Abstract
Background
Crack cocaine use undermines adherence to highly active antiretroviral therapy (HAART). This pilot randomized clinical trial tested the feasibility and efficacy of 2 interventions based on the Information-Motivation-Behavioral Skills model to improve HAART adherence and reduce crack cocaine problems.
Methods
Participants were 54 adults with crack cocaine use and HIV with <90% HAART adherence. Most participants were African-American (82%) heterosexual (59%), and crack cocaine dependent (92%). Average adherence was 58% in the past 2 weeks. Average viral loads (VL) were detectable (log VL 2.97). The interventions included 6 sessions of Motivational Interviewing plus feedback and skills building (MI+), or Video information plus debriefing (Video+) over 8 weeks. Primary outcomes were adherence by 14-day timeline follow-back and Addiction Severity Index (ASI) Drug Composite Scores at 3 and 6 months. Repeated measures ANOVA assessed main effects of the interventions and interactions by condition.
Results
Significant increases in adherence and reductions in ASI Drug Composite Scores occurred in both conditions by 3 months and were maintained at 6 months, representing medium effect sizes. No between group differences were observed. No VL changes were observed in either group. Treatment credibility, retention, and satisfaction were high and not different by condition.
Conclusions
A counseling and a video intervention both improved adherence and drug problems durably among people with crack cocaine use and poor adherence in this pilot study. The interventions should be tested further among drug users with poor adherence. Video interventions may be feasible and scalable for people with HIV and drug use.
Keywords: HIV, Adherence, Crack Cocaine, Motivational Interviewing plus Feedback, Video Intervention
1. Introduction
Poor adherence to highly active antiretroviral therapy (HAART) and frequent crack cocaine use may result in a faster progression of disease, morbidity, and mortality among HIV positive individuals (Baum et al., 2008, Malta et al., 2008a; Malta et al., 2008b). Adherence to HAART medications must be nearly perfect to prolong health and to avoid antiviral medication resistance and treatment failure, although there is emerging evidence that some newer formulations of HAART medications may allow for slightly more nonadherence (Menendez-Arias 2010; Nieuwkerk and Oort, 2005; Paredes and Clotet, 2010; Bangsberg et al., 2006; Tozzi et al., 2006). Crack cocaine use has a particularly negative effect on HAART adherence (Arnsten et al., 2002; Ingersoll, 2004; Lucas et al., 2002; Lucas et al., 2007; Sharpe et al., 2004). Crack cocaine is an independent predictor of running out of HIV medications (Ingersoll, 2004). The binge pattern typical of crack cocaine use is especially problematic and can lead to the suspension of medication adherence during obtainment, use, and recovery from the drug (Harzke et al., 2009). In addition to its effects on adherence, cocaine use itself is independently associated with HIV accelaration and disease progression (Baum et al., 2008; Cook et al., 2008). HIV positive substance users often enter HIV care and treatment in more advanced states of disease (Celentano et al., 2001; Wang et al., 2004), making them more vulnerable to poor treatment outcomes and associated morbidity and mortality (Zolopa, 2010).
While some modest improvements in HAART adherence have been achieved by behavioral interventions (Amico, et al., 2006; Fogarty et al., 2002; Ickovics and Meade, 2002; Rueda et al., 2006; Sandelowski et al., 2009; Simoni et al., 2003; Simoni et al., 2006), little research has targeted high risk subpopulations such as crack cocaine users on HAART. Although there are no published intervention studies targeting both HAART adherence and cocaine, two clinical trials have applied Motivational Interviewing (MI, Miller and Rollnick, 2002) combined with Cognitive Behavioral Therapy (CBT) to dually target both alcohol use and HAART adherence. Parsons et al., (2007) developed an intervention using the Information Motivation Behavioral Skills (IMB) model of behavior change, which suggests that change occurs when the person becomes well-informed, highly motivated, and skilled (Amico et al., 2009; Fisher et al., 2006; Fisher et al., 2008). They compared an 8-session MI and CBT intervention to an 8-session video educational condition. Individuals in the counseling intervention showed improvements in adherence compared to the education condition at the post-treatment follow-up. However, adherence gains were not maintained three months later, and neither condition improved drinking outcomes (Parsons et al., 2007). Another multi-component intervention study tested MI plus problem solving and a medication timer device against treatment as usual for drinking and HAART adherence (Samet et al., 2005). This study yielded no main effects, and the intervention and treatment as usual groups did not differ on adherence, or alcohol consumption at 6 and 13 month follow-up points.
It is possible that crack cocaine users will respond differently than drinkers to interventions targeting both drug use and adherence. We developed the first dually-targeted intervention to address both crack cocaine use and HAART adherence using MI plus personalized feedback and relapse prevention skills building consistent with the IMB model. We developed the intervention following formative research in which we gathered input from the targeted patient population about their preferences for the content and delivery of interventions targeting cocaine use and HIV care (Cohen and Ingersoll, 2004; Cohen et al., 2004; Cohen and Ingersoll, 2005). The purpose of this pilot study was to test the feasibility and promise of the intervention (MI+) against an information condition (Video+) to improve two target behaviors: HAART adherence and crack cocaine problems, and to investigate the persistence of effects. We hypothesized that those assigned to the MI+ condition would show higher HAART adherence and lower Addiction Severity Index (ASI) Drug Composite Scores (primary outcomes) and lower percent of days using crack cocaine and HIV log VL (log VL) (secondary outcomes), than the those in the Video+ condition. Because this was a pilot study, we were also interested in whether treatments were feasible, administered with good fidelity to protocols, and were credible and able to retain and satisfy participants.
2. Methods
2.1 Participants
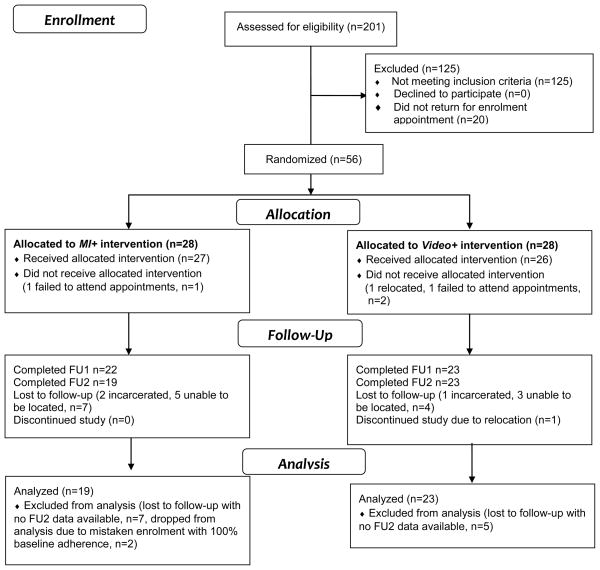
English speaking HIV positive adults with current crack cocaine use or a crack cocaine use disorder (abuse or dependence) and less than 90% self-reported adherence to a current prescription for HAART over the preceding 14 days were eligible to participate in the study. No specific history of lifetime HAART medications was required. Exclusion criteria included: (1) severe cognitive impairment, (2) inability to provide informed consent, (3) current participation in another adherence-enhancing intervention, (4) active suicidality, (5) current incarceration or hospitalization, (8) an inability to provide urine samples (as in end stage renal disease), or (9) plans to leave the area prior to study completion. Participants responded to flyers or provider referrals at community substance abuse treatment programs and HIV care sites. Interested volunteers were screened in person or by telephone to determine eligibility. Participant demographics are shown in Table 1 and the study flow chart is shown in Figure 1. Participants provided written informed consent. Study protocols were approved by university institutional review boards. Participants received up to $300 for their time spent completing measurements at each study visit, prorated by visit completed. Transportation to and from study sites, which were research clinics located in three university research buildings in two cities, was provided if needed.
Table 1.
Demographic, HIV, Drug Use, and Psychiatric Characteristics of the Sample
| Characteristic | Full Sample at Baseline n=54 | Final Sample at FU2 n=42 | MI+ at FU2 n=19 | Video+ at FU2 n=23 | t-test value |
|---|---|---|---|---|---|
| Continuous variables | Mode, Mean (SD) | Mode, Mean(SD) | Mean (SD) | Mean (SD) | |
| Age | 41, 44.7 (6.4) | 41, 45, (5.9) | 44.1 (5.1) | 45.2 (7.5) | t=−.55 |
| Years since HIV diagnosis | 18, 11.3 (6.3) | 18, 12.1 (6.5) | 8.9 (6.0) | 13.4 (5.9) | t=−1.86 |
| Immune functioning | |||||
| Log VL | 2.97 (1.18) | 2.99 (1.20) | 2.51(1.05) | 2.88 (1.0) | t=−1.16 |
| CD4 count | 433 (317.3) | 441 (320) | 548.5(330.7) | 448.1(324.8) | t=.99 |
| Categorical variables | n (%) | χ2 value | |||
| Sex | χ2 (1df)=.51 | ||||
| Men | 25 (46.3%) | 18 (42.9%) | 7 (36.8%) | 11 (47.8%) | |
| Women | 28 (51.9%) | 24 (57.1%) | 12 (63.2%) | 12 (52.2%) | |
| Transgender | 1 (1.9%) | 0 | |||
| Race | χ2 (3df)=3.48 | ||||
| Black | 44 (81.5%) | 35 (83.3%) | 17 (89.5%) | 18 (78.3%) | |
| White | 7 (13%) | 5 (11.9%) | 1 (5.3%) | 4 (17.4%) | |
| Other | 2 (3.7%) | 1 (2.4%) | 0 | 1 (4.4%) | |
| Native American | 1 (1.9%) | 1 (2.4%) | 1 (5.3%) | 0 | |
| Education | χ2 (2df)=2.19 | ||||
| Less than high school | 20 (37%) | 17 (40.5%) | 7 (36.8%) | 10 (43.5%) | |
| High school/equivalent | 17 (31.5%) | 13 (31%) | 8 (42.1%) | 5 (21.7%) | |
| Some college or more | 17 (31.5%) | 12 (28.6%) | 4 (21.1%) | 8 (34.8%) | |
| Employment | χ2 (2df)=2.98 | ||||
| Unemployed | 44(81.5%) | 36 (85.7%) | 18 (94.7%) | 18 (78.3%) | |
| Working full time | 6 (11.1%) | 3 (7.1%) | 1 (5.3%) | 2 (8.7%) | |
| Working part time | 4 (7.4%) | 3 (7.1%) | 0 | 3 (13%) | |
| Sexual Orientation | χ2 (4df)=2.22 | ||||
| Heterosexual | 32 (59.3%) | 23 (54.8%) | 11 (57.9%) | 12 (52.2%) | |
| Homosexual | 13 (24.1%) | 12 (28.6%) | 5 (26.3%) | 7 (30.4%) | |
| Bisexual | 7 (13%) | 5 (11.9%) | 2 (10.5%) | 3 (13%) | |
| Undecided | 1 (1.9%) | 1 (2.4%) | 0 | 1 (4.4%) | |
| Refused to answer | 1 (1.9%) | 1 (2.4%) | 1 (5.3%) | 0 | |
| Criminal Justice Involved | χ2(4df)=4.73 | ||||
| Current | 2 (3.8%) | 2 (4.9%) | 1 (5.3%) | 1 (4.6%) | |
| Ever | 42 (79.3%) | 32 (78.1%) | 13 (68.4%) | 19 (86.4%) | |
| Never | 7 (13.2%) | 5 (12.2%) | 4 (21.1%) | 1 (4.6%) | |
| VL | |||||
| Detectable | 20 (37.7%) | 12 (28.6%) | 4 (21.1%) | 8 (34.8%) | χ2(1df)=.96 |
| Undetectable | 33 (62.3%) | 30 (71.4%) | 15 (78.9%) | 15 (65.2%) | |
| Categorical variables | n (%) | ||||
| HIV Risk Behaviors(ever) | |||||
| Sex work | 18 (34%) | 13 (31.7%) | 5 (26.3%) | 7 (31.8%) | χ2(3df)=1.12 |
| Contracted other STI | 39 (73.6%) | 30 (73.2%) | 15 (78.9%) | 15 (68.2%) | χ2(4df)=4.34 |
| Needle sharing | 16 (30.2%) | 13 (31.7%) | 6 (31.6%) | 7 (31.8%) | χ2(1df)=.0003 |
| Unprotected w/men | 37 (72.6%) | 30 (75%) | 15 (83.3%) | 15 (68.2%) | χ2(4df)=2.42 |
| Unprotected w/women | 29 (54.7%) | 21 (51.2%) | 8 (42.1%) | 11 (50%) | χ2(2df)=2.47 |
| Sex with IDU(s) | 26 (47.2%) | 22 (43.7%) | 11 (57.9%) | 11 (50%) | χ2(3df)=1.60 |
| Sex with known HIV+ partner | 35 (66%) | 26 (63.4%) | 12 (63.2%) | 14 (63.6%) | χ2(3df)=.74 |
| Positive urine toxicology | |||||
| Cocaine | 26 (50%) | 23 (57.5%) | 11 (57.9%) | 12 (57.1%) | χ2(1df)=.002 |
| Marijuana | 20 (38.5%) | 19 (47.5%) | 9 (47.4%) | 10 (47.6%) | χ2(1df)=.003 |
| Benzodiazepines | 3 (5.9%) | 3 (7.9%) | 1 (5.6%) | 2 (9.5%) | χ2(1df)=.21 |
| Opioids | 4 (7.7%) | 3 (7.5%) | 1 (5.3%) | 2 (9.5%) | χ2(1df)=.26 |
| Barbiturates | 2 (3.9%) | 1 (2.5%) | 0 | 1 (4.8%) | χ2(1df)=.93 |
| Methamphetamine | 1 (1.9%) | 1 (2.5%) | 1 (5.3%) | 0 | χ2(1df)=.1.13 |
| DSM-IV Substance Use Disorders | |||||
| Cocaine Dependence | 48 (92.3%) | 37 (90.2%) | 16 (84.2%) | 21 (95%) | χ2(1df)=1.46 |
| Cocaine Abuse | 2 (3.9%) | 2 (4.9%) | 1 (5.3%) | 1 (4.6%) | χ2(1df)=. 01 |
| Alcohol Dependence | 13 (25%) | 10 (24.4%) | 7 (36.8%) | 3 (13.6%) | χ2(1df)=2.98 |
| Alcohol Abuse | 7 (13.5%) | 5 (12.2%) | 2 (10.5%) | 3 (13.6%) | χ2(1df)=.09 |
| DSM-IV Psychiatric Disorders | |||||
| Current MDD | 25 (48.1%) | 21 (51.2%) | 9 (47.4%) | 12 (54.6%) | χ2(1df)=.21 |
| Recurrent MDD | 19 (36.5%) | 15 (36.6%) | 9 (47.4%) | 6 (27.3%) | χ2(1df)=1.77 |
| Current Dysthymia | 11 (21.2%) | 8 (19.5%) | 3 (15.8%) | 5 (22.7%) | χ2(1df)=.31 |
| Current Panic D/O | 3 (5.8%) | 3 (7.3%) | 3 (15.8%) | 0 | χ2(1df)=3.45 |
| Lifetime Panic D/O | 8 (15.4%) | 6 (14.6%) | 3 (15.8%) | 3 (13.6%) | χ2(1df)=..04 |
| Current Agoraphobia | 12 (23.1%) | 7 (17.1%) | 3 (15.8%) | 4 (18.2%) | χ2(1df)=.04 |
| Current Social Phobia | 8 (15.4%) | 6 (14.6%) | 2 (10.5%) | 4 (18.2%) | χ2(1df)=.48 |
| Current OCD | 10 (19.2%) | 8 (31.7%) | 4 (21.1%) | 4 (18.2%) | χ2(1df)=.05 |
| Current Gen. Anxiety | 16 (30.8%) | 13 (31.7%) | 8 (42.1%) | 5 (22.7%) | χ2(1df)=1.77 |
| Current PTSD | 6 (11.5%) | 5 (12.2%) | 2 (10.5%) | 3 (13.6%) | χ2(1df)=.09 |
| Ideation | |||||
| Suicidal | 18 (35.3%) | 15 (38.5%) | 7 (38.9%) | 8 (36.4%) | χ2(3df)= −2.12 |
| Homicidal | 7 (13.5%) | 7 (17.1%) | 4 (21.1%) | 3 (13.6%) | χ2(1df)=.40 |
p<.05
Note: Full Sample is the sample at baseline when 2 mistaken enrollments were removed. Sample at FU2 is the sample completing FU2. Comparisons of MI+ and Video+ conditions are those participants included in the final sample. Some n’s are lower than the full sample due to missing data for that variable. Some percentages do not total to 100% due to rounding, or due to responses such as “refused” or “don’t know,” (data not shown). STI is new sexually transmitted infection after diagnosis with HIV. Unprotected w/men is unprotected sex with men. Unprotected w/women is unprotected sex with women. Sex with IDU(s) is sex with injection drug user(s). D/O is Disorder. MDD is Major Depressive Disorder. OCD is Obsessive Compulsive Disorder. Gen. Anxiety is Generalized Anxiety Disorder. PTSD is Post Traumatic Stress Disorder.
Figure 1.
Study Flow Diagram
2.2. Measures
2.2.1. Screening measures
Researchers conducted screening verbally using a structured interview guide. Researchers asked potential participants to recall HAART adherence behavior and crack cocaine use over the previous 14 days using the timeline follow-back (TLFB) method, a guided method using a calendar to prompt accurate recollection of daily behavior in clinical populations with excellent validity and reliability among substance users (Sobell and Sobell, 1992). A preliminary study showed good agreement of the 14 day TLFB for adherence with prospective, phoned-in daily adherence reports (Hettema and Ingersoll unpublished pilot data) and a previous study showed the utility of the TLFB for assessing proportion of HAART medications taken as prescribed (Ingersoll, 2004). Researchers screened for crack cocaine abuse and dependence using a DSM-IV checklist
2.2.2. Primary outcome measures
Primary outcomes were mean 14-day HAART adherence and ASI Drug Composite Scores (McLellan et al., 1985). Adherence was defined as the percent of prescribed pills taken and was assessed using the TLFB. Researchers used visual aids to guide collection of self-reported information on each participant’s prescribed regimen, including names of all prescribed medications, pills per dose, and doses per day. Total pills prescribed per day served as the denominator, while pills taken that day was the numerator to calculate adherence per day. We calculated Cocaine-specific Drug Composite Scores from data collected via the ASI, a brief semi-structured interview with accepted reliability and validity for assessing the existence, duration, and severity of substance-use-related problems in seven areas (drug, alcohol, medical, employment, legal, family/social, and psychiatric problems) over the previous 30 days (McLellan et al., 1985). The Drug Composite Scores from the ASI can show change in drug use problems over time, are considered more objective than ASI Severity Ratings, and offer an internally consistent estimate of drug use problems with higher scores reflecting more problems related to drug use (McGahan et al., 1986).
2.2.3. Secondary outcome measures
Secondary outcomes were HIV log VL and percent days using crack cocaine. We quantified plasma HIV-1 RNA (VL) using the Roche Amplicor® HIV-1 Monitor which has an assay range of 49–750,000 copies/mL. Undetectable VL was a result below the assay limit, or <49 copies/mL. To adjust for skew, we log-transformed VL results and we considered a change of +/−.5 log VL to be a useful clinical indicator (Holodniy, 2010). We calculated percent days using crack cocaine from the TLFB.
2.2.4. Demographic and other variables
Measured characteristics included age, sex, race, ethnicity, education, employment, sexual orientation, criminal justice involvement, lifetime HIV risk behaviors, years since HIV diagnosis, immune health status, and comorbid health conditions. Researchers also evaluated cocaine and alcohol use, including abuse and dependence, and comorbid psychopathology with the Mini International Neuropsychiatric Interview (M.I.N.I., (Sheehan et al., 1997; Sheehan et al., 1998). The M.I.N.I. is a short, structured, diagnostic interview with adequate validity and reliability for identifying DSM-IV and ICD-10 psychiatric disorders (Sheehan et al., 1998).
2.2.5. Treatment fidelity
Treatment fidelity is the extent to which an intervention was delivered as planned. The supervisors (S.C. and C.H.) and principal investigator (K.I.), all members of the Motivational Interviewing Network of Trainers, facilitated the training and supervision of therapists. Therapists also attended external MI workshops and trainings. All sessions were audio taped or videotaped and these recordings were used in weekly group and individual supervision. Immediately after each treatment session, therapists completed the study’s Therapist Checklist, a 39-item instrument containing all of the major components that appeared in the MI+ or Video+ treatment manuals. The form included two activities relevant to the Video+ condition, including showing the video and asking debriefing stem questions, 35 activities relevant to the MI+ condition, including providing personalized feedback, completing a decisional balance worksheet, and using MI-consistent conversational strategies, and 2 activities that were relevant to both conditions, including role induction and providing informational handouts and pamphlets. Additionally, fidelity was assessed during weekly supervision of therapists conducting the interventions via review of videotaped sessions to ensure that the conditions were delivered consistent with treatment manuals, and were adequately differentiated.
2.2.6. Treatment credibility
To assess treatment credibility of both interventions, we adapted a 4-item, 1–10 scale instrument that assessed participants’ self-report of how much the assigned intervention made sense to them, how successful they believed the intervention would be in helping them adhere HAART, how confident they would be in recommending the intervention to a friend with HIV, and how successful the intervention would be in helping them to reduce cocaine use. Treatment credibility measured before a treatment has been found to be a predictor of outcome in the area of chronic pain (Dennis Turk, 2000, personal communication). When asking participants to rate credibility before treatment, therapists (who were unblinded to assignment) explained the assigned intervention to the participant following randomization. For example, therapists stated “You have been randomly assigned to the Counseling intervention. That means that you will meet with a therapist for six sessions who will discuss your crack cocaine use and HIV medication adherence with you.” Alternatively, they stated “You have been randomly assigned to the Video intervention. That means that you will view a series of six videos and answer some questions about them in discussion with a therapist.” Following randomization and this discussion, blinded researchers administered the measure. Total scores could range from 4–40, with higher scores indicating higher credibility of the assigned intervention.
2.2.7.Treatment satisfaction
We assessed satisfaction with a 6-item, 1–5 scale that asked participants to rate their overall satisfaction with their treatment, their overall satisfaction with their therapist, whether the treatment had an effect on their cocaine use, whether the program had an effect on their HAART adherence, their overall satisfaction with the intervention setting (researchers, therapists, facility, etc.) and whether they would recommend the program to others. Some items were reverse scored. The scores could range from 5–30, with 5 representing the highest satisfaction and 30 the lowest.
2.3. Interventions
Intervention components are shown in Table 2. When delivering the MI+ intervention, therapists used MI to explore participants’ thoughts, feelings, motivations and behaviors related to adherence and cocaine use using a variety of strategies, including providing personalized feedback, emphasizing choice and control, goal setting, and change planning. Issues addressed in the personalized feedback included rate of crack cocaine use and its cost, rate of HAART adherence, recent urine drug screen results, general health status indicators that can be affected by cocaine use such as pulse rate and blood pressure, VL, and CD4 cell count, a marker of the number of immune helper cells remaining in the body. Data for the feedback were drawn from the baseline assessment. Sessions also explicitly incorporated strategies such as self-monitoring, exploring triggers for drug use or nonadherence with behavior chain analysis for relapse prevention, and development of problem-solving plans. Some of these strategies are often used in CBT, but study therapists did not directly target cognitive errors in this intervention. Rather, these strategies were delivered using the MI counseling style, with a focus on evoking the participant’s ideas about change using extensive reflective listening. Therapists also offered participants educational handouts to take home with them that contained further information on the topics discussed in the sessions.
Table 2.
Intervention Components by Treatment Session1
| MI+: Used a collaborative, evocative style to acknowledge autonomy and provide support while completing the following: | Video+: Used a friendly, matter-of-fact style to provide information while completing the following |
|---|---|
| Session One Activities | |
|
|
| Session Two Activities | |
|
|
| Session Three Activities | |
|
|
| Session Four Activities | |
|
|
| Session Five Activities | |
|
|
| Session Six Activities | |
|
|
An expanded version of this table containing more detail on intervention components including video stem questions is available in online supplementary material.
We developed a comparison condition equivalent for time and attention that provided information about HAART adherence and crack cocaine use. This comparison condition (Video+) included watching a 30–45 minute video, plus debriefing and reading materials. We selected videos that were accurate, included diverse peer role models, contained no “scare tactics” or counter-motivational communication strategies, and contained at least some personal narrative in addition to didactic information presented by peer role models and medical experts. Additionally, the videos selected addressed either crack cocaine use or drug use generally, HIV treatment, or both. Video titles are presented in Table 2. After participants viewed each video, therapists facilitated a 10-minute debriefing discussion by asking a brief list of questions intended to check that participants had watched and understood the information in the video. Therapists were trained to keep these conversations neutral and focused on checking that the participant had watched the video, and providing information instead of using reflective listening. Therapists also offered participants educational handouts that contained additional information on the topics discussed in the videos.
2.4. Procedures
Researchers facilitated a 15-minute screening of interested potential participants in person or on the telephone. If the person met initial eligibility criteria, researchers obtained releases of information and confirmed HIV+ and HAART status with medical records. Researchers then offered eligible individuals participation and scheduled a baseline assessment visit at their convenience within the following 2 weeks.
During the baseline visit, researchers verified locator information and faciliated the informed consent process, then administered interview and self-report measures. Participants provided urine, blood samples, and vital signs to assess drug use, VL, CD4 count, blood pressure, and pulse rate. Immediately after the baseline assessment, a therapist informed the participant of their random assignment and scheduled the first treatment session for approximately 1 week later. Baseline assessment visits took 4–5 hours including a lunch break, with lunch provided for participants.
The first 4 intervention sessions were held on a weekly basis, and the final 2 sessions were scheduled biweekly. All sessions took place at university research clinics and not in HIV clinics. Study therapists were responsible for providing both interventions. Both interventions were delivered by 9 racially, ethnically, and culturally diverse therapists (7 women, 2 men) who had master’s or doctoral degrees in clinical or counseling psychology, social work, or counseling. The same therapist met with each participant for all intervention sessions. In 2 cases, a new therapist completed the sessions with a participant who had initially begun with a therapist who departed during that participant’s treatment. Each session lasted between 45 and 60 minutes.
Follow-up one (FU1) occurred 2–3 months post randomization (usually directly following the sixth treatment session in week 8 or as late as week 12), and follow-up two (FU2) occurred 5–6 months post randomization, which was 3 months after treatment completion. Researchers blinded to participants’ assigned conditions administered the ASI, the HIV Medication Chart and Adherence Questions and the TLFB for HAART adherence and crack cocaine use, and treatment satisfaction forms. Participants provided urine to assess drug use at FU1 and at FU2, with the addition of blood to assess VL at FU2. At FU2, participants also completed treatment credibility forms. The FU1 session was typically completed in 1 hour and the FU2 session was typically completed in 2 hours.
2.5. Data Analysis
We used descriptive statistics, t-tests, and chi-square tests to characterize the sample and the intervention measures including participant satisfaction, treatment credibility, and activities in each session reported on Therapist Checklists (i.e., treatment fidelity). We assessed the relationship between baseline adherence and viral load, and whether participant characteristics like age, years since HIV diagnosis, race, employment, and sexual orientation were related to outcomes using univariate analyses to determine if they should be considered as covariates. We examined the relationships between baseline ASI Drug Composite Scores and cocaine using days and adherence. We examined the relationships between baseline adherence and baseline log VL. We used a repeated measures mixed model analysis of variance (ANOVA) to assess main effects and between-group effects on the primary and secondary dependent variables. We tested for the normality of the distributions, homogeneity of covariance matrices, and sphericity. When violations of the sphericity assumptions were found, we used the Greenhouse Geyser Epsilon to adjust the probability of F (Lix and Keselman 2010). Omnibus F testing was followed by univariate tests of our a priori hypothesis that adherence and ASI Drug Composite Scores would differ by condition. Analyses were conducted using SAS Proc GLM, which allows for maximum use of observations across time even when some data are missing. To determine whether there was an effect of group, time, or a group × time interaction on the 2 primary outcome variables specified a priori: 1) HAART adherence, and 2) ASI Drug Composite Scores, and on the 2 secondary outcomes variables specified a priori: 1) percentage days using cocaine, and 2) log VL, we conducted 4 repeated measures ANOVAs with 2 levels of condition (MI+ and Video+) and 3 levels of time (baseline, FU1, FU2). When the null was rejected in omnibus testing, we conducted further univariate tests of hypotheses for between-groups and main effects and calculated effect sizes for the effects observed across time (Eta squared; ή2). Generally, ή2 is considered small at .02, medium at .13, and large at .26 (Bakeman, 2005). In addition, for each primary outcome variable at each follow up point, effect sizes and 95% confidence intervals (CIs) were computed. Between-group unbiased estimators of effect size (d) and 95% CIs were calculated. These effect sizes represent MI+ versus Video+ condition differences in post-treatment scores. Cohen’s (1988) criteria for identifying the magnitude of d were used, where d = .2 is a small effect, d = .5 is a medium effect, and d = .8 is a large effect.
In addition to analyzing the effects of time and group on log VL, we calculated difference scores in log VL from randomization to FU2 and classified participants with a decrease of −.5 log VL or greater (meaning a larger decrease in log VL) as clinically relevant decreased log VL, those with log VL difference score between −.5 and +.5 as unchanged log VL, and those with an increase of +.5 log VL or greater clinically relevant increased log VL (Holodniy, 2010). We analyzed these log VL difference score categories using a chi-square analysis to determine if the proportion of decreased log VL, unchanged log VL, and increased log VL differed between the MI+ and Video+ conditions. Additionally, we examined the proportion of participants whose VL were detectable and undetectable in each group at randomization and FU2 to determine if there were differences between the MI+ and Video+ conditions.
3. Results
3.1. Sample
Baseline characteristics of the sample are shown in Table 1 for the full sample (n=54) and for the final sample (n=42). There were no significant differences between conditions at baseline for the full sample or the final sample on demographic, HIV risk, VL or CD4 counts, drug use, psychiatric, adherence, percentage of days of cocaine use, or ASI Drug Composite Scores. Fifty-four patients on HAART participated in the study, including 28 women, 25 men, and 1 transgendered individual; 82% were African-American, and 1 participant was Hispanic. Their mean age was 45 (SD=6.4); the modal age was 41. Thirty-seven percent of the sample had less than a high school education, but 31.5% had high school and 31.5% had some college education. Most (82%) were not employed. Most (44; 82%) had a history of involvement with the criminal justice system. More than half of this sample was heterosexual (32, 59.3%). On average, participants had been diagnosed with HIV 11.3 years ago (SD=6.5); the most recent diagnosis was four months before, and the most distant diagnosis was 22 years before. On average, participants took 58.27% (SD =27.5) of their prescribed HAART pills in the two weeks prior to enrollment. At baseline, there was a significant inverse relationship between adherence and viral load (r = −.34, p<.05). The participants had detectable HIV VL (mean log VL 2.97, SD = 1.18) and compromised immune functioning as indicated by low CD4 cells (mean 433, SD = 317). While not shown in the table, the full sample reported significant health problems beyond HIV; 31.5% had a current diagnosis of AIDS, while other common current co-morbidities included Sexually Transmitted Infections (93%), Hepatitis A (5.6%), B (18.5%), or C (35.2%), hypertension (42.6%) and Type 2 Diabetes Mellitus (7.4%). Participants were prescribed 6.4 medications per day, with 2.5 for HIV disease. Despite high unemployment, 94.4% reported stable housing.
Table 1 also shows the drug use and positive psychiatric screens for the sample by assignment condition. On average, participants used crack cocaine on a third of days in the past month prior to enrollment in the study. On urine toxicology screens at randomization, half screened positive for cocaine, consistent with their self-report of using crack cocaine on a third of days. Some also screened positive for marijuana, benzodiazepines, opioids, barbiturates, and methamphetamine. Most were classified as crack cocaine dependent on the M.I.N.I., and 38.5% were also classified with alcohol use disorders. Psychiatric problems were common, with nearly half experiencing current Major Depression. Current suicidal and homicidal ideations were also present in the sample; whenever these were endorsed, study therapists and supervisors reviewed responses while the participant was there, and assessed current risk using a standardized protocol.
3.2 Treatment and Follow-Up Retention
Retention rates did not vary by group at any of the assessment or treatment sessions. The 2 mistaken enrollments had both been randomized to the MI+ condition and had completed the study, but were dropped from analyses. Forty of 54 enrolled participants (74.1%%) received all 6 treatment sessions, 45 of 54 (83.3%%) completed FU1, and 42 of 54 (77.8%) completed FU2 (see Figure 1 for the study flow chart and completion by condition).
3.3. Primary Outcomes
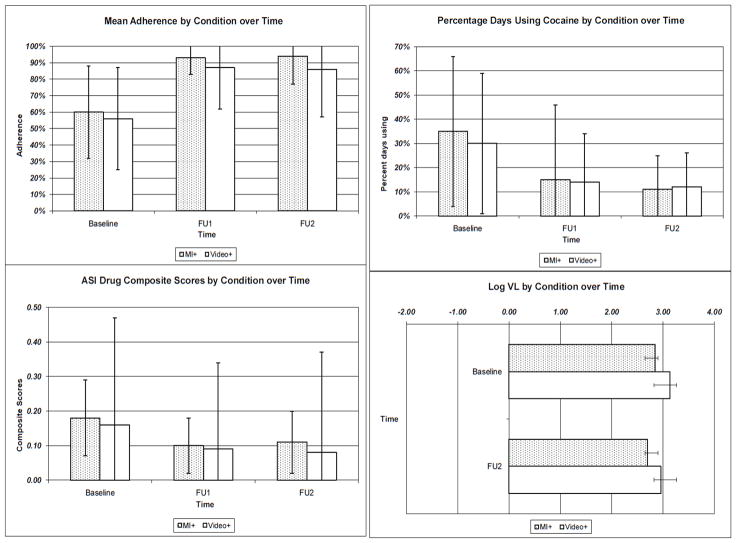
Figure 2 shows the mean differences for the primary dependent variables (mean adherence and ASI Drug Composite Scores) and secondary dependent variables (days using cocaine and log VL) across time. There were main effects of both interventions on HAART adherence and ASI Drug Composite Scores. Table 3 shows the ANOVA results for the sample of 54 at baseline, 45 at FU1, and 42 at FU2. In some instances, missing data resulted in changes to the n’s when variables for that analysis were missing as noted below.
Figure 2.
Mean differences between conditions across time for adherence, ASI Drug Composite Scores, days using cocaine, and logVL
Table 3.
Analysis of Variance Results for Condition and Time Effects on Dependent Variables
| Source |
df |
SS |
MS |
F value |
Effect size ή2 |
|---|---|---|---|---|---|
| Adherence Between subjects | |||||
| Condition | 1 | 0.013 | 0.013 | 0.32 | .008 |
| Error 1 | 37 | 1.54 | 0.042 | ||
| Adherence within subjects | |||||
| Time | 2 | 1.987 | 0.993 | 29.74+**** | .45 |
| Time × Condition | 2 | 0.055 | 0.028 | 0.83+ | |
| Error 2 | 74 | 2.472 | 0.033 | ||
| ASI Drug Composite Score Between subjects | |||||
| Condition | 1 | 0.027 | 0.027 | 2.07 | .056 |
| Error 1 | 35 | 0.462 | 0.013 | ||
| ASI Drug Composite Score within subjects | |||||
| Time | 2 | 0.154 | .0.077 | 12.25++**** | .259 |
| Time × Condition | 2 | 0.005 | 0.003 | .40++ | |
| Error 2 | 70 | 0.440 | 0.006 | ||
| Percentage days using cocaine Between subjects | |||||
| Condition | 1 | 0.0004 | .0004 | .00 | .001 |
| Error 1 | 37 | 3.693 | .0998 | ||
| Percentage days using cocaine within subjects | |||||
| Time | 2 | 1.505 | 0.753 | 22.72+++**** | .38 |
| Time × Condition | 2 | 0.018 | 0.009 | 0.27 | |
| Error 2 | 74 | 2.45 | 0.033 | ||
| log VL Between subjects | |||||
| Condition | 1 | 2.530 | 2.530 | 1.32 | .014 |
| Error 1 | 37 | 70.8 | 1.914 | ||
| log VL within subjects | |||||
| Time | 1 | 0.168 | 0.168 | 0.27 | .007 |
| Time × Condition | 1 | 0.182 | 0.182 | 0.29 | |
| Error 2 | 37 | 23.078 | 0.624 | ||
Df=degrees of freedom
SS=Sums of squares
MS=Mean squares
values adjusted by Greenhouse Geyser Epsilon=.8963
values adjusted by Greenhouse Geyser Epsilon=.9008
values adjusted by Greenhouse Geyser Epsilon=.6287.
p<.05
p<.01
p<.001
p<.0001
There was a moderately negatively skewed distribution of adherence at baseline (skewness = −.837), but this deviation from the normal distribution was within the acceptable range to proceed with the repeated measures ANOVA. There was a significant main effect of time on adherence. In the MI+ condition, adherence improved from 60.2% (SD =26.7%; n =26) at baseline to 93.3% (SD =10%; n =22) at FU1 and 93.9% (SD =13.7%; n =19) at FU2. In the Video+ condition, adherence improved from 56.4% (SD =28.5%; n =28) at baseline to 87% (SD =25.3%; n =23) at FU1 and 86% (SD =29.1%; n =23) at FU2. There was no significant between-groups effect on adherence. Between groups effect sizes for adherence were small and nonsignificant at FU1 [d =.33 (−.26, .91); p =.28] and FU2 [d =.34 (−.28, .95); p =.28]. When collapsing across conditions, participants improved their adherence from 58.27% (SD =27.5%) at baseline (n =54) to 90% (SD =19.5%) at FU1 (n =45) and 90% (SD =24%) at FU2 (n =42). Follow-up contrasts showed that mean adherence was lower at baseline than at FU1, F (1, 37) =32.11, p<.0001 and FU2, F (1, 37) =43.81, p<.0001; repeated measures effect sizes are shown in Table 3.
There was also a significant main effect on ASI Drug Composite Scores, which range from 0–1, with lower scores indicating fewer problems from drug use. In the MI+ condition, ASI Drug Composite Scores decreased from .18 (SD =.11) at baseline (n =26) to .10 (SD =.08) at FU1 (n =20) and rose slightly to .11 (SD =.09) at FU2 (n =18). In the Video+ condition, ASI Drug Composite Scores decreased from .16 (SD =.09) at baseline (n =28) to .09 (SD =.09) at FU1 (n =21) and .08 (SD =.08) at FU2 (n =22). Mean composite scores were higher at baseline than at FU1, F (1, 35) =17.27, p<.001, and FU2, F (1,35) =14.32, p<.001. There was no significant between-groups effect on ASI Drug Composite Scores. Between groups effect sizes for composite scores were small, nonsignificant, and in different directions at FU1 (d =.12 (−.72, .48); p =.72) and FU2 (d =−.12 (−.72, .48); p =.70). Across conditions, participants decreased their ASI Drug Composite Scores from .17 (SD =.10) at baseline (n =54) to .09 (SD =.09) at FU1 (n =41) and .09 (SD =.09) at FU2 (n =40).
3.4. Secondary Outcomes
There was a significant main effect of the interventions on percent of days using cocaine, but not on log VL or the proportion of participants with detectable or undetectable VL. In the MI+ condition, percent days using cocaine decreased from 35.4% (SD =31%) at baseline (n =26) to 15.4% (SD =15.1%) at FU1 (n =20) and 10.9% (SD =14.7%) at FU2 (n =19). In the Video+ condition, percent days using cocaine decreased from 30.4% (SD 29%) at baseline (n =28) to 13.8% (SD =19.7%) at FU1 (n =23) and 12.1% (SD =20.4%) at FU2 (n =23). Percent days using cocaine was higher at baseline than at FU1, F (1, 37) =22.32, p<.0001, and FU2, F (1, 37) =26.49, p<.0001. No significant between-groups effects were identified for any of these secondary outcomes. Across conditions, participants decreased the percent days they used cocaine from 32.8% (SD =30%) at baseline (n =54) to 14.6% (SD =17%) at FU1 (n =45) and 11.6% (SD =18%) at FU2 (n =42).
There was no significant main effect of time on log VL and no significant between-groups effect. Thirty-five percent of 26 MI+ participants and 41% of 27 Video+ participants had detectable VL at baseline, (χ2(1 df) =.21, p=.65), while 26% of the 19 MI+ and 43% of the 23 Video+ had detectable VL at FU2, (χ2(2 df) =.2.32, p=.31). Similarly, the proportion of people in each condition with decreased, unchanged, or increased log VL did not differ. Only 1 MI+ participant and 3 Video+ participants had significantly decreased log VL, while 14 MI+ and 14 Video+ participants had unchanged log VL, and 4 MI+ and 3 Video+ participants had increased log VL (χ2(2 df) =1.12, p =.57).
3.5. Feasibility
Both recruitment and retention were feasibility challenges. It required over 2 years to enroll 56 participants, 2 of whom were mistakenly enrolled despite reporting 100% adherence at baseline. Screening 201 potential participants yielded 76 eligible participants, a 38% gross eligibility rate and a net rate of participants to screens of 28%. The accrual rate was 2 new enrolled participants per month with approximately 8 screened per month. The primary reason for ineligibility was that potential enrollees reported over 85% HAART adherence, and in many cases, reported 100% adherence.
An additional challenge with this crack cocaine-using population was that it took considerable effort to retain participants in the study. Several participants were incarcerated long enough that they timed out of the study and missed either intervention or follow-up visits. Given both the drug dependence and psychiatric disorders that were present in the sample, we often engaged in crisis management (including two emergency hospitalizations for homicidality) and referrals to psychiatric care. Retention was quite good despite these challenges.
3.5.1. Treatment fidelity
As discussed previously, all sessions were reviewed weekly by therapists and supervisors to ensure that each condition was delivered as planned, and that drift across conditions was prevented. In addition, post-session Therapist Checklists indicated that the activities conducted within the 2 conditions differed as planned. Specifically, all of the activities occurred in the MI+ condition, varying as planned by session. In contrast, only role induction, education about HIV adherence, education about cocaine, showing videos, using stem questions to guide debriefing, and providing tip sheets and reading materials occurred in the Video+ condition, consistent with the protocol for that condition. In the Video+ condition, therapists showed no use of reflective listening or other MI skills or strategies; rather, they asked scripted questions as written. All Therapist Checklist items differed significantly by condition (data not shown for χ2 analysis of each activity by each session by condition; all p values <.05).
3.5.2. Treatment credibility
Pre-treatment, both MI+ and Video+ participants rated their assigned interventions favorably. MI+ participants scored a mean of 35.5 (SD = 5.4) out of 40, while Video+ participants had a mean score of 33.3 (SD = 7.5). Post-treatment, both groups rated their condition higher, with a mean for MI+ of 36.8 (SD = 4.3) and a mean for Video+ of 35.6 (SD = 4.8). These scores indicate that participants rated treatments as equally credible before and after they received them.
3.5.3. Treatment satisfaction
Both MI+ and Video+ participants reported total satisfaction scores indicating excellent satisfaction. MI+ participants’ satisfaction with their condition was a mean of 8.1 (SD = 3.0; 5 is most satisfied; 30 is least satisfied) at FU1, and was a mean of 8.3 (SD = 2.5) at FU2. Video+ participants’ satisfaction with their condition was a mean of 9.3 (SD = 2.7) at FU1 and 9 (SD =1.3) at FU2; none of these means were significantly different. These scores indicate that participants were satisfied with their assigned condition immediately and three months after completing treatment, and that the satisfaction rates did not differ by condition.
4. Discussion
In this pilot randomized control trial (RCT), a Motivational Interviewing plus feedback and skills building intervention (MI+) and a Video information plus scripted debriefing intervention (Video+) significantly increased HAART adherence and decreased drug-related problems and days of cocaine use among crack cocaine users with poor adherence. Effect sizes were medium to large. Moreover, these improvements were attained quickly (by the post-treatment follow-up), and were maintained across time (3 months post-treatment), in contrast to prior studies targeting adherence among drinkers in which effects faded after treatment. Additionally, these interventions were credible and satisfying to participants.
4.1. Promise of MI and Video Interventions
We had expected significant differences by condition, but both MI+ and Video+ interventions resulted in significant improvements to both targeted behaviors. As we expected, the MI+ condition was associated with large effects on cocaine use and HAART adherence. We did not anticipate significant main effects in the Video+ condition, because early studies of video education interventions were associated with changes in knowledge and attitudes, but not with changes in treatment compliance (Healton and Messeri 1993). Additionally, Parsons et al., (2007) had used a video information condition as the control condition and found no significant benefit for nonadherent HIV+ drinkers. However, other studies published after this study was underway found that video interventions may be more potent than previously thought. Recent RCTs (Brock and Smith, 2007; Purcell et al., 2007; Sampaio-Sa et al., 2008) and quasi-experimental studies (Brock and Smith, 2007; Wong et al., 2006) have found that informational videos can lead to improvements in HAART knowledge (Brock and Smith 2007; Wong et al., 2006), adherence behavior (Brock and Smith, 2007; Purcell et al., 2007; Sampaio-Sa et al., 2008), and substance use (Gilbert et al., 2008; Purcell et al., 2007). Some studies have found durable effects, with video interventions reducing drug use (Gilbert et al., 2008; Purcell et al., 2007) among HIV+ (Gilbert et al., 2008; Purcell et al., 2007) and mixed serostatus (Chiasson et al., 2009) samples for three to twelve months.
In this study, therapists delivering the Video+ intervention adhered to debriefing scripts and asked stem questions designed to elicit participants’ new factual knowledge. However, in response to these questions, participants often gave answers that personalized their own risk, demonstrated that they were considering or planning change, and that they had learned some behavioral skills through vicarious learning. Some participants gave change talk, a correlate of positive outcomes in previous MI research (Miller and Rollnick, 2002). Thus, participants may have received Information, Motivation, and Behavioral Skills benefits from the Video+ intervention despite our intent for it to be primarily informational, and despite the therapists succeeding in delivering it to be completely distinguishable from the MI+ condition.
4.2. Limitations
The results of this pilot study should be considered in light of several limitations. Preliminary power analysis of a similar MI-based intervention for cigarette smoking among HIV+ patients were conducted before the trial. Those analyses indicated that 50 participants would allow more than 90% power to show differences between conditions, but the methodological and analytical differences between that pilot study and this pilot trial encouraged us to revisit the issue of insufficient power to detect real differences between conditions, or the probability of type II error.
In the current trial, given the mean differences and within-group variability observed, 202 participants would be needed to have an 80% chance of rejecting the null hypothesis at the p<.05 level of significance for adherence rates at FU1 and 470 participants would be needed at FU2. Thus, it is possible that the study was underpowered to discern between-groups differences in adherence. Increased power would not likely influence the probability of type II error for the ASI Drug Composite Scores outcomes as effects were not in the predicted direction. To further assess the probability of type II error, we examined between-groups effect sizes because their magnitude is not affected by sample size. Overall, the between-group effect sizes we observed were small and nonsignificant. The high similarity in treatment effect sizes across conditions, and adherence and substance use rates that are clinically comparable across conditions, increase our confidence in the real lack of between-group differences. Furthermore, as a stage 1a/2b project, this pilot study was designed to provide information on the feasibility and promise of the interventions, in order to determine whether either or both interventions should be tested further in a larger clinical trial. Data from this investigation will provide future researchers with important parameter estimates on which to base the methodology of future investigations.
It is possible that time alone led to improvements rather than either intervention. However, in examining the data, there was considerable individual variability in both adherence and cocaine use, and history alone does not seem a likely reason for mean improvements in both behaviors. Even though patient demographic characteristics were unrelated to outcome and therefore were not used as covariates in repeated measures analyses, it is possible that study findings may generalize only to other patient groups including primarily people living with HIV who are unemployed, African-Americans, and heterosexuals. Another limitation was that, once enrolled, some participants received intervention sessions spaced over more weeks than we planned, up to a limit of 12, due to the acuity of health problems, mental disorders, and incarceration in this sample The extended time-frame might have changed the impact of either intervention. Additionally, providing elements such as transportation may limit the generalizability of findings beyond a clinical trial setting. Specifically, it is unknown whether the setting of a clinical trial is required to yield the benefits seen in the Video+ condition. Simply showing videos without the accoutrements of the trial, including personal attention, payment, time spent in an attractive and congenial setting, and other intangible factors may not yield the same benefits.
Neither intervention was related to significant changes in immune health (CD4 count) or reductions in VL. There are several potential explanations for this discrepancy. Little methodological evidence is available regarding the specific level or duration of adherence improvements required to improve VL at specific follow-up time points (Gross et al., 2008). The relationship between adherence level and VL and the “lag time” required to observe biological changes are likely highly confounded by baseline adherence and VL. Additionally, while adherence and VL were significantly inversely related at baseline, adherence and logVL were inversely related, but the relationship was not significant. This may be in part because the relationship between VL and logVL is imperfectly but significantly correlated (r =.66; p<.0001), as expected due to the nature of the log transformation. Therefore, while it is appropriate to examine logVL as the outcome, the truncation of the overall range of values and variance makes it more difficult to observe change. It also is possible that while adherence improved across time, it was insufficient in level or duration to reliably improve these biomarkers. It is also possible that the absolute level of adherence obtained was insufficient to overcome the high VLs and active HIV disease experienced by most participants. This is a limitation of our interventions, because ultimately, adherence interventions must translate improvements in biomarkers. Lastly, we used a self-report measure of adherence. Self-report can result in increased estimates of adherence compared to electronic monitoring, but TLFB methods improve self-report over simpler quantity-frequency measures in the area of alcohol use, and have been useful in other studies of adherence among drug users and drinkers (Parsons et al., 2007; Arnsten et al., 2001; Ingersoll, 2004). While it is possible that self-reported levels of adherence in this study are inflated, there was variability in reported adherence, reducing concern that there was a universal bias toward social desirability. However, it is possible that while adherence appeared to improve significantly in both conditions, the absolute level of improvement may not be accurate.
We required poor adherence for study entry, and this was a challenge for our study. While this may have reduced the risk of ceiling effects, the cost was in feasibility. Recruitment was slow and it required an extended period of time to recruit participants given that the settings were a medium sized city and a small city, neither of which has a large population of HIV+ cocaine users. Additionally, we found that some referral sources “prescreened” their patients, assuming they did not use crack cocaine. Thus, in retrospect, we might have disguised the specific type of drug user being sought; these tactics might have improved our flow of referrals.
4.3. Strengths
Our study had several strengths. The sample included a good proportion of women, African-Americans, and heterosexual participants compared to most other adherence studies. The design was a longitudinal RCT with blinded assessments occurring up to 6 months after randomization. Other strengths included participants’ perceptions that both interventions were credible and satisfying. Lastly, the study retained the majority of study participants despite the challenges they presented.
4.4. Summary and Future Directions
Both the MI+ and Video+ interventions were efficacious at improving adherence and crack cocaine use at post-treatment and they maintained their efficacy 3 months later. This means that this study has developed 2 useful interventions targeting 2 behaviors with better durability than others tested so far. The Video+ intervention should be tested further among underserved sub-populations of drug users taking HAART. The MI+ intervention may also merit further testing among nonadherent patients who have drug use disorders other than cocaine abuse or dependence. In addition, future investigations could provide valuable information by identifying factors that may moderate the impact of treatment on outcome, including baseline severity of drug use and psychiatric disorders and readiness for change.
In the present report, we provided evidence of treatment fidelity based on therapist checklists. Future studies should endeavor to explicate the relationship between treatment processes and outcomes. Currently, our team is objectively coding taped visits for future process-outcomes analyses that will include measures of interpersonal characteristics completed by therapists and participants.
The finding that the Video+ intervention resulted in significant, sustained behavior change is surprising, and has implications for future intervention research. While we employed therapists with master’s and doctoral degrees as study therapists, it may be possible to utilize less highly trained staff or even peers to deliver the Video+ intervention, and this is a fruitful area for further investigation. Compared to the MI+ intervention, the Video+ intervention requires fewer resources for implementation and can potentially be made portable to allow patients to view video content at the time and place of their choosing. While recently a few others have found similar results, most adherence and drug use treatment studies have not included video as an active intervention. As a field, we may be overlooking the promise of video-based interventions based on older findings that video-delivered information alone was not enough to achieve behavior change. Future dual-focused adherence and substance use intervention studies could include cost-effectiveness analyses (Gold et al., 1996; Goldie et al., 2003; Tate et al., 2009). Future studies should investigate how, when, and where best to deliver video-based interventions to address HAART adherence and drug use problems that undermine adherence.
Supplementary Material
Footnotes
A table showing the components of treatment sessions and overlap with the Therapist Checklist Activities can be found as supplementary materials by accessing the online version of this paper at http://dx.doi.org by entering doi:…
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41:285–97. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- Amico KR, Barta W, Konkle-Parker DJ, Fisher JD, Cornman DH, Shuper PA, Fisher WA. The information-motivation-behavioral skills model of ART adherence in a deep south HIV+ clinic sample. AIDS Behav. 2009;13:66–75. doi: 10.1007/s10461-007-9311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, Buono D, Eckholdt H, Howard AA, Schoenbaum EE. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–23. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37:379–84. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Acosta EP, Gupta R, Guzman D, Riley ED, Harrigan PR, Parkin N, Deeks SG. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–31. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–9. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Brock TP, Smith SR. Using digital videos displayed on personal digital assistants (PDAs) to enhance patient education in clinical settings. Int J Med Inform. 2007;76:829–35. doi: 10.1016/j.ijmedinf.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, Gallant JE. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–15. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- Chiasson MA, Shaw FS, Humberstone M, Hirshfield S, Hartel D. Increased HIV disclosure three months after an online video intervention for men who have sex with men (MSM) AIDS Care. 2009;21:1081–9. doi: 10.1080/09540120902730013. [DOI] [PubMed] [Google Scholar]
- Cohen J, Ingersoll KS. Cocaine use interferes with HIV antiretroviral medication adherence: Focus group results. Oral presentation to the College on Problems of Drug Dependence Annual Scientific Meeting; Orlando, FL. 2005. [Google Scholar]
- Cohen J, Ingersoll KS. Identifying mental health issues and service needs of HIV+ lesbians. Poster presented to the American Psychological Association convention; Honolulu, HI. 2004. [Google Scholar]
- Cohen J, Ingersoll KS, Fox S. Service gaps reported by HIV+ cocaine users: Focus group results. Poster presented to the American Psychological Association Convention; July; Honolulu, HI. 2004. [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Levine AM, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–63. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGerome A. Positively: Adults coping with HIV and AIDS. State of the Art, Inc; Washington, D.C: 2001. [Google Scholar]
- Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5:193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–73. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: A review of published and abstract reports. Patient Educ Couns. 2002;46:93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, Calderon SH, Bogetz A, Gerbert B. Interactive “video doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3:e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness In Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- Goldie SJ, Paltiel AD, Weinstein MC, Losina E, Seage GR, Kimmel AD, Walensky RP, Sax PE, Freedberg KA. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003;115:632–41. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Gross R, Bilker WB, Wang H, Chapman J. How long is the window of opportunity between adherence failure and virologic failure on Efavirenz-based HAART? HIV Clin. Trials. 2008;9:202–06. doi: 10.1310/hct0903-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzke AJ, Williams ML, Bowen AM. Binge use of crack cocaine and sexual risk behaviors among African-American, HIV-positive users. AIDS Behav. 2009;13:1106–18. doi: 10.1007/s10461-008-9450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema J, Ingersoll KS. Pilot study of prospective telephone adherence reporting and timeline followback adherence recall unpublished data. [Google Scholar]
- Holodniy M. Ask the experts about: Understanding your labs. [Accessed on May 15, 2010.];The Body: The Complete HIV/AIDS Resource. 2010 http://www.thebody.com/Forums/AIDS/Labs/Archive/TestResults/
- Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care. 2002;14:309–18. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16:199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- Kaiser Permanente. Now That You Know: Living Healthy with HIV. Concept Media; Irvine, CA: 1990. [Google Scholar]
- Levin M, Casciato T, Hughes K, Pellett G, Wagner Mason P. Close to Home: Moyers on Addiction- Portrait of Addiction. Films Media Group, Films for the Humanities and Sciences; New York, NY: 1998. [Google Scholar]
- Lix LM, Keselman HJ. Analysis of Variance Repeated Measures Designs. In: Hancock GR, Mueller RO, editors. The Reviewer’s Guide to Quantitative Methods in the Social Sciences. Routledge; New York, NY: 2010. pp. 15–28. [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, McCaul ME, Weidle PJ, Hader S, Moore RD. Adherence, drug use, and treatment failure in a methadone-clinic-based program of directly administered antiretroviral therapy. AIDS Patient Care STDS. 2007;21:564–74. doi: 10.1089/apc.2006.0192. [DOI] [PubMed] [Google Scholar]
- Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008a;103:1242–57. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2008b;14:731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- McGahan PL, Griffith JA, Parente R, McLellan AT. Addiction severity index, composite score manual. The University of Pennsylvania/Veterans Administration Center for Studies of Addiction; Philadelphia: 1986. [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the addiction severity index reliability and validity in three centers . J Nerv Ment Dis. 1985;173:412–23. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Menendez-Arias L. Molecular basis of human immunodeficiency virus drug resistance: an update. Antiviral Res. 2010;85:210–31. doi: 10.1016/j.antiviral.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J Acquir Immune Defic Syndr. 2005;38:445–8. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- Northwest AIDS Education and Training Center. Taking Control: Adherence and HIV/AIDS Medication. Northwest AIDS Education and Training Center; Seattle, WA: 1998. [Google Scholar]
- Paredes R, Clotet B. Clinical management of HIV-1 resistance. Antiviral Res. 2010;85:245–65. doi: 10.1016/j.antiviral.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:443–50. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DW, Latka MH, Metsch LR, Latkin CA, Gomez CA, Mizuno Y, Arnsten JH, Wilkinson JD, Knight KR, Knowlton AR, Santibanez S, Tobin KE, Rose CD, Valverde EE, Gourevitch MN, Eldred L, Borkowf CB for the INSPIRE Study Team. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46:S35–47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- Rueda S, Park-Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, Glazier RH. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Dukes K, Tripps T, Sullivan L, Freedberg KA. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10:83–93. doi: 10.1177/135965350501000106. [DOI] [PubMed] [Google Scholar]
- Sampaio-Sa M, Page-Shafer K, Bangsberg DR, Evans J, Dourado Mde L, Teixeira C, Netto EM, Brites C. 100% adherence study: educational workshops vs. video sessions to improve adherence among ART-naive patients in Salvador, Brazil. AIDS Behav. 2008;12:S54–62. doi: 10.1007/s10461-008-9414-0. [DOI] [PubMed] [Google Scholar]
- Sandelowski M, Voils CI, Chang Y, Lee EJ. A systematic review comparing antiretroviral adherence descriptive and intervention studies conducted in the USA. AIDS Care. 2009;21:953–66. doi: 10.1080/09540120802626212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. J Community Health. 2004;29:117–27. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Bonora LI, Keskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC. Reliability and validity of the MINI international neuropsychiatric interview (M.I.N.I.): according to the SCID-P. Eur Psychiatry. 1997;12:232–241. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Simoni JM, Frick PA, Pantalone DW, Turner BJ. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med. 2003;11:185–98. [PubMed] [Google Scholar]
- Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43:S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A Technique for Assessing Self-reported Ethanol Consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Tate DF, Finkelstein EA, Khavjou O, Gustafson A. Cost effectiveness of internet interventions: review and recommendations. Ann Behav Med. 2009;48:40–45. doi: 10.1007/s12160-009-9131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Zaccarelli M, Bonfigli S, Lorenzini P, Liuzzi G, Trotta MP, Forbici F, Gori C, Bertoli A, Bellagamba R, Narciso P, Perno CF, Antinori A. Drug-class-wide resistance to antiretrovirals in HIV-infected patients failing therapy: prevalence, risk factors and virological outcome. Antivir Ther. 2006;11:553–60. [PubMed] [Google Scholar]
- University of Washington. High Impact: Substance Abuse and HIV Care. Cascade Health; Seattle, WA: 2000. [Google Scholar]
- Wang C, Vlahov D, Galai N, Bareta J, Strathdee SA, Nelson KE, Sterling TR. Mortality in HIV-seropositive versus -seronegative persons in the era of highly active antiretroviral therapy: implications for when to initiate therapy. J Infect Dis. 2004;190:1046–54. doi: 10.1086/422848. [DOI] [PubMed] [Google Scholar]
- Wong IY, Lawrence NV, Struthers H, McIntyre J, Friedland GH. Development and assessment of an innovative culturally sensitive educational videotape to improve adherence to highly active antiretroviral therapy in Soweto, South Africa. J Acquir Immune Defic Syndr. 2006;43:S142–8. doi: 10.1097/01.qai.0000248345.02760.03. [DOI] [PubMed] [Google Scholar]
- Zolopa AR. The evolution of HIV treatment guidelines: current state-of-the-art of ART. Antiviral Res. 2010;85:241–4. doi: 10.1016/j.antiviral.2009.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.