Abstract
InlB is a Listeria monocytogenes protein that promotes entry of the bacterium into mammalian cells by stimulating tyrosine phosphorylation of the adaptor proteins Gab1, Cbl and Shc, and activation of phosphatidyl– inositol (PI) 3-kinase. Using affinity chromatography and enzyme-linked immunosorbent assay, we demonstrate a direct interaction between InlB and the mammalian protein gC1q–R, the receptor of the globular part of the complement component C1q. Soluble C1q or anti-gC1q–R antibodies impair InlB-mediated entry. Transient transfection of GPC16 cells, which are non-permissive to InlB-mediated entry, with a plasmid-expressing human gC1q–R promotes entry of InlB-coated beads. Furthermore, several experiments indicate that membrane recruitment and activation of PI 3-kinase involve an InlB–gC1q–R interaction and that gC1q–R associates with Gab1 upon stimulation of Vero cells with InlB. Thus, gC1q–R constitutes a cellular receptor involved in InlB-mediated activation of PI 3-kinase and tyrosine phosphorylation of the adaptor protein Gab1. After E–cadherin, the receptor for internalin, gC1q–R is the second identified mammalian receptor promoting entry of L.monocytogenes into mammalian cells.
Keywords: gC1q-R/InlB/invasion/Listeria/signaling
Introduction
Most infectious diseases involve binding of a microorganism to a host cell as a critical step in tissue colonization, resulting in either localization of the microorganism on the surface of the host cell or internalization into an intracellular niche. Some intracellular pathogens have the ability to induce their own entry into mammalian cells that are non-phagocytic. A variety of strategies to promote their entry into host cells have been described that often imply participation of both bacterial factors and host cell surface components. In the case of Salmonella or Shigella, invasion is a multifactorial process (Galan, 1996; Nhieu and Sansonetti, 1999), and increasing evidence suggests that bacterial factors directly translocated in the host cell play a critical role. Some receptors of these proteins have been identified (Watarai et al., 1996; Pier et al., 1998). In contrast, entry of Yersinia or Listeria into cultured cells implies direct interaction between a bacterial ligand and a mammalian receptor (reviewed in Finlay and Cossart, 1997; Ireton and Cossart, 1998). For Yersinia, the outer membrane protein invasin binds to β1 integrin receptors, leading to bacterial entry (Isberg and Leong, 1990). Listeria monocytogenes has developed a similar strategy to enter the human enterocyte-like epithelial cell line Caco-2 and some other epithelial cells. In these cells, E–cadherin, a cell surface molecule normally involved in calcium-dependent cell–cell adhesion, is the receptor for the bacterial protein internalin (InlA) (Mengaud et al., 1996a; Lecuit et al., 1999). Interestingly, entry of L.monocytogenes into most cell lines is not promoted by internalin but requires InlB, a bacterial protein that does not use E–cadherin as a receptor (Cossart and Lecuit, 1998).
InlB is a 630 amino acid surface protein that promotes bacterial internalization into a wide variety of cultured cell lines including Vero, HEp-2, HeLa and some hepatocytes and endothelial cells (Dramsi et al., 1995; Lingnau et al., 1995; Ireton et al., 1996; Parida et al., 1998). InlB is not only associated with the bacterial surface, but also found in culture supernatants of L.monocytogenes, indicating that a fraction of this protein is secreted or released from the bacterial surface (Lingnau et al., 1995; Braun et al., 1997; Jonquières et al., 1999). The loose association of InlB with the bacterial surface is mediated by the so-called GW repeats located in the 232 amino acid C-terminal region of InlB, which bind to the bacterial membrane component lipoteichoic acid (Braun et al., 1997; Jonquières et al., 1999). InlB, present on the surface of non-invasive bacteria or on latex beads, is sufficient to induce uptake (Braun et al., 1998). The contribution of released InlB to the entry process is unknown. While the role of InlA in virulence remains to be established, several reports indicate that InlB plays a role in the hepatic phase of the infection (Dramsi et al., 1995; Gaillard et al., 1996; Gregory et al., 1997).
The InlB-mediated entry of L.monocytogenes into cultured cells requires bacterial stimulation of phosphatidyl– inositol (PI) 3-kinase (Ireton et al., 1996). Activation of this lipid kinase appears to occur through tyrosine phosphorylation of three adaptor proteins Gab1, Cbl and Shc that may help recruitment of the kinase to the InlB receptor (Ireton et al., 1999). InlB is sufficient to activate PI 3-kinase in mammalian cells since a recombinant InlB protein stimulates accumulation of the lipid products of this kinase and tyrosine phosphorylation of the three adaptor proteins.
gC1q–R is a ubiquitous protein, originally identified as a membrane protein that binds to the globular ‘heads’ of C1q (Ghebrehiwet et al., 1994). This receptor now appears as a multifunctional protein with affinity for diverse ligands (reviewed in Ghebrehiwet and Peerschke, 1998). Here we identify gC1q–R as a receptor for InlB and demonstrate that it plays a role in bacterial invasion of non-phagocytic cells.
Results
Mammalian cells bind to InlB
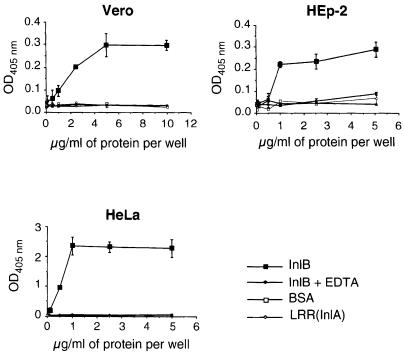
We have shown previously that InlB bound to non-invasive bacteria or latex beads promoted entry into InlB-permissive cell lines and that pre-treatment of Vero cells with purified InlB inhibited entry of L.monocytogenes, indicating that interaction between InlB and mammalian cells is essential for the entry process (Braun et al., 1998, 1999). A simple hypothesis for how this entry process could be induced would be that InlB binds to a cellular receptor. To address this issue, we determined whether mammalian cells were able to bind purified InlB. Microtiter plates (96-well) were coated with increasing concentrations of InlB and incubated with Vero cells. The number of bound cells was quantitated by assaying lysosomal hexosaminidase (Figure 1). Vero cells were able to bind to wells coated with InlB. In contrast, no cells bound to uncoated wells or wells coated with bovine serum albumin (BSA) or LRR(InlA), a recombinant protein containing the LRR region of InlA (Lecuit et al., 1997). Binding was concentration dependent and saturable and was totally inhibited with 10 mM EDTA, suggesting that divalent cations were required for interaction of InlB with Vero cells. Similar results were obtained with HEp-2 and HeLa cells, two cell lines permissive to InlB-mediated entry (Figure 1).
Fig. 1. Mammalian cells bind to purified InlB. Microtiter plates were coated with increasing concentrations of InlB, LRR(InlA) or BSA, and a suspension of Vero, HEp-2 or HeLa cells was added in either the absence or the presence of 10 mM EDTA. After incubation for 1 h, wells were washed and the number of bound cells was determined by assaying for lysosomal hexosaminidase. Values represent the mean ± SEM of three independent experiments.
InlB binds to a 33 kDa protein
In order to detect a putative InlB receptor, we used a ligand overlay assay. Solubilized Vero cell proteins separated by SDS–PAGE and transferred onto a nitrocellulose membrane were probed with purified InlB. Bound InlB was detected with the InlB-specific monoclonal antibody H15.1 (Braun et al., 1999) and horseradish peroxidase (HRP)-conjugated secondary antibody. InlB bound predominantly to a protein with an apparent mol. wt of ∼33 kDa (Figure 2). We verified that H15.1 antibody did not cross-react with Vero components and that no binding was observed when the membrane was overlaid with LRR(InlA) (data not shown).
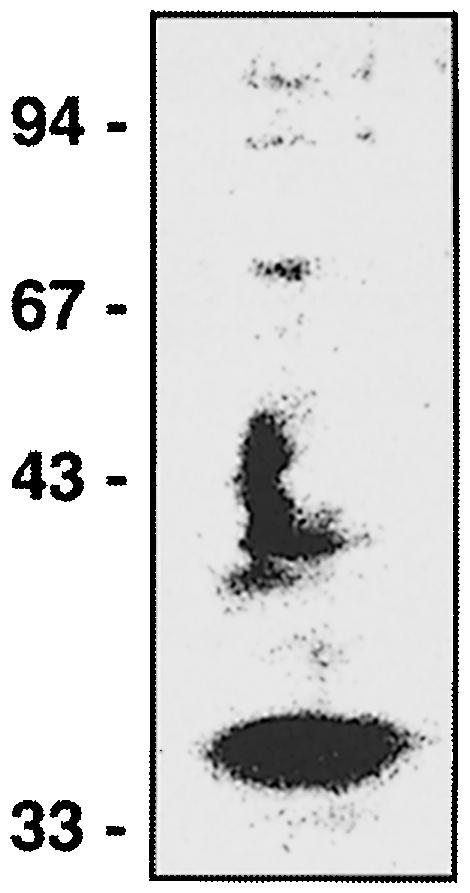
Fig. 2. InlB binding to electrophoretically separated soluble proteins from Vero cells by ligand overlay assay. Soluble proteins from Vero cells were separated on an 8% polyacrylamide gel and transferred onto a nitrocellulose membrane. Proteins on nitrocellulose were then probed with 50 μg/ml InlB and subsequently detected with the InlB-specific monoclonal antibody H15.1 (Braun et al., 1999).
gC1q–R binds to InlB
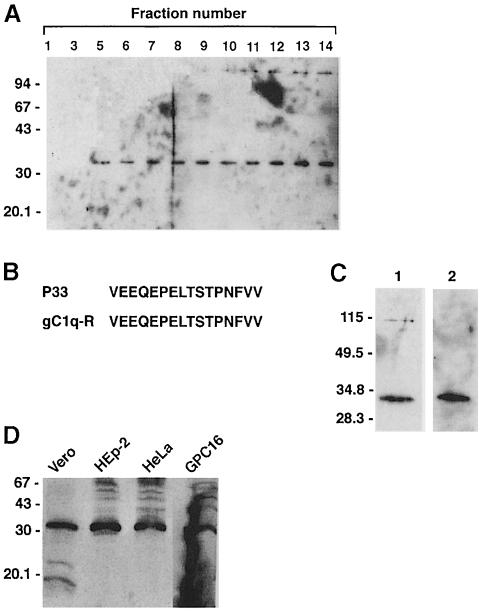
To identify the InlB receptor, we used a purification protocol based on preparation of affinity matrices of purified InlB (Isberg and Leong, 1990; Mengaud et al., 1996a). Vero cell surface proteins were labeled using the membrane-impermeant biotinylation reagent NHS-LC-biotin. After labeling, detergent-soluble proteins were extracted with n-octyl glucoside, and loaded onto an InlB agarose column. After extensive washes, proteins bound to InlB were eluted with EDTA, separated by electrophoresis, transferred onto a nitrocellulose membrane and detected using streptavidin covalently coupled to HRP. This technique revealed a major biotinylated protein with an apparent mol. wt of 33 kDa (P33) (Figure 3A). As a control, we used a column of BSA and passed Vero cell extracts over it. No biotinylated protein was detected after EDTA elution. Moreover, when the flow-through of unbound proteins from the BSA column was loaded onto the InlB column, the same protein of 33 kDa was eluted, indicating that this protein interacts specifically with InlB (data not shown).
Fig. 3. gC1q–R is a ligand for InlB. (A) N-octyl glucoside extracts were prepared from surface biotin-labeled Vero cell extracts and loaded onto an InlB affinity column. After extensive washing, proteins were eluted with 10 mM EDTA, and 200 μl fractions were collected. Fraction samples (10 μl) were analyzed by SDS–PAGE, transferred onto nitrocellulose and probed with streptavidin coupled to peroxidase to detect biotinylated proteins by chemiluminescence. (B) Amino acid sequence comparison of internal sequences from P33 isolated on the displayed column and gC1q–R encoded by human lymphocytes (Ghebrehiwet et al., 1994). (C) Western blot analysis of an elution fraction (15 μl) with streptavidin (1) or with a polyclonal antibody directed against gC1q–R (2). (D) Western blot analysis of the cellular expression of gC1q–R. A 100 μg aliquot of solubilized Vero, HEp-2, HeLa or GPC16 cell membrane proteins was separated by SDS–PAGE and transferred onto nitrocellulose. gC1q–R was revealed with an anti-gC1q–R polyclonal antibody.
In order to identify the 33 kDa protein, the pooled EDTA eluate was loaded on a 12% polyacrylamide gel. The 33 kDa polypeptide was then subjected to internal amino acid sequencing. A 16 amino acid sequence was obtained. A search in the protein databanks showed that this sequence was identical to an internal sequence of a known protein, gC1q–R, the receptor for the globular heads of the complement component C1q, which has an apparent mol. wt of 33 kDa (Ghebrehiwet et al., 1994) (Figure 3B). In Western blot experiments, the 33 kDa protein reacted specifically with a polyclonal antibody directed against gC1q–R (Figure 3C), indicating that this protein is antigenically related to gC1q–R. Taken together, these results demonstrate that InlB interacts with a cellular receptor named gC1q–R.
Since InlB-mediated entry takes place in a wide variety of cultured cell lines, and since Vero, HEp-2 and HeLa cells bind to InlB, it was essential to verify that gC1q–R was expressed in these cells. Membrane protein extracts of Vero, HEp-2 and HeLa cells were prepared and analyzed by Western blotting using an anti-gC1q–R polyclonal antibody. For the three cell lines, the antibody recognized the same protein with an approximate mol. wt of 33 kDa (Figure 3D). These results indicate that gC1q–R is expressed in the InlB-permissive Vero, HEp-2 and HeLa cells, in agreement with the reported ubiquitous expression of this protein (Ghebrehiwet et al., 1994).
InlB interacts with purified gC1q–R
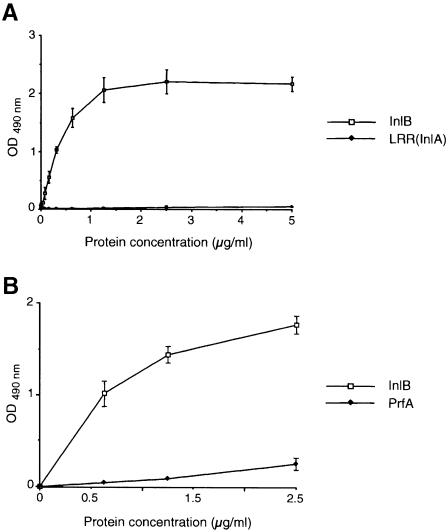
Interaction of InlB with gC1q–R was established definitively by an enzyme-linked immunosorbent assay (ELISA). Wells of microtiter plates were coated with recombinant gC1q–R and incubated with increasing concentrations of purified InlB or LRR(InlA) used as a control. Bound protein was detected using the InlB-specific monoclonal antibody B4.6 (Braun et al., 1999) for InlB and the InlA-specific monoclonal antibody G6.1 (Mengaud et al., 1996b) for LRR(InlA) (Figure 4A). InlB was able to bind to gC1q–R in a concentration-dependent and saturable manner. In contrast, LRR(InlA) was unable to bind gC1q–R. To analyze further the specificity of the interaction between InlB and gC1q–R, gC1q–R-coated wells were incubated with different concentrations of PrfA, a cytoplasmic protein of L.monocytogenes with an isoelectric point close to that of InlB (9.1 versus 9.8, respectively). PrfA was unable to bind gC1q–R efficiently (Figure 4B). Taken together, these results indicate that the interaction between InlB and gC1q–R is direct and specific.
Fig. 4. InlB binds to gC1q–R. Wells of a microtiter plate were coated with a solution of 1 μg/ml gC1q–R. After blocking with a 1% BSA solution, wells were incubated with increasing concentrations of purified proteins, either InlB, LRR(InlA) (A) or PrfA (B), and then analyzed by ELISA as described in Materials and methods.
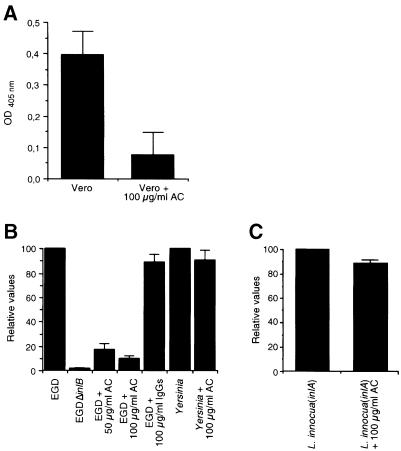
C1q competes with InlB for binding to gC1q–R and inhibits entry of L.monocytogenes into mammalian cells
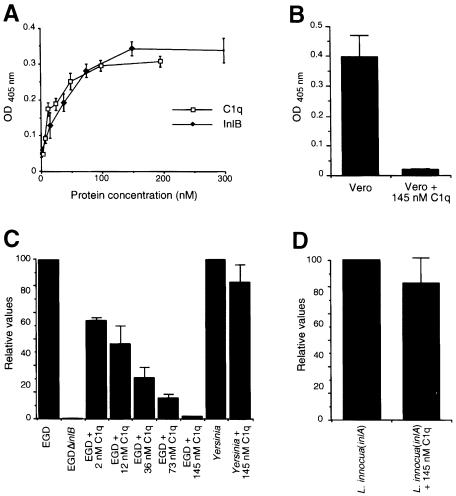
To gain further insight into the relevance of gC1q–R in the InlB-mediated entry process, we used soluble C1q, a ligand of gC1q–R, as a potential competitive inhibitor. We first studied the ability of Vero cells to bind to C1q-coated wells using the hexosaminidase assay (Figure 5A). Vero cells were able to bind to wells coated with C1q in a saturable and C1q concentration-dependent manner, as was observed with InlB-coated wells.
Fig. 5. C1q inhibits entry of EGD into Vero cells. (A) Comparison of the binding of Vero cells to wells coated with increasing concen- trations of InlB or C1q using the colorimetric hexosaminidase assay. (B) Effect of C1q on the binding of Vero cells to InlB. Microtiter wells coated with a 10 μg/ml concentration of InlB were incubated with a Vero cell suspension that had been treated or not for 5 min at 37°C with 145 nM C1q. After allowing 1 h for attachment of the Vero cells to immobilized InlB, wells were washed, and cell attachment was quantified using the colorimetric hexosaminidase assay. (C and D) Effect of C1q on entry of L.monocytogenes EGD and Yersinia into Vero cells (C) or on entry of L.innocua(inlA) into Caco-2 cells (D). The inhibitory effect of C1q was tested by incubating the cells with different concentrations of C1q, 5 min before adding the bacteria. The number of intracellular bacteria was determined using gentamicin survival assays as described in Materials and methods. Values along the vertical axis are given relative to efficiencies of invasion of L.monocytogenes EGD in Vero cells or L.innocua(inlA) in Caco-2 cells (D), which are fixed arbitrarily at 100.
Pre-treatment of a Vero cell suspension for 5 min at 37°C with 145 nM soluble C1q, a concentration determined to be maximal, resulted in a 95% reduction of Vero cell binding to InlB (Figure 5B). Similar results were obtained with HEp-2 and HeLa cells (data not shown), suggesting that InlB and C1q interact with the same sites, or sites located close by in gC1q–R.
We then studied the effect of C1q on entry of L.monocytogenes strain EGD into Vero cells. Pre-treatment of Vero cells with different concentrations of C1q for 5 min at 37°C prior to infection inhibited entry of EGD (Figure 5C). The inhibition was concentration dependent and maximal at 145 nM (98% inhibition). At this con– centration, C1q has no effect on entry of a Yersinia pseudotuberculosis strain YPIIIc cured of its virulence plasmid and which is internalized due to the interaction between invasin and its cellular receptor of the integrin β1 family (Isberg and Leong, 1990). The same results were obtained with HEp-2 and HeLa cells (data not shown). In contrast, pre-treatment of Caco-2 cells, which express gC1q–R (data not shown) and in which entry is mostly InlA dependent, with 145 nM C1q had no inhibitory effect on entry of Listeria innocua(inlA) (Figure 5D), indicating that C1q inhibition of Listeria entry is specific for the InlB-mediated entry. Taken together, these results indicate that the binding of InlB to gC1q–R is required for InlB-mediated entry.
Antibodies directed against gC1q–R inhibit entry of L.monocytogenes into cells
To investigate further the role of gC1q–R in bacterial internalization, we examined the ability of a polyclonal antibody directed against the 18 amino acid N-terminal peptide of gC1q–R to inhibit the InlB-mediated entry of L.monocytogenes EGD. We first analyzed whether binding of Vero cells to InlB was gC1q–R dependent by examining whether anti-gC1q–R antibodies had inhibitory effects using the microtiter plate binding assay. Pre-treatment of a Vero cell suspension for 60 min at 37°C with 100 μg/ml antibodies resulted in an 81% reduction of Vero cell binding (Figure 6A), indicating that the binding to gC1q–R is required for Vero cell attachment to InlB.
Fig. 6. Anti-gC1q–R antibodies inhibit entry of EGD into Vero cells. (A) Effect of anti-gC1q–R antibodies on the binding of Vero cells to InlB. Microtiter wells coated with a 10 μg/ml concentration of InlB were incubated with a Vero cell suspension that had been treated or not with 100 μg/ml antibodies (AC) for 60 min at 37°C. Cell attachment to InlB was quantitated using the colorimetric hexos- aminidase assay. (B and C) Effect of anti-gC1q–R antibodies on entry of EGD and Yersinia into Vero cells (B) or L.innocua(inlA) into Caco-2 cells (C). Vero or Caco-2 cells were incubated with anti-gC1q–R antibodies or pre-immune rabbit IgGs for 60 min before adding the bacteria. The inhibitory effect of the antibodies was tested by incubating the cells with the antibodies for 60 min before adding the bacteria. The number of intracellular bacteria was determined using gentamicin survival assays as described in Materials and methods. Values along the vertical axis are given relative to efficiencies of invasion of EGD in Vero cells or L.innocua(inlA) in Caco-2 cells (C), which are the reference (value of 100).
In a second step, we determined whether these antibodies that reduce attachment to InlB could also interfere with InlB-mediated entry. Incubation of Vero cells with anti-gC1q–R antibodies reduced entry of wild-type L.monocytogenes (Figure 6B). Inhibition of entry was specific since it was observed neither with the Y.pseudotuberculosis strain YPIIIc nor when Vero cells were pre-treated with rabbit IgGs used at the same concentration (Figure 6B). Similar results were obtained with HEp-2 and HeLa cells (data not shown). This inhibition was specific for the InlB-mediated pathway of entry since pre-treatment of Caco-2 cells with these antibodies had no effect on entry of L.innocua(inlA) (Figure 6C). These results strongly suggest that gC1q–R is acting as a receptor for the InlB protein of the L.monocytogenes strain EGD.
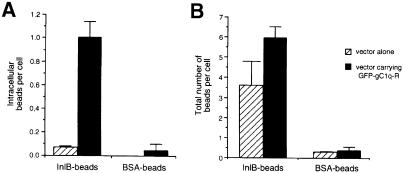
Adhesion and entry of InlB-coated beads into gC1q–R-transfected GPC16 cells
GPC16 cells are guinea-pig cells of epithelial origin, which exhibit a high level of InlA-dependent entry but no significant InlB-dependent entry (Lecuit et al., 1999; M.Lecuit and P.Cossart, unpublished results). In order to determine whether the absence of InlB-dependent entry was due to the absence of gC1q–R, we analyzed the presence of gC1q–R in membrane protein extracts of GPC16 cells by Western blotting using anti-gC1q–R polyclonal antibodies. A cross-reacting protein was present in these extracts, in amounts similar to those found in Vero, HEp-2 or HeLa cells (Figure 3D), suggesting that gC1q–R is not accessible or is not in the proper conformation for InlB-mediated entry or that the residues critical for interaction with InlB are absent or that other factors are required. To investigate whether expression of human gC1q–R in GPC16 cells would affect adhesion and entry of InlB-coated beads, we transfected GPC16 cells with a plasmid expressing a green fluorescent protein (GFP)-tagged human gC1q–R. The GFP intrinsic fluorescence allows direct visualization of transfected cells. Transfected cells were tested for their ability to promote adhesion and entry of InlB-coated beads. Results reported in Figure 7A provide evidence that transfected GPC16 cells promote entry of InlB-coated beads. Entry was 14 times more efficient in transfected cells than in cells transfected with the vector alone. Interestingly, adhesion of InlB-coated beads was not significantly affected in cells expressing the GFP–gC1q–R fusion protein (p = 0.122) (Figure 7B). This effect of GFP–gC1q–R fusion protein on entry seemed to be InlB specific since it was not observed with BSA-coated beads (Figure 7A and B). Taken together, the transfection experiments indicate that human gC1q–R plays a critical role in the internalization step.
Fig. 7. InlB-dependent entry into gC1q–R-transfected GPC16 cells. GPC16 cells were transiently transfected with a control plasmid (hatched bars) or with a plasmid carrying a GFP–gC1q–R fusion protein (black bars) at a multiplicity of ∼100 beads per cell for 1 h. Internalized (A) and associated (B) beads were identified and quantified using immunofluorescence as described previously (Braun et al., 1998). The efficiency of entry is expressed as the average number of intracellular beads per cell. Association corresponds to the number of beads either bound or internalized. The results are expressed as the mean ± SD of three independent experiments.
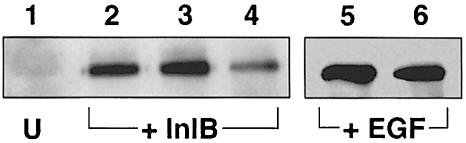
Antibodies against gC1q–R reduce association of p85 with tyrosine-phosphorylated proteins
Entry of L.monocytogenes into Vero cells activates the p85/p110 PI 3-kinase and tyrosine phosphorylation of three mammalian adaptor proteins, Gab1, Cbl and Shc (Ireton et al., 1999). InlB plays a direct role in this process since purified soluble InlB is able to activate this kinase (Ireton et al., 1999). In order to determine whether gC1q–R was implicated in the PI 3-kinase activation pathway, we analyzed the effect of anti-gC1q–R antibodies on the InlB-dependent stimulation of association of p85 with tyrosine-phosphorylated proteins in Vero cells. Rabbit IgGs were used as a control. As shown in Figure 8, the amount of p85 decreased when cells were pre-treated with the antibodies (compare lanes 2 and 4). In contrast, rabbit IgGs used at the same concentration had no effect (lane 3). Moreover, anti-gC1q–R antibodies had no significant effect on the association of p85 in anti-phosphotyrosine immunoprecipitates prepared from epidermal growth factor (EGF)-treated cells (compare lanes 5 and 6). These results suggest that recruitment of p85 involves an interaction between InlB and gC1q–R.
Fig. 8. Anti-gC1q–R antibodies decrease the InlB-dependent association of p85 with tyrosine-phosphorylated proteins. Vero cells were pre-treated for 1 h with DMEM (lanes 1, 2 and 5), with 100 μg/ml rabbit IgGs (lane 3) or with 100 μg/ml anti-gC1q–R antibodies (lanes 4 and 6). Cells were then left untreated (U) (lane 1), or treated with 3 nM InlB (lanes 2–4) or 17 nM EGF for 1 min (lanes 5 and 6). The cells were then washed with PBS and lysed in the immunoprecipitation buffer. Tyrosine-phosphorylated proteins were immunoprecipitated with anti-P-tyr antibodies, separated by SDS–PAGE, and p85 was detected by immunoblotting with anti-p85 antibodies. The anti-gC1q–R antibodies decrease the InlB-induced association of p85 with tyrosine-phosphorylated proteins (lane 4), but had no effect on EGF-induced association of p85 with tyrosine-phosphorylated proteins (lane 6). Similar amounts of material were used for each immunoprecipitation. The results are representative of three experiments.
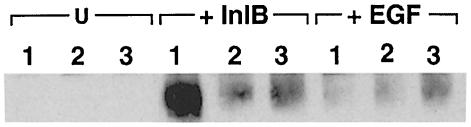
Interaction of gC1q–R with the adaptor protein Gab1
The adaptor protein Gab1 was shown to be tyrosine phosphorylated and to co-precipitate with p85 in response to InlB in Vero cells (Ireton et al., 1999). In order to examine the possible association of gC1q–R with Gab1, we investigated the ability of a recombinant His6-gC1q–R protein to associate with Gab1. Extracts from Vero cells treated with InlB or EGF used as a control were incubated with His6-gC1q–R and protein complexes were precipitated with agarose–nickel beads. The presence of tyrosine-phosphorylated Gab1 in the precipitates was then analyzed by ‘re-immunoprecipitation’ experiments with anti-Gab1 antibodies as described previously (Ireton et al., 1999). As shown in Figure 9, tyrosine-phosphorylated Gab1 was able to co-precipitate with gC1q–R upon stimulation with InlB but not upon stimulation with EGF. Tyrosine-phosphorylated Gab1 was barely detectable when cell lysates were incubated without recombinant protein or with LRR(InlA)-His6, used as a control (Figure 9). These results suggest that there is a significant gC1q–R–Gab1 association in Vero cells stimulated with InlB.
Fig. 9. Association of gC1q–R with tyrosine-phosphorylated Gab1. Vero cells were left untreated (U), or were treated with 3 nM InlB or 17 nM EGF for 1 min. Solubilized lysates were then prepared by addition of immunoprecipitation buffer, and 2.5 mg of lysate were incubated for 2 h at 4°C in the presence of 5 μg His6-gC1q–R (1), 5 μg LRR(InlA)-His6 (2) or buffer alone (3). After addition of Ni-NTA–agarose beads, the adsorbed proteins were dissociated by boiling in the presence of 0.5% SDS/5 mM dithiothreitol, diluted in re-immunoprecipitation buffer and re-precipitated with antibodies against Gab1. Tyrosine-phosphorylated proteins in the final immunoprecipitates were detected with anti-P-Tyr antibodies. Similar amounts of starting material were used for each re-precipitation. The results are represen- tative of two experiments.
Discussion
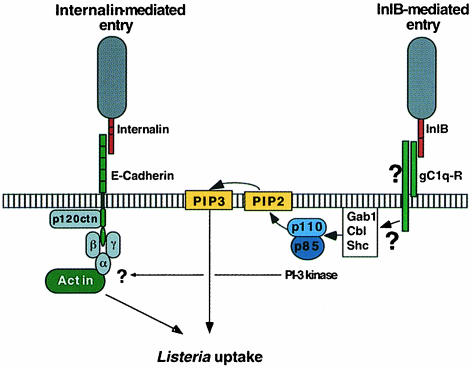
This study is the first report describing the identification of an InlB receptor. After E–cadherin, the receptor for internalin, gC1q–R is the second identified cellular protein used by L.monocytogenes for entry into cells (Figure 10). This 33 kDa protein (also called p32) is a highly acidic and ubiquitous cell membrane protein that binds to the globular heads of C1q, the first component of complement present in the serum. Although gC1q–R was first identified as a protein displaying affinity for the globular ‘heads’ of C1q, it is also able, as shown recently, to bind multiple ligands including thrombin, vitronectin, high molecular weight kininogen and the HIV-1 Tat transactivator (Fridell et al., 1995; Yu et al., 1995; Herwald et al., 1996; Joseph et al., 1996; Lim et al., 1996). InlB represents a new ‘ligand’ for gC1q–R. We have demonstrated that gC1q–R binds to InlB in a divalent cation-dependent manner. However, a requirement for divalent cations is not a general feature of the binding of gC1q–R to its various ligands. Indeed, binding of C1q to gC1q–R is not metal ion dependent (Ghebrehiwet et al., 1994) while the binding of high molecular weight kininogen to gC1q–R on endothelial cells is strictly zinc dependent (Joseph et al., 1996). A requirement for divalent cations seems to be a common theme in bacterial induced phagocytosis. Receptors for the Y.pseudotuberculosis invasin, i.e. β1 integrins, and for the L.monocytogenes internalin, E–cadherin, also require divalent cations. β1 integrins and E–cadherins are members of large families and are found on a variety of cell types. gC1q–R is not a member of a large family but is expressed on a wide range of cell types including lymphocytes, neutrophils, hepatocytes and endothelial cells (Ghebrehiwet et al., 1994; Eggleton et al., 1995; Peerschke et al., 1996). No cell line devoid of gC1q–R has yet been reported. This ubiquitous distribution correlates well with the role of InlB for entry of L.monocytogenes into a wide range of cell types.
Fig. 10. The two pathways of entry of L.monocytogenes into mammalian cells.
Binding to gC1q–R appears to be critical for InlB-dependent entry into mammalian cells because both C1q, a ligand of gC1q–R, and a polyclonal anti-gC1q–R antibody were able to inhibit attachment of mammalian cells to purified InlB. In line with these results, the wild-type L.monocytogenes strain EGD was unable to enter Vero, HEp-2 or HeLa cells pre-treated with C1q or anti-gC1q–R antibody. These data are consistent with a direct interaction between InlB and gC1q–R during entry, and illustrate that a first requirement for entry is bacterial attachment to gC1q–R. That InlB-coated beads enter into GPC16 cells transfected with a GFP–gC1q–R fusion protein definitively establishes the critical role of gC1q–R in the entry process. Why the endogenous gC1q–R of the GPC16 cells was unable to promote entry remains unclear. It is possible that InlB could interact with other surface structures in addition to gC1q–R and that these other factors could be required for entry. The hypothesis of an additional receptor is highly probable in view of the gC1q–R amino acid sequence itself. Indeed, gC1q–R is devoid of a typical hydrophobic transmembrane-spanning region or of a consensus site for glycosylphosphatidylinositol anchoring. The mode of attachment of gC1q–R to the surface membrane is unclear (Ghebrehiwet et al., 1994). The crystal structure of human gC1q–R has recently been determined (Jiang et al., 1999) but did not shed light on the understanding of cell surface attachment. In addition, gC1q–R localizes not only to the cell surface (Eggleton et al., 1995) but also to intracellular compartments (Dedio et al., 1998). However, the functional role of cytosolic gC1q–R, if any, remains to be determined. It has been proposed that gC1q–R would be tethered to the cell surface by as yet unknown intrinsic membrane proteins. These docking proteins, by interacting with gC1q–R, could also be implicated in InlB-dependent entry and could also associate with InlB. The possibility of multiple protein–protein interactions is well served by the structure of the InlB LRR domain, whose crystal structure has been determined recently (Marino et al., 1999). Its elongated shape could provide an extended surface for protein–protein interactions. Identification of an eventual InlB co-receptor is currently under investigation.
The regions of gC1q–R and that of InlB involved in the gC1q–R–InlB interaction are unknown. The highly acidic N-terminal part of gC1q–R has been shown to contain a binding site for C1q (Ghebrehiwet et al., 1994) and to mediate the binding of gC1q–R to the heparin-binding forms of vitronectin (Lim et al., 1996). It probably also contains a binding site for InlB since C1q or anti-gC1q–R antibodies directed against this region inhibit InlB-dependent entry into mammalian cells.
Activation of PI 3-kinase and tyrosine phosphorylation of the adaptor proteins Gab1, Cbl and Shc are among the early signal transduction events that take place during InlB-mediated entry of L.monocytogenes (Ireton et al., 1996, 1999). In this work, we show that anti-gC1q–R antibody decreases association of PI 3-kinase with tyrosine-phosphorylated proteins induced by InlB and that gC1q–R is associated with tyrosine-phosphorylated Gab1. Whether this association is direct or not remains to be determined. These findings favor the hypothesis for a role of gC1q–R in the signaling pathway leading to activation of PI 3-kinase. Signal transduction events associated with gC1q–R responses including inositol triphosphate production on platelets (Peerschke et al., 1993), chemo– taxis on fibroblasts (Oiki and Okada, 1988) or Fc-mediated phagocytosis by neutrophils (Bobak et al., 1987) are poorly understood. It will be important to analyze PI 3-kinase activation during these events.
The identification of gC1q–R as an InlB receptor provides an important insight into understanding the different steps leading to L.monocytogenes uptake by mammalian cells. Whether other receptors in addition to gC1q–R are required for InlB-mediated entry and, if any, how these molecules are involved in the infectious process of L.monocytogenes remain challenging issues.
Materials and methods
Bacterial strains and cell lines
Listeria strains were cultured in brain heart infusion broth or plates at 37°C. For invasion assays, we used the wild-type L.monocytogenes strain EGD (BUG 600) and its ΔinlB isogenic mutant (BUG 947), and a recombinant L.innocua strain expressing internalin (BUG 991) and its isogenic counterpart harboring the plasmid vector alone (BUG 994) (Dramsi et al., 1995). Yersinia pseudotuberculosis strain YPIIIc was grown in Luria–Bertani broth.
The African green monkey kidney cell line Vero, the human laryngeal epithelial cell line HEp-2, the human cervical epithelial cell line HeLa and the human enterocyte-like cell line Caco-2 were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco-BRL) supplemented with 10% fetal calf serum (FCS; Valbiotech), 2 mM l-glutamine (Sigma) and 1% non-essential amino acids (Gibco-BRL) at 37°C in 10% CO2. The guinea-pig epithelial cell line GPC16 (ATCC CCL-242) was cultured in minimal essential medium (MEM) with non-essential amino acids supplemented with 10% FCS, 2 mM l-glutamine (Sigma) and 1 mM sodium pyruvate (Gibco-BRL).
Antibodies and purified proteins
Polyclonal antiserum against rat p85α was purchased from Upstate Biotechnology. Rabbit pre-immune IgG was from Sigma. Monoclonal anti-Tyr(P) antibodies (clone RC20) coupled to peroxidase (E120H) were from Transduction Laboratories. Streptavidin covalently linked to HRP was purchased from Biosys. Monoclonal antibodies against phosphotyrosine (clone 4G10) used for immunoprecipitation were purified using Affi-Prep protein A support from Bio-Rad following the manufacturer's instructions. Polyclonal rabbit antiserum against gC1q–R was raised as described below. The InlA-specific monoclonal antibodies I4.4 and G6.1 and the InlB-specific monoclonal antibody H15.1 have been described previously (Mengaud et al., 1996b; Braun et al., 1999). Recombinant InlB, internalin LRRs [LRR(InlA)] and PrfA were purified as described previously (Sheehan et al., 1996; Braun et al., 1997; Lecuit et al., 1997). Recombinant gC1q–R was purified as described below. Recombinant human EGF was from Upstate Biotechnology. BSA and complement component C1q were puchased from Sigma.
Purification of gC1q–R
A 648 bp PCR fragment encoding the mature form of human gC1q–R (amino acids 74–282) was produced using pBluescript containing the gC1q–R cDNA insert (Ghebrehiwet et al., 1994) as a template and the primers 5′-(CTA)GCTAGC.CTGCACCGACGGAGAC-3′ and 5′-(CGC)GGATCC.TTTCAGCATCTGTCTGCTCTA-3′ with Vent DNA polymerase (Biolabs). The purified amplified fragment was cloned in-frame downstream from the His tag sequence, between the NheI and BamHI sites of the pET28a expression vector (Novagen), yielding plasmid pET28a-5. The nucleotide sequence of the amplified fragment was verified by sequencing. For expression of the recombinant protein, pET28a-5 was transformed into Escherichia coli strain BL21(DE3). The recombinant protein was purified as described previously (Braun et al., 1997) except that, after the elution step with imidazole, purified recombinant protein was dialyzed against 20 mM Tris pH 8.5, 0.15 M NaCl and loaded onto a HiTrap Q column. The protein was eluted by a continuous salt gradient from 0.15 to 1 M NaCl. Pure fractions were concentrated to the desired volume using Centriprep 10 devices (Amicon), and then stored at –20°C.
Cell-binding assays
Binding of InlB-permissive cells to InlB was determined by the hexos– aminidase assay as described previously (Landegren, 1984; Mengaud et al., 1996a).
To determine the effect of C1q, 145 nM protein was added to the cell suspension for 5 min at 37°C before and during the cell-binding assay. The effect of anti-gC1q–R antibodies was tested by adding 100 μg/ml antibodies or 100 μg/ml rabbit IgG used as a control to the cell suspension for 60 min at 37°C before and during the cell-binding assay.
Ligand overlay assays
Vero cell components recognized by InlB were detected using a ligand overlay of Western blotted proteins. Approximately 8 × 105 Vero cells were seeded in 75 cm2 tissue culture flasks and grown for 48 h. Cells were washed three times with 10 ml of phosphate-buffered saline (PBS), and then scraped into 1.5 ml of PBS, spun down (60 s at 4000 g) and lysed in 0.2 ml of lysis solution [1% NP-40, 50 mM Tris pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/ml pepstatin, 2.5 μg/ml leupeptin, 2 μg/ml aprotinin]. The suspension was incubated for 15 min on ice and spun (5 min at 15 000 g). The supernatant was mixed with Laemmli sample buffer and boiled for 5 min. Solubilized proteins were separated by SDS–PAGE on a 10% polyacrylamide gel, electroblotted onto nitrocellulose membrane and then blocked overnight at room temperature in TBS-T (50 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween-20) containing 2% BSA, 3% milk and 1% ovalbumin. The nitrocellulose was incubated overnight at 4°C with the purified proteins [50 μg/ml InlB or LRR(InlA)] diluted in blocking solution. Following extensive washes, the membrane was incubated with primary antibody, followed by washing, and a second incubation with HRP conjugate. Bound proteins were subsequently detected using the chemiluminescent ECL detection kit (Amersham).
Preparation of cell membrane proteins from Vero, HEp-2 and HeLa cells
Approximately 1 × 107 cells were washed three times and resuspended in 1 ml of 5 mM phosphate buffer pH 7.5 containing 0.5 mM EDTA, 150 mM NaCl and 0.5 mM PMSF. Cell membranes were prepared by freeze–thawing (five times) at –80°C and centrifugation at 30 000 g for 1 h at 4°C. The pelleted membranes were then solubilized by suspension in 0.5 ml of the above buffer containing 1% NP-40 and stirred for 20 h at 4°C. The solubilized membrane proteins were freed from insoluble material by centrifugation at 30 000 g for 60 min at 4°C. After dialysis against 5 mM phosphate buffer pH 7.5 containing 0.5 mM EDTA, 20 mM NaCl, 0.5 mM PMSF and 0.1% NP-40, 100 μg of the dissolved membrane proteins were applied to a 12% polyacrylamide gel.
Purification of the InlB receptor
InlB or BSA was covalently coupled to Affi-gel 10 (Bio-Rad) in 50 mM HEPES pH 7.5, following the manufacturer's instructions. A total of 5 × 108–5 × 109 Vero cells were grown to 90% confluence. Biotinylation, extraction of surface proteins and affinity chromatography of the InlB receptor(s) were performed as described (Isberg and Leong, 1990; Mengaud et al., 1996a). Proteins eluted with EDTA were analyzed by SDS–PAGE, transferred to nitrocellulose membrane and detected with streptavidin covalently coupled to HRP and the chemiluminescent ECL detection kit (Amersham).
Protein sequencing
EDTA-eluted proteins were pooled and loaded on a 10% polyacrylamide gel. Protein bands were visualized by Amido black staining of the gel. The 33 kDa band present on the gel was cut out and subjected to internal sequencing using an Applied Biosystems 473A sequencer.
ELISA
A standard ELISA was performed using microtiter plates (MaxiSorb, Nunc) coated overnight at 4°C with 50 μg/well of 1 μg/ml gC1q–R diluted in 50 mM carbonate buffer pH 9.5. After blocking the unreacted sites with 1% BSA in TBS (20 mM Tris–HCl pH 7.5, 150 mM NaCl), the plates were incubated with 50 μl of various concentrations of either InlB, LRR(InlA) or PrfA in TBS containing 0.1% BSA. Bound InlB and LRR(InlA) proteins were detected using the monoclonal antibodies B4.6 and G6.1, respectively (Mengaud et al., 1996b; Braun et al., 1999), while PrfA protein was detected using an anti-PrfA polyclonal antibody (Sheehan et al., 1996). Antibodies were detected using HRP-conjugated anti-mouse or anti-rabbit antibody and the chromogenic substrate 1,2-phenylenediamine dihydrochloride (Dako). The absorbance of the resulting color development was measured at 490 nm. Washes between all reactions were carried out three times in TBS containing 0.05% Tween-20.
Production of anti-gC1q–R antibodies
Antibodies to an 18 amino acid synthetic peptide (TDGDKAFV– DFLSDEIKEE), spanning residues 76–93 of gC1q–R, were generated by injection of keyhole limpet hemocyanin (KLH)-conjugated peptide following the immunization protocol described previously (Friederich et al., 1995). Antibodies were affinity purified as described (Friederich et al., 1995).
Invasion assays
Invasion assays in Vero, HEp-2, HeLa and Caco-2 cells were performed in 24-well plates using the gentamicin survival assay as described previously (Braun et al., 1998). Invasion assays were carried out in three independent experiments, each time in duplicate.
To test the ability of anti-gC1q–R antibodies to inhibit entry of the L.monocytogenes strain EGD, antibodies were added to cells in 0.5 ml of DMEM in 24-well tissue culture dishes for 1 h before and during the cell infection step. To test the ability of C1q to inhibit entry of the L.monocytogenes strain EGD, different concentrations of purified C1q were added to cells in 0.5 ml of DMEM in 24-well tissue culture dishes for 5 min before and during the cell infection step. At the concentrations used, anti-gC1q–R antibodies and C1q had no effect on the viability of cells, as determined by staining with Trypan blue.
Transient transfection experiments, immunofluorescence labeling and quantification of entry of InlB-coated beads
gC1q–R was fused to the C-terminus of EGFP by cloning human gC1q–R full-length cDNA at XhoI and BamHI sites in pEGFP-C1 (Clontech), giving rise to pEGFP-gC1q–R. Expression of the fusion protein was verified by Western blotting experiments using the gC1q–R polyclonal antibody. pEGFP-gC1q–R was purified using the Nucleobond AX kit (Macherey-Nalgel) and transfections were carried out using the Lipofectamine Plus Reagent (Gibco-BRL) with 5 × 104 GPC16 cells, grown for 36 h on coverslips. At 24 h post-transfection, cells were incubated with 2 × 107 InlB- or BSA-coated beads, prepared as described previously (Braun et al., 1998), with centrifugation at 200 g for 1 min. Cells were then incubated at 37°C in 10% CO2 for 1 h, washed in PBS and fixed with 3% paraformaldehyde in PBS. Adhesion and entry of beads coated with InlB or BSA were analyzed as described previously (Braun et al., 1998). Briefly, extracellular beads were labeled with an anti-InlB polyclonal antibody or anti-rabbit BSA antiserum, followed by a second labeling step with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG antibodies. Cells were then permeabilized and intracellular beads were allowed to react with an anti-InlB polyclonal antibody or anti-rabbit BSA antiserum. Cells were then incubated with rhodamine-conjugated antibodies against rabbit IgGs, a step that labels both extra- and intracellular beads.
For enumeration of total and intracellular beads in transfected cells, four or five coverslips were observed. On each coverslip, 50 transfected cells were selected randomly by immunofluorescence. The number of total beads per transfected cell was evaluated under phase contrast observation and the number of intracellular beads by enumerating among these beads those that were rhodamine labeled.
Immunoprecipitation
Approximately 1.5 × 105 Vero cells were seeded in 35 mm tissue culture dishes and grown for 40 h. Cells were then pre-treated with 100 μg/ml anti-gC1q–R antibodies for 1 h before the addition of 3 nM (200 ng/ml) of purified InlB or 17 nM (100 ng/ml) of EGF for 1 min. Cells were then washed with cold PBS, and incubated in lysis buffer [50 mM NaCl, 50 mM Tris pH 8, 1% NP40, 1 mM EDTA, 1 mM aminoethylbenzenesulfonyl fluoride (AEBSF), 3 mM sodium vanadate, and 5 μg/ml aprotinin, leupeptin, pepstatin and chymostatin]. Pre-clearance and immunoprecipitations with anti-phosphotyrosine antibody 4G10 were performed as described (Ireton et al., 1996). After the pre-clearance step, protein concentrations were determined using the BCA system (Pierce) and equal quantities of total protein were used for immunoprecipitations. Samples were subjected to electrophoresis (7.5% SDS–PAGE), and p85 was detected using immunoblotting with a polyclonal antibody to p85α.
Pull-down assay
Vero cells were starved for 5 h in serum-free DMEM and cell lysates were prepared from untreated, InlB- or EGF-treated cells in lysis buffer as described above. The extracts were clarified by centrifugation at 15 000 g for 15 min at 4°C. The supernatants were pre-cleared for 16 h at 4°C with Ni-NTA–agarose (Qiagen). Protein concentrations of the lysates were determined using the BCA system and equal quantities of total protein were incubated with His6-gC1q–R, LRR(InlA)-His6 or buffer alone, followed by addition of Ni-NTA–agarose beads for 16 h. The beads were then washed and proteins retained on beads were dissociated by boiling for 2 min in the presence of buffer containing 50 mM Tris–HCl pH 7.5, 0.5% SDS and 5 mM dithiothreitol. ‘Re-immunoprecipitation’ experiments with antibodies against Gab1 were performed as described (Ireton et al., 1999).
Acknowledgments
Acknowledgements
We thank M.Lecuit for critical advice and the gift of LRR(InlA), E. Gouin for production of the polyclonal anti-gC1q–R antibody, F.Nato for constant help with ELISAs, R.Hurme for purification of the monoclonal antibody 4G10, and R.Jonquières for critical reading of the manuscript. This work received financial support from the European Economic Community (grant BMH4-CT 96-0659), the DGA 97/069 and the Ministère de l'Education Nationale, de la Recherche et de la Technologie: programme de Microbiologie 1998. L.B. is a recipient of a fellowship from the CANAM.
References
- Bobak D., Gaither, T., Frank, M. and Tenner, A.J. (1987) Modulation of FcR function by complement subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J. Immunol., 138, 1150–1156. [PubMed] [Google Scholar]
- Braun L., Dramsi, S., Dehoux, P., Bierne, H., Lindahl, G. and Cossart, P. (1997) InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol., 25, 285–294. [DOI] [PubMed] [Google Scholar]
- Braun L., Ohayon, H. and Cossart, P. (1998) The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol., 27, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Braun L., Nato, F., Payrastre, B., Mazié, J.-C. and Cossart, P. (1999) The 213-amino acid leucine-rich repeat region of the Listeria monocytogenes InlB protein is sufficient for entry into mammalian cells, stimulation of PI 3-kinase and membrane ruffling. Mol. Microbiol., 34, 10–23. [DOI] [PubMed] [Google Scholar]
- Cossart P. and Lecuit, M. (1998) Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J., 17, 3797–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedio J., Jahnen-Dechent, W., Bachmann, M. and Müller-Esterl, W. (1998) The multiligand-binding protein gC1q–R, putative C1q receptor, is a mitochondrial protein. J. Immunol., 160, 3534–3542. [PubMed] [Google Scholar]
- Dramsi S., Biswas, I., Maguin, E., Braun, L., Mastroeni, P. and Cossart, P. (1995) Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol. Microbiol., 16, 251–261. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Ghebrehiwet, B., Sastry, K.N., Coburn, J.P., Zaner, K.S., Reid, K.B.M. and Tauber, A.I. (1995) Identification of a gC1q-binding protein (gC1q–R) on the surface of human neutrophils. J. Clin. Invest., 95, 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. and Cossart, P. (1997) Exploitation of mammalian host cell functions by bacterial pathogens. Science, 276, 718–725. [DOI] [PubMed] [Google Scholar]
- Fridell R.A., Harding, L.S., Bogerd, H.P. and Cullen, B.R. (1995) Identification of a novel human zinc finger protein that specifically interacts with the activation domain of lentiviral Tat protein. Virology, 209, 347–357. [DOI] [PubMed] [Google Scholar]
- Friederich E., Gouin, E., Hellio, R., Kocks, C., Cossart, P. and Louvard, D. (1995) Targeting of Listeria monocytogenes ActA protein to the plasma membrane as a tool to dissect both actin-based cell morphogenesis and ActA function. EMBO J., 14, 2731–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J.L., Jaubert, F. and Berche, P. (1996) The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J. Exp. Med., 183, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J.E. (1996) Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol., 20, 263–271. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B. and Peerschke, E.I.B. (1998) Structure and function of gC1q–R: a multiligand binding cellular protein. Immunobiology, 199, 225–238. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B., Lim, B.L., Peerschke, E.I.B., Willis, A.C. and Reid, K.B.M. (1994) Isolation, cDNA cloning, and overexpression of a 33-kDa cell surface glycoprotein that binds to the globular ‘heads’ of C1q. J. Exp. Med., 179, 1809–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S.H., Sagnimeni, A.J. and Wing, E.J. (1997) Internalin B promotes the replication of Listeria monocytogenes in mouse hepatocytes. Infect. Immun., 65, 5137–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwald H., Dedio, J., Kellner, R., Loos, M. and Müller-Esterl, W. (1996) Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. J. Biol. Chem., 271, 13040–13047. [DOI] [PubMed] [Google Scholar]
- Ireton K. and Cossart, P. (1998) Interaction of invasive bacteria with host signaling pathways. Curr. Opin. Cell Biol., 10, 276–283. [DOI] [PubMed] [Google Scholar]
- Ireton K., Payrastre, B., Chap, H., Ogawa, W., Sakaue, H. and Cossart, P. (1996) A role for phosphoinositide 3-kinase in bacterial invasion. Science, 274, 780–782. [DOI] [PubMed] [Google Scholar]
- Ireton K., Payrastre, B. and Cossart, P. (1999) The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem., 274, 17025–17032. [DOI] [PubMed] [Google Scholar]
- Isberg R.R. and Leong, J.M. (1990) Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell, 60, 861–871. [DOI] [PubMed] [Google Scholar]
- Jiang J., Zhang, Y., Krainer, A.R. and Xu, R.-M. (1999) Crystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein. Proc. Natl Acad. Sci. USA, 96, 3572–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonquières R., Bierne, H., Fiedler, F., Gounon, P. and Cossart, P. (1999) Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of Gram-positive bacteria. Mol. Microbiol., 34, 902–914. [DOI] [PubMed] [Google Scholar]
- Joseph K., Ghebrehiwet, B., Peerschke, E.I.B., Reid, K.B.M. and Kaplan, A.P. (1996) Identification of the zinc-dependent endothelial cell binding protein for high molecular weight kininogen and factor XII: identity with the receptor that binds to the globular ‘heads’ of C1q (gC1q–R). Proc. Natl Acad. Sci. USA, 93, 8552–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U. (1984) Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J. Immunol. Methods, 67, 379–388. [DOI] [PubMed] [Google Scholar]
- Lecuit M., Ohayon, H., Braun, L., Mengaud, J. and Cossart, P. (1997) Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun., 65, 5309–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M., Dramsi, S., Gottardi, C., Fedor-Chaiken, M., Gumbiner, B. and Cossart, P. (1999) A single amino acid in E–cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J., 18, 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B.L., Reid, K.B.M., Ghebrehiwet, B., Peerschke, E.I.B., Leigh, L.A.E. and Preissner, K.T. (1996) The binding protein for globular heads of complement C1q, gC1q–R: functional expression and characterization as a novel vitronectin binding factor. J. Biol. Chem., 271, 26739–26744. [DOI] [PubMed] [Google Scholar]
- Lingnau A., Domann, E., Hudel, M., Bock, M., Nichterlein, T., Wehland, J. and Chakraborty, T. (1995) Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun., 63, 3896–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M., Braun, L., Cossart, P. and Ghosh, P. (1999) Structure of the InlB leucine-rich repeats, a domain that triggers host cell invasion by the bacterial pathogen L. monocytogenes. Mol. Cell, 4, 1063–1072. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Ohayon, H., Gounon, P., Mège, R.M. and Cossart, P. (1996a) E–cadherin is the receptor for internalin, a surface protein required for entry of L.monocytogenes into epithelial cells. Cell, 84, 923–932. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Lecuit, M., Lebrun, M., Nato, F., Mazié, J.-C. and Cossart, P. (1996b) Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E–cadherin. Infect. Immun., 64, 5430–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhieu G.T. and Sansonetti, P.J. (1999) Mechanism of Shigella entry into epithelial cells. Curr. Opin. Microbiol., 2, 51–55. [DOI] [PubMed] [Google Scholar]
- Oiki S. and Okada, Y. (1988) C1q induces chemotaxis and K+ conductance activation coupled to increased cytosolic Ca2+ in mouse fibroblasts. J. Immunol., 141, 3177–3185. [PubMed] [Google Scholar]
- Parida S.K., Domann, E., Rohde, M., Müller, S., Darji, A., Hain, T., Wehland, J. and Chakraborty, T. (1998) Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol. Microbiol., 28, 81–93. [DOI] [PubMed] [Google Scholar]
- Peerschke E.I.B., Reid, K.B.M. and Ghebrehiwet, B. (1993) Platelet activation by C1q results in the induction of αIIb/β3 integrins (GPIIb-IIIa) and the expression of P-selectin and procoagulant activity. J. Exp. Med., 178, 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke E.I.B., Smyth, S.S., Teng, E., Dalzell, M. and Ghebrehiwet, B. (1996) Human umbilical vein endothelial cells possess binding sites for the globular domain of C1q. J. Immunol., 157, 4154–4158. [PubMed] [Google Scholar]
- Pier G.B., Grout,M., Zaidi,T., Meluleni,G., Mueschenborn,S.S., Banting,G., Ratcliff,R., Evans,M.J. and Colledge,W.H. (1998) Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature, 393, 79–82. [DOI] [PubMed] [Google Scholar]
- Sheehan B., Klarsfeld, A., Ebright, R. and Cossart, P. (1996) A single substitution in the putative helix–turn–helix motif of the pleiotropic activator PrfA attenuates Listeria monocytogenes virulence. Mol. Microbiol., 20, 785–797. [DOI] [PubMed] [Google Scholar]
- Watarai M., Funato, S. and Sasakawa, C. (1996) Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med., 183, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Zhang, Z., Loewenstein, P.M., Desai, K., Tang, Q., Mao, D., Symington, J.S. and Green, M. (1995) Molecular cloning and characterization of a cellular protein that interacts with the human immunodeficiency virus type 1 Tat transactivator and encodes a strong transcriptional activation domain. J. Virol., 69, 3007–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]