death or hospital discharge. The most common exclusion criteria were severe exercise-limiting lung disease, longer than 48 hours since initiation of mechanical ventilation, expected death within 48 hours, and chronic liver disease. Exclusions for enteral nutrition contraindications or parenteral nutrition were infrequent and only 13 patients were excluded for either partial or complete bowel obstruction, bowel ischemia or infarction.
Figure 2. Patient Screening, Enrollment, and Follow-up.
Baseline characteristics were similar in both groups (Table 1). On average, patients were 53.5 ± 17.7 years old, largely Caucasian and with a slight female preponderance. Comorbidities were similar between groups with hypertension the most common. Acute lung injury represented the most common ICU admitting diagnosis. The majority of patients were overweight with an average BMI of 28.7 ± 9.7. Over a third of the patients were on vasopressors at enrollment, with an average APACHE II score of 26.9 ± 7.3 and PaO2/FiO2 ratio of 182 ± 116. Mean baseline serum creatinine and albumin levels were also similar between groups.
Table 1. Baseline Demographics.
Baseline demographics, respiratory parameters, and labs for patients randomized to initial trophic vs. full-energy enteral nutrition.
| Characteristic | Trophic (N=98) |
Full-Energy (N=102) |
P-Value |
|---|---|---|---|
| Age (years) | 53 ± 19 | 54 + 17 | 0.71 |
| Female (%) | 60.2 | 53.9 | 0.37 |
| Caucasian (%) | 84.7 | 91.2 | 0.25 |
| ICU Diagnosis (%) | 0.56 | ||
| ALI | 21 | 20 | |
| Pneumonia | 15 | 19 | |
| Altered Mental Status/Neurological | 14 | 15 | |
| Sepsis | 10 | 12 | |
| Overdose | 10 | 7 | |
| Comorbidities (%) | |||
| Hypertension | 42 | 37 | 0.51 |
| Cardiac Disease | 24 | 23 | 0.75 |
| Diabetes | 22 | 23 | 0.98 |
| Chronic Renal Insufficiency | 18 | 12 | 0.19 |
| COPD | 16 | 18 | 0.80 |
| Immunosuppression | 14 | 16 | 0.48 |
| Peptic Ulcer Disease | 4 | 4 | 0.96 |
| Gastroesophageal Reflux | 4 | 4 | 0.96 |
| APACHE II Score | 26.9 ± 8.1 | 26.9 ± 6.6 | 0.94 |
| Vasopressors (%) | 35 (35.7%) | 42 (41.2%) | 0.43 |
| FiO2 (%) | 40.8 ± 25.0 | 42.3 ± 23.1 | 0.65 |
| PEEP (cm H20) | 7.6 ± 3.6 | 7.7 ± 3.7 | 0.82 |
| PaO2/ FiO2 | 181 ± 110 | 183 ± 122 | 0.91 |
| Creatinine (mg / dL) | 1.7 ± 1.4 | 1.8 ± 1.4 | 0.52 |
| Albumin (g / dL) | 2.8 ± 0.6 | 2.8 ± 0.7 | 0.99 |
| Total Bilirubin (mg / dL) | 1.7 ± 2.9 | 1.6 ± 4.1 | 0.84 |
| Aspartate Transaminase (AST) U/L | 184 ± 531 | 200 ± 485 | 0.85 |
| Platelets (× 10ˆ9 / mmˆ3) | 213 ± 122 | 215 ± 125 | 0.91 |
| Height (in) | 66.6 ± 4.0 | 67.0 ± 4.7 | 0.54 |
| Weight (kg) | 83.3 ± 28.7 | 81.9 ± 29.3 | 0.73 |
| Predicted Body Weight (PBW) (kg) | 62.8 ± 10.2 | 64.3 ± 11.1 | 0.32 |
| BMI (kg/m2) | 29.2 ± 10.2 | 28.2 ± 9.4 | 0.48 |
| On Enteral Feeds at Randomization (%) | 22.4 | 21.6 | 0.88 |
| Ventilation to Start of Enteral Feeds (days) | 1.0 ± 1.0 | 1.1 ± 1.2 | 0.72 |
| Goal (REE by Harris Benedict) | 1694 ± 435 | 1675 ± 451 | 0.763 |
| Goal (25 kcal / kg PBW) | 1570 ± 255 | 1608 ± 277 | 0.32 |
| Hours of Enteral Nutrition on day 0 | 14.1 ± 4.3 | 12.8 ± 4.6 | 0.06 |
Enteral Nutrition
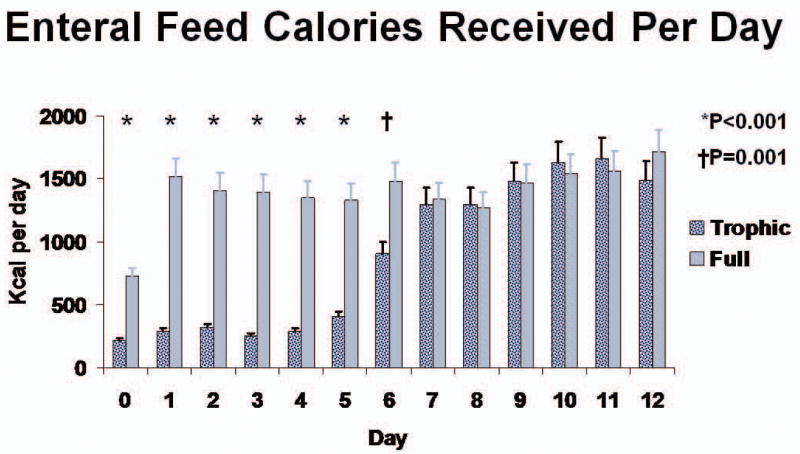
Calculated energy requirements were approximately 1600 kcal/day for both groups (Table 1). Enteral nutrition was initiated a day after intubation in both groups, with slightly less than a quarter of patients having already been started on enteral nutrition at the time of randomization. On average, patients in both groups received enteral nutrition for just over 5 days (5.5 ± 3.6 for trophic vs. 5.1 ± 3.3 for full-energy; P=0.51). After the day of enrollment both groups were fed for about 19 hours per day. Only 9 patients (9%) in the trophic group and 8 patients (8%) in the full-energy group were still ventilated and receiving enteral nutrition on study day 12. Patients in the full-energy group received significantly more volume and calories from daily enteral nutrition for study days 0-6 (Figure 3). For study days 1-5, patients receiving enteral nutrition in the full-energy group averaged 1418 ± 686 kcal per day compared to 300 ± 149 kcal per day for the trophic group (P<0.001), representing an average delivery of 74.8 ± 38.5% and 15.8 ± 11% of targeted goal daily calories, respectively. A similar separation was observed for daily grams of protein received (54.4 ± 33.2 vs. 10.9 ± 6.8; P<0.001). Both groups received similar amounts of calories and protein daily for study days 7 through 12. Only 19 of the 31 (61.3%) patients in the trophic group still receiving mechanical ventilation on study day 6 achieved goal enteral nutrition rates compared to 94 of the original 102 (94%) full-energy patients (P<0.001). The full-energy group reached goal feeding rates on average 13.3 ± 12.6 hours compared to 141 ± 32 hours after randomization for the trophic patients (P<0.001). Although infrequent, prokinetic use did not differ between groups (13.3% of trophic patients vs. 18.8% of full-energy; P=0.44).
Figure 3. Calories of Enteral Nutrition Received per Day in Patients Receiving Enteral Feeds.
Daily calories for patients receiving trophic vs. full-energy enteral nutrition for study days 0-12. * P<0.001; † P=0.001
Episodes of Gastrointestinal Intolerance
Overall, 66 patients had at least one episode of intolerance, with a trend toward a higher incidence in patients fed full-energy nutrition (39.2% vs. 26.5%; P=0.08). Over the first 6 days, the full-energy group had a trend toward more patient feeding days with any episode of GI intolerance (34.6 ± 37.3% vs. 24.6 ± 35.5%; P=0.06). The incidence of patient feeding days with GI intolerances did not differ between groups for days 7-12 and the trend for more feeding days with episodes of GI intolerances in the full-energy group disappeared when the full 12 days of enteral feeding was evaluated (34.4 ± 36.7% vs. 26.5 ± 36.1%; P=0.14). Diarrhea represented the most common GI intolerance in both groups, with a trend for more in the full-energy fed patients (24.1% vs. 19.1% of patient-fed days; P=0.08). Although infrequent in both groups, GRV > 300 cc were also more common in the full-energy group (7.5 vs. 2.1% of patient-fed days; P<0.001). Abdominal distention or cramping (8.2% vs. 5.5%; P=0.14) and constipation (9.1% vs. 6%; P=0.10) were similar in both groups. Vomiting occurred rarely (1.8 vs. 2.1%), one episode of regurgitation occurred in each group and one episode of aspiration occurred in the trophic group.
Clinical Outcomes
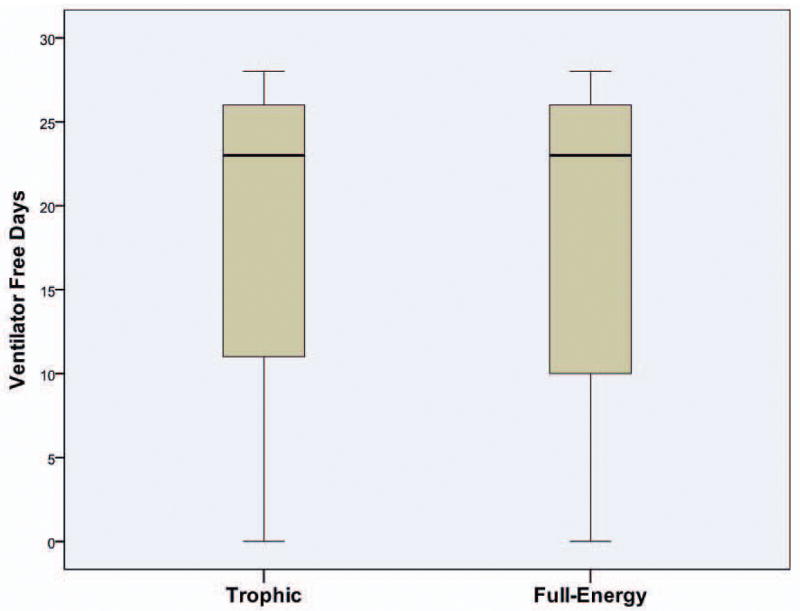
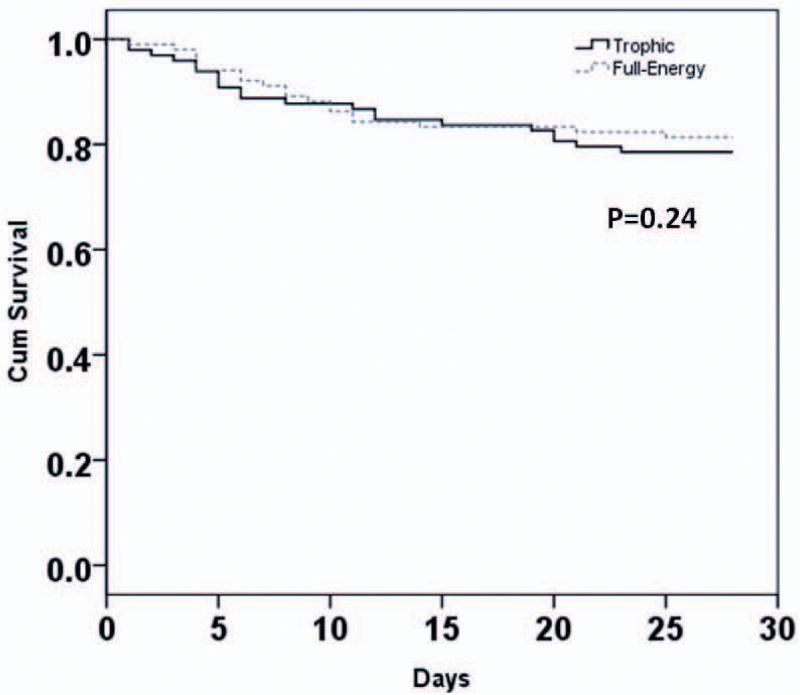
Patients receiving initial trophic enteral nutrition had similar clinical outcomes as those receiving initial full-energy enteral nutrition (Table 2). Specifically, patients from both groups had a similar number of days alive and free from mechanical ventilation to study day 28 (Difference in means 0.1 day; 95% CI -2.6 to 2.9 days; P=0.90) (Figure 4). Overall, 22 / 98 patients (22.4%) in the trophic group and 20 / 102 patients (19.6%) in the full-energy group died prior to hospital discharge (P=0.62). Kaplan-Meier curves demonstrated similar 28-day survival plots for both groups (P=0.24) (Figure 5).
Table 2. Clinical Outcomes.
The Number of ventilator-free, ICU-free, and organ failure-free days andhospital mortality for the trophic and full-energy groups. Subgroup analyses of clinical outcomes in patients with acute lung injury, pneumonia, or sepsis and those with BMI ≥ 35.
| Outcome | Trophic (N=98) |
Full-Energy (N=102) |
P-Value |
|---|---|---|---|
| Ventilator-Free Days (median; IQR) | 23.0 (10.5-26) | 23.0 (9.3-26) | 0.90 |
| Ventilator-Free Days (mean ± s.d.) | 17.9 ± 10.4 | 17.8 ± 10.5 | 0.94 |
| All-Cause Hospital Mortality (%) | 22 (22.4%) | 20 (19.6%) | 0.62 |
| ICU-Free Days (median; IQR) | 21.0 (6.5-24) | 21.0 (9.3-24) | 0.64 |
| Hospital-Free Days (median; IQR) | 12.0 (0-21) | 16.5 (0-21) | 0.36 |
| Shock-Free Days (mean ± s.d.) | 22.1 ± 11.7 | 22.5 ± 11.0 | 0.84 |
| Renal Failure-Free Days (mean ± s.d.) | 18.4 ± 13.1 | 18.3 ± 12.9 | 0.97 |
| Hepatic Failure-Free Days (mean ± s.d.) | 20.0 ± 13.0 | 22.0 ± 12.2 | 0.32 |
| Hematologic Failure-Free Days (mean ± s.d.) | 20.2 ± 12.5 | 22.1 ± 11.6 | 0.27 |
| Ventilator Days in survivors (mean ± s.d.) | 5.5 ± 5.4 | 5.7 ± 6.4 | 0.85 |
| Subgroup (ALI, Sepsis, or Pneumonia) | N=46 | N=51 | |
| Ventilator-Free Days (mean ± s.d.) | 16.3 ± 10.6 | 17.5 ± 11.1 | 0.59 |
| ICU-Free Days (mean ± s.d.) | 14.9 ± 9.9 | 15.5 ± 10.0 | 0.76 |
| All-Cause Hospital Mortality (%) | 12 (26.1%) | 13 (25.5%) | 0.95 |
| Subgroup (BMI > 35) | N=19 | N=16 | |
| Ventilator-Free Days (mean ± s.d.) | 20.8 ± 8.8 | 17.9 ± 9.6 | 0.36 |
| ICU-Free Days (mean ± s.d.) | 19.3 ± 8.3 | 15.4 ± 8.9 | 0.18 |
| All-Cause Hospital Mortality (%) | 2 (10.5%) | 4 (25%) | 0.26 |
Figure 4. Distribution of VFD to Study Day 28 by Treatment.
The black line represents the median, the shaded box the IQR and the error bars 95% CI.
Figure 5. Kaplan-Meier Survival Curve.
Survival curves for patients receiving initial trophic vs full-energy enteral nutrition. P=0.24
Both groups had a median of 21 ICU-free days (P=0.64) and a similar number of hospital-free days (12.0 trophic vs. 16.5 full-energy; P=0.36). There were no differences in the number of other organ failure-free days between groups (Table 2). The two groups were also similar in the percentage of patients who developed any infection after enrollment (30.6% trophic vs. 32.4% full-energy; P=0.79) or nosocomial pneumonia (14.3% trophic vs. 17.6% full-energy; P=0.73)
Subgroup Analyses
About half the patients (48.5%) had acute lung injury, sepsis, or pneumonia as their ICU admitting diagnosis. Analysis of this a priori defined subgroup found similar results for VFD, ICU-free days, and mortality in patients fed initial trophic compared to full-energy enteral nutrition (Table 2). Similarly, the subgroup of patients with BMI greater than or equal to 35 also had similar outcomes in both groups. Although not defined as an a priori endpoint, survivors who received initial full-energy enteral nutrition were more likely to be discharged home with or without help as compared to a rehabilitation facility (68.3% full-energy vs. 51.3% for trophic; P=0.04).
Discussion
Consensus guidelines recommend enteral over parenteral nutrition in patients with acute respiratory failure who are unable to eat conventionally (13-15). Although the same guidelines recommend advancing enteral nutrition to full-energy rates over the first 48-72 hours, they acknowledge the data supporting this latter recommendation are weak (14). The results of this randomized trial demonstrate that providing trophic enteral nutrition for the first 6 days of ventilation resulted in similar clinical outcomes as a strategy of advancing enteral nutrition to full-energy rates as quickly as possible. Initial trophic feeds did result in fewer episodes of gastrointestinal intolerance over the first 6 days, with fewer elevated GRV and a trend toward less diarrhea. Unlike previous data (19-21), this study did not find an increased risk of infection with either feeding strategy.
Enteral nutrition supports the structural and functional integrity of the intestine, helping to prevent increased gut permeability and associated bacterial translocation (5-7). Specifically, enteral nutrition stimulates epithelial cell growth and proliferation, maintains mucosal mass and microvilli height (22,23), preserves tight junctions between epithelial cells, and promotes blood flow (24). The intestine responds to intraluminal contents by producing and secreting a variety of endogenous agents which have a trophic effect on the intestinal epithelium (25,26).
Although the benefits of enteral nutrition are well documented (5-12,22-26), the amount needed to confer these benefits, especially in humans, remains unknown. In animal studies, continuous enteral nutrition of very small volumes, commonly termed trophic feedings for the nourishing effect that they have on the intestinal mucosal, preserve the intestinal microvilli and maintain enteric function (23, 27-29). However, data concerning the effect of different volumes of enteral nutrition on outcomes in humans are conflicting. Although confounded by better feeding tolerance in less sick patients, some observational studies have found that higher volumes of enteral nutrition are associated with reduced mortality (30) and lower rates of bloodstream infections (19). Additional studies demonstrated that increasing enteral nutrition adequacy by implementing a nutrition protocol shortened the duration of mechanical ventilation (31) and hospital length of stay with a trend toward lower mortality (32). By contrast, other observational data found patients who received 33-65% of goal calories had higher rates of survival and being liberated from the ventilator, with a lower likelihood of developing sepsis (20). A separate cluster randomized trial demonstrated no improvement in clinical outcomes despite use of a protocol resulting in greater nutritional delivery (33). A prospective controlled trial in 150 medical ICU patients fed either low volume or goal calorie bolus enteral nutrition for the first 4 days of ventilation found similar hospital mortality. However, the patients assigned low volume enteral nutrition experienced significantly less ventilator associated pneumonia and shorter ICU and hospital lengths of stay (21).
Our study is the first randomized study comparing different volumes of enteral nutrition by continuous infusion in mechanically ventilated patients. Similar to the findings in animals (28,29), these data demonstrate that initial trophic feeds, providing about 15% of goal calories daily, result in similar clinical outcomes as initial full-energy enteral nutrition. Although designed similarly to Ibrahim's study, important differences beyond the results should be noted. This study used concealed randomization to allocate patients and continuous enteral nutrition, as opposed to bolus feedings which may increase the risk of aspiration (34). Furthermore, our study achieved excellent separation of treatment arms with the full-energy group in our study receiving 70-75% of goal calories daily compared to only 20% in Ibrahim's study.
All patients in this study were at least initially fed via tubes terminating in the stomach. This likely resulted in higher GRV than seen with post-pyloric feeding. In addition, pro-kinetic agents were utilized in only about one of every six patients, less frequently than reported in observational studies of regular practice (4,33,35). Despite this, 94% of patients in the full-energy group still reached goal rates in a little over half a day, as fast or faster than previously reported (4,16,31,33,35). Our decision to utilize higher than historic GRV thresholds for holding enteral nutrition likely helped facilitate rapid advancement. However, more recent findings and recommendations suggest that utilizing higher thresholds is both safe and promotes delivery of enteral nutrition (14,34,36-39). Despite the higher threshold, this study had very little vomiting, regurgitation, or aspiration, although this rapid advancement to goal feeds may have contributed to the higher incidence of diarrhea in the full-energy group (40,41). More frequent checking of GRVs while enteral feeds were being advanced to goal rates (q6h vs. q12h) may have contributed to more episodes of elevated GRV in the full-energy group.
This study has some limitations. It is a single center study largely conducted in a medical ICU. However, the sample size, which represents one of the largest published among human enteral nutrition studies, was adequate to detect a clinically significant difference in VFDs of 3 days. However, the study is underpowered to detect smaller differences in VFDs or to determine whether small differences in mortality or other clinical outcomes between the two groups are significant. Furthermore, the study enrolled a heterogeneous medical population with good representation of acute lung injury, pneumonia, sepsis, and overdose. Patients with GI hemorrhage were underrepresented due to clinicians' reluctance to enterally feed these patients early in their ICU course. However, patients with resuscitated shock, who constituted almost 40% of the study population, tolerated enteral nutrition with no documented episodes of intestinal ischemia or infarction.
Although sometimes used, indirect calorimetry was not undertaken to determine energy requirements. Consistent with guideline recommendations (13-15) and standard practice in our ICU, energy requirements were instead calculated using a target of 25-30 kcal/kg/d. Furthermore, no patients received added protein or micronutrients, such as anti-oxidants or vitamins, beyond that provided in the enteral nutrition formulation. Protein supplementation has become popular in clinical practice since standard enteral formulas tend to have a low nitrogen to non-protein calorie ratio (14). Despite receiving five times as much protein (54 vs. 11 grams/day) for the first 6 days, the full-energy group had similar outcomes as the trophic group. However, neither group received the goal of 1.5 g / kg of daily protein. Parenteral nutrition or supplemental intravenous dextrose was not initiated in any study patient, including those unable to tolerate 100% of goal calories after 7-10 days (14).
This study also has a number of strengths. The feeding protocol delivered average daily amounts comparable to the best data from other protocolized enteral nutrition strategies (4,16,31,33,35), rendering the full-energy group a representative control. Randomization was done via concealed envelopes with complete follow-up of all patients. Similar clinical outcomes with initial trophic compared to full-energy enteral nutrition were found in both subgroups analyzed. Observational data have suggested that increased intakes of energy are associated with improved outcomes in patients with BMI < 25 or ≥ 35 (42). Since underweight and malnourished patients were excluded from our study, the number of patients with BMI < 25 was too small for subgroup analysis. However, full-energy nutrition did not result in significantly more ventilator-free or ICU-free days nor lower mortality in the subgroup of patients with BMI ≥ 35.
Guidelines recommend initiating enteral nutrition within 24 to 48 hours of intubation (13-15) and a recent meta-analysis suggests improved survival in critically ill patients when enteral nutrition is initiated within 24 hours of injury or admission (12). This study does not address the question of whether early administration of enteral nutrition improves outcomes, nor does it address the question of what is the optimal composition. Although advancement to full-energy rates was later in the trophic group, all patients received some enteral nutrition within 48 hours of ventilation. In fact, enteral nutrition was initiated on average about 24 hours after intubation in all study patients which is considerably earlier than reported in routine clinical practice (4,16,31,33,35).
Conclusions
Providing initial trophic enteral nutrition in mechanically ventilated patients with acute respiratory failure, results in similar clinical outcomes as early advancement to full-energy enteral nutrition with fewer episodes of gastrointestinal intolerance. Overall, these data suggest that a less aggressive feeding strategy during the initial stages of mechanical ventilation is not demonstrably worse than early advancement to full-energy enteral nutrition, although larger studies are needed to better determine the risks and benefits.. Further study is needed to determine both optimal composition and timing of initiation of enteral nutrition in these patients and to clarify whether protein or micronutrient supplementation may confer added benefit.
Supplementary Material
Acknowledgments
DSMB Members – John Newman, MD; Pratik Pandharipande MD, MSCI; Chang Yu, PhD; Timothy Girard, MD, MSCI
Funding Support: K23HL81431 (TWR); P30DK058404 (TWR); 1 UL1 RR024975 (TWR; GRB) Clinicaltrials.gov registration: NCT00252616
References
- 1.Linko R, Okkonen M, Pettila V, et al. Acute Respiratory Failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Med. 2009;35:1352–61. doi: 10.1007/s00134-009-1519-z. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Akca S, de Mendonca A, et al. The epidemiology of Acute Respiratory Failure in critically ill patients. Chest. 2002;121:1602–9. doi: 10.1378/chest.121.5.1602. [DOI] [PubMed] [Google Scholar]
- 3.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: An analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 4.Cahill NE, Dhaliwal R, Day AG, et al. Nutrition therapy in the critical care setting: what is “best achievable” practice? An international multicenter observational study. Crit Care Med. 2010;38:395–401. doi: 10.1097/CCM.0b013e3181c0263d. [DOI] [PubMed] [Google Scholar]
- 5.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185–90. [PubMed] [Google Scholar]
- 6.MacFie J, Reddy BS, Gatt M, Jain PK, Sowdi R, Mitchell CJ. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2006;93:87–93. doi: 10.1002/bjs.5184. [DOI] [PubMed] [Google Scholar]
- 7.Kane TD, Alexander JW, Johannigman JA. The detection of microbial DNA in the blood: A sensitive method for diagnosing bacteremia and/or bacterial translocation in surgical patients. Ann Surg. 1998;227:1–9. doi: 10.1097/00000658-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore E, Jones T. Benefits of Immediate Jejunostomy Feeding After Major Abdominal Trauma- A Prospective, Randomized Study. J Trauma. 1986;26:874–81. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, controlled trial to determine the effect of early enhanced enteral nutrition on clinical outcome in mechanically ventilated patients suffering head injury. Crit Care Med. 1999;27:2525–31. doi: 10.1097/00003246-199911000-00033. [DOI] [PubMed] [Google Scholar]
- 10.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–83. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–5. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doig GS, Heighes PT, Simpson F, et al. Early enteral nutrition, provided within 24h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomized controlled trials. Intensive Care Med. 2009;35:2018–27. doi: 10.1007/s00134-009-1664-4. [DOI] [PubMed] [Google Scholar]
- 13.Martindale RG, McClave SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757–61. doi: 10.1097/CCM.0b013e3181a40116. [DOI] [PubMed] [Google Scholar]
- 14.McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–315. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 15.Heyland DK, Dhaliwal R, Drover JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–73. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 16.Rice TW, Swope T, Bozeman S, Wheeler AP. Variation in enteral nutrition delivery in mechanically ventilated patients. Nutrition. 2005;21:786–92. doi: 10.1016/j.nut.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 17.McClave SA, Sexton LK, Spain DA, et al. Enteral tube feeding in the intensive care unit: factors impeding adequate delivery. Crit Care Med. 1999;27:1252–6. doi: 10.1097/00003246-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kollef MH. Ventilator-associated pneumonia. A multivariate analysis. JAMA. 1993;270:1965–70. [PubMed] [Google Scholar]
- 19.Rubinson L, Diette GB, Song X, et al. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32:350–7. doi: 10.1097/01.CCM.0000089641.06306.68. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan JA, Parce PB, Martinez A, et al. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest. 2003;124:297–305. doi: 10.1378/chest.124.1.297. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim EH, Mehringer L, Prentice D, Sherman G, Schaiff R, Fraser V, et al. Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. JPEN J Parenter Enteral Nutr. 2002;26:174–81. doi: 10.1177/0148607102026003174. [DOI] [PubMed] [Google Scholar]
- 22.Levine GM, Deren JJ, Steiger E, Zinno R. Role of oral intake in maintenance of gut mass and disaccharide activity. Gastroenterology. 1974;67:975–82. [PubMed] [Google Scholar]
- 23.Zaloga GP, Black KW, Prielipp R. Effect of rate of enteral nutrient supply on gut mass. JPEN J Parenter Enteral Nutr. 1992;16:39–42. doi: 10.1177/014860719201600139. [DOI] [PubMed] [Google Scholar]
- 24.Ammori BJ, Leeder PC, King RF, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–62. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- 25.Hanna MK, Kudsk KA. Nutritional and pharmacological enhancement of gut-associated lymphoid tissue. Can J Gastroenterol. 2000;14(Suppl D):145D–51D. doi: 10.1155/2000/308787. [DOI] [PubMed] [Google Scholar]
- 26.Kang W, Gomez FE, Lan J, et al. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg. 2006;244:392–9. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg LM, Pusateri JP, Jr, Levine GM. Comparison of different caloric substrates on intestinal adaptation in the rat. Gastroenterology. 1989;96:1514–20. doi: 10.1016/0016-5085(89)90520-9. [DOI] [PubMed] [Google Scholar]
- 28.Owens L, Burrin DG, Berseth CL. Minimal enteral feeding induces maturation of intestinal motor function but not mucosal growth in neonatal dogs. J Nutr. 2002;132:2717–22. doi: 10.1093/jn/132.9.2717. [DOI] [PubMed] [Google Scholar]
- 29.Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH, Jr, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–10. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 30.Haddad SH, Arabi Y, Sakkijha M, et al. Relation between caloric intake and outcome of the critically ill patients. Am J Resp Crit Care Med. 2003;31(Suppl):A83. [Google Scholar]
- 31.Barr J, Hecht M, Flavin KE, et al. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125:1446–57. doi: 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 32.Martin CM, Doig GS, Heyland DK, et al. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT) Can Med Assoc J. 2004;170:197–204. [PMC free article] [PubMed] [Google Scholar]
- 33.Doig GS, Simpson F, Finfer S, et al. Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–41. doi: 10.1001/jama.2008.826. [DOI] [PubMed] [Google Scholar]
- 34.McClave SA, DeMeo MT, DeLegge MH, et al. North American summit on aspiration in the critically ill patient: consensus statement. JPEN J Parenter Enteral Nutr. 2002;26(6 Suppl):S80–5. doi: 10.1177/014860710202600613. [DOI] [PubMed] [Google Scholar]
- 35.Heyland DK, Dhaliwal R, Day A, et al. Validation of the Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients: results of a prospective observational study. Crit Care Med. 2004;32:2260–6. doi: 10.1097/01.ccm.0000145581.54571.32. [DOI] [PubMed] [Google Scholar]
- 36.McClave SA, Lukan JK, Stefater JA, et al. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med. 2005;33:324–30. doi: 10.1097/01.ccm.0000153413.46627.3a. [DOI] [PubMed] [Google Scholar]
- 37.Fanny P, Dimet J, Martin-Lefevre L, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr. 2010;34:125–30. doi: 10.1177/0148607109344745. [DOI] [PubMed] [Google Scholar]
- 38.Davies AR. Gastric residual volume in the ICU: Can we do without measuring it? JPEN Parenter Enteral Nutr. 2010;34:160–2. doi: 10.1177/0148607109357626. [DOI] [PubMed] [Google Scholar]
- 39.Montejo JC, Minambres E, Bordeje L, et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med. 2010;36:1386–93. doi: 10.1007/s00134-010-1856-y. [DOI] [PubMed] [Google Scholar]
- 40.Barrett JS, Shepherd SJ, Gibson PR. Strategies to manage gastrointestinal symptoms complicating enteral feeding. JPEN J Parenter Enteral Nutr. 2009;33:21–6. doi: 10.1177/0148607108325073. [DOI] [PubMed] [Google Scholar]
- 41.Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999;27:1447–53. doi: 10.1097/00003246-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–37. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.