Abstract
Neuropathy is frequently a late complication of diabetes mellitus. Auditory neuropathy and microangiopathy of inner ear are the possible causes of hearing loss in diabetics. To study the correlation between glycaemic control and hearing threshold in patients with type 2 diabetes mellitus and to determine the differences of hearing threshold between groups treated with different modality. This single blind randomized controlled study was performed at the Department of Medicine and Department of Otorhinolaryngology, Hospital Universiti Kebangsaan Malaysia (UKM) between 1st May 2003 and 31st September 2004. This study was approved by Research Ethics Committee (code number FF-137). Subjects were randomized into two groups. Group 1 were patients treated with conventional oral hypoglycemic agents. The patients in group 2 were those treated with insulin injection. The subjects were seen 4 weekly for 3 months. Audiometric test were performed in all subjects at each visit. Blood were taken for fasting blood glucose, Hb1Ac, and fructosamine at every visit to determine the glycaemic controls of the subject. They were 11 patients (22 ears) treated with oral hypoglycemic agents and 17 patients treated (34 ears) with subcutaneous insulin. There is no significant difference between mean pure tone threshold before and after treatment at all frequencies in both groups. There is also no significance different in fasting glucose level and fructosamine. However, there is significant difference HbA1c levels between the two groups after treatment (P < 0.05). This study has shown that glycaemic control does not have significant impact on hearing. The hearing threshold is neither affected by insulin treatment nor by the glycaemic control.
Keywords: Diabetes mellitus, Hearing loss, Glycemic control, Insulin, Fructosamine
Introduction
Hearing loss is one of the most common health problems among elderly. The major risk factors for hearing loss are age, noise exposure and vascular disease. Since both Type 1 (insulin dependent diabetes mellitus, IDDM) and type 2 (non insulin dependent diabetes mellitus, NIDDM) diabetes are known for their widespread microvascular lesion, their relationship with hearing loss has been widely studied but findings are contradictory. Several studies have reported a positive correlation between diabetes and hearing loss [1, 2] others have failed to confirm such association [3, 4]. Some investigators have shown that only diabetics with severe peripheral neuropathy or retinopathy are at increased risk of hearing loss [5, 6].
There are several theories on how diabetes mellitus may cause hearing loss. Most authors agreed that microangiopathy of inner ear is the main cause of hearing loss in diabetics as reported in animal and temporal bone studies [7, 8]. The other possible theory that diabetes can cause hearing loss is due to auditory neuropathy, as it is widely known that diabetes can results in peripheral neurothy. Whether the neuropathy is caused by microangiopathy or metabolic effect is not known.
Some researchers suggest that diabetes may affect both central and peripheral nervous system. Marullo found evidence of cochlear and retro cochlear pathology in 60 diabetic subjects [9]. A problem in all the electrophysiological studies is that none of them demonstrated significant hearing loss that correlates with the electrophysiological changes that were found. Therefore, the clinical significance of these changes must be in doubt. In addition, not all studies of brainstem-evoked responses have shown abnormalities in diabetic patients. Verma et al. showed no difference between the responses of 22 diabetic subjects and 14 healthy controls [10].
This study was aimed at determining the correlation between diabetes mellitus and hearing threshold and to compare objectively the hearing threshold of subjects with type 2 diabetes mellitus treated with oral hypoglycemic agents against subjects treated with intensive short-term insulin therapy.
Material and Methods
This single blind randomized controlled study was performed at the Department of Medicine and Department of Otorhinolaryngology, Center A between 1st May 2003 and 31st September 2004. The ethical committee approved this study with code number FF-137. Patients with type 2 diabetes mellitus who attended the outpatient clinic of endocrine unit during the study period. They have type 2 diabetes mellitus within 10 years of diagnosis with a glycated hemoglobin (Hb1Ac) in between 8 to 11%. Their age between 18 to 70 yrs old with body mass index less than 30. Those with known specific causes for sensorineural hearing loss (SNHL) such as post major trauma to the ear, history of ototoxicity, history of ear surgery, conductive hearing loss (CHL) such as otosclerosis will be excluded.
Subjects selected for this study were randomized into two groups. Group 1 were patients treated with conventional oral hypoglycemic agents. The patients in group 2 were treated with insulin injection. In this study, diabetes is diagnosed when fasting blood glucose level is more than 6.7 mmol/l. Patients in both groups were screened for factors that might have predetermined effect on auditory function. A complete history and physical examination that include otoscopic examination were carried out at the beginning of the study. Demographic data were then obtained from all patients. The subjects were seen every 4 weeks for 3 months. Audiometric test was performed in all subjects at each visit. Blood was taken for fasting blood glucose, Hb1Ac and fructosamine every visit as well to determine the glycaemic controls of the subject.
Audiometric Test
In all subjects, air and bone conduction thresholds were determined using GSI 21 Audiometer (Gaston-Stradler Inc) at the ORL Clinic. The pure tone audiograms were obtained in a soundproof cabin using Hughson Westlake ascending method. Sound stimulus was delivered through TDH49 headphones for air conduction and bone oscillator B-27 for bone conduction threshold measurements. All patients used a conventional patient response button. Tests frequencies included 0.25–8 kHz at octave interval. Hearing threshold was defined as the lowest intensity of a pure tone that was just audible to the subject. The threshold recorded for each frequency was the lowest decibel (dB) level at which 50% or more positive responses were obtained. Masking was applied to non-test ear when indicated.
The presence of air bone gap might indicate middle ear pathology and thus excluded from further analysis. In this study, we will refer the air conduction threshold determination as the pure tone-hearing threshold. Average thresholds were used for analysis of hearing levels. However, in order to obtain more detailed results, several averages of the test frequencies were obtained:
Average low frequency is an arithmetic average of thresholds of 0.25 and 0.5 kHz.
Average mid frequency is an arithmetic average of thresholds at 1 and 2 kHz.
Average high frequency is an arithmetic average of thresholds at 4 and 8 kHz.
The hearing threshold of both ears of each subjects were analyzed separately and therefore statistical analysis for each group were based on number of ears and not on number of subjects. The analysis was performed using statistical program from SPSS version 11.0.
Results
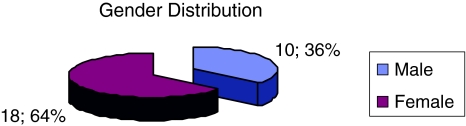
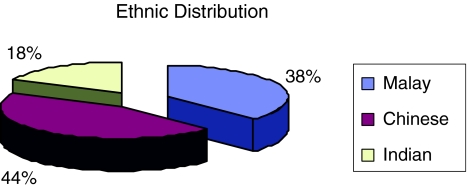
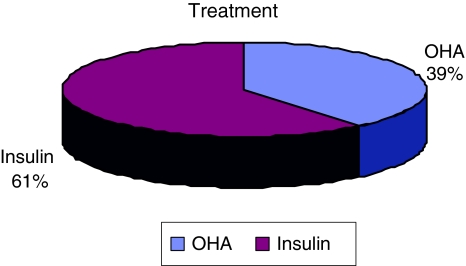
A total of 28 individuals were recruited for this study. There were 10 males (35.7%) and 18 females (64.3%) (Fig. 1). Among them 42.9% were Chinese, 39.3% were Malays and the rest were Indians (Fig. 2). The age group of the subject was between 37 years old to 70 years old, with mean age being 53.2 years. They were 11(22 ears) patients treated with oral hypoglycemic agents and 17(34 ears) patients treated with subcutaneous insulin (Fig. 3).
Fig. 1.
Gender distribution of the subjects
Fig. 2.
Ethnic distribution of the subjects
Fig. 3.
Treatment received by the subjects
Audiometric Findings
The mean average threshold at the start and after treatment for low frequencies (250 and 500 Hz), mid frequencies (1000 and 2000 Hz) and high frequencies for the study groups are tabulated in Tables 1, 2 and 3 respectively. There is no significant difference of mean hearing threshold between two groups except at low frequencies at the initial phase of the study. There is no significant difference between mean pure tone threshold before and after treatment in all frequencies in both groups.
Table 1.
Mean average pure tone hearing threshold in insulin and OHA groups before treatment
| Insulin | OHA | P value | |
|---|---|---|---|
| Low frequencies + SE (dB) | 19.6 + 1.2 | 15.5 + 1.3 | 0.04 |
| Mid frequencies + SE (dB) | 21.7 + 1.2 | 19.0 + 1.3 | 0.25 |
| High frequencies + SE (dB) | 36.4 + 3.7 | 29.1 + 3.2 | 0.33 |
SE = standard error
Table 2.
Mean average pure tone hearing threshold in insulin and OHA groups after treatment
| Insulin | OHA | P value | |
|---|---|---|---|
| Low frequencies + SE (dB) | 19.4 + 1.2 | 16.0 + 1.1 | 0.07 |
| Mid frequencies + SE (dB) | 21.0 + 1.4 | 17.0 + 1.6 | 0.08 |
| High frequencies + SE (dB | 32.6 + 3.2 | 28.9 + 3.2 | 0.5 |
Table 3.
Comparison of fasting blood glucose level between groups after treatment
| Group | N | Mean | Test | P |
|---|---|---|---|---|
| Insulin | 34 | 8.6647 | Mann–Whitney | P > 0.05 |
| OHA | 22 | 7.6745 |
Measures of Glycaemic Controls
An analysis of glycaemic control between two groups was carried out. There is no significant different in fasting glucose level and fructosamine (Tables 3 and 4). However, there is significant different in level of HbA1c between two groups after the treatment (P < 0.05) (Table 5).
Table 4.
Mean fructosamine level after treatment
| Group | N | Mean | Test | P |
|---|---|---|---|---|
| Insulin | 34 | 321.2353 | Mann–Whitney | P > 0.05 |
| OHA | 22 | 340.2727 |
Table 5.
Mean HbA1c level after the treatment
| Group | N | Mean | Test | P |
|---|---|---|---|---|
| Insulin | 34 | 7.9235 | Mann–Whitney | P < 0.05 |
| OHA | 22 | 7.1091 |
Correlation of pure tone threshold and diabetics control
Further analysis of pure tone threshold and diabetic control was carried out. Glycaemic control parameters in this study are HbA1c and fructosamine. Pearson correlation was used to determine the correlation between glycaemic control and pure tone threshold. There were no correlation noted regarding glycaemic control parameters and all frequencies of pure tone threshold (Table 6 and 7).
Table 6.
Correlation between pure tone threshold and HbA1c
| Variable | r | P |
|---|---|---|
| HbA1c | ||
| Low frequency threshold | 0.201 | 0.138 |
| Mid frequency threshold | 0.159 | 0.243 |
| High frequency threshold | 0.123 | 0.366 |
Table 7.
Correlation between pure tone threshold and fructosamine
| Variable | r | P |
|---|---|---|
| Fructosamine | ||
| Low frequency threshold | 0.113 | 0.407 |
| Mid frequency threshold | 0.232 | 0.086 |
| High frequency threshold | 0.170 | 0.209 |
Discussion
Our study has shown that glycaemic control does not have significant impact on hearing. This finding does not contradict or agree with previous studies. Previous studies have shown mixed result regarding association of diabetes mellitus and hearing impairment. Dalton et al. found there is weak association between NIDDM and hearing impairment after corrected for age and presbycusis in a population based longitudinal studies of aging in Beaver Dam, Wisconsin [11]. However, they did not find any association between duration of diabetes and glycaemic control. However, in Framingham heart study which examined a possible association between diabetes and hearing loss, found no association between hearing threshold and presence or absence of diabetes or impaired glucose tolerance [12]. This was a cross sectional study which examined hearing threshold in a number of population and used the worse hearing ear as indication of hearing loss.
However, Venkata et al. [13] found the prevalence of SNHL in diabetic is significantly raised (13.1% of 12,575 diabetics vs. 10.3% of 53,461 non diabetics). They also concluded that severity of hearing loss seemed to correlate with progression of the disease as reflected in serum creatinine. Raised serum creatinine was used as an indicator of microangiopathy in the renal vasculature. Thus, they concluded that hearing loss in these subjects might be due to microangiapathic changes in the inner ear.
Our study also does not show any association between hearing threshold and the type of treatment received (insulin or oral hypoglycemic agent). Ma et al. [14] studied hearing impairment and diabetes in 1740 Mexican–Americans to examine their relationship. They found that diabetics had a significantly higher mean threshold than non-diabetics only at 500 Hz. Their data also showed that diabetics who were not using insulin had significantly higher threshold at 2,000 and 4,000 Hz than those who were using insulin. They concluded that association between diabetes and decreased hearing acuity in higher frequencies only present in subject who do not use insulin. However, their study was cross-sectional study and the sequence of event cannot be known from the data. Thus, it cannot be taken to prove that insulin has a protective effect on hearing.
Several studies have investigated the relationship between insulin and hearing loss. Taylor and Irvin [15] found that dosage of insulin was not related to the degree of hearing loss. However Kurien et al. [16] showed that poorly controlled diabetics had worse threshold than those who were well controlled [16]. Ma et al. data suggested that insulin use might limit the progression of hearing impairment especially at higher frequencies [14]. Another unexpected finding in Ma’s study was that diabetics taking insulin had significantly lower threshold than non-diabetics. However, in Ma’s study they did not measure any parameters of glycaemic control such as fructosamine and HbA1C [14].
Our study shows that hearing threshold was neither affected by insulin treatment nor by the glycaemic control. In the studies that support the hypothesis that there is possible association between hearing loss and diabetes [1, 2, 5, 8, 15], the authors noted the association was predominantly present at higher frequencies. However, these study mention little about the controls and other important factors such as noise exposure. Chapman et al. studied the association of hearing loss and diabetic in rat model [17]. Their study included noise exposure control to non-insulin dependent diabetes mellitus rats in an attempt to extrapolate the same disease process in human. Cochlear histology was examined at the end of the study to show microangiopathy of the inner ear related to NIDDM. The authors found that NIDDM alone did not cause statistically significant basement membrane thickening; however, NIDDM in combination with noise exposure did show significant basement membrane thickening.
Nageris et al. attempted to correlate hearing loss and diabetes mellitus using rat model by examining cochlear histology [18]. The parameters that were used to measure cochlear damage were outer and inner hair cells and atrophy of stria vascularis. They did not find any statistical difference in the inner and outer hair cells or in the stria vascularis between genetically induced diabetic rats and control subjects. They concluded if there is any hearing loss in diabetes the pathogenesis does not involve damage to the hair cells or stria vascularis. However, extrapolation of findings in genetically induced diabetic rats into human scenario might prove irrelevant. However, Fowler and Jones argued that changes in cochlea may represent genetic predisposition to cochlear damage rather than the effect of diabetes per se [19].
Limitation
The complexity of hearing mechanism and various confounding factors make it difficult to design a single study to examine the association of hearing impairment and diabetes. A long-term longitudinal study in diabetes mellitus patients with meticulous records of other possible factors that cause hearing impairment (e.g. noise exposure, ototoxic drugs) is needed. A larger histological study of temporal bone in correlation with documented hearing threshold may shed a light on how true is this microangiopathic hypothesis causing hearing impairment.
Conclusion
This study has shown that glycaemic control does not have significant impact on hearing.
The hearing threshold neither affected by insulin treatment nor by the glycaemic control.
There was significant correlation between age and pure tone threshold in all frequencies (P < 0.01).
Acknowledgments
We are grateful to the all our staff at ENT and Endocrine Clinic especially to Associate Professor Norlinah, Mr Borhan, Miss Ann, Puan Jamilah, Puan Roslin, Mr Almyzan and Miss Wan for their assistance.
References
- 1.Ishii EK, Talbott E, Findlay RC, D’Antonio JA, Kuller LH. Is NIDDM a risk factor for noise-induced hearing loss in occupationally noise exposed Cohort. Sci Total Environ. 1992;127:155–165. doi: 10.1016/0048-9697(92)90474-7. [DOI] [PubMed] [Google Scholar]
- 2.Cullen JR, Cinnamond MJ. Hearing loss in diabetics. J Laryngol Otol. 1993;107:179–182. doi: 10.1017/s0022215100122571. [DOI] [PubMed] [Google Scholar]
- 3.Harner SG. Hearing in adult onset diabetes mellitus. Otolaryngol Head Neck Surg. 1991;89:322–327. doi: 10.1177/019459988108900235. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson MJ, Talbott E, Helmkamp JC, Kuller LH. Diabetes, noise exposure and hearing loss. J Occup Med. 1987;29:576–579. [PubMed] [Google Scholar]
- 5.Friedman SA, Schulman RH, Weiss S. Hearing and diabetic neuropathy. Arch Intern Med. 1975;135:573–576. doi: 10.1001/archinte.135.4.573. [DOI] [PubMed] [Google Scholar]
- 6.Miller JJ, Beck L, Davis A, Jones DE, Thomas AB. Hearing loss in patient with diabetic neuropathy. Am J Otolaryngol. 1983;4:342–346. doi: 10.1016/S0196-0709(83)80021-0. [DOI] [PubMed] [Google Scholar]
- 7.Wackym PA, Lintchicum FH. Diabetes mellitus and hearing loss: clinical and histopathological relationships. Am J Otol. 1986;7(3):176–182. [PubMed] [Google Scholar]
- 8.Jorgensen MB, Buch NH. Studies on inner ear functions and cranial nerves in diabetics. Acta Otolaryngol. 1961;53:350–364. doi: 10.3109/00016486109126500. [DOI] [PubMed] [Google Scholar]
- 9.Marullo T. Contribution a letude des hypoacouses perceptives du diabete sucre. Rev Laryngol Otol Rhinol. 1975;95:253–271. [PubMed] [Google Scholar]
- 10.Verma A, Bisht M, Ahuja CK. Involvement of central nervous system in diabetes mellitus. J Neurol Neurosurg Psychiatr. 1984;47:414–416. doi: 10.1136/jnnp.47.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton DS, Cruickshanks KJ, Klein R, Klein BEK, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care. 1998;21:1540–1544. doi: 10.2337/diacare.21.9.1540. [DOI] [PubMed] [Google Scholar]
- 12.Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- 13.Venkata K, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol. 2003;24:382–386. doi: 10.1097/00129492-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Ma F, Gomez-Marin O, Lee DJ, Balkany T. Diabetes and hearing impairment in Mexican American adults: a population based study. J Laryngol Otol. 1998;112:835–839. doi: 10.1017/S0022215100141842. [DOI] [PubMed] [Google Scholar]
- 15.Taylor IG, Irwin J. Some audiological aspect of diabetes mellitus. J Laryngol Otol. 1978;92:99–113. doi: 10.1017/S0022215100085108. [DOI] [PubMed] [Google Scholar]
- 16.Kurien M, Thomas K, Bhanu TS. Hearing threshold in patient with diabetes mellitus. J Laryngol Otol. 1989;103:164–168. doi: 10.1017/s0022215100108345. [DOI] [PubMed] [Google Scholar]
- 17.Chapman TM, Baxter A, Timothy LS, Raynor E. Non-insulin-dependent diabetic microangiopathy in the inner ear. J Laryngol Otol. 1999;113:13–18. doi: 10.1017/s0022215100143051. [DOI] [PubMed] [Google Scholar]
- 18.Nageris B, Hadar T, Feinmesser M, Elidan J. Cochlear histopathologic analysis in diabetic rats. Am J Otol. 1998;19(1):63–65. [PubMed] [Google Scholar]
- 19.Fowler PD, Jones NS. Diabetes and hearing loss. Clin Otolaryngol. 1998;24:3–8. doi: 10.1046/j.1365-2273.1999.00212.x. [DOI] [PubMed] [Google Scholar]