Abstract
Objective
The purpose of this phase I open label non-randomized trial was to assess the safety and efficacy of autologous bone marrow mononuclear cell (ABMNC) therapy in promoting amputation free survival (AFS) in patients with critical limb ischemia (CLI).
Methods
Between September 2005 and March 2009 twenty-nine patients (30 limbs), with a median age of 66 (range 23–84) (14 male,15 female) with CLI were enrolled . Twentyone limbs presented with rest pain (RP), six with RP and ulceration, and three with ulcer only. All patients were not candidates for surgical bypass due to absence of a patent artery below the knee and/or endovascular approaches to improving perfusion was not possible as determined by an independent vascular surgeon. Patients were treated with an average dose of 1.7 ± 0.7 × 109 ABMNC injected intramuscularly in the index limb distal to the anterior tibial tuberosity. The primary safety endpoint was accumulation of serious adverse events and the primary efficacy endpoint was AFS at one year. Secondary endpoints at 12 weeks post-treatment were changes in first toe pressure (FTP), toe-brachial index (TBI), ankle-brachial index (ABI), and transcutaneous oxygen measurements (TcPO2). Perfusion of the index limb was measured with PET-CT with intra-arterial infusion of H2O15. Rest pain (RP), using a 10-cm visual analog scale, quality of life using the VascuQuol questionnaire, and ulcer healing were assessed at each follow-up interval. Subpopulations of endothelial progenitor cells were quantified prior to ABMNC administration using immunocytochemistry and fluorescent activated cell sorting.
Results
There were two serious adverse events however there no procedure related deaths. Amputation-free survival at one-year was 86.3%. There was a significant increase in FTP (10.2+ 6.2 mmHg, P=.02) and TBI (0.10± 0.05, P=.02) and a trend in improvement in ABI (0.08±0.04, P=.73). Perfusion Index by PET-CT H2O15 increased by 19.3 ± 3.1 and RP decreased significantly by 2.2 ± 0.6 cm. (P=.02). The VascuQol questionnaire demonstrated significant improvement in QOL and three of 9 ulcers (33%) healed completely. KDR+ but not CD34+ or CD133+ subpopulations of ABMNC were associated with improvement in limb perfusion.
Conclusion
This phase I study has demonstrated safety and the AFS rates suggest efficacy of ABMNC in promoting limb salvage in “no option” CLI. Based on these results we plan to test the concept that ABMNCs improve AFS at one year in a phase III randomized, double-blinded multicenter trial.
Critical Limb Ischemia (CLI) is defined as insufficient arterial blood flow to maintain tissue viability of the lower extremities due to advanced peripheral arterial disease (PAD)1,2. The clinical manifestations consist of rest pain and/or tissue necrosis with ulceration and gangrene, all of which herald eventual limb loss. The incidence of CLI in Western societies is approximately 220 new cases per million per year , and with an aging population, persistent rates of tobacco abuse, and an increase in diabetes a steady growth of the population at risk is expected2. Revascularization of the ischemic leg with surgical bypass has provided a combination of low mortality and exceptional amputation-free survival in the treatment of CLI3,4, and more recently endovascular approaches have demonstrated equivalent limb salvage efficacy in this challenging patient population5–9. Despite these advances in surgical and endovascular techniques approximately 20–40% of patients with CLI will not be candidates for either of these approaches due to a lack of autogenous vein or extensive atherosclerotic disease in the tibial and peroneal arteries10–12. For these patients the only remaining alternative for relief of rest pain and infection is amputation of the affected extremity. It is estimated that between 120 to 500 amputations are performed each year per million population and one fourth of all patients who undergo below knee amputation will fail rehabilitation and require chronic institutional care or professional assistance at home2,13. Consequently the median cost of managing a patient after amputation is estimated to be almost twice that of successful limb salvage14. Beyond the physical disability and financial cost, there is a significant emotional toll as well; psychological testing of patients with CLI shows quality of life indices similar to patients with terminal malignancy15,16. Thus there is a critical need to develop novel strategies to promote limb salvage in patients with CLI with no options for conventional revascularization.
Cell mediated angiogenesis was born a decade ago from the observation that CD34+ cells isolated from peripheral blood had the capacity to differentiate into fully differentiated and functional endothelial cells in culture17. These putative stem cells, termed endothelial progenitor cells (EPC), can also be isolated from bone marrow (BM) and express the surface markers CD34, CD133, and kinase insert domain receptor (KDR)17–19. Most importantly BM derived EPCs have been shown to participate in blood vessel development in preclinical models of hindlimb ischemia, providing evidence that EPCs may possess therapeutic utility in treating ischemic tissue19–21. The Therapeutic Angiogenesis using Cell Transplantation (TACT) study demonstrated that intramuscular (IM) injection of the mononuclear fraction of autologous BM (ABMNCs) into critically ischemic legs increased perfusion with significant improvement in ankle-brachial indices, transcutaneous oxygen pressures , and rest pain22. Subsequent to this vanguard study there have been multiple phase I clinical reports on the efficacy of ABMNCs in improving limb salvage in patients with CLI23–27, however currently there is no pivotal proof of this concept. The primary objective of this phase I/II clinical trial was to assess the safety and efficacy of ABMNC transplantation in promoting amputation free survival (AFS) in patients with CLI. We further provide a novel measure of cell mediated changes in perfusión and an analysis of the impact of ABMNC subpopulations on clinical outcome.
Methods
A detailed description is available in the supplemental Methods section.
Patient Selection
Based on the adverse events reported in the TACT trial22 and communication with the authors we determined that a sample size of twenty patients completing the one year follow-up interval (anticipating a 10–15% drop out rate) would provide sufficient data to assess safety of ABMNC treatment. Recruits were between the ages of 21 and 85 with CLI defined as symptoms of rest pain and/or ulceration with an ankle brachial index (ABI) ≤ 0.55 and/or toe brachial index (TBI) ≤ 0.40 (Table I). Enrolled patients had “no options” for reperfusion of the index limb defined as absence of a patent artery below the knee that was continuous to the foot and thus excluding bypass and/or endovascular approaches to improving perfusion were not possible as determined by an independent vascular surgeon. All patients had to have been offered amputation as an eventual treatment option pior to enrollment. The TBI was used to include the subset of CLI patients with lower extremity vasculitic syndromes that have profound ischemia of the foot despite patency of the tibial arteries proximal to the malleoli. Patients were instructed to continue current medical regimens and activity levels (including exercise regimens) throughout the course of the study. Any changes in medications and activity levels were noted. Patients with wounds were instructed to continue with the regimen used at the time of enrollment or therapies considered the standard of care for ischemic ulceration. The trial protocol and informed consents were approved by the Institutional Review Boards of the institutions and all patients gave written informed consent to participate in the study.
Table I.
Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 21–85 years of age | History or evidence of malignancyc |
| Rest pain and /or ulcerationa | HbA1C > 8.5% |
| ABI ≤ 0.55, TBI ≤ 0.40 | Infected wound on the index limbd |
| Unreconstructable arterial diseaseb | Renal insufficiency or failuree |
| Non-pregnant women | Left ventricular failuref |
| Competent to give consent | Proliferative retinopathy on retinal exam |
| Myeloproliferative disorder | |
For those patients with equivalent arterial insufficiency in both legs, the most symptomatic with regards to rest pain and tissue loss was designated as the index limb.
Enrolled patients were not candidates for surgical bypass or endovascular revascularization as determined by an independent vascular surgeon.
History other than squamous or basal cell carcinoma of the skin. Evidence based on screening studies established by the American Cancer Society (chest x-ray, mammography, Papanicolaou smear, prostate specific antigen).
Evidence of fever, purulence and/or cellulitis of the index limb with an elevated white blood count.
Insufficiency defined as a creatinine ≥ 2.0 , failure as requiring hemodialysis.
Ejection fraction on echocardiography ≤ 30%
Study Design
This was an open-label, single dose prospective study that consisted of two sequential parts: (1) injection of ABMNCs isolated using conventional Ficoll density centrifugation (FC) (14 patients) and (2) injection of ABMNCs using the MarrowStim™ (MS) closed centrifugation system (Biomet Biologics, Warsaw, IN) (15 patients, 16 limbs).
Safety Analysis
Medical records, concomitant medication use, electrocardiogram (EKG) results, blood chemistry and hematology results, vital signs, and physical examination findings were tabulated and reviewed for evidence of adverse and serious adverse events at each follow up visit at post-procedure day 1, weeks 4, 8, and 12, and at 6 and 12 months. The safety of ABMNC administration was determined by estimating the risks of the procedure for major complications including death by review of serious adverse events (SAE) accumulated during the study.
Amputation Free Survival
The primary efficacy outcome was time to above-ankle amputation of the index leg or death from any cause, whichever occurred first. Viability of the index limb was determined at each follow-up visit at weeks 4, 8, 12 and at 6 and 12 months. All cause mortality was accrued through hospital records and communication with referring health care providers.
Measures of Perfusion
First toe pressure (FTP), TBI, ABI, and transcutaneous oxygen pressures (TcPO2) were measured bilaterally at baseline and at weeks 4, 8, and 12. Patients were evaluated for response to therapy by the parameter (ABI or TBI) by which they were enrolled. For patients that met both ABI and TBI inclusion criteria, response to therapy was measured by ABI. Contrast arteriography (CA) and magnetic resonance arteriography (MRA) were performed at baseline and at 12 weeks in four patients to score neovascularization in the index limb. Both of these procedures were abandoned due to a lack of interpretable data and associated risks. An experimental Positron Emission Tomography (PET) based protocol with computed tomography (CT) was developed to directly quantify perfusion of the limb using radiolabeled water (H2O15)28. PET-CT perfusion images were generated by intra-arterial injection of H2O15 and then quantifying the peak tracer uptake level of H2O15 in both lower extremities for a period of 120 seconds. A Blood Perfusion Index (BPI) was calculated as the ratio of H2O15 peak tracer uptake level of the untreated:treated leg. The BPI was measured at baseline and at 12 weeks in four patients.
Additional Efficacy Assessments
Rest pain (RP) was measured with a 10-cm visual analog scale (VAS) at baseline and each follow-up visit. Patients were instructed to place a mark on a line from 0 (no pain) to 10-cm (worst pain previously experienced) that would best describe the level of pain in the index limb during the previous twenty four hours prior to examination. The distance from the 0- cm mark was then measured and recorded. The Vascular Quality of Life Questionnaire (VascuQol) was used to assess quality of life at baseline, and each follow up visit. The questionnaire focuses on the domains of pain, symptoms, emotional well-being, social function, and activity. The responses were then scored 1 to 7 (all the item scores divided by 25) for each evaluation. Ulcer status was assessed at each follow up interval and compared to baseline. Complete healing of the index ulcer with re-epithelialization was considered a successful response to therapy.
Flow Cytometric Characterization of Endothelial Progenitor Cell Subpopulations
Fluorescent activated cell sorting (FACS) analysis was used to quantify subpopulations of EPCs in the mononuclear fraction of the BM aspirate of eight patients. Antibodies for CD34 (a hematopoietic progenitor marker), CD133 (a nonspecific stem cell marker), and KDR (an endothelial and monocyte surface marker) were used with techniques described in the supplemental methods section18.
Statistical Analysis
The primary efficacy outcome of AFS at one year was assessed using the product-limit estimate. The four measures of perfusion (FTP, TBI, ABI, and TcPO2) were analyzed by a repeated measures model that accounts for possible heterogeneous variances among observations obtained on the same patient. Correlations between measures of perfusion and cell counts were analyzed by the Pearson product moment correlation coefficient. Differences in EPC subpopulations between groups and changes in the 10-cm VAS and in the domains of the VascuQol were evaluated using the paired t-test.
Results
Patient Baseline Characteristics
Between September 2005 and March 2009 we enrolled and treated 29 patients (30 limbs, 14 men/15 women) with CLI with a median age of 66 (range, 23–84) (Table II). Twenty-two patients (76%) had a previous surgical bypass or endovascular procedure in the index limb. The mean baseline TBI was 0.15 ± 0.037 for those limbs enrolled on TBI criteria (n=14) and mean baseline ABI was 0.32 ± 0.042 for those limbs enrolled on ABI criteria (n=16). Seven patients (24%) did not complete the one year assessment of limb viability due to amputation (2), dropped out of the study for alternative therapies (3), or death (2).
Table II.
Characteristics of the Study Population at Baseline
| Variable | n (29) | % |
|---|---|---|
| Age, mean ± SE (range) | 61.3± 12.6 (23–84) | |
| Gender | ||
| Male | 14 | 48 |
| Female | 15 | 52 |
| Body Mass Indexa (mean ± SE) | 25±4 | |
| Diabetes | 6 | 21 |
| HbA1C (mean, range) | 6.6 (5.8–7.6) | |
| Tobacco use | ||
| Active | 2 | 7 |
| Former | 17 | 59 |
| Hypertension | 15 | 52 |
| Hyperlipidemia | 18 | 62 |
| Hypercoagulable disorder | 1 | 3 |
| Coronary artery disease | 11 | 38 |
| Cardiac arrhythmia | 5 | 17 |
| COPD | 5 | 17 |
| Renal Insufficiencyb | 3 | 10 |
| Buerger’s disease | 6 | 21 |
| Autoimmune disorder | 7 | 24 |
| Previous bypass | 16 | 55 |
| Previous PTA/stent | 6 | 21 |
| Ulceration | 9 | 31 |
| Medications | ||
| ACE inhibitor | 11 | 38 |
| Statin | 17 | 59 |
| ASA | 17 | |
| Clopidogrel | 15 | 52 |
| Warfarin | 10 | 35 |
| Calcium channel blocker | 8 | 26 |
| Insulin | 3 | 10 |
| Oral AHG agent | 3 | 10 |
Abbreviations: SE, standard error of the mean, COPD, chronic obstructive pulmonary disease, Cr, creatinine, PTA, percutaneous transluminal angioplasty, ACE, angiotensin converting enzyme, ASA, acetylsalicylicacid, AHG, antihypergylycemic, ABI, ankle brachial index, TBI, toe-brachial index
Body mass index calculated as weight in kilograms divided by height in meters squared.
Renal insufficiency defined as (Cr ≥1.5 mg./dL)
ABMNC Preparation and Injection
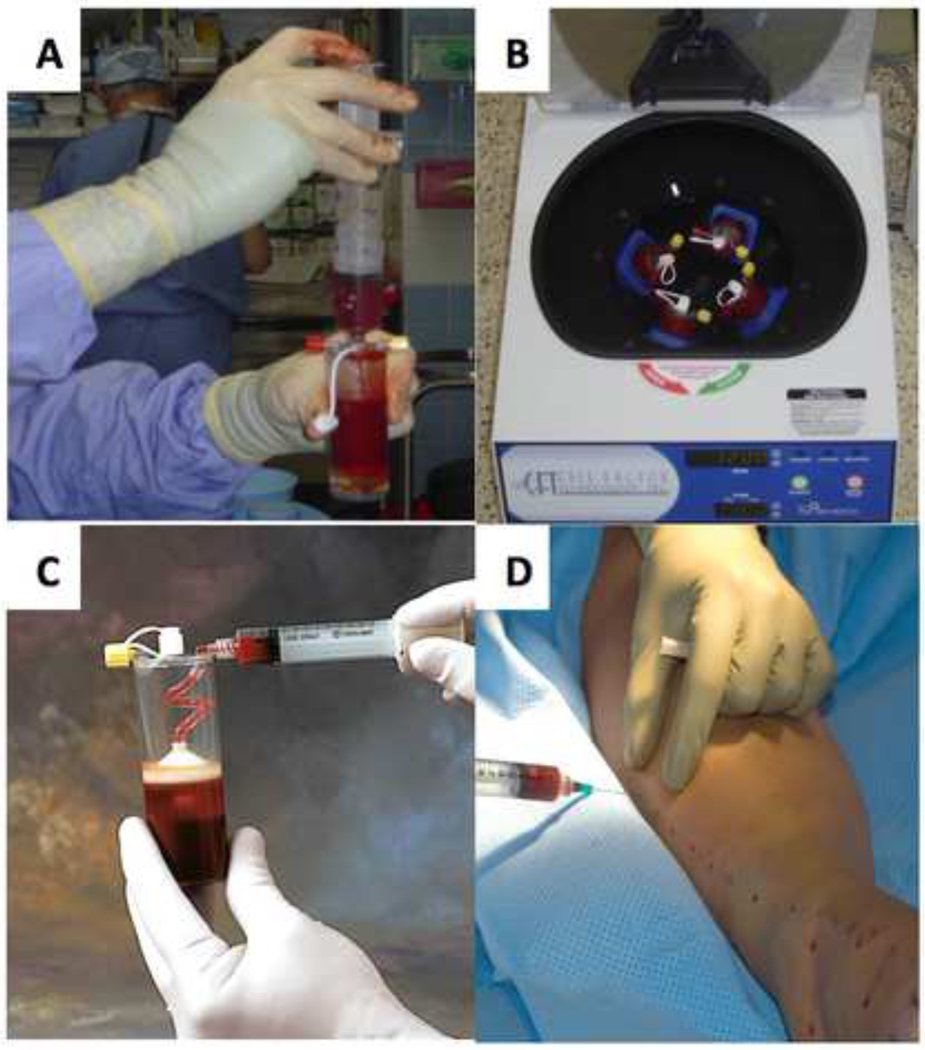
The average number of ABMNCs obtained after FC isolation was 1.3± 0.7 × 109 and for the MS isolation was 2.0 ± 1.6 × 109, respectively. Cell viability was greater than 97% for both FC and MS. The ABMNC suspension was concentrated to a volume of 30-mL and 0.75-mL aliqouts were delivered via 40 IM injections (mean ±SE=37 ± 6) at 2-cm intervals, 1.5-cm deep into the gastrocnemius muscle, along the medial and lateral aspect of the index limb. In those patients with distal occlusive disease extending below the malleoli (based on imaging studies prior to enrollment) injections were extended onto the dorsal and medial foot, in the distribution of the dorsalis pedal and posterior tibial arteries and terminal arcades (Fig.1A–D).
Figure 1.
Bone marrow aspirate is loaded into a closed sterile 60-mL MarrowStim™ centrifugation tube in the operating room (A) and placed into the centrifuge device where the buffy coat is separated at 1500 rpm for 15 min (B). The ABMNC rich buffy coat is aspirated from the tube (C) and and 0.75 ml. aliquots are delivered via IM injections at 2-cm intervals, 1.5-cm deep into the gastrocnemius muscle, along the medial and lateral aspect of the index limb (D).
Safety Analysis
There were two procedure-related SAEs. A 71 year-old patient with CAD experienced angina with ST segment depression on an electrocardiogram (EKG) on post-procedure day 1. The patient’s hemoglobin had dropped from 11.0-gm/dL pre- to 8.8-gm/dL post-BM aspiration. The patient was transfused two units of packed red blood cells and the anginal symptoms and EKG changes resolved without elevation of cardiac enzymes. The protocol was subsequently modified to decrease the volume of BM aspirated from 500 to 360-mL to reduce precipitous shifts in hemoglobin levels in this high cardiac risk population. A procedure-related SAE occurred in one patient during CA of the index limb with entrapment of a guidewire in the wall of the common femoral artery requiring open exploration and extraction. There were no wound complications at the BM aspiration site or ABMNC injection sites in the index limb. Additionally there were no adverse events associated with administration of general anesthesia. There were no serum chemistry or hematologic abnormalities other than anemia. There were two non-treatment related deaths in the one year follow-up interval, at 8 weeks and at 8 months.
Amputation Free Survival
AFS at one year was 86.3%. There were three amputations during the one-year follow up period: day 2, week 5, and week 25 post-treatment. A below knee amputation (BKA) was performed for severe ischemic rest pain in a 25 year old-male who had a guillotine transmetatarsal amputation for gangrene secondary to Buerger’s disease and IM ABMNC two days later. A BKA was performed 2 days post-ABMNC treatment for intractable rest pain. A BKA was performed at 5 weeks after ABMNC injection in a 60 year-old diabetic male who was found to have osteomyelitis in the first metatarsal bone of the index limb that was not discovered on plain x-rays at the time of screening. An above-knee amputation (AKA) was performed in an 80 year-old woman with aortoiliac, femoral, and tibial artery occlusive disease. The baseline ABI of the index limb was 0.38 and decreased to 0.22 by week 12. There were two deaths during the study , at 7 and 26 weeks post-treatment. The first death occurred in a 55 year old male with ulcerative colitis, rheumatoid arthritis, and insulin dependent diabetes mellitus with severe bilateral lower extremity ischemia with gangrene (FTP recordings were absent in both feet). Despondent over his prognosis the patient committed suicide at 7 weeks post-treatment. The second death occurred at 26 weeks post-treatment from complications ten days after an AKA for gangrene of the index limb, as previously described.
Changes in Measures of Perfusion
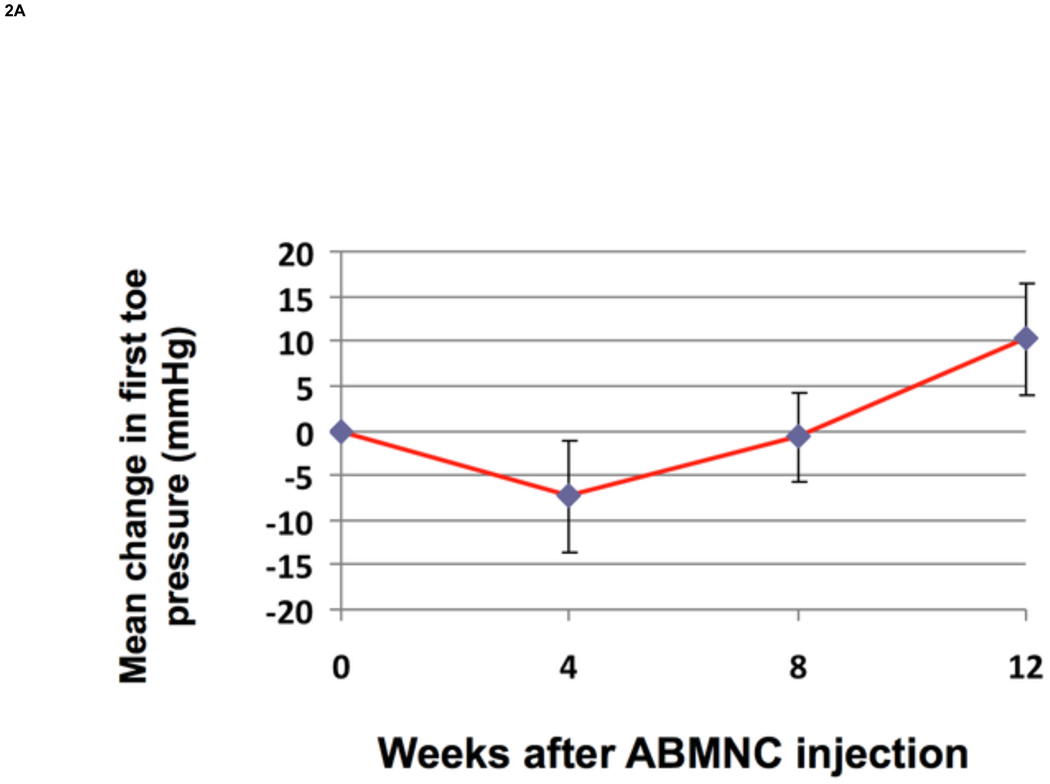
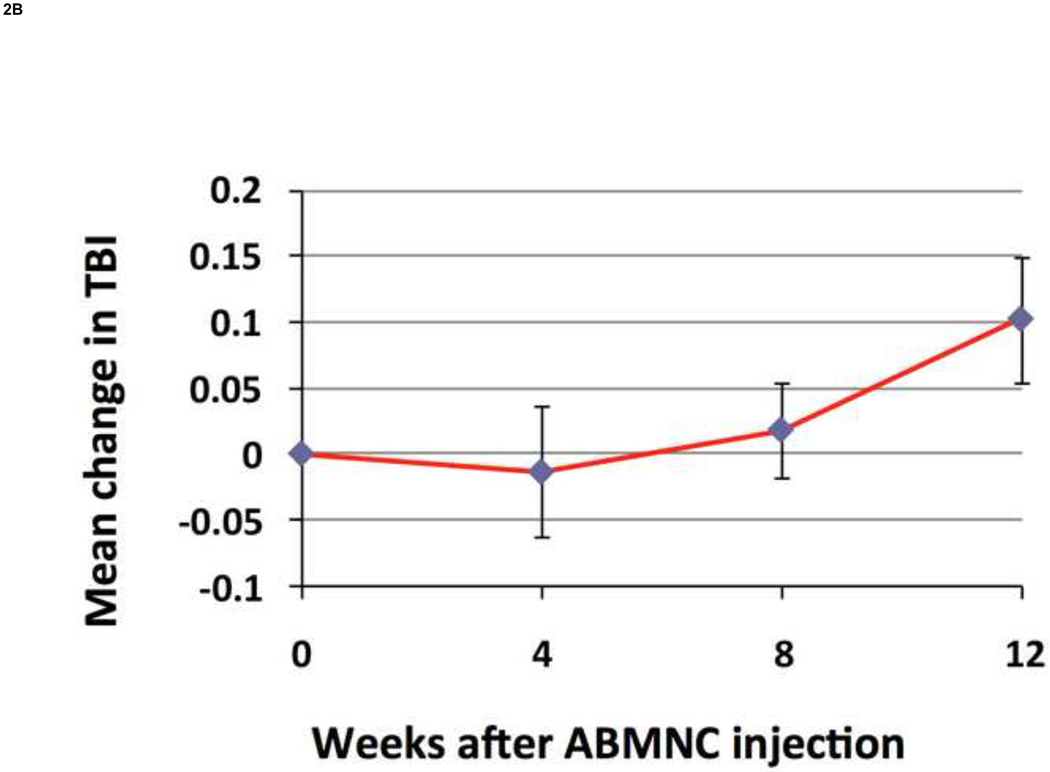
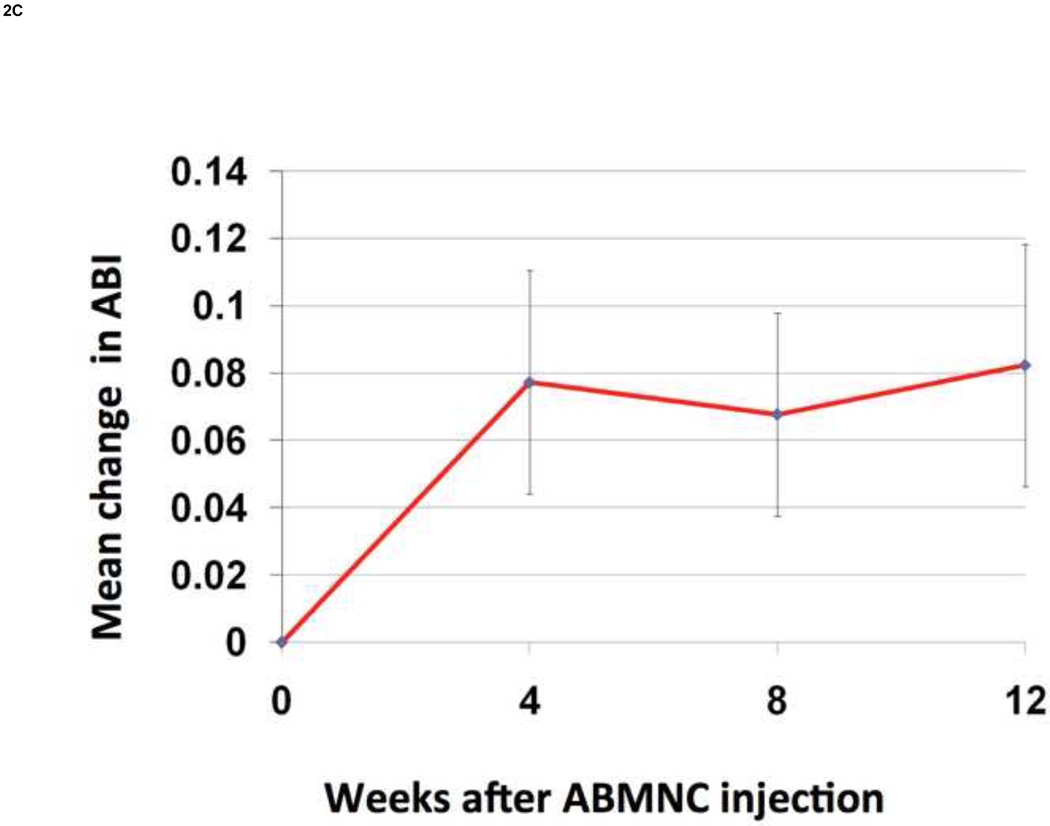
There was a significant increase from baseline in FTP by 10.2± 6.2 mmHg (P=.02) (Fig.2A) and in TBI by 0.10 + 0.05 (P=.02) (Fig. 2B) at week 12. There was a trend in improvement in ABI at week 12 by 0.08 + 0.04 (P=.73) (Fig.2C). Applying recognized clinical thresholds in these hemodynamic indices we found that 5 of 10 limbs (50%) enrolled on TBI criteria and 5 of 15 limbs (33%) enrolled on ABI criteria “responded” to ABMNC treatment by a margin of ≥ 0.15 from baseline. There were no significant differences between the “responder” and “non-responder” groups in age however most responders were female (70%). All four of the diabetic patients enrolled that completed the 12 week assessment of perfusion had an increase ≥ 0.15 in TBI (3) or ABI (1) (Table III). Only one patient had a decrease in ABI by ≥ 0.15 at week 12 and eventually had an AKA for gangrene, as detailed previously. There was an insignificant increase in TcPO2 at week 12 by 4.1 ± 6.8 mmHg (P=.63) . The BPI in the treated limb, as quantified by H2O15 PET-CT perfusion data (Fig.3) increased by 19.3 ± 3.1 from baseline (n=4).
Figure 2. Change in FTP, TBI, and ABI from baseline (mean ± SE).
FTP increased by 10.2±6.2 mmHg (P=.02) (A) and TBI increased by 0.10 ± 0.05 (P=.02) (B) at week 12 from baseline. The ABI increased by 0.08 ± 0.04 at week 12 but was not significant (P=.73) (C).
Table III.
Characteristics of Responders.
| Responders: ABI or TBI ≥ 0.15 (n=10) |
Non-Responders: ABI or TBI < 0.15 (n=20) |
|
|---|---|---|
| Age (years, ±SE) | 61.7 ± 17.7 | 67.5 ± 17.2 years |
| Gender (n/%) | Female 7/70 Male 3/30 | Female 8/57 Male 6/43 |
| Baseline ABI (± SE)a | 0.18 ± 0.05 | 0.38 ± 0.04 |
| Baseline TBI (± SE)b | 0.09 ± 0.04a P=.01 | 0.27 ± 0.05 |
| Diabetes (n/%) | 4/40 | 0 |
| Decrease in RPc | 4.2 ±1.3 cm | 2.5 ± 0.8 cm |
P = .008 ,
P = .01 , and
P =.02.
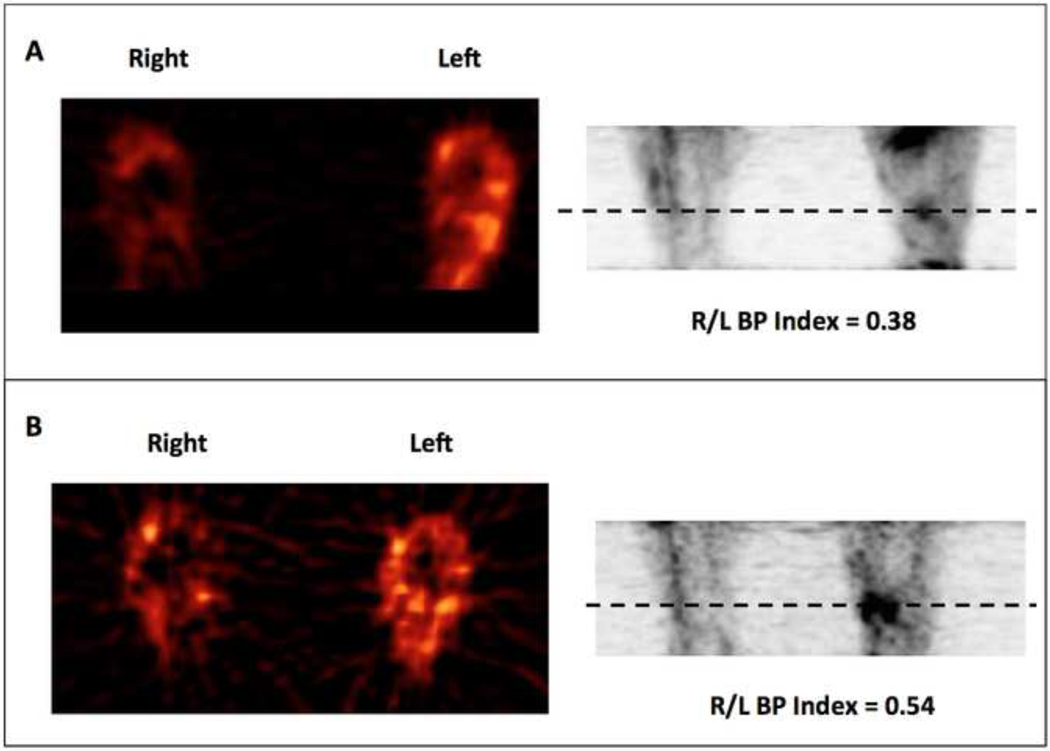
Figure 3. Radiolabeled H2O15 Positron Emission Tomogaphy-CT Scan.
A representative image of blood perfusion (BP) quantified with intra-arterial injection of H2O15 before ABMC treatment (A) in the left leg and after (B). A Blood Perfusion Index (BPI) was calculated by comparing the ratio of H2O15 peak tracer uptake level of the untreated:treated in the segment of the leg denoted by the dashed line. In this patient the BPI increased from 0.38 at baseline to 0.54 (42%) at 12 weeks , concurrent with an increase in the ABI from 0 to 0.32 in the same period.
Correlation of Cell Counts and Changes in Perfusion
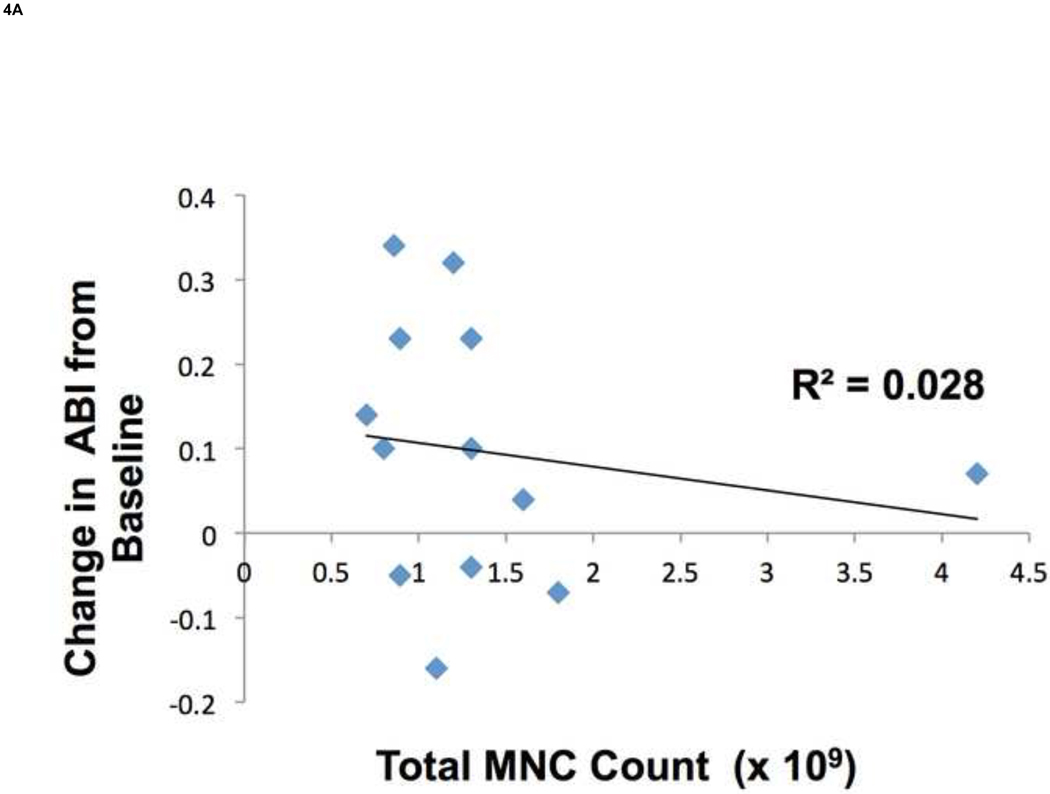
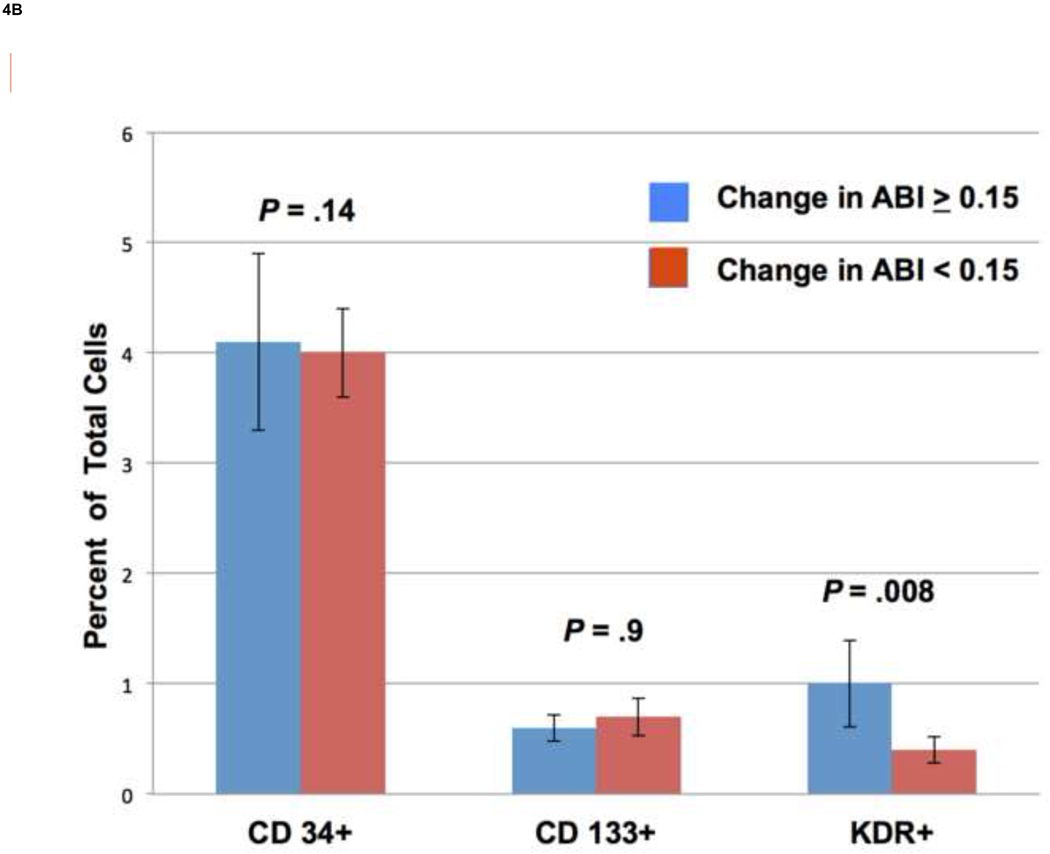
We found that there was no correlation between total MNC count and changes in ABI (Fig. 4A). There was a significant difference in counts of KDR+ cells between the treatment responders and non-responders; however there were no differences in counts of CD34+ or CD133+ cells between these two groups (Fig. 4B) . It should be noted that these subpopulations isolated based on CD34, CD133, and KDR expression represented a small portion of the total MNC population as compared to cells expressing the hematopoietic and monocyte marker CD45 (mean 89.46 ± 3.2%). There was no difference in the relative composition of CD45+ cells between responders and non-responders (data not shown).
Figure 4. Correlation of bone marrow subpopulations and changes in perfusion.
There was no correlation between total MNC count and changes in ABI (R2 = 0.028) (A). Using FACS analysis of EPC surface markers in ABMNCs we found a significant difference in KDR+ cells between responders to treatment as compared to non-responders (1.02 ± 0.39% vs. 0.36 ± 0.12 %, P = .008), respectively. There were no differences in CD34+ (4.21± 0.80% vs. 3.97 ± 0.40%, P = .14) or CD133+ (0.59 ± 0.12% vs. 0.68 ± 0.17%, P= .9) subpopulations between responders and nonresponders, respectively.
Additional Efficacy Assessments
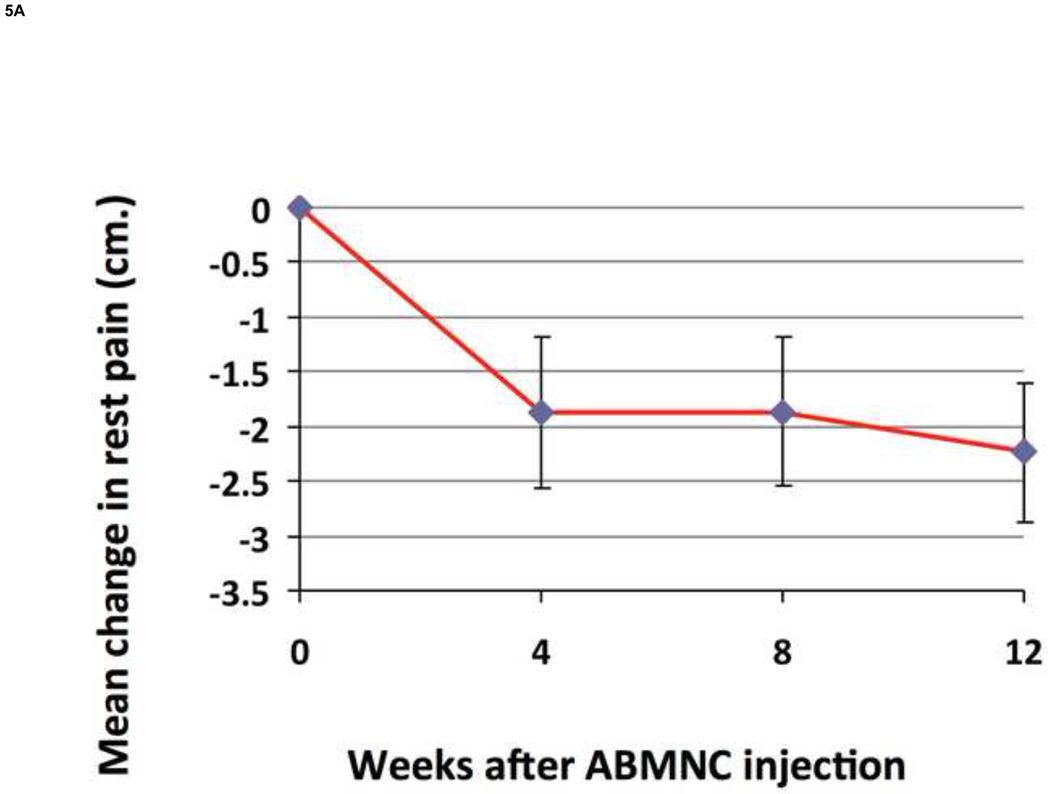
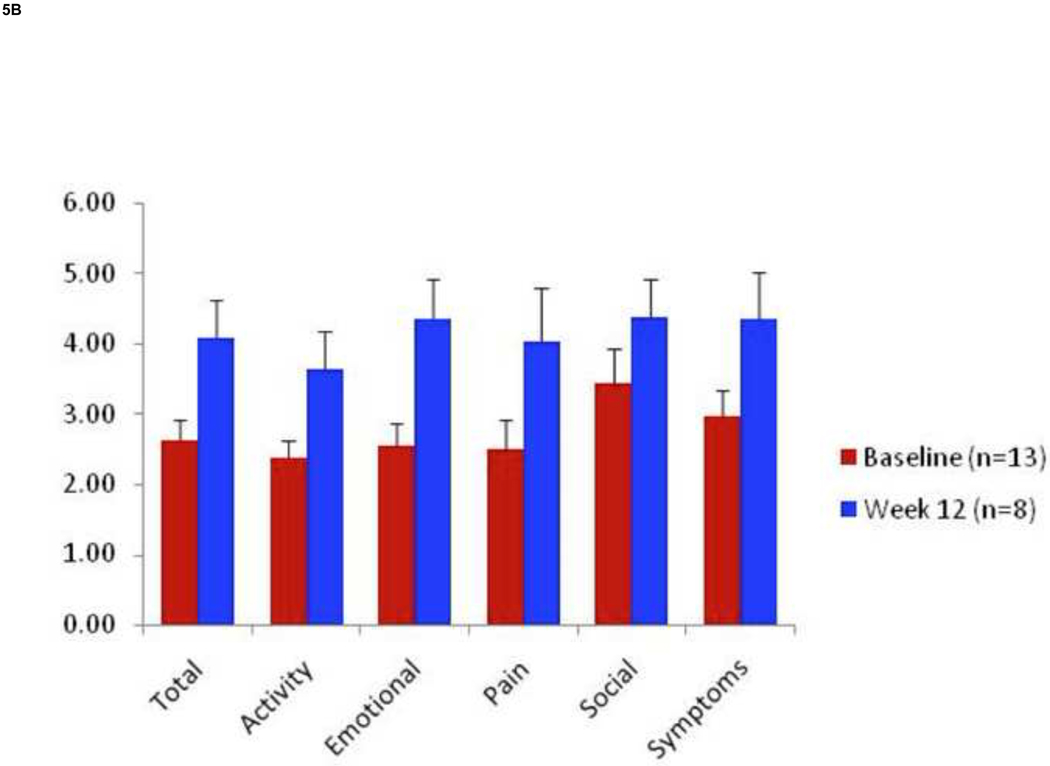
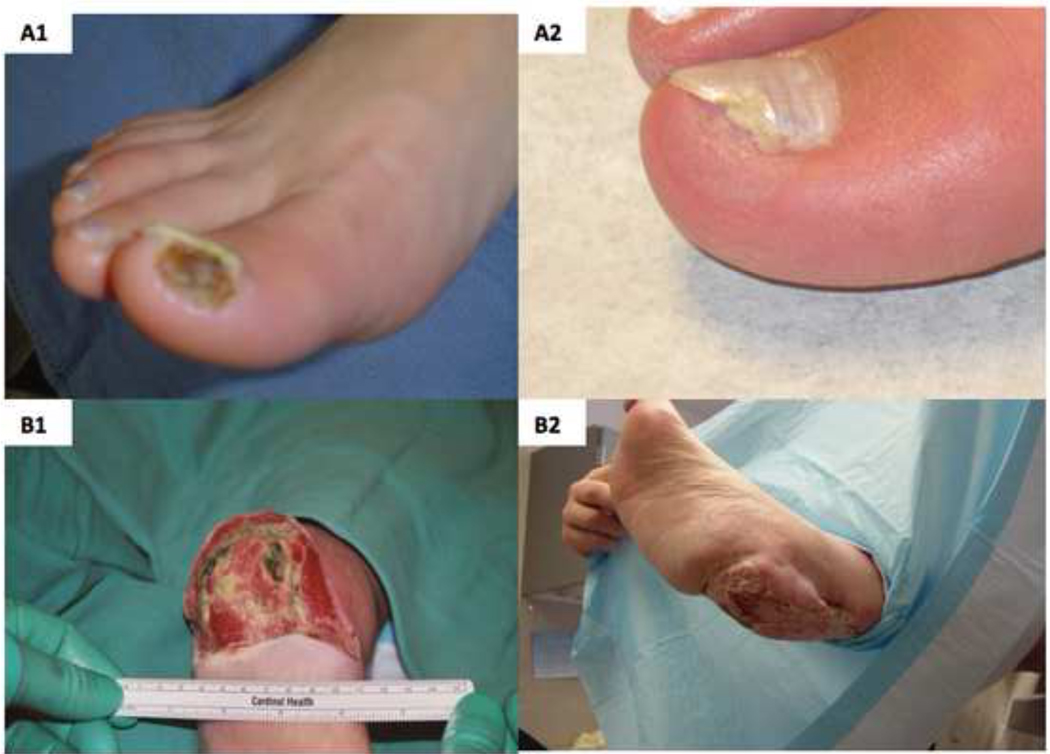
Rest pain decreased significantly at 12 weeks from baseline with 8 of 15 (53%) patients enrolled on ABI criteria and 8 of 10 (80%) patients enrolled on TBI criteria reporting improvement in symptoms (Fig. 5A). RP decreased from baseline to a greater degree in treatment responders than non-responders (Table III). Within the ABI group 4 patients (27%) reported an increase in RP and within the TBI group 2 patients (20%) reported an increase in RP. Interestingly both of these patients in the TBI group improved by ≥ 0.15. The VascuQol total score increased in all categories, especially in the domains of emotion (2.5 ± 0.3 to 4.3 ± 0.5) and pain (2.5 ± 0.4 to 4.0 ± 0.7). (Fig.5B). Three of 9 ulcers (33 %) healed completely by week 12 (Fig.6).
Figure 5. Changes in Rest Pain and Quality of Life.
Using a 10-cm visual analog scale RP significantly decreased at 12 weeks by 2.2 ± 0.6 cm. (P=.02) (A). The VascuQol total score increased from 2.62 to 4.07 at 12 weeks (P= .008) (B).
Figure 6. Ulcer healing.
Nine patients with ulceration of the index limb were enrolled. Complete healing of the ulcer with re-epithelialization was achieved in three patients (33%) at 12 weeks. Representative photographs are shown of a 46 year old woman with Buerger’s disease and an ulcer on the right first toe at baseline (A1) and at 12 weeks (A2). FTP concurrently increased from 2 to 25 mmHg in this period. A 73 year old male with ulceration of the right heel with exposed calcaneus bone at baseline (B1) and at 12 weeks (B2). There was sufficient growth of granulation tissue to cover the calcaneus and support a split thickness skin graft (STSG). Although not considered a success of trial therapy alone, as a STSG was required for re-epithelialization, - this case is presented to illustrate the potential of ABMNC in promoting wound healing, including via enablement of other procedures such as STSG.
Discussion
The report by the TACT investigators was the first clinical assessment of ABMNCs in treating CLI, demonstrating that IM injection of ABMNCs significantly improved ABI, TcPO2, rest pain, and pain free walking time at 24 weeks after treatment22. Intrigued by these results and motivated by a need in our own clinic population, we used the TACT report as a template for an open label, non-randomized phaseI/II clinical trial that was the first FDA approved cell-based trial for PAD in the United States. In the current study there were no deaths within thirty days of treatment. There were two procedure-related SAEs - anemia related myocardial ischemia and entrapment of a guidewire during CA. Similar CLI studies have also reported anemia in patients after large volume BM aspiration with various adverse events26,31 and modification of the study protocol prevented further anemia related SAEs. There were no adverse events associated with administration of general anesthesia, which was a major concern in this high cardiac risk patient population. There were two deaths during the 12 month follow-up period neither of which was procedure related. The thirty-day perioperative morbidity rate of ABMNC therapy in this trial, with two serious adverse events (no deaths) in thirty limbs treated, was 7%. The perioperative morbidity and mortality of lower extremity amputation ranges from 7 – 17% 1,2,13. Based on a review of SAEs by an independent data and safety monitoring board we conclude that IM administration of ABMNCs is safe and presents less of a risk to the CLI patient than amputation.
The second objective of this phase I/II trial was to assess the ability of IM injection of ABMNCs to promote AFS, a composite of limb salvage and all cause mortality, in patients with no option CLI. There were three amputations during the twelve month follow–up, and including the two deaths that occurred, the one year AFS rate for this study is 86.3%. Similar studies have reported AFS rates of 59–66.7%29,30. Our enthusiasm for the results we report is tempered by the fact this was a non-randomized open-label trial and we excluded patients with a hemoglobin A1c > 8.5% and renal failure. Thus the patient population of this trial was not representative of the more morbid CLI population at large. When we consider the fate of the contralateral limb of those patients with bilateral CLI by inclusion criteria (n=12), although less symptomatic with regards to rest pain or tissue loss, none of the contralateral limbs required amputation during the twelve month follow up. In a larger trial assessing the effect of iloprost in patients with CLI, patients randomized to the placebo arm had a one year limb salvage rate of 87%31. Thus any conclusions regarding efficacy of ABMNC in promoting AFS in CLI will await a larger randomized trial with broader inclusion criteria.
In the present study we measured FTP, TBI, ABI, and TcPO2 to assess changes in perfusion of the index limb to provide an objective measure of hemodynamic improvement. We also incorporated ABI and TBI values, in addition to the clinical findings of rest pain and ulceration, to more succinctly define CLI for our inclusion criteria as compared to the broader Fontaine and Rutherford classifications. Our objective was to provide a more defined metric by which to quantify response to therapy, consistent with the recent recommendations of Conte for assessing responses to intervention in CLI32. In this study we found that 76% of patients had some degree of improvement in ABI or TBI and over one third increased by a clinically significant margin of ≥ 0.15. These results support the concept that ABMNC improves perfusion in ischemic tissue, however the exact mechanism by which this occurs remains to be defined in more elaborate preclinical and clinical studies. We did find an association between content of KDR+ cells in the ABMNC fraction and improvement in hemodynamic parameters in a small sample population. This finding will be borne out by current investigations in our laboratory focusing on BM and peripheral blood derived EPCs from PAD patients in in vitro and in vivo experiments.
There was a significant improvement in RP with 64% of patients reporting some degree of improvement from baseline, findings that are consistent with other trials focusing on cell therapy in the context of CLI22–25. The patients enrolled in this open label trial had severe symptoms, were highly motivated, and consequently had expectations for clinical benefit from an unproven therapeutic agent, a scenario associated with a 30–40% placebo effect with a subjective endpoint such as RP33–35. Intractable pain is a major indication for lower extremity amputation and consequently it will be imperative to blind patients to treatment in the design of a phase III efficacy trial that includes major amputation as a component of the endpoint.
The conduct of this study was modified several times in response to pitfalls that we encountered. Inclusion of the sterile centrifugation MS system significantly reduced operative time as the ABMNCs were isolated in the operating room immediately after BM aspiration. The average procedure time for aspiration, ABMNC separation, and cell injection using FC was 527±38.9 minutes and for the MS was 114±18.8 minutes. The closed MS system did not require gram stains, endotoxin assays, and bacterial and fungal cultures necessary to ensure sterility as with FC. CA was initially incorporated in the study however required exclusion of patients with renal insufficiency and provided highly subjective data at considerable expense and risk. We also included MRA for the same purpose as CA. Unfortunately imaging of vessels of the calf was frequently obscured by venous contamination and restricted inclusion of patients with implanted devices, notably pacemakers and defibrillators, which constituted 20% of our study population. Therefore we eliminated both of these tests and we developed a novel PET-CT protocol to directly measure blood volume and perfusion in the treated limb. This protocol was not standardized at the time of this report however measurements of perfusion correlated with improvements in ABI. The results for TcPO2 in this study were widely disparate despite rigorous control of elements involved in this test, a problem noted by other investigators as well36–38, and we will not use this parameter as a measure of efficacy in future studies.
In conclusion this phase I/II trial has demonstrated that ABMNC therapy for CLI is safe. In addition our results provide compelling evidence suggesting that IM ABMNC administration promotes AFS survival. Based on these results we have moved forward with a pivotal multicenter randomized, double blinded, phase III trial which will ultimately test the concept that IM ABMNC improves AFS at one year in patients with no option CLI .
Supplementary Material
Acknowledgements
Rafat Abonour, MDe, and Nelson Chao, MDc, for assistance with bone marrow aspiration. Pat G’Sell, RNd , for assistance in patient recruitment and data collection.
This study was supported by the National Institutes of Health Grant MO1 00750 , Biomet Biologics Inc., Warsaw, IN., the Cryptic Masons’ Medical Research Foundation, and the IUPUI Vascular and Cardiac Center for Adult Stem Cell Therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competition of Interest: Drs Wilson and March serve as consultants to Biomet Biologics, Inc., Warsaw, IN.
ClinicalTrials.gov Identifier: NCT00113243
Presented at the Annual Meeting of the Midwestern Vascular Surgical Society, Indianapolis, IN, Sept 10, 2010.
References
- 1.Dormandy J, Heeck L, Vig S. Predicting which patients will develop chronic critical leg ischemia. Semin Vasc Surg. 1999;12:138–141. [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, M R, Harris KA, Fowkes FG TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45 doi: 10.1016/j.jvs.2006.12.037. S5-S-67. [DOI] [PubMed] [Google Scholar]
- 3.Tretinyak AS, Lee ES, Kuskowski MM, Caldwell MP, Santilli SM. Revascularization and quality of life for patients with limb-threatening ischemia. Ann Vasc Surg. 2001;15:84–88. doi: 10.1007/s100160010007. [DOI] [PubMed] [Google Scholar]
- 4.Pomposelli FB, Kansal N, Hamdan AD, Belfield A, Sheahan M, Campbell DR, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37:307–315. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- 5.Haider SN, Kavanagh EG, Forlee M, Colgan MP, Madhavan P, Moore DJ, et al. Two-year outcome with preferential use of infrainguinal angioplasty for critical ischemia. J Vasc Surg. 2006;43:504–512. doi: 10.1016/j.jvs.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, Ruckley CV, Raab GM. BASIL trial Participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 7.Giles KA, Pomposelli FB, Hamdan AD, Blattman SB, Panossian H, Schermerhorn ML. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic InterSociety Consensus class to outcome in 176 limbs. J Vasc Surg. 2008;48:128–136. doi: 10.1016/j.jvs.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Bosiers M, Peeters P, Elst FV, Vermassen F, Maleux G, Forneau I, et al. Excimer laser assisted angioplasty for critical limb ischemia: results of the LACI Belgium Study. Eur J Vasc Endovasc Surg. 2005;29:613–619. doi: 10.1016/j.ejvs.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Zeller T, Rastan A, Schwarzwalder U, Frank U, Burgelin K, Amantea P, et al. Percutaneous peripheral atherectomy of femoropopliteal stenoses with a new generation device: six-month results from a single center experience. J Endovasc Ther. 2004;11:676–685. doi: 10.1583/04-1316R.1. [DOI] [PubMed] [Google Scholar]
- 10.Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–147. [PubMed] [Google Scholar]
- 11.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 12.Marston W, Davies S, Armstrong B, Farber M, Mendes R, Fulton J, Keagy B. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44:108–114. doi: 10.1016/j.jvs.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Feinglass J, Pearce WH, Martin GJ, et al. Postoperative and late survival outcomes after major amputation: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program. Surgery. 2001;130:21–29. doi: 10.1067/msy.2001.115359. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Evans L, Datta D, et al. The costs of managing lower limb-threatening ischaemia. Eur J Vasc Endovasc Surg. 1996;12:359–362. doi: 10.1016/s1078-5884(96)80257-7. [DOI] [PubMed] [Google Scholar]
- 15.Albers M, Fratezi AC, DeLuccia N. Assessment of quality of life of patients with severe ischemia as a result of infrainguinal arterial occlusive disease. J Vasc Surg. 1992;16:54–59. [PubMed] [Google Scholar]
- 16.de Vries M, Ouwendijk R, Kessels AG, et al. Comparison of generic and disease-specific questionnaires for the assessment of quality of life in patients with peripheral arterial disease. J Vasc Surg. 2005;41(2):261–268. doi: 10.1016/j.jvs.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 18.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker P, Verfaillie C. Origin of endothelial progenitors in human post natal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 20.Shintani S, Murohara T, Ikeda H, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circ. 2001;103:895–897. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 21.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone marrow cells: a pilot study and a randomized controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 23.Esato K, Hamano K, Li TS, et al. Neovascularization induced by autologous bone marrow cell transplantation in peripheral arterial disease. Cell Transplant. 2002;11:747–752. [PubMed] [Google Scholar]
- 24.Miyamoto M, Yasutake M, Takano H, et al. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transplant. 2004;13:429–437. doi: 10.3727/000000004783983837. [DOI] [PubMed] [Google Scholar]
- 25.Saigawa T, Kato K, Ozawa T, et al. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J. 2004;68:1189–1193. doi: 10.1253/circj.68.1189. [DOI] [PubMed] [Google Scholar]
- 26.Durdu S, Akar AR, Arat M, et al. Autologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II–III thromboangiitis obliterans. J Vasc Surg. 2006;44:732–739. doi: 10.1016/j.jvs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto K, Nishigami K, Nagaya N, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–2684. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 28.Fischman AJ, Hsu H, Carter EA, Yu YM, Tompkins RG, Guerrero JL, Young VR, Alpert NM. Regional measurement of canine skeletal muscle blood flow by positron emission tomography with H215O. J Appl Physiol. 2002;92:1709–1716. doi: 10.1152/japplphysiol.00445.2001. [DOI] [PubMed] [Google Scholar]
- 29.Amann B, Luedemann C, Ratei R, Schmidt-Lucke J. Autologous bone marrow cell transplantation increases leg perfusion and reduced amputations in patients with advanced critical limb ischemic due to peripheral artery disease. Cell Transplantation. 2009;18:371–380. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 30.Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. J Vasc Surg. 2009;50:1378–1390. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 31.Brass EP, Anthony R, Dormandy J, Hiatt WR, Jiao J, Nakanishi A, et al. Parenteral therapy with lipo-ecraprost, a lipid-based formulation of a PGE1 analog, does not alter six-month outcomes in patients with critical leg ischemia. J Vasc Surg. 2006;43:752–759. doi: 10.1016/j.jvs.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 32.Conte M. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) and the (hoped for) dawn of evidence-based treatment for advanced limb ischemia. J Vasc Surg. 2010;51:69S–75S. doi: 10.1016/j.jvs.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AG. Surgery as a placebo. Lancet. 1994;344:1140–1142. doi: 10.1016/s0140-6736(94)90637-8. [DOI] [PubMed] [Google Scholar]
- 34.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 35.Leon MB, Kornowski R, Downey WE, Weisz G, Baim DS, Bonow RO, Hendel RC, Cohen DJ, Gervino E, Laham R, Lembo NJ, Moses JW, Kuntz RE. A blinded, randomized, placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J. Am. Coll. Cardiol. 2005;46:1812–1819. doi: 10.1016/j.jacc.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 36.Bunt T, Holloway G. TcPO2 as an accurate predictor of therapy in limb salvage. Ann Vasc Surg. 1996;10:224–227. doi: 10.1007/BF02001886. [DOI] [PubMed] [Google Scholar]
- 37.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH. Results of a Double-Blind, Placebo-Controlled Study to Assess the Safety of Intramuscular Injection of Hepatocyte Growth Factor Plasmid to Improve Limb Perfusion in Patients With Critical Limb Ischemia. Circulation. 118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 38.Padberg F, Back T, Thompson P, Hobson R. Transcutaneous oxygen (TcPO2) estimates probability of healing in the ischemic extremity. J Surg Res. 1996;60:365–369. doi: 10.1006/jsre.1996.0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.