Abstract
Electrographic seizures are common in neonates with hypoxic-ischemic encephalopathy, but detailed data are not available regarding seizure incidence during therapeutic hypothermia. The objective of this prospective study was to determine the incidence and timing of electrographic seizures in term neonates undergoing whole-body therapeutic hypothermia for hypoxic-ischemic encephalopathy as detected by conventional full-array electroencephalography for 72 hours of therapeutic hypothermia and 24 hours of normothermia. Clinical and electroencephalography data were collected from 26 consecutive neonates. Electroencephalograms were reviewed by 2 pediatric neurophysiologists. Electrographic seizures occurred in 17 of 26 (65%) patients. Seizures were entirely nonconvulsive in 8 of 17 (47%), status epilepticus occurred in 4 of 17 (23%), and seizure onset was in the first 48 hours in 13 of 17 (76%) patients. Electrographic seizures were common, were often nonconvulsive, and had onset over a broad range of times in the first days of life.
Keywords: hypothermia, induced, infant, seizures, electroencephalography, hypoxia-ischemia, brain
Hypoxic-ischemic encephalopathy is a major cause of morbidity and mortality among term newborns, with both acute and chronic consequences. Most literature describing hypoxicischemic encephalopathy predates therapeutic hypothermia, which is increasingly employed to improve outcomes among children with moderate or severe neonatal encephalopathy.1-3 In addition to changing long-term outcomes, there is evidence that hypothermia modifies the clinical evolution and neuroimaging findings in treated neonates.4,5 Thus, data from the “prehypothermia” era must be reconsidered.
Neonates with moderate or severe hypoxic-ischemic encephalopathy are at risk for acute symptomatic seizures, with estimates of incidence ranging from 22% to 64% using routine electroencephalography (EEG) or amplitude-integrated EEG.1,2,6 Among neonates with hypoxic-ischemic encephalopathy, seizures have been correlated with biomarkers of brain injury, as well as worse clinical outcomes.7-12 However, there are limited data regarding seizure occurrence during therapeutic hypothermia, and it is unknown whether or how hypothermia affects the incidence or timing of seizures in neonates with hypoxic-ischemic encephalopathy. A recent report of 20 term neonates receiving selective head cooling for hypoxic-ischemic encephalopathy found 19 (95%) had seizures on amplitude-integrated EEG during cooling, though none had overt clinical signs.13 This result was substantially higher than previous reports and would suggest additional studies are needed to better understand occurrence of seizures in this group. In other patient populations, reports conflict as to hypothermia's impact on activity reflected on EEG and on occurrence of seizures.14-22 Further complicating issues related to seizure detection is the relative difficulty of detecting neonatal seizures on the basis of clinical signs alone; up to 80% of electrographic seizures are clinically unrecognized.23
To date, the incidence and evolution of neonatal seizures during cooling has not been described using the gold standard for seizure detection: conventional, full-array continuous video-EEG. This study employs continuous EEG monitoring to examine the incidence and timing of seizures in consecutive term neonates receiving whole-body cooling for hypoxicischemic encephalopathy.
Patients and Methods
The Children's Hospital of Philadelphia Institutional Review Board approved the conduct of this study. A consecutive cohort of neonates undergoing clinically indicated therapeutic hypothermia were prospectively enrolled. Patients met institution-specific clinical eligibility criteria for cooling, based on guidelines derived from the 2005 National Institute for Child Health and Development protocol. Exclusion criteria for cooling included gestational ages less than 36 weeks or birth weight under 1800 grams. All neonates were cooled to 34°C-35°C (skin temperature), initiated within 6 hours of life and for a total duration of 72 hours, using the Blanketrol II (Cincinnati Sub-Zero, Cincinnati, OH) cooling device. Demographic and clinical data were obtained by chart review and through a database of neonates with hypoxic-ischemic encephalopathy.
According to our customary clinical practice protocols, all neonates treated with therapeutic hypothermia were monitored with continuous EEG using a modified neonatal 10-20 electrode placement system and Grass Telefactor Software (Astro-Med, West Warwick, RI). Electroencephalograhic monitoring was initiated as soon as possible after patient admission and with a goal of continuation for at least 24 hours beyond rewarming. Two pediatric neurophysiologists (CW and NA) reviewed all continuous EEG recordings by consensus to confirm the presence of electrographic seizures. Video monitoring was used to determine whether clinical signs were associated with electrographic events. Electroencephalograhic tracings were segmented into 12-hour epochs. Each epoch was scored for the presence or absence of electrographic seizures, and for the presence or absence of electrographic status epilepticus.
Electrographic seizures were defined as abnormal electrographic events lasting more than 10 seconds with a plausible electrographic distribution that evolved in morphology and frequency. Electro-graphic status epilepticus was defined as the presence of seizures for more than 50% of a 1-hour epoch. Thus, status epilepticus was present if there was a single, long seizure lasting more than 30 minutes or multiple recurrent brief seizures totaling 30 minutes or more during a 1-hour period. Nonconvulsive seizures were defined as electro-graphic seizures without clinical change detected on video. Electroclinical seizures were defined as electrographic seizures associated with an abnormal clinical event apparent on video.
Magnetic resonance imaging (MRI) including diffusion weighted imaging was performed as clinically indicated. Based on neuroradiologist interpretation, MRI findings were classified based on the extent of diffusion changes into categories of (1) none, (2) cortex alone, (3) deep grey alone, and (4) cortex and deep grey structures.
Results
Thirty potentially eligible consecutive term neonates were identified between March 2008 and January 2010. Four died prior to initiation of continuous EEG, leaving 26 consecutive subjects enrolled. Demographic characteristics are summarized in Table 1.
Table 1.
Characteristics of Included Neonates and EEG Records (N = 26)
| Male sex, n (%) | 15 (57.6) |
| Gestational age, wk, mean (± SD) | 39 (± 1.5) |
| Birth weight, g, mean (± SD) | 3254 (± 555) |
| First h arterial pH <7, n (%) | 16 (61.5) |
| Apgar scores, mean (± SD) | |
| 1 min | 1.4 (± 1.7) |
| 5 min | 3.34 (± 2.2) |
| 10 min | 4.2 (± 2.3) |
| Given phenobarbital for clinical seizure prior to EEG initiation, n (%) | 10 (38.5) |
| Time from birth to hypothermia achieved, h, mean (± SD)a | 4.83 (± 5.75) |
| Time from birth to initiation of video EEG monitoring, h, mean (± SD) | 9.1 (± 5.9) |
| Died prior to hospital discharge, n (%) | 6 (23.1) |
| Electrographic seizures, n (%)b | 17 (65.4) |
| Electroclinical seizures, n (%)c | 9 (34.6) |
Abbreviations: EEG, electroencephalography; SD, standard deviation; h, hour.
Time of hypothermia achieved is first documented body temperature below 34° C.
One or more seizures on EEG during recording.
One or more seizures on EEG during recording with clinical signs seen on video.
Continuous EEG was initiated urgently upon transfer, within a mean of 9.1 hours of birth (± 5.9 h), and within a mean of 4.3 hours (± 5.8 h) of achieving hypothermia. Although the goal duration of recording was 92 hours, in some cases clinical circumstances required early continuous EEG termination (ie, patient transported for neuroimaging, patient transferred, or intensive care discontinued). The mean total duration of cEEG was 85.1 hours (± 21.8 h) per patient.
Electrographic seizures occurred in 17 of 26 (65.4%) patients. In 4 of 26 (15.4%) patients, at least some of the 12-hour epochs during monitoring included electrographic status epilepticus. Among those with electrographic seizures, 8of17(47%) had exclusively nonconvulsive seizures, whereas 9 of 17 (53%) had at least some electroclinical seizures admixed with nonconvulsive seizures. No neonate had exclusively electroclinical seizures.
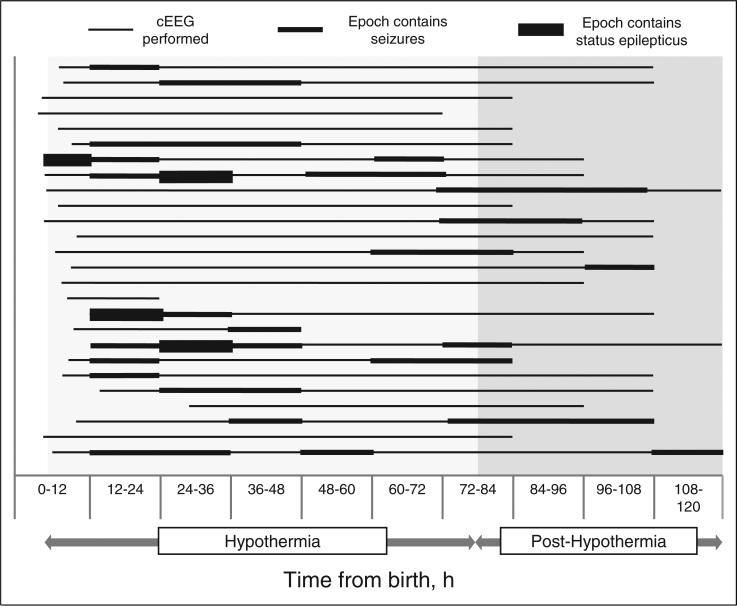
Among the 17 subjects with electrographic seizures, the earliest occurrence was at 5 hours, 42 minutes of life. The mean time of first electrographic seizure was 9 hours and 30 minutes (interquartile range 16 h, 45 min to 40 h, 31 min). The latest time of first electrographic seizure for any subject was 97 hours, 54 minutes. Seizure onset occurred at 0-24 hours in 9 subjects, 24-48 hours in 4 subjects, 48-72 hours in 1 subject, and more than 72 hours in 3 subjects. No subjects had just a single seizure. Five of 26 patients (19.2%) experienced temporary cessation of seizures for more than 24 consecutive hours, only to have seizures later relapse. Overall EEG results are summarized in Table 1 and Figure 1.
Figure 1.
Seizures and status epilepticus by subject over time.
Eighteen of 26 patients (69.2%) received antiepileptic drugs during cooling, and 10 of 26 (38.5%) received at least 1 dose of phenobarbital prior to initiation of continuous EEG for suspected clinical seizures. No patients received other antiepileptic drugs prior to continuous EEG initiation. Of these 10 who received 1 dose of phenobarbital, 7 had electro-graphic confirmed seizures during monitoring. An additional 8 patients (30.8%) received antiepileptic drugs owing to electrographic seizures detected during continuous EEG. Continuous EEG led to the discontinuation of phenobarbital in 3 (11.6%) patients owing to the absence or resolution of electrographic seizures. To treat seizures refractory to phenobarbital alone, fosphenytoin was also administered to 3 patients, levetiracetam was added for 3, and 1 received both fosphenytoin and levetiracetam in addition to phenobarbital. By the time of return to normothermia, 4 patients continued to have uncontrolled seizures.
Magnetic resonance imaging was performed in 25 subjects at a mean age of 107 hours ± 38 hours. Diffusion changes were absent in 7, present in cortex and deep structures in 15, present in cortex alone in 2, and present in deep structures alone in 1. Our study size did not allow for correlation between seizure occurrence and MRI findings. However, all 4 subjects with status epilepticus had diffusion changes in both cortex and deep structures.
Discussion
This study uses conventional, continuous EEG monitoring to describe the incidence of electrographic seizures among term neonates receiving therapeutic hypothermia for hypoxicischemic encephalopathy. Using the definitive standard for seizure detection, we found 65% of subjects had electrographic seizures during or immediately after treatment with hypothermia. This finding is consistent with the “pre-hypothermia” literature, which describes seizures in 22%-64%, suggesting that hypothermia as employed for hypoxic-ischemic encephalopathy does not substantially affect the incidence of seizures.1,2,6
Among neonates receiving hypothermia, initial data from 20 term neonates receiving selective head cooling for hypoxicischemic encephalopathy found 19 had seizures on amplitude-integrated EEG during cooling, all of which were nonconvulsive seizures.13 We did not identify as high an incidence of seizures in our group. The reason for the discrepant results is not obvious. This finding may be a result of differences in study populations, as each study was a single-institution group, and thus there may be inherent differences in the patient populations between hospitals. We also cannot exclude the possibility that the results diverge because of differences between how whole-body cooling and selective head cooling influence seizure pathophysiology; these results could occur if selective head cooling were somehow to elicit an increase in electrographic seizures as compared to whole-body cooling or historical normothermia. Finally, our results may be different because of differences between seizure detection with amplitude-integrated EEG and conventional video EEG recording. Although amplitude-integrated EEG systems generally allow access to a raw single-channel EEG tracing for further review, these records overall can be subject to higher degrees of artifact than conventional multichannel EEG.24,25 Such artifact may mislead the bedside clinician in the diagnosis of seizures. Further research is needed to resolve the difference between the findings, as the results have important clinical management implications. If seizures are nearly universal, as previously reported,13 then prophylactic administration of anti-seizure medications might be considered for any neonate undergoing therapeutic hypothermia. Conversely, if seizures are common but not universal, as our data suggest, management might involve EEG monitoring with anti-seizure medication administered only when seizures are detected.
This study demonstrated a relatively wide range in the time of first seizure onset, from about 6-95 hours of life, with a mean time of first seizure at 35 hours. although about half of patients with seizures had the onset of seizures within 24 hours after birth, a substantial number had seizure onset much later. Furthermore, approximately a third of those with seizures experienced temporary cessation of seizures for more than 24 consecutive hours, only to have seizures later recur. This finding is a departure from previous reports. Later seizure recurrence may happen because hypothermia delays injury and thus seizure occurrence, but seizures may also have occurred because we performed continuous EEG monitoring for a longer duration than previous studies and thus may have been more sensitive for detection of seizure recurrence. Further work is needed to confirm rates of seizure recurrence after 24 hours in high-risk neonates. If our findings are generalized, they would suggest that limiting continuous EEG to the first 24 hours after birth or discontinuing EEG upon initial seizure resolution could miss important clinical information.
This study has several limitations. First, this was an observational study. All subjects received hypothermia, and so a basis for comparison can be drawn only from historical data in the literature. These historical data suggest seizures occur in 22%-64% of neonates with hypoxic-ischemic encephalopathy without cooling.1,2,6 However, we do not have a direct comparison group, and thus we cannot conclusively demonstrate whether hypothermia influences seizure occurrence or influences the timing of seizure occurrence. Furthermore, although consecutive enrollment and prospective data collection protect against selection bias, there was no control for clinical care administered during the period in question. For example, a substantial number of patients received anti-seizure medications either before or during continuous EEG at the discretion of the treating physician, which could influence both the incidence and timing of seizures. The general practice in our institution is to treat with anti-seizure medications with the goal of electro-graphic seizure termination. Other treatment algorithms might have resulted in different occurrences of seizures. Second, this was a single institution study with a limited number of subjects; larger studies including patients at other sites under different treatment algorithms are needed to confirm and generalize our findings. Third, this study examined only the presence and time of onset of electrographic seizures. Further research is needed to characterize the exact seizure burden and other EEG features in this population, and to better understand their effects on clinical outcomes. Finally, MRIs were not performed in a standardized manner, thereby precluding systematic analysis of neuroimaging findings and seizure occurrence.
In conclusion, this prospective study of a consecutive cohort identified electrographic seizures in 65% of neonates receiving therapeutic hypothermia for hypoxic-ischemic encephalopathy. Of those with seizures, 47% had exclusively nonconvulsive seizures, and 23% had status epilepticus. The range of time to seizure onset was wide, spanning several days, including during hypothermia, rewarming, and upon return to normothermia. Many patients had only nonconvulsive seizures, which would not have been detected even with careful clinical observation. Although electrographic seizures were common, they were not universal, suggesting standard administration of anti-seizure medications to neonates with hypoxic-ischemic encephalopathy is not warranted anticipatorily. At the same time, given the relatively high incidence of seizures in this group, EEG monitoring should be carefully considered. The optimal duration of monitoring is unclear, though our results suggest it may be beneficial to extend EEG monitoring beyond the first 24 hours for neonates receiving hypothermia for hypoxic-ischemic encephalopathy.
Financial Disclosure/Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work is supported by the NINDS Neurological Sciences Academic Development Award (NSADA) NS049453 to Dr. Abend.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
Ethical Approval
The Children's Hospital of Philadelphia Institutional Review Board approved the conduct of this study.
References
- 1.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 4.Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–58. 58 e1. doi: 10.1016/j.jpeds.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxicischemic encephalopathy. Pediatrics. 2005;116:1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 6.Murray DM, Ryan CA, Boylan GB, Connolly S. Prediction of seizures in asphyxiated neonates: correlation with continuous video-electroencephalographic monitoring. Pediatrics. 2006;118:41–46. doi: 10.1542/peds.2005-1524. [DOI] [PubMed] [Google Scholar]
- 7.Brunquell PJ, Glennon CM, DiMario FJ, Jr, Lerer T, Eisenfeld L. Prediction of outcome based on clinical seizure type in newborn infants. J Pediatr. 2002;140:707–712. doi: 10.1067/mpd.2002.124773. [DOI] [PubMed] [Google Scholar]
- 8.Legido A, Clancy RR, Berman PH. Neurologic outcome after electroencephalographically proven neonatal seizures. Pediatrics. 1991;88:583–596. [PubMed] [Google Scholar]
- 9.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–513. doi: 10.1212/wnl.55.4.506. [DOI] [PubMed] [Google Scholar]
- 10.Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 12.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yap V, Engel M, Takenouchi T, Perlman JM. Seizures are common in term infants undergoing head cooling. Pediatr Neurol. 2009;41:327–331. doi: 10.1016/j.pediatrneurol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Elting JW, Naalt JV, Fock JM. Mild hypothermia for refractory focal status epilepticus in an infant with hemimegalencephaly. Eur J Paediatr Neurol. 2010;14:452–455. doi: 10.1016/j.ejpn.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9:189–197. doi: 10.1007/s12028-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 17.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 18.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: II. Changes in electroencephalogram and evoked potentials during rewarming. Ann Thorac Surg. 2001;71:22–28. doi: 10.1016/s0003-4975(00)02021-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Kim SH, Yoo SK, Kim JY, Nam YT. The effects of mild hypothermia on thiopental-induced electroencephalogram burst suppression. J Neurosurg Anesthesiol. 1998;10:137–141. doi: 10.1097/00008506-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Levy WJ. Hypothermia and the electroencephalogram. Anesthesiology. 1983;58:396–397. doi: 10.1097/00000542-198304000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Hicks RG, Poole JL. Electroencephalographic changes with hypothermia and cardiopulmonary bypass in children. J Thorac Cardiovasc Surg. 1981;81:781–786. [PubMed] [Google Scholar]
- 22.Reilly EL, Brunberg JA, Doty DB. The effect of deep hypothermia and total circulatory arrest on the electroencephalogram in children. Electroencephalogr Clin Neurophysiol. 1974;36:661–667. doi: 10.1016/0013-4694(74)90233-8. [DOI] [PubMed] [Google Scholar]
- 23.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–261. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 24.Hagmann CF, Robertson NJ, Azzopardi D. Artifacts on electroencephalograms may influence the amplitude-integrated EEG classification: a qualitative analysis in neonatal encephalopathy. Pediatrics. 2006;118:2552–2554. doi: 10.1542/peds.2006-2519. [DOI] [PubMed] [Google Scholar]
- 25.de Vries LS, Toet MC. Amplitude integrated electroencephalography in the full-term newborn. Clin Perinatol. 2006;33:619–632, vi. doi: 10.1016/j.clp.2006.06.002. [DOI] [PubMed] [Google Scholar]