Abstract
Background
Guidelines recommend that sepsis be treated with an early resuscitation protocol, such as early goal directed therapy (EGDT). Our objective was to assess the cost-effectiveness of implementing EGDT as a routine protocol.
Design
Prospective before and after study.
Setting
Large urban hospital ED with >110,000 visits/year.
Patients
The target population was patients with consensus criteria for septic shock. We excluded those with age <18 yrs, no aggressive care desired, or need for immediate surgery.
Interventions
Clinical and cost data were prospectively collected on two groups: 1) patients from 1 yr before and 2) 2 yrs after implementing EGDT as standard-of-care. Before phase patients received nonprotocolized care at attending discretion. The primary outcomes were one year mortality, discounted life expectancy, and quality adjusted life years (QALYs). Using costs and QALYs, we constructed an incremental cost-effectiveness ratio and performed a net monetary benefit (NMB) analysis, producing the probability that the intervention was cost-effective given different values for the willingness to pay for a QALY.
Results
285 subjects, 79 in the before and 206 in the after phases, were enrolled. Treatment with EGDT was associated with an increased hospital cost of $7028 and an increase in both discounted sepsis-adjusted life expectancy and QALYs of 1.5 and 1.3 yrs, respectively. EGDT use was associated with a cost of $5397 per QALY gained and the NMB analysis indicates a 98% probability (p = .038) that EGDT is cost-effective at a willingness to pay of $50,000 per QALY.
Conclusion
Implementation of EGDT in the ED care of severe sepsis patients is cost effective.
Sepsis remains a common and deadly public health issue in modern medicine. Severe sepsis has been estimated to affect three quarters of a million patients in the United States (US) each year and carries a mortality rate of approximately 30% (1). The rate of severe sepsis hospitalizations in the US has doubled during the last decade (2). Although much of the care of severe sepsis occurs in the intensive care unit, the majority of hospital cases of sepsis originate in the emergency department (ED) with an average ED length of stay of almost 5 hrs (3)
In 2001, Rivers et al reported a striking improvement in mortality among patients with severe sepsis treated with an early structured resuscitation protocol, termed early goal-directed therapy (EGDT) (4). The aim of EGDT is to achieve the predefined physiologic goals through the implementation of various therapeutic interventions in a stepwise manner. In the randomized controlled trial performed by Rivers et al, the EGDT protocol was instituted early in the patient’s hospital course, specifically upon recognition of sepsis in the ED. The study found that patients who received the protocol had a 16% absolute reduction in in-hospital mortality as compared to those patients who received standard care. Since its original publication, several observational studies have supported the use of EGDT in the ED as a method of improving mortality in severe sepsis (5–7). Furthermore, the international surviving sepsis campaign guidelines recommend the use of EGDT in the earliest phases of the patient’s clinical course (8).
Unfortunately, the interventions comprising EGDT have documented barriers to adoption including inadequate staffing and equipment, and lack of education, training and procedural competency.(9,10) These barriers may also relate to costs, value and resource use and thus evaluating these aspects of EGDT are important before widespread implementation. From an intuitive standpoint, offering more aggressive, time intensive and resource intensive care would result in higher costs of delivery. However, given the findings of improvement in outcomes, examining both the costs and health consequences of EGDT (i.e., cost-effectiveness) is important and of high priority. Thus the aim or our study was to assess the cost-effectiveness of implementing EGDT as a routine protocol in the ED care of severe sepsis.
METHODS
Study Design and Setting
We performed an economic analysis using data collected prospectively for two groups of sepsis patients: 1) patients from 1 yr before and 2) 2 yrs after implementing EGDT as standard-of-care. The clinical study methods and effectiveness results have been previously published. (5,11) Patients were enrolled in the ED at Carolinas Medical Center, an urban teaching hospital with >100,000 ED patient visits per year. This study was approved and informed consent waived by the institutional review board and privacy board of Carolinas Healthcare System.
Selection of Subjects
Eligible subjects were identified by emergency physicians in the ED, and inclusion criteria were identical for both phases: 1) age > 17 yrs; 2) suspected or confirmed infection; 3) two or more systemic inflammatory response syndrome (SIRS) criteria (12): heart rate > 90 beats per minute, respiratory rate > 20 breaths per minute, temperature >38 or < 36°C, white blood cell count > 12,000 or < 4000 cells/mm3 or > 10% bands; 4) systolic blood pressure < 90 mm Hg or mean arterial pressure < 65 mm Hg after a 20 mL/kg isotonic fluid bolus OR anticipated need for ICU care and a serum lactate concentration ≥ 4.0 mM. Exclusion criteria were the need for immediate surgery or a contraindication to chest central venous catheterization.
Interventions
The study was comprised of two distinct periods. The before phase encompassed a 13 month period, from August 2004 to September 2005. During this time emergency physicians identified candidates with the inclusion and exclusion criteria and provided care at their discretion. In this phase no formal resuscitation protocol was utilized. The after phase encompassed two years, from November 2005 to October 2007. During this phase identification of an ED patient eligible for our EGDT protocol triggered an alphanumeric page to ancillary staff and the protocol quality assurance physician. In all cases, the ED physicians identified the patients and directed the EGDT protocol. At the time of patient transport to an intensive care unit (ICU) bed, clinical care was then transferred from the ED physicians to the admitting physicians.
Our full EGDT protocol has been described in detail elsewhere (5). In brief, our protocol was the similar to that reported by Rivers et al (4) In our resuscitation protocol we targeted three physiologic end points of resuscitation: central venous pressure (central venous pressure), mean arterial pressure (MAP) and central venous oxygen saturation (ScvO2). Our protocol differed from that of Rivers et al in that these patients were cared as a part of routine care in the ED without additional staff and we did not mandate the entire resuscitation occur in the ED. During the after phase, there were no other formal order sets or bundles of therapies that were instituted for the care of severe sepsis.
Data Collection and Variables
Baseline characteristics, demographics, co-morbid conditions, vital signs, site of infection, and laboratory data were collected in both phases. The sequential organ failure assessment (SOFA) score was calculated on all patients at the time of identification (13). We also recorded the number of days spent in both the ICU and hospital during the index hospitalization for all patients. Hospital costs for each patient were taken from the hospital’s cost accounting system(HBOC Star Navigator, McKesson, San Francisco, CA), where each patient’s stay is associated with a number of charge codes. The charge codes come from a master list of chargeable items in the hospital, with different charge codes for items in each department. The cost allocation to each charge code is based on multiple relative value units, which are typically labor minutes or supply costs for a specific item in a specific department. In addition, overhead is allocated to revenue producing departments within the hospital.
We performed follow-up on all patients at the time of hospital discharge and at 3, 6, and 9 months and 1 yr after hospital discharge. These follow-ups were established through a comprehensive methodology that we have previously described in depth (11). To calculate life expectancy for those who died in the hospital, we divided the length of stay in the hospital by 365. For those patients who lived to discharge but were found to be dead at one of the follow up intervals, we assigned the midpoint life expectancy between measurement points. Thus, for those who lived to discharge but died within in 3 months were assigned 0.123288 yrs (1.5 months*30.4 average days in a month/365 days in a year), those who lived to 3 months but died within 6 months were assigned 0.374795 yrs (or 4.5 months), those who lived to 6 months but died within 9 months were assigned 0.624658 yrs (or 7.5 months), and those who lived to 9 month but died within a 1 yr were assigned 0.874521 yrs (or 10.5 months). To calculate life expectancy beyond one year, we estimated age and gender specific expected life years using 2005 US life tables. Life expectancy values beyond one year were then decreased according the methodology of Quartin et al (14) by multiplying by 0.51 to account for increased relative risk of death among sepsis survivors, to give us a measure of sepsis-adjusted life expectancy for each individual.
Our construction of quality adjusted life years (QALYs) for each patient requires us to consider both sepsis-adjusted life expectancy (as described above) and the utility attached to each additional year (or partial year) of life. Previous investigators have documented that the overall quality of life is diminished after a hospitalization for severe sepsis (15). This requires one to adjusted down utility estimates postsepsis to properly characterize the quality of life for these “marginal survivors.” Therefore, we estimated QALYs by first assigning a utility value representing the quality of life in each year of life after sepsis, where 0 indicates death and 1 indicates perfect health. To generate the utility value for a particular year of life for an individual, we assigned each patient the average utility level of a person in the general population with the same sepsis-adjusted life expectancy (rather than the same age), same gender, same race, and same ethnicity using parameters from a model presented by Sullivan et al (16), where estimates are based on utilities derived from a nationally representative sample from the U.S. population from 2000–2002. In essence, our utility values are based on an artificially aged population patient population to account for the fact that sepsis survivors tend to be “marginal survivors” and are likely to have quality of life closer to that of someone from the general population with a similar life expectancy rather than someone from the general population with a similar age. After obtaining a utility value for each year of sepsis-adjusted expected life for each sepsis survivor, the utilities were summed to get a measure of QALYs for each individual. All QALYs and sepsis-adjusted life expectancy were discounted using a rate of 3%, as suggested by the Public Health Services Panel on Cost-Effectiveness in Health and Medicine (17).
All costs associated with in-hospital treatment were adjusted to 2006 U.S. dollar values using the US GDP Implicit Deflator. The cost associated with implementation of the protocol was estimated based on the recorded time spent by the physician director (30 hrs), nurse director (30 hrs), and staff training (1 hr for all ED nurses). This translates into an added cost of $43.69 per patient in the after phase.
Data Analysis
Continuous data are presented as means ± SD, and categorical data are reported as proportions rounded to the nearest whole number. Statistically significant differences between the before and after groups were considered using unpaired t tests or Mann-Whitney U tests for continuous variables and chi-square or Fisher’s exact tests for categorical data. For all statistical tests, p < .05 was considered significant.
We used two related approaches to consider the cost-effectiveness of the after phase arm as compared to the before phase arm: computation of incremental cost-effectiveness ratio and net monetary benefit analyses. The most common approach for determining cost-effectiveness involves the calculation of incremental cost-effectiveness ratios (ICERs) (18). The incremental cost-effectiveness ratio considers the added cost of EGDT relative to the added health effect. We calculated the incremental cost-effectiveness ratios by dividing the difference between the average costs of treating a patient in the after phase (C1) and the average cost in the before phase (C0) by the difference between average health outcomes (QALYs) gained in the after phase (O1) and those gained in the before phase (O0). Thus the incremental cost-effectiveness ratio is calculated by the following equation: (C1 - C0)/(O1 - O0). We also calculated incremental cost-effectiveness ratios using life years gained as the health outcome of interest.
One of the shortcomings of incremental cost-effectiveness ratios is that they provide no guidance regarding whether added costs are worth any gains obtained. Thus the medical system is left choosing some threshold (λ) below which a project is deemed cost-effective, where λ is the maximum acceptable willingness to pay for an outcome, such as one QALY gained. Using the notation above and QALYs as the outcome, a project is considered cost-effective if (C1 - C0)/(O1 - O0) < λ, i.e., if the added costs per QALY gained (the ICER) is below the maximum society is willing to pay to gain an additional year of life at full health. Additionally, incremental cost-effectiveness ratios based on sample mean values do not consider significant baseline differences between groups, leading to potentially confounded results (18). To address these shortcomings, we also performed a net monetary benefit (NMB) analysis for determining cost-effectiveness.(19,20) The NMB approach controls for baseline group differences using multivariate modeling and allows for the calculation of confidence intervals. To perform NMB analysis, net benefit at the individual level is computed for each participant, given that participant’s QALYs, costs, and a value for the maximum willingness to pay for an additional QALY by society (λ). More specifically, for individual i, NMBi = λOi - Ci, where λOi reflects the value of the QALYs gained by individual i and Ci reflects the costs of the intervention and directly related medical costs for individual i. NMB is modeled, using ordinary least squares, as a function of whether the individual received the intervention while controlling for baseline individual covariates. In this context, the coefficient on the binary indicator for whether the individual was in the after group that received EGDT provides an estimate of the average net monetary benefit of the intervention, while the p-value for the estimated coefficient allows one to judge whether the effect of the intervention is statistically significant and the standard error on the estimated coefficient can be used to construct a confidence interval around the coefficient estimate.
Rather than arbitrary selection of willingness-to-pay for a QALY (λ), it is recommended that a range of values for λ be investigated. This approach has the benefit of allowing for a graphical presentation of cost-effectiveness through cost-effectiveness acceptability curves, which display the probability that the intervention is cost-effective at different levels of willingness-to-pay for a QALY. In our analysis, we allowed λ to vary from $0 (unwilling to pay anything per QALY gained) to $300,000. Previous investigators have provided convincing evidence that the plausible range for the value of a QALY in the U.S. is $109,000 to $297,000 per QALY saved (21).
Sensitivity Analysis
We performed a sensitivity analysis to determine the impact of different assumptions on calculation of life expectancy and QALYs. In our primary analysis we multiplied life years by a factor of 0.51 to take into account the decreased life expectancy of sepsis survivors. For the sensitivity analysis we applied a worst case penalty of 0.39 as has been previously described (22). We also considered a best case penalty of .63. Likewise, in our primary analysis we estimated a utility weight based on sepsis-adjusted life expectancy, gender, race and ethnicity. Previous studies have reported estimates of utility values of survivors of acute respiratory distress syndrome or sepsis to be between 0.6 and 0.8 (22–24) with two studies using 0.68 and 0.69.(22,25) The average utility weight derived from these studies is 0.69, which is the value we used in our sensitivity analysis to calculate an alternative measure of QALYs. Finally, we varied the discount rate for QALYs from 0% (no discounting) to 6%.
RESULTS
We enrolled 285 patients, 79 subjects in the before phase and 206 in the after phase. Table 1 shows the demographics, co-morbidities, clinical variables, severity of illness score, and source of suspected infections between the groups. The groups were well matched for demographics and co-morbidities. There were significantly more subjects in the before phase with dialysis dependent renal failure compared with the after phase. Subjects in the after phase had variables suggesting a higher severity of illness with a lower initial systolic blood pressure, higher initial respiratory rate and higher initial SOFA score, as compared to before phase subjects. Table 2 shows the resuscitative interventions utilized in the initial 6 hrs of EGDT between the groups. Patients in the after phase were intubated more frequently, received a significantly larger crystalloid volume and more frequent infusion of vasopressors, as compared to the before phase. We observed no significant differences in the rate of packed red blood cell transfusion, dobutamine administration, or median time to antibiotic administration. Among subjects with positive blood cultures, there was no difference in the proportion of patients who received an initial dose of antibiotics that was not sensitive to the cultured organism in the before phase (3/37, 8%) compared with the after phase (4/68, 6%; proportion difference 2%, 95% CI −8 to 16%).
Table 1.
Patient demographics, clinical characteristics, and physiologic measurements.
| Variable | Before Phase n = 79 | After Phase n = 206 | P value |
|---|---|---|---|
| Age (mean years ± sd) | 58 ± 16 | 56 ± 18 | 0.58 |
| Race n, (%) | |||
| Caucasian | 40 (51) | 110 (54) | 0.68 |
| Black American | 38 (48) | 84 (41) | 0.27 |
| Gender n, (%) | |||
| Male | 47 (59) | 101 (49) | 0.12 |
| Female | 32 (41) | 105 (51) | 0.12 |
| Co-morbidities n, (%) | |||
| Diabetes Mellitus | 23 (29) | 53 (26) | 0.56 |
| COPD | 12 (15) | 41 (20) | 0.37 |
| HIV | 8 (10) | 24 (12) | 0.74 |
| DD-End stage renal disease | 25 (32) | 28 (14) | 0.0008 |
| Cancer | 9 (11) | 33 (16) | 0.33 |
| Organ transplant | 3 (4) | 4 (2) | 0.40 |
| Indwelling vascular line | 7 (9) | 27 (13) | 0.33 |
| Nursing home resident | 18 (23) | 39 (19) | 0.47 |
| Do not resuscitate | 5 (6) | 5 (2) | 0.14 |
| ED Vital Signs (mean ± sd) | |||
| Lowest SBP (median, (IQR) mm Hg) | 85 (73–91) | 72 (65–79) | <0.0001 |
| Highest Pulse (beats/min) | 118 ± 27 | 120 ± 25 | 0.50 |
| Highest RR (breaths/min) | 26 ± 9 | 30 ± 11 | 0.008 |
| Highest Temp(°F) | 101 ± 3 | 100 ± 3 | 0.04 |
| Lowest O2 Sat (%) | 94 ± 7 | 92 ± 7 | 0.35 |
| Lowest CVP (mm Hg) | - | 7 ± 4 | - |
| Highest CVP (mm Hg) | - | 13 ± 6 | - |
| Lowest ScVO2 (%) | - | 67 ± 13 | - |
| Highest ScVO2 (%) | - | 80 ± 11 | - |
| ED SOFA score (mean ± sd) | 5 ± 3 | 7 ± 4 | 0.0004 |
Some patients had more than 1 suspected source, thus the total is > 100%.
Abbreviations: COPD -- chronic obstructive pulmonary disease; HIV -- human immunodeficiency virus; DD -- dialysis dependent; ED -- emergency department; SBP --systolic blood pressure; RR -- respiratory rate; O2 -- oxygen; mm Hg -- millimeters of mercury; F -- Fahrenheit; CVP -- central venous pressure; ScvO2 -- central venous oxygen saturation; SOFA -- sequential organ failure assessment; sd --sd; IQR -- interquartile range.
Table 2.
Resuscitation interventions utilized in the initial 6 hrs
| Intervention | Before Phase n = 79 | After Phase n = 206 | P value* |
|---|---|---|---|
| Endotracheal intubation n, (%) | 7 (9) | 55 (27) | 0.0006 |
| Crystalloid volume (median, (IQR) liters) | 2.0 (1.0–3.4) | 5.0 (3.8–7.2) | <0.0001 |
| Vasopressor administration n, (%) | 27 (34) | 149 (72) | <0.0001 |
| Dobutamine administration n, (%) | 1 (1) | 9 (4) | 0.22 |
| PRBC transfusion n, (%) | 1 (1) | 13 (6) | 0.07 |
| Other | |||
| Time to initial antibiotics (median, (IQR) minutes) | 85 (50–190) | 90 (55–156) | 0.62 |
| Activated protein C n, (%) | 3 (4) | 5 (2) | 0.54 |
PRBC -- packed red blood cell; IQR -- interquartile range.
Table 3 shows the outcomes, costs, discounted sepsis-adjusted life expectancy and discounted quality adjusted life years of the two groups. Mortality at 1 yr was observed in 49% (39/79) of the before and 37% (77/206) of the after phase subjects (log-rank test p = .04). Patients in the after phase had higher ICU and hospital lengths of stay, resulting in total hospital costs that were on average $7028 higher than the before phase patients. Patients in the after phase had an increase in discounted sepsis-adjusted life expectancy of 1.5 yrs and an increase in discounted quality adjusted life years of 1.3 yrs. Calculated unadjusted cost-effectiveness ratios were favorable with an increased cost of $5397 per quality adjusted life year gained.
Table 3.
Outcomes, costs and predicted life years.
| Outcome | Before phase (n = 79) | After phase (n = 206) | Difference |
|---|---|---|---|
| In-hospital mortality, n (%) | 21 (27%) | 37 (18%) | 9% |
| 1 yr mortality, n (%) | 39 (49%) | 77 (37%) | 12% |
| In-hospital costs, adjusted to 2006 US dollars using the GDP Implicit Deflator, mean (sd) | $13,261 (13,893) | $20,289 (19,403) | $7028 |
| ICU length of stay, days, mean (sd) | 2.0 (2.67) | 4.1 (4.38) | 2 |
| Hospital length of stay, mean (sd), days | 8.0 (8.11) | 10.0 (9.41) | 2 |
| Discounted sepsis-adjusted life expectancy, years, mean (sd) | 5.7 (9.20) | 7.2 (9.32) | 1.5 |
| Discounted Quality adjusted life years, mean (sd) | 5.1 (5.98) | 6.4 (5.95) | 1.3 |
| Incremental cost-effectiveness | $4667/LY gained $5397/QALY gained |
||
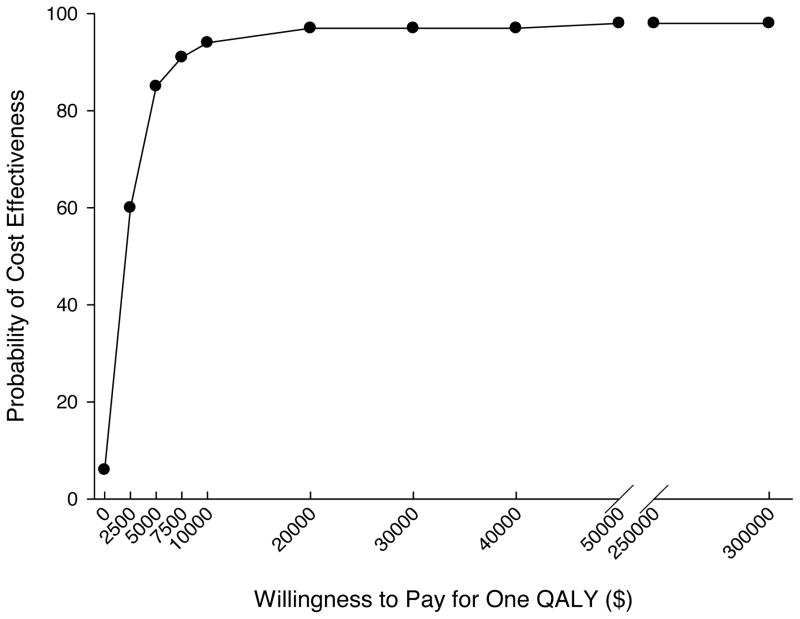
The results of the NMB analysis for determining cost-effectiveness, using different levels of willingness to pay for an additional QALY (λ), are shown in Table 4. Positive signs on the estimated net monetary benefit due to the intervention indicate positive net benefit, while negative signs indicate that costs outweigh benefits. If no value is placed on extending life (λ = $0), the costs of EGDT outweigh the benefits, when controlling for patient level covariates. At a willingness to pay for a QALY of $50,000, the NMB is $92,729 and statistically significant. The p-values from the coefficients were used construct a cost-effectiveness acceptability curve (Fig. 1) that indicates the probability that treatment in the after group is cost-effective relative to the before group at different levels of λ. At a willingness to pay of $7500 per QALY, when both intervention costs and medical costs directly related to the use of EGDT are considered, the probability that the after group was cost-effective was 91%, at a willingness to pay of $20,000 per QALY the probability of cost-effectiveness was 97% and at a willingness to pay of $50,000 per QALY the probability of cost-effectiveness was over 98%.
Table 4.
Net Monetary Benefit Estimates.
| Willingness to Pay for One QALY (λ) | Estimated Net Monetary Benefit | P-value |
|---|---|---|
| $0 | −$4,623 | 0.0869 |
| $2,500 | $194 | 0.9561 |
| $5,000 | $5,012 | 0.3373 |
| $10,000 | $14,647 | 0.1142 |
| $20,000 | $33,918 | 0.0578 |
| $30,000 | $53,188 | 0.0456 |
| $40,000 | $72,459 | 0.0405 |
| $50,000 | $92,729 | 0.0377 |
| $75,000 | $139,905 | 0.0344 |
| $100,000 | $188,082 | 0.0328 |
| $150,000 | $284,434 | 0.0313 |
| $200,000 | $380,787 | 0.0306 |
| $250,000 | $477,139 | 0.0301 |
| $300,000 | $573,491 | 0.0299 |
Figure 1.
Cost-effectiveness acceptability curve generated from the net monetary benefit analysis of the after phase vs. the before phase. At higher willingness to pay values for a quality adjusted life year, there is a higher probability that use of early goal directed therapy is cost-effective.
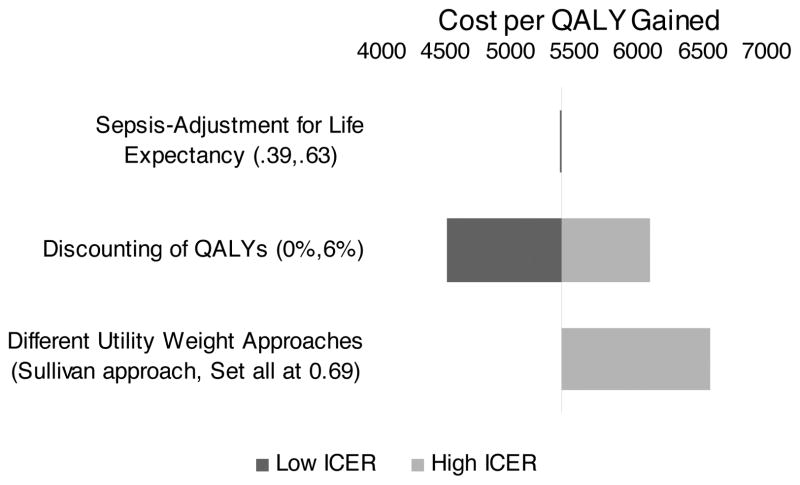
Figure 2 provides a tornado diagram that illustrates the effect of modifying three key assumptions regarding QALYs on the cost per QALY gained relative to the base case, where cost per QALY gained is $5397. First, the variation in the sepsis-adjustment to life expectancy from 0.39 to 0.63 yields very little change in the cost per QALY gained. Second, the variation in the discount rate resulted in a range of costs per QALY gained of $4508 to $6086. Calculating discounted QALYs using a utility weight of 0.69 instead of the Sullivan et al model also resulted in a decrease in QALYs in both groups (4.3 yrs in the before group and 5.4 yrs in the after group, difference 1.1 yrs) and increased the cost effectiveness ratio to $6556 per QALY gained.
Figure 2.
Tornado plot for the assumptions for life expectancy and QALYs that gives the range of the cost per QALY gained by EGDT when the assumptions are varied. Values to the left of the center line indicate costs per QALY gained lower than the baseline case, and values to the right of the center line indicate costs per QALY gained higher than the baseline case.
DISCUSSION
In this study we report that implementation of EGDT as a standard resuscitation protocol for the care of severe sepsis patients in the ED is cost effective. Although the cost for treating a patient with EGDT was $7028 higher than for treating patients before the use of EGDT, both the life expectancy and QALYs were increased in patients treated with EGDT. EGDT use was associated with a cost of $5397 per QALY gained, and in NMB modeling, a willingness to pay threshold of $50,000 per QALY resulted in an over 98% probability that EGDT is cost-effective. It is important to note that these findings use a conservative measure of QALYs that is constructed using sepsis-adjusted life expectancy, conservative utility weights, and discounting of future health outcomes. This conservative measure would tend to understate the benefits of EGDT.
A previous systematic review of the cost effectiveness of critical care treatments for severe sepsis reported cost-effectiveness ratios ranging from $7800 to $995,740 per QALY gained (26). The determination of the cost-effectiveness of an intervention requires a decision about society’s willingness to pay for prolonging and enhancing the quality of life. Although there is no absolute cutoff for cost effectiveness, most consider $50,000 per QALY gained to be good value for the money. In fact, the $50,000 per QALY decision rule, which came into use in 1982, has been used by many authors without adjusting for inflation (27). In 2006 dollars, this rule would suggest a willingness to pay for a QALY of over $100,000. In addition, recent investigators have provided support for a range of $109,000 to $297,000 for the value for a year of life at full health in the US (21). In this report, we found the estimated cost effectiveness ratio for implementing EGDT in the ED to be only $5397 per QALY gained before adjusting for baseline differences. After adjusting for baseline differences between the groups using NMB analysis, using a willingness to pay of $50,000 per QALY, we find that EGDT had an over 98% probability of being cost effective. These results suggest that, when compared to convention, implementation of EGDT for the care of severe sepsis patients in the ED is highly cost effective.
Despite calls for cost-effectiveness evaluations of EGDT (26), to date there is little literature describing the economic impact of implementing EGDT in the ED. Only two previous full length manuscripts have reported economic analyses of early sepsis care. First, in a theoretical decision analysis, Huang et al (28) retrospectively analyzed economic data from the original EGDT trial (4) and concluded that despite important startup and delivery costs, EGDT may be cost saving to hospitals and cost effective to society in the long run. A drawback to this report was that it was a simulation analysis of an “average” emergency department and thus is prone to the many limitations and assumptions regarding patient volume and care delivery associated with EGDT. Additionally, these authors used economic data from the original EGDT clinical trial and thus analyzed a very homogeneous population that was likely not representative of severe sepsis populations encountered in most hospitals. In a second paper, Talmor et al (25) examined the cost effectiveness of treating patients with a comprehensive sepsis protocol, which included not only EGDT but other aggressive treatments such as intensive insulin for glucose control, low tidal volume ventilation, and activated protein C administration. In 130 subjects (cases and controls) they found this comprehensive protocol to be cost-effective. Our study differs from this study in several important ways. First, Talmor et al used a retrospective control group potentially introducing selection bias into their study. Second, Talmor et al did not use methods to control for baseline difference between their groups, leading to potential underestimation of the cost-effectiveness of the intervention. And finally, the Talmor et al study included a protocol with many more therapies than just EGDT and thus there is no way of knowing if individual components of this protocol are cost effective when implemented in isolation. Two additional reports provided economic data on the implementation of EGDT with one reporting that protocol implementation was cost saving (29) and the other reporting lower hospital charges associated with EGDT use (6). Neither of these latter two reports performed formal cost effectiveness analyses.
From a resource utilization standpoint, we found a 2 day increase in both the ICU and hospital lengths of stay in our after phase patients as compared to the before phase. Although we did not compare costs according to hospital service, it is likely that the majority of the $7028 increase costs observed in the after group were the result of prolongation of both intensive care services and hospital stay. Our explanatory hypotheses regarding these observations are that for patients who derived the most benefit from the EGDT protocol (i.e., EGDT was life saving) were more likely to have prolonged ICU and hospital stays compared to those who would have died before the use of EGDT and in the patients who received the protocol but eventually died in the hospital (i.e., the protocol prolonged but did not save life) also had longer ICU and hospital stays compared to patients who would have had a more rapid hospital death. Unfortunately, we were unable to obtain data on costs for patients after hospitalization, and we are unable to determine whether patients in the EGDT group would have had higher or lower posthospitalization costs due to potential differences for the two groups in discharge location and follow-up care. However, given the very low incremental cost effectiveness ratio from our analysis, these cost differences would have to be quite large to change the ultimate finding that EGDT is cost-effective.
There are several unique features about our report that highlight its strengths. First, we present data on hospital costs rather than hospital charges. Often researchers report economic analyses on charges, which tend to overestimate the true cost of care. Second, our EGDT protocol was implementing in a large, urban ED as a standard-of-care rather than under the auspices of a research protocol. Thus our results likely have more external validity given our implementation of EGDT in a nonresearch, real world setting. Third, we followed patients after discharge for one year, to determine actual life expectancy during the first year. Fourth, we used two methods of determining cost-effectiveness, computation of incremental cost-effectiveness ratios and NMB analyses. Utilizing the NMB analysis allows for controlling for group differences, which was important in our study as our after phase group had a higher severity of illness and a lower proportion of important co-morbid diseases (i.e., dialysis dependent end stage renal disease). Not controlling for such factors might lead to the conclusion that EGDT is less cost-effective than it really is, given that it was applied to a potentially different risk profile group. Finally, we followed published recommendations for reporting of cost effectiveness analyses (30).
This report has several limitations that warrant discussion. First, this is a single center study that was not conducted as a tightly controlled experimental investigation. As such our results may not be generalizable to other populations. Second, since our cohorts are not contemporaneous but actually divided along a time continuum, it is important to note that some of the clinical impact may be due to change in technology, skill or other factors during the study period. Third, while we had methods in place to attempt to enroll consecutive eligible patients, it is possible that we missed cases that qualified for the study. Forth, our study examined the cost-effectiveness of an invasive early sepsis resuscitation protocol. As new scientific discoveries in resuscitation science are published, such as the use of lactate clearance to guide resuscitative interventions (31), the present findings may not be necessarily be extrapolated to such changes and thus future studies will need to reexamine cost-effectiveness. Fifth, the costs we examined were generated by our hospital using a specific methodology. As such, these costs may not be generalizable to other hospital systems. Sixth, it might be argued that our design may have been influenced by a “Hawthorne-like” effect, caused by heightened awareness of the clinical staff about the protocol resulting in both earlier and more aggressive response to physiologic abnormalities. Finally we did not quantify, explore, or exclude deviations from the EGDT protocol, as this protocol was implemented into a real world clinical setting.
In conclusion, we found that implementation of EGDT in the ED care of severe sepsis patients is cost- effective. Use of EGDT was associated with an increase of 1.3 QALYs and at a willingness to pay threshold of $50,000 per QALY the probability of EGDT being cost-effectiveness was 98%.
Acknowledgments
Dr. Jones received funding from NIH and received a grant from Hutchinson Technology. Dr. Kline received funding from NIH. Dr. Troyer has not disclosed any potential conflicts of interest.
This project was supported by a grant from the National Institutes of Health/National Institute of General Medical Sciences to Dr. Jones (GM76652).
Footnotes
Authors Contributions: All authors had contributed to study design, obtaining the data, data analysis and interpretation and drafting and revising the manuscript. Dr Jones had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Angus D, Linde-Zwirble W, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–36. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Jones AE, Focht A, Horton JM, et al. Prospective external validation of the clinical effectiveness of an emergency department-based early goal directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–32. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trzeciak S, Dellinger RP, Abata NL, et al. Translating research to clinical practice: A 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129:225–32. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34:1025–32. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 9.Jones AE, Kline JA. Use of goal-directed therapy for severe sepsis and septic shock in academic emergency departments. Crit Care Med. 2005;33:1888–89. doi: 10.1097/01.ccm.0000166872.78449.b1. [DOI] [PubMed] [Google Scholar]
- 10.Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department-Results of a national survey. Crit Care Med. 2007;35:2525–35. doi: 10.1097/01.ccm.0000298122.49245.d7. [DOI] [PubMed] [Google Scholar]
- 11.Puskarich MA, Marchick MR, Kline JA, et al. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: A before and after study. Crit Care. 2009;13:R167. doi: 10.1186/cc8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bone R, Balk R, Cerra F, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 14.Quartin AA, Schein RM, Kett DH, et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–63. [PubMed] [Google Scholar]
- 15.Lazosky A, Young GB, Zirul S, Phillips R. Quality of life after septic illness. J Crit Care. 2009;25:406–12. doi: 10.1016/j.jcrc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43:736–49. doi: 10.1097/01.mlr.0000172050.67085.4f. [DOI] [PubMed] [Google Scholar]
- 17.Gold M, Siegel J, Russell L, et al. Cost-effectiveness in health and medicine. 1. New York: Oxford University Press, USA; 1996. [Google Scholar]
- 18.Drummond MF, O’Brien B, et al. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2000. [Google Scholar]
- 19.Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue. A framework for the marriage of health econometrics and cost-effectiveness analysis. Health Economics. 2002;11:415–30. doi: 10.1002/hec.678. [DOI] [PubMed] [Google Scholar]
- 20.Stinnett AA, Mullahy J. Net health benefits. A new framework for the analysis of uncertainty in cost-effectiveness analysis. Medical Decision Making. 1998;18:S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 22.Angus DC, Musthafa AA, Clermont G, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care. 2001;163:1389–94. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 23.Manns BJ, Lee H, Doig CJ, et al. An economic evaluation of activated protein C treatment for severe sepsis. N Engl J Med. 2002;347:993–100. doi: 10.1056/NEJMsa020969. [DOI] [PubMed] [Google Scholar]
- 24.Fowler RA, Hill-Popper M, Stasinos J, et al. Cost-effectiveness of recombinant human activated protein C and the influence of severity of illness in the treatment of patients with severe sepsis. J Crit Care. 2003;18:181–94. doi: 10.1016/j.jcrc.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Talmor D, Greenberg D, Howell MD, et al. The costs and cost-effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36:1168–74. doi: 10.1097/CCM.0b013e318168f649. [DOI] [PubMed] [Google Scholar]
- 26.Talmor D, Shapiro N, Greenberg D, et al. When is critical care medicine cost-effective? A systematic review of the cost-effectiveness literature. Crit Care Med. 2006;34:2738–47. doi: 10.1097/01.CCM.0000241159.18620.AB. [DOI] [PubMed] [Google Scholar]
- 27.Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 28.Huang DT, Clermont G, Dremsizov TT, et al. Implementation of early goal-directed therapy for severe sepsis and septic shock: A decision analysis. Crit Care Med. 2007;35:1–12. doi: 10.1097/01.ccm.0000281636.82971.92. [DOI] [PubMed] [Google Scholar]
- 29.Shorr AF, Micek ST, Jackson WL, Jr, et al. Economic implications of an evidence-based sepsis protocol: Can we improve outcomes and lower costs? Crit Care Med. 2007;35:1257–62. doi: 10.1097/01.CCM.0000261886.65063.CC. [DOI] [PubMed] [Google Scholar]
- 30.Siegel JE, Weinstein MC, Russell LB, et al. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 31.Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA. 2010;303:739–46. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]