Abstract
Using a molecular genetic approach, we try to confirm the molecular alterations of inverted papilloma and clarify its status as a putative precursor lesion of sinonasal squamous cell carcinoma. To better understand its genetics, we investigated the immunohistochemical protein expression patterns of cell-cycle-regulators p53, p63, p21, p27 and proliferation marker Ki-67 in 22 inverted papilloma and 9 squamous cell carcinoma of the sinonasal tract. Significantly elevated levels of p53 and p63 in squamous cell carcinoma of sinonasal tract compared with inverted papilloma were revealed. Ki-67-stained neoplastic cell nuclei were found in a significantly higher percentage of squamous cell carcinoma of sinonasal tract than in inverted papilloma, whereas no variation of p21 and p27 expression was identified. This work first examined the immunohistochemical overexpression of p63 in sinonasal inverted papilloma and squamous cell carcinoma. In conclusion, this is a first study shedding light on the expression of p63 in tumors of paranasal sinuses.
Keywords: Inverted papilloma, p63, p53, Sinonasal squamous cell carcinoma, p21, Cell cycle regulators, MIB-1
Introduction
Inverted papilloma (IP) is a benign epithelial neoplasm that arises from the outlining Schnederian respiratory membrane. It is a rare sinonasal tumor comprising only 0.5–4% of all nasal tumors [1]. Although this tumor is benign in nature, it exhibits the characteristics of local invasiveness, recurrence, and malignant transformation [2–5].The incidence of malignant change in individual series of inverted papillomas ranges from 2 to 27% [6]. Recently, it was reported that there is a clear relationship between paranasal IP and squamous cell carcinoma (SCC), and that nearly 10% of inverted papillomas are associated with SCC [7]. Malignant transformation within an IP may take on the histologic appearance of all grades of SCC [7]. Clinical relevance and possible association of IP with sinonasal carcinoma, if IP is considered to be a premalignant lesion, remain a matter of debate.
A complex network of various intracellular proteins regulates cell cycle and apoptosis. Cyclin and cyclin-dependent kinase (CDK) complexes induce a transition between different cell cycle phases, whereas Cdk inhibitors, such as p21 and p27, restrain the function of these complexes and arrest the cell cycle [8]. Its gene product (p21Waf1/Cip1 protein) acts as an inhibitor of G1 CDKs and regulates entry of the cells into S phase. Cells lacking functional p53 express only low levels of p21Waf1/Cip1. The p21Waf1/Cip1 promoter contains a p53-binding site, suggesting that expression of p21Waf1/Cip1 depends on p53 function [9, 10]. However, it has been shown that expression of p21Waf1/Cip1 is also induced by p53-independent pathways [11]. p27Kip1 is another CDKI that shares partial homology with p21Waf1/Cip1 and binds to CDK complexes. The p27Kip1 protein combines with cyclin E-CDK2, cyclin A-CDK2, and cyclin D1-CDK4 complexes, and prevents their activation or inhibits previously activated complexes [12]. There is considerable evidence that p27Kip1 plays a crucial role in several cellular processes, including proliferation and apoptosis [13].
The proliferation marker Ki67 antigen, detected with monoclonal antibody MIB-1, is expressed in all phases of the cell cycle except G0 [14]. Ki-67 has been utilized to demonstrate proliferating cells in G1, S, G2 and M phases of the cell cycle [15]. Ki-67 has been used to study the growth fraction and cytokinetic activity in various carcinomas [15]. It was found that a high Ki-67 score correlates with histological tumour grade and early recurrence in carcinoma.
The immunohistochemical protein expression patterns of cell-cycle-regulators p53, p21, p27 and proliferation marker Ki-67 have been studied in IP and sinonasal carcinoma, which gave clues to this progression [16–19].
p63, which is becoming increasingly recognized as an important player in human tumorigenesis, is a new member of the p53 tumor suppressor family. Like p53 and p63 codes for proteins with an amino(N)-terminal transcription-activating region, a middle DNA-binding region, and a carboxyl terminal region are responsible for oligomerization [20]. p63 helps regulate differentiation and proliferation in epithelial progenitor cells. Its expression is often higher in malignant tissue compared with normal tissue, and poorly differentiated carcinomas often show a larger number of p63-positive cells than well-differentiated tumors [21].
Very few reports have investigated the role of cell cycle regulators as biomarkers in IP and SCC. To date the role of p63 protein has not been studied in IP and SCC. The aim of this study is to investigate what role cell-cycle-regulators p53, p21, p27, p63, and proliferation marker Ki-67 have in predicting malignancy.
Materials and Methods
In a retrospective study conducted from January 2000–June 2006, 22 cases of IP and 9 cases of SCC were collected and reviewed from the files of the Department of Pathology of the Izmir Training and Research Hospital.
Tissue fixed in 10% formalin and embedded in paraffin was available in all cases. Immunohistochemical studies were performed using the avidin–biotin-peroxidase complex method. Mouse monoclonal antibodies (mAb), anti-p21 (Neomarkers, MS 387-P, 200 mg/L), anti-p27 (Neomarkers, MS 256-P, 200 mg/L), anti-p63 (Neomarkers, MS 1081-P, 200 mg/L), anti-p53 (Neomarkers, RM 9105-S), and Ki-67 (Neomarkers RM 9106-S) were used for immunohistochemistry through the streptavidin–biotin peroxidase method. The tissue sections were deparaffinized in xylene, rehydrated in an alcohol series, and immersed in distilled water. Endogen peroxidase activity was blocked using a 0.3% solution of hydrogen peroxidase in phosphate-buffered saline at room temperature for 10 min and rinsed with Tris buffer. The sections were then boiled in citrate buffer solution (10 mmol/L; pH, 6.0) in a microwave oven for 15 min for epitope retrieval in staining with p53, p21, and p27. Primary antibodies were applied for 60 min at room temperature and were washed in Tris buffer. The linking antibody and streptavidin peroxidase complex (DAKO LSAB Kit, K-0675; Carpintera, CA) were added consecutively for 15 min at room temperature and washed in Tris buffer. The sections were stained for 15 min with 0.05% 3′3-diaminobenzidine tetrahydrochloride (DAB), freshly prepared in 0.05 M tris (hydroxymethyl)-aminomethane (Tris) buffer at pH 7.6, containing 0.024% H2O2 and washed twice in The Tris buffer. The sections were then washed in deionized water, counterstained with Mayer’s hematoxylin, and mounted. For each antibody, sections were stained as positive controls (tonsilla palatina for Ki-67, SCC for p21, and colorectal adenocarcinoma for p27, p21, and p53), while the primary antibody was replaced by The Tris buffer in the case of negative controls. The immunostained nuclei were quantified in each case. All counting was performed under a standard light microscope in 1000× field to evaluate positive nuclei/total number of cells. Ten fields or at least 500 cells were counted on each section. Tumor sections were considered negative if staining was absent, present in <10% of tumor cells. A score of 1+ was given when 10–30% of the cells were positive to the reaction. A score of 2+ was given when 30–50% of the cells were positive to the reaction. A score of 3+ was given when >50% of the cells were positive to the reaction, respectively (Tables 1, 2).
Table 1.
Relationship between immunohistochemical parameters and patients with inverted papilloma
| Case No | Sex | Age | p63 (%) | p21 (%) | p27 (%) | Ki-67 (%) | p53 (%) |
|---|---|---|---|---|---|---|---|
| 1 | E | 37 | 12 | 70 | 0 | 14 | 0 |
| 2 | E | 73 | 80 | 40 | 0 | 5 | 0 |
| 3 | K | 61 | 10 | 0 | 0 | 0 | 0 |
| 4 | E | 37 | 20 | 40 | 0 | 0 | 0 |
| 5 | K | 66 | 15 | 30 | 5 | 17 | 6 |
| 6 | E | 60 | 50 | 20 | 15 | 25 | 3 |
| 7 | E | 70 | 12 | 4 | 0 | 8 | 5 |
| 8 | E | 47 | 40 | 35 | 1 | 8 | 1 |
| 9 | E | 58 | 35 | 15 | 12 | 14 | 0 |
| 10 | E | 42 | 25 | 70 | 0 | 15 | 0 |
| 11 | E | 40 | 12 | 12 | 15 | 8 | 5 |
| 12 | E | 42 | 40 | 25 | 20 | 13 | 0 |
| 13 | E | 67 | 55 | 65 | 15 | 8 | 0 |
| 14 | E | 59 | 45 | 55 | 25 | 12 | 0 |
| 15 | E | 44 | 65 | 85 | 40 | 12 | 0 |
| 16 | E | 59 | 20 | 75 | 12 | 5 | 0 |
| 17 | K | 49 | 70 | 8 | 0 | 0 | 0 |
| 18 | E | 56 | 12 | 35 | 0 | 2 | 0 |
| 19 | E | 59 | 40 | 60 | 2 | 15 | 12 |
| 20 | E | 50 | 8 | 50 | 0 | 25 | 0 |
| 21 | E | 57 | 65 | 70 | 2 | 17 | 0 |
| 22 | E | 54 | 11 | 15 | 4 | 14 | 0 |
Table 2.
Relationship between immunohistochemical parameters and patients with squamous cell carcinoma
| Case No | Sex | Age | p63 (%) | p 21 (%) | p27 (%) | Ki-67 (%) | p53 (%) |
|---|---|---|---|---|---|---|---|
| 1 | E | 65 | 60 | 30 | 0 | 30 | 80 |
| 2 | E | 69 | 55 | 80 | 0 | 65 | 60 |
| 3 | K | 35 | 80 | 80 | 0 | 1 | 0 |
| 4 | E | 48 | 65 | 0 | 0 | 30 | 0 |
| 5 | K | 63 | 15 | 12 | 0 | 25 | 0 |
| 6 | E | 73 | 52 | 35 | 0 | 12 | 0 |
| 7 | E | 67 | 0 | 20 | 20 | 25 | 2 |
| 8 | E | 54 | 55 | 90 | 0 | 55 | 0 |
| 9 | E | 59 | 90 | 70 | 12 | 75 | 85 |
Statistical significance was analyzed using the χ2 test or the Mann–Whitney U nonparametric test. Results were considered statistically significant when the P value was <0.05.
Results
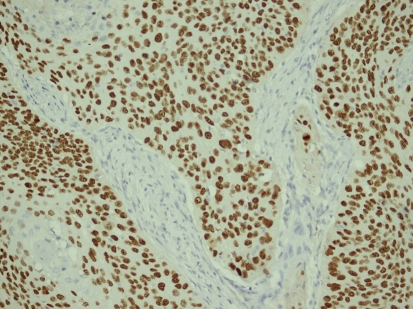
The stains revealed significantly elevated levels of p53 and p63 in The SCC of sinonasal tract compared with IP. In the statistical analysis using the χ2 test, p63 positivity (≥50%) was significantly higher in the SCC group (77.8%) when compared to the IP group (22.7%) (P = 0.019 < 0.05) (Fig. 1) (Table 3).
Fig. 1.
Diffuse and high positivity for p63 in squamous cell carcinoma (Peroxidase stain; orginal magnification ×220)
Table 3.
Expression of p63 in inverted papilloma and squamous cell carcinoma
| p63 | Inverted papillom | Squamous cell carcinoma | P | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| − | 1 | 4.5 | 1 | 1.1 | 0.019* |
| + | 10 | 45.5 | 1 | 11.1 | |
| ++ | 6 | 27.3 | 0 | 0 | |
| +++ | 5 | 22.7 | 7 | 77.8 | |
| 22 | 100 | 9 | 100 | ||
* p < 0.005 Statistically significant
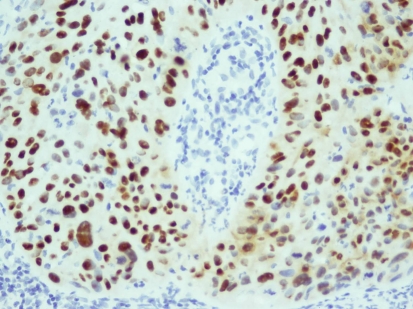
p53 positivity (+++) was absent in the IP group, whereas 33.3% of The SCC group showed p53 positivity; this was found to be statistically significant (P = 0.015 < 0.05) (Fig. 2) (Table 4).
Fig. 2.
Squamous cell carcinoma with nuclear positivity for p53 protein in most of its cells (Peroxidase stain; original magnification ×220)
Table 4.
Expression of p53 in inverted papilloma and squamous cell carcinoma
| p53 | Inverted papilloma | Squamous cell carcinoma | P | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| − | 21 | 95.5 | 6 | 66.7 | 0.015* |
| + | 1 | 4.5 | 0 | 0 | |
| ++ | 0 | 0 | 0 | 0 | |
| +++ | 0 | 0 | 3 | 33.3 | |
| 22 | 100 | 9 | 100 | ||
* p < 0.005 Statistically significant
There was a marked decrease in the expression of p27Kip1 in the SCC group compared to IP group, but this was not statistically significant.
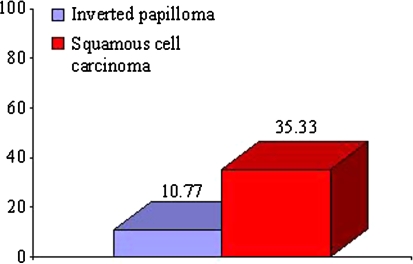
The mean immunohistochemical staining percentage of Ki-67 was significantly higher in the SCC group compared to The IP group with Mann–Whitney test (P < 0.05) (Fig. 3). In the statistical analysis using the χ2 test, Ki-67 proliferation index was significantly higher in the SCC group when compared to the IP group (P = 0.009 < 0.05).
Fig. 3.
Percentage of Ki-67 expression
Statistical analysis between the IP and SCC in terms of p21 expression revealed that there was no significant difference between the groups.
Discussion
Inverted papillomas are rare lesions that have the propensity for local aggresiveness, recurrence, and malignant transformation. Establishment of IP as a putative precursor lesion of sinonasal SCC has allowed analyses of the molecular changes associated with stepwise progression of SCC carcinogenesis. Recent studies demonstrated crucial dysregulations in the cell cycle along with dysplastic and neoplastic transformation in IP [22, 23]. Furthermore, genetic instabilty or some alterations, which induce p53 mutation in IP may be associated with carcinogenesis of sinonasal SCC [22, 23]. To resolve the mechanism by which sinonasal SCC develops from IP, which is a multistep process that involves activation of oncogenes and inactivation of tumor suppressor genes, we evaluated p53, p63, p21, and p27 mutations in sinonasal IP and SCC.
p63 is a close relative of the tumor supressor gene p53, but overexpression of p63 in many SCCs suggests that it could act as an oncogene. Alterations of p63 have been reported in human cancers including digestive tract, head and neck, cervix, lung, and also in urothelial carcinomas, some thymomas and non-Hodgkin lymphomas [24], but to this date the role of this protein has never been studied in paranasal tumors.
Tonon et al. [25] found that genomic locus of p63 is consistently amplified in squamous cell carcinoma, suggesting that upregulation of p63 contributes to tumorigenesis.
Guo et al. [26] evaluated the immunohistochemical expression of p63 in nasopharyngeal carcinoma, nasopharyngeal inflammation, and noncancerous nasaopharyngeal tissue and found that all 202 undifferentiated nasopharyngeal carcinomas showed strong positive staining, but 29 cases with nasopharyngeal inflammation and 17 with noncancerous epithelial tissue were negatively stained. They suggested that p63 might be used as an adjunct diagnostic marker of nasopharyngeal carcinoma.
p63 immunostaining data shows a progressive increase of IP throughout the depth of the epithelium moving from metaplasia to severe dysplasia which relates to the pathology. These findings confirm the data previously reported by Sniezek et al. [27] and Pelosi et al. [28] in a small series of head and neck, lung tumors.
In the study of Sniezek et al. [29] p63 is overexpressed in head and neck SCC when compared with normal tissue control specimens. They indicated that p63 plays an undifferentiating and antiapoptotic role in the mucosal epithelium of the head and neck region, possibly leading to tumor formation.
Massion et al. [30] found a significant increase in the p63 gene copy number in preinvasive lesions graded as severe dysplasia or higher. Their data demonstrate that there is early and frequent genomic amplification of p63 in the development of squamous carcinoma of the lung and that patients with non-small cell lung carcinoma showing amplification and overexpression of p63 have a prolonged survival. They suggested that p63 genomic amplification has an early role in lung tumorigenesis and deserves additional evaluation as a biomarker for lung cancer progression. In our study, p63 expression showed a significant difference between IP and paranasal SCC.
Immunostaining for the p53 protein is generally accepted as a marker of malignant transformation, and p53 overexpression has been found to be associated with malignant potential in premalignant lesions of the head and neck. Gujrathi et al. [31] found that four of the five cases of malignancy associated with IP demonstrated overexpression of p53 and none of the benign cases of IP demonstrated overexpression. They indicated that overexpression of p53 may serve as a marker for malignant transformation of IP.
In Katori et al’s [32] study, significantly increased staining of p53 was observed in IP with severe dysplasia, IP with carcinoma, and invasive carcinoma compared with control nasal mucosa. They suggested that testing for p53 may help to screen out papilloma lesions with a potential for dysplasia or carcinoma. In the present study, p53 expressions were significantly different between IP and paranasal SCC.
Increased proliferative activity of tumor cells is also associated with malignancy and is an important prognostic marker in many human tumors. Increasing cell proliferation seems to be a very important factor in the development of IP. Recent data suggested of the biologic significance of Ki-67 as a proliferative index in paranasal tumors. Katori et al. [32] investigated the Ki-67 index in IP and invasive SCC and found significantly higher Ki-67 in IP with severe dysplasia, IP with carcinoma and SCC compared with IP with mild and moderate dysplasia. Ikegawa et al. evaluated Ki-67 reactivity and p53 protein expression in paranasal tumors including nasal polyps (NP), IP, IPs accompanied with SCC or dysplastic lesions (DL). The authors [33] showed that the p53 scores of SCC areas and Ki-67 index were significantly larger than those of IPs without SCC/DL or those of NPs. This study clearly demonstrated that Ki-67 proliferative index values were significantly higher in the paranasal SCC compared with the values in IPs.
The p53, p21, and p27 tumor suppressor genes act by modulating cell proliferation via control of the G1 arrest checkpoint of the cell cycle. The p21 and p27 genes produce proteins that are activated by p53 and induce cell-cycle arrest by inhibition of kinase activity of cyclin/cyclin-dependent kinase complexes which regulate cell-cycle progression [34]. The clinical roles of p21 and p27 expression in the biological behaviour of human malignancies, especially head and neck SCC, are still controversial. Furthermore, there is little information regarding the expression of these regulatory molecules in IP and paranasal SCC. Katori et al. [32] indicated that significantly increased staining of p21 and p53 was observed in IP with severe dysplasia, IP with carcinoma and invasive carcinoma compared with control nasal mucosa. They suggested that testing for p21 and p53 may help to screen out papilloma lesions with a potential for dysplasia or carcinoma. Keleş et al. [18] found that p21 expression was not associated with dysplasia and the degree of dysplasia in IP in their study. There was no significant correlation with tumor differentiation in spite of increased p21 expression in paranasal sinus SCCs that Saegusa et al. [35] observed. Similarly, we found no significant difference in the level of p21 expression between IPs and paranasal SCCs.
p27 is a negative regulator of the cell cycle and putative tumor suppressor gene. Loss of p27 expression was reported to be correlated with the high degree of malignancy in many human cancers. Choi et al. [36] evaluated p27 and p21 protein expression by immunohistochemistry in non-dysplastic squamous epithelium, premalignant lesions and oral squamous carcinomas. Their study indicated that down-regulation of p27 and up-regulation of p21 were associated with early progression of head and neck SCC. Saegusa et al. demonstrated that the average p27 scores decreased from normal to malignant lesions. They suggested that p27 expression may be a useful marker for the disregulation of cell kinetics in these tumors. However, in contrast to this study, we found no significant differences between IPs and sinonasal SCCs in terms of p27 expression.
In conclusion, this is a first study shedding light on the expression of p63 in tumors of paranasal sinuses. Furthermore, it is the first description of p63 in these tumor types.
References
- 1.Lampertico P, Russel WO, Maccomb WS. Squamous papilloma of upper respiratory epithelium. Arch Pathol. 1963;75:293–302. [PubMed] [Google Scholar]
- 2.Batsakis JG. Tumours of the head and neck. 2. Baltimore: Williams &Wilkins; 1979. [Google Scholar]
- 3.Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206. doi: 10.1177/000348947108000205. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald C, Franzmann M-B, Tos M. Sinonasal papillomas: a report of 82 cases in Copenhagen County, including a longitudinal epidemiological and clinical study. Laryngoscope. 1995;105:72–79. doi: 10.1288/00005537-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Lawson W, Kaufman MR, Biller HF. Treatment outcomes in the management of inverted papillomas: an analysis of 160 cases. Laryngoscope. 2003;113:1548–1556. doi: 10.1097/00005537-200309000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Barnes L (2002) Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol 15(3):279–297. Review [DOI] [PubMed]
- 7.von Buchwald C, Bradley PJ (2007) Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr Opin Otolaryngol Head Neck Surg 15(2):95–98. Review [DOI] [PubMed]
- 8.Sherr CJ. Roberts JM CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 9.Harper JW, Adami GR, Wie N, et al. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-G. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y, Hannon GJ, Zang H, Casso D, Kobayashi R. Beach D p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 11.Michieli P, Chedid M, Lin D, et al. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54:3391–3395. [PubMed] [Google Scholar]
- 12.Steeg PS, Abrams JS. Cancer prognostics: past, present and p27. Nat Med. 1997;3:152–154. doi: 10.1038/nm0297-152. [DOI] [PubMed] [Google Scholar]
- 13.Sgambato A, Cittadini A, Faraglia B, Weinstein IB. Multiple functions of p27 (Kip1) and its alterations in tumor cells: a review. J Cell Physiol. 2000;183:18–27. doi: 10.1002/(SICI)1097-4652(200004)183:1<18::AID-JCP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 15.Phillips PP, Gustafson RO, Facer GW. The clinical behavior of inverting papilloma of the nose and paranasal sinuses: Report of 112 cases and review of the literature. Laryngoscope. 1996;100:463–469. doi: 10.1288/00005537-199005000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Katori H, Nozawa A, Tsukuda M (2005) Markers of malignant transformation of sinonasal inverted papilloma. [Comparative Study. Journal Article] Eur J Surg Oncol 31(8):905–911 [DOI] [PubMed]
- 17.Califano J, Koch W, Sidransky D, Westra WH. Inverted sinonasal papilloma: a molecular genetic appraisal of its putative status as a precursor to squamous cell carcinoma. Am J Pathol. 2000;156(1):333–337. doi: 10.1016/S0002-9440(10)64734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keles N, Erdamar B, Kaur A, Deger K. p21, p53, and p27 Kip1 alterations in benign and malignant tumors of sinonasal epithelium. Otolaryngol Head Neck Surg. 2003;129(1):77–84. doi: 10.1016/S0194-5998(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 19.Garavello W, Vigano P, Romagnoli M, Sordo L, Berti E, Tredici G, Gaini RM. Expression of cell cycle regulatory proteins and analysis of apoptosis in normal nasal mucosa and in nasal polyps. Am J Rhinol. 2005;19(6):549–553. [PubMed] [Google Scholar]
- 20.Yang A, Kaghad M, Caput D, et al. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/S0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 21.Ramalho FS, Ramalho LN, Della Porta L, et al. Comparative immunohistochemical expression of p63 in human cholangiocarcinoma and hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21(8):1276–1278. doi: 10.1111/j.1440-1746.2006.04309.x. [DOI] [PubMed] [Google Scholar]
- 22.Fang SY, Yan JJ, Ohyama M. Assessment of p53 protein expression in normal mucosa and benign and malignant lesions of the nasal cavity. Oncology. 1998;55:168–173. doi: 10.1159/000011852. [DOI] [PubMed] [Google Scholar]
- 23.Mirza N, Montone K, Sato Y, Kroger H, Kennedy DW. Identification of p53 and human papillomavirus in schneiderian papillomas. Laryngoscope. 1998;108:497–501. doi: 10.1097/00005537-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Di Como CJ, Urist MJ, Babayan I, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8(2):494–501. [PubMed] [Google Scholar]
- 25.Tonon G, Wong KK, Maulik G et al (2005) High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci USA 102(27):9625–9630. Epub 2005 June 27 [DOI] [PMC free article] [PubMed]
- 26.Guo C, Pan ZG, Li DJ. The expression of p 63 is associated with the differential stage in nasophayngeal carcinoma and EBV infection. J Transl Med. 2006;4:23. doi: 10.1186/1479-5876-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sniezek JC, Matheny KE, Burkey BB, et al. Expression of p63 and 14-3-3 in normal and hyperdifferentiated mucosa of the upper aerodigestive tract. Otolaryngol. Head Neck Surg. 2002;126:598–601. doi: 10.1067/mhn.2002.125302. [DOI] [PubMed] [Google Scholar]
- 28.Pelosi G, Pasini F, Olsen Stenholm C, et al. p63 immunoreactivity in lung cancer: yet another player in the development of squamous cell carcinomas? J Pathol. 2002;198:100–109. doi: 10.1002/path.1166. [DOI] [PubMed] [Google Scholar]
- 29.Sniezek JC, Matheny KE, Westfall MD, Pietenpol JA. Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope. 2004;114(12):2063–2072. doi: 10.1097/01.mlg.0000149437.35855.4b. [DOI] [PubMed] [Google Scholar]
- 30.Massion PP, Taflan PM, Jamshedur Rahman SM, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63(21):7113–7121. [PubMed] [Google Scholar]
- 31.Gujrathi C, Pathak I, Freeman J, Asa S. Expression of p53 in inverted papilloma and malignancy associated with inverted papilloma. J Otolaryngol. 2003;32(1):48–50. doi: 10.2310/7070.2003.35293. [DOI] [PubMed] [Google Scholar]
- 32.Katori H, Nozawat A, Tsukuda M. Relationship between p21 and p53 expression, human papilloma virus infection and malignant transformation in sinonasal-inverted papilloma. Clin Oncol (R Coll Radiol) 2006;18(4):300–305. doi: 10.1016/j.clon.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Ikegawa K, Matsukuma S (2005) Immunohistochemical study of p53 and Ki-67 in inverted papillomas and nasal polyps arising from nasal or paranasal regions. Rinsho Byori 53(6):499–503 (In article) [PubMed]
- 34.Michieli P, Chedid M, Lin D, et al. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54(13):3391–3395. [PubMed] [Google Scholar]
- 35.Saegusa M, Nitta H, Hashimura M, Okayasu I. Down-regulation of p27Kip1 expression is correlated with increased cell proliferation but not expression of p21waf1 and p53, and human papillomavirus infection in benign and malignant tumours of sinonasal regions. Histopathology. 1999;35(1):55–64. doi: 10.1046/j.1365-2559.1999.00688.x. [DOI] [PubMed] [Google Scholar]
- 36.Choi HR, Tucker SA, Huang Z, et al. Differential expressions of cyclin-dependent kinase inhibitors (p27 and p21) and their relation to p53 and Ki-67 in oral squamous tumorigenesis. Int J Oncol. 2003;22(2):409–414. [PubMed] [Google Scholar]