Abstract
SpoIIE is a bifunctional protein with two critical roles in the establishment of cell fate in Bacillus subtilis. First, SpoIIE is needed for the normal formation of the asymmetrically positioned septum that forms early in sporulation and separates the mother cell from the prespore compartment. Secondly, SpoIIE is essential for the activation of the first compartment-specific transcription factor σF in the prespore. After initiation of sporulation, SpoIIE localizes to the potential asymmetric cell division sites near one or both cell poles. Localization of SpoIIE was shown to be dependent on the essential cell division protein FtsZ. To understand how SpoIIE is targeted to the asymmetric septum we have now analysed its interaction with FtsZ in vitro. Using the yeast two-hybrid system and purified FtsZ, and full-length and truncated SpoIIE proteins, we demonstrate that the two proteins interact directly and that domain II and possibly domain I of SpoIIE are required for the interaction. Moreover, we show that SpoIIE interacts with itself and suggest that this self-interaction plays a role in assembly of SpoIIE into the division machinery.
Keywords: Bacillus subtilis/cell division/oligomerization/protein–protein interaction/sporulation
Introduction
A fundamental question in developmental biology is how cell division and cell fate are linked together. In organisms like the fly Drosophila melanogaster, the nematode Caenorhabditis elegans, the green alga Volvox carteri and the budding yeast Saccharomyces cerevisiae, the formation of an asymmetrically positioned division septum gives rise to two unequally sized progeny with different developmental potentials (reviewed by Horvitz and Herskowitz, 1992; Jan and Jan, 1998). In the Gram-positive bacterium Bacillus subtilis, asymmetric division is a key feature of spore formation. Early in sporulation, the developing cell divides asymmetrically into a smaller compartment, the prespore, which becomes the spore, and a larger compartment, the mother cell, which participates in the maturation of the spore and finally lyses to release it. The developmental fates of the two cells are dictated by the localized activation of sporulation-specific transcription factors. The first transcription factor, σF, which only becomes active in the prespore, governs the activation and expression of the remaining cell-specific transcription factors (as reviewed by Errington, 1996; Losick and Dworkin, 1999).
σF is made before asymmetric septation, together with two coordinately expressed regulatory proteins, SpoIIAA and SpoIIAB (Gholamhoseinian and Piggot, 1989). At first, σF is held in an inactive complex by the anti-σ factor SpoIIAB (Duncan and Losick, 1993; Min et al., 1993; Alper et al., 1994). SpoIIAB is also a protein kinase, which phosphorylates the anti-anti-σ factor SpoIIAA on a specific serine residue, thereby maintaining it in an inactive state (Min et al., 1993; Diederich et al., 1994; Najafi et al., 1995; Duncan et al., 1996; Magnin et al., 1996). After asymmetric septation, σF remains inactive in the mother cell, but in the prespore it is released from SpoIIAB (Margolis et al., 1991). This release is triggered by SpoIIE, a membrane-bound phosphatase, which dephosphorylates SpoIIAA-P and allows it to bind SpoIIAB, thereby releasing σF (Duncan et al., 1995; Arigoni et al., 1996; Feucht et al., 1996). σF is then free to associate with core RNA polymerase and direct transcription. Thus, SpoIIE plays a key role in σF activation by regulating the phosphorylation state of SpoIIAA.
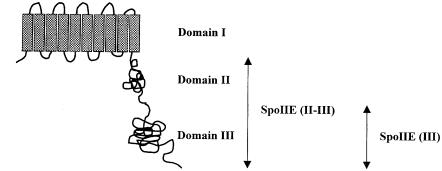
SpoIIE is an integral membrane protein and has a multi-domain structure (Figure 1) (Barak et al., 1996; Arigoni et al., 1999). It contains 10 membrane-spanning segments in its N–terminal domain (domain I), which targets the protein to the membrane. The large central domain (domain II) is poorly conserved and its function is unknown. The C–terminal domain (domain III) contains serine phosphatase activity and is structurally related to eukaryotic PP2C protein phosphatases (Adler et al., 1997). PP2C phosphatases are involved in regulating stress response pathways in both prokaryotes and eukaryotes (Das et al., 1996). SpoIIE is synthesized before asymmetric division and is localized initially in ring-like structures at one or both potential sites of asymmetric division near the cell poles (Arigoni et al., 1995). During asymmetric septation, most of the SpoIIE from the ring at the prespore is sequestered to the prespore side of the asymmetric septum, which could favour the activation of σF in the prespore (Wu et al., 1998). The prespore-distal SpoIIE ring (in the mother cell) then disappears (Arigoni et al., 1995; Pogliano et al., 1997).
Fig. 1. Topological model for the multi-domain structure of SpoIIE protein. The membrane-spanning segments are shown as grey rectangles and the soluble part of the protein is shown as two adjacent globules. The cytoplasmic domain of SpoIIE consists of domains II and III (residues 326–827) and is referred to as SpoIIE (II–III) in the text. Domain III (residues 568–827) contains the PP2C phosphatase and is referred to as SpoIIE (III).
In nearly all bacteria, cytokinesis begins with the polymerization of the essential GTPase FtsZ into a ring structure at the nascent division site (reviewed by Bramhill, 1997; Lutkenhaus and Addinall, 1997; Rothfield and Justice, 1997). The FtsZ ring provides a cytoskeletal scaffold that recruits other division proteins and directs the ingrowth of the septum (Bi and Lutkenhaus, 1991; Addinall and Lutkenhaus, 1996a,b; Addinall et al., 1996, 1997; Ma et al., 1996; Hale and de Boer, 1997; Wang et al., 1998; Weiss et al., 1997; Yu et al., 1998). The crystal structure of FtsZ closely resembles that of eukaryotic α- and β–tubulin (Löwe and Amos, 1998; Nogales et al., 1998). Like tubulin, FtsZ undergoes GTP/GDP-dependent polymerization, forming protofilaments, sheets and minirings in vitro (Bramhill and Thompson, 1994; Mukherjee and Lutkenhaus, 1994, 1998; Erickson et al., 1996; Yu and Margolin, 1997). In B.subtilis, FtsZ localization shifts from a medial to a bipolar pattern after the onset of sporulation, forming a ring near each pole (Levin and Losick, 1996). Immunofluorescence microscopy has shown that localization of SpoIIE also requires FtsZ and is independent of other known division proteins like FtsA, FtsL, DivIC and DivIB (Levin et al., 1997; Feucht et al., 1999). Recently, it has been suggested that the phosphatase activity of SpoIIE is partially dependent on the FtsZ-dependent assembly of SpoIIE (King et al., 1999). In turn, in a spoIIE deletion mutant, polar FtsZ ring formation is impaired, suggesting that SpoIIE contributes to the switch from medial to polar septation (Khvorova et al., 1998). Additionally, it has been shown that SpoIIE plays a role in septal morphogenesis (Piggot, 1973; Illing and Errington, 1991). The precise role of SpoIIE in septation is still not clear, but the finding that the protein has two distinct and independent functions provides a fascinating example of co-ordination between morphogenesis and transcriptional regulation (Barak and Youngman, 1996; Feucht et al., 1996).
In this study, we have examined how SpoIIE is targeted to the asymmetric septum by analysing its interaction with FtsZ. Using purified FtsZ and full-length and truncated SpoIIE proteins, we demonstrate that the two proteins interact directly and that domain II and possibly domain I of SpoIIE are required for the interaction. Moreover, our data suggest that SpoIIE self-interaction plays an important role in the assembly of SpoIIE into the division machinery.
Results
Purified SpoIIE interacts with SpoIIE and FtsZ from a B.subtilis cell extract
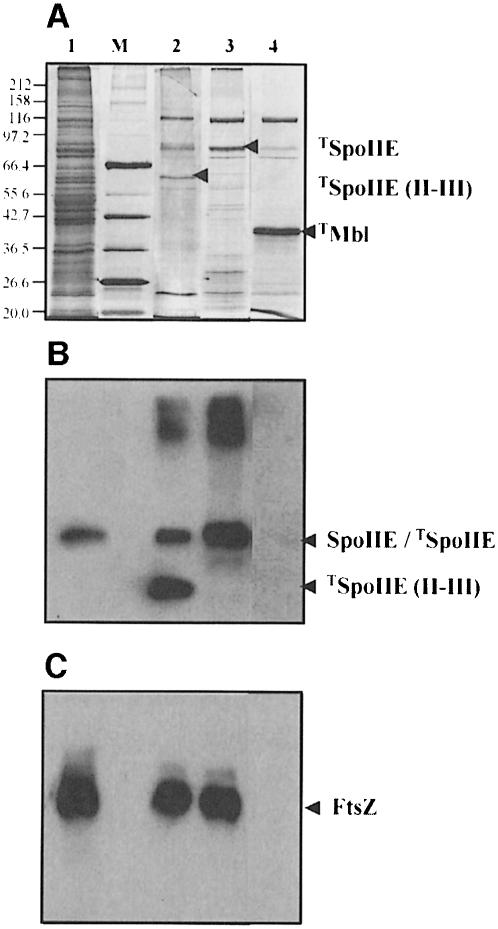
To find interacting partners of SpoIIE that might be involved in regulation or targeting, we purified full-length SpoIIE and its cytoplasmic domain, SpoIIE (II–III) (see Figure 1), both fused to a strep-tag, by using streptavidin-coated magnetic beads. The beads carrying either full-length SpoIIE or SpoIIE (II–III) were incubated with cell extract taken 1.5 h after the initiation of sporulation. As a negative control we used beads coated with an unrelated strep-tagged protein Mbl (Abhayawardhane and Stewart, 1995). After extensive washing, bound proteins were separated by SDS–PAGE and visualized by silver staining (Figure 2A). Several bands were retained specifically by the SpoIIE proteins, but not by Mbl (Figure 2A, lanes 2–4). Based on their Mr, it was possible that two of the proteins retained by strep-tagged SpoIIE (II–III) might be SpoIIE (91 kDa) and FtsZ (40 kDa). To test this possibility we used Western blotting analysis with anti-SpoIIE antibodies (Figure 2B) and anti-FtsZ antibodies (Figure 2C). Neither antibody reacted with the proteins retained by strep-tagged Mbl (Figure 2B and C, lane 4). In contrast, in the sample containing the purified strep-tagged SpoIIE (II–III) strong bands of both full-length SpoIIE and FtsZ were detected (Figure 2B and C, lane 2). FtsZ was also bound to the purified strep-tagged full-length SpoIIE (Figure 2C, lane 3). It was not possible to determine whether SpoIIE from the sporulating cell extract was also retained because the two proteins ran at the same position.
Fig. 2. Interaction of purified full-length SpoIIE or purified SpoIIE (II–III) with SpoIIE and FtsZ from sporulating cell extracts. Cell extract was incubated with strep-tagged protein on magnetic beads and washed. Proteins from the beads were separated by SDS–PAGE and (A) silver stained or blotted (B) with anti-SpoIIE antibodies and (C) with anti-FtsZ antibodies. Lane 1, total cell extract; lane 2, cell extract incubated with purified strep-tagged SpoIIE (II–III); lane 3, cell extract incubated with purified strep-tagged full-length SpoIIE; lane 4, cell extract incubated with purified strep-tagged Mbl. In (A), the arrowhead for each lane points to the protein that is bound to the magnetic beads. Strep-tagged proteins are referred as to TSpoIIE, TSpoIIE (II–III) and TMbl. In (B) and (C), the positions of full-length SpoIIE, SpoIIE (II–III) and FtsZ are indicated by arrows. M, Biolabs broad range marker. The sizes of the proteins (in kilodaltons) are indicated on the left side of the figure.
Interaction of SpoIIE with itself as detected by the yeast two-hybrid system
To confirm that SpoIIE interacts with itself, we exploited the yeast two-hybrid system. Residues 326–827 (domains II and III) were fused both to the activation and to the binding domain of the GAL4 transcription factor. If the fusion proteins interacted, expression of a lacZ reporter gene in the yeast host strain would be induced. As shown in Table IA, when only one of the fusion proteins was produced in the yeast, no β–galactosidase activity was observed. By contrast, a positive interaction was detected when both fusion proteins were produced, suggesting that the soluble SpoIIE (II–III) interacts with itself. No interaction with SpoIIE (II–III) was detected when the full-length SpoIIE was fused to the binding domain of GAL4 (results not shown); we attribute this result to problems caused by the expression of the membrane domain (domain I) of SpoIIE in yeast.
Table I. Interactions detected by the yeast two-hybrid system.
| Binding domain fusiona | Activation domain fusion | β-Galactosidase activityb |
|---|---|---|
| (A) | ||
| SpoIIE (II–III) | None | – |
| None | SpoIIE (II–III) | – |
| SpoIIE (II) | None | – |
| None | SpoIIE (II) | – |
| SpoIIE (III) | None | – |
| None | SpoIIE (III) | – |
| SpoIIE (II–III) | SpoIIE (II–III) | ++ |
| SpoIIE (II) | SpoIIE (II) | – |
| SpoIIE (III) | SpoIIE (III) | – |
| SpoIIE (II–III) | SpoIIE (III) | – |
| SpoIIE (III) | SpoIIE (II–III) | – |
| SpoIIE (II) | SpoIIE (II–III) | – |
| SpoIIE (II–III) | SpoIIE (II) | – |
| (B) | ||
| None | FtsZ | – |
| SpoIIE (II–III) | FtsZ | + |
| SpoIIE (II) | FtsZ | – |
| SpoIIE (III) | FtsZ | – |
aSpoIIE (II–III), SpoIIE (II) and SpoIIE (III) contain residues 326–827, 326–567 and 568–827, respectively.
bDetected by colour development using X-gal. ++, detectable colour development within 2 h; +, detectable colour development within 3 h; –, no significant colour development after 8 h.
The cytoplasmic domain of SpoIIE is composed of a large central domain (domain II) and the C–terminal phosphatase domain (domain III). To determine whether either of these domains alone was capable of supporting a protein–protein interaction, we fused residues 568–827 (domain III) or residues 326–567 (domain II) to the activation and binding domain of the GAL4 transcription factor. As shown in Table IA, none of the pairwise tests involving these fusion proteins gave a positive result.
These results demonstrate that the cytoplasmic part of SpoIIE interacts with itself, and this interaction may require both domain II and domain III.
Domain II of SpoIIE is involved in oligomerization in vitro
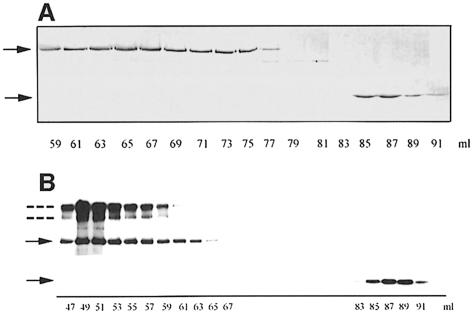
To characterize further the interaction of SpoIIE with itself, we investigated full-length and truncated SpoIIE proteins. Purification of the full-length SpoIIE was performed as described by Lucet et al. (1999). To obtain SpoIIE (II–III), we used a fusion to an Intein-chitin binding domain and purified the overproduced protein precursor on a chitin column. Self-cleavage of Intein with dithiothreitol (DTT) unexpectedly yielded two major products with an apparent Mr of 55 and 28 kDa by SDS–PAGE; the Intein-chitin binding domain fusion remained bound to the column. These two products were further purified by gel filtration on Superdex 200 and their N–terminal sequences were determined. The 55 kDa fragment corresponded to SpoIIE (II–III) and eluted with an apparent Mr from 50 to 300 kDa (Figure 3A), indicating the presence of multimers. The 28 kDa fragment corresponded to SpoIIE (III) and eluted as a monomer with an apparent Mr of 28 kDa (Figure 3A). The N–terminal sequence analysis of this 28 kDa fragment revealed methionine instead of valine as the first amino acid (position 568), suggesting that this polypeptide is generated by de novo initiation and is not a degradation product. These data confirmed that the phosphatase domain is a monomer and suggested that SpoIIE (II–III) can oligomerize, but SpoIIE (III) cannot.
Fig. 3. Interaction of SpoIIE, SpoIIE (II–III) and SpoIIE (III). (A) SDS–PAGE of SpoIIE (II–III) and SpoIIE (III) after separation on a Superdex 200 gel filtration column and staining with Coomassie Blue. SpoIIE (II–III) was eluted between 59 and 77 ml, corresponding to an apparent Mr between 300 and 50 kDa. SpoIIE (III) was eluted between 84 and 90 ml, corresponding to an apparent Mr of ∼28 kDa. The upper and lower arrows indicate the position of SpoIIE (II–III) and SpoIIE (III), respectively. (B) Purified full-length SpoIIE (3.2 μM) and SpoIIE (III) (3 μM) were mixed and applied to a Superdex 200 gel filtration column equilibrated with buffer C. Elution was performed in the same buffer and fractions of 1 ml were collected and monitored by Western blotting with anti-SpoIIE antibodies. Full-length SpoIIE was eluted between 46 and 66 ml, corresponding to an apparent Mr between 700 and 170 kDa. SpoIIE (III) was eluted between 84 and 91 ml, corresponding to an apparent Mr of ∼28 kDa. The positions of full-length SpoIIE and SpoIIE (III) are indicated by solid arrows. The additional immunoreactive bands (indicated by dotted lines) correspond to dimeric and higher molecular mass (possibly tetrameric) forms of SpoIIE.
To confirm that no interaction occurs between domain II of one SpoIIE molecule and domain III of another (see Table IA), we incubated the full-length SpoIIE with SpoIIE (III) and applied the mixture to a gel filtration column. Each fraction was monitored by Western blotting using anti-SpoIIE antibodies. As shown in Figure 3B, the two proteins eluted separately. In addition, we observed that purified full-length SpoIIE eluted from Superdex 200 with an apparent Mr of 170–700 kDa, suggesting the presence of molecular species higher than a dimer in solutions with a high concentration of SpoIIE (Lucet et al., 1999). Some of the dimeric and higher molecular mass forms of SpoIIE are found to be SDS resistant and are easily detected on the Western blot, as has been found for other protein complexes (Patricelli et al., 1998). These results confirm that SpoIIE interacts with itself and suggest that oligomerization is one function of the previously uncharacterized domain II.
Domains I and II do not affect the phosphatase activity of SpoIIE
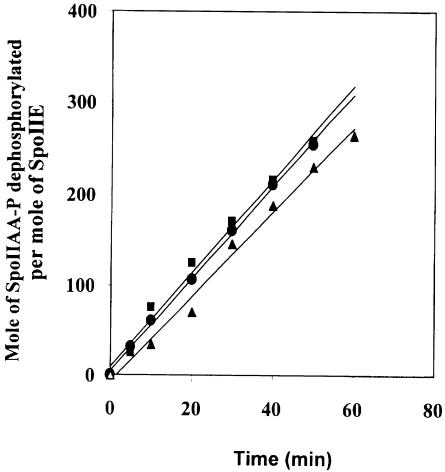
To analyse whether oligomerization of SpoIIE affects its phosphatase activity, we determined the rate of dephosphorylation of SpoIIAA-P by full-length SpoIIE, SpoIIE (II–III) and SpoIIE (III). As shown in Figure 4, no significant difference between the phosphatase activities was detected. The turnover number (moles of SpoIIAA-P dephosphorylated per mole of SpoIIE) was 7.6 × 10–2 s–1 for full-length SpoIIE, 8.6 × 10–2 s–1 for SpoIIE (II–III) and 8.5 × 10–2 s–1 for SpoIIE (III) at 30°C within the concentration range used (25–180 nM). These results suggest that in vitro the membrane-associated N–terminal domain and the central domain of SpoIIE do not regulate the enzymic activity of the phosphatase domain.
Fig. 4. Time course of dephosphorylation of SpoIIAA-P by full-length SpoIIE (▴), SpoIIE (II–III) (•) and SpoIIE (III) (▪). The assays were as described in Materials and methods.
SpoIIE and FtsZ form a stable complex
Immunofluorescence microscopy has shown that localization of SpoIIE to the asymmetric division septum requires the FtsZ division protein but not the later assembling division proteins FtsA, DivIC, FtsL or DivIB (Levin et al., 1997; Feucht et al., 1999). Our data suggested that there is a direct interaction between FtsZ and SpoIIE (see Figure 2). To confirm such an interaction, we again employed the yeast two-hybrid system and fused FtsZ to the activation domain of GAL4 (FtsZ fused to the binding domain of GAL4 expressed the lacZ reporter gene and therefore could not be used in this test). The GAL4 fusion containing SpoIIE (II–III) interacted with FtsZ, but again no interaction was detected with the fusions containing isolated domains II or III (Table IB).
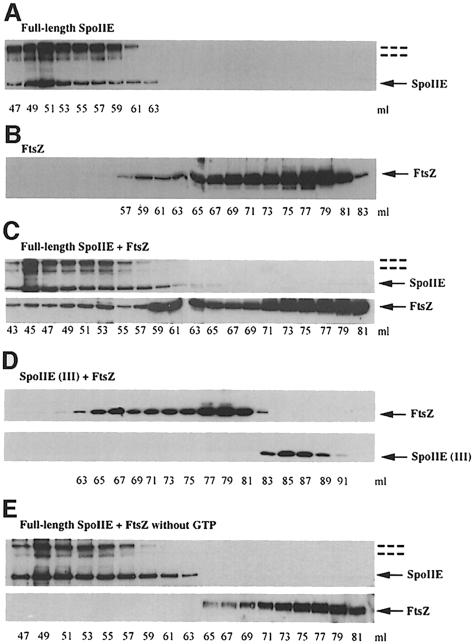
To investigate the interaction between SpoIIE and FtsZ in more detail, purified full-length SpoIIE and purified FtsZ were applied to a gel filtration column separately or after mixing in the presence of GTP. The elution patterns for SpoIIE, FtsZ and the SpoIIE–FtsZ mixture were monitored by Western blotting each fraction with anti-SpoIIE or anti-FtsZ antibodies. As shown in Figure 5A, purified SpoIIE again eluted with an apparent Mr of 170–700 kDa. When purified FtsZ was filtered alone, the majority of the protein eluted with an apparent Mr of 45 kDa, with a smaller fraction, presumably corresponding to multimers of FtsZ, eluting with a higher apparent Mr (Figure 5B). In the presence of full-length SpoIIE, however, ∼30% of the FtsZ was displaced to a high molecular weight and co-eluted with SpoIIE (Figure 5C). By contrast, when FtsZ was mixed with purified SpoIIE (III), no change in the elution pattern of FtsZ was found, and the two proteins eluted separately (Figure 5D). To check whether GTP (i.e. polymerization of FtsZ) is required for SpoIIE–FtsZ interaction, the experiment with full-length SpoIIE and FtsZ was repeated without GTP. As shown in Figure 5E, no significant change in the elution pattern of FtsZ was found, suggesting that SpoIIE interacts only with FtsZ in a polymeric or GTP-bound form.
Fig. 5. Interaction between SpoIIE and FtsZ as detected by gel filtration chromatography. Full-length SpoIIE, SpoIIE (III) and FtsZ were applied to a Superdex 200 gel filtration column equilibrated with buffer D. Elution was performed in the same buffer, and fractions of 1 ml were collected and monitored by Western blotting with anti-SpoIIE or anti-FtsZ antibodies. (A) Purified full-length SpoIIE (3.2 μM) was applied to the column. SpoIIE was eluted between 47 and 64 ml. (B) Purified FtsZ (7 μM) was applied to the column. FtsZ was eluted between 59 and 84 ml. (C) Full-length SpoIIE (3.2 μM) pre-mixed with FtsZ (7 μM) was applied to the column. Note the shift of the FtsZ peak. (D) SpoIIE (III) (3.2 μM) pre-mixed with FtsZ (7 μM) was applied to the column. The elution patterns of SpoIIE (III) and FtsZ were unchanged. (E) Full-length SpoIIE (3.2 μM) pre-mixed with FtsZ (7 μM) without GTP was applied to the column. The elution patterns of full-length SpoIIE and FtsZ were unchanged. SpoIIE proteins and FtsZ are indicated by solid arrows. Dashed lines indicate dimeric and higher molecular mass (possibly tetrameric) forms of SpoIIE.
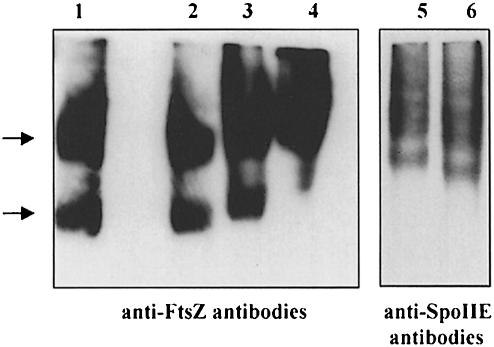
We could not analyse SpoIIE (II–III) by gel filtration because of its tendency to interact tightly with the column material at low ionic strength. Higher ionic strength could not be employed because of its interference with the formation of SpoIIE–FtsZ complexes. We therefore incubated full-length SpoIIE, SpoIIE (II–III) or SpoIIE (III) with purified FtsZ, and subjected the mixtures to non-denaturing PAGE. The gels were analysed by Western blotting with anti-FtsZ antibodies. As seen in Figure 6, when FtsZ was incubated with SpoIIE (III), no change in the FtsZ pattern was observed (lane 2). However, after incubation with SpoIIE (II–III), a small but reproducible shift occurred in the position of FtsZ (lane 3). This shift became more striking when FtsZ was incubated with full-length SpoIIE (lane 4). To confirm the formation of complexes between SpoIIE (II–III) and FtsZ, we repeated the experiment and blotted the non-denaturing PAGE with anti-SpoIIE antibodies. SpoIIE (II–III) alone ran in a ladder-like pattern, suggesting the presence of a series of discrete oligomeric forms (lane 6). In the presence of FtsZ, these bands were shifted to a higher position (lane 5). Thus, our data demonstrate that SpoIIE and FtsZ form a stable complex and that domain II and possibly domain I of SpoIIE are involved in the interaction with FtsZ.
Fig. 6. Interaction of SpoIIE proteins and FtsZ as detected by non-denaturing PAGE. FtsZ (7 μM) (lane 1) was incubated with SpoIIE (III) (3.5 μM; lane 2), SpoIIE (II–III) (3.5 μM; lane 3) or full-length SpoIIE (3.5 μM; lane 4) and the mixture was subjected to 8% non-denaturing PAGE. The proteins were transferred to a nitrocellulose membrane and probed with anti-FtsZ antibodies. SpoIIE (II–III) alone (lane 6) or incubated with FtsZ (lane 5) was analysed as described above and the nitrocellulose membrane was probed with anti-SpoIIE antibodies. Arrows on the left side of the figure indicate two different stages of polymerization of FtsZ.
Discussion
The results of Levin et al. (1997) showed that depletion of the key division protein FtsZ resulted in delocalization of SpoIIE, which suggested that FtsZ is required to target SpoIIE to the potential division sites. SpoIIE is also recruited to FtsZ rings in cells lacking functional FtsA, FtsL, DivIC and DivIB, suggesting that SpoIIE is probably attracted to the division sites at an early stage in their development (Levin et al., 1997; Feucht et al., 1999). How then does FtsZ recruit SpoIIE to potential division sites? In this study, we have provided biochemical and genetic evidence that SpoIIE interacts directly with FtsZ. Using gel filtration chromatography and non-denaturing PAGE we showed that purified FtsZ was displaced by incubation with purified full-length SpoIIE or SpoIIE (II–III) to a higher apparent molecular weight (Figures 5 and 6). Interestingly, SpoIIE binds only to FtsZ in the GTP-bound, presumably polymeric form (Figure 5E). In Escherichia coli, FtsZ is capable of interacting directly with several other division proteins, including FtsA, SulA and ZipA (Addinall et al., 1996; Huang et al., 1996; Hale and de Boer, 1997). In B.subtilis, only FtsA and now SpoIIE have been shown to interact directly with FtsZ. Wang et al. (1997) reported that FtsA interacts with the C–terminal domain of FtsZ, and it will therefore be useful to investigate whether SpoIIE binds to the same domain.
The interaction between SpoIIE and FtsZ requires domain II of SpoIIE. This was shown by two independent methods: the yeast two-hybrid system and gel filtration/non-denaturing PAGE. In the yeast two-hybrid system, FtsZ interacted with SpoIIE (II–III), but not with SpoIIE (II) or SpoIIE (III) (Table I). Moreover, full-length SpoIIE (Figures 5 and 6) and SpoIIE (II–III) (Figure 6), but not SpoIIE (III) (Figures 5 and 6), shifted FtsZ to a higher apparent molecular weight. These results show that at least part of domain II is required for interaction with FtsZ (and for oligomerization; see below). At the moment, we favour the notion that this domain of SpoIIE is responsible for the protein–protein interactions and that domain III, which functions independently as a phosphatase, is required indirectly, e.g. to stabilize domain II or control its folding.
It is also possible that domain I contributes to the interaction with FtsZ in vivo. Insertion of SpoIIE into the membrane might impose a topology that favours interaction with FtsZ as it assembles at the membrane. King et al. (1999) reported that replacement of domain I of SpoIIE with the first two membrane-spanning domains of E.coli MalF protein localizes the fusion protein to the cytoplasmic membrane. It may be that the presence of the membrane-spanning segments of MalF overrides localization signals in domain II of SpoIIE, or else that domain I of SpoIIE also plays a role in the proper localization of SpoIIE. The latter suggestion is supported by the finding that a SpoIIE mutant protein with a deletion of all membrane segments was dispersed throughout the cytoplasm (Arigoni et al., 1999). Possibly the SpoIIE membrane anchor helps to bring SpoIIE to where FtsZ ring formation is taking place.
Until now not much was known about the function of the poorly conserved domain II (Arigoni et al., 1999). The present study reveals that it is involved not only in binding FtsZ, but also in self-oligomerization. The yeast two-hybrid system showed that SpoIIE (II–III) interacts with itself. Moreover, purified full-length SpoIIE and SpoIIE (II–III) behaved like large oligomeric species on gel filtration chromatography, whereas SpoIIE (III) behaved as a monomer. Preliminary sedimentation equilibrium analysis of purified SpoIIE (II–III) suggests that the sample has, like full-length SpoIIE, a heterogeneous distribution, from monomeric species to tetramer (I.Lucet and M.D.Yudkin, unpublished results).
In contrast to FtsZ polymerization, which is regulated by GTP hydrolysis (Mukherjee and Lutkenhaus, 1998), oligomerization of SpoIIE seems to depend only on the concentration of the protein. However, more studies will be needed to distinguish between self-association and non-specific aggregation. Preliminary results from sedimentation equilibrium analysis indicate that detergent–protein complexes of full-length SpoIIE protein exist as monomeric, dimeric and higher molecular weight species (up to tetramers) depending on the concentration of the protein (I.Lucet and M.D.Yudkin, unpublished results).
SpoIIE protein has two distinct and independent functions: (i) a morphogenic activity needed for the formation of the asymmetric septum; and (ii) a phosphatase activity needed for release of the critical first cell-specific sigma factor σF in the prespore. It seems that domain I and domain II are involved in the first function by sequestering the protein to the membrane and targeting it to FtsZ rings. The finding of a direct interaction with FtsZ provides new insight into the function of SpoIIE in asymmetric septum formation. One reasonable hypothesis is that interaction of the membrane-bound SpoIIE protein with FtsZ facilitates recruitment of FtsZ to the membrane. This recruitment, together with the oligomerization of SpoIIE, could strongly promote formation of the FtsZ ring. In fact, Khvorova et al. (1998) have shown that in a spoIIE deletion strain, FtsZ ring formation is delayed and the proportion of bacteria with FtsZ rings is substantially reduced. Moreover, Wu et al. (1998) have reported that SpoIIE tends to localize sequentially to the potential division sites, and that the prespore septum forms at the pole where SpoIIE appears first.
The second function of SpoIIE seems to depend only on domain III. There was no significant difference between the phosphatase activities of full-length SpoIIE, SpoIIE (II–III) and SpoIIE (III) (Figure 4), showing that at least in vitro domains I and II do not regulate the phosphatase activity of SpoIIE. This result might also imply that targeting of SpoIIE to the membrane, or oligomerization, is required only for its role in septum formation, and not for its activity in regulating the release of σF in the prespore. On the other hand, it has been shown that expression of a SpoIIE mutant protein without membrane-spanning segments leads to a severe reduction in sporulation frequency (Arigoni et al., 1999), demonstrating the importance of co-ordination of the two functions.
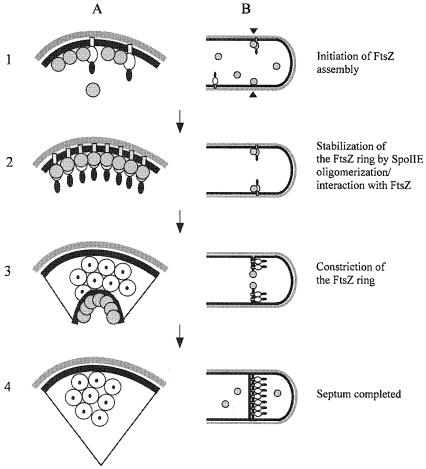
Our data are summarized in the schematic model for SpoIIE–FtsZ interaction shown in Figure 7. FtsZ localizes to potential division sites and recruits SpoIIE by interaction with domain II and possibly also domain I. The local concentration of SpoIIE is thereby increased, which leads to the oligomerization of SpoIIE. In turn, SpoIIE provides a membrane anchor for the FtsZ polymer. When the septum has formed, most of the SpoIIE is sequestered into the membrane on the prespore side by an unknown mechanism. SpoIIE remains localized in the asymmetric septum, whereas FtsZ depolymerizes and becomes dispersed throughout the cytoplasm, suggesting either that SpoIIE remains associated with the septum because of its membrane domain and its oligomerization state or that it has an affinity for some feature of the septum other than FtsZ. This leaves us with the interesting questions how is SpoIIE sequestered onto the prespore face of the septum as it forms, and is FtsZ part of the mechanism for this sequestration?
Fig. 7. Schematic model of the interactions between FtsZ and SpoIIE. The outline of a pre-septational cell soon after initiation of sporulation is shown in cross-section (A) and longitudinal section (B). (1) FtsZ (grey circles) starts to assemble at a potential site for asymmetric division (black arrowheads). SpoIIE (domain I, open rectangle; domain II, open oval; domain III, black oval) begins to be made and is recruited to this site by direct interaction with FtsZ. (2) FtsZ ring formation is stabilized by its interaction with SpoIIE. (3) The FtsZ ring constricts at the leading edge of the developing septum and SpoIIE is released into the septum. (4) When septum formation is completed, FtsZ depolymerizes, whereas SpoIIE stays behind in the septum. Previous work has suggested that the SpoIIE protein from the ring is retained mainly on the prespore face of the septum (Wu et al., 1998; King et al., 1999).
Materials and methods
Strains and plasmids
The strains and plasmids used in this study are described in Table II. All plasmids were constructed in E.coli strain DH5α. DNA manipulations and E.coli transformations were carried out using standard methods (Sambrook et al., 1989). The sequence of all cloned PCR products was confirmed by DNA sequencing. Bacillus subtilis chromosomal DNA was prepared by a scaled-down method based on the one described by Errington (1984).
Table II. Strains and plasmids.
| Strain/plasmid | Relevant genotypea | Construction, source or reference |
|---|---|---|
| B.subtilis | ||
| SG38 | trpC2 amyE | Errington and Mandelstam (1986) |
| E.coli | ||
| DH5α | F– endA1 hsdR17 supE44 λ– thi-1 recA1 gyrA96 relA1 Δ(lacZYA-argF) U169 φ80dlac Δ(lacZ)M15 | Gibco-BRL |
| C41 (DE3) | BL21 (DE3) derivative | Miroux et al. (1996) |
| S.cerevisiae | ||
| Y187 | MATα ura3-52 his3-200 ade 2-101 trp 1-901 leu 2-3 112 gal4Δ met– gal80Δ URA3::GAL1UAS-Gal1TATA-lacZ | Clontech |
| Plasmids | ||
| pAS2-1 | GAL4 DNA-binding domain vector | Clontech |
| pACT2 | GAL4 activation domain vector | Clontech |
| pSG1711 | pACT2 containing ftsZ | A.Marston (unpublished) |
| pSG1904 | pAS2-1 containing spoIIE(976–2481) | this work |
| pSG1905 | pACT2 containing spoIIE(976–2481) | this work |
| pSG1906 | pAS2-1 containing spoIIE(1702–2481) | this work |
| pSG1907 | pACT2 containing spoIIE(1702–2481 | this work |
| pSG1916 | pAS2-1 containing spoIIE(976–1701) | this work |
| pSG1917 | pACT2 containing spoIIE(976–1701) | this work |
| pET-11a | T7 expression vector | Novagen |
| pET-21d | T7 expression vector | Novagen |
| pTYB3 | expression vector for Intein-chitin binding domain fusion protein | New England BioLabs |
| pRB1011 | pET-11a containing spoIIE | Lucet et al. (1999) |
| pIL1 | pET-11a containing strep′-′spoIIE | this work |
| pSG1909 | pET-21d containing strep′-′spoIIE(961–2481) | this work |
| pSG1911 | spoIIE(961–2481)′-′VMA intein′-′chitin-binding domain | this work |
| pSG4600 | pET-3a containing strep′-′mbl | R.Carballido-López (unpublished) |
aNumbers in parentheses after spoIIE refer to the first and last nucleotides of the insert from the spoIIE coding sequence.
Growth media
Nutrient agar (Oxoid) was used as a solid medium for growing B.subtilis. Sporulation was induced by growth in a hydrolysed casein medium, followed by resuspension in a starvation medium (Sterlini and Mandelstam, 1969; Partridge and Errington, 1993). Times (hours) after resuspension of cells in the starvation medium were denoted t0, t1, t2 and so on. Media used for growing E.coli were 2xTY (Sambrook et al., 1989) and nutrient agar (Oxoid) supplemented with ampicillin (100 μg/ml).
Yeast two-hybrid analysis
Plasmid pSG1904 was constructed by cloning a 1506 bp PCR amplified spoIIE fragment from SG38 chromosomal DNA. The oligonucleotides used for PCR, 5′-ATGGCCATGGAGGCCAGGAAAGTGGCGAGATAT-3′ and 5′-GCGGATCCCATATATTCCCATCTTCGCCAGAAG–3′, introduced SfiI and BamHI sites for insertion into vector plasmid pAS2–1, which was digested with SfiI and BamHI. The SfiI–BamHI spoIIE insert from pSG1904 was subcloned into SfiI–BamHI-digested pACT2 to generate pSG1905. Plasmids pSG1906 and pSG1907 were constructed as above, except that a 780 bp spoIIE fragment was amplified by PCR, using the following oligonucleotide to introduce a SfiI site: 5′-ATGGCCATGGAGGCCGTGAAAGCCGAACAGCACTC-3′. Plasmids pSG1916 and pSG1917 were constructed similarly, except that a 726 bp spoIIE fragment was amplified by PCR, using the following oligonucleotide to generate a BamHI site: 5′-CGGGATCCTTAAAGAATTTGTTCCTCCAG-3′. Plasmids were transformed into S.cerevisiae strain Y187 and β–galactosidase activity was estimated by the colony-lift filter assay with X-gal as a substrate as described in the Clontech protocol.
Construction of strep-tagged SpoIIE fusions and purification of the recombinant proteins
To join the 5′ end of spoIIE in-frame with a strep-tag, oligonucleotides 5′-GGAATTCCATATGAGCGCTTGGCGTCACCCGCAGTTCGGTGGTGAAAAAGCAGAAAGAAGAGTGAAC-3′ and 5′-TCATTAAGTCATATGTTTTATCAAAGTTCTGTACCC-3′, generating a strep-tag (bold) at the 5′ end and a NdeI site at each end of the PCR product, were used to amplify a 1280 bp spoIIE fragment from pRB1011. The PCR product was digested with NdeI and subcloned into NdeI-digested and gel-purified pRD1011, thereby replacing the 5′ end of spoIIE and yielding plasmid pIL1. To generate an N–terminal strep-tag fusion to only the cytoplasmic domain of SpoIIE, a 1521 bp spoIIE fragment was amplified by PCR from SG38 chromosomal DNA. The oligonucleotides were 5′-CATGCCATGGCTAGCGCTTGGCGTCACCCGCAGTTCGGTGGTCCTCAATCTATTACGAGGAAAGTGG-3′ and 5′-GCGGATCCCATATATTCCCATCTTCGCCAGAAG-3′, introducing a strep-tag (bold) and NcoI and BamHI sites for insertion into NcoI–BamHI-digested pET-21d to give plasmid pSG1909.
Plasmids pIL1 and pSG1909 were transformed into E.coli C41 (DE3) and the proteins were overexpressed for 4 h at 30°C after induction with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The cells from 1 l culture were resuspended in 25 ml of 50 mM Tris–HCl pH 8 containing 250 mM NaCl, 1 mM EDTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1% Triton X-100, 0.5 mg/ml lysozyme, 0.5 mg/ml DNase, 0.5 mg/ml RNase (buffer A) and disrupted by sonication (three times for 10 s). The membrane fractions were recovered by centrifugation at 29 000 g for 1 h and washed three times with the same buffer. The washed membranes were resuspended in 20 ml of buffer A, supplemented with 350 mM NaCl and 5% Triton X-100 for the full-length SpoIIE, or with 1 M NaCl and 10% Triton X-100 for the cytoplasmic domain, and stirred at 4°C for at least 3 h. After centrifugation at 29 000 g for 30 min, the supernatants were incubated overnight at 4°C with 200 μl of streptavidin-coated magnetic beads (DYNAL) washed in the same buffer. The magnetic beads were then washed with 10 ml of the same buffer to remove unspecific binding. The binding of the strep-tagged protein to the magnetic beads was checked by SDS–PAGE.
Interaction between strep-tagged SpoIIE proteins and SpoIIE and FtsZ from sporulating B.subtilis cell extract
The strep-tagged SpoIIE fusion proteins [full-length SpoIIE and SpoIIE (II–III)] bound to magnetic beads were incubated overnight at 4°C with cell extract taken 1.5 h after the initiation of sporulation. The cells were broken in 50 mM Tris–HCl pH 7 containing 250 mM KCl, 0.1 mM DTT, 1 mM MnCl2, 2 mM MgCl2, 10% glycerol, 1% Triton X-100, 1 mM GTP. After extensive washes, aliquots were loaded onto SDS–PAGE gels, silver stained and Western blotted using anti-SpoIIE antibodies or anti-FtsZ antibodies.
Construction and purification of C–terminal SpoIIE protein by a self-cleavage affinity tag
To generate a C–terminal fusion of the cytoplasmic domain of SpoIIE to Intein, a 1521 bp spoIIE fragment was amplified by PCR from SG38 chromosomal DNA. The oligonucleotides used, 5′-CATGCCATGGCTCCTCAATCTATTACGAGG-3′ and 5′-CCACCAGCTCTTCCGCATGAAATTTCTTGTTTG-3′, introduced NcoI and SapI sites for insertion into NcoI–SapI-digested pTYB3. The resulting plasmid pSG1911 was transformed into E.coli C41 (DE3) and the fusion protein was overproduced overnight at 16°C after induction with 0.3 mM IPTG. The cells from 1 l of culture were resuspended in 20 ml of 20 mM Tris–HCl pH 8 containing 250 mM NaCl, 1 mM EDTA, 1 mM PMSF, 0.1% Triton X-100 (buffer B), and disrupted by sonication on ice (three times for 10 s). After centrifugation at 29 000 g for 30 min, the clarified extract was loaded onto a 6 ml chitin column equilibrated in buffer B. The column was washed with 200 ml of the same buffer supplemented with 250 mM NaCl at a flow rate of 1 ml/min. The column was flushed quickly with 18 ml of 50 mM DTT freshly diluted in buffer B supplemented with 250 mM NaCl and left overnight at 4°C. The protein of interest was eluted using buffer B without DTT, and 1 ml fractions were collected and analysed by SDS–PAGE. SpoIIE (II–III) and SpoIIE (III) were purified further on a Superdex 200 gel filtration column equilibrated in 100 mM Tris–HCl pH 8 containing 500 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM DTT, 10% glycerol (buffer C). Fractions of 1 ml were collected and analysed by SDS–PAGE.
Assay for dephosphorylation of SpoIIAA-P
Non-radioactive phosphatase assays were performed using the Ser/Thr phosphatase assay system (Promega) as described by the manufacturer. Dephosphorylation was carried out at 30°C in 100 μl volume containing 20 μM SpoIIAA-P. The reaction was started by the addition of full-length SpoIIE, SpoIIE (II–III) or SpoIIE (III) (25–180 nM).
Gel filtration chromatography assay
Bacillus subtilis FtsZ was purified according to the method published previously (Wang and Lutkenhaus, 1993). Purified full-length SpoIIE, SpoIIE (III) and FtsZ were dialysed overnight at 4°C in 50 mM Tris–HCl pH 7 containing 250 mM KCl, 0.1 mM DTT, 1 mM MnCl2, 2 mM MgCl2, 10% glycerol, 0.5% Triton X-100, 1 mM GTP (buffer D), and applied separately or after mixing to a FPLC Sephacryl S200 gel filtration column previously equilibrated in the same buffer. Elution was performed with the same buffer at 0.5 ml/min and fractions of 1 ml were collected. Each fraction (20 μl) was tested by Western blotting with either anti-SpoIIE antibodies or anti-FtsZ antibodies. Standard proteins used to calibrate the column were thyroglobulin (670 kDa), γ–globulin (158 kDa), ovalbumin (44 kDa), myoglobulin (17 kDa) and vitamin B12 (1.3 kDa) (gel filtration calibration kit, Bio-Rad). Gel filtration data are presented either as elution volumes or as apparent molecular weight, calculated from the molecular sieve coefficient kav, itself calculated as (Ve – Vo)/(Vt – Vo), where Vo represents the elution volume corresponding to the peak concentration of a protein, Vo is the void volume of the column and Vt is the total volume of the gel bed. The void volume was determined by measuring the elution volume with blue dextran. The apparent molecular weight of the proteins was determined from a graph of log molecular weight versus Kav constructed for the above mentioned standard proteins.
Gel shift experiment on non-denaturing PAGE
Purified SpoIIE proteins and purified FtsZ were incubated together at 4°C in buffer D. Samples were added to sample buffer and subjected to non-denaturing 8% PAGE overnight. GTP was added to the running buffer at 1 mM. Proteins were detected by immunoblotting.
Acknowledgments
Acknowledgements
We thank Julie Wickson for outstanding technical assistance, S.Lee for the assistance with photography, A.Marston and R.Carballido-López for providing plasmid pSG1711 and pSG4600, respectively, A.Taylor for her help with FtsZ purification and providing the anti-FtsZ antibodies, and P.Crellin for introducing the Intein purification system. This work was supported by the Biotechnology and Biological Sciences Research Council.
References
- Abhayawardhane Y. and Stewart,G.C. (1995) Bacillus subtilis possesses a second determinant with extensive sequence similarity to the Escherichia coli mreB morphogene. J. Bacteriol., 177, 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addinall S.G. and Lutkenhaus, J. (1996a) FtsZ spirals and arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol., 22, 231–238. [DOI] [PubMed] [Google Scholar]
- Addinall S.G. and Lutkenhaus, J. (1996b) FtsA is localised to the septum in an FtsZ dependent manner. J. Bacteriol., 178, 7167–7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addinall S.G., Bi, E. and Lutkenhaus, J. (1996) FtsZ ring formation in fts mutants. J. Bacteriol., 178, 3877–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addinall S.G., Cao, C. and Lutkenhaus, J. (1997) FtsN, a late recruit to the septum in E.coli. Mol. Microbiol., 25, 303–310. [DOI] [PubMed] [Google Scholar]
- Adler E., Donella-Deana, A., Arigoni, F., Pinna, L.A. and Stragier, P. (1997) Structural relationship between a bacterial developmental protein and eukaryotic PP2C protein phosphatases. Mol. Microbiol., 23, 57–62. [DOI] [PubMed] [Google Scholar]
- Alper S., Duncan, L. and Losick, R. (1994) An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell, 77, 195–205. [DOI] [PubMed] [Google Scholar]
- Arigoni F., Pogliano, K., Webb, C.D., Stragier, P. and Losick, R. (1995) Localisation of protein implicated in establishment of cell type to sites of asymmetric division. Science, 270, 637–640. [DOI] [PubMed] [Google Scholar]
- Arigoni F., Duncan, L., Alper, S., Losick, R. and Stragier, P. (1996) SpoIIE governs the phosphorylation state of a protein regulating transcription factor σF during sporulation in Bacillus subtilis. Proc. Natl Acad. Sci. USA, 93, 3238–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arigoni F., Guerout-Fleury, A.M., Barak, I. and Stragier, P. (1999) The SpoIIE phosphatase, the sporulation septum and the establishment of forespore-specific transcription in Bacillus subtilis: a reassessment. Mol. Microbiol., 31, 1407–1415. [DOI] [PubMed] [Google Scholar]
- Barak I. and Youngman, P. (1996) SpoIIE mutants of Bacillus subtilis comprise two distinct phenotypic classes consistent with a dual functional role for the SpoIIE protein. J. Bacteriol., 178, 4984–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak I., Behari, J., Olmedo, G., Guzman, P., Brown, D.P., Castro, E., Walker, D., Westpheling, J. and Youngman, P. (1996) Structure and function of the Bacillus SpoIIE protein and its localisation to sites of sporulation septum assembly. Mol. Microbiol., 19, 1047–1060. [DOI] [PubMed] [Google Scholar]
- Bi E. and Lutkenhaus, J. (1991) FtsZ ring structure associated with cell division in Escherichia coli. Nature, 354, 161–164. [DOI] [PubMed] [Google Scholar]
- Bramhill D. (1997) Bacterial cell division. Annu. Rev. Cell Dev. Biol., 13, 395–424. [DOI] [PubMed] [Google Scholar]
- Bramhill D. and Thompson, C.M. (1994) GTP-dependent polymerisation of Escherichia coli FtsZ protein to form tubules. Proc. Natl Acad. Sci. USA, 91, 5813–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A.K., Helps, N.R., Cohen, P.T.W. and Barford, D. (1996) Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 Å resolution. EMBO J., 15, 6798–6809. [PMC free article] [PubMed] [Google Scholar]
- Diederich B., Wilkinson, J.F., Magnin, T., Najafi, S.M.A., Errington, J. and Yudkin, M.D. (1994) Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor σF of Bacillus subtilis. Genes Dev., 8, 2653–2663. [DOI] [PubMed] [Google Scholar]
- Duncan L. and Losick, R. (1993) SpoIIAB is an anti-σ factor that binds to and inhibits transcription by regulatory protein σF from Bacillus subtilis. Proc. Natl Acad. Sci. USA, 90, 2325–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L., Alper, S., Arigoni, F., Losick, R. and Stragier, P. (1995) Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science, 270, 641–644. [DOI] [PubMed] [Google Scholar]
- Duncan L., Alper, S. and Losick, R. (1996) SpoIIAA governs the release of cell-type specific transcription by a serine phosphatase at the site of asymmetric division. J. Mol. Biol., 260, 147–164. [DOI] [PubMed] [Google Scholar]
- Erickson H.P., Taylor, D.W., Taylor, K.A. and Bramhill, D. (1996) Bacterial cell division protein FtsZ assembles into protofilament sheet and minirings, structural homologs of tubulin polymers. Proc. Natl Acad. Sci. USA, 93, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. (1984) Efficient Bacillus subtilis cloning system using bacteriophage vector φ105J9. J. Gen. Microbiol., 130, 2615–2628. [DOI] [PubMed] [Google Scholar]
- Errington J. (1996) Determination of cell fate in Bacillus subtilis. Trends Genet., 12, 31–34. [DOI] [PubMed] [Google Scholar]
- Errington J. and Mandelstam, J. (1986) Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIAA in spo mutants of Bacillus subtilis. J. Gen. Microbiol., 132, 2967–2976. [DOI] [PubMed] [Google Scholar]
- Feucht A., Magnin, T., Yudkin, M.D. and Errington, J. (1996) Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes Dev., 10, 794–803. [DOI] [PubMed] [Google Scholar]
- Feucht A., Daniel, R.A. and Errington, J. (1999) Characterisation of a morphological checkpoint coupling cell-specific transcription to septation in Bacillus subtilis. Mol. Microbiol., 33, 1015–1026. [DOI] [PubMed] [Google Scholar]
- Gholamhoseinian A. and Piggot, P.J. (1989) Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis. J. Bacteriol., 171, 5747–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C.A. and de Boer, P.A.J. (1997) Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E.coli. Cell, 88, 175–185. [DOI] [PubMed] [Google Scholar]
- Horvitz H.R. and Herskowitz, I. (1992) Mechanisms of asymmetric cell division: Two Bs or not two Bs, that is the question. Cell, 68, 237–255. [DOI] [PubMed] [Google Scholar]
- Huang J., Cao, C. and Lutkenhaus, J. (1996) Interaction between FtsZ and inhibitors of cell division. J. Bacteriol., 178, 5080–5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing N. and Errington, J. (1991) Genetic regulation of morphogenesis in Bacillus subtilis: role of σE and σF in prespore engulfment. J. Bacteriol., 173, 3159–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y.N. and Jan, L.Y. (1998) Asymmetric cell division. Nature, 392, 775–778. [DOI] [PubMed] [Google Scholar]
- Khvorova A., Zhang, L., Higgins, M.L. and Piggot, P. (1998) The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J. Bacteriol., 180, 1256–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N., Dreesen, O., Stragier, P., Pogliano, K. and Losick, R. (1999) Septation, dephosphorylation and the activation of σF during sporulation in Bacillus subtilis. Genes Dev., 13, 1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin P. and Losick, R. (1996) Transcription factor Spo0A switches the localisation of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev., 10, 478–488. [DOI] [PubMed] [Google Scholar]
- Levin P.A., Losick, R., Stragier, P. and Arigoni, F. (1997) Localisation of the sporulation protein SpoIIE in Bacillus subtilis is dependent upon the cell division protein FtsZ. Mol. Microbiol., 25, 839–846. [DOI] [PubMed] [Google Scholar]
- Losick R. and Dworkin, J. (1999) Linking asymmetric division to cell fate: teaching an old microbe new tricks. Genes Dev., 13, 377–381. [DOI] [PubMed] [Google Scholar]
- Löwe J. and Amos, L.A. (1998) Crystal structure of the bacterial cell-division protein FtsZ. Nature, 391, 203–206. [DOI] [PubMed] [Google Scholar]
- Lucet I., Borris, R. and Yudkin, M.D. (1999) Purification, kinetic properties and intracellular concentration of SpoIIE, an integral membrane protein that regulates sporulation in Bacillus subtilis. J. Bacteriol., 181, 3242–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. and Addinall, S.G. (1997) Bacterial cell division and the Z ring. Annu. Rev. Biochem., 66, 93–116. [DOI] [PubMed] [Google Scholar]
- Ma X., Ehrhardt, D.W. and Margolin, W. (1996) Colocalisation of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc. Natl Acad. Sci. USA, 93, 12998–13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnin T., Lord, M., Errington, J. and Yudkin, M.D. (1996) Establishing differential gene expression in sporulating Bacillus subtilis: Phosphorylation of SpoIIAA (anti-anti σF) alters its conformation and prevents formation of a SpoIIAA/SpoIIAB/ADP complex. Mol. Microbiol., 19, 901–907. [DOI] [PubMed] [Google Scholar]
- Margolis P., Driks, A. and Losick, R. (1991) Establishment of cell type by compartmentalized activation of a transcription factor. Science, 254, 562–565. [DOI] [PubMed] [Google Scholar]
- Min K.-T., Hilditch, C.M., Diederich, B., Errington, J. and Yudkin, M.D. (1993) σF, the first compartment-specific transcription factor of Bacillus subtilis, is regulated by an anti-σ factor that is also a protein kinase. Cell, 74, 735–742. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. and Lutkenhaus, J. (1994) Guanine nucleotide-dependent assembly of FtsZ into filaments. J. Bacteriol., 176, 2754–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A. and Lutkenhaus, J. (1998) Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J., 17, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi S.M.A., Willis, A.C. and Yudkin, M.D. (1995) Site of phosphorylation of SpoIIAA, the anti-anti-σ factor for sporulation specific σF of Bacillus subtilis. J. Bacteriol., 177, 2912–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E., Wolf, S.G. and Downing, K.H. (1998) Structure of the αβ tubulin dimer by electron crystallography. Nature, 391, 199–203. [DOI] [PubMed] [Google Scholar]
- Partridge S.R. and Errington, J. (1993) The importance of morphological events and intracellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol. Microbiol., 8, 945–955. [DOI] [PubMed] [Google Scholar]
- Patricelli M.P., Lashuel, H.A., Giang, D.K., Kelly, J.W. and Cravatt, B.F. (1998) Comparative characterisation of a wild type and transmembrane domain-deleted fatty acid amide hydrolase: identification of the transmembrane domain as a site for oligomerization. Biochemistry, 37, 15177–15187. [DOI] [PubMed] [Google Scholar]
- Piggot P.J. (1973) Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporulation operons. J. Bacteriol., 114, 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano K., Hofmeister, A.E. and Losick, R. (1997) Disappearance of the σE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol., 179, 3331–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L.I. and Justice, S.S. (1997) Bacterial cell division: the cycle of the ring. Cell, 88, 581–584. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sterlini J.M. and Mandelstam, J. (1969) Commitment to sporulation in Bacillus subtilis and its relationship to the development of actinomycin resistance. Biochem. J., 113, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. and Lutkenhaus, J. (1993) The FtsZ protein of Bacillus subtilis is localised at the division site and has a GTPase activity that is dependent upon FtsZ concentration. Mol. Microbiol., 9, 435–442. [DOI] [PubMed] [Google Scholar]
- Wang X., Huang, J., Mukherjee, A., Cao, C. and Lutkenhaus, J. (1997) Analysis of the interaction of FtsZ with itself, GTP and FtsA. J. Bacteriol., 179, 5551–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Khattar, M.K., Donachie, W.D. and Lutkenhaus, J. (1998) FtsI and FtsW are localised to the septum in Escherichia coli. J. Bacteriol., 180, 2810–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D.S., Pogliano, K., Carson, M., Guzman, L.M., Fraipont, C., Nguyen-Disteche, M., Losick, R. and Beckwith, J. (1997) Localisation of the Escherichia coli cell division protein FtsI (PBP3) to the division site and cell pole. Mol. Microbiol., 25, 671–681. [DOI] [PubMed] [Google Scholar]
- Wu L.J., Feucht, A. and Errington, J. (1998) Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes Dev., 12, 1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.-C. and Margolin, W. (1997) Ca2+ mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J., 16, 5455–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.-C., Tran, A.H., Sun, Q. and Margolin, W. (1998) Localisation of cell division protein FtsK to the Escherichia coli septum and identification of a potential N–terminal targeting domain. J. Bacteriol., 180, 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]