Abstract
Sodium-coupled neutral amino acid transporter 2 (SNAT21) belongs to the SLC38 family of solute transporters. Transport of 1 amino acid molecule into the cell is driven by the co-transport of 1 Na+ ion. The functional significance of the C-terminus of SNAT2, which is predicted to be located in the extracellular space, is currently unknown. Here, we removed 13 amino acid residues from the SNAT2 C-terminus and studied the effect of the deletion on transporter function. The truncation abolished amino acid transport currents at negative membrane potentials (< 0 mV), as well as substrate uptake. However, transport currents were observed at positive membrane potentials, demonstrating that transport was accelerated while the driving force decreased. Membrane expression levels were normal in the truncated transporter. SNAT2Del C-ter showed 3-fold higher apparent affinity for alanine, and 2-fold higher Na+ affinity compared to SNAT2WT, suggesting that the C-terminus is not required for high-affinity substrate and Na+ interaction with SNAT2. pH sensitivity of amino acid transport was partially retained after the truncation. In contrast to the truncation after the final trans-membrane domain, TM11, deletion of TM11 resulted in an inactive transporter, most likely due to a defect in cell surface expression. Together, the results demonstrate that the C-terminal domain of SNAT2 is an important voltage regulator that is required for a normal amino acid translocation process at physiological membrane potentials. However, the C-terminus appears not to be involved in regulation of membrane expression.
Keywords: Amino acid transport, SNAT, SLC38, C-terminus, transport mechanism, uptake
INTRODUCTION
Sodium-coupled neutral amino acid transporters (SNATs) play an important role in transporting small, neutral amino acids, such as glutamine and alanine, across cellular membranes. SNATs belong to the solute carrier 38 (SLC38) family. The family member SNAT2 has been cloned [1–3] and is found in every tissue tested using Northern analysis [2–5]. The stoichiometry of the coupling of SNAT2 amino acid transport is proposed to be 1 neutral amino acid to 1 Na+ ion. Therefore, 1 net positive charge is transported into the cell for each transported neutral amino acid [6–7], rendering the process electrogenic. SNAT2 also mediates an anion leak conductance [8]. SNAT2 may play an important role in the brain, where it is believed to be involved in the process of transporting glutamine from astrocytes to neurons via the glutamate-glutamine cycle [9].
Membrane proteins are complex proteins with distinct structural domains mediating divergent functions. Recent research shows that the C-terminus of membrane proteins can play important roles in the structure-function and the regulation of expression of these proteins, as indicated by the following three examples: 1) Large movements in the C-terminus of CLC-0 chloride channels are associated with a slow gating process [10]. 2) The cytoplasmic C-terminal domain of the vesicular acetylcholine transporter (vAChT) contains signals targeting this transporter to synaptic vesicles (SVs) [11–12]. 3) The C-terminus of the L-type voltage-gated calcium channel CaV1.2 encodes a transcription factor, which regulates the expression of a wide variety of endogenous genes important for neuronal signaling and excitability [13]. Based on hydropathy analysis [14] and experimental evidence from related plant transporters [15–17], the predicted topology of SNAT2 shows that SNAT2 includes 11 transmembrane domains (TMs) with an intracellular N terminus and a relatively short extracellular C-terminus (Fig. 1A), as shown previously by Hyde et al. using an epitope-tagged transporter [18]. This predicted topology is supported by recent evidence suggesting structural homology with a large class of amino acid transporters with known structure [19–20]. It is reported that a highly conserved C-terminal histidine residue (H504) contributes to the pH-sensitivity of SNAT2 [21]. However, the overall effect of the C-terminus on the function of SNAT2 is poorly understood.
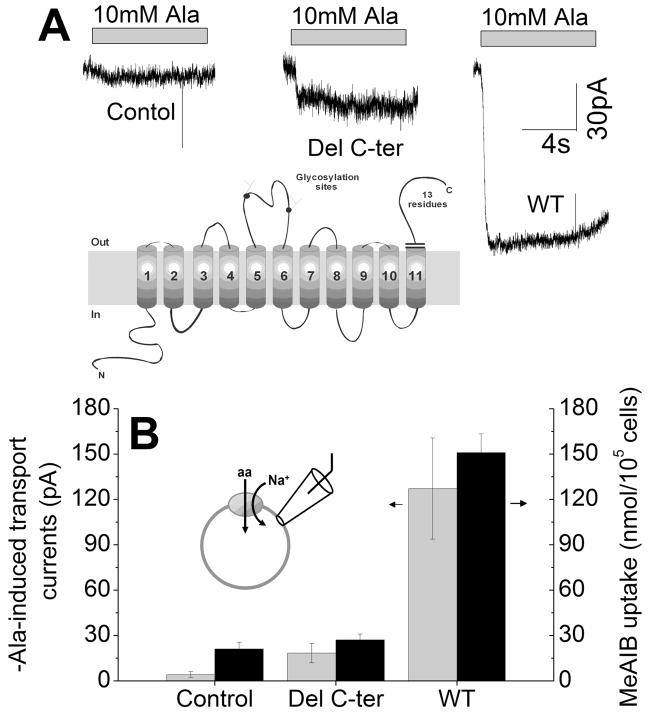
Figure 1. Deletion of the C-terminus of SNAT2 inhibits transport current and amino acid uptake.
(A) Typical transport currents induced by application of 10 mM alanine to non-transfected (control), SNAT2Del C-ter and SNAT2WT-expressing cells. The bath solution contained 140 mM NaMes, and the pipette solution contained 140 mM KMes at 0 mV transmembrane potential. (B) Average alanine-induced transport currents (gray bars, left axis) and MeAIB uptake (black bars, right axis) in HEK293 cells transiently transfected with vecter pBK-CMV(Δ(1098–1300)), SNAT2Del C-ter, and SNAT2WT cDNA. The inset shows the predicted SNAT2 trans-membrane topology and the truncation site.
To identify the importance of the C-terminus of SNAT2, we deleted 13 amino acid residues of the C-terminus of SNAT2, as well as the 11th trans-membrane domain (TM11, Figs. 1 and 6), and determined the function of the truncated SNAT2s. Our results show that transporters with the C-terminal deletions express normally in the membrane of cells, but that transport is absent after TM11 deletion, and strongly inhibited after deletion of the extracellular C-terminus. The apparent affinities for both amino acid and Na+ are not impaired by the C-terminus deletion. Furthermore, pH dependence of transport is reduced, but not eliminated by the deletion. Together, the results suggest that the C-terminus of SNAT2 plays an important role for amino acid translocation and its voltage dependence, but not amino acid, Na+ or proton binding, by the transporter.
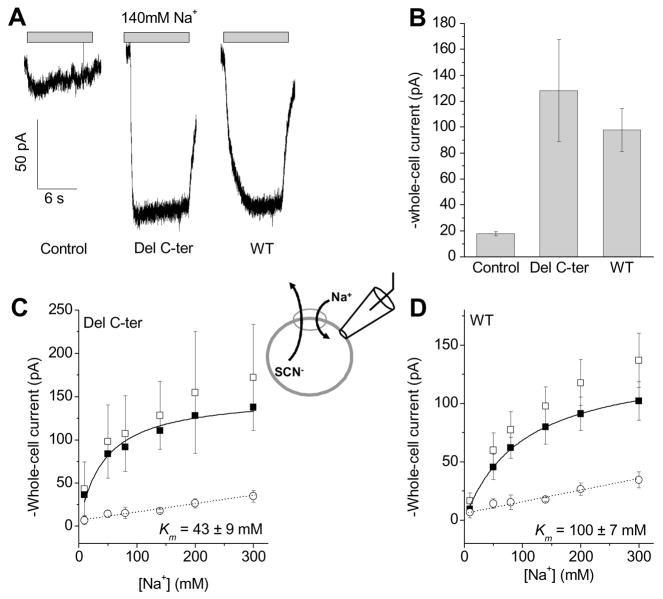
Figure 6. SNAT2Del C-ter retains normal apparent affinity for Na+.
Whole-cell current recordings were performed with a KSCN-based pipette solution (140 mM) and at 0 mV trans-membrane potential. (A), Comparison of typical leak anion currents induced by the application of 140 mM extracellular Na+ among non-transfected cells (control), SNAT2Del C-ter, and SNATWT -expressing cells, and indicated by the gray bars. (B), Statistical analysis of average Na+-induced currents as the ones shown in (A). (C) and (D) show leak anion currents as a function of extracellular [Na+] for SNAT2Del C-ter (C) and SNATWT (D), respectively (solid squares, after subtracting the unspecific currents, open circles, determined from non-transfected cells). The open squares in (C) and (D) are results from the original experiments before subtraction of the unspecific leak currents (open circles) determined in non-transfected control cells.
EXPERIMENTAL
Molecular biology and transient expression
The cDNA coding for rat SNAT2, which was kindly provided by H. Varoqui, was subcloned into the SacI and NheI sites of a modified pBK-CMV vector (Δ[1098–1300]) (Stratagene, La Jolla, CA), containing the CMV promoter for mammalian expression. The 13 amino acid residues of the SNAT2 C-terminus (VLDWVHDASAGGH) and the 54 amino acid residues (from the position 451 to 503) of the SNAT2 TM11-C-terminus fragment (LPSAFYIKLVKKEPMRSVQKIGALCFLLSGVVV-MIGSMGLIVLDWVHDASAGGH) were removed by site-directed mutagenesis, respectively, according to the QuikChange protocol (Stratagene), as described by the supplier. The primers for mutation experiments were obtained from the DNA core lab, Department of Biochemistry at the University of Miami Miller School of Medicine. The complete coding sequences of mutated SNAT2 clones were subsequently sequenced. Wild-type and mutant transporter constructs were used for transient transfection of sub-confluent human embryonic kidney cell (HEK293T/17, ATCC number CRL 11268) cultures using FuGENE 6 Transfection Reagent (Roche, Indianapolis, IN) according to the instructions of the supplier. The 293T/17 cell line is a derivative of the 293T cell line into which the gene for SV40 T-antigen was inserted. Electrophysiological recordings were performed between 1 and 2 days post transfection.
Electrophysiology
SNAT2-mediated currents were recorded with an Adams & List EPC7 amplifier (Heka Elektronik, Lambrecht, Germany) under voltage-clamp conditions in the whole-cell current-recording configuration. The typical resistance of the recording electrode was 2–3 MΩ; the series resistance was 5–8 MΩ. Because the currents induced by substrate, anion, or cation application were small (typically < 500 pA), series resistance (Rs) compensation had a negligible effect on the magnitude of the observed currents (< 4% error). Therefore, Rs was not compensated. The extracellular bath buffer solution contained (mM): 140 sodium methanesulfonate (NaMes), 2 magnesium gluconate (MgGlu2), 2 calcium gluconate (CaGlu2), 30 Tris, pH 8.0. Except for the pH dependent experiments, all other experiments were conducted at an extracellular pH 8.0 because amino acid transport by SNAT2 is pH dependent and the transport rate is maximal at pH 8.0. For testing the [Na+] dependence of the currents, Na+ in the extracellular solution was replaced with NMG+ (N-methylglucamine). The pipette solution contained (in mM): 140 KMes or KSCN, 2 MgGlu2, 10 EGTA, 30 HEPES, pH 7.3. For determining the voltage dependence of SNAT2 alanine transport, a combined voltage ramp/solution exchange protocol was used. In this protocol, the cell membrane was initially held at 0 mV, before ramping the voltage to its final value (−90 to +60 mV) within 2 s. Two seconds after establishing the new voltage, the extracellular solution was changed from no alanine to the final concentration of alanine, followed by ramping the voltage back to 0 mV. The currents were low-pass filtered at 1–10 KHz (Krohn-Hite 3200) and digitized with a digitizer board (Axon Instruments (Foster City, CA) Digidata 1200) at a sampling rate of 10–15 kHz, which was controlled by software (Axon PClamp). All the experiments were performed at room temperature. Solutions were applied to the cells as described previously [8, 20, 22].
Amino Acid Uptake Assay
HEK293 cells were plated on collagen-coated 12-well plates (1×105 cells/well) in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, penicillin (100 units/ml), streptomycin (100 mg/ml), and glutamine (4 mM). 48 h after transfection with vector, wild-type SNAT2, or truncated SNAT2 cDNA, the cells were washed with uptake buffer two times. The uptake buffer contained 140 mM sodium methanesulfonate (NaMes), 2 mM MgMes2, 2 mM calcium gluconate (CaGlu2), 30 mM Tris/Mes, pH 8.0, 5 mM glucose. The cells were then pre-incubated in the same buffer for 5 min at 37 °C before the buffer was replaced with fresh buffer containing unlabeled α-methylamino-isobutyric acid (MeAIB) and 0.4 μCi of [14C]MeAIB (PerkinElmer Life Sciences; total concentration, 40 μM). After 1 min of incubation at room temperature, uptake was terminated by washing twice with 1 ml of uptake buffer on ice (after 1 min uptake was in the linear range, as determined by quantifying the time dependence of uptake for times up to 5 min). The cells were then solubilized in 0.5 ml of 1% SDS, and radioactivity was measured by scintillation counting in 3 ml of scintillation fluid. The MeAIB uptake measurements were performed in duplicate.
Data analysis
Nonlinear regression fits of experimental data were performed with Origin (Microcal Software, Northampton, MA) or Clampfit (pClamp8 software, Axon Instruments). Dose response relationships of currents were fitted with a Michealis-Menten-like equation, yielding Km and Imax. Endogenous alanine transport activity in HEK293T cells, as measured by current recording from non-transfected cells, is minimal (see Results section). For the Na+ concentration dependence of the leak current, the dose-response data was corrected by subtraction of the nonspecific component of the current, which increases linearly with increasing [Na+]. The non-specific component was determined from non-transfected HEK293 cells. Each experiment was repeated at least three times with at least two different cells. The error bars represent mean ± SD, unless stated otherwise. Maximum SNAT2-dependent currents, Imax, vary approximately by a factor of 3 between different cells, depending on the expression levels of each individual cell. Such changes in expression levels did not affect the Km for the amino acid. The Imax values were obtained by averaging the Imax values from these individual cells.
Confocal microscopy
Confocal microscopy was performed after expressing a AcGFP-SNAT2 fusion construct in HEK293 cells. Rat SNAT2 cDNA was sub-cloned from the pBK-CMV vector into a pAcGFP1-C In-Fusion Ready Vector (Clontech, Mountain View, CA) (primers: sense - AAGGCCTCTGTCGACACACCGTTCCTCCGGATCAGC, antisense – AGAATTCGCAAGCTTTCTGAGCGAGTTGAGTGGACCCAA), to add a GFP protein sequence in frame to SNAT2’s N-Terminus. After a thirty hour incubation post-transfection, the AcGFP-SNAT2-expressing HEK293T cells were wet mounted and visualized using an Axiovert inverted microscope (Carl Zeiss Inc., Thornwood, NY) using a 40× oil-immersion objective lens. Single optical sections were taken and recorded digitally using a Zeiss LSM 510 META confocal imaging system. The images were taken upon excitation with an argon laser. Images of non-transfected HEK293 cells were also recorded in bright field mode.
RESULTS
Amino acid transport is impaired after deletion of SNAT2 C-terminus
Application of 10 mM alanine to SNAT2WT-expressing cells resulted in inwardly-directed transport currents (Fig. 1A) in the presence of 140 mM Na+ at the extracellular side of the membrane (V = 0 mV, average current of −127 ± 30 pA, n = 28; gray bars in Fig. 1B). In contrast, application of 10 mM alanine to the C-terminally-truncated SNAT2 (SNAT2Del C-ter) resulted in small inward currents (Fig. 1A), which was on average −18 ± 6 pA (n = 7) and 11 % of the SNAT2WT response (corrected for nonspecific currents, Fig. 1B). These results suggest that deletion of the SNAT2 C-terminus strongly interferes with the ability of SNAT2 to catalyzed alanine transport current. Although the current carried by SNAT2Del C-ter was small, it was significantly larger (4.5-fold) than that observed in non-transfected cells (−4 ± 2 pA, n = 19, Fig. 1B).
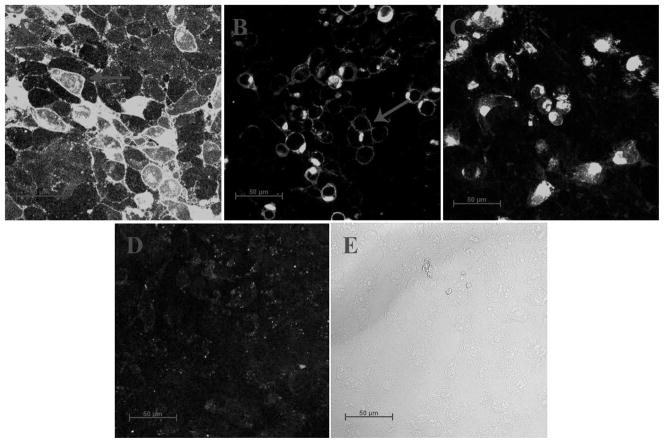
The lack of alanine-induced transport current for SNAT2Del C-ter may be caused by reduced expression. To test this possibility, we determined sub-cellular localization by using confocal microspcopy with a green-fluorescent protein (AcGFP) tagged transporter (Fig. 2). Current recording experiments show that the N-terminal AcGFP tag does not affect function of the transporter (data not shown). Non-transfected cells showed no significant fluorescence (Fig. 2D and E), whereas both SNAT2WT- and SNAT2Del C-ter-expressing cells exhibited intracellular fluorescence, as well as significant fluorescence at the cell boundaries (Fig. 2A and B, arrows). Although small differences in the expression levels of SNAT2WT and SNAT2Del C-ter cannot be quantified using this method, these results indicate that the deletion of the C-terminus of SNAT2 does not affect the transporter localization in the membrane in a major way. This result is consistent with transport current measurements at + 60 mV (Fig. 3C and 3D), which clearly indicate that SNAT2Del C-ter is expressed in the membrane.
Figure 2.
Confocal microscopy images testing for cellular localization of truncated SNAT2-AcGFP fusion constructs. (A) SNAT2WT, (B) SNAT2Del C-ter, and (C) SNAT2Del TM11. Panel (D) shows control, non-transfected cells, and (E) is the bright-field control image, showing that cells were present. The arrows indicate regions of intense cell boundary fluorescence. AcGFP was attached in frame to the N-terminus of SNAT2.
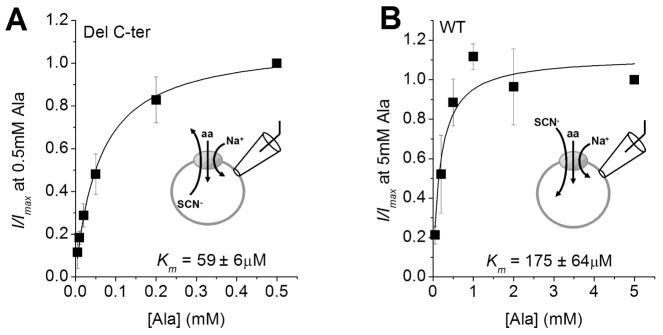
Figure 3. The C-terminal deletion does not interfere with substrate binding.
Apparent affinities for the substrate L-alanine of SNAT2WT (A) and SNAT2Del C-ter (B) were determined by recording substrate-inhibited anion leak currents as a function of [alanine] at 0 mV in the presence of 140 mM intracellular KSCN (SNAT2Del C-ter) and 140 mM extracelluar NaSCN (SNAT2WT).
In order to directly test whether amino acid transport by SNAT2Del C-ter is impaired, we performed amino acid uptake experiments. SNAT2WT transfected cells showed significant specific methylamino-α-isobutyric acid (MeAIB, a specific SNAT substrate) uptake activity (7-fold higher than vector-transfected cells), whereas uptake was insignificant in SNAT2Del C-ter and vector-transfected cells (Fig. 1B). These results support the electrophysiological analysis of SNAT2Del C-ter, indicating that deletion of SNAT2 C-terminus results in a loss of electrogenic transport current, as well as uptake activity.
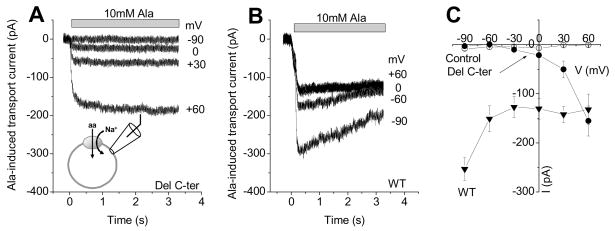
The loss of alanine transport activity of SNAT2Del C-ter may be caused by impaired binding of alanine to the truncated transporter. However, alanine application induced large outward currents in the presence of intracellular SCN− for SNAT2Del C-ter (Fig. 5), which allowed us to determine the apparent affinity of SNAT2Del C-ter for alanine (internal SCN− generates an inward current by moving outward through the uncoupled leak anion conductance, thus generating alanine-dependent current in the absence of electrogenic transport current). The apparent alanine Km values were 175 ± 64 μM for SNAT2WT (Fig. 3B, n = 4) and 59 ± 6 μM for SNAT2Del C-ter (Fig. 3A, n= 4). This result indicates that removal of SNAT2 C-terminus does not interfere with alanine binding. Therefore, the impairment of alanine transport activity cannot be caused by an increase in the Km for amino acid.
Figure 5. Deletion of the C-terminus decreases, but does not eliminate anion leak current.
Voltage dependence of alanine-sensitive currents mediated by SNAT2Del C-ter (A) and SNAT2WT (B), in the presence of 140 mM intracelluare KSCN and 140 mM extracellular NaMes. 10 mM L-alanine was applied at t = 0 s, as indicated by the bar. C, current-voltage relationships of 10 mM L-alanine-sensitive currents in nontransfected cells (open circles), SNAT2Del C-ter (up triangles), and SNAT2WT (squares) expressing cells in the presence of 140 mM intracellular KSCN.
The C-terminus is involved in controlling the voltage dependence of amino acid transport
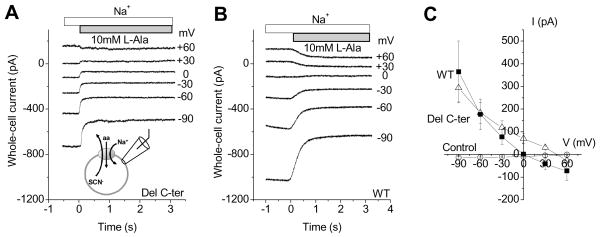
To test whether transport activity of SNAT2Del C-ter is restored by an increase in the electrical driving force, we determined the voltage dependence of L-alanine induced transport currents. As shown previously, application of L-alanine (10 mM) evoked inward transport currents in SNAT2WT. These transport currents showed a relatively small dependence on the membrane potential from −60 mV to +60 mV but increased steeply at a potential of −90 mV (1.95 times the current at 0 mV; Fig. 4B and C). The reason for this steep current increase at −90 mV is not known, but may be related to a shift of the rate limiting step at very negative potentials. The voltage-dependence of alanine-induced (10 mM) transport currents mediated by SNAT2Del C-ter was opposite to that of SNAT2WT. Transport currents were only observed at positive membrane potentials, but not at potentials more negative that 0 mV (4A and C). The transport current was restored to the same level as SNAT2WT at +60 mV (−150 ± 30 pA for SNAT2Del C-ter, −132 ± 30 pA for SNAT2WT). These results indicate that the SNAT2 C-terminus plays an important role in regulating the voltage dependent processes in the transport cycle.
Figure 4. The deletion of the C-terminus affects the voltage dependence of substrate transport.
I-V relationships of L-alanine-induced transport currents at a concentration of 10 mM for SNAT2Del C-ter (A) and SNAT2WT (B). C, average current-voltage relationships of alanine-induced transport currents in nontransfected cells (control, open circles), SNAT2Del C-ter (solid squares), and SNAT2WT (solid triangles).
Anion leak current is unaffected by the C-terminal truncation
In a previous publication [8], we reported that SNAT2 mediates an anion leak conductance, which is inhibited by the transported substrate. In order to test if deletion of the C-terminus of SNAT2 affects this anion leak conductance, we performed experiments with the highly-permeant anion SCN− on the intracellular side of the membrane. Application of 10 mM L-alanine induced outwardly-directed currents in SNAT2WT at negative membrane potentials (Fig. 5B), which is caused by inhibition of a tonic inward anion current carried by SCN− leaving the cell. As expected, the outward currents increased at more negative transmembrane potentials, which increase the driving force for SCN− efflux. The total current was a sum of transport current and anion leak current with a reversal potential for SNAT2WT of −1 ± 4 mV (Fig. 5C, n = 6), as reported previously [8]. Application of 10 mM alanine also induced outwardly-directed currents in SNAT2Del C-ter with a similar magnitude to that of the wild-type transporter at −90 mV (+360 ± 130 pA for SNAT2WT and +290 ± 60 pA for SNAT2Del C-ter). The reversal potential for SNAT2Del C-ter current was shifted to a positive value (+46 ± 2 mV, Fig. 5C, n = 7). This result is consistent with the idea that the alanine-induced current is dominated by the anion component in SNAT2Del C-ter, due to the small transport current at voltages more negative than +30 mV.
Na+ binding to transporters is unaltered by the C-terminal truncation
Na+ activates an anion leak conductance in SNAT2, which we used as a tool to determine the apparent affinity of the wild-type and truncated transporters for [Na+] [8, 20, 22]. Upon application of extracellular Na+ in the presence of intracellular SCN−, large inward currents were observed in HEK293T cells expressing SNAT2WT (Fig. 6A and B, −98 ± 16 pA, n=8, 0 mV) or SNAT2Del C-ter (Fig. 6A and B, −130 ± 40 pA, n = 7, 0 mV, in the absence of amino acid, original traces are shown in Fig. 6A). Non-transfected control cells showed only small current responses to [Na+] jumps at the same conditions (Fig. 6A and B, −18 ± 2 pA, n = 10), and current evoked by SNAT2WT was 5-fold over control cells. Within experimental error, the current evoked by SNAT2Del C-ter was in the same range as that of SNAT2WT. The leak current was Na+ concentration dependent (Fig. 6C and D). Kinetic analysis indicated that the apparent affinity of the transporters for Na+ was 2-fold higher for SNAT2Del C-ter (Km = 43 ± 9 mM, n = 8) compared to SNAT2WT (Km = 100 ± 7 mM, n = 5) at pH 8.0. This result indicates that Na+ affinity is not reduced by deletion of the C-terminus, excluding the possibility that the loss of transport activity of SNAT2Del C-ter is caused by a decrease of the Na+ Km of the transporter.
In the absence of amino acid substrate, SNAT2WT induces transient currents to step changes of the transmembrane potential, which are believed to be caused by electrogenic Na+ binding to and dissociation from the transporters [22]. As shown in Suppl. Fig. 1A, SNAT2Del C-ter exhibited similar transient currents in response to voltage jumps as SNATWT, although the transient currents evoked by SNAT2 Del C-ter were smaller in magnitude (Suppl. Fig. 1C and D). Both wild-type and SNAT2Del C-ter transient currents significantly exceeded non-transfected control current levels (Suppl. Fig. 1B). The charge movement, Q, obtained by integrating the on and the off response of these currents, was similar to each other in wild-type and truncated transporters, within experimental error (Suppl. Fig. 1E and F), suggesting that this charge movement is capacitive in nature. The voltage dependence of the charge movement was unchanged by the truncation (Suppl. Fig. 1). Overall, these results are consistent with the data from Na+-induced leak currents, indicating that removal of the SNAT2 C-terminus does not interfere with Na+ binding.
Effect of extracellular pH
As demonstrated in the literature, amino acid transport by SNAT2 is highly pH dependent [3, 23], with a maximum transport rate observed around pH 8 (+60 mV, Suppl. Fig. 2). Because a histidine in the C-terminus (H504A) was proposed to be involved in this pH effect [21], we tested the pH dependence of alanine transport currents of SNAT2 Del C-ter at +60 mV, where transport current is substantial. As shown in Suppl. Fig. 2, SNAT2 Del C-ter transport current was maximally activated at pH 8.0, in analogy to the wild-type transporter. However, the pH dependence was somewhat reduced compared to that of SNAT2WT, but not eliminated. This result supports the previously-published data on the H504A mutant transporter, which has decreased pH sensitivity [21].
Truncation before TM11 results in a non-functional transporter
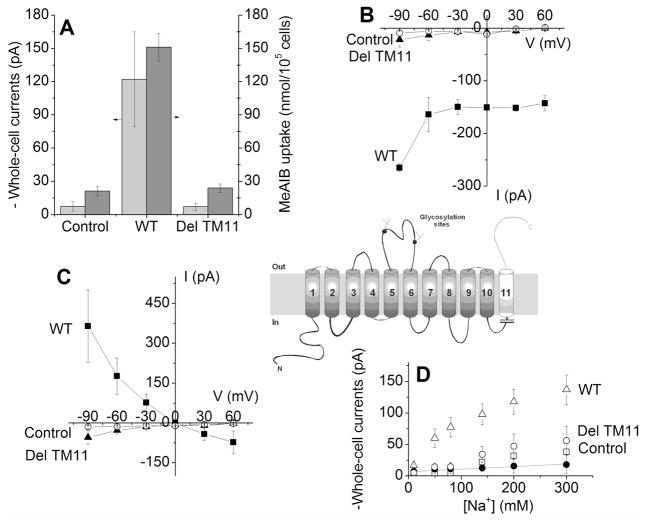
The crystal structures of transporters thought to be homologous to SNAT2 suggest that TM11 is not an integral part of the transport pathway [24]. In fact, members of the spore germinating protein (SGP) family, which are distant relatives of SNAT2, have only 10 TM domains, but still bind amino acid [25]. Therefore, we generated and tested a transporter that was C-terminally truncated after leucine 451, also deleting TM11 (SNAT2Del TM11). The transport current induced by SNAT2Del TM11 in the presence of 10 mM of alanine was −7 ± 3 pA at the membrane potential 0 mV (Fig. 7A, n=5), within experimental error the same as that in non-transfected cells (−4 ± 4 pA, n=10, Fig. 7A), suggesting together with MeAIB uptake data (Fig. 7A) that the amino acid transport function was eliminated by removing TM11. Transport cannot be restored by an increase of the electrical driving force (Fig. 7B). Alanine-inhibited anion leak currents (Fig. 7C) as well as Na+-induced anion leak currents were not significantly larger than those in control cells (n = 6, Fig. 7D). All of these results indicate that SNAT2Del TM lost transport function. Cell surface expression of SNAT2Del TM11 transporter was significantly reduced (Fig. 2C), compared to SNAT2WT (Fig. 2A). Therefore, the functional impairment of the SNAT2Del TM11 transporter is most likely caused by a defect in cell surface targeting of the transporter.
Figure 7. Deletion of TM11 results in a non-functional transporter.
(A) Transport currents induced by nontransfected cells, SNAT2WT- and SNAT2Del TM11-expressing cells at 10 mM of alanine and MeAIB uptake by vector (pBK-CMV (Δ[1098–1300])), SNAT2Del C-ter and SNAT2WT in transiently transfected HEK293 cells. Transport of [14C]MeAIB (40 μM) was measured at 1 min in NaMes-containing buffer. (B) Voltage-dependence of transport currents induced by SNAT2Del TM11 in the presence of 10 mM of alanine in nontranfected cells (open circles), SNAT2WT- (squares) and SNAT2Del TM11-expressing cells (triangles). (C) Average current-voltage relationships of anion leak current in the presence of 10 mM of alanine among nontransfected cells (contol, open circles), SNAT2WT- (squares) and SNAT2Del TM11- expressing cells (triangles). (D) Anion leak currents induced by 140 mM of extracellular Na+. The open triangles (SNAT2WT) and open circles (SNAT2Del TM11) are the currents from original experiments before subtraction of the unspecific leak currents (open squares, control, nontransfected cells). The solid circles are the anion leak currents induced by SNAT2Del TM11 after subtracting the unspecific currents (open circles). The inset shows the truncation site.
DISCUSSION
The sodium-dependent neutral amino acid transporter SNAT2, the ubiquitous member of the SLC38 family, accounts for the activity of transport system A for neutral amino acids in most mammalian tissue [1–3]. Based on the predicted topology of SNAT2 from related plant transporters [15–17], and a previously published homology model [20], SNAT2 includes 11 transmembrane domains (TMs) with an intracellular N terminus and an extracellular C-terminus (Fig. 1 inset). For most sodium-dependent amino acid transporters, such as glutamate transporters (EAATs) [26], the leucine transporter (LeuT) [24], and the γ-aminobutyric acid (GABA) transporter [26–27], etc, the C-terminus is located in the cytoplasm. Studies on various members of the Na+-dependent neurotransmitter transporter family regarding the cellular mechanisms of their functional expression have highlighted that the C-terminus is critical for the regulation of trafficking to the plasma membrane [28–29]. However, the C-terminus of SNAT2 is extracellular, which likely precludes it from playing roles in mechanisms that regulate membrane expression/targeting. Consistent with this idea, truncation of the C-terminus did not cause a defect in expression of the transporter in the plasma membrane, suggesting a lack of involvement of the C-terminus in processes that regulate intracellular trafficking.
The main findings of the present study are twofold: 1) The C-terminus is critical for the amino acid transport function of the transporter, as demonstrated by the defective alanine transport at voltages ≤ 0 mV, as well as defective MeAIB uptake. 2) The C-terminus contributes to controlling the voltage dependence of amino acid transport. At positive voltages, alanine-induced transport current was observed, indicating a dramatic change in the rate limitation by voltage dependent steps in the transport cycle. In SNAT2Del C-ter the transport rate may be limited by a step that is accelerated at positive membrane potentials. Alternatively, negative membrane potential may accelerate transition into an inactive state that does not allow amino acid transport. We currently cannot differentiate between these possibilities. Together, these results suggest that the C-terminal region plays an important role in the amino acid translocation process, or relocation of the empty transporter. A similar behavior was reported for the human noradrenaline (norepinephrine) transporter (hNET1), in which deletion of the last seven amino acids of hNET1 C-terminal region did not affect cell surface expression, but severely affected the uptake of [3H] noradrenaline (NE) [28]. Overall, the strong influence of the C-terminal domain on the transport rate and its voltage dependence is somewhat surprising, because this domain is thought to be outside of the core membrane domain of the transporter, on which the membrane potential is expected to have its major effect. Therefore, it is likely that the C-terminal domain affects substrate transport and its voltage dependence through a regulatory, possibly allosteric, process. This interpretation is consistent with results from a transporter with the C-terminal H504A mutation, for which an allosteric regulation of the transport rate by protonation was proposed [21].
In addition to H504, the C-terminus of SNAT2 contains two potentially charged residues, D494 and D498, which may contribute to regulation of the voltage dependence of amino acid transport. To test this possibility, we neutralized both aspartate residues by replacement with alanine (Suppl. Fig. 3). However, within experimental error, the mutant transporters displayed the same alanine transport activity as the wild-type transporter. Therefore, these two acidic residues are unlikely to play a major part in the modulatory process. We hypothesize that regulation of transport by the C-terminus is not caused by electrostatic interaction. The identification of other C-terminal amino acid residues that do contribute to the modulatory effect awaits further experimentation.
In contrast to the amino acid transport activity, anion leak currents, induced by Na+ application or inhibited by alanine application, were little affected by the C-terminal truncation. These results suggests that the anion leak pathway, which most likely is associated with the core translocation domain of the transporter, is not modulated by the C-terminal domain. Interestingly, this points to the possibility of modulating transport activity and anion leak activity independently from each other, as has been previously shown for the anion conductance of the structurally different transporters from the EAAT family [30–32]. The result also suggests that amino acid translocation is not required for the anion conductance to work.
SNAT2Del C-ter exhibits about 3-fold higher apparent alanine affinity (Fig. 3) and 2-fold higher apparent Na+ affinity (Fig. 6) compared to the wild-type transporter. Voltage-jump-induced transient currents, which are caused by the binding/dissociation of Na+ to/from the transporter are also not affected by the truncation. These results indicate that the C-terminal truncation does not disrupt the structure of the amino acid and Na+ binding sites, and does not interfere with Na+ access to its binding site. These binding sites are most likely deeply buried in the membrane, as suggested by the structural homology model that we developed on the basis of the crystal structures of several bacterial amino acid transporters [20]. In fact, the Na+ binding site is constituted of TM domains 1 and 8, which are predicted to be located on the side opposite from the C-terminal domain of the transporter [20].
The pH dependence of amino acid transport by SLC38 members is clearly established [23, 33–35]. While SNAT3 and 5 couple amino acid movement to proton transport [33, 36–37], the effect of H+ on SNAT1 and 2 is modulatory, inhibiting transport at pH < 8 [3, 38–39]. Truncation of the C-terminus results in a decrease of sensitivity of SNAT2 to pH, although inhibition of transport at pH 7.0 and 6.0 is still observed. These results are consistent with a previous report that linked the pH sensitivity to histidine 504 in the C-terminal region of SNAT2 [21]. This histidine is deleted in the truncated transporter studied here. While a modulatory role of H504, depending on its protonation state, is likely, this cannot account for the total pH sensitivity, since the transporter remains somewhat pH sensitive even after C-terminal truncation. Therefore, it is likely that other protonation sites exist that contribute to the modulation of the amino acid transport rate by the external pH.
We also tested the properties of a construct in which the C-terminus was removed after TM10. TM11 is thought to be removed from the catalytic core of the transport machinery. Despite this fact, deletion of TM11 rendered the transporter non-functional in any aspects tested. Because deletion of TM11 resulted in a defect in cell surface expression, it is likely that membrane targeting is disrupted in the absence of TM11.
In conclusion, in the present study, we have demonstrated that the C-terminal domain of SNAT2 is not essential for trafficking and recruitment to the plasma membrane. However, removal of the C-terminal region severely impairs amino acid transport, suggesting that the C-terminal domain affects the functional states of SNAT2, which switches between outward- and inward-facing conformations during the transport cycle. Therefore, the C-terminal domain of SNAT2 probably plays an important role in modulating the rate of translocation, as well as the voltage dependence of this rate, most likely through an allosteric mechanism.
Supplementary Material
Acknowledgments
We thank Dr. Diez-Sampedro for help with the MeAIB uptake. This work was supported by a grant from the NIH (7R01NS049335-05) awarded to CG, by grants from NSFC (30870560 to ZZ), Innovation Program of Shanghai Municipal Education Commission (09ZZ139 to ZZ), Shanghai Normal University (DZL808, DYL810 and DRL804 to ZZ), and Shanghai Leading Academic Discipline (S30406).
Footnotes
Abbreviations: CLC: Chloride channel; EAAT: Excitatory amino acid transporter; EGTA: ethylene glycol tetraacetic acid; GABA: γ-aminobutyric acid; Glu: Gluconate; HEPES: (4-(2-hydroxyethyl)--1-piperazineethanesulfonic acid); HEK: Human embryonic kidney; LeuT: Leucine transporter; MeAIB: N-methylaminoisobutyric acid; Mes: Methanesulfonic acid; NE: Norepinephrine; NET: Norepinephrine transporter; NMG: N-methylglucamine; SDS: Sodium dodecyl sulfate; SLC: Solute carrier; SGP: Spore germinating protein; SNAT2: Sodium-coupled neutral amino acid transporter; TM: Transmembrane domain; vAChT: Vesicular acetylcholine transporter.
References
- 1.Reimer RJ, Chaudhry FA, Gray AT, Edwards RH. Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc Natl Acad Sci U S A. 2000;97:7715–7720. doi: 10.1073/pnas.140152797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugawara M, Nakanishi T, Fei YJ, Huang W, Ganapathy ME, Leibach FH, Ganapathy V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 3.Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD. A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem. 2000;275:22790–22797. doi: 10.1074/jbc.M002965200. [DOI] [PubMed] [Google Scholar]
- 4.Hatanaka T, Huang W, Wang H, Sugawara M, Prasad PD, Leibach FH, Ganapathy V. Primary structure, functional characteristics and tissue expression pattern of human ATA2, a subtype of amino acid transport system A. Biochim Biophys Acta. 2000;1467:1–6. doi: 10.1016/s0005-2736(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 5.Varoqui H, Zhu H, Yao D, Ming H, Erickson JD. Cloning and functional identification of a neuronal glutamine transporter. J Biol Chem. 2000;275:4049–4054. doi: 10.1074/jbc.275.6.4049. [DOI] [PubMed] [Google Scholar]
- 6.Jauch P, Petersen OH, Lauger P, Jauch P, Lauger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: I. Tight-seal whole-cell recordings. J Membr Biol. 1986;94:99–115. doi: 10.1007/BF01871191. [DOI] [PubMed] [Google Scholar]
- 7.Jauch P, Lauger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: II. Comparison with transport models. J Membr Biol. 1986;94:117–127. doi: 10.1007/BF01871192. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Grewer C. The sodium-coupled neutral amino acid transporter SNAT2 mediates an anion leak conductance that is differentially inhibited by transported substrates. Biophys J. 2007;92:2621–2632. doi: 10.1529/biophysj.106.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- 10.Bykova EA, Zhang XD, Chen TY, Zheng J. Large movement in the C terminus of CLC-0 chloride channel during slow gating. Nat Struct Mol Biol. 2006;13:1115–1119. doi: 10.1038/nsmb1176. [DOI] [PubMed] [Google Scholar]
- 11.Varoqui H, Erickson JD. Dissociation of the vesicular acetylcholine transporter domains important for high-affinity transport recognition, binding of vesamicol and targeting to synaptic vesicles. J Physiol Paris. 1998;92:141–144. doi: 10.1016/S0928-4257(98)80152-6. [DOI] [PubMed] [Google Scholar]
- 12.Varoqui H, Erickson JD. The cytoplasmic tail of the vesicular acetylcholine transporter contains a synaptic vesicle targeting signal. J Biol Chem. 1998;273:9094–9098. doi: 10.1074/jbc.273.15.9094. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swarup R, Kargul J, Marchant A, Zadik D, Rahman A, Mills R, Yemm A, May S, Williams L, Millner P, Tsurumi S, Moore I, Napier R, Kerr ID, Bennett MJ. Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell. 2004;16:3069–3083. doi: 10.1105/tpc.104.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang AB, Lin R, Keith Studley W, Tran CV, Saier MH., Jr Phylogeny as a guide to structure and function of membrane transport proteins. Mol Membr Biol. 2004;21:171–181. doi: 10.1080/09687680410001720830. [DOI] [PubMed] [Google Scholar]
- 16.Saier MH., Jr Families of transmembrane transporters selective for amino acids and their derivatives. Microbiology. 2000;146 (Pt 8):1775–1795. doi: 10.1099/00221287-146-8-1775. [DOI] [PubMed] [Google Scholar]
- 17.Young GB, Jack DL, Smith DW, Saier MH., Jr The amino acid/auxin:proton symport permease family. Biochim Biophys Acta. 1999;1415:306–322. doi: 10.1016/s0005-2736(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 18.Hyde R, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem. 2007;282:19788–19798. doi: 10.1074/jbc.M611520200. [DOI] [PubMed] [Google Scholar]
- 19.Broer S, Schneider HP, Broer A, Deitmer JW. Mutation of asparagine 76 in the center of glutamine transporter SNAT3 modulates substrate-induced conductances and Na+ binding. J Biol Chem. 2009;284:25823–25831. doi: 10.1074/jbc.M109.031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Albers T, Fiumera HL, Gameiro A, Grewer C. A conserved Na(+) binding site of the sodium-coupled neutral amino acid transporter 2 (SNAT2) J Biol Chem. 2009;284:25314–25323. doi: 10.1074/jbc.M109.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baird FE, Pinilla-Tenas JJ, Ogilvie WL, Ganapathy V, Hundal HS, Taylor PM. Evidence for allosteric regulation of pH-sensitive System A (SNAT2) and System N (SNAT5) amino acid transporter activity involving a conserved histidine residue. Biochem J. 2006;397:369–375. doi: 10.1042/BJ20060026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Gameiro A, Grewer C. Highly conserved asparagine 82 controls the interaction of Na+ with the sodium-coupled neutral amino acid transporter SNAT2. J Biol Chem. 2008;283:12284–12292. doi: 10.1074/jbc.M706774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shotwell MA, Jayme DW, Kilberg MS, Oxender DL. Neutral amino acid transport systems in Chinese hamster ovary cells. J Biol Chem. 1981;256:5422–5427. [PubMed] [Google Scholar]
- 24.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 25.Jack DL, Paulsen IT, Saier MH. The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology. 2000;146:1797–1814. doi: 10.1099/00221287-146-8-1797. [DOI] [PubMed] [Google Scholar]
- 26.Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol. 2006;213:89–100. doi: 10.1007/s00232-006-0877-5. [DOI] [PubMed] [Google Scholar]
- 27.Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- 28.Sogawa C, Kumagai K, Sogawa N, Morita K, Dohi T, Kitayama S. C-terminal region regulates the functional expression of human noradrenaline transporter splice variants. Biochem J. 2007;401:185–195. doi: 10.1042/BJ20060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao J, Hersh LB. The vesicular monoamine transporter 2 contains trafficking signals in both its N-glycosylation and C-terminal domains. J Neurochem. 2007;100:1387–1396. doi: 10.1111/j.1471-4159.2006.04326.x. [DOI] [PubMed] [Google Scholar]
- 30.Melzer N, Biela A, Fahlke C. Glutamate modifies ion conduction and voltage-dependent gating of excitatory amino acid transporter-associated anion channels. J Biol Chem. 2003;278:50112–50119. doi: 10.1074/jbc.M307990200. [DOI] [PubMed] [Google Scholar]
- 31.Ryan RM, Vandenberg RJ. Distinct conformational states mediate the transport and anion channel properties of the glutamate transporter EAAT-1. J Biol Chem. 2002;277:13494–13500. doi: 10.1074/jbc.M109970200. [DOI] [PubMed] [Google Scholar]
- 32.Torres-Salazar D, Fahlke C. Neuronal glutamate transporters vary in substrate transport rate but not in unitary anion channel conductance. J Biol Chem. 2007;282:34719–34726. doi: 10.1074/jbc.M704118200. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhry FA, Krizaj D, Larsson P, Reimer RJ, Wreden C, Storm-Mathisen J, Copenhagen D, Kavanaugh M, Edwards RH. Coupled and uncoupled proton movement by amino acid transport system. N Embo J. 2001;20:7041–7051. doi: 10.1093/emboj/20.24.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 35.McGivan JD, Pastor-Anglada M. Regulatory and molecular aspects of mammalian amino acid transport. Biochem J. 1994;299 (Pt 2):321–334. doi: 10.1042/bj2990321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broer A, Albers A, Setiawan I, Edwards RH, Chaudhry FA, Lang F, Wagner CA, Broer S. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J Physiol. 2002;539:3–14. doi: 10.1113/jphysiol.2001.013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhry FA, Reimer RJ, Krizaj D, Barber D, Storm-Mathisen J, Copenhagen DR, Edwards RH. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell. 1999;99:769–780. doi: 10.1016/s0092-8674(00)81674-8. [DOI] [PubMed] [Google Scholar]
- 38.Albers A, Broer A, Wagner CA, Setiawan I, Lang PA, Kranz EU, Lang F, Broer S. Na+ transport by the neural glutamine transporter ATA1. Pflugers Arch. 2001;443:92–101. doi: 10.1007/s004240100663. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhry FA, Schmitz D, Reimer RJ, Larsson P, Gray AT, Nicoll R, Kavanaugh M, Edwards RH. Glutamine uptake by neurons: interaction of protons with system a transporters. J Neurosci. 2002;22:62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.