Abstract
OBJECTIVE
To determine risk factors for development of recurrent acute lung injury (ALI).
DESIGN
A population-based case-control study.
SETTING
The study was conducted in Olmsted County, Minnesota from 1999 to 2008.
PATIENTS
Using a validated electronic screening protocol, investigators identified intensive care patients with acute hypoxemia and bilateral pulmonary infiltrates.
MEASUREMENTS
The presence of ALI was independently confirmed according to American-European Consensus Conference (AECC) criteria. Recurrent ALI cases were subsequently matched (1:1:1) with two controls (single ALI and no ALI) on age, gender, duration of follow-up, and predisposing conditions. Risk factors evaluated included gastroesophageal reflux disease (GERD), alcohol consumption, smoking, chronic opioid use, and transfusions.
MAIN RESULTS
We identified 917 patients with ALI, of which 19 developed a second episode, yielding an incidence of 2.02 (95%CI 1.10–2.93) per 100,000 person years. The median time to development of the second episode was 264 days (IQR 80 – 460) days, with a mortality of 47% during the episode. The history of GERD was highly prevalent in patients who developed recurrent ALI: 15/19 patients (79%), compared to 5/19 (26%) matches with single episode of ALI (p=0.006) and 8/19 (42%) matches without ALI (p=0.016). Other exposures were similar between the cases and the two matched controls.
CONCLUSIONS
Recurrent ALI is not a rare phenomenon in the ICU, and may continue to increase with improvements in survival following ALI. GERD was identified as an important risk factor for recurrent ALI and may suggest an important role of gastric aspiration in the development of this syndrome.
Keywords: epidemiology, incidence, recurrent acute lung injury, acute lung injury, acute respiratory distress syndrome, gastroesophageal reflux disease
INTRODUCTION
Acute lung injury (ALI) is a common and important cause of acute respiratory failure that typically develops after severe injury or illness [1–5]. The incidence of ALI reported on prior studies ranges from 1.5 to 78.9 per 100,000 person-years [6]. Despite recent advances in our understanding of the pathophysiology and treatment of ALI, it is not known why some patients develop this syndrome, and mortality has remained high [6–14]. However, survival has improved to the point that there have been case reports of recurrent ALI [15–18].
A systematic study of this phenomenon has not been reported. The characteristics of such patients may point to specific environmental or genetic factors important in the development of this complication. Savici and Katzenstein identified a history of gastroesophageal reflux disease (GERD) and chronic pain treated with opioids in a series of patients with diffuse alveolar damage [17]. We sought to identify the incidence and risk factors for recurrent ALI in Olmsted County, Minnesota.
MATERIALS AND METHODS
After receiving institutional review board approval, we performed a population-based, retrospective case-control study of Olmsted County, Minnesota residents (≥18 years) from 1999 to 2008. The demographics of Olmsted County residents are typical of a suburban community in the Midwestern United States (US). The population was 124,277 consisting largely of middle-class Caucasians with minorities representing thirteen percent of the population according to 2000 US census reports. Because of its geographic isolation, critical care services are provided exclusively by the two Mayo Clinic hospitals in Rochester, MN. No other critical care services are available in the surrounding communities.
Cases and Case-finding
The healthcare system utilizes an integrated electronic medical record system. Combined with multiple comprehensive, prospectively collected clinical research databases (Rochester Epidemiology Project [REP], Mayo Clinic Life Sciences System [MCLSS], Acute Physiology and Chronic Health Evaluation [APACHE] database), the research environment enables easy and extensive access to detailed clinical information from this patient population [19]. A previously validated screening tool (“ARDS screening tool”) with excellent negative predictive value (0.99) [20] screened all patients who received intensive care services from 1999 to 2008. The screening tool was designed to identify all patients who met the following criteria within a single 24-hour period:
Qualifying arterial blood gas analysis: ratio of partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300.
Qualifying chest radiograph report: free text Boolean query containing words – “edema” OR “bilateral” and “infiltrate”
Trained critical care fellows reviewed digital chest radiographs, hemodynamic monitoring data (PAOP≤18 cm H2O or CVP<15 cm H2O in the absence of pulmonary hypertension), echocardiography results (E/E’<15), brain natriuretic peptide levels (BNP<250 pg/mL in the absence of renal failure), and response to therapy (brisk response to diuretics and positive pressure ventilation favors hydrostatic edema) of all patients identified by the ALI screening tool. The presence or absence of ALI was determined according to AECC definition [21]. Reviewers were blinded to the presence or absence of a prior episode or history of ALI. Patients identified as having ALI were also reviewed by a second investigator (GL). Further, those individuals with more than one episode of ALI were reviewed by a third investigator (TB) and identified as “Cases”. These recurrent cases must have been separated by 30 days, and all patients must have been discharged prior to occurrence of second episode. Any discrepancies were resolved by group discussion until consensus was achieved.
Controls and Matching (Figure 2)
The two control groups were identified from the same cohort. The first control labeled “Single ALI” represents those patients who had experienced only one episode of ALI, and had at least the same duration of survival as the matched case. For example, if a case developed a second episode of ALI 260 days after their initial case, the matched control was still alive and had not developed a second episode of ALI 260 days after their initial hospitalization. The second control labeled “No ALI” was identified at the time of initial hospitalization to have multiple risk factors for the development of ALI, but was discharged without developing ALI. Additionally, the Single ALI control matched with the above example case was still alive 260 days after their initial hospitalization and never developed ALI. Both controls were individually matched to the index case by age, gender, lung injury prediction score (LIPS) [22–25], the duration of follow up, the year in which their initial hospitalization occurred, and their predisposing conditions at the time of their initial hospitalization including pneumonia, sepsis, trauma, or shock.
Exposures (Risk factors) and Clinical data
We systematically examined clinical records for a history of GERD, chronic opioid use, smoking, chronic alcohol use, obstructive or restrictive lung diseases, immunocompromised state, diabetes mellitus, the body mass index (BMI), and exposure to transfusions. These variables were chosen on the basis of literature review. GERD was defined according to the American College of Gastroenterology Guidelines [26, 27] and required a clinical history of GERD at least 3 months prior to the initial admission, and improvement or resolution of symptoms with treatment. The use of opioids, proton pump inhibitors (PPI) and H2-antagonists were also captured from the electronic medical record and must have been present prior to the initial hospitalization for the first episode of ALI to be included in risk factor analysis. Chronic alcohol use was defined by more than 14 drinks per week as documented in the electronic medical record. Smoking history was defined as > 20 pack-year smoking history, regardless of current smoking status. Obstructive and restrictive lung diseases were defined as a pre-hospitalization diagnosis of chronic obstructive lung disease, or interstitial lung disease or other restrictive lung disease, respectively, as documented in the electronic medical record.
Immunosuppression was defined as use of immunosuppressive medications (e.g. greater than 20 mg of prednisone) or a disease state associated with immunosuppression, and was captured from the electronic medical record. Transfusion was defined as multiple transfusions of blood products (greater than 15 units within 24 hours) [3] known to increase risk of ALI prior to diagnosis of ALI, and was captured from the electronic medical record. The determination of these risk factors was performed by the primary investigator.
Statistical Analysis
Data were reported as percentages, medians, and interquartile ranges (IQR). The denominator age- and sex-specific person-years for the population of Olmsted County residents aged 18 years or older were estimated from decennial census data, with interpolation between 1999 and 2008. 95% CIs were calculated for the incidence, assuming that they follow a Poisson distribution. Incidence rates were directly adjusted for age or age and sex to the population structure of white persons in the United States in 2000. Wilcoxon signed rank test was used to examine differences between cases and controls for continuous variables, McNemar’s tests for dichotomous variables. Exact P values from the McNemar’s test were used to compare controls and cases on those risk-factor variables and P<0.05 was considered to be a statistically significant difference. SAS statistical software was used for all analyses (SAS version 9.2; SAS Institute, Inc., Cary, NC).
RESULTS
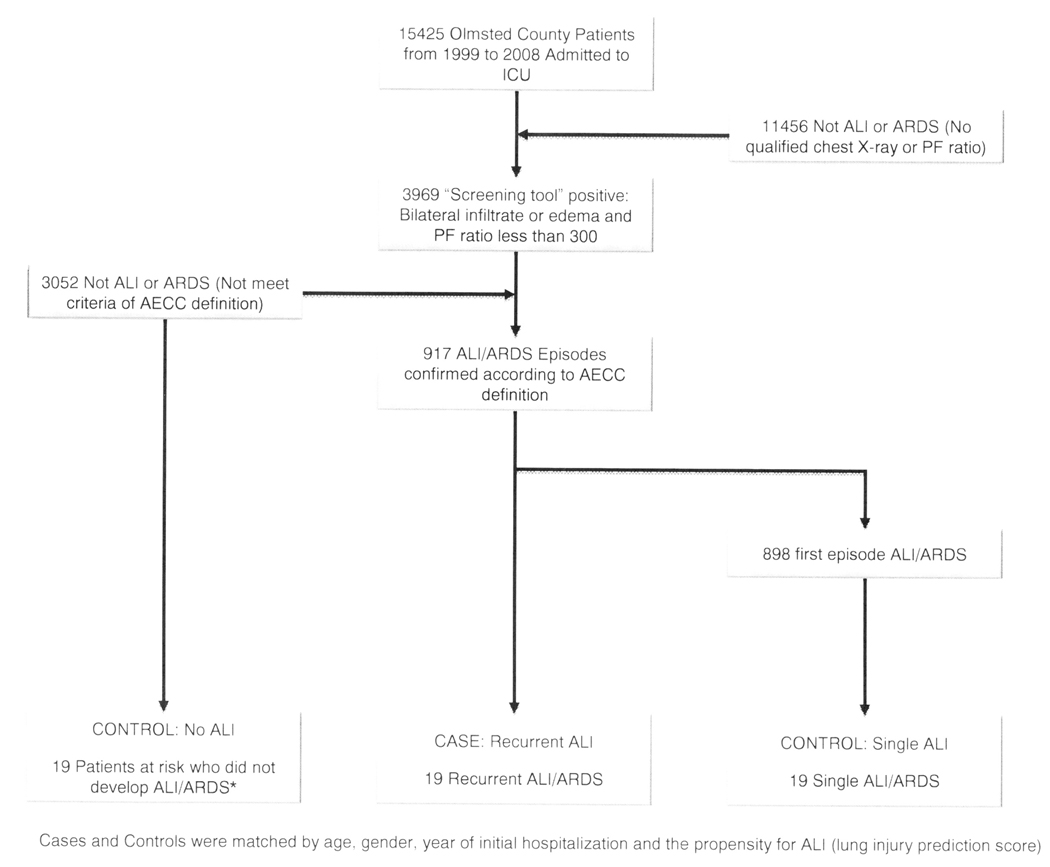
A total of 15,425 Olmsted County ICU admissions were screened from 1999 to 2008. Of these admissions, 917 episodes were identified as ALI among which 19 (2%) were identified as having a second episode (Figure 1). Thus, the incidence of recurrent ALI was 2.02 (95% CI 1.10–2.93) per 100,000 person years. No patients were identified with more than two episodes. Each case of Recurrent ALI was matched with two controls selected from the Single ALI and No ALI groups (Figure 1). The baseline characteristics and the propensity for ALI (LIPS) are presented in Table 1. Other important factors associated with the development of Recurrent ALI are presented in Table 2.
Figure 1.
Outline of the ARDS screening protocol and case ascertainment
*Patients had to have survived the initial episode of critical illness and were followed for the same duration as patients who developed recurrent ALI/ARDS
Table 1.
Baseline demographics and characteristics
| Variable | Recurrent ALI (n = 19) |
Single ALI (n = 19) |
No ALI (n = 19) |
|
|---|---|---|---|---|
| Gender, n Female (%) | 11 (57.9) | 9 (47.4) | 12 (63.2) | |
| Race, n (%) | ||||
| White | 17 (89.5) | 18 (94.7) | 16 (84.2) | |
| Black | 0 (0) | 0 (0) | 2 (10.5) | |
| Asian/Pacific Islander | 2 (10.5) | 1 (5.3) | 1 (5.3) | |
| Age, years, median (IQR) | 69 (54–80) | 62 (47–74) | 67 (55–79) | |
| BMI, kg/m2, median (IQR) | 25.7 (22.4–31.2) | 23.8 (21.0–29.0) | 27.6 (25.4–29.9) | |
| Risk factor for ALI | 1st episode | 2nd episode | ||
| Sepsis, n (%) | 13 (68.4) | 14 (73.6) | 13 (68.4) | 11 (57.9) |
| Pneumonia, n (%) | 9 (47.4) | 13 (68.4) | 10 (52.6) | 7 (36.8) |
| Shock, n (%) | 8 (42.1) | 6 (31.6) | 8 (42.1) | 6 (31.6) |
| Surgery, n (%) | 8 (42.1) | 0 (0) | 3 (15.7) | 8 (42.1) |
| Transfusion, n (%) | 5 (26.3) | 2 (10.5) | 6 (31.6) | 1 (5.3) |
| DIC, n (%) | 2 (10.5) | 1 (5.3) | 0 (0) | 0 (0) |
| Trauma, n (%) | 1 (5.3) | 0 (0) | 2 (10.5) | 0 (0) |
| LIPS, median (IQR) | 3.0 (2.0–4.5) | 3.0 (2.0–4.0) | 3.0 (2.5–4.5) | |
| Obstructive Lung Disease, n (%) | 6 (31.6) | 4 (21.1) | 4 (21.1) | |
| Restrictive Lung Disease, n (%) | 2 (10.5) | 0 (0) | 0 (0) | |
| Immunocompromised, n (%) | 2 (10.5) | 1 (5.3) | 3 (15.7) | |
| Diabetes Mellitus, n (%) | 6 (31.6) | 3 (15.7) | 3 (15.7) | |
Abbreviations: BMI = body mass index; LIPS = lung injury prediction score; DIC = disseminated intravascular coagulopathy
Table 2.
Associations with Recurrent ALI and Single ALI
| Exposure Frequency | Discordant pairs* | Concordant pairs* | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Recurrent ALI n (%) |
Single ALI n (%) |
+/− | −/+ | +/+ | −/− | Odds ratio** | p Value |
| GERD | 15 (79) | 5 (26) | 11 | 1 | 4 | 3 | 11 | 0.006 |
| PPI use | 8 (42) | 3 (16) | 6 | 1 | 2 | 10 | 6 | 0.13 |
| H2 blocker use | 6 (32) | 2 (11) | 5 | 1 | 1 | 12 | 5 | 0.22 |
| Aspiration of gastric contents | 7 (37) | 3 (16) | 5 | 1 | 2 | 11 | 5 | 0.22 |
| Chronic pain | 1 (5) | 0 (0) | 1 | 0 | 0 | 18 | - | 1 |
| Chronic opioid use | 4 (21) | 0 (0) | 4 | 0 | 0 | 15 | - | 0.13 |
| Smoking | 13 (68) | 9 (47) | 8 | 4 | 5 | 2 | 2 | 0.39 |
| Chronic alcohol use | 2 (10) | 2 (10) | 2 | 2 | 0 | 15 | 1 | 1 |
| Obstructive lung disease | 6 (31.6) | 4 (21.1) | 4 | 2 | 2 | 11 | 2 | 0.69 |
| Immunocompromise | 2 (10.5) | 1 (5.3) | 2 | 1 | 0 | 16 | 2 | 0.99 |
| Diabetes mellitus | 6 (31.6) | 3 (15.7) | 5 | 2 | 1 | 11 | 2.5 | 0.45 |
| BMI≥30 | 6 (31.6) | 4 (21) | 5 | 3 | 1 | 10 | 1.7 | 0.73 |
| Transfusion | 5 (26) | 6 (32) | 3 | 4 | 2 | 10 | 0.75 | 1 |
Abbreviations: GERD, ALI, PPI, BMI = body mass index
+/− = matched pair with Recurrent exposed, Single unexposed; −/+ = Recurrent unexposed, Single exposed; +/+ = both Recurrent and Single exposed; −/− = both Recurrent and Single unexposed
Odds ratio of – represents an incalculable odds ratio, requiring division by zero
Median time to second episode was 264 (IQR 80 – 460) days. Nine of the 19 patients died during the second episode (47%). Overall, 17 out of the 19 patients had died by the time of this study (89%) with a median survival after the second episode of 22 (IQR 4 – 371) days.
There was a significantly higher prevalence (79%) of GERD in the Recurrent ALI group than in the Single ALI group (26%, p=0.006), or the No ALI group (42%, p=0.016) (Table 2 and Table 3). Witnessed or suspected aspiration was more commonly documented in patients with recurrent ALI. The chronic use of acid suppression did not modify the risk of recurrent ALI. No significant differences were observed in chronic opioid use, alcohol, smoking, or transfusions,
Table 3.
Associations with Recurrent ALI and No ALI
| Exposure Frequency | Discordant pairs* | Concordant pairs* | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Recurrent ALI n (%) |
No ALI n (%) |
+/− | −/+ | +/+ | −/− | Odds ratio** | p Value |
| GERD | 15 (79) | 8 (42) | 7 | 0 | 8 | 4 | - | 0.016 |
| PPI use | 8 (42) | 8 (42) | 6 | 6 | 2 | 5 | 1 | 0.99 |
| H2 blocker use | 6 (32) | 1 (5) | 5 | 0 | 1 | 13 | - | 0.06 |
| Aspiration of gastric contents | 7 (37) | 1 (5) | 7 | 1 | 0 | 11 | 7 | 0.07 |
| Chronic pain | 1 (5) | 1 (5) | 1 | 1 | 0 | 17 | 1 | 0.99 |
| Chronic opioid use | 4 (21) | 1 (5) | 4 | 1 | 0 | 14 | 4 | 0.38 |
| Smoking | 13 (68) | 9 (47) | 7 | 3 | 6 | 3 | 2.3 | 0.34 |
| Chronic alcohol use | 2 (10) | 1 (5) | 1 | 0 | 1 | 17 | - | 0.99 |
| Obstructive lung disease | 6 (31.6) | 4 (21.1) | 6 | 4 | 0 | 9 | 1.5 | 0.75 |
| Immunocompromise | 2 (10.5) | 3 (15.7) | 1 | 2 | 1 | 15 | 0.5 | 0.99 |
| Diabetes mellitus | 6 (31.6) | 3 (15.7) | 5 | 2 | 1 | 11 | 2.5 | 0.45 |
| BMI≥30 | 6 (31.6) | 4 (21) | 4 | 2 | 2 | 11 | 2 | 0.69 |
| Transfusion | 5 (26) | 1 (5) | 5 | 1 | 0 | 13 | 5 | 0.22 |
+/− = matched pair with Recurrent exposed, No ALI unexposed; −/+ = Recurrent unexposed, No ALI exposed; +/+ = both Recurrent and No ALI exposed; −/− = both Recurrent and No ALI unexposed
Odds ratio of – represents an incalculable odds ratio, requiring division by zero
DISCUSSION
With the improvement of ALI survival in this population-based study we observed a significant number of patients who had more than one episode of ALI. GERD was identified as the most important risk factor for recurrent ALI, potentially suggesting an important role of gastric aspiration in the development of this syndrome.
ALI represents a specific injury pattern resulting from a variety of insults in a susceptible host. Aspiration is an important recognized mechanism for acute lung injury (ALI) found to be a risk factor in 11% with an associated mortality of 44% [6]. Unfortunately, aspiration in both clinical practice and in research are limited by its clinical definition of a “witnessed aspiration” event, raising the concern that many aspiration events that lead to ALI/ARDS are unrecognized. In those with witnessed gastric aspiration, we observed a “dose dependent” pattern from 5% in those who had no ALI, to 16% in those with a single episode of ALI, to finally 37% in those who had recurrent ALI. This is consistent with our understanding of aspiration and its potential to cause lung injury, but it further underscores that it is a risk factor for recurrent episodes of acute lung injury.
With a lack of a proven standard for identifying gastric to pulmonary aspiration, we looked for other signals that may indicate that silent aspiration occurs more frequently as a cause of ALI and ARDS. Mechanistically, for gastric to pulmonary aspiration to occur, there must first be reflux of gastric contents. This may occur acutely as can be seen in the critically ill (e.g. emesis and aspiration in an obtunded patient), but more commonly it is seen in the chronic condition of gastroesophageal reflux disease. GERD could act as the necessary prerequisite risk for aspiration, and furthermore, because it is chronic, it may be a risk for repeated episodes of silent aspiration and acute lung injury. Our data support this plausible mechanism showing GERD as the strongest risk factor for recurrent ALI. With a prevalence of GERD of 20% in our population in Olmsted County and worldwide [28, 29], the at-risk population may be significant. Notably, the risk for ALI as a result of having GERD was higher than for aspiration itself. However, this is not unexpected as aspiration is often insensitively defined clinically, and suggests that potentially, many aspiration events are unrecognized. This highlights the dire need for a more objective determination of aspiration.
Chronic use of acid suppressive medications did not alter the susceptibility of ALI development, but our study design did not allow us to determine the timing of medication use when compared to the initial insult. In the critically ill, acid suppression is typically utilized for the prevention of upper intestinal bleeding [30]. There is increasing concern as to whether the empiric use of potent acid suppression may increase the risk for pneumonia [31, 32] and clostridium difficile infection [33, 34]. However, there is no data on how acid suppression might modify the risk for ALI, whether favorably or unfavorably. Although we expect the acid suppression would confer a protective effect, its concomitant increase in risk for pneumonia and subsequent lung injury may be unfavorable. The limited sample size did not allow us to evaluate these risks in a multivariate fashion to formally assess for confounding and interaction. Additionally, the retrospective nature of the study did not allow us to quantify adherence or actual use of medications.
Only a limited number of reports are available on this unique group of patients with recurrent ALI without an identifiable cause [15–18]. Savici and Katzenstein described 6 cases of pathologically confirmed diffuse alveolar damage without a clear causative agent, as identified in these other reports [17]. Interestingly, 5 of their 6 patients were noted to have a history of GERD or a hiatal hernia, and they proposed that perhaps this in combination with the use of chronic opiates seen in 4 of their cases predisposed the patients to an aspiration mechanism for lung injury. Our results support the possibility of an aspiration mechanism, though we did not identify an independent risk with the chronic use of opiates. It is possible this risk is now becoming more evident with the improvement in survival from ALI as a result of advances in supportive care [3, 35, 36].
The incidence of recurrent ALI in our population was 2.02 per 100,000 person years. When affected with a second episode of ALI, mortality was high at 47%, with a median survival of 22 days. No previous study has reported the incidence of recurrent ALI. Cely et al reported 10 cases of recurrent ALI among their series of veterans with ALI, with a similar mortality of 50% and a median time to recurrence of 192 days. They also reported a higher frequency of 16% per year of developing a recurrent episode of ALI [15].This difference may be explained by the differences in study populations. Our study subjects were from a regional population in the Midwest while Cely’s study population was from a Veterans Affairs Medical Center. Their study population tended to be older, and they also included referred patients who tend to have a higher severity of illness than in a population-based study. Interestingly, both of our studies noticed the recurrent episodes usually happened around 200 days after the initial one. This finding needs to be confirmed in a larger cohort in the future.
Our study is susceptible to the typical weakness of a case-control study utilizing existing clinical records, as well as the limitations of a small sample size. We have avoided some of the sampling bias inherent to case-control studies by using incident cases and drawing our sample from the complete health-linkage records system of our population in Olmsted County. There are still some concerns towards measurement bias since the exposures, including GERD, was not systematically derived and, although unlikely, clinicians may have heightened their suspicion for GERD and aspiration as a result of a diagnosis of ALI or recurrent ALI. Because of the small sample size, our study was potentially underpowered to detect other clinically important risk factors. For example, both aspiration of gastric contents and H2 blocker use had much higher rates in the recurrent ALI group, but this was not statistically significant in either case. Additional limitations inherent to even systematic research are the subjective and limited definition of exposures such as “aspiration.” All patients with recurrent ALI did undergo echocardiogram during hospitalization, but it is still difficult to exclude the possibility that cardiac dysfunction played a role in lung injury. The strength of our study lies in the sampling of two control groups drawn from the same base population, with consistency in the findings across the comparisons. As noted, an apparent “dose-response” effect was observed between documented aspiration and ALI, further supporting that this biologically plausible relationship is genuine.
In conclusion, recurrent ALI without a clear identifiable trigger is rare, but is expected to be seen with increasing frequency with improved survival in ALI. Our study is the first systematic study exploring the incidence and risks associated with recurrent ALI. Our data suggests that GERD is the strongest risk factor for recurrent ALI, likely as a more sensitive marker for aspiration. How acid suppression might modify this apparent risk is unknown at this time. Given the prevalence of GERD and our limited ability to treat ALI once it occurs, further investigation in a prospective study is necessary to determine the true risk of GERD in ALI and whether any specific interventions will moderate this risk.
Acknowledgments
Funding: Supported in part by NIH grant LM10468Z-01
Dr. Bice and Dr. Li received funding from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors have not disclosed any potential conflicts of interest.
References
- 1.Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, Petty TL, Hyers TM. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 Pt 1):593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 2.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Critical care medicine. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 3.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 4.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 5.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 6.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 7.The Acute Respiratory Distress Syndrome N: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GDPP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. New England Journal of Medicine. 1998;vol. 338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 9.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. New England Journal of Medicine. 2004;vol. 351 doi: 10.1056/NEJMoa032193. 327-336+411. [DOI] [PubMed] [Google Scholar]
- 10.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. American Review of Respiratory Disease. 1985;vol. 132:485–489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- 12.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. The New England journal of medicine. 2002;346(17):1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 13.Rubenfeld G. Looking beyond 28-day all-cause mortality. Critical care (London, England) 2002;6(4):293–294. doi: 10.1186/cc1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomsen GE, Morris AH. Incidence of the adult respiratory distress syndrome in the State of Utah. American journal of respiratory and critical care medicine. 1995;vol. 152:965–971. doi: 10.1164/ajrccm.152.3.7663811. [DOI] [PubMed] [Google Scholar]
- 15.Cely CM, Rojas JT, Maldonado DA, Schein RM, Quartin AA. Use of intensive care, mechanical ventilation, both, or neither by patients with acute lung injury. Critical care medicine. 2010;38(4):1126–1134. doi: 10.1097/CCM.0b013e3181d56fae. [DOI] [PubMed] [Google Scholar]
- 16.Gonzolez ER, Cole T, Grimes MM, Fink RA, Fowler AA., 3rd Recurrent ARDS in a 39-year-old woman with migraine headaches. Chest. 1998;114(3):919–922. doi: 10.1378/chest.114.3.919. [DOI] [PubMed] [Google Scholar]
- 17.Savici D, Katzenstein A-LA. Diffuse alveolar damage and recurrent respiratory failure: Report of 6 cases. Human Pathology. 2001;32(12):1398–1402. doi: 10.1053/hupa.2001.29670. [DOI] [PubMed] [Google Scholar]
- 18.Suarez M, Krieger BP. Bronchoalveolar lavage in recurrent aspirin-induced adult respiratory distress syndrome. Chest. 1986;90(3):452–453. doi: 10.1378/chest.90.3.452. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 20.Herasevich V, Afessa B, Chute CG, Gajic O. Designing and testing computer based screening engine for severe sepsis/septic shock; AMIA Annual Symposium proceedings / AMIA Symposium; 2008. p. 966. [PubMed] [Google Scholar]
- 21.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Kojicic M, Herasevich V, Gajic O. Early identification of patients with or at risk of acute lung injury. Neth J Med. 2009;67(9):268–271. [PubMed] [Google Scholar]
- 23.Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, Kojicic M, Kashyap R, Thakur S, Thakur L, Herasevich V, Malinchoc M, Gajic O. Acute lung injury prediction score: derivation and validation in a population based sample. Eur Respir J. doi: 10.1183/09031936.00036810. [DOI] [PubMed] [Google Scholar]
- 24.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson Iii H, Hoth JJ, Mikkelsen ME, Gentile NT, et al. Early Identification of Patients at Risk of Acute Lung Injury: Evaluation of Lung Injury Prediction Score in a Multicenter Cohort Study. American journal of respiratory and critical care medicine. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakur SJ, Trillo-Alvarez CA, Malinchoc MM, Kashyap R, Thakur L, Ahmed A, Reriani MK, Cartin-Ceba R, Sloan JA, Gajic O. Towards the prevention of acute lung injury: a population based cohort study protocol. BMC emergency medicine. 10:8. doi: 10.1186/1471-227X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The American journal of gastroenterology. 2005;100(1):190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 27.Hirano I, Richter JE. ACG practice guidelines: esophageal reflux testing. The American journal of gastroenterology. 2007;102(3):668–685. doi: 10.1111/j.1572-0241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 28.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 29.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastrooesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Winton TL, Rutledge F, Todd TJ, Roy P, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. The New England journal of medicine. 1994;330(6):377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 31.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. Jama. 2009;301(20):2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 32.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. Jama. 2004;292(16):1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 33.Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 34.Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772–778. doi: 10.1001/archinternmed.2010.73. [DOI] [PubMed] [Google Scholar]
- 35.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 36.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. American journal of respiratory and critical care medicine. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]