Abstract
Imiquimod is an immune response modifier prescribed as a topical medication for a number of viral and neoplastic conditions. We evaluated the antiviral activity of imiquimod against vaccinia virus (WR strain) cutaneous infections in immunosuppressed (with cyclophosphamide) hairless mice when administered after virus exposure. Primary lesions progressed in severity, satellite lesions developed, and infection eventually killed the mice. Once daily topical treatment with 1% imiquimod cream for three, four, or five days were compared to twice daily topical treatment with 1% cidofovir cream for seven days. Survival time of mice in all treated groups was significantly prolonged compared to placebo controls. The mean day of death for the placebo group, three-day imiquimod, four day imiquimod, five-day imiquimod, and cidofovir groups were 15.5, 20.0, 20.5, 19.5, and 20.5 days post-infection, respectively. All treatment groups showed significant reductions in primary lesion size and in the number of satellite lesions. The cidofovir and 4-day imiquimod treatments delayed the appearance of lung virus titers by 3 and 6 days, respectively, although cutaneous lesion and snout virus titers were not as affected by treatment. Benefits in survival and lesion reduction were observed when imiquimod treatment was delayed from 24, 48 and 72 hours post-infection. However, increasing the treatment dose of imiquimod from 1% to 5% led to a significant decrease in antiviral efficacy. These results demonstrate the protective effects of topically administered imiquimod against a disseminated vaccinia virus infection in this mouse model.
Keywords: Poxvirus, Imiquimod, Immunomodulator, Cidofovir, Antiviral, Cyclophosphamide
1. Introduction
The threat of smallpox has reemerged, due to the potential use of variola virus as a biological weapon for bioterrorism (Breman and Henderson, 1998; Peters, 2002; Bossi et al., 2006; Kman and Nelson, 2008). Therefore, smallpox virus vaccination using live vaccinia virus has increased in response to this perceived threat (Fulginiti et al., 2003). Occasionally, complications may arise following smallpox vaccination. These may include dissemination of the infection to other sites on the body (such as other areas of the skin or the eyes), progressive vaccinia, eczema vaccinatum, and/or generalized vaccinia virus infection (Bray, 2003; Bray and Wright, 2003). Bacterial superinfection of the vaccination site is the most common nonviral complication. Many of the more serious viral complications are the result of the individual being immunosuppressed, owing to genetic immunodeficiency, age, or chemotherapy (Bray, 2003; Bray and Wright, 2003). In addition, the current population has a larger number of immunosuppressed individuals, owing to HIV infection and AIDS, and increases in organ transplantations and chemotherapy.
Current treatment regimens for vaccinia virus infections include vaccinia immune globulin (VIG) for people with serious reactions to the smallpox vaccine (Kesson et al., 1997; Cono et al., 2003), and cidofovir (Bray et al., 2000; Smee et al., 2001; De Clercq, 2002; Kern et al., 2002; Quenelle, et al., 2003) for secondary treatment of vaccinia-related complications that do not respond to VIG treatment. Although still experimental, ST-246 is a newer antiviral with promising activity against orthopoxviruses (Yang, et al., 2005; Vora et al., 2008; Kern et al., 2009).
Imiquimod, an imidazoquinoline amine, is an immune response modifier prescribed as a topical medication for a number of viral and neoplastic conditions, including basal cell and superficial squamous cell carcinomas, actinic keratosis, and genital warts (condylomata acuminata) (Chang et al., 2005). The major biological effects of imiquimod are mediated through toll-like receptors, TLR-7 and TLR-8, with the subsequent induction of pro-inflammatory activity, including pro-apoptotic activity toward tumor cells and interference with adenosine receptor signaling pathways (Schön and Schön, 2007). Imiquimod induces innate immune factors such as interferon α, tumor necrosis factor α, and interleukin-12. Adaptive immune factors that are induced include interleukin-6, interleukin-8, granulocyte-colony stimulating factor, and granulocyte macrophage-colony stimulating factor, along with certain pro-inflammatory chemokines (Schön and Schön, 2007). The induction of these factors favor the mounting of a profound Th1 type cellular immune response.
A search of the literature did not identify any reports demonstrating the use of imiquimod for treatment of vaccinia virus infections in experimental animals, even though it has been used successfully in humans against another member of the Poxviridae family, Molluscum contagiosum (Barba et al., 2001; Chang et al., 2005; Schön and Schön 2007). Primary herpes simplex virus infections in guinea pigs have been effectively treated with topically applied imiquimod, with efficacy similar to that of acyclovir (Miller et al., 1999).
While the present studies were being completed, a report indicating the use of imiquimod for treatment of progressive vaccinia in a military smallpox vaccinee was released (Centers for Disease Control and Prevention, 2009). On January 13, 2009, a 20 year-old military service member received a smallpox vaccination. Twelve days later, the patient was admitted to a local hospital with fever and headache. Following transfer to a U.S. Navy tertiary-care facility, he was diagnosed with acute myelogenous leukemia. The patient later underwent chemotherapy with cytarabine, idarubicin, and dexamethasone, and after the vaccination site failed to heal, the patient received direct application of imiquimod to the lesion. Following confirmation of progressive vaccinia, the patient also received VIG intravenously and both oral and topical ST-246. The patient survived, however further complications necessitated amputation of both feet (Centers for Disease Control and Prevention, 2009). Conclusions regarding the efficacy of the combination treatment could not be drawn from the results of this single case.
Several mouse models have been developed to study antiviral treatments of severe progressive vaccinia virus infections. Severe combined immunodeficient (SCID) mice lacking both B- and T-cell immunity are highly susceptible to infection, and die in spite of antiviral treatment with cidofovir (Neyts et al., 1993; Bray et al., 2000), although the time to death in treated animals is considerably prolonged. We developed a progressive cutaneous vaccinia virus infection model using immunosuppressed hairless (normally-immunocompetent) mice (Smee et al., 2004). Severe immunosuppression was accomplished by treating mice every four days with cyclophosphamide (100 mg/kg/day) starting one day prior to virus exposure. Treatment with cidofovir, either topically or parenterally, can reduce primary and satellite lesions and delay the time to death. Because of the aggressive nature of the vaccinia virus infection and the severity of the cyclophosphamide-induced immunosuppression that affects both B- and T-cell immunity (Worthington et al., 1972), antiviral treatment cannot cure the animals and they die from infection. Only small, non-progressive lesions develop in these animals if they are not immunosuppressed (Quenelle et al., 2004; Smee et al., 2004), and no animals will die from infection. Athymic nude mice (lacking T-cell immunity) that are infected cutaneously with vaccinia virus also develop lesions and die from infection (Neyts et al., 2004; Quenelle et al., 2004). In that model the lesions are not quite as severe as with cyclophosphamide immunosuppression. The present experiments determined the protective activity of topically applied imiquimod compared to cidofovir and placebo in the more severe cyclophosphamide-induced immunosuppression model of cutaneous vaccinia virus infection (Smee et al., 2004). Effects of treatment on delaying mortality, suppressing cutaneous lesions, and reducing virus titers in various tissues (skin, snout, and lung) were determined.
2. Materials and Methods
2.1. Mice
Female, specific pathogen–free, immune-competent, 7–8 weeks old (about 24 g) SKH-1 hairless mice were obtained from Charles River Labs (Wilmington, MA). The animals were quarantined 48 h before use and were maintained on Teklad Rodent Diet (Harlan Teklad, Indianapolis, IN) and tap water at the Laboratory Animal Research Center of Utah State University (Logan).
2.2. Virus
Vaccinia virus (WR strain) was purchased from the American Type Culture Collection (Manassas, VA). The virus was propagated in African green monkey kidney (MA-104; Cambrex Bio Science, Charles City, IA) cells for use in these studies.
2.3. Compounds
Aldara® (5% imiquimod) cream and Dermovan (Owen Laboratories, San Antonio, TX) were purchased from a local pharmacy. Aldara was combined with Dermovan to prepare a 1% cream formulation. Cidofovir (Gilead Sciences, Foster City, CA) was dissolved in water at a concentration of 25 mg/ml and then combined with Dermovan to prepare a 1%-cidofovir cream formulation (Toro et al., 2000). Dermovan combined with water served as the placebo control.
2.4. Experiment design
Mice were anesthetized with ketamine (100 mg/kg) by intraperitoneal (i.p.) injection. They were scratched with a 25-gage needle to penetrate the dermal layer in the hip and shoulder areas on 1 side of the body. The area of each scratched site was roughly 25 mm2 (5 mm × 5 mm) and consisted of 4–5 scratches in one direction. A 25-μl volume of vaccinia virus (containing approximately 2.5 × 105 pfu) was placed on each wound area and remained there while the mice rested under the influence of the anesthesia. Immunosuppression was accomplished by i.p. treatment of the mice with cyclophosphamide (100 mg/kg/day) every 4 days, starting 1 day before virus challenge, until the end of the study (Smee et al., 2004). Uninfected hairless mice have been treated with cyclophosphamide in this manner for up to 45 days and remained healthy (Smee et al., 2004). Creams (∼50–100 μl) were applied with a spatula to each lesion site beginning 24 hours post-infection. Effects of delaying treatment were determined by applying creams at 48, 72, or 96 hours post-infection. Three parameters were used to evaluate disease progression. 1) Mortality was recorded daily through day 27 post-infection. 2) The primary lesion area was measured in square millimeters (length × width). Satellite lesions that merged with the primary lesion were not included in the measurement. 3) The number of satellite lesions per mouse was counted each day. Mice were ear tagged and accounted for individually. The final scores for primary lesion area and the number of satellite lesions were maintained for comparison purposes through day 21 post-infection. Ten mice per group were treated with either imiquimod or cidofovir, and there were twenty placebo-treated mice per group per experiment.
2.5. Virus titration from infected tissues
Determinations of virus titers were made from tissue samples obtained on different days after infection using five mice per treatment group per time point. Tissue homogenization and plaque assay titrations were performed as described previously (Smee et al., 2001).
2.6. Statistical evaluations
Kaplan-Meier survival curves were generated and analyzed. The primary lesion area, number of satellite lesions, and virus titers were analyzed by two-way ANOVA followed by Bonferroni post-tests. All analyses were performed using Prism 5.0b (GraphPad Software Inc., La Jolla, CA). Most pairwise analyses compared drug-treated to placebo groups, with certain comparisons made between the drug-treated groups themselves.
3. Results
3.1. Topical treatment with 1% imiquimod for 3, 4, or 5 days
Initially, Aldara cream (5% imiquimod) was combined with Dermovan to prepare a 1% imiquimod cream formulation for testing against a vaccinia virus infection in eight-week old, hairless mice. A 1% imiquimod preparation was used to minimize local skin reactions, as was the regimen of skipping days between treatments. Treatment with 1% imiquimod was well tolerated in uninfected animals. None of the mice lost weight, and no adverse events such as skin rash were detected (data not shown).
The first experiment was conducted to determine the efficacy of 1% imiquimod cream against vaccinia virus cutaneous infections in immunosuppressed mice when treatment began 1 day after infection. Topical treatment with a 1% cream formulation of cidofovir has been shown to be effective in reducing the severity of primary lesions and the number of satellite lesions following infection with vaccinia virus (Smee et al., 2004), thus topical imiquimod treatment was compared to topical cidofovir treatment. Once daily topical treatment with 1% imiquimod for three days (D1, 3, and 5), four days (D1, 4, 7, and 10), or five days (D1, 3, 5, 7, and 9) were compared to twice daily topical treatment with 1% cidofovir cream or placebo for seven days (D1-7). All mice were immunosuppressed by treatment with cyclophosphamide (100 mg/kg/day) every four days starting one day before vaccinia virus exposure to wounded skin in order to induce a disseminating infection (Smee et al., 2004).
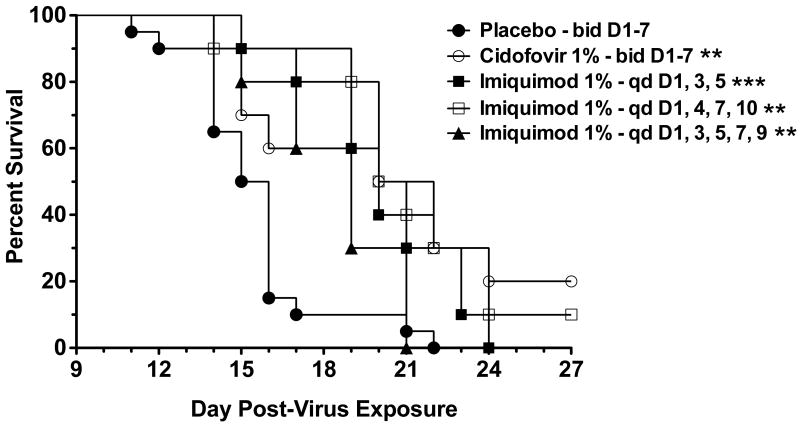
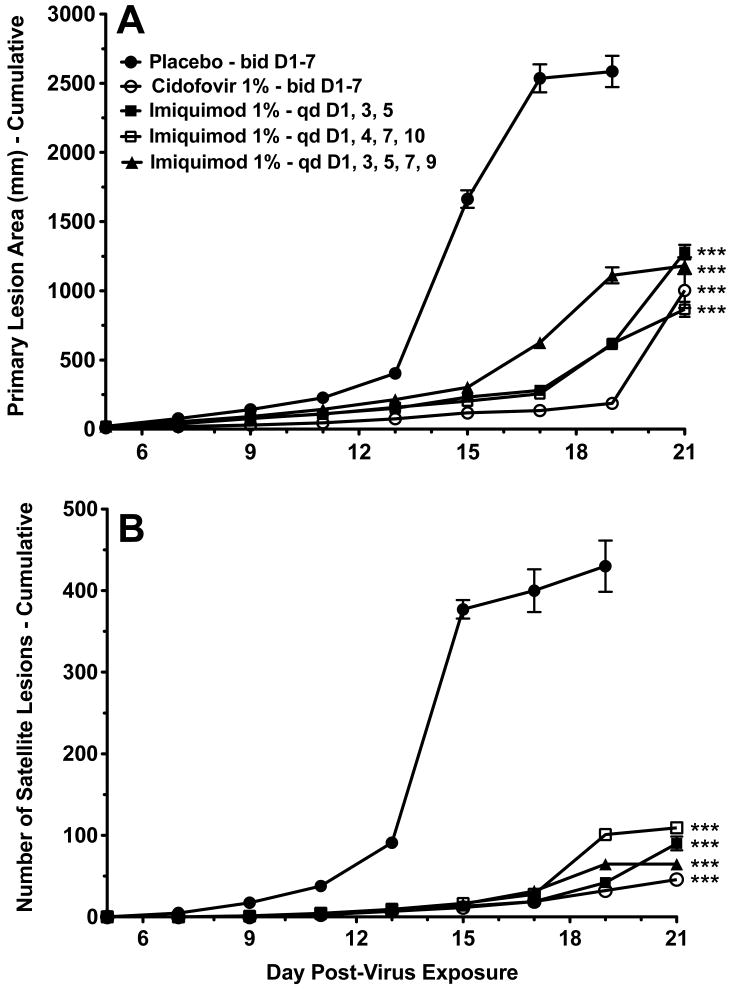
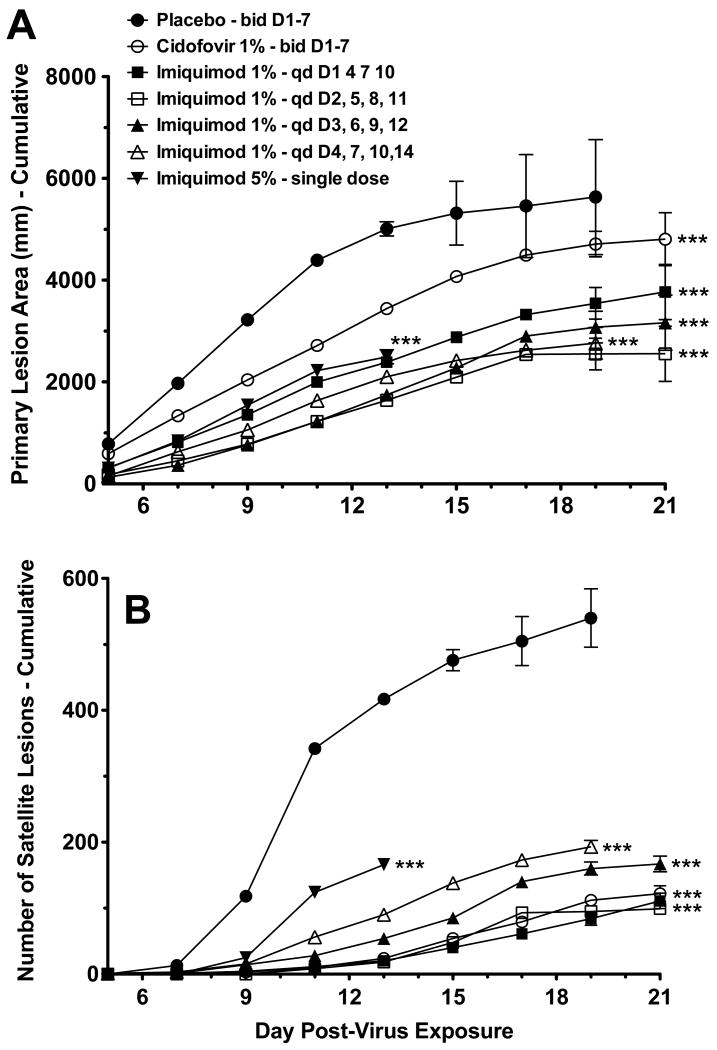
Primary lesions progressed in severity, satellite lesions developed, and infection eventually killed the mice. Kaplan-Meier survival curves for all treated groups were significantly different from placebo controls (Fig. 1). The mean day of death for the placebo group, three-day imiquimod, four-day imiquimod, five-day imiquimod, and cidofovir groups were 15.5, 20.0, 20.5, 19.5, and 20.5 days post-infection, respectively. The cidofovir and imiquimod treatment groups showed significant reductions in the primary lesion size (Fig. 2A), and significant reductions in the number of satellite lesions (Fig. 2B) compared to placebo controls. In this experiment cidofovir treatment was more effective in reducing primary lesion size than the three imiquimod treatments (P<0.001 for days 11-19), and was also more effective in reducing the number of satellite lesions (P<0.001 for days 19 and 21).
Fig. 1.
Survival of immunosuppressed hairless mice following 3-, 4-, or 5-day topical imiquimod treatments beginning 1 day after infection with vaccinia virus. ** P<0.01, *** P<0.001, compared to placebo.
Fig. 2.
Effects of 3-, 4-, or 5-day topical imiquimod treatments beginning 1 day after infection of immunosuppressed hairless mice with vaccinia virus. (A) Primary lesion area (cumulative). (B) Number of satellite lesions (cumulative). *** P<0.001 for days 9-19, compared to placebo. Lines of data that do not extend to day 21 are due to complete mortality in the group of mice.
3.2. Topical treatment with 1% or 5% imiquimod for 4 days
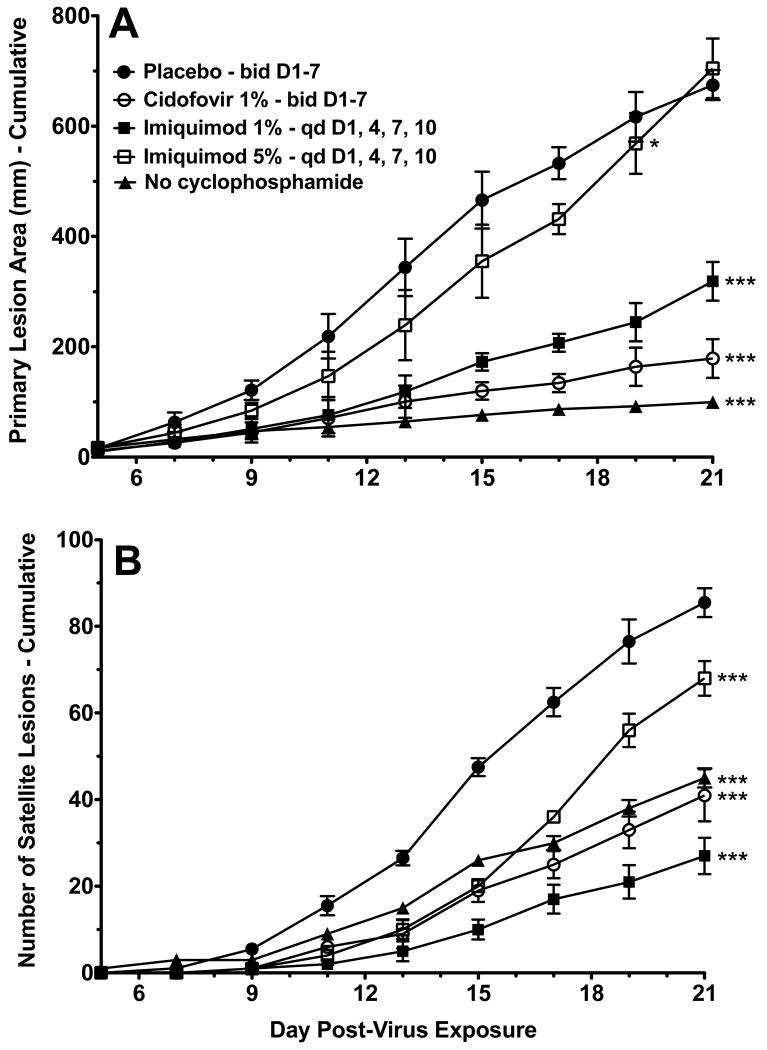
The antiviral activity of once daily topical treatment with 1% imiquimod cream for four days (D1, 4, 7, and 10) was compared to 5% imiquimod cream (Aldara) under the same dosing regimen. In order to confirm the necessity of using cyclophosphamide-treated mice in this cutaneous infection model, one group of mice that had not been treated with cyclophosphamide were infected with vaccinia virus and observed for signs of infection. Mice that were not immunosuppressed by cyclophosphamide treatment developed smaller primary lesions and fewer satellite lesions following infection than immunosuppressed placebo controls (Fig. 3). In addition, lesions on mice that were not immunosuppressed began to heal within the observation period of the study. No mortality was observed in the group of mice that were not immunosuppressed (data not shown). The cidofovir and imiquimod treatment groups showed significant reduction in primary lesion size (Fig. 3A) and in the number of satellite lesions (Fig. 3B). Surprisingly, increasing the dose of imiquimod from 1% to 5% resulted in significantly less protection. Cidofovir treatment was significantly more effective in reducing lesion size compared to 1% imiquimod (P<0.01 for days 15-21) or 5% imiquimod (P<0.001 for days 11-21). Cidofovir treatment was also significantly more effective in reducing the number of satellite lesions compared to 5% imiquimod (P<0.01 for days 17-21). In this test significantly fewer satellite lesions were seen on mice treated with 1% imiquimod compared to cidofovir (P<0.05 for days 15, 19, and 21).
Fig. 3.
Effects of 1% or 5% imiquimod treatments starting 1 day after infection of immunosuppressed hairless mice with vaccinia virus. (A) Primary lesion area (cumulative). (B) Number of satellite lesions (cumulative). * P<0.05 for days 9-19, compared to placebo. *** P<0.001 for days 9-21 (Fig. 3A) or 11-21 (Fig. 3B), compared to placebo. Statistically significant differences (P<0.01) were also found between 1% and 5% imiquimod groups for days 11-21 (Fig. 3A) and 15-21 (Fig. 3B).
3.3. Single dose imiquimod pre-treatment or 2 to 4 day delayed treatment
Additional studies were conducted in immunosuppressed mice to determine the effect of a single dose of 5% imiquimod administered 24 hours prior to viral infection, and the effect of delaying initiation of treatment with 1% imiquimod for 24 to 96 hours. Once daily topical treatment with 1% imiquimod cream for four days, beginning 24 hours (D1, 4, 7, and 10), 48 hours (D2, 5, 8, and 11), 72 hours (D3, 6, 9, and 12), or 96 hours (D4, 7, 10, and 14) after infection were compared to twice daily topical treatment with 1% cidofovir cream for seven days (D1-7). In addition to the three parameters used to evaluate disease progression previously mentioned, virus titers in tissue samples (skin, snout, and lung) were determined from selected groups.
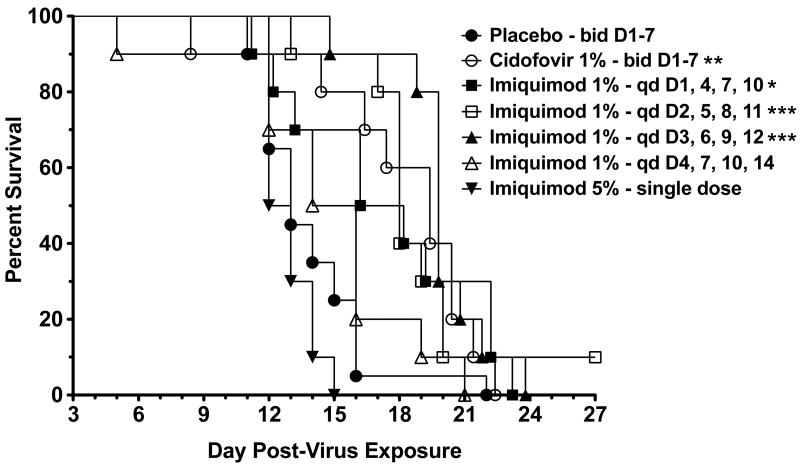
Survival of mice in the imiquimod treated groups, when treatment began 24, 48, and 72 hours post-infection, and the cidofovir treated group were significantly different from placebo controls (Fig. 4). No significant increases in survival were found with the 96-hour treatment group nor with the group treated a single time pre-infection with 5% imiquimod. The mean day of death for mice treated with placebo, 5% imiquimod, and cidofovir were 13.9, 12.9, and 17.6 days, respectively. The mean day of death for the 1% imiquimod treatments beginning 24, 48, 72, or 96 hours post-infection were 17.2, 18.9, 19.1, and 14.5 days post-infection, respectively. The groups receiving imiquimod treatment beginning 24, 48, 72, or 96 hours post-infection or once with 5% imiquimod showed significant reductions in the primary lesion area (Fig. 5A) and in numbers of satellite lesions (Fig. 5B) for as long as the mice were alive. The inhibitory effects of imiquimod on lesions were similar regardless of treatment initiation time. In this experiment cidofovir treatment was significantly less effective than all four imiquimod treatments in reducing lesion areas (P<0.001 for days 7-19). However, cidofovir treatment was more effective in reducing the number of satellite lesions than for imiquimod groups that started treatment on days 3 and 4 (P<0.001 for days 11-19).
Fig. 4.
Survival of immunosuppressed hairless mice following 1% imiquimod treatment beginning 1, 2, 3, or 4 days following infection with vaccinia virus, or with a single 5% dose given 24 hours prior to infection. * P<0.05, **P<0.01, ***P<0.001, compared to placebo.
Fig. 5.
Effects of 1% imiquimod treatment beginning 1, 2, 3, or 4 days following infection, or a 5% dose given 24 hours prior to infection of immunosuppressed hairless mice with vaccinia virus. (A) Primary lesion area (cumulative). (B) Number of satellite lesions (cumulative). * P<0.05, ** P<0.01, *** P<0.001 for days 7-19 (Fig. 5A) or 9-19 (Fig. 5B) or through 13 days (5% imiquimod group), compared to placebo. Lines of data that do not extend to day 21 are due to complete mortality in the group of mice.
3.4. Virus titers in tissues
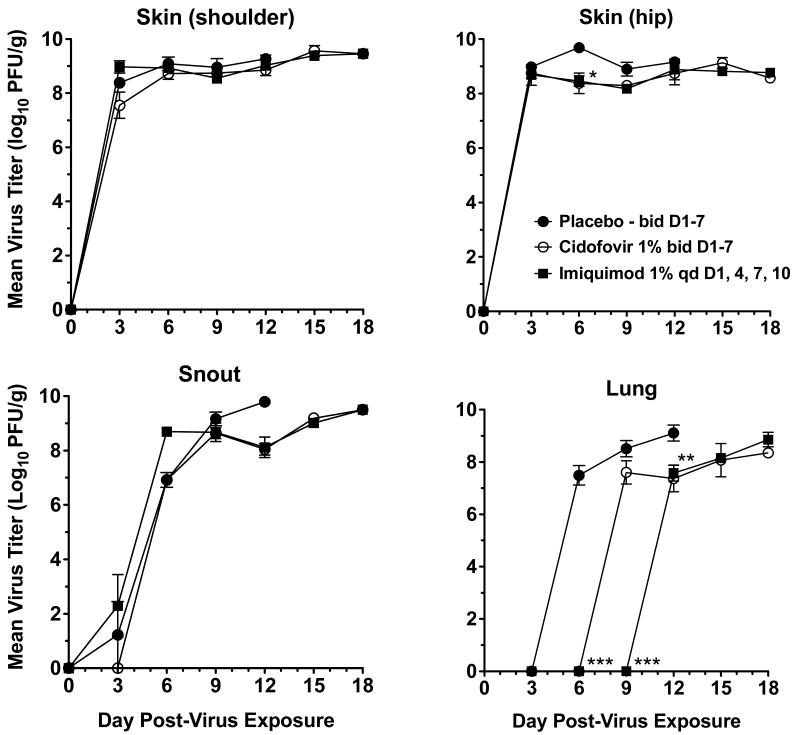
Tissue samples were collected from imiquimod treated, cidofovir treated, and placebo groups from day 3 to 18 post-infection for determination of skin, snout, and lung virus titers (Fig. 6). Although virus infection was initiated in the skin, the virus soon spread to the snout and lungs. Transmission from skin to snout may have been through orofacial contact with the lesions. Lung infection may have been due to transmission from snout to lungs via inhalation. Alternatively or concurrently, the virus may have been transmitted systemically via the blood stream. Virus titers climbed rapidly to high levels in each of these tissues, exceeding 108 pfu/g. Treatments with imiquimod and cidofovir did not cause significant reductions in cutaneous and snout virus titers compared to placebo, except at one time point (day 6, hip lesions). Virus titers in the lungs were first detected (and at high levels) on day 6 post-infection in the placebo group, then on day 9 in the cidofovir treated group, and finally on day 12 in the imiquimod treated group. Thus, there was a 3-day delay in viral spread to the lungs of cidofovir-treated mice (P<0.001) and a 6-day delay to the lungs of imiquimod-treated animals (P<0.001). In addition, the difference in lung virus titers on day 9 between imiquimod and cidofovir groups was statistically significant (P<0.001).
Fig. 6.
Effects of imiquimod treatment of immunosuppressed hairless mice on virus titers from skin (shoulder or hip), snout, or lung samples after infection with vaccinia virus. * P<0.05, ** P<0.01, *** P<0.001 for day 6, 9, or 12 as indicated, compared to placebo.
4. Discussion
These studies demonstrate that a once daily topical treatment with 1% imiquimod cream under various treatment regimens and 1% cidofovir cream provided significant antiviral efficacy against vaccinia virus cutaneous infections in immunosuppressed hairless mice, and delayed the time to death. Viral titers in skin, snouts, and lungs of all treatment groups reached high levels, but at different times, and indications were that the drug treatments only slowed the infection but could not arrest it. Notably, virus dissemination and replication in the lung could be delayed by topical treatment with cidofovir or imiquimod for 3 and 6 days, respectively. However, suppression of skin virus titers by topical treatments was nominal, and may have been negatively impacted by starting treatment one day after infection. In this immunosuppression model, viral titers take a relentlessly upward course in a number of tissues (Smee et al., 2004), which the present results show are difficult to suppress by drug treatment.
Cidofovir treatment was more effective in reducing cutaneous lesion size than imiquimod in two experiments (Figs. 2A and 3A) but not in a third experiment (Fig. 5A). Reduction in the number of satellite lesions was more pronounced with cidofovir in the same studies (Figs. 2B and 3B) but not in the third experiment except for comparisons made to 3- and 4-day delayed imiquimod treatments (Fig. 5B). However, a significant delay in lung virus titer occurred with imiquimod treatment compared to cidofovir. Because of these mixed results, it is not possible to conclude from the studies which compound is superior to the other in efficacy. Surprisingly, increasing the treatment dose of imiquimod from 1% to 5% led to a decrease in antiviral efficacy. It is possible that the antiviral effect of imiquimod is separate from its pro-inflammatory effects and the higher dose has a negative impact on antiviral activity. Alternatively, the high dose of imiquimod may have been less effective than the lower dose at stimulating the immune system, which is not unusual for immune response modifiers.
The necessity of using cyclophosphamide-treated mice in this vaccinia virus cutaneous infection model (Smee et al., 2004) was also confirmed by these studies. No mortality was observed in non-treated mice following infection, and the infected mice developed smaller primary lesions and fewer satellite lesions compared to cyclophosphamide-treated placebo controls.
Miller et al. (1999) described the use of imiquimod for treatment of a herpes simplex virus type 2 (HSV-2) infection in guinea pigs. Twice daily intravaginal treatment with 1% imiquimod for 5 days caused statistically significant inhibition of lesion development when initiated 48 to 72 hours after inoculation. However, if initiation of imiquimod treatment was delayed until day 4 when visible signs were present, it was no longer effective at reducing lesion severity (Miller et al., 1999). In addition, a single dose of resiquimod (a compound related to imiquimod) applied subcutaneously, dermally, or intravaginally 24 hours before inoculation gave complete protection from HSV-2 lesions (Tomai et al., 1995). Based upon these results, the effect of a single prophylactic dose of 5% imiquimod administered 24 hours prior to infection were determined. The prophylactic dose had less of an effect on primary lesion area and the number of satellite lesions, and did not improve survival of treated mice compared to the 1% dose given multiple times. In addition, topical treatment with 1% imiquimod that was delayed until 4 days after infection began to lose its protective ability. Smee et al. (2004) reported that the best efficacy for topical cidofovir treatment were observed when treatment was initiated 1 day after infection, with efficacy decreasing when treatments started on day 3 or 5 post-infection.
Because cyclophosphamide treatment suppresses both T- and B-cell responses (Worthington et al., 1972), one would anticipate that part of the immune response activated by imiquimod in normal mice would not occur in cyclophosphamide-treated animals. This suggests that imiquimod will provide better protection to humans because they are not expected to be as immunosuppressed. We suspect that only innate immunity is activated by imiquimod in this mouse model, possibly interferon α (Schön and Schön, 2007). Exogenously administered interferon α is known to provide protection to immunocompetent mice infected intranasally with vaccinia virus when administered up to 1 day after infection (Liu et al., 2004). It remains to be determined which immune factors are induced by imiquimod in cyclophosphamide-treated mice.
Only one vehicle, Dermovan, was used for formulating 1% imiquimod and cidofovir. The 5% imiquimod (Aldera) was administered as the pre-formulated drug. We recognize that the vehicle used for topical application of antiviral or immunomodulatory agents can greatly influence activity (Spruance et al., 1984a, 1984b; Sidwell et al., 1990). Factors that affect uptake into the skin include compound size, solubility, and ability to diffuse from the topical vehicle. Dermovan appeared to be a suitable vehicle for both compounds since antiviral activity was evident, although it may not be ideal. Evaluating multiple vehicles with each of these compounds was beyond the scope of this research.
The studies presented here explored only the topical effects of imiquimod and cidofovir, and did not address activity resulting from systemic treatment or exposure. Systemic activation of the immune system by imiquimod could occur from absorption of the drug through the skin or possibly by ingestion as a result of grooming the lesions. We did not observe animals grooming the cream away from lesions but such activity was not prevented by restraint either. Because the snouts of mice became infected (Fig. 6), this suggests that orofacial contact with the lesions was made. Imiquimod is known to be orally active in mice (Sidky et al., 1992) and humans (Savage et al., 1996; Goldstein et al., 1998), where it induces systemic interferon levels. Cidofovir is poorly absorbed by the oral route in mice (Wachsman et al., 1996), thus, grooming would not contribute to systemic uptake. Although, application of topical cidofovir to abraded skin leads to significant systemic exposure in rabbits (Cundy et al., 1997). The drug has also been shown to be absorbed by the topical route in humans (Toro et al., 2000). Systemic absorption through the skin is a likely scenario for both imiquimod and cidofovir, and may account for the antiviral effect observed by the delay in production of lung virus titers (Fig. 6), and the delay in the time to death (Figs. 1 and 4).
The use of topical cidofovir is not without risk to humans. A report in the literature indicated that a bone marrow transplant recipient with genital condylomas who was treated topically with 4% cidofovir for 12 days experienced renal failure, which fortunately was reversible upon cessation of treatment (Bienvenu et al., 2002). In addition, cidofovir is known to cause renal toxicity when administered intravenously (Wachsman et al., 1996). Although, co-administration of probenecid moderates this toxicity (Polis et al., 1995). In light of these concerns, topical imiquimod may provide a relatively safe alternative treatment to topical cidofovir. Importantly, imiquimod is already an approved drug for topical treatment of certain neoplasias and genital warts (Chang et al., 2005). To date, the toxic effects associated with 5% imiquimod treatment are mainly adverse skin reactions, primarily erythema and swelling at the site of application (Miranda-Verástegui et al., 2005; Tangheti and Werschler, 2007; Demirci et al., 2010). In a study where children with Molluscum contagiosum were treated with 5% imiquimod cream, the investigators found no evidence of systemic toxicity (Barba et al., 2001). However, potential toxicity of imiquimod as a result of systemic absorption through vaccinia lesions would need to be closely monitored, particularly if the drug is applied over a broad area of skin. Oral imiquimod treatment of patients leads to flu-like symptoms, nausea, and lymphopenia, which are side effects associated with systemic levels of interferon (Savage et al., 1996; Goldstein et al., 1998).
The use of the topically applied immunomodulatory drug, imiquimod, may not be practical for severe smallpox and monkeypox virus infections because the lesions are distributed over the entire body. However, topical treatment should be considered for localized infections, particularly for complications arising from smallpox vaccination, as was recently employed (Centers for Disease Control and Prevention, 2009). The use of imiquimod either alone or in combination with compounds such as cidofovir or ST-246 to treat vaccinia cutaneous infections warrants further study.
Acknowledgments
These studies were conducted with approval of the Institutional Animal Care and Use Committee and completed in the AAALAC-accredited Laboratory Animal Research Center of Utah State University. Financial support was received from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA (Contract N01-AI-30063) awarded to Southern Research Institute, Birmingham, Alabama.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barba AR, Kapoor S, Berman B. An open label safety study of topical imiquimod 5% cream in the treatment of Molluscum contagiosum in children. Dermatol Online J. 2001;7(1):20. [PubMed] [Google Scholar]
- Bienvenu B, Martinez F, Devergie A, Rybojad M, Rivet J, Bellenger P, Morel P, Gluckman E, Lebbé C. Topical use of cidofovir induced acute renal failure. Transplantation. 2002;73:661–662. doi: 10.1097/00007890-200202270-00033. [DOI] [PubMed] [Google Scholar]
- Bossi P, Garin D, Guihot A, Gay F, Crance JM, Debord T, Autran B, Bricaire F. Bioterrorism: management of major biological agents. Cell Mol Life Sci. 2006;63:2196–2212. doi: 10.1007/s00018-006-6308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101–114. doi: 10.1016/s0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766–774. doi: 10.1086/374244. [DOI] [PubMed] [Google Scholar]
- Bray M, Martinez M, Smee DF, Kefauver D, Thompson E, Huggins JW. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J Infect Dis. 2000;181:10–19. doi: 10.1086/315190. [DOI] [PubMed] [Google Scholar]
- Breman JG, Henderson DS. Poxvirus dilemmas—monkeypox, smallpox and biological terrorism. N Engl J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Progressive vaccinia in a military smallpox vaccinee - United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532–536. [PubMed] [Google Scholar]
- Chang YC, Madkan V, Cook-Norris R, Sra K, Tyring S. Current and potential uses of imiquimod. South Med J. 2005;98:914–920. doi: 10.1097/01.smj.0000176712.01491.98. [DOI] [PubMed] [Google Scholar]
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm Rep. 2003;52(RR-4):1–28. [PubMed] [Google Scholar]
- Cundy KC, Lynch G, Lee WA. Bioavailability and metabolism of cidofovir following topical administration to rabbits. Antiviral Res. 1997;35:113–122. doi: 10.1016/s0166-3542(97)00022-3. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 2002;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci H, Shields CL, Bianciotto CG, Shields JA. Topical imiquimod for periocular lentigo maligna. Ophthalmology. 2010;117:2424–2429. doi: 10.1016/j.ophtha.2010.03.049. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part I: background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241–250. doi: 10.1086/375824. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Hertzog P, Tomkinson E, Couldwell D, McCarville S, Parrish S, Cunningham P, Newell M, Owens M, Cooper DA. Administration of imiquimod, an interferon inducer, in asymptomatic human immunodeficiency virus-infected persons to determine safety and biologic response modification. J Infect Dis. 1998;178:858–861. doi: 10.1086/515343. [DOI] [PubMed] [Google Scholar]
- Kern ER, Hartline C, Harden E, Keith K, Rodriguez N, Beadle JR, Hostetler KY. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob Agents Chemother. 2002;46:991–995. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ER, Prichard MN, Quenelle DC, Keith KA, Tiwari KN, Maddry JA, Secrist JA., III Activities of certain 5-substituted 4′-thiopyrimidine nucleosides against orthopoxvirus infections. Antimicrob Agents Chemother. 2009;53:572–579. doi: 10.1128/AAC.01257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesson AM, Ferguson JK, Rawlinson WD, Cunningham AL. Progressive vaccinia treated with ribavirin and vaccinia immune globulin. Clin Infect Dis. 1997;25:911–914. doi: 10.1086/515534. [DOI] [PubMed] [Google Scholar]
- Kman NE, Nelson RN. Infectious agents of bioterrorism: a review for emergency physicians. Emerg Med Clin North Am. 2008;26:517–547. doi: 10.1016/j.emc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhai Q, Schaffner DJ, Wu A, Yohannes A, Robinson TM, Maland M, Wells J, Voss TG, Bailey C, Alibek K. Prevention of lethal respiratory vaccinia infections in mice with interferon-alpha and interferon-gamma. FEMS Immunol Med Microbiol. 2004;40:201–206. doi: 10.1016/S0928-8244(03)00358-4. [DOI] [PubMed] [Google Scholar]
- Miller RL, Imbertson LM, Reiter MJ, Gerster JF. Treatment of primary herpes simplex virus infection in guinea pigs by imiquimod. Antiviral Res. 1999;44:31–42. doi: 10.1016/s0166-3542(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Miranda-Verástegui C, Llanos-Cuentas A, Arévalo I, Ward BJ, Matlashewski G. Randomized, double-blind clinical trial of topical imiquimod 5% with parenteral meglumine antimoniate in the treatment of cutaneous leishmaniasis in Peru. Clin Infect Dis. 2005;40:1395–1403. doi: 10.1086/429238. [DOI] [PubMed] [Google Scholar]
- Neyts J, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SID) mice. J Med Virol. 1993;41:242–246. doi: 10.1002/jmv.1890410312. [DOI] [PubMed] [Google Scholar]
- Neyts J, Leyssen P, Verbeken E, De Clercq E. Efficacy of cidofovir in a murine model of disseminated progressive vaccinia. Antimicrob Agents Chemother. 2004;48:2267–2273. doi: 10.1128/AAC.48.6.2267-2273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CJ. Many viruses are potential agents of bioterrorism. ASM News. 2002;68:168–173. [Google Scholar]
- Polis MA, Spooner KM, Baird BF, Manischewitz JF, Jaffe HS, Fisher PE, Falloon J, Davey RT, Jr, Kovacs JA, Walker RE, Whitcup SM, Nussenblatt RB, Lane HC, Masur H. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1995;39:882–886. doi: 10.1128/aac.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Collins DJ, Kern ER. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob Agents Chemother. 2003;47:3275–3280. doi: 10.1128/AAC.47.10.3275-3280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle DC, Collins DJ, Kern ER. Cutaneous infections of mice with vaccinia or cowpox viruses and efficacy of cidofovir. Antiviral Res. 2004;63:33–40. doi: 10.1016/j.antiviral.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage P, Horton V, Moore J, Owens M, Witt P, Gore ME. A phase I clinical trial of imiquimod, an oral interferon inducer, administered daily. Br J Cancer. 1996;74:1482–1486. doi: 10.1038/bjc.1996.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157(Suppl.2):8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- Sidky YA, Borden EC, Weeks CE, Reiter MJ, Hatcher JF, Bryan GT. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 1992;52:3528–3533. [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH, Schafer TW, Shipman C. Influence of vehicle on topical efficacy of 2-acetylpyridine thiosemicarbazone and related derivatives on in vivo type 2 herpes simplex virus infections. Chemotherapy. 1990;36:58–69. doi: 10.1159/000238749. [DOI] [PubMed] [Google Scholar]
- Smee DF, Bailey KW, Sidwell RW. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antivir Chem Chemother. 2001;12:71–76. doi: 10.1177/095632020101200105. [DOI] [PubMed] [Google Scholar]
- Smee DF, Bailey KW, Wong MH, Wandersee MK, Sidwell R. Topical cidofovir is more effective than is parenteral therapy for treatment of progressive vaccinia in immunocompromised mice. J Infect Dis. 2004;190:1132–1139. doi: 10.1086/422696. [DOI] [PubMed] [Google Scholar]
- Spruance SL, McKeough MB, Cardinal JR. Penetration of guinea pig skin by acyclovir in different vehicles and correlation with the efficacy of topical therapy of experimental cutaneous herpes simplex virus infection. Antimicrob Agents Chemother. 1984a;25:10–15. doi: 10.1128/aac.25.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruance SL, McKeough M, Sugibayashi K, Robertson F, Gaede P, Clark DS. Effect of azone and propylene glycol on penetration of trifluorothymidine through skin and efficacy of different topical formulations against cutaneous herpes simplex virus infections in guinea pigs. Antimicrob Agents Chemother. 1984b;26:819–823. doi: 10.1128/aac.26.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanghetti E, Werschler P. Comparison of 5% 5-fluorouracil cream and 5% imiquimod cream in the management of actinic keratoses on the face and scalp. J Drugs Dermatol. 2007;6:144–147. [PubMed] [Google Scholar]
- Tomai MA, Gobson SJ, Imbertson LM. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antiviral Res. 1995;28:253–264. doi: 10.1016/0166-3542(95)00054-p. [DOI] [PubMed] [Google Scholar]
- Toro JR, Wood LV, Patel NK, Turner ML. Topical cidofovir: a novel treatment for recalcitrant Molluscum contagiosum in children infected with human immunodeficiency virus 1. Arch Dermatol. 2000;136:983–985. doi: 10.1001/archderm.136.8.983. [DOI] [PubMed] [Google Scholar]
- Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, Gerber SI, Garcia-Houchins S, Lederman E, Hruby D, Collins L, Scott D, Thompson K, Barson JV, Regnery R, Hughes C, Daum RS, Li Y, Zhao H, Smith S, Braden Z, Karem K, Olson V, Davidson W, Trindade G, Bolken T, Jordan R, Tien D, Marcinak J. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555–1561. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- Wachsman M, Petty BG, Cundy KC, Jaffe HS, Fisher PE, Pastelak A, Lietman PS. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antiviral Res. 1996;29:153–161. doi: 10.1016/0166-3542(95)00829-2. [DOI] [PubMed] [Google Scholar]
- Worthington M, Rabson AS, Baron S. Mechanism of recovery from systemic vaccinia virus infection. I. The effects of cyclophosphamide. J Exp Med. 1972;136:277–290. doi: 10.1084/jem.136.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, Burns C, Rhodes G, Tohan S, Huggins JW, Baker RO, Buller RL, Touchette E, Waller K, Schriewer J, Neyts J, De Clercq E, Jones K, Hruby D, Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]