Abstract
Intracellular protozoan parasites are potent stimulators of cell-mediated immunity. The induction of macrophage proinflammatory cytokines by Trypanosoma cruzi is considered to be important in controlling the infection and the outcome of Chagas' disease. Here we show that the potent tumour necrosis factor–α-, interleukin–12- and nitric oxide-inducing activities of T.cruzi trypomastigote mucins were recovered quantitatively in a highly purified and characterized glycosylphosphatidylinositol (GPI) anchor fraction of this material. The bioactive trypomastigote GPI fraction was compared with a relatively inactive GPI fraction prepared from T.cruzi epimastigote mucins. The trypomastigote GPI structures were found to contain additional galactose residues and unsaturated, instead of saturated, fatty acids in the sn–2 position of the alkylacylglycerolipid component. The latter feature is essential for the extreme potency of the trypomastigote GPI fraction, which is at least as active as bacterial endotoxin and Mycoplasma lipopeptide and, therefore, one of the most potent microbial proinflammatory agents known.
Keywords: Chagas' disease/cytokines/glycosylphosphatidylinositol/inflammation/nitric oxide
Introduction
The protozoan parasite Trypanosoma cruzi is transmitted to mammals in the faeces of the reduviid bug insect vector. The metacyclic trypomastigotes that enter the mammalian host, via skin lesions and/or mucous membranes, invade host cells and differentiate into small amastigote forms that replicate in the cytoplasm. Following differentiation into non-dividing bloodstream form trypomastigotes, the parasites escape and re-invade host cells (predominantly cardiac and smooth muscle cells) to propagate the infection. Ingestion of bloodstream form trypomastigotes by a reduviid bug results in their differentiation to dividing epimastigote forms that subsequently differentiate into non-dividing metacyclic forms, thus completing the life cycle. The different life cycle stages of T.cruzi synthesize common and stage-specific cell surface macromolecules throughout the life cycle.
Macrophages are triggered by certain microbial components to induce phagocytosis, microbicidal activity and the release of cytokines, such as interleukin (IL)–12 and other co-stimulatory cytokines, which are crucial for the stimulation of innate immunity (Gazzinelli et al., 1993; Vespa et al., 1994; Biron and Gazzinelli, 1995; Silva et al., 1995; Aliberti et al., 1996; Fearon and Locksley, 1996; Trinchieri and Scott, 1996). In addition, the early stimulation of macrophages and natural killer (NK) cells appears to have an important role in directing the development of cell-mediated immunity (CMI) (Hsieh et al., 1993; Seder et al., 1993; Abbas et al., 1996; Fearon and Locksley, 1996). During infection with T.cruzi, the induction of parasite-specific CMI is likely to be involved in at least two aspects of Chagas' disease pathophysiology (Vespa et al., 1994; Fearon and Locksley, 1996; Brener and Gazzinelli, 1997). The first is the control of parasite replication and its spread in the vertebrate host tissues. The second is the inflammatory reaction observed in the infected host tissues, which is likely to be a major cause of cardiac tissue damage during the acute and chronic phases of the disease.
Relatively little is known about the protozoan parasite molecules that initiate the synthesis of pro-inflammatory cytokines and nitric oxide (NO) by macrophages. Recent studies have suggested a role for glycosylphosphatidylinositol (GPI) anchors from Plasmodium falciparum (Schofield and Hackett, 1993; Schofield et al., 1996; Tachado et al., 1996, 1997) and Trypanosoma brucei (Magez et al., 1998) in this process. However, no evidence of the biochemical purity of the P.falciparum GPIs was provided, making estimates of their concentrations dubious. Furthermore, the possibility of Mycoplasma lipopeptide (Mühlradt et al., 1997) contamination (that has confounded other studies into proinflammatory factors) cannot be formally excluded. In the case of the T.brucei GPI work, highly purified and structurally characterized fractions were used, but cytokine induction was only seen when these molecules were used at micromolar concentrations that may not be physiologically relevant.
We have described previously the ability of GPI-anchored mucin-like glycoproteins from the trypomastigote (but not the epimastigote or metacyclic) life cycle stage of T.cruzi to trigger the synthesis of tumour necrosis factor (TNF)–α, IL–12 and NO by mouse inflammatory macrophages (Camargo et al., 1997a, b). The present study was undertaken to isolate and define the active component(s) of the T.cruzi trypomastigote mucins, establish their purity and structure, compare their structure with similar yet relatively inactive material for T.cruzi epimastigote and metacyclic forms, look directly for possible Mycoplasma lipopeptide contamination and establish their potency in precise molar terms. A detailed knowledge of the structure–activity relationships of parasite cytokine-inducing factors may provide insights into the processes controlling the inflammatory responses responsible for the immunopathology associated with many protozoan parasite infections.
Results
Purification and analysis of T.cruzi trypomastigote and epimastigote mucins
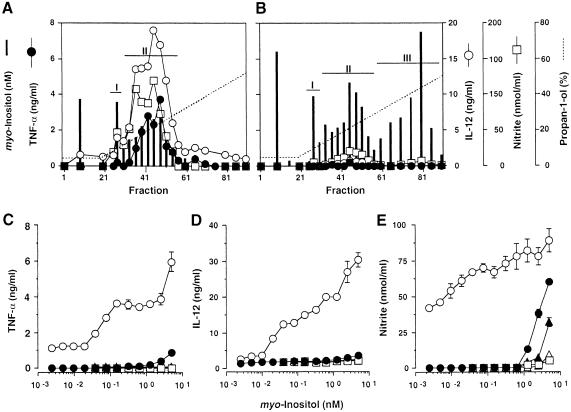
We purified trypomastigote and epimastigote mucins from Y strain T.cruzi as described before (Almeida et al., 1994; Serrano et al., 1995) and analysed the fractions eluting from the final octyl-Sepharose chromatography step for myo-inositol content, by selected ion-monitoring gas chromatography–mass spectrometry (GC–MS) (Ferguson, 1993), and for the induction of TNF–α, IL–12 and NO when presented to interferon (IFN)–γ-primed C3H/HeJ mouse macrophages (Camargo, 1997a, b) (Figure 1). Essentially all of the cytokine- and NO-inducing activity of the trypomastigote preparation co-eluted with a peak of myo-inositol-containing material (Figure 1A, peak II). Another earlier eluting peak containing myo-inositol was also observed (Figure 1A, peak I), but this material had little or no NO- or cytokine-inducing activity. The equivalent preparation from epimastigotes produced three myo-inositol-containing peaks (peaks I–III) that had little or no cytokine- or NO-inducing activity (Figure 1B).
Fig. 1. Fractionation and bioactivity of T.cruzi GPI-anchored glycoconjugates. Octyl-Sepharose chromatograms of T.cruzi trypomastigote (A) and epimastigote (B) glycoconjugate extracts from 1.5 × 1010 and 2.0 × 1010 cells, respectively. Aliquots of each fraction were presented to 106 thioglycollate-elicited, IFN–γ-primed, murine peritoneal macrophages and the amounts of TNF–α (•), IL–12(p40) (○) and NO (□) (measured as nitrite) produced in 24 h were measured. Vertical bars indicate the molarity of the glycoconjugate used in each assay, based on the myo-inositol content of each fraction eluted from the column. The data shown in (A) and (B) are representative of three different fractionation experiments. Peak fractions were pooled as indicated and titrated for TNF–α- (C), IL–12(p40)- (D) and NO– (E) inducing activity. Trypomastigote-derived peak I and II materials (•, ○); epimastigote-derived peak I, II and III materials (□, ▴, ▵). The data shown in (C–E) are representative of three separate titration experiments with three different sets of fractions. Each cytokine and NO determination was carried out in duplicate, and the error bars (where visible) indicate the standard error of the mean.
The epimastigote peak III material was analysed by electrospray-mass spectrometry (ES–MS) (data not shown) and found to contain the previously described GPI-related glycoinositol phospholipids (GIPLs) (De Lederkremer et al., 1976, 1991; Mendonça–Previato et al., 1983; Previato et al., 1990; Carreira et al., 1996) that are much less abundant in trypomastigotes (Golgher et al., 1993). The peak I and peak II fractions from the trypomastigote and epimastigote preparations were analysed by SDS–PAGE and silver staining (not shown), which revealed the characteristic high and low apparent molecular weight mucins of these life cycle stages, respectively (Alves and Colli, 1975; Previato et al., 1985; Schenkman et al., 1993; Almeida et al., 1994; Camargo et al., 1997a). The trypomastigote peak I and II fractions also reacted strongly with total serum and anti-α–galactosyl antibodies isolated from patients with chronic Chagas' disease (Almeida et al., 1991), which is synonymous with the presence of trypomastigote mucins (Almeida et al., 1994).
The phosphatidylinositol (PI) moieties of the GPI anchors of the purified trypomastigote and epimastigote mucins (peaks I and II) and the epimastigote GIPLs (peak III) were released by nitrous acid deamination and analysed by ES–MS and ES–MS–collision-induced dissociation (CID)–MS (Table I). These data were consistent with those previously published for the epimastigote mucins (Previato et al., 1995; Serrano et al., 1995) and GIPLs (Previato et al., 1990; De Lederkremer et al., 1991, 1993; Carreira et al., 1996) and trypomastigote mucins (Camargo et al., 1997a), and further showed that the peak I fractions contained mainly lyso–PI species.
Table I. Phosphatidylinositols released from purified T.cruzi GPI-anchored glycoconjugates by nitrous deamination.
| Pseudomolecular iona [M-H]– m/z | Octyl-Sepharose fraction (see Figure 1A and B) |
Lipid component of the PI moiety | ||||
|---|---|---|---|---|---|---|
| Trypomastigote (%) |
Epimastigote (%) |
|||||
| I | II | I | II | III | ||
| 557 | 95 | 11 | 29 | 13 | 2 | 1-O-(C16:0)lyso-alkylglycerol |
| 571 | trb | 0 | 17 | 5 | 7 | (C16:0)lyso-acylglycerol |
| 599 | tr | 0 | 12 | tr | tr | (C18:0)lyso-acylglycerol |
| 627 | tr | 0 | 12 | 0 | 0 | (C20:0)lyso-acylglycerol |
| 655 | tr | 0 | 6 | 0 | 0 | (C22:0)lyso-acylglycerol |
| 778 | 0 | 0 | 0 | 0 | 6 | ceramide, (C16:0)fatty acid-(C18:1)sphingosine |
| 780 | 0 | 0 | 0 | 0 | 8 | ceramide, (C16:0)fatty acid-(C18:0)sphinganine |
| 795 | 5 | 37 | 24 | 77 | 16 | 1-O-(C16:0)alkyl-2-O-(C16:0)acylglycerol |
| 819 | tr | 21 | 0 | 0 | 0 | 1-O-(C16:0)alkyl-2-O-(C18:2)acylglycerol |
| 821 | 0 | 31 | 0 | 0 | 0 | 1-O-(C16:0)alkyl-2-O-(C18:1)acylglycerol |
| 823 | 0 | 0 | 0 | 2 | 0 | 1-O-(C16:0)alkyl-2-O-(C18:0)acylglycerol |
| 890 | 0 | 0 | 0 | 0 | 18 | ceramide, (C24:0)fatty acid-(C18:1)sphingosine |
| 892 | 0 | 0 | 0 | 3 | 43 | ceramide, (C24:0)fatty acid-(C18:0)sphinganine |
aOther minor pseudomolecular ion species that could not be characterized by ES-MS–CID-MS as inositolphospholipids were not included in this table. btr = trace.
The trypomastigote and epimastigote peak materials from Figure 1 were pooled separately, quantified by total myo-inositol content and, using these values to estimate the molarity of the fractions, titrated for the induction of TNF–α, IL–12 and NO when presented to macrophages (Figure 1C–E). The trypomastigote peak II material was extremely active, giving significant induction of TNF–α and IL–12 at concentrations >30 pM, and significant induction of NO at the lowest concentration tested (2 pM). In contrast, the trypomastigote peak I material and all of the epimastigote materials were at least 100–fold less active in these assays. Although all of the data shown here were obtained using IFN–γ-primed macrophges, similar results were obtained using unprimed macrophages except that the levels of induction were lower, producing ∼40, 20 and 10% of the TNF–α, IL–12 and NO levels shown in Figures 1 and 2.
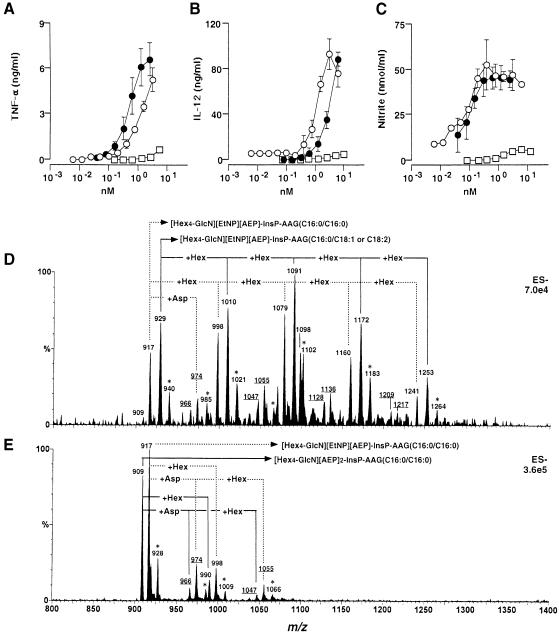
Fig. 2. Bioactivity and mass spectra of purified GPI anchors released from T.cruzi mucins. Samples were presented to 106 thioglycollate-elicited, IFN–γ-primed, murine peritoneal macrophages and titrations of the TNF–α- (A), IL–12(p40)- (B) and NO- (C) inducing activities of intact trypomastigote mucin (•), purified GPI released from trypomastigote mucin (○) and purified GPI released from epimastigote mucin (□) are shown. The data shown in (A–C) are representative of four separate titration experiments. Each cytokine and NO determination was carried out in duplicate, and the error bars (where visible) indicate the standard error of the mean. The purified trypomastigote GPI (D) and epimastigote GPI (E) fractions were analysed by negative-ion ES–MS. The compositions corresponding to each [M–2H]2– pseudomolecular ion are indicated. Ions marked with asterisks are [M+Na–3H]2– pseudomolecular ions, and the underlined ions correspond to GPI anchors attached to Asp. EtNP, ethanolamine phosphate; AEP, 2–aminoethylphosphonate; InsP, inositol-phosphate; AAG, alkylacylglycerol. The numbers following the AAG abbreviation correspond to the chain length and degree of unsaturation of the alkyl/acyl chains. Note: m/z differences of 81 correspond to mass differences of 162 [equivalent to a hexose (Hex), i.e. mannose or galactose] because the charge state of the ions (z) is 2.
Purification and analysis of the mucin GPI anchors
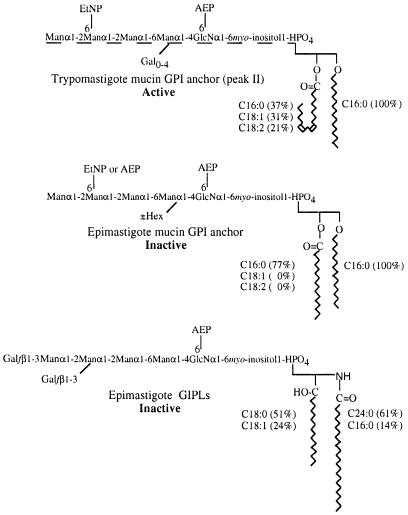
In order to establish the structural basis for the bioactivity of the trypomastigote versus the epimastigote peak II mucins, these materials were digested with proteinase K, and the GPI anchors of each repurified by octyl-Sepharose chromatography. After pooling the myo-inositol-containing fractions, the purified GPI anchors were titrated for NO- and cytokine-inducing activity alongside the intact trypomastigote peak II mucins (Figure 2A–C). The purified trypomastigote GPI anchors had essentially the same specific activity (relative to myo-inositol content) as the intact trypomastigote peak II mucins and, as expected, the purified epimastigote GPI anchors had relatively little activity. The purified GPI anchors were analysed by ES–MS (Figure 2D and E). Both preparations showed ion clusters corresponding to doubly charged pseudomolecular ions. In the case of the epimastigote mucin GPI anchors, the m/z values of these ions can be interpreted readily in terms of the known primary structures and compositions of these GPI anchors (Figures 2E and 5) (Previato et al., 1995; Serrano et al., 1995). Taking into account these assignments, and the data on the PI molecular species in Table I, the doubly charged ions of the trypomastigote GPI anchors can also be interpreted (Figures 2D and 5). In essence, the epimastigote anchors contain 4–5 hexoses and fully saturated alkylacyl–PI moieties, whereas the trypomastigote anchors contain 4–8 hexoses and mainly unsaturated (C18:1 or C18:2) fatty acids in their alkylacyl–PI moieties. The precise arrangement of the hexoses in the trypomastigote mucins was not determined, but it is reasonable to assume that it is based on the Manα1-2Manα1-2Manα1-6Manα1-4GlcNα1-6PI structure common to all T.cruzi GPI/GIPL structures thus far identified (De Lederkremer et al., 1991; Güther et al., 1992; Couto et al., 1993; Previato et al., 1995; Serrano et al., 1995; Carreira et al., 1996). Monosaccharide composition analysis by GC–MS revealed the presence of only mannose and galactose, in the ratio 4.0:2.5, suggesting that the trypomastigote GPI anchors are substituted with, on average, 2.5 galactose residues.
Fig. 5. Comparison of active and representative relatively inactive T.cruzi GPI structures. The epimastigote mucin (peak II) GPI structure (Previato et al., 1995; Serrano et al., 1995) is consistent with the ES–MS spectrum in Figure 2E and the ES–MS analysis of the PI moiety (Table I). Only the identity and linkage position of the additional hexose residue (±Hex) are unknown (Previato et al., 1995; Serrano et al., 1995). The proposed trypomastigote peak II GPI structure is based on the epimastigote structure and is consistent with the ES–MS spectrum in Figure 2D, the GC–MS monosaccharide analysis and the ES–MS analysis of the PI moiety (Table I). The dotted line indicates that the linkage position(s) of the uncharacterized galactose substituents (Gal0–4) is unknown. The types and proportions of the major alkyl and acyl (fatty acid) chains are indicated. The exact isomer of the C18:2 fatty acid (shown here as linoleic acid) has not been determined. The ceramide-containing epimastigote (peak III) GIPL structure is taken from De Lederkremer et al. (1991) and Previato et al. (1990), and is consistent with an ES–MS spectrum of this fraction (data not shown) and the ES–MS analysis of the PI moiety (Table I).
It is worth noting that proteinase K, unlike Pronase (Ferguson et al., 1988), appears to be capable of cleaving between the C–terminal amino acid and the ethanolamine phosphate bridge. The removal of amino acids from the GPI anchors was not complete, and some molecular species containing aspartic acid were observed in the mass spectra. Furthermore, amino acid sequencing of the GPI fraction revealed Asp and a trace of Ile–Asp. These observations are consistent with the predicted amino acid sequences of the T.cruzi mucins, many of which contain a conserved IDGS sequence next to a C–terminal hydrophobic sequence (Salazar et al., 1996; Di Noia et al., 1998; Freitas–Junior et al., 1998), such that D would be the predicted peptide cleavage/GPI attachment site (Udenfriend and Kodukula, 1995).
The bioactive trypomastigote mucin GPI anchors are free from Mycoplasma lipopeptides
Mycoplasma lipopeptides are among the most potent known proinflammatory microbial products (Mühlradt et al., 1997, 1998). Since Mycoplasma are common tissue culture contaminants, it was important to exclude the possibility of Mycoplasma lipopeptide contamination from the bioactive T.cruzi GPI preparations. As mentioned above, Edman sequencing (five cycles) of the purified trypomastigote GPI preparation revealed only D and a trace of I in the first cycle, and only a trace of D in the second cycle, consistent with D and ID linked to the GPI ethanolamine phosphate bridge. No further residues were detected in the third, fourth and fifth cycles. In contrast, five cycles of Edman sequencing of synthetic Mycoplasma fermentans lipopeptide (MALP–2) produced the expected XGNND sequence (Muhlradt et al., 1997) in good yield, where X (an empty cycle) is due to the thioether glycerolipid-modified N–terminal cysteine residue. Other proinflammatory Mycoplasma lipopeptides (e.g. those of M.hyorhinis) have N–terminal sequences of XGQTD or XGQTN (Muhlradt et al., 1998). Thus, the lack of G in the second cycle and the lack of any residues in the third, fourth and fifth Edman cycles of the T.cruzi trypomastigote GPI fraction strongly suggested that the preparation was devoid of contaminating Mycoplasma lipopeptides.
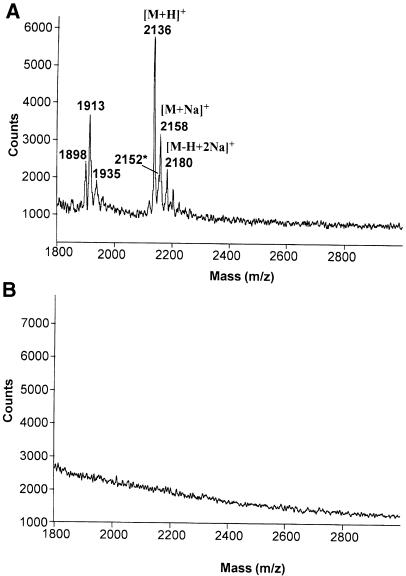
Finally, positive-ion matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) analysis of 10 pmol of the GPI preparation did not reveal any peptides or lipopeptides under conditions where 10 pmol of a synthetic MALP–2 were clearly visible (Figure 3).
Fig. 3. Purified trypomastigote GPIs are free of lipopeptides. A sample of 10 pmol of synthetic Mycoplasma lipopeptide (A) and 10 pmol of purified T.cruzi trypomastigote mucin GPI fraction (B) were analysed by positive-ion MALDI-TOF under identical conditions. The absence of ions in (B) reflects the absence of any lipopeptides or peptides in the preparation and the inability of GPIs to produce positive ions under the conditions used. In (A), the ions at m/z 2136, 2158 and 2180 are the [M+H]+, [M+Na]+ and [M–H+2Na]+ ions of S–(2,3–bishexa- decanoyloxypropyl)cysteine-GNNDESNISFKEK, respectively and the minor ion at m/z 2152* is most probably the [M+H]+ ion of the corresponding sulfoxide. The ions at lower m/z values may be due to degradation products.
Epimastigote GPI-mucins and GIPLs from different strains of T.cruzi are inactive and do not inhibit the activity of trypomastigote GPI-mucins
To investigate whether the lack of cytokine and NO induction by Y strain GPI-mucins and GIPLs was unique to this strain of T.cruzi, GPI-mucins and GIPLs were purified from CL and Tulahuen strains, and, together with Y strain materials, tested at concentrations up to 400 nM (Figure 4A). The chemical compositions of the epimastigote GIPLs and GPI-mucin GPI anchors (released by proteinase K) were analysed by ES–MS and ES–MS–CID–MS, as described above, and the data are summarized in Table II.
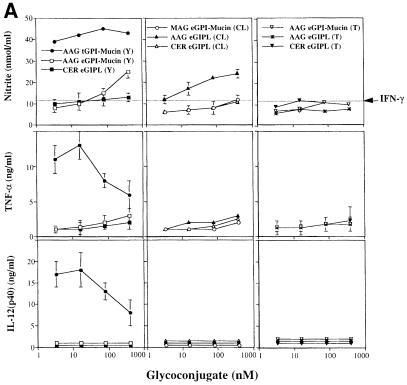
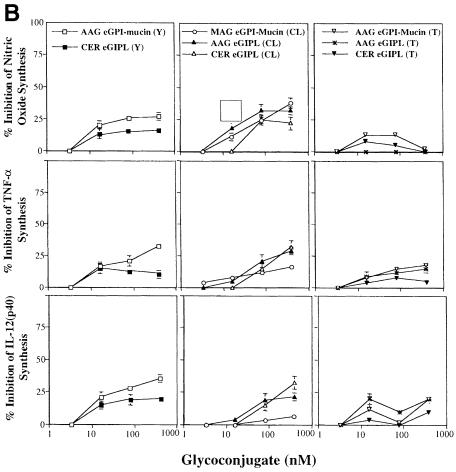
Fig. 4. Bioactivities of Y, CL and Tulahuen strain epimastigote GPI-mucins and GIPLs. (A) Titration of NO (top panels), TNF–α (middle panels) and IL–12(p40) (bottom panels) induction by intact GPI-mucins or GIPLs purified from the epimastigote forms of Y, CL and Tulahuen (T) strains of T.cruzi. The partial structure and purity of each glycoconjugate were evaluated by ES–MS and ES-MS–CID-MS (Table II); AAG, MAG and CER indicate the presence of alkylacylglycerol, monoalkylglycerol or ceramide lipids in the PI moieties of the GPI structures. Macrophages were primed with IFN–γ (100 U/ml) overnight and then stimulated with 3.3, 16, 80 and 400 nM epimastigote-derived glycoconjugate. As a positive control, Y strain trypomastigote GPI-mucin was used at the same concentrations. TNF–α was measured in the macrophage culture supernatants 24 h after stimulation, and IL–12(p40) and nitrite were measured 48 h after stimulation. Horizontal lines indicate the macrophage response to IFN–γ alone. (B) Inhibitory activities of the epimastigote GPI-mucins and GIPLs. Titration of the inhibition of NO (top panels), TNF–α (middle panels) and IL–12(p40) (bottom panels) induction by 1 nM trypomastigote GPI-mucin by pre-incubation (2 h) with epimastigote GPI-mucins and GIPLs.
Table II. Mucin- and GIPL-derived GPIs isolated from different strains of T.cruzi epimastigotes.
| Epimastigote strain | GPI origina | Major ion speciesb [M-2H]2– | Molecular mass (Da) | Proposed assignment for the GPI |
|
|---|---|---|---|---|---|
| Glycan core | Lipid component of the PI moiety | ||||
| Y | GIPLs | 1038.3 | 2078.6 | [Hex5-GlcN][AEP]2 | CER, (C24:0)fatty acid-(C18:0)sphinganine |
| 1046.5 | 2095.0 | [Hex5-GlcN][EtNP][AEP] | CER, (C24:0)fatty acid-(C18:0)sphinganine | ||
| 1065.6 | 2133.2 | [Hex6-GlcN][AEP] | CER, (C24:0)fatty acid-(C18:0)sphinganine | ||
| mucins | 908.9 | 1819.8 | [Hex4-GlcN][AEP]2 | AAG, (C16:0)alkyl-(C16:0)acylglycerol | |
| 916.8 | 1835.6 | [Hex4-GlcN][EtNP][AEP] | AAG, (C16:0)alkyl-(C16:0)acylglycerol | ||
| 997.9 | 1997.8 | [Hex5-GlcN][EtNP][AEP] | AAG, (C16:0)alkyl-(C16:0)acylglycerol | ||
| CL | GIPLs | 989.8 | 1981.6 | [Hex5-GlcN][AEP]2 | AAG, (C16:0)alkyl-(C16:0)acylglycerol |
| 997.8 | 1997.6 | [Hex5-GlcN][EtNP][AEP] | AAG, (C16:0)alkyl-(C16:0)acylglycerol | ||
| 1017.1 | 2036.2 | [Hex6-GlcN][AEP] | AAG, (C16:0)alkyl-(C16:0)acylglycerol | ||
| 1038.3 | 2078.6 | [Hex5-GlcN][AEP]2 | CER, (C24:0)fatty acid-(C18:0)sphinganine | ||
| 1046.5 | 2095.0 | [Hex5-GlcN][EtNP][AEP] | CER, (C24:0)fatty acid-(C18:0)sphinganine | ||
| 1065.6 | 2133.2 | [Hex6-GlcN][AEP] | CER, (C24:0)fatty acid-(C18:0)sphinganine | ||
| mucins | 789.8c | 1581.6 | [Hex4-GlcN][AEP]2 | MAG, (C16:0)mono(lyso)alkyl-glycerol | |
| 797.8c | 1597.6 | [Hex4-GlcN][EtNP][AEP] | MAG, (C16:0)mono(lyso)alkyl-glycerol | ||
| Tulahuen | GIPLs | 908.9 | 1819.8 | [Hex4-GlcN][AEP]2 | AAG, (C16:0)alkyl-(C16:0)acylglycerol |
| 965.2 | 1932.4 | [Hex4-GlcN][EtNP][AEP] | CER, (C24:0)fatty acid-(C18:0)sphinganine | ||
| mucins | 916.8 | 1835.6 | [Hex4-GlcN][EtNP][AEP] | AAG, (C16:0)alkyl-(C16:0)acylglycerol | |
aThe GPI anchor was obtained after proteinase K digestion of the purified mucin as described in Materials and methods.
bThe most abundant GPI species within the mixture is underlined.
cThe monoalkyglycerol-PI species was obtained from alkylacylglycerol-PI (m/z 909 or 917) species after alkaline hydrolysis (6 M ammonium hydroxide, 37°C, 1 h), to remove the fatty acid at the sn-2 position of the glycerol, followed by purification on an octyl-Sepharose column.
CER, ceramide; AAG, alkylacylglycerol; MAG, monoalkylglycerol.
The trypomastigote GPI-mucins produced maximal NO, TNF–α and IL–12 synthesis at the lowest concentration used in this experiment (3.3 nM), and some reduction in the synthesis of TNF–α and IL–12 was observed at 80 and 400 nM trypomastigote GPI-mucin. In contrast, the epimastigote materials showed little or no bioactivity across the whole concentration range. The ceramide-containing GIPLs from Y, CL and Tulahuen strains were particularly inactive in all of the assays, as was the CL strain GPI-mucin containing an lyso-alkyl-PI moiety in the GPI anchor. Only the alkylacyl-PI-containing Y strain GPI-mucins and CL strain GIPLs showed significant activity: at 400 nM these materials produced ∼30% of the NO response induced by 1 nM trypomastigote GPI-mucin (Figure 4A).
To investigate whether the epimastigote fractions only appeared to lack activity due to the presence of inhibitory components, the induction of IL–12, TNF–α and NO by 1 nM trypomastigote GPI-mucin was also measured after pre-incubation with the purified epimastigote GPI-mucin and GIPL fractions (Figure 4B). No inhibition of IL–12, TNF–α or NO synthesis was observed with 3.3 nM epimastigote GPI-mucins and GIPLs. Although some inhibition was observed at epimastigote GPI-mucin and GIPL concentrations of 16 nM and above (rising to a maximum of 30% inhibition at 400 nM), it seems unlikely that the lack of activity in the epimastigote GPI-mucins and GIPLs is due to the presence of an inhibitory agent.
Discussion
The unsaturated fatty acid of the trypomastigote GPI alkylacyl-PI moiety is likely to be important for bioactivity since the trypomastigote peak I mucins, which appear to be identical to the peak II mucins except that they contain mainly lyso-alkyl-PI moieties (Table I), are relatively inactive. This is also consistent with the known alkali lability of the trypomastigote mucin bioactivity (Camargo et al., 1997a). The trypomastigote GPI PI moieties alone (isolated after deamination of the trypomastigote peak II mucin) were inactive at concentrations up to 25 nM, suggesting that other features of the intact GPI anchor are also necessary for bioactivity. Although it is possible that the differences in the carbohydrate structures of the epimastigote and trypomastigote GPIs are crucial for bioactivity, it is also conceivable that the precise structure of the GPI anchor glycan may not be critical and that it serves, in this context, to solubilize the unsaturated alkylacyl-PI moiety to make it available to its macrophage receptor. Despite considerable efforts, we were unable to confirm certain chemical (e.g. catalytic hydrogenation and mild acid hydrolysis) and enzymatic (e.g. α- and β–galactosidases) modifications to the trypomastigote GPI fraction by ES–MS. This was due to the small amounts of material available from trypomastigotes (Almeida et al., 1994), which are non-dividing cells that are cultured by the infection of mammalian cell monolayers (Andrews and Colli, 1982). In the absence of mass spectrometric confirmation of the predicted modifications to trypomastigote GPIs, the effects of these treatments on bioactivity are inadmissible. Therefore, in the long term, we plan to resolve the minimum structural features required for bioactivity by chemical synthesis.
An important feature of the data presented here is the extremely low concentration of the trypomastigote GPI anchors required for cytokine induction. Previous work using highly purified GPI material from T.brucei variant surface glycoproteins has shown cytokine induction at micromolar rather than sub-nanomolar concentrations (Magez et al., 1998). Similarly, Schofield and colleagues have estimated that their P.falciparum and T.brucei GPI anchor preparations are active in the 0.1–10 μM range (Tachado and Schofield, 1994; Schofield et al., 1996; Tachado et al., 1996). These low specific activities are similar to those observed here for the relatively inactive T.cruzi epimastigote GIPLs and epimastigote mucin GPI anchor structures, and one might question the physiological relevance of effects using micromolar concentrations of GPIs.
Dual requirements for the carbohydrate and lipid components have been proposed to account for cytokine induction by protozoal GPI anchors. Tachado et al. (1997) suggest a receptor for the GPI glycan and the translocation of the PI moiety to the cytoplasm to act as a second messenger, whereas Magez et al. (1998) suggest the involvement of two distinct receptors for the glycan and lipid components of T.brucei-derived GPI anchors. In contrast, we were unable to detect any cytokine or NO induction using the purified PI component of the trypomastigote GPI anchor, even when presented to macrophages at concentrations up to 25 nM (∼1000–fold higher than that required for the induction of TNF–α and IL–12 by the intact GPI). Therefore, we suggest that intact trypomastigote GPI anchors bind to some, as yet undefined, macrophage receptor that leads to transmembrane signalling and subsequent transcription of the genes encoding certain cytokines and inducible nitric oxide synthase. The mechanism by which trypomastigote mucins might reach a putative macrophage receptor in vivo is unknown. However, the passive shedding from, and re-incorporation into, cell plasma membranes by GPI-anchored proteins is a well known phenomenon, and mono-molecular, micellar and micro-vesicular transport have all been discussed in this context (reviewed in Ilangumaran et al., 1996; Medof et al., 1996).
In conclusion, we have established that highly purified GPI anchors isolated from GPI-anchored mucins from one life cycle stage (trypomastigote) of T.cruzi, and having unique structural features (Figure 5), are extremely potent inducers of NO, IL–12 and TNF–α when presented to macrophages. On a molar basis, this material is at least as active as bacterial lipopolysaccharide (LPS) (Camargo et al., 1997a) and Mycoplasma lipopeptides (Mühlradt et al., 1997, 1998), establishing it as one of the most potent microbial proinflammatory molecules known. Since the NO and cytokine induction experiments were performed with LPS-hyporesponsive (C3H/HeJ) macrophages, contamination of the T.cruzi GPIs with bacterial LPS can be ruled out as an explanation for their potency. Furthermore, contamination with Mycoplasma lipopeptide can be ruled out because: (i) MALDI-TOF-MS analysis of the GPI preparation did not reveal any peptides or lipopeptides under conditions where MALP–2 was clearly visible; (ii) Edman peptide sequencing of the GPI preparations did not reveal any Mycoplasma lipopeptide-related amino acid sequences; and (iii) N–acetylation of MALP–2 abolished its NO-inducing activity, as expected (Mühlradt et al., 1998), but had no significant effect on the NO-inducing activity of trypomastigote mucin (data not shown).
Finally, it is possible that T.cruzi trypomastigote GPI anchors may be crucial for the potentiation of innate cellular immunity, which controls the parasite population and drives T-cell development during the early stages of infection (Aliberti et al., 1996; Fearon and Locksley, 1996; Brener and Gazzinelli, 1997). Moreover, despite the controversy about the mechanism of immunopathogenesis of Chagas' disease (Brener and Gazzinelli, 1997), recent studies suggest that T.cruzi parasites are physically present at the sites of inflammation (Higuchi et al., 1993; Jones et al., 1993; Vago et al., 1996). Thus, the local release of trypomastigote GPI-anchored mucins could play an important role in parasite-elicited inflammation and the genesis of cardiac and/or oesophagus and/or colon pathology observed during chronic Chagas' disease.
Materials and methods
Animals
Male LPS-hyporesponsive C3H/HeJ mice (FIOCRUZ, Rio de Janeiro), 6–7 weeks old, were used as the source of inflammatory macrophages.
Mammalian cells and parasites
Green monkey kidney-derived LLC-MK2 cells (American Type Culture Collection, Rockville, MD) were maintained at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l–glutamine, 20 μg/ml gentamicin sulfate, 0.3% sodium bicarbonate and 5% CO2. Trypomastigote forms of T.cruzi (Y strain) were obtained from the supernatant of LLC–MK2 cells 6–8 days after primary infection with 10 trypomastigotes per cell (Andrews and Colli, 1982). Parasites were harvested daily by centrifugation (700 g, 15 min, 4°C), washed three times in ice-cold phosphate-buffered saline (PBS) pH 7.4 and kept at –70°C. Epimastigote forms of T.cruzi (Y strain) were grown at 28°C in cell-free liver infusion tryptose (LIT) medium supplemented with 10% heat-inactivated FCS (Camargo, 1964).
Purification of epimastigote and trypomastigote mucins
Trypanosoma cruzi mucin-like glycoproteins from trypomastigote and epimastigote forms (Y strain) were purified using a slight modification of previous procedures (Almeida et al., 1994; Serrano et al., 1995). In brief, parasite pellets containing in total 1.5 × 1010 trypomastigotes and 2.0 × 1010 epimastigotes were freeze-dried and sequentially delipidated with 10 vols of each of the following solvent mixtures: (i) chloroform: methanol (2:1, v/v); (ii) chloroform:methanol (1:1, v/v); (iii) chloroform: methanol (1:2, v/v); and (iv) chloroform:methanol:water (1:2:0.8, by vol.). Between each extraction, insoluble cell debris was separated from the organic phase by centrifugation (1500 g, 15 min, 10°C). The final delipidated parasite debris was dried under N2 and extracted three times with 10 vols of butan–1–ol-saturated water (9% butan–1–ol) (4 h, at room temperature). The resulting extracts were combined, dried under vacuum, dissolved in 5% propan–1–ol, 0.1 M ammonium acetate (buffer A), and applied onto an octyl-Sepharose (Amersham-Pharmacia Biotech, Uppsala, Sweden) column (1.0 × 10 cm) at a flow rate of 4 ml/h, at room temperature. After washing with 10 vols of buffer A and 10 vols of 5% propan–1–ol, the column was eluted using a propan–1–ol gradient (5–60%), at a flow rate of 12 ml/h. Eighty 1 ml fractions were collected. For detection of the trypomastigote mucin-containing fractions, 2–5 μl aliquots of each fraction were used for Western blotting and chemiluminescent-enzyme-linked immunosorbent assay (CL-ELISA) using anti-α–galactosyl (anti-Gal) antibodies purified from chronic Chagas' disease patients (Almeida et al., 1991, 1994). Epimastigote and trypomastigote mucins were also detected by silver staining of the SDS–polyacrylamide gel (Camargo et al., 1997a). GIPLs were identified by ES–MS analysis (see below) of 5 μl aliquots of each octyl-Sepharose-purified fraction. The alkylacylglycerol- and ceramide-containing GIPLs were enriched in different fractions of peak III, with the former eluting slightly earlier than the latter. Note: the appearance of substantial amounts of GIPLs in the 9% butan–1–ol fraction was a reproducible phenomenon that shows that the T.cruzi GIPLs (particularly the alkylacylglycerol-containing species) are not extracted quantitatively into chloroform: methanol:water (1:2:0.8, by vol.). This is different from the situation in other trypanosomatids, such as the Leishmania, and suggests that the T.cruzi GIPLs and mucins may co-extract as a complex.
Isolation of the epimastigote and trypomastigote mucin GPIs
Briefly, ∼1 and 3 nmol (with respect to myo-inositol content) of trypomastigote and epimastigote mucins, respectively, were dried in a SpeedVac, redissolved in 90 μl of 10 mM Tris–HCl pH 7.8, 1 mM CaCl2, and incubated with 10 μl of proteinase K (5 mg/ml; Promega, Madison, WI) for 16 h at 37°C. The incubation was terminated by heating at 100°C for 5 min. The sample was then extracted three times with 200 μl of water-saturated butan–1–ol (91% butan–1–ol). The released GPI moiety was recovered in the butanolic phases, which were combined and washed further (three times) with water to remove any residual glycopeptides and/or salts. The sample was purified further on an octyl-Sepharose column as described above. Five microlitres of each 1 ml fraction were used for myo-inositol and ES–MS analyses.
Myo-inositol and monosaccharide analyses
Native mucins and isolated GPIs were quantified by their myo-inositol content using selective ion monitoring at m/z 305, 307, 318 and 321 of trimethylsilyl derivatives (Ferguson, 1993). Monosaccharides were quantified as methyl-glycoside-trimethylsilyl derivatives as decribed in Ferguson (1993), except that selected ion-monitoring GC–MS (for m/z 173, 204, 217, 272, 298, 305 and 318) was used.
Mass spectrometry of GPI fractions
Trypomastigote and epimastigote GPI samples (2.5 and 4.9 pmol/μl, respectively) were introduced into the Micromass Quattro electrospray source (Micromass, Manchester, UK) at 5 μl/min in 50% propan–1–ol, 0.2% formic acid. Negative ion spectra were collected. The capillary voltage was 2.3 kV, the cone voltage was 40 V and cone/skimmer offset was 5 V.
Positive-ion MALDI-TOF spectra of GPIs and synthetic MALP–2 lipopeptide were recorded in linear mode using a PerSeptive Biosystems Voyager DE-STR spectrometer and α–cyano–4–hydroxycinnamic acid as the matrix.
Preparation and analysis of PI components
Epimastigote and trypomastigote mucins (peaks I and II; 4.8 and 1.8 nmol with respect to myo-inositol content, respectively) were dissolved in 200 μl of water and extracted three times with 200 μl of butan–1–ol saturated with water to remove any contaminating lipids. The aqueous phases were dried and redissolved in 40 μl of 0.1 M sodium acetate buffer pH 4.0, and deaminated by three additions (at 1 h intervals) of 10 μl of 0.5 M sodium nitrite, at 60°C. The samples were mixed with 250 μl of 9% butan–1–ol and the released PIs were recovered by three extractions with 250 μl of butan–1–ol saturated with water. The combined butanolic phases were dried, redissolved in 200 μl of chloroform:methanol:5 mM ammonium acetate (10:10:3, by vol.) and introduced into the electrospray source at 5 μl/min. Negative-ion spectra were collected. The capillary voltage was 2.5 kV, the cone voltage was 60 V and cone/skimmer offset was 5 V. Collision-induced dissociation (CID) experiments were performed using 2.5 × 10–3 torr argon and an accelerating voltage of 50 V. Epimastigote GIPLs (peak III; 5.6 nmol myo–inositol) were also submitted (without pre-extraction with butan–1–ol) to nitrous deamination and PI analysis as described above.
N-acetylation
Samples were N-acetylated at 0°C in 40 μl of 1 M ammonia by two additions of 1 μl of acetic anhydride, 10 min apart. After 30 min at room temperature, 160 μl of water were added and the samples were desalted by freeze–drying twice.
Macrophage culture
C3H/HeJ mice were inoculated intraperitoneally with 2 ml of 3% thioglycollate. Four days later, the elicited peritoneal exudate cells were harvested in cold FCS-free DMEM (Life Technologies, Grand Island, NY) and centrifuged at 700 g for 10 min at 4°C. Cells were resuspended in DMEM supplemented with 5% heat-inactivated FCS, 2 mM l–glutamine and 40 μg/ml gentamicin sulfate (MacMed), at a final concentration of 2 × 106/ml, and 0.1 ml aliquots were transferred into wells of a 96–well plate. Cells were allowed to adhere at 37°C and 5% CO2 for 3 h, and were then washed once with serum-free DMEM. One hundred microlitres of MacMed were added to the macrophage cultures in a final volume of 200 μl/well. Purified parasite preparations were incubated with macrophages in the presence or absence of IFN–γ (100 U/ml; Genzyme Corp., Cambridge, MA). Aliquots of the supernatant (50 and 100 μl) were collected after 24 and 48 h of culture for TNF–α and IL–10 as well as IL–12(p40) measurements, respectively (Camargo et al., 1997a,b).
Cytokine and nitric oxide measurements
All samples were analysed in duplicate. TNF–α was quantified employing an ELISA kit (Genzyme, Duoset kit). IL–12(p40) was assayed by sandwich ELISA, as described previously (Camargo et al., 1997a). C17.1.5 (1510) rat monoclonal antibody (mAb) (5 μg/ml) and biotinylated C 15.6 (676) rat mAb (Genzyme) (1:750 dilution) were used for capture and detection of bound IL–12, respectively. Recombinant murine IL–12 was used as standard. The nitrite concentration in the culture supernatants was assayed in a 98–well microplate by mixing 0.1 ml of culture supernatant with 0.1 ml of Griess reagent. The absorbance at 540 nm was read 10 min later and the NO2 concentration was determined by reference to a standard curve of 1–100 mM NaNO2 (Camargo et al., 1997b).
Acknowledgments
Acknowledgements
We are grateful to Peter F.Mühlradt for kindly providing MALP–2 synthetic lipopeptide, and Nick Morrice for peptide sequencing. We thank Terry K.Smith and Alvaro Acosta–Serrano for helpful discussions, and S.J.Tadeu for support. This work was supported by grants from The Wellcome Trust (054491), WHO–TDR (Id No. 970506), FAPESP (98/10495-5) and CNPq. I.C.A. was a recipient of a FAPESP fellowship (No. 96/04260-0).
References
- Abbas A.K., Murphy, K.M. and Sher, A. (1996) Functional diversity of helper T lymphocytes. Nature, 383, 787–793. [DOI] [PubMed] [Google Scholar]
- Aliberti J.C.S., Cardoso, M.A., Martins, G.A., Gazzinelli, R.T., Vieira, L.Q. and Silva, J.S. (1996) Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect. Immun., 64, 1961–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida I.C., Milani, S.R., Gorin, P.A. and Travassos, L.R. (1991) Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-α-galactosyl antibodies. J. Immunol., 146, 2394–2400. [PubMed] [Google Scholar]
- Almeida I.C., Ferguson, M.A.J., Schenkman, S. and Travassos, L.R. (1994) Lytic anti-α-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J., 304, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M.J.M. and Colli, W. (1975) Glycoproteins from Trypanosoma cruzi: partial purification by gel chromatography. FEBS Lett., 52, 188–198. [DOI] [PubMed] [Google Scholar]
- Andrews N. and Colli, W. (1982) Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J. Protozool., 29, 264–269. [DOI] [PubMed] [Google Scholar]
- Biron C.A. and Gazzinelli, R.T. (1995) Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr. Opin. Immunol., 7, 485–496. [DOI] [PubMed] [Google Scholar]
- Brener Z. and Gazzinelli, R.T. (1997) Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol., 114, 103–110. [DOI] [PubMed] [Google Scholar]
- Camargo E.P. (1964) Growth and differentiation in Trypanosoma cruzi. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. S. Paulo, 6, 93–100. [PubMed] [Google Scholar]
- Camargo M.M., Almeida, I.C., Pereira, M.E., Ferguson, M.A.J., Travassos, L.R. and Gazzinelli, R.T. (1997a) Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J. Immunol., 158, 5890–5901. [PubMed] [Google Scholar]
- Camargo M.M., Andrade, A.C., Almeida, I.C., Travassos, L.R. and Gazzinelli, R.T. (1997b) Glycoconjugates isolated from Trypanosoma cruzi but not from Leishmania species membranes trigger nitric oxide synthesis as well as microbicidal activity in IFN-γ-primed macrophages. J. Immunol., 159, 6131–6139. [PubMed] [Google Scholar]
- Carreira J.C., Jones, C., Wait, R., Previato, J.O. and Mendonça-Previato, L. (1996) Structural variation in the glycoinositolphospholipids of different strains of Trypanosoma cruzi. Glycoconj. J., 13, 955–966. [DOI] [PubMed] [Google Scholar]
- Couto A.S., de Lederkremer, R.M., Colli, W. and Alves, M.J. (1993) The glycosylphosphatidylinositol anchor of the trypomastigote-specific Tc–85 glycoprotein from Trypanosoma cruzi. Metabolic-labeling and structural studies. Eur. J. Biochem., 217, 597–602. [DOI] [PubMed] [Google Scholar]
- De Lederkremer R.M., Alves, M.J., Fonseca, G.C. and Colli, W. (1976) A lipopeptidophosphoglycan from Trypanosoma cruzi (epimastigota). Isolation, purification and carbohydrate composition. Biochim. Biophys. Acta, 444, 85–96. [DOI] [PubMed] [Google Scholar]
- De Lederkremer R.M., Lima, C., Ramirez, M.I., Ferguson, M.A.J., Homans, S.W., Thomas-Oates, J. (1991) Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi epimastigotes. J. Biol. Chem., 266, 23670–23675. [PubMed] [Google Scholar]
- De Lederkremer R.M., Lima, C.E., Ramirez, M.I., Gonçalvez, M.F. and Colli, W. (1993) Hexadecylpalmitoylglycerol or ceramide is linked to similar glycophosphoinositol anchor-like structures in Trypanosoma cruzi. Eur. J. Biochem., 218, 929–936. [DOI] [PubMed] [Google Scholar]
- Di Noia J.M., D'Orso, I., Aslund, L., Sanchez, D.O. and Frasch, A.C.C. (1998) The Trypanosoma cruzi mucin family is transcribed from hundreds of genes having hypervariable regions. J. Biol. Chem., 273, 10843–10850. [DOI] [PubMed] [Google Scholar]
- Fearon D.T. and Locksley, R.M. (1996) The instructive role of innate immunity in the acquired immune response. Science, 272, 50–54. [DOI] [PubMed] [Google Scholar]
- Ferguson M.A.J. (1993) GPI-membrane anchors: isolation and analysis. In Fukuda,M. and Kobata,A. (eds), Glycobiology: A Practical Approach. Oxford University Press, New York, NY, pp. 349–383. [Google Scholar]
- Ferguson M.A.J., Homans, S.W., Dwek, R.A. and Rademacher, T.W. (1988) Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science, 239, 753–759. [DOI] [PubMed] [Google Scholar]
- Freitas-Junior L.H.G., Briones, M.R.S. and Shenkman, S. (1998) Two distincts groups of mucin-like genes are differentially expressed in the developmental stages of Trypanosoma cruzi. Mol. Biochem. Parasitol., 93, 101–114. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R.T., Hieny, S., Wynn, T.A., Wolf, S. and Sher, A. (1993) Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl Acad. Sci. USA, 90, 6115–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgher D.B., Colli, W., Souto-Padron, T. and Zingales, B. (1993) Galactofuranose-containing glycoconjugates of epimastigote and trypomastigote forms of Trypanosoma cruzi. Mol. Biochem. Parasitol., 60, 249–264. [DOI] [PubMed] [Google Scholar]
- Güther M.L., Cardoso de Almeida, M.L., Yoshida, N. and Ferguson, M.A.J. (1992) Structural studies on the glycosylphosphatidylinositol membrane anchor of Trypanosoma cruzi 1G7-antigen. The structure of the glycan core. J. Biol. Chem., 267, 6820–6828. [PubMed] [Google Scholar]
- Higuchi M.L., Gutierrez, P.S., Aiello, V.D., Palomino, S., Bocchi, E., Kalil, J., Bellotti, G. and Pileggi, F. (1993) Immunohistochemical characterization of infiltrating cells in human chronic chagasic myocarditis: comparison with myocardial rejection process. Virchows Arch. A. Pathol. Anat. Histopathol., 423, 157–160. [DOI] [PubMed] [Google Scholar]
- Hsieh C.S., Macatonia, S.E., Tripp, C.S., Wolf, S., O'Garra, A. and Murphy, K.M. (1993) Development of Th1 CD4+ T cells through IL–12 produced by Listeria-induced macrophages. Science, 260, 547–549. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S., Robinson, P.J. and Daniel, C.H. (1996) Transfer of exogenous glycosylphosphatidylinositol (GPI)-linked molecules to plasma membranes. Trends Cell Sci., 6, 163–167. [DOI] [PubMed] [Google Scholar]
- Jones E.M., Colley, D.G., Tostes, S., Lopes, E.R., Vnencak-Jones, E.M. and McCurley, T.L. (1993) Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am. J. Trop. Med. Hyg., 48, 348–357. [DOI] [PubMed] [Google Scholar]
- Magez S., Stijlemans, B., Radwanska, M., Pays, E., Ferguson, M.A.J. and De Baetselier, P. (1998) The glycosyl-inositol-phosphate and dimyristoylglycerol moieties of the glycosylphosphatidylinositol anchor of the trypanosome variant-specific surface glycoprotein are distinct macrophage-activating factors. J. Immunol., 160, 1949–1956. [PubMed] [Google Scholar]
- Medof M.E., Nagarajan, S. and Tykocinski, M.L. (1996) Cell-surface engineering with GPI-anchored proteins. FASEB J., 10, 574–586. [DOI] [PubMed] [Google Scholar]
- Mendonça-Previato L., Gorin, P.A., Braga, A.F., Scharfstein, J. and Previato, J.O. (1983) Chemical structure and antigenic aspects of complexes obtained from epimastigotes of Trypanosoma cruzi. Biochemistry, 22, 4980–4987. [DOI] [PubMed] [Google Scholar]
- Mühlradt P.F., Kiess, M., Meyer, H., Sussmuth, R. and Jung, G. (1997) Isolation, structure elucidation and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma acting at picomolar concentration. J. Exp. Med., 185, 1951–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P.F., Kiess, M., Meyer, H., Sussmuth, R. and Gunther, J. (1998) Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect. Immun., 66, 4804–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previato J.O., Andrade, A.F., Pessolani, M.C. and Mendonça-Previato, L. (1985) Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol. Biochem. Parasitol., 16, 85–96. [DOI] [PubMed] [Google Scholar]
- Previato J.O., Gorin, P.A., Mazurek, M., Xavier, M.T., Fournet, B., Wieruszesk, J.M. and Mendonça-Previato, L. (1990) Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J. Biol. Chem., 265, 2518–2526. [PubMed] [Google Scholar]
- Previato J.O., Jones, C., Xavier, M.T., Wait, R., Travassos, L.R., Parodi, A.J. and Mendonça-Previato, L. (1995) Structural characterization of the major glycosylphosphatidylinositol membrane-anchored glycoprotein from epimastigote forms of Trypanosoma cruzi Y-strain. J. Biol. Chem., 270, 7241–7250. [DOI] [PubMed] [Google Scholar]
- Salazar N.A., Mondragon, A. and Kelly, J.M. (1996) Mucin-like glycoprotein genes are closely linked to members of the trans-sialidase super-family at multiple sites in the Trypanosoma cruzi genome. Mol. Biochem. Parasitol., 78, 127–136. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Ferguson, M.A.J., Heise, N., Cardoso de Almeida, M.L., Mortara, R.A. and Yoshida, N. (1993) Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol. Biochem. Parasitol., 59, 293–303. [DOI] [PubMed] [Google Scholar]
- Schofield L. and Hackett, F. (1993) Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J. Exp. Med., 177, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Novakovic, S., Gerold, P., Schwarz, R.T., McConville, M.J. and Tachado, S.D. (1996) Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J. Immunol., 156, 1886–1896. [PubMed] [Google Scholar]
- Seder R.A., Gazzinelli, R.T., Sher, A. and Paul, W.E. (1993) Interleukin 12 acts directly on CD4+ cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc. Natl Acad. Sci. USA, 90, 10188–10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A.A., Schenkman, S., Yoshida, N., Mehlert, A., Richardson, J.M. and Ferguson, M.A.J. (1995) The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J. Biol. Chem., 270, 27244–27253. [DOI] [PubMed] [Google Scholar]
- Silva J.S., Vespa, G.N., Cardoso, M.A., Aliberti, J.C. and Cunha, F.Q. (1995) Tumor necrosis factor α mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected γ interferon-activated macrophages. Infect. Immun., 63, 4862–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachado S.D. and Schofield, L. (1994) Glycosylphosphatidylinositol toxin of Trypanosoma brucei regulates IL-1 α and TNF-α expression in macrophages by protein tyrosine kinase-mediated signal transduction. Biochem. Biophys. Res. Commun., 205, 984–991. [DOI] [PubMed] [Google Scholar]
- Tachado S.D., Gerold, P., McConville, M.J., Baldwin, T., Quilici, D., Schwarz, R.T. and Schofield, L. (1996) Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J. Immunol., 156, 1897–1907. [PubMed] [Google Scholar]
- Tachado S.D., Gerold, P., Schwarz, R., Novakovic, S., McConville, M. and Schofield, L. (1997) Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. Proc. Natl Acad. Sci. USA, 94, 4022–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. and Scott, P. (1995) Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res. Immunol., 146, 423–431. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. and Kodukula, K. (1995) How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu. Rev. Biochem., 64, 563–591. [DOI] [PubMed] [Google Scholar]
- Vago A.R., Macedo, A.M., Adad, S.J., Reis, D.D. and Correa-Oliveira, R. (1996) PCR detection of Trypanosoma cruzi DNA in oesophageal tissues of patients with chronic digestive Chagas' disease. Lancet, 348, 891–892. [DOI] [PubMed] [Google Scholar]
- Vespa G.N., Cunha, F.Q. and Silva, J.S. (1994) Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect. Immun., 62, 5177–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]