Abstract
Objective
To assess the predictive accuracy of serum procalcitonin (PCT) in distinguishing bacterial aspiration pneumonia from aspiration pneumonitis.
Design
Prospective observational study.
Setting
Intensive care unit of a University-affiliated hospital.
Patients
Sixty five consecutive patients admitted with pulmonary aspiration and 7 controls intubated for airway protection.
Interventions and results
Quantitative cultures from bronchoalveolar lavage (BAL) fluid were conducted on all participants at the time of admission. Serial serum PCT levels were measured on day 1 and day 3 using the PCT enzyme-linked fluorescent assay. There were no differences in the median serum concentrations of PCT between patients with positive BAL cultures (n=32) and patients with negative BAL cultures (n=33) on either day 1 or day 3 post admission. The areas under the ROC curves were 0.59 (95% CI 0.47–0.72) and 0.63 (95% CI 0.5–0.75), respectively (p=0.74). However, duration of mechanical ventilation and antibiotic therapy were shorter in those who had a decrease in their PCT levels on day 3 from baseline compared to those who did not (6.7±7.1 days and 11.1±13.5 days, p=0.03; and 8.2±2.6 days versus 12.8±4.6 days; p<0.001, respectively). Hospital mortality was associated with radiographic multilobar disease (adjusted odds ratio (AOR) 1.14; 95% CI 1.01 to 1.31; p=0.04) and increasing procalcitonin levels (AOR 5.63; 95% CI 1.56 to 20.29; p= 0.008).
Conclusion
Serum PCT levels had poor diagnostic value in separating bacterial aspiration pneumonia from aspiration pneumonitis based on quantitative BAL culture. However, serial measurements of serum PCT may be helpful in predicting survival from pulmonary aspiration.
Keywords: procalcitonin, aspiration pneumonia, aspiration pneumonitis, outcome, intensive care unit
INTRODUCTION
The clinical presentation of aspiration syndromes falls within a wide spectrum ranging from subclinical manifestations such as dry cough or dysphonia to rapidly progressing disease such as the acute respiratory distress syndrome (ARDS). The two most common consequences resulting from aspiration of gastric or oropharyngeal content are chemical pneumonitis and bacterial aspiration pneumonia. Both entities present with comparable clinical signs and symptoms. In the absence of a reliable marker to differentiate between these two conditions, most patients with suspected aspiration are treated empirically with antimicrobial agents. The widespread and often unnecessary use of antibiotics in these instances is fueling a wave of emerging drug resistant bacteria.
The use of biomarkers has been suggested as a possible tool to distinguish chemical aspiration pneumonitis from bacterial aspiration pneumonia. Procalcitonin (PCT) is a 116 amino acid peptide with a sequence identical to that of pro-hormone of calcitonin but devoid of hormonal activity. Since PCT determination was first performed by Assicot et al (1) to differentiate between bacterial and non bacterial causes of sepsis, it has become a marker of bacterial infection and used to provide rapid evidence of bacterial origin of shock or respiratory illness. Furthermore, the finding that PCT is released into the circulation within 3 h after endotoxin injection, plateaus at 6 h, and remains elevated for 24 h, makes PCT a promising new agent for early and sensitive identification of severe infection (2). Recently, PCT has been used to shorten duration of antibiotic therapy in patients hospitalized with community-acquired pneumonia and sepsis (3–9). However, PCT can be increased in noninfectious conditions (10,11) and may remain low in bacterial infections (12,13). There are relatively few data available on the utility of PCT in patients with aspiration syndromes. Therefore, we investigated the utility of plasma levels of PCT to differentiate between aspiration pneumonia and aspiration pneumonitis.
MATERIALS AND METHODS
Study design
A prospective, outcome blind observational study was performed in the intensive care unit (ICU) of a tertiary university teaching hospital between September 2007 and December 2009. The study was approved by the ethical committee of the hospital and informed consent was obtained from all participants or their health care proxy. Some of the patients were part of a previous investigation (14). All patients who were admitted with the following criteria were considered for enrollment: 1) the presence of risk factors for aspiration; 2) symptoms and signs suggestive of lower respiratory tract pathology; 3) new radiographic infiltrate on chest roentgenogram; and 4) need for mechanical ventilation. Exclusion criteria included: 1) discharge from a hospital within the prior 10 days; 2) an episode of pneumonia within the past 30 days; 3) prior antibiotics; 4) documented bacteremia; 5) chronic mechanical ventilation; 6) immunosuppressive therapy; 7) positive HIV antibody titer; and 8) post cardiopulmonary arrest. A control group devoid of underlying inflammatory disorders who were intubated for airway protection was included.
Data collection
Demographic data collected included age, gender, comorbid illnesses, and the Charlson Index (15). Clinical data were obtained on ICU admission and comprised of clinical symptoms, daily vital signs, laboratory and radiographic data, and the Acute Physiology and Chronic Health Evaluation (APACHE II) score (16). The duration of mechanical ventilation, the length of stay and the outcome (death or discharge) were also recorded. Drug selection and final decision about stopping antibiotics were at the treating physician's discretion.
Biological samples
A bronchoalveolar lavage (BAL) was performed on all participants within 6 hours of hospital admission. Samples for microbiologic analysis were processed as described previously (17). Pneumonia was considered present when at least one pathogenic bacteria grew at concentration ≥ 104 CFU/ml. For all patients, serum from blood drawn daily for routine biologic tests was collected on day 1 and on day 3 of hospitalization. Samples were frozen immediately after blood was drawn, then stored at −80°C. Assays were performed in batches at the end of the study period. Procalcitonin was measured using the bioMerieux’s VIDAS BRAHMS PCT assay. The assay has a functional assay sensitivity of 0.09 ng/ml, and a coefficient of variation of 6.2% at a mean concentration of 1.33 ng/ml and 6.4% at a mean concentration of 13.2 ng/ml. All PCT measurements were performed in duplicate, and the mean of these duplicate results was considered.
Statistical analysis
Data are expressed as means ± standard deviation (SD) unless specified otherwise. Categorical variables were compared using Chi-squared and Fisher's exact tests when appropriate. Comparison of numerical and categorical variables was performed with unpaired t-tests or Mann–Whitney U-tests when the latter were dichotomous, and ANOVA or Kruskal–Wallis H-tests were carried out for variables with more than two categories. Correlation was assessed using Spearman’s rank test. Cook’s distance was used to assess for potential outliers. Values > 1 were considered influential. To examine the univariate effects of patients' clinical characteristics and physiologic variables on unfavorable outcome, a logistic-regression model was used to test each characteristic. Risk factors with p values of 0.10 or less in our univariate analysis were entered into the multivariate model. Interactions were tested in the model; variables strongly associated with another(s) were not included in the multivariate analysis. For all analysis, a two-tailed p value <0.05 was considered statistically significant.
RESULTS
Sixty five consecutive patients with clinical suspicion for aspiration and 7 controls were enrolled. All patients were intubated in the emergency department and were hemodynamically stable on admission to the ICU. The mean age of the study population was 57.3±20.7 years. The main underlying causes for aspiration were neurodegenerative disorders (n=24), drug overdose (n= 15), cerebrovascular accident (n=14), and seizure (n=11).. The characteristics of the study population are shown in Table 1. Patients with pulmonary aspiration were older, had higher burden of comorbidities, and were more severely ill than the control group.
Table 1.
Characteristics of the study population
| Variable | Control | Aspiration syndromes |
P value |
|---|---|---|---|
| (n=7) | (n=65) | ||
| Age, years | 48.2±12.6 | 57.3±20.7 | 0.15 |
| Gender, no. (%) | 1.0 | ||
| Male | 3 (43) | 26 (40) | |
| Female | 4 (57) | 39 (60) | |
| Nursing home residents, no. (%) | 0 | 35 (54) | 0.012 |
| Charlson Index, no. (%) | 0.039 | ||
| 0–1 | 3 (43) | 7 (11) | |
| 2–3 | 3 (43) | 25 (38) | |
| ≥4 | 1 (14) | 33 (51) | |
| APACHE II score | 21.1±3.8 | 28.0±5.7 | <0.001 |
| Glasgow Coma Scale | 12.4±1.8 | 10.3±2.5 | 0.02 |
| Body temperature, (oF) | 97.8±1.3 | 99.0±3.7 | 0.053 |
| Leukocyte count, 109 cells/L | 11.5±3.7 | 16.1±6.3 | 0.007 |
| Radiographic pattern, no. (%) | |||
| Unilobar infiltrates | 0 | 45 (69) | |
| Multilobar infiltrates | 0 | 20 (31) |
Microbiological analysis of the BAL fluid confirmed the presence of pneumonia in 32 cases. The microbial etiology of aspiration pneumonia is shown in Table 2. Staphylococcus aureus and Escherichia coli were the predominant pathogens isolated. Table 3 displays the clinical characteristics between patients with BAL positive and BAL negative cultures. All participants received a parenteral antimicrobial coverage consisting of ampicillin/clavulanic acid plus a macrolide (n= 29), a quinolone plus clindamycin (n=17), or vancomycin plus ciprofloxacin plus piperacillin/tazobactam (n=19).
Table 2.
Microbial etiology of pulmonary aspiration
| Pathogens | N |
|---|---|
| Streptococcus pneumoniae | 1 |
| Staphylococcus aureus | 6 |
| Streptococcus species | 5 |
| Hemophilus influenzae | 2 |
| Serratia species | 1 |
| Escherichia coli | 6 |
| Klebsiella pneumoniae | 3 |
| Enterobacter sp. | 2 |
| Prevotella species | 5 |
| Fusobacterium species | 1 |
| Peptostreptococcus species | 4 |
Table 3.
Comparison of clinical characteristics of patients with bronchoalveolar lavage (BAL) culture positive and culture negative pulmonary aspiration
| Variable | BAL positive | BAL negative | P value |
|---|---|---|---|
| (n=32) | (n=33) | ||
| Age, years | 61.2±17.7 | 55.2±19.6 | 0.26 |
| APACHE II score | 29.0±4.7 | 26.9±6.4 | 0.12 |
| Glasgow Coma Scale | 12.1±2.4 | 10.3±3.9 | 0.14 |
| Body temperature, (oF) | 100.1±3.2 | 98.1±3.8 | 0.02 |
| Leukocyte count, 109 cells/L | 16.6±7.3 | 15.8±5.2 | 0.23 |
| PaO2/FIO2 | 219.1±55.9 | 206.8±53.9 | 0.37 |
| Radiographic pattern, no. (%) | |||
| Unilobar infiltrates | 23 (72) | 15 (45) | 0.06 |
| Multilobar infiltrates | 9 (28) | 18 (55) | |
| Procalcitonin levels†, ng/ml | 2.4 (1.7–4.6) | 2.9 (2.2–4.9) | 0.17 |
median (Interquartile range)
Diagnostic value of procalcitonin
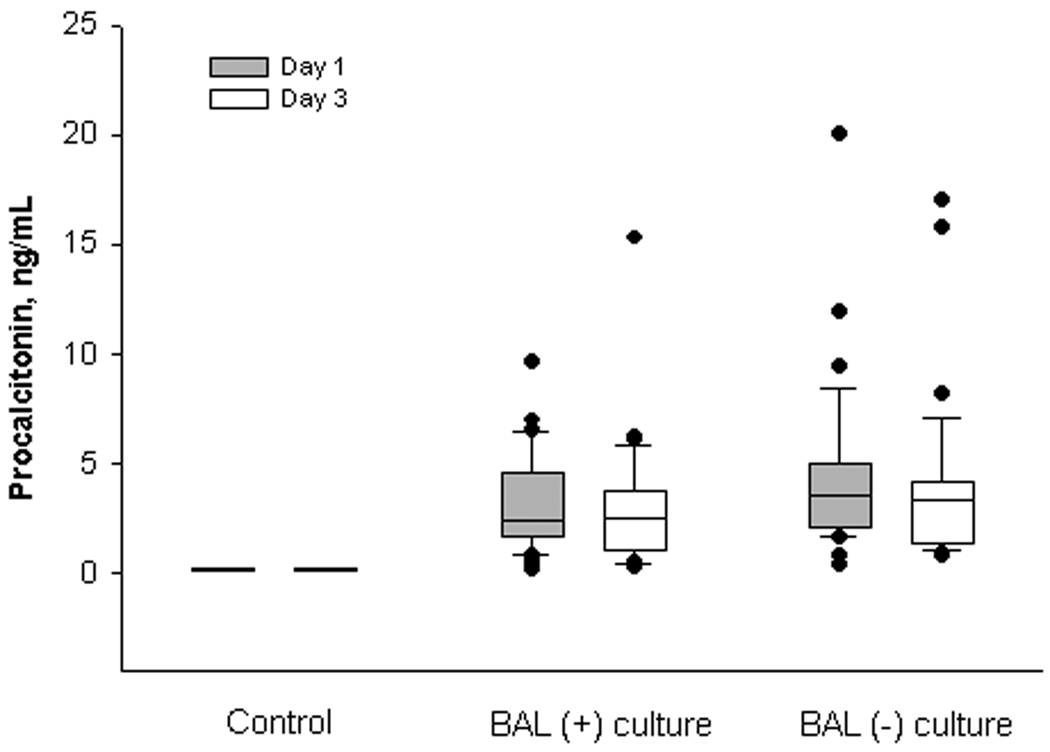
The differences in the plasma levels of PCT among the three groups (positive BAL culture, negative BAL culture, and control) on day 1 of aspiration were statistically significant (p<0.001 by ANOVA) with the highest values corresponding to patients with culture negative BAL samples. However, there was no significant difference in PCT levels when comparing patients with culture positive and culture negative BAL specimens (p=0.17) (Figure 1).
Figure 1.
Serial serum procalcitonin in patients with pulmonary aspiration. Box plots represent the 25th and 75th centiles. The internal horizontal line shows the median and whiskers show the 10th and 90th centiles. Circles represent outliers.
There was also no correlation between alveolar arterial gradient, PaO2/FiO2, or extent of radiographic disease and serum procalcitonin levels (p=0.42; p=0.66; and p=0.8, respectively). No correlation was found also between the species type and quantity of microorganisms cultured in the BAL fluid and the serum levels of PCT.
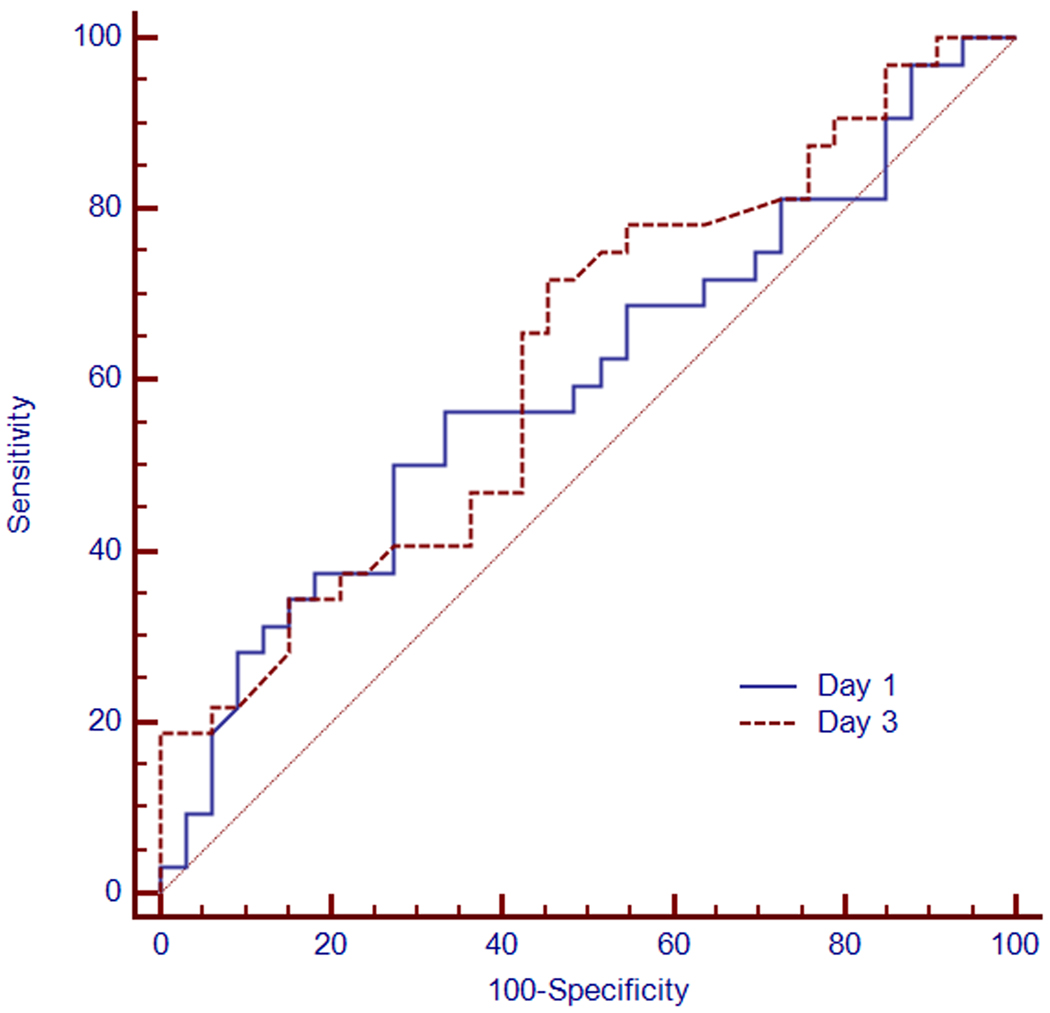
On day 1 of aspiration, no best cutoff PCT value for the diagnosis of aspiration pneumonia could be established. Using a threshold of 2.0 ng/ml yielded a sensitivity of 76%, specificity of 38%, positive predictive value of 56%, and a negative predictive value of 60% (Table 4). The median serum PCT concentration values obtained on day 3 did not change significantly from day 1 for all three groups (Figure 1). The predictive ability of PCT to differentiate between culture positive pulmonary aspiration and culture negative pulmonary aspiration was assessed using the receiver operator characteristic (ROC) curves on day 1 and day 3 (Figure 2). The areas under the ROC curves (AUCs) were 0.59 (95% CI 0.47–0.72) and 0.63 (95% CI 0.5–0.75), respectively (p=0.74).
Table 4.
Predictive value for serum procalcitonin levels in pulmonary aspiration
| ng/mL | Sensitivity (95% CI) |
Specificity (95% CI) |
Positive predictive value (95% CI) |
Negative predictive value (95% CI) |
Positive likelihood ratio (95% CI) |
Negative likelihood ratio (95% CI) |
|---|---|---|---|---|---|---|
| Day 1 | ||||||
| ≥ 0.5 | 97 (84–99) | 3 (0.1–16) | 51 (38–64) | 50 (0.1–99) | 1.0 (0.1–6.9) | 0.97 (0.1–6.7) |
| ≥ 1 | 94 (79–99) | 9 (2–25) | 52 (38–65) | 60 (11–97) | 1.04 (0.4–3.1) | 0.65 (0.2–2.5) |
| ≥ 2 | 76 (58–89) | 38 (21–56) | 56 (40–71) | 60 (35–81) | 1.21 (0.7–2.0) | 0.65 (0.3–1.3) |
| ≥ 3 | 49 (31–67) | 59 (41–76) | 57 (37–76) | 53 (35–70) | 1.19 (0.8–1.9) | 0.87 (0.5–1.5) |
| ≥ 4 | 30 (16–49) | 72 (53–86) | 53 (29–76) | 51 (36–65) | 1.08 (0.6–1.9) | 0.97 (0.5–1.8) |
| Day 3 | ||||||
| ≥ 0.5 | 97 (84–100) | 18 (7–36) | 56 (42–69) | 86 (38–100) | 1.16 (0.6–2.5) | 0.16 (0.02–1.1) |
| ≥ 1 | 91 (76–98) | 22 (9–40) | 54 (40–68) | 70 (35–93) | 1.18 (0.7–2.1) | 0.42 (0.1–1.2) |
| ≥ 2 | 63 (45–79) | 44 (26–62) | 53 (37–70) | 54 (33–74) | 1.2 (0.8–1.9) | 0.78 (0.4–1.4) |
| ≥ 3 | 55 (36–72) | 69 (50–84) | 64 (44–82) | 59 (42–75) | 1.75 (1.2–2.6) | 0.66 (0.4–1.2) |
| ≥ 4 | 42 (26–61) | 78 (60–91) | 67 (42–86) | 57 (41–72) | 1.94 (1.3–3.0) | 0.74 (0.4–1.5) |
Figure 2.
Receiver operating characteristic (ROC) curves of procalcitonin concentration obtained from patients with pulmonary aspiration on day 1 (continuous line) and day 3 (broken line) from admission with respective areas under the curves of 0.59 (95% CI 0.47–0.72) and 0.63 (95% CI 0.5–0.75).
Outcome
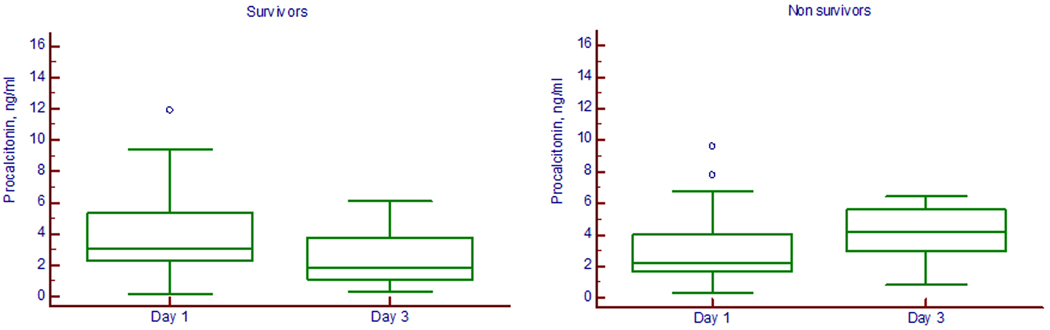
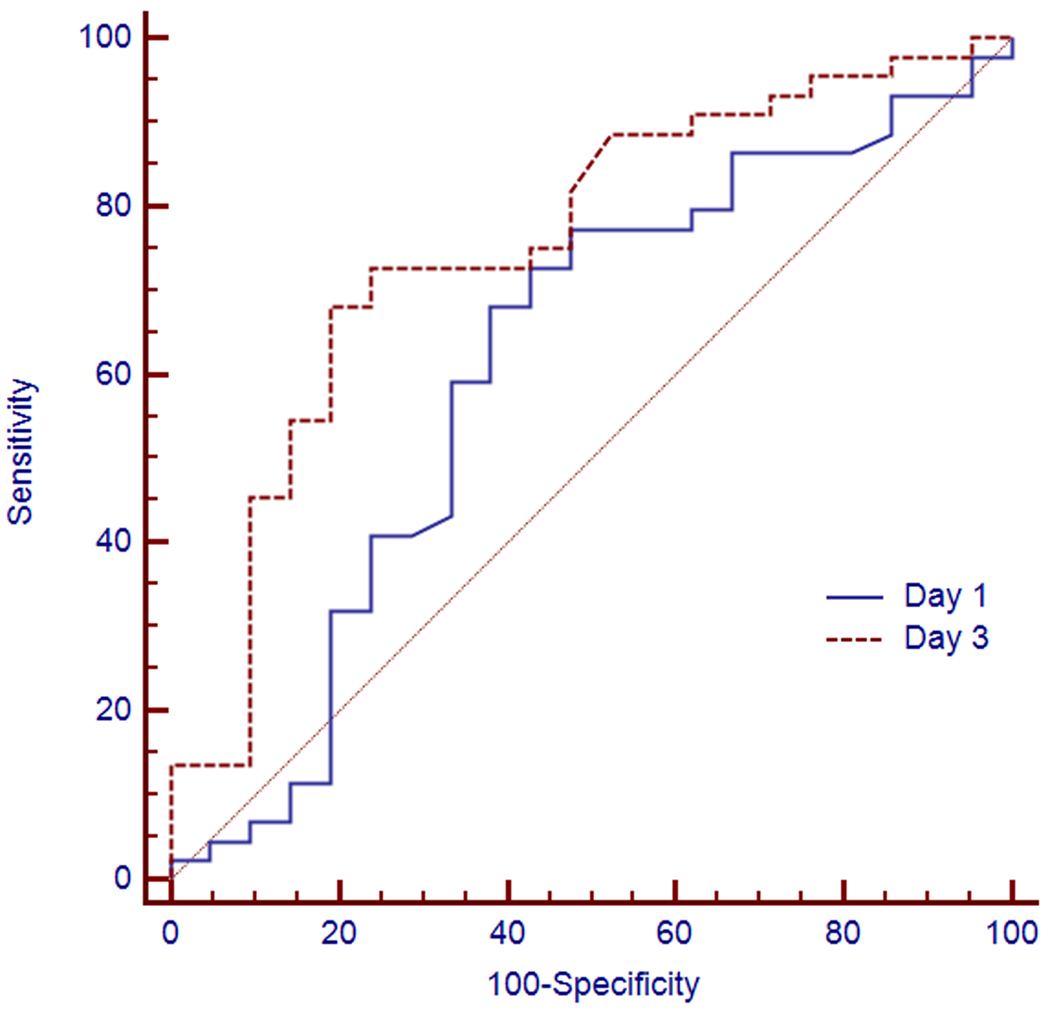
The in-hospital mortality rates for BAL culture positive and culture negative groups were 36% and 28%, respectively (p=0.85). On day 3, the median serum PCT concentrations were significantly higher in patients whose outcomes would be unfavorable (4.20 [IQR 2.98–5.60] ng/mL) than in those with subsequent favorable outcome (1.84 [IQR 1.10–3.79] ng/mL) (p=0.001) (Figure 3). A procalcitonin cutoff value of 2 ng/ml on Day 1 had a sensitivity of 77% (95% CI 62%–89%) and a specificity of 47% (95% CI 26%–70%) in predicting survival with an AUC of 0.62 (95% CI 0.48–0.73). A procalcitonin threshold of 2 ng/ml on Day 3 had a sensitivity of 86% (95% CI 64%–97%) and a specificity of 52% (95% CI 37%–68%) with an AUC of 0.76 (95% CI 0.67–0.88) (Figure 4).
Figure 3.
Box plots of serial serum procalcitonin levels on day 1 and day 3 post admission in survivors and nonsurvivors of pulmonary aspiration (p=0.001 for survivors and p=0.04 for nonsurvivors). The box represents the values from the lower to the upper quartile. The middle line represents the median. The open circles indicate outlier values.
Figure 4.
Receiver operating characteristic (ROC) curves of procalcitonin concentration obtained on day 1 (continuous line) and day 3 (broken line) in predicting outcome with respective areas under the curves of 0.67 (95% CI 0.54–0.78) and 0.76 (95% CI 0.63–0.85) (p=0.07).
Duration of mechanical ventilation and length of ICU stay were also shorter in those who had a drop in serum PCT levels in day 3 compared to day 1 (6.7±7.1 days and 11.1±13.5 days versus 10.3±12.6 days and 14.3±11.2 days; respectively) (p=0.03 and p=0.02). There was also significant difference in the duration of antimicrobial therapy between those patients who had a decline in PCT and those who did not (8.2±2.6 days versus 12.8±4.6 days; p<0.001). Logistic regression analysis identified radiographic multilobar disease on admission (AOR 1.14; 95% CI 1.01 to 1.31; p=0.04) and a one unit increase in PCT levels between day 1 and day 3 (AOR 5.63; 95% CI 1.56 to 20.29; p=0.008) as independent predictor of hospital mortality.
DISCUSSION
The results of this study indicate that serum PCT was not a good indicator of bacterial aspiration pneumonia. A drop in the serum procalcitonin levels between day 1 and day 3 predicted a favorable outcome in pulmonary aspiration syndromes.
This is the largest study to assess the utility of serum PCT levels in pulmonary aspiration syndromes. Currently, the diagnosis of pulmonary aspiration is established by history and presence of underlying factors. This approach is severely limited by the fact that the clinical presentation and the laboratory work up would not distinguish aspiration pneumonitis from aspiration pneumonia. Our findings reaffirm this conclusion. Nylen and colleagues (18) were the first to study the kinetics of serum calcitonin in 12 patients with infectious pneumonitis and 3 with witnessed episodes of aspiration more than a decade ago as a biomarker of pulmonary infection. Following admission, total serum calcitonin increased rapidly in all patients and progressively declined with clinical resolution. Interestingly, gel filtration chromatography confirmed at that time that the predominant circulating forms of calcitonin were the precursor forms including PCT. No single cutoff however could be established that would separate the two entities from each other. Subsequently, the utility of PCT as a diagnostic marker of gastric aspiration was investigated in 23 patients who presented with closed head injury (19). Nine patients had evidence of aspiration of gastric content, defined as the presence of persistent infiltrates on CXR in the first 24 hours after admission and/or signs of aspiration on bronchoscopy. The initial serum PCT levels in these patients were significantly higher compared to those who had no evidence of aspiration. PCT was also significantly higher in non-survivors. However, the study did not look at the role of PCT in differentiating bacterial aspiration pneumonia from chemical aspiration pneumonitis.
Overall, the accuracy of procalcitonin in diagnosing pneumonia in critically ill patients has been inconsistent (20–22). In fact, Luyt and colleagues (20) showed that for a cutoff of 0.5 ng/ml, procalcitonin had a sensitivity of 72% and a specificity of 24% for the diagnosis of VAP. Earlier, Gibot and coworkers (21) reported significant overlap in procalcitonin levels between patients with ventilator-associated pneumonia and those without pneumonia. Schuetz and colleagues (23) noted also in a retrospective study involving patients with therapeutic hypothermia following cardiac arrest, that the increase in PCT was unspecific and paralleled the intensity of inflammatory reaction rather than the underlying infection. We found that the case for using PCT in pulmonary aspiration syndromes is no different. In our study population, there was a significant overlap in the levels of PCT in both BAL positive and BAL negative cultures making any distinction between the two entities unachievable. In few cases of aspiration with negative BAL cultures, the levels of PCT were much higher than those with documented bacterial infection. This disparity could be related to a time lag of 24 to 48 hours which can exist between onset of bacterial infection and peak PCT release (24). Alternatively, acid-related lung injury may result in instantaneous extravasation of calcitonin precursor eclipsing the rise of PCT in bacterial aspiration pneumonia.
Our study results are in line with previous investigations pointing to a significant association between decreasing levels of PCT and favorable outcome. Seligman and coworkers (25) showed that repeated measurement of PCT at onset and on the fourth day of treatment can predict survival in patients with ventilator-associated pneumonia. A drop in either PCT or CRP was indicative of lower mortality. Similarly, Luyt et al (26) established in a comparable population that serial PCT levels may provide an early indication of unfavorable outcome. In the absence of immediate therapeutic modality for aspiration, prognostic biomarkers can be used as a guide in triaging patients to a lower level of care, directing utilization of scare resources, and approaching patients for participating in clinical trials. We should be careful however from overstating the role of PCT in prognosticating outcome of patients with the aspiration syndromes until further validation studies are performed.
More recently, PCT has been advocated as a clinical tool to guide antimicrobial therapy in patients presenting at the emergency department or for the treatment of lower respiratory tract infection (5,6,27,28). In a randomized controlled trial, Schuetz et al. (5) demonstrated that administration of antibiotics based on serial PCT determination with predefined cutoff ranges for initiating or stopping antibiotics for lower respiratory tract infections reduced antibiotic usage by 3 days. The reduction in antibiotics duration was achieved predominantly by early discontinuation of antibiotic therapy. In contrast, Stolz et al. (6) reported a 27% reduction in duration of antibiotics in the procalcitonin group mostly because the control group had received 15 days of antimicrobial therapy. Using a similar design, Bouadma and colleagues (9) extended these observations to patients with suspected infections on admission or during their stay in intensive care units. For patients in the procalcitonin group, there was a 23% reduction in antibiotic exposure compared to the standard approach. However, there were several concerns about the design of the trial that pertained to the 10% non-inferiority margin for mortality and the lack of adherence to the protocol in discontinuing antibiotic therapy.. Whether a similar algorithm-guided intervention can be developed for patients with suspected aspiration requires further investigations.
Some limitations of the study should be mentioned. In the absence of a “gold standard” diagnostic tool, the distinction between bacterial aspiration pneumonia and aspiration pneumonitis remains an arbitrary one. Even after relying on BAL cultures, the possibility of misclassification of either type of aspiration could have still occurred. Second, we have not measured BAL procalcitonin because previous studies have suggested that the local production of PCT within the alveoli is limited (29,30) and hence it plays a limited role in the diagnosis of lower respiratory tract infections. Third, the study population comprised of patients with high acuity of disease requiring mechanical ventilation. It is plausible that the range of PCT values in less severely ill patients may be different from the one observed in this study. Moreover, the study is limited by the inclusion of a control group of relatively small size with different baseline characteristics. Fourth, we have not assessed other biomarkers in the current study; however previous investigations found CRP to be of limited value in aspiration syndromes (14,31). Fifth, we have not determined the cost effectiveness of routine PCT measurements. Pragmatic evidence of the comparative and cost-effectiveness of different point-of-care strategies is needed.
In conclusion, serum PCT may not be useful as a diagnostic marker of bacterial pulmonary aspiration. However, serial serum procalcitonin may provide an early indication of outcome in pulmonary aspiration syndromes.
Acknowledgments
Grant support: National Institutes of Health- RO1HL48889 (PRK)
Dr. Knight has received funding/grant support from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors do not have any potential conflicts of interest to disclose.
REFERENCES
- 1.Assicot M, Bohuon C, Gendrel D, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gendrel D, Bohuon C. Procalcitonin as a marker of bacterial infection. Pediatr Infect Dis J. 2000;19:679–687. doi: 10.1097/00006454-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 4.Simon P, Milbrandt E, Emlet L. Procalcitonin-guided antibiotics in severe sepsis. Critical Care. 2008;12:309. doi: 10.1186/cc7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 6.Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomized study. Eur Respir J. 2009;34:1364–1375. doi: 10.1183/09031936.00053209. [DOI] [PubMed] [Google Scholar]
- 7.Hochreiter M, Köhler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13:R83. doi: 10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 9.Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomized controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 10.Remskar M, Horvat M, Hojker S, et al. Procalcitonin in patients with acute myocardial infarction. Wien Klin Woschenschr. 2002;114:205–210. [PubMed] [Google Scholar]
- 11.Kallio R, Surcel HM, Bloigu A, et al. C-reactive protein, procalcitonin, and interleukin-8 in the primary diagnosis of infections in cancer patients. Eur J Cancer. 2000;36:889–894. doi: 10.1016/s0959-8049(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 12.Martinot M, Hansmann Y, De Martino S, et al. Procalcitonin in pyelonephritis and acute community-acquired pneumonia in adults. Press Med. 2001;30:1091–1096. [PubMed] [Google Scholar]
- 13.Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta. 2002;323:17–29. doi: 10.1016/s0009-8981(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 14.El-Solh AA, Akinnusi ME, Peter M, et al. Triggering receptors expressed on myeloid cells in pulmonary aspiration syndromes. Intensive care Med. 2008;34(6):1012–1019. doi: 10.1007/s00134-008-1087-7. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 17.El-Solh AA, Aquilina AT, Dhillon RS, et al. Impact of invasive strategy on management of antimicrobial treatment failure in institutionalized older people with severe pneumonia. Am J Respir Crit Care Med. 2002;166(8):1038–1043. doi: 10.1164/rccm.200202-123OC. [DOI] [PubMed] [Google Scholar]
- 18.Nylén ES, Snider RH, Thompson KA, et al. Pneumonitis-associated hyperprocalcitonemia. Am J Med Sci. 1996;312(1):12–18. doi: 10.1097/00000441-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Pusch F, Wildling E, Freitag H, et al. Procalcitonin as a diagnostic marker in patients with aspiration after closed head injury. Wien Klin Wochenschr. 2001;113(17–18):676–680. [PubMed] [Google Scholar]
- 20.Luyt CE, Combes A, Reynaud C, et al. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 2008;34(8):1434–1440. doi: 10.1007/s00134-008-1112-x. [DOI] [PubMed] [Google Scholar]
- 21.Gibot S, Cravoisy A, levy B, et al. Soluble triggering receptor expressed on myeloid cell and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 22.Jung B, Embriaco N, Roux F, et al. Microbiogical data, but not procalcitonin improve the accuracy of the clinical pulmonary infection score. Intensive Care Med. 2010;36:790–798. doi: 10.1007/s00134-010-1833-5. [DOI] [PubMed] [Google Scholar]
- 23.Schuetz P, Affolter B, Hunziker S, et al. Serum procalcitonin, C-reactive protein and white blood cell levels following hypothermia after cardiac arrest: a retrospective cohort study. Eur J Clin Invest. 2010;40(4):376–381. doi: 10.1111/j.1365-2362.2010.02259.x. [DOI] [PubMed] [Google Scholar]
- 24.Meisner M. Procalcitonin: experience with a new diagnostic tool for bacterial infection and systemic inflammation. J Lab Med. 1999;23:263–272. [Google Scholar]
- 25.Seligman R, Meisner M, Lisboa TC, et al. Decreases in procalcitonin and C-reactive protein are strong predictors of survival in ventilator-associated pneumonia. Crit Care. 2006;10(5):R125. doi: 10.1186/cc5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luyt CE, Guerin V, Combes A, et al. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2005;171:48–53. doi: 10.1164/rccm.200406-746OC. [DOI] [PubMed] [Google Scholar]
- 27.Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:3–5. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 28.Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168:2000–2007. doi: 10.1001/archinte.168.18.2000. [DOI] [PubMed] [Google Scholar]
- 29.Linssen CF, Bekers O, Drent M, et al. C-reactive protein and procalcitonin concentrations in bronchoalveolar lavage fluid as a predictor of ventilator-associated pneumonia. Ann Clin Biochem. 2008;45(pt 3):293–298. doi: 10.1258/acb.2007.007133. [DOI] [PubMed] [Google Scholar]
- 30.Duflo F, Debon R, Monneret G, et al. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology. 2002;96:74–79. doi: 10.1097/00000542-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Mylotte J, Goodnough S, Gould M. Pneumonia versus pneumonitis in nursing home residents: prospective application of a clinical algorithm. J Am Geriatr Soc. 2005;53:755–761. doi: 10.1111/j.1532-5415.2005.53258.x. [DOI] [PubMed] [Google Scholar]