Abstract
Objective
Aspiration of oropharyngeal or gastric contents into the lower respiratory tract is a common event in critically ill patients, and can lead to pneumonia or pneumonitis. Aspiration pneumonia is the leading cause of pneumonia in the intensive care unit and is one of the leading risk factors for acute lung injury and acute respiratory distress syndromes. Despite its frequency, it remains largely a disease of exclusion, characterized by ill defined infiltrates on the chest radiograph and hypoxia. An accurate ability to diagnose aspiration is paramount as different modalities of therapy, if applied early and selectively, could change the course of the disease. This article reviews definitions, diagnosis, epidemiology, pathophysiology, including animal models of aspiration-induced lung injury, and evidence-based clinical management. Additionally, a review of current and potential biomarkers which have been tested clinically in humans is provided.
Data Sources
Data were obtained from a PubMed search of the medical literature. PubMed “related articles” search strategies were employed.
Summary and Conclusions
Aspiration in the intensive care unit is a clinically relevant problem requiring expertise and awareness. A definitive diagnosis of aspiration pneumonitis or pneumonia is challenging to make. Advances in specific biomarker profiles and prediction models may enhance the diagnosis and prognosis of clinical aspiration syndromes. Evidence-based management is supportive, including mechanical ventilation, bronchoscopy for particulate aspiration, consideration of empiric antibiotics for pneumonia treatment, and lower respiratory tract sampling to define pathogenic bacteria that are causative.
Keywords: Aspiration, aspiration pneumonitis, aspiration pneumonia, acute lung injury, acute respiratory distress syndrome, intensive care unit
Aspiration induced lung injury is often under diagnosed in the clinical setting in the care of the critically ill and accounts for a significant proportion of acute pulmonary dysfunction(1). Moreover, it is recognized as an independent risk factor for subsequent development of pneumonia or acute lung injury or acute respiratory distress syndrome (ALI/ARDS). Despite the clinical importance of gastric aspiration-induced lung injury, the underlying mechanisms responsible for progression to severe inflammation and ALI/ARDS are not fully understood. This concise review focuses on various clinical aspects of this form of acute lung injury in order to provide a guide for the bedside clinician in the intensive care unit. The lack of well-designed, randomized clinical studies on this subject has lead to a number of generalizations and clinical paradigms that are clearly misleading. In this regard, the guidelines that are based on existing data and consensus statements are provided.
Additionally, since a major emphasis of our laboratory has been to elucidate mechanisms and identify distinct characteristics of the inflammation in gastric aspiration lung injury in animal models, we will include this new information to supplement these guidelines in this review. Particular studies highlighted in coverage include research in rodent models (rats, mice) on how mild acid-induced aspiration injury can be greatly exacerbated if gastric food particles are also present (2–7). The potential for using statistical predictive modeling (8) to “diagnose” different forms of gastric aspiration (acid, gastric particles, and a combination of the two) based on inflammatory mediator and biomarker profiles, is also discussed. Definition, epidemiology, diagnosis and the basic guidelines for clinical management of gastric aspiration-induced lung injury are provided.
1. Definitions
Aspiration is defined as the inhalation of foreign material into the airways beyond the vocal cords(1,9). The content of the aspirate is variable and may comprise secretions, blood, bacteria, liquids and food particles. Aspiration may be silent (or unwitnessed) or witnessed. Additionally, aspiration could involve repeated episodes of micro-aspiration that rarely cause acute symptoms(9). Aspiration is different from regurgitation where the reflux of gastric contents into the oropharynx or the esophagus is not associated with entry into the lungs.
Aspiration events can also be categorized as aspiration pneumonitis (chemical pneumonitis) or aspiration pneumonia (infectious process secondary to an aspiration event), though the differentiation between these two processes can be very difficult(1). The precise timing and nature of the aspiration of bacteria is currently not well understood.
2. Incidence and Epidemiology
Gastric aspiration is a recognized complication of general anesthesia occurring in 1 of every 2–3 thousand anesthetics (1,10–12). Gastric aspiration also frequently occurs in trauma or ICU patients with altered states of consciousness (e.g., head trauma, alcohol or drug-induced alterations in sensorium, cerebrovascular accidents). The inhalation of low pH gastric fluid and/or particulate food material leads to an initial pneumonitis that may or may not become complicated by subsequent bacterial pneumonia (1,10,13). Unwitnessed gastric aspiration is thought to be potentially important in many unexplained cases of perioperative pulmonary dysfunction. However, a majority of gastric aspiration pneumonitis cases can be difficult to diagnose and is frequently mistaken for bacterial pneumonia leading to inappropriate treatment (1,14,15). The most challenging task for medical practitioners, is the ability to differentiate between the two most common presentations of the aspiration syndromes: the inflammatory lung injury with aspiration pneumonitis and the bacterial aspiration pneumonia.
The true incidence of aspiration induced lung injury is difficult to estimate considering that most aspiration events are silent or unwitnessed. In a prospective study of critically ill, Methany and colleagues, using BAL levels of pepsin as a surrogate marker of aspiration, estimated that at least one aspiration event occurred in 88.9% of patients(16).
The severity of lung injury following gastric aspiration ranges from a mild, subclinical pneumonitis to a progressive respiratory failure with significant morbidity and mortality. Gastric aspiration is a major direct cause of ALI and the more severe ARDS (1,10,17,18). ALI/ARDS typically involves a sudden, severe pulmonary inflammation and alveolar-capillary permeability injury that includes proteinaceous edema, hypoxemia, loss of lung compliance, and is also frequently associated with multi-organ system failure (18, 19). Approximately a third of patients with aspiration pneumonitis develop a more severe, protracted course associated with ALI/ARDS (12,13,20–22). The incidence of ALI/ARDS has been variably reported to be 50,000–150,000 cases per year in the United States, with high associated mortality and morbidity (17,23–29). An analysis by Goss et al (27) has estimated the incidence of clinical ALI in the United States as 22–64 cases per 100,000 persons per year. In addition to severe acute respiratory failure, ALI/ARDS can also progress to a later “fibroproliferative” phase of disease that involves chronic lung injury with tissue remodeling and the initiation of fibrosis (18). It has been hypothesized that one of the pathogenic mechanisms responsible for many cases of idiopathic pulmonary fibrosis is repeated chronic micro-aspiration. Aside from the substantial medical consequences, the economic cost of ALI/ARDS is significant, with estimates of 3.5 – 6 billion dollars and ~$4,150 per patient per day in the U.S. (29). Mortality from ARDS has decreased somewhat since the initial description of this syndrome in 1967(30), but remains unacceptably high at 30 to 40% despite sophisticated medical intensive care (17,23–26,29). ALI/ARDS associated with aspiration pneumonitis carries a 30% mortality, and accounts for up to 20% of all deaths attributable to anesthesia (31–33).
3. Risk Factors
Aspiration is a frequently reported event in patients with altered levels of consciousness such as patients undergoing general anesthesia (11), elderly and nursing home residents (14,15), people with gastro-intestinal (GI) and esophageal abnormalities (34), as well as patients with neurologic trauma and neuro-muscular diseases (35). The clinical manifestation of an aspiration event falls within a wide spectrum ranging from subclinical manifestations such as dry cough or dysphonia to a fulminant life threatening failure such as ARDS. The pathogenic process can be further complicated by superimposed secondary infection, lung abscess, airway obstruction, exogenous lipoid pneumonia, and progression into chronic interstitial fibrosis (1). The distinction between the two entities is important because while bacterial aspiration pneumonia needs to be treated with antibiotics, treatment of aspiration pneumonitis is supportive (1,15). Patients at greatest risk for aspiration-associated ALI/ARDS in the peri-operative period include those with reduced glottic competency due to extreme age, esophageal neuromuscular disease, endotracheal tube intubation, or impaired consciousness from ethanol intoxication, anesthesia, head trauma, or cerebrovascular accidents (1,10). Patients with low gastric pH levels, decreased gastro-esophageal sphincter tone, or increased gastric pressure are also susceptible, with additional predisposing conditions ranging from sepsis to pregnancy to diet. A complete list of potential risk factors is enumerated in Table 1.
Table 1.
Risk Factors for Aspiration-Induced Lung Injury
|
4. Pathogenesis of different forms of gastric aspiration: What have we learned from animal models?
A. Acid aspiration
The acid component of gastric aspirates is frequently modeled by intratracheal instillation of hydrochloric acid (HCl) in animal models. Lung injury in adult rats following the aspiration of dilute hydrochloric acid (ACID = normal saline (NS) plus HCl at a final pH of 1.25) is characterized by a bi-phasic response comprising an early insult that is characterized by stimulation of capsaicin-sensitive neurons and direct caustic actions of low pH on airway epithelium followed by an acute neutrophilic inflammatory response at 4–6 hr (36). These pathogenic mechanisms lead to the loss of pulmonary microvascular integrity and extravasation of fluid and protein into the airways and alveoli (36,37). In addition to mechanically increasing the work of breathing by increasing airway resistance and inhibiting the diffusion of oxygen, edema fluid contains plasma proteins and other substances that can directly interfere with the function of alveolar surfactant [for a comprehensive review of pulmonary surfactant activity and dysfunction in lung injury see (38)]. One indication of the importance of surfactant dysfunction in the pathophysiology of acid aspiration pneumonitis is the finding in rats (39) that treatment with exogenous surfactant improves pulmonary function only after inhibitory plasma proteins are removed by lavage. Neutrophils are the inflammatory cellular response following aspiration of low pH gastric contents. Additionally a number of local (pulmonary lavage) and systemic (blood) inflammatory mediators have been demonstrated in acid-induced lung injury (4,5,7,40–44). Levels of the proximal proinflammatory cytokine, tumor necrosis factor (TNF)-α, are elevated following gastric aspiration (4,44,45), and concentrations of several important chemotactic cytokines (i.e., chemokines) in BAL are also increased. This includes elevated levels of the neutrophil chemotactic chemokine interleukin (IL)-8 in rabbits, or the rodent homologs macrophage inflammatory protein - 2(MIP-2) and cytokine-induced neutrophil chemoattractant - 1 (CINC-1) in rats, during acid-induced lung injury (5,7,43). TNF-α and MIP- 2 are required for the pathogenesis of acute pulmonary injury in rats given ACID (HCl, pH 1.25) (4,7). In addition, increases in leukocyte-derived oxidants and proteinases have been demonstrated in acid-induced lung injury (3,40,46). Eicosanoids also play a role in acute acid-induced pulmonary injury, and act in conjunction with cytokines to promote neutrophil infiltration and activation (41). Activation of complement is additionally known to be important in the systemic response following acid aspiration (47).
B. Gastric food particle-induced lung injury
Small non-acidified gastric particles (SNAP) for animal studies can be prepared from the stomach contents of rodents by a combination of washing in normal saline, filtration through gauze, autoclaving, and centrifugation (e.g., (2,5,48)). The typical distribution of particle sizes in preparations of SNAP is bimodal, with diameter peaks at approximately 4.5 and 13 μm (average diameter <10 μm) (2). Following tracheal instillation of SNAP in rats, acute neutrophilic inflammation can be detected at 4–6 hr (2), but there is no initial edema as is observed in ACID. A monocytic response peaks at about 48 hr post-aspiration, at which time lung tissue exhibits signs of early granuloma formation. TNF-α, MIP-2, and CINC-1 are among the acute inflammatory mediators elevated in lavage from rats following the aspiration of SNAP (4,5,7). Monocyte chemoattractant protein - 1 (MCP-1) is also elevated in rats following SNAP aspiration (5) and the importance of this mediator has been documented in several aspects of the innate pulmonary inflammatory response including the development of granulomas (e.g., (49–53)). MCP-1 is produced by multiple cell types in lung injury, including pulmonary vascular endothelial cells and alveolar type II epithelial cells (e.g., (52,53)).
C. Combined acid/gastric food particle (CASP) aspiration lung injury
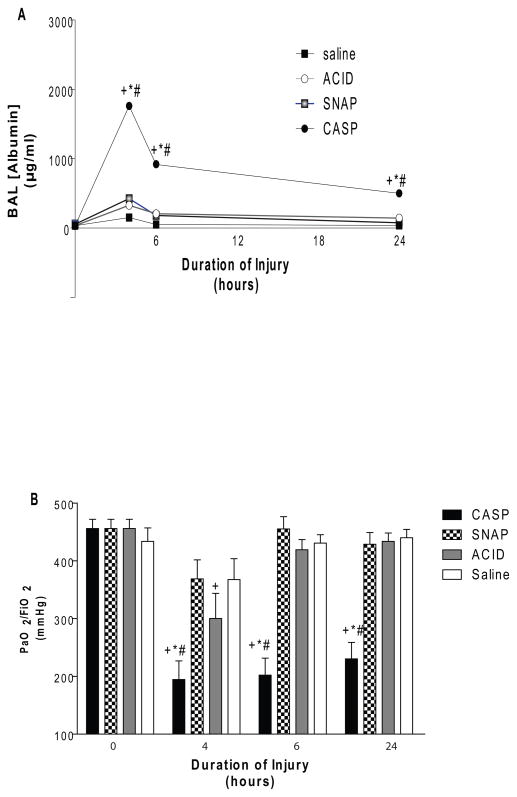
One emphasis of aspiration-related research noted earlier has been on animal models that involve two-hit insults (based on the contents of the aspirates themselves or additional stresses i.e., hyperoxia, bacterial seeding, lung contusion, or anesthesia). This work has shown that multiple insults can generate substantially more than additive severe pulmonary pathology than the sum of the individual component insults alone (3–5,54–59). In terms of two-hit gastric aspirates, attention has been focused on a combination of acid plus small non-acidified gastric particles (CASP = ACID + SNAP). In both the rats and mice, the severity of lung injury from CASP aspiration is more severe than for either SNAP or ACID alone (4,5,56,58). Levels of albumin in BAL, which are indicative of loss of alveolar-capillary membrane integrity, are significantly higher in rats given CASP compared to ACID or SNAP (Figure 1A). Arterial oxygenation is also dramatically reduced following intratracheal administration of CASP, with PaO2/FiO2 ratios meeting the criteria for clinical ARDS in the 24 hr period following aspiration (Figure 1B) (5). Increased lung injury based on BAL albumin levels following CASP aspiration in rodents is synergistic (greater than additive) compared to the individual component injuries of ACID or SNAP alone.
Figure 1. Relative severity of acute pulmonary injury in rats instilled with different gastric aspirates.
The severity of lung injury in rats was measured as a function of time after tracheal instillation of ACID, SNAP, CASP, or normal saline (NS). Lung injury was assessed by ELISA measurements of albumin levels in bronchoalveolar lavage (BAL) (Panel A), and by the ratio of the arterial partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) (Panel B). Rats breathed 98% O2 for a 15 min period prior to measurements of arterial oxygenation in order to emphasize the relative severity of intrapulmonary shunting. Data are Mean ± SEM for n= 9–2. Statistical symbols are: p<0.0001 compared to NS (+), ACID (*) and SNAP (#). Adapted from (5).
Data on leukocyte influx and cytokine/chemokine production demonstrate an over-exuberant proinflammatory response following the instillation of CASP compared to ACID or SNAP alone (4–7,48). Numbers of neutrophils (PMN’s) are dramatically increased in BAL from rodents given CASP compared to ACID or SNAP alone, and remain high in the airspaces for 48 hr post-aspiration indicating continuing leukocytic infiltration (5,6). The degree of synergistic inflammation in CASP depends on the time delay between the administration of the first component injury (ACID) and the administration of the second (SNAP). The window of time for a synergistic pro-inflammatory lung injury exists for at least 8 hr following ACID, but if SNAP is administered at 24 hr following ACID the severity of acute lung injury is decreased and the inflammatory response is suppressed compared to SNAP alone. Standard conditions for studies of CASP aspiration in our laboratory have involved either simultaneous instillation of ACID with SNAP, or SNAP following ACID within 4 hr (4–7,48).
A broad spectrum of cytokines and chemokines have been studied to examine different patterns of inflammatory lung injury in the three forms of aspiration, and examples from rat studies are given in Figure 1 (5). Levels of the CXC chemokine CINC-1 (homolog of IL-8) in BAL were increased at both 4 and 6 hr in rats given CASP, and were higher than found in animals receiving SNAP or ACID (Figure 2). BAL levels of the monotactic chemokine MCP-1 were also elevated following CASP injury compared to SNAP or ACID at 6 hr, and continued to increase at 24 hr. Levels of the modulatory cytokine IL-10 in BAL were increased at 6 hr following either CASP or SNAP, but only rats that received CASP had increased IL-10 at 24 hr. IL-10 levels were the single best predictor of lung injury severity based on albumin concentrations in BAL across all forms of aspiration studied (ACID, SNAP, CASP) at both 6 and 24 hr (5). A statistical correlation model that incorporated both IL-10 and MCP-1 levels improved the prediction of the severity of lung injury at both time points.
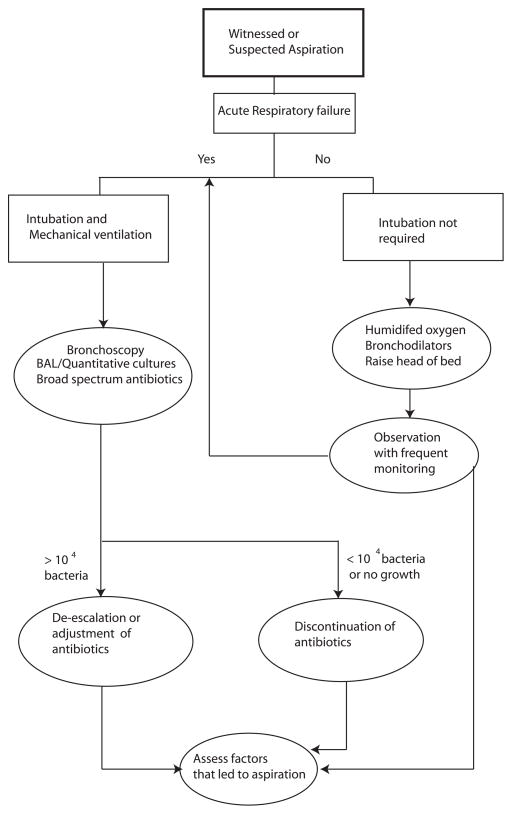
Figure 2. Proposed Algorithm for suspected or witnessed aspiration event.
An algorithm, based on the available evidence to date, is presented. For practical purposes and based on current practice patterns, it is difficult to differentiate aspiration pneumonitis from aspiration pneumonia. The indication to perform endotracheal intubation and mechanical ventilation is not different from other clinical scenarios. Additionally the institution of the type of antibiotics should be based on local ecology of the ICU and should follow the practice guidelines (74).
These cytokines have been extensively studied in the context of ALI/ARDS. However, to date there is no available data regarding the nature and pattern of cytokines that are elaborated specifically in the BAL or serum of patients with aspiration syndromes.
5. Diagnosis of gastric aspiration induced lung injury
a. Clinical assessment
Aspiration may be completely asymptomatic or present with dramatic signs and symptoms that include wheezing, shortness of breath, cyanosis, hypotension and hypoxia(1,9). A witnessed aspiration event is evident with the documentation of food particles or oral contents in the trachea-bronchial tree(9). For instance, in patients, who have sustained significant trauma, the aspirate may be made of blood and saliva(60). These patients may subsequently deteriorate into ALI/ARDS or develop superimposed bacterial infection. The timing of the bacterial infection whether related to the initial aspiration event or progressive migration of the bacteria into the distal bronchial tree, is a subject of intense controversy (1,55).
Unwitnessed gastric aspiration is one of the most difficult entities to diagnose. There are no gold standards for diagnosis of aspiration induced lung injury. Often times it is a disease of exclusion, where other etiologies of hypoxia such as pulmonary edema, pulmonary embolism or community or hospital acquired bacterial pneumonia have been ruled out. Aspiration pneumonitis is diagnosed by a combination of hypoxia with a chest infiltrate that typically involves the dependant portions of the lungs. The portions of the lung affected may depend on the position in which the patient aspirates. Initially, the respiratory distress is much more severe than the radiographic changes would indicate, and many times the radiographic picture becomes more pronounced as the hypoxia and the clinical symptoms resolve. However it is the differentiation of aspiration pneumonitis and aspiration pneumonia that poses the greatest challenge to the intensivist.
b. Specific Biomarkers
Numerous biomarkers have been studied in the context of aspiration-induced lung injury in humans and animals(61). Additionally, similar markers have been investigated in the context of other lung injuries such as community acquired pneumonia, hospital acquired pneumonia and sepsis, though not examined in human subjects with aspiration pneumonitis. A detailed discussion of these biomarkers is outside the scope of this review. Table 2 lists specific and potential biomarkers for aspiration that have been studied in humans and specific data pertaining to animal studies have been excluded.
Table 2.
Biomarkers of Gastric Aspiration
| Biomarker | Summary of Clinical Data | Advantages | Disadvantages |
|---|---|---|---|
| BAL pepsin | Elevated in ICU population with subsequent pneumonia(16). Linked to broncho-pulmonary dyplasia in lung transplant patients with increased pepsin.(78) Never compared to gold standards of witnessed gastric aspiration |
Easy to perform | Can be detected for a very short time No standardization for positive result |
| Lipid laden macrophage | Has been linked with gastric aspiration in a number of studies; Shown to be semi- quantitative(79–83) | Semi-quantitative | Non specific |
| Soluble-TREM-1 | Single study reported higher levels in aspiration vs. non aspiration patient groups(84) | Standardized measurement available for serum | Non specific; Increased in trauma, infectious pneumonia(85, 86) |
| c-Reactive Protein | Elevated in aspiration pneumonitis and pneumonia but cannot be used to distinguish them(15) | Easy to measure | Non specific |
| Procalcitonin | Tested positive in aspiration patients vs. non aspirated group(87) N-terminal procalcitonin elevated in aspiration patients(88) |
Widely available and easy to use | Lack of sensitivity Non specific – increased to much higher levels in infections |
| Receptor for advanced Glycation end product (RAGE) | Elevated in sepsis and ALI/ARDS patients compared to normal(89) ; Marker of Type I alveolar cell injury?(90) Not tested in aspiration | Specific marker of lung injury | Not tested in aspiration |
| Exhaled breath condensates Leukotriene B4 concentration | Shown to be higher in children with community acquired pneumonia compared to healthy controls(91) | Non invasive | Difficult to collect and analyze; Never tested in aspiration |
| Carbamoyl phosphate synthase -1 | Increased in healthy volunteers after LPS infusion(92) | Early and quick response in 4–5 hr following LPS | Not tested in aspiration |
| Endothelin-1 or precursors | Elevated in community acquired pneumonia(93); correlated with severity of injury and mortality | Levels predicted mortality | Not tested in aspiration |
| Copeptin | Elevated in patients with lower respiratory tract infection and correlated with severity of disease(94, 95); Correlated with SIRS in noncardiac surgery patients(96) | Levels related to severity of illness | Not tested in aspiration |
c. Biomarker Profiles and Prediction models
In addition to correlation analyses relating cytokine levels to lung injury severity, a complementary hierarchical cluster analysis was performed to summarize the degree of relationship among different cytokines and inflammatory cells in BAL at 6 and 24 hr post aspiration (5). Because of the complexity and interactions of different facets of the inflammatory response, correlational analyses involving cytokine and chemokine levels as noted here provide information that are suggestive rather than definitive. Nonetheless, these analyses support the potential of utilizing information on local or systemic inflammatory mediators to better understand mechanisms and pathways that contribute to different forms of aspiration lung injury(62). Moreover, the existence of differences in inflammatory mediator responses in rats to various forms of aspiration (ACID, SNAP, CASP) suggests that it may ultimately be feasible to identify specific inflammatory mediator “signatures” to enhance the diagnosis and prognosis of clinical aspiration syndromes as described later(62).
6. Management Principles
Even though aspiration is considered a common event in the critically ill, the clinical consequences can vary. For the purpose of future discussion, it is assumed that aspiration pneumonitis and aspiration pneumonia are different clinical scenarios though it is often difficult to separate the two entities. A proposed algorithm based on existing evidence and common practice patterns is highlighted in figure 2. It is additionally emphasized that the management principles adopted are based more on symptomatology and progression of the disease rather than the strict differentiation between aspiration pneumonitis and pneumonia.
Aspiration Pneumonitis
Most aspirates in the clinical scenarios are liquid in nature. It is the composition of the aspirate that determines the extent and progression of the injury on the pulmonary parenchyma. The course of pneumonitis can be broadly differentiated into 2 clinical phases. Phase 1 involves intense coughing or bronchospasm that occur immediately following the aspiration event where as the second phase characterized by the onset of inflammation in the pulmonary occurs over the next 4–6 hrs(36).
Although there are limited data from randomized clinical trials regarding definitive therapy for aspiration events, there are certain guidelines that can be followed in managing these patients. As it is impossible to enumerate all the therapeutic maneuvers that have been tested, only a few are discussed in detail.
a. Positioning and aggressive pulmonary care
Following a witnessed aspiration event, the patient should be positioned so that further aspiration of gastric contents is significantly reduced. In an awake patient, this is best achieved by turning the head laterally and suctioning the oral and pharyngeal cavity(63). The patient’s bed can also be raised by 45 degrees with the head up. The decision to intubate the patient is based on general neurological status, degree of hypoxia, and hemodynamic stability of the patient. Additionally, patients with large volume particulate aspiration may require intubation, to facilitate future bronchoscopy (63,64). Nebulized bronchodilators may be administered for bronchospasm. In view of the recent aspiration event, attempts to institute non-invasive ventilation should be avoided. Mechanical ventilation should be continued ascribing to the current standards of lung protective strategy. Maneuvers to prevent recurrent aspiration episodes with gastric decompression such as placement of naso-gastric tubes and connecting gastrostomy tubes to suction or gravity drainage, should also be instituted.
b. Role of bronchoscopy
Aspirated material is frequently liquid in nature and disperses rapidly. Hence routine bronchoscopy with lavage is not indicated. However, in the event that the aspirate is predominantly particulate in nature with clear radiographic evidence of lobar collapse or major atelectasis, a therapeutic bronchoscopy is helpful(64). The other major advantage of bronchoscopy is the sampling of the lower respiratory tract. The quantitative bacteriology obtained from the BAL samples can not only guide definitive therapy and de-escalation of antibiotics but also can result in discontinuation of antibiotics if cultures do not show significant bacterial growth(65).
c. Role of empiric antibiotics
Aspiration is characterized in the early phase as an acute pneumonitis. This inflammatory episode is often characterized by fever and leukocytosis. Though antibiotics are not necessary in the treatment of pneumonitis(1), it is often difficult to distinguish aspiration pneumonitis from pneumonia. Additionally, bacteria from colonization of the stomach due to the common use of proton pump inhibitors or from the oropharynx may be aspirated with the gastric contents. Although there are no randomized trials on this subject, it is worthwhile to consider the following facts. In the context of treating ventilator associated Pneumonia (VAP), a short course of antibiotics has not been shown to have adverse effects as long as the antibiotics are deescalated or discontinued based on quantitative microbiology. Moreover, a recent survey suggested that a vast majority of intensivists were prescribing antibiotics in patients with suspected aspiration events(66). A rational strategy would be to start antibiotics appropriate to cover VAP (depending on the duration of stay in the ICU) and de-escalate the antibiotic use based on appropriate definitive, quantitative cultures (usually within 72 hrs). It is emphasized that it is equally important to eliminate the antibiotics if the culture results do not show significant bacterial growth or contamination of the aspirated material. Antibiotics should also be considered in patients who have documented aspiration secondary to small bowel obstruction or in patients with colonization of gastric contents(1). It has been noted that anaerobic coverage was routinely instituted in the past for aspiration-induced lung injury. However, to date, available data do not indicate that anaerobic bacteria are a concern (67,68).
d. Role of Steroids
Based on available data, steroid administration cannot be recommended for aspiration events. Though a single study showed early radiologic resolution of infiltrates following administration of steroids(69), subsequently, two large multicenter randomized studies have failed to show any benefit(70,71). Moreover, at least one study reported a higher incidence of gram negative pneumonia in the patient arm that received steroids(72).
Aspiration Pneumonia
Aspiration induced lung injury is a clear risk factor for development of pneumonia (16,55). Whether the aspiration of bacteria occurs at the initial time period or is a result of altered host-bacterial interaction, is currently not well understood. Available evidence indicates that the bacteriology of aspiration pneumonia is not different from that of hospital or ventilator acquired pneumonia(16,73). Once the diagnosis of aspiration pneumonia is definitively established, early administration of antibiotics is strongly recommended(74). These may represent patients with persistent leukocytosis, fever, and infiltrates 48hr following the initial aspiration event. Additionally, identification of pathogenic bacteria in significant amount (>104 CFU/ml in most ICU), either by protected brush specimen or BALs is usually confirmatory for a diagnosis of aspiration pneumonia (65). The choice of antibiotics may vary based on the local ecology of the ICU. However, certain broad principles can be instituted (75). It is appropriate to initiate early, empiric, broad spectrum antibiotics with activity against Gram-negative bacteria (75). Routine use of antibiotics with anaerobic coverage is not needed unless there is evidence of severe periodontial disease, necrotizing pneumonia, or lung abscess visualized in a CT scan (67,68). The duration of the antibiotics is similar to what is described for VAP and although this subject is of intense debate, the recommended duration should be based on the patients clinical response to antibiotics and may extend from 3–13 days(75,76).
7. Future Directions
Despite major advances in understanding the pathophysiology of aspiration-induced lung injury in small animal models, there remains a significant gap in diagnosing unwitnessed gastric aspiration events, as well as the ability to predict the likelihood of progression of the pulmonary insult to ALI/ARDS. One of the major problems in this regard is the absence of distinct diagnostic or prognostic signatures to diagnose this entity. With the development of microarray technology, there have been a number of studies that have focused on the identification of single or multiple gene products as biomarkers for diagnosis or prognosis. Recently the emerging field of whole body or organ genomics has provided a methodology to develop classification models such as those applied in the field of acute lung injury (77). The ability to do so in combination with animal and well-designed human studies in the near future, will likely result in the development of biomarkers that are useful in identifying the etiology and determining the potential pulmonary injury severity of aspiration events.
8. Conclusions
Aspiration induced lung injury is a frequent problem that particularly affects the critically ill and is often misdiagnosed, or a disease of exclusion. Patients following an aspiration event can present with a reversible non-infectious chemical pneumonitis (aspiration pneumonitis) or aspiration-related bacterial pneumonia (aspiration pneumonia), either of which could progress to ALI/ARDS. Animal studies have confirmed that a combination of acid and particulate matter aspirate results in severe, progressive lung injury compared to the individual gastric components of acid or particulate matter individually. Being able to distinguish the different aspiration syndromes could have significant prognostic and therapeutic implications. However, the basic mechanistic understanding of aspiration-induced lung injury from small animal studies has yet to produce clinically valid distinct “signatures” of aspiration pneumonitis versus bacterial aspiration pneumonia. Investigations examining individual biomarkers in aspiration have come up with a number of shortcomings and the search for the clinically useful biomarker(s) continues. Finally, analysis of cytokines profiles has shown promise in animal models, but its relevance in clinical decision-making needs to be validated in well designed clinical studies.
Acknowledgments
Grant Support: RO1-HL102013 (KR) and RO1-HL 48889 (PRK) from the NIH.
Dr. Raghavendran received funding from grants from NIH, NIGMS, and NHLBI. Dr. Knight received funding from a grant from NIH.
Footnotes
The other authors have not disclosed any potential conflicts of interest.
Bibliography
- 1.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 2.Knight PR, Rutter T, Tait AR, et al. Pathogenesis of gastric particulate lung injury: a comparison and interaction with acidic pneumonitis. Anesth Analg. 1993;77(4):754–760. doi: 10.1213/00000539-199310000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Nader-Djalal N, Knight PR, Bacon MF, et al. Alterations in the course of acid-induced lung injury in rats after general anesthesia: volatile anesthetics versus ketamine. Anesth Analg. 1998;86:141–146. doi: 10.1097/00000539-199801000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Davidson BA, Knight PR, Helinski JD, et al. The role of tumor necrosis factor-alpha in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology. 1999;91:486–499. doi: 10.1097/00000542-199908000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Knight PR, Davidson BA, Nader ND, et al. Progressive, severe lung injury secondary to the interaction of insults in gastric aspiration. Exp Lung Res. 2004;30:535–557. doi: 10.1080/01902140490489162. [DOI] [PubMed] [Google Scholar]
- 6.Raghavendran K, Davidson BA, Mullan BA, et al. Acid and particulate induced aspiration injury in mice: Role of MCP-1. Am J Physiol: Lung Cell Mol Physiol. 2005;289:L134–L143. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- 7.Shanley TP, Davidson BA, Nader ND, et al. Role of macrophage inflammatory protein-2 in aspiration-induced lung injury. Crit Care Med. 2000;28(7):2437–2444. doi: 10.1097/00003246-200007000-00041. [DOI] [PubMed] [Google Scholar]
- 8.Hutson AD. A semiparametric bootstrap approach to correlated data analysis problems. Comput Methods Programs Biomed. 2004;73(2):129–134. doi: 10.1016/s0169-2607(03)00021-x. [DOI] [PubMed] [Google Scholar]
- 9.Zaloga GP. Aspiration-related illnesses: definitions and diagnosis. JPEN J Parenter Enteral Nutr. 2002;26(6 Suppl):S2–7. doi: 10.1177/014860710202600602. discussion S7–8. [DOI] [PubMed] [Google Scholar]
- 10.Healy T, Knight PR. Wylie and Churchill- Davidson Practice of Anesthesia. 7. London: Edward Arnold; 2003. [Google Scholar]
- 11.Olsson GL, Hallen B, Hambraeus-Jonzon K. Aspiration during anaesthesia: a computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30(1):84–92. doi: 10.1111/j.1399-6576.1986.tb02373.x. [DOI] [PubMed] [Google Scholar]
- 12.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Britto J, Demling RH. Aspiration lung injury. New Horiz. 1993;1(3):435–439. [PubMed] [Google Scholar]
- 14.Mylotte JM, Naughton B, Saludades C, et al. Validation and application of the pneumonia prognosis index to nursing home residents with pneumonia. J Am Geriatr Soc. 1998;46:1538–1544. doi: 10.1111/j.1532-5415.1998.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 15.Mylotte JM, Goodnough S, Naughton BJ. Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51:17–23. doi: 10.1034/j.1601-5215.2002.51004.x. [DOI] [PubMed] [Google Scholar]
- 16.Metheny NA, Clouse RE, Chang YH, et al. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit Care Med. 2006;34(4):1007–1015. doi: 10.1097/01.CCM.0000206106.65220.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1348. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 18.Raghavendran K, Davidson BA, Helinski JD, et al. A rat model for isolated bilateral lung contusion from blunt chest trauma. Anesthesia and Analgesia. 2005;101:1482–1489. doi: 10.1213/01.ANE.0000180201.25746.1F. [DOI] [PubMed] [Google Scholar]
- 19.Notter RH, Finkelstein JN, Holm BA, editors. Lung Injury: Mechanisms, Pathophysiology and Therapy. New York: Marcel Dekker; 2005. [Google Scholar]
- 20.Turnstall MEOG. Obstetrics, general anesthesia. 5. London: Butterworth and co; 1989. [Google Scholar]
- 21.Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 Pt 1):593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 22.Pepe PE, Potkin RT, Reus DH, et al. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 23.Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 24.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 25.Doyle RL, Szaflarski N, Modin GW, et al. Identification of patients with acute lung injury: Predictors of mortality. Am J Respir Crit Care Med. 1995;152:1818–1824. doi: 10.1164/ajrccm.152.6.8520742. [DOI] [PubMed] [Google Scholar]
- 26.Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003;31(4 Suppl):S276–284. doi: 10.1097/01.CCM.0000057904.62683.2B. [DOI] [PubMed] [Google Scholar]
- 27.Goss CH, Brower RG, Hudson LD, et al. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31(6):1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 28.Knight PR, Rotta AT. Acute lung injury: Etiologies and basic features. In: Notter RH, Finkelstein JN, Holm BA, editors. Lung injury: Mechanisms, pathophysiology, and therapy. Boca Raton: Taylor & Francis Group; 2005. pp. 67–110. [Google Scholar]
- 29.Treggiari MM, Hudson LD, Martin DP, et al. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med. 2004;32(2):327–331. doi: 10.1097/01.CCM.0000108870.09693.42. [DOI] [PubMed] [Google Scholar]
- 30.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 31.Bannister WK, Sattilaro AJ. Vomiting and aspiration during anesthesia. Anesthesiology. 1962;23:251–264. doi: 10.1097/00000542-196203000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Bodlander FM. Deaths associated with anaesthesia. Br J Anaesth. 1975;47(1):36–40. doi: 10.1093/bja/47.1.36. [DOI] [PubMed] [Google Scholar]
- 33.Marx GF, Mateo CV, Orkin LR. Computer analysis of postanesthetic deaths. Anesthesiology. 1973;39(1):54–58. doi: 10.1097/00000542-197307000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Ravelli AM, Panarotto MB, Verdoni L, et al. Pulmonary aspiration shown by scintigraphy in gastroesophageal reflux-related respiratory disease. Chest. 2006;130(5):1520–1526. doi: 10.1378/chest.130.5.1520. [DOI] [PubMed] [Google Scholar]
- 35.Daniels SK, Brailey K, Priestly DH, et al. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy TP, Johnson KJ, Kunkel RG, et al. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesth Analg. 1989;69(1):87–92. [PubMed] [Google Scholar]
- 37.Grimbert FA, Parker JC, Taylor AE. Increased pulmonary vascular permeability following acid aspiration. J Appl Physiol. 1981;51(2):335–345. doi: 10.1152/jappl.1981.51.2.335. [DOI] [PubMed] [Google Scholar]
- 38.Notter RH. Lung surfactants: Basic science and clinical applications. New York: Marcel Dekker, Inc; 2000. [Google Scholar]
- 39.Eijking EP, Gommers D, So KL, et al. Surfactant treatment of respiratory failure induced by hydrochloric acid aspiration in rats. Anesthesiology. 1993;78(6):1145–1151. doi: 10.1097/00000542-199306000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Knight PR, Druskovich G, Tait AR, et al. The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology. 1992;77(4):772–778. doi: 10.1097/00000542-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Goldman G, Welbourn R, Klausner JM, et al. Neutrophil accumulations due to pulmonary thromboxane synthesis mediate acid aspiration injury. J Appl Physiol. 1991;70(4):1511–1517. doi: 10.1152/jappl.1991.70.4.1511. [DOI] [PubMed] [Google Scholar]
- 42.Goldman G, Welbourn R, Klausner JM, et al. Leukocytes mediate acid aspiration-induced multiorgan edema. Surgery. 1993;114(1):13–20. [PubMed] [Google Scholar]
- 43.Folkesson HG, Matthay MA, Hebert CA, et al. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest. 1995;96(1):107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudoh I, Ohtake M, Nishizawa H, et al. The effect of pentoxifylline on acid-induced alveolar epithelial injury. Anesthesiology. 1995;82(2):531–541. doi: 10.1097/00000542-199502000-00023. [DOI] [PubMed] [Google Scholar]
- 45.Goldman G, Welbourn R, Kobzik L, et al. Tumor necrosis factor-alpha mediates acid aspiration-induced systemic organ injury. Ann Surg. 1990;212:513–519. doi: 10.1097/00000658-199010000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman G, Welbourn R, Kobzik L, et al. Reactive oxygen species and elastase mediate lung permeability after acid aspiration. J Appl Physiol. 1992;73:571–575. doi: 10.1152/jappl.1992.73.2.571. [DOI] [PubMed] [Google Scholar]
- 47.Nishizawa H, Yamada H, Miyazaki H, et al. Soluble complement receptor type 1 inhibited the systemic organ injury caused by acid instillation into a lung. Anesthesiology. 1996;85(5):1120–1128. doi: 10.1097/00000542-199611000-00021. [DOI] [PubMed] [Google Scholar]
- 48.Davidson BA, Knight PR, Wang Z, et al. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L699–708. doi: 10.1152/ajplung.00229.2004. [DOI] [PubMed] [Google Scholar]
- 49.Flory CM, Jones ML, Warren JS. Pulmonary granuloma formation in the rat is partially dependent on monocyte chemoattractant protein 1. Lab Invest. 1993;69(4):396–404. [PubMed] [Google Scholar]
- 50.Kilgore KS, Imlay MM, Szaflarski JP, et al. Neutrophils and reactive oxygen intermediates mediate glucan-induced pulmonary granuloma formation through the local induction of monocyte chemoattractant protein-1. Lab Invest. 1997;76(2):191–201. [PubMed] [Google Scholar]
- 51.Flory CM, Jones ML, Miller BF, et al. Regulatory roles of tumor necrosis factor-alpha and interleukin-1 beta in monocyte chemoattractant protein-1-mediated pulmonary granuloma formation in the rat. Am J Pathol. 1995;146(2):450–462. [PMC free article] [PubMed] [Google Scholar]
- 52.Standiford TJ, Kunkel SL, Phan SH, et al. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266(15):9912–9918. [PubMed] [Google Scholar]
- 53.Standiford TJ, Kunkel SL, Greenberger MJ, et al. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24–28. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 54.Nader-Djalal N, Knight PR, Davidson BA, et al. Hyperoxia exacerbates microvascular lung injury following acid aspiration. Chest. 1997;112:1607–1614. doi: 10.1378/chest.112.6.1607. [DOI] [PubMed] [Google Scholar]
- 55.Rotta AT, Shiley KT, Davidson BA, et al. Gastric acid and particulate aspiration injury inhibits pulmonary bacterial clearance. Crit Care Med. 2004;32:747–754. doi: 10.1097/01.ccm.0000114577.10352.46. [DOI] [PubMed] [Google Scholar]
- 56.Manderscheid PA, Bodkin RP, Davidson BA, et al. Bacterial clearance and cytokine profiles in a murine model of postsurgical nosocomial pneumonia. Clin Diagn Lab Immunol. 2004;11:742–751. doi: 10.1128/CDLI.11.4.742-751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson BA, Stewart CC, Russo TA, et al. Discrimination of resident and infiltrated alveolar macrophages by flow cytometry in influenza A virus-infected mice. Exp Lung Res. 2004 doi: 10.1080/01902140590918524. in press. [DOI] [PubMed] [Google Scholar]
- 58.Bless NM, Huber-Lang M, Guo RF, et al. Role of CC chemokines (macrophage inflammatory protein-1 beta, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J Immunol. 2000;164(5):2650–2659. doi: 10.4049/jimmunol.164.5.2650. [DOI] [PubMed] [Google Scholar]
- 59.Knight PR, Holm BA. The three components of hyperoxia. Anesthesiology. 2000;93:3–5. doi: 10.1097/00000542-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Lockey DJ, Coats T, Parr MJ. Aspiration in severe trauma: a prospective study. Anaesthesia. 1999;54:1097–1098. doi: 10.1046/j.1365-2044.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 61.Jaoude PA, Knight PR, Ohtake P, et al. Biomarkers in the diagnosis of aspiration syndromes. Expert Rev Mol Diagn. 10(3):309–319. doi: 10.1586/erm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hutson AD, Davidson BA, Raghavendran K, et al. Statistical Prediction of the Type of Gastric Aspiration Lung Injury Based on Early Cytokine/Chemokine Profiles. Anesthesiology. 2006;104(1):73–79. doi: 10.1097/00000542-200601000-00013. [DOI] [PubMed] [Google Scholar]
- 63.Moore FA. Treatment of aspiration in intensive care unit patients. JPEN J Parenter Enteral Nutr. 2002;26(6 Suppl):S69–74. doi: 10.1177/014860710202600611. discussion S74. [DOI] [PubMed] [Google Scholar]
- 64.Haenel JB, Moore FA, Moore EE, et al. Efficacy of selective intrabronchial air insufflation in acute lobar collapse. Am J Surg. 1992;164(5):501–505. doi: 10.1016/s0002-9610(05)81189-4. [DOI] [PubMed] [Google Scholar]
- 65.Kollef MH, Bock KR, Richards RD, et al. The safety and diagnostic accuracy of minibronchoalveolar lavage in patients with suspected ventilator-associated pneumonia. Ann Intern Med. 1995;122:743–748. doi: 10.7326/0003-4819-122-10-199505150-00002. [DOI] [PubMed] [Google Scholar]
- 66.Rebuck JA, Rasmussen JR, Olsen KM. Clinical aspiration-related practice patterns in the intensive care unit: a physician survey. Crit Care Med. 2001;29(12):2239–2244. doi: 10.1097/00003246-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178–183. doi: 10.1378/chest.115.1.178. [DOI] [PubMed] [Google Scholar]
- 68.Mier L, Dreyfuss D, Darchy B, et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med. 1993;19(5):279–284. doi: 10.1007/BF01690548. [DOI] [PubMed] [Google Scholar]
- 69.Sukumaran M, Granada MJ, Berger HW, et al. Evaluation of corticosteroid treatment in aspiration of gastric contents: A controlled clinical trial. Mt Sinai J Med. 1980;47(4):335–340. [PubMed] [Google Scholar]
- 70.Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317(25):1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 71.Bone RC, Fisher CJ, Jr, Clemmer TP, et al. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987;92(6):1032–1036. doi: 10.1378/chest.92.6.1032. [DOI] [PubMed] [Google Scholar]
- 72.Wolfe JE, Bone RC, Ruth WE. Effects of corticosteroids in the treatment of patients with gastric aspiration. Am J Med. 1977;63(5):719–722. doi: 10.1016/0002-9343(77)90157-7. [DOI] [PubMed] [Google Scholar]
- 73.Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129(6):433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 74.Iregui M, Ward S, Sherman G, et al. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122(1):262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 75.Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 76.Singh N, Rogers P, Atwood CW, et al. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 77.Howrylak JA, Dolinay T, Lucht L, et al. Discovery of the gene signature for acute lung injury in patients with sepsis. Physiol Genomics. 2009;37(2):133–139. doi: 10.1152/physiolgenomics.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ward C, Forrest IA, Brownlee IA, et al. Pepsin like activity in bronchoalveolar lavage fluid is suggestive of gastric aspiration in lung allografts. Thorax. 2005;60(10):872–874. doi: 10.1136/thx.2004.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahrens P, Noll C, Kitz R, et al. Lipid-laden alveolar macrophages (LLAM): a useful marker of silent aspiration in children. Pediatr Pulmonol. 1999;28(2):83–88. doi: 10.1002/(sici)1099-0496(199908)28:2<83::aid-ppul2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 80.Bryn T, Mahic M, Aandahl EM, et al. Inhibition of protein kinase A improves effector function of monocytes from HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24(7):1013–1015. doi: 10.1089/aid.2008.0071. [DOI] [PubMed] [Google Scholar]
- 81.Corwin RW, Irwin RS. The lipid-laden alveolar macrophage as a marker of aspiration in parenchymal lung disease. Am Rev Respir Dis. 1985;132(3):576–581. doi: 10.1164/arrd.1985.132.3.576. [DOI] [PubMed] [Google Scholar]
- 82.Parameswaran K, Anvari M, Efthimiadis A, et al. Lipid-laden macrophages in induced sputum are a marker of oropharyngeal reflux and possible gastric aspiration. Eur Respir J. 2000;16(6):1119–1122. doi: 10.1034/j.1399-3003.2000.16f17.x. [DOI] [PubMed] [Google Scholar]
- 83.Vejar L, Le Cerf P. Pulmonary aspiration in children. Quantification of lipid laden alveolar macrophages. Rev Med Chil. 1997;125(2):191–194. [PubMed] [Google Scholar]
- 84.El Solh AA, Akinnusi ME, Peter M, et al. Triggering receptors expressed on myeloid cells in pulmonary aspiration syndromes. Intensive Care Med. 2008;34(6):1012–1019. doi: 10.1007/s00134-008-1087-7. [DOI] [PubMed] [Google Scholar]
- 85.Giamarellos-Bourboulis EJ, Mouktaroudi M, Tsaganos T, et al. Evidence for the participation of soluble triggering receptor expressed on myeloid cells-1 in the systemic inflammatory response syndrome after multiple trauma. J Trauma. 2008;65(6):1385–1390. doi: 10.1097/TA.0b013e31814699cc. [DOI] [PubMed] [Google Scholar]
- 86.Gibot S, Cravoisy A. Soluble form of the triggering receptor expressed on myeloid cells-1 as a marker of microbial infection. Clin Med Res. 2004;2(3):181–187. doi: 10.3121/cmr.2.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pusch F, Wildling E, Freitag H, et al. Procalcitonin as a diagnostic marker in patients with aspiration after closed head injury. Wien Klin Wochenschr. 2001;113(17–18):676–680. [PubMed] [Google Scholar]
- 88.Nylen ES, Snider RH, Jr, Thompson KA, et al. Pneumonitis-associated hyperprocalcitoninemia. Am J Med Sci. 1996;312(1):12–18. doi: 10.1097/00000441-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Bopp C, Hofer S, Weitz J, et al. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008;147(1):79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carraro S, Andreola B, Alinovi R, et al. Exhaled leukotriene B4 in children with community acquired pneumonia. Pediatr Pulmonol. 2008;43(10):982–986. doi: 10.1002/ppul.20889. [DOI] [PubMed] [Google Scholar]
- 92.Struck J, Uhlein M, Morgenthaler NG, et al. Release of the mitochondrial enzyme carbamoyl phosphate synthase under septic conditions. Shock. 2005;23(6):533–538. [PubMed] [Google Scholar]
- 93.Schuetz P, Stolz D, Mueller B, et al. Endothelin-1 precursor peptides correlate with severity of disease and outcome in patients with community acquired pneumonia. BMC Infect Dis. 2008;8:22. doi: 10.1186/1471-2334-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seligman R, Papassotiriou J, Morgenthaler NG, et al. Copeptin, a novel prognostic biomarker in ventilator-associated pneumonia. Crit Care. 2008;12(1):R11. doi: 10.1186/cc6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muller B, Morgenthaler N, Stolz D, et al. Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. Eur J Clin Invest. 2007;37(2):145–152. doi: 10.1111/j.1365-2362.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- 96.Jochberger S, Zitt M, Luckner G, et al. Postoperative vasopressin and copeptin levels in noncardiac surgery patients: a prospective controlled trial. Shock. 2009;31(2):132–138. doi: 10.1097/SHK.0b013e31817fd1d6. [DOI] [PubMed] [Google Scholar]