Abstract
Membrane proteins located on vesicles (v–SNAREs) and on the target membrane (t–SNAREs) mediate specific recognition and, possibly, fusion between a transport vesicle and its target membrane. The activity of SNARE molecules is regulated by several soluble cytosolic proteins. We have cloned a bovine brain cDNA encoding a conserved 117 amino acid polypeptide, denoted Golgi-associated ATPase Enhancer of 16 kDa (GATE–16), that functions as a soluble transport factor. GATE–16 interacts with N–ethylmaleimidesensitive factor (NSF) and significantly stimulates its ATPase activity. It also interacts with the Golgi v–SNARE GOS–28 in an NSF-dependent manner. We propose that GATE–16 modulates intra-Golgi transport through coupling between NSF activity and SNAREs activation.
Keywords: Golgi/membrane transport/NSF/SNAREs
Introduction
Vesicular transport of proteins is mediated by coated vesicles that bud from membrane-bound compartments and are then targeted and fused with the appropriate acceptor organelle (Palade, 1975; Rothman, 1994). This process is highly conserved from yeast to man (Ferro–Novick and Jahn, 1994).
Docking of a vesicle at the appropriate target membrane involves the interaction between integral membrane proteins located on the vesicle, the v–SNAREs, and the t–SNAREs at the target membrane (Söllner et al., 1993b; Pfeffer, 1996). Prior to pairing between v– and t–SNAREs, initial docking of a vesicle with its target organelle is mediated by a peripheral membrane protein, p115 (Nakamura et al., 1997), originally identified as a protein needed for intra-Golgi transport (Waters et al., 1992). P115 interacts with two integral membrane proteins, giantin, located on COPI vesicles, and GM130, a Golgi matrix protein, thus providing a bridge between vesicles and their target membrane (Sonnichsen et al., 1998). Uso1p, the yeast homolog of the mammalian p115, has been suggested to act together with the small GTPase Ypt1p prior to the formation of SNARE pairs (Lupashin et al., 1996; Sapperstein et al., 1996; Lupashin and Waters, 1997) and initiate docking of endoplasmic reticulum (ER)-derived COPII vesicles to the Golgi (Cao et al., 1998). Ypt7p was also shown to have a role in the initial docking (tethering) between two vacuoles (Ungermann et al., 1998).
The events that follow vesicles docking and lead to membrane fusion are still being investigated. It has been argued that the SNARE complex allows the cell to overcome the energy barrier required for membrane fusion (Fasshauer et al., 1997; Hanson et al., 1997). Furthermore, using liposomes reconstituted with t– or v–SNAREs, Rothman and co-workers showed that the v–SNARE–t–SNARE complex per se fulfills the minimal requirement for fusion between two membranes (Weber et al., 1998; Nickel et al., 1999; Parlati et al., 1999). Yet, based on an in vitro system that reconstitutes homotypic fusion of yeast vacuoles, Ungermann and co-workers (1998) deduced that the formation of the SNARE complex is only an intermediate step in the overall fusion reaction. According to this view, SNARE molecules are involved in docking between donor and acceptor membranes, while another set of proteins participates in subsequent stages of the fusion process. This notion is supported by Peters and Mayer (1998), who suggested that calmodulin and other as yet unidentified factors are involved in mediating late stages of vacuolar fusion.
Following its formation, the v–SNARE–t–SNARE complex binds two soluble factors: N–ethylmaleimide-sensitive fusion protein (NSF) and soluble NSF attachment protein (SNAP). These in turn catalyze the disassembly of the SNARE complex (Söllner et al., 1993a) after a round of fusion, thus allowing a new round to take place (Mayer et al., 1996; Otto et al., 1997; Ungermann et al., 1998).
A variety of cell-free systems that reconstitute distinct transport steps were utilized to identify many proteins implicated in intracellular vesicular transport. A well studied transport system that reconstitutes transport of proteins between early Golgi cisternae was developed originally by Rothman and co-workers (Balch et al., 1984). NSF, SNAP, p115, p16 (therein termed GATE–16), phosphatidylinositol transfer protein α and a 13S Golgi transport complex were isolated as cytosolic factors essential for this assay (Block et al., 1988; Clary et al., 1990; Waters et al., 1992; Legesse–Miller et al., 1998; Paul et al., 1998; Walter et al., 1998). However, additional soluble factors are required to reconstitute intra-Golgi transport (Clary et al., 1990; Waters et al., 1992; Elazar et al., 1994b; Legesse–Miller et al., 1998).
Bet3p, a hydrophilic factor originally identified as a synthetic lethal together with the bet1–1 mutant (Rossi et al., 1995), is apparently part of a large peripheral membrane protein complex localized in the cis–Golgi, which is involved in docking and fusion of ER-derived vesicles (Sacher et al., 1998). In addition, the low Mr activity (LMA1), a heterodimer composed of thioredoxin and IB2, participates in vacuolar homotypic fusion (Xu and Wickner, 1996; Xu et al., 1997) as well as in fusion of ER-derived COPII vesicles with the Golgi (Barlowe, 1997; Cao et al., 1998). A mechanism for the function of LMA1 in vacuolar fusion was described recently (Xu et al., 1998; Ungermann et al., 1999). Accordingly, LMA1 first interacts with Sec18 and then, in an ATP-dependent manner, it is transiently transferred to Vam3p, a vacuolar t–SNARE.
We recently have identified and purified a 16 kDa polypeptide from bovine brain cytosol on the basis of its activity in a cell-free intra-Golgi transport system (Legesse–Miller et al., 1998). Utilizing the amino acid sequence derived from the 16 kDa polypeptide, we describe here the cloning of a novel, highly conserved soluble protein, localized predominantly in the Golgi, which we tentatively term GATE–16 (Golgi-associated ATPase Enhancer of 16 kDa). This protein exhibits high amino acid sequence homology to the human GABAA receptor-associated protein (GABAA-RAP) (Wang et al., 1999) and to the light chain 3 (LC3) of neuronal microtubule-associated proteins (MAPs) (Mann and Hammarback, 1994, 1996). A GATE–16 homolog in Saccharomyces cerevisiae, Aut7/Apg8, recently was reported to be involved in the autophagic process (Lang et al., 1998; Kirisako et al., 1999).
In this study, we demonstrate that GATE–16 interacts with NSF, enhancing its ATPase activity. In addition, GATE–16 interacts specifically with GOS–28, a Golgi-specific v–SNARE, in an NSF- and SNAP-dependent manner. We suggest that GATE–16 is transferred from NSF to GOS–28 in an ATP-dependent manner, stabilizing GOS–28 in a ‘primed’ form. We hypothesize that GATE–16 links between NSF activity and SNARE activation.
Results
Cloning the cDNA encoding GATE–16
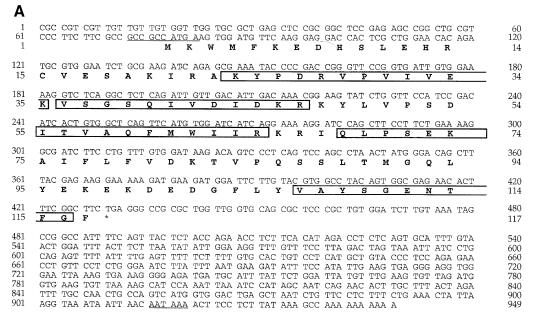
Previously we have purified a 16 kDa polypeptide from bovine brain cytosol based on its activity in a cell-free intra-Golgi transport assay (Legesse-Miller et al., 1998). The identity of the pure protein was determined by the amino acid sequence analysis of five tryptic peptides derived from the 16 kDa polypeptide band. Two of these peptides were used to synthesize degenerate oligonucleo– tides. A PCR product of 150 bp was used to screen a λZAP bovine brain cDNA library, leading to the identification of a complete open reading frame (ORF) of a 117 amino acid protein, denoted GATE–16 (Figure 1A). A Kozak conserved sequence for translation initiation is present at the beginning of the ORF and a polyadenylation signal is located 486 bases downstream of the last amino acid of GATE–16, followed by the polyadenylation site (Figure 1A). All tryptic peptides obtained from the purified protein were detected in the GATE–16 ORF (framed in Figure 1A).
Fig. 1. Sequence of bovine GATE–16. (A) Sequence of the cloned GATE–16 cDNA from bovine brain and the deduced amino acid sequence. Boxed amino acids correspond to sequences obtained from tryptic fragments of the purified protein. Underlined nucleotides at the 5′–untranslated region represent the ‘Kozak’ sequence for initiation of translation; underlined nucleotides at the 3′–untranslated region represent the signal for poly– adenylation. (B) GATE–16 aligned with its homologs using the ClustalW multiple sequence alignment program version 1.7. Sequence alignment is depicted by the SeqVu 1.0.1 program, identity is represented by a black frame and homology by a gray background. Mammalian GATE–16 from bovine (accession No. AF20262), rat (AB 003515), mouse (AA124324) and human (AJ010569) (full-length EST sequences found at the NCBI) are 100% identical in their amino acid sequences. (C) To determine the expression pattern of GATE–16, homogenates were prepared from different organs of a freshly sacrificed rat, and 42 μg of each sample were separated by 14% SDS–PAGE. GATE–16 was visualized by Western blotting using affinity-purified anti-GATE–16 antibodies. No differences were observed in the total protein between the different samples, as determined by amido black staining of the nitrocellulose filters.
GATE–16 exhibits significant homology to proteins found in eukaryotes ranging from yeast to human. For example, the S.cerevisiae GATE–16 homolog (Aut7p/Apg8) shows 56% identity and 75% similarity to the mammalian protein (Figure 1B). A search of the expressed sequence tag database (dbEST) at the NCBI (National Center for Biotechnology Information) yielded cDNA sequences for mouse, rat and human GATE–16 that are 100% identical to the bovine protein. Taken together, these findings indicate that GATE–16 is a highly conserved protein. Two other GATE–16-related mammalian proteins were reported previously: LC3 of neuronal MAPs (Mann and Hammarback, 1994) and GABA-RAP (Wang et al., 1999). These proteins exhibit 38 and 57% identity, and an overall 68 and 88% similarity to GATE–16, respectively (Figure 1B), alluding to the existence of a GATE–16-related protein family.
We determined the expression pattern of GATE–16 by Western blot analysis using affinity-purified anti-GATE–16 polyclonal antibodies. GATE–16 was found in all secretory organs studied (Figure 1C). The expression of GATE–16 was significantly higher in brain tissue, suggesting a specific role for this protein in neurons. Although in most tissues the anti-GATE–16 antibodies recognized predom– inantly the 16 kDa polypeptide (GATE–16), in tissues such as intestine and brain these antibodies recognized an additional minor 18 kDa polypeptide. The identity of this minor cross-reacting polypeptide is as yet unknown.
Recombinant GATE–16 is active in intra-Golgi transport in vitro
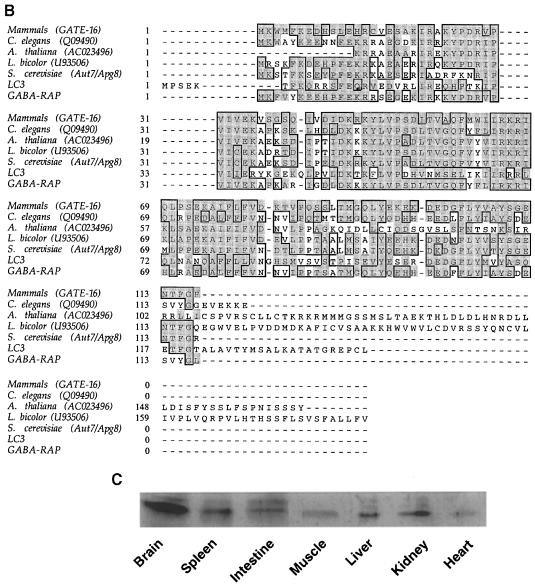
To establish that the isolated cDNA encodes an active GATE–16, we subcloned the coding region into a pRSET–C vector to produce a protein tagged with six histidine residues at its N–terminus (His6GATE–16). The protein was expressed in Escherichia coli and purified on nickel-nitrilotriacetic acid (Ni-NTA)–agarose and Mono-S columns (Figure 2A). The activity of the recombinant GATE–16 was tested in the GATE–16-dependent cell-free transport assay (Legesse–Miller et al., 1998). In this assay, each sample contained saturating levels of the known cytosolic factors such as αSNAP, NSF and p115, as well as a cytosolic fraction termed β, obtained by fractionating crude cytosol on an anion exchange column (Leggesse et al., 1998). As shown in Figure 2B, His6GATE–16 is active in the GATE–16-dependent intra-Golgi transport assay, similarly to the endogenous GATE–16 isolated from bovine brain. When αSNAP was not added to the reaction mixture, His6GATE–16 failed to stimulate the transport activity (Figure 2C), indicating that GATE–16 acts as part of the known transport machinery. Notably, addition of higher levels of αSNAP in the absence of GATE–16 failed to stimulate the assay signal (data not shown). Since most of the soluble transport factors are associated peripherally with the membrane, we tested the activity of GATE–16 in a GATE–16-dependent assay using Golgi membranes washed with 1 M KCl. Although the assay signal was significantly lower in comparison with standard Golgi membranes, GATE–16 appears active under these conditions too (Figure 2D).
Fig. 2. Recombinant GATE–16 is active in a cell-free intra-Golgi transport assay. GATE–16 cDNA was cloned into a pRSET–C vector and expressed in E.coli to create a His6-tagged recombinant protein as described in Materials and methods. (A) Proteins eluted from an Ni2+-NTA column (lane 1), unbound material (lane 2) and purified His6GATE–16 eluted from a Mono–S column (lane 3). Proteins were run on a 14% SDS–polyacrylamide gel and visualized by Coomassie blue staining. (B) Increasing amounts of GATE–16 purified from bovine brains (○) or His6GATE–16 (•) were added to the GATE–16-dependent cell-free transport assay (see Materials and methods). (C) His6GATE–16 (25 ng) was added to the GATE–16-dependent transport assay in the presence or absence of 30 ng of αSNAP. Assays were carried out in duplicate, and the mean is plotted with the error bar representing the higher value. (D) Salt-washed Golgi membranes (1 M KCl) were used in the GATE–16-dependent transport assay in the absence or presence of 25 ng of His6-GATE–16.
We next used polyclonal anti-GATE–16 antibodies, purified on nitrocellulose strips containing pure His6GATE–16, to assay the role of the endogenous protein in the cell-free transport assay reconstituted with crude bovine brain cytosol. These antibodies inhibited up to 90% of the transport activity, with half-maximal inhibition in the presence of ∼90 ng of antibodies (Figure 3A); immunoglobulins obtained from a pre-immune serum did not affect the transport. Furthermore, the inhibitory effect of anti-GATE–16 antibodies was reversed specifically by His6GATE–16 (Figure 3B) but not by αSNAP (data not shown), again demonstrating the specificity of this inhibition.
Fig. 3. Anti-GATE–16 antibodies inhibit intra-Golgi transport in vitro. (A) Increasing amounts of anti-GATE–16 antibodies (•) or IgGs from pre-immune serum (○) were added to the cell-free transport assay in the presence of 50 μg of crude bovine brain cytosol. (B) Anti-GATE–16 antibodies (150 ng) and 2.5 μg of His6GATE–16 were added to the standard transport reaction as indicated. (C) A standard intra-Golgi transport assay was carried out at 30°C for 2 h. At the indicated time points (marked by the symbols), anti-GATE–16 antibodies (150 ng, ○), Rab-GDI (1 μg, ▪) or GTPγS (50 μM, □) were added and the reaction was terminated after 2 h. The progression of transport in the absence of inhibitors was measured by transferring samples to ice at the indicated time points (•).
In the experiment described in Figure 3C, the transport assay was either terminated at different time points by ice, or each of the inhibitors, anti-GATE–16, Rab-GDI or GTPγS, was added at these time points, after which the reaction was allowed to proceed for a 2 h incubation period. The resistance of the reaction during a distinct time interval may be indicative of the sequence of events during the intra-Golgi transport assay. Standard samples, treated with buffer only, were incubated further at 30°C until the end of the 2 h incubation period and served as control (100% transport). All three inhibitors, when added at the onset of the reaction, produced ∼90% inhibition of transport. The reaction became resistant to the anti-GATE–16 antibodies when these were added 40–60 min after transport was initiated. Apparently GATE–16 is acting prior to the glycosylation step represented by the ice samples. The assumption that GATE–16 operates late in the transport process is supported by the finding that the transport became resistant to the anti-GATE–16 antibodies after the inhibition by GTPγS and Rab-GDI, which block vesicle uncoating or vesicle tethering, respectively.
GATE-16 interacts with NSF
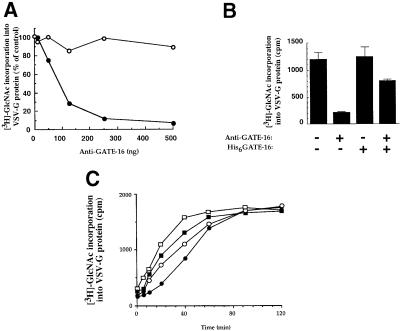
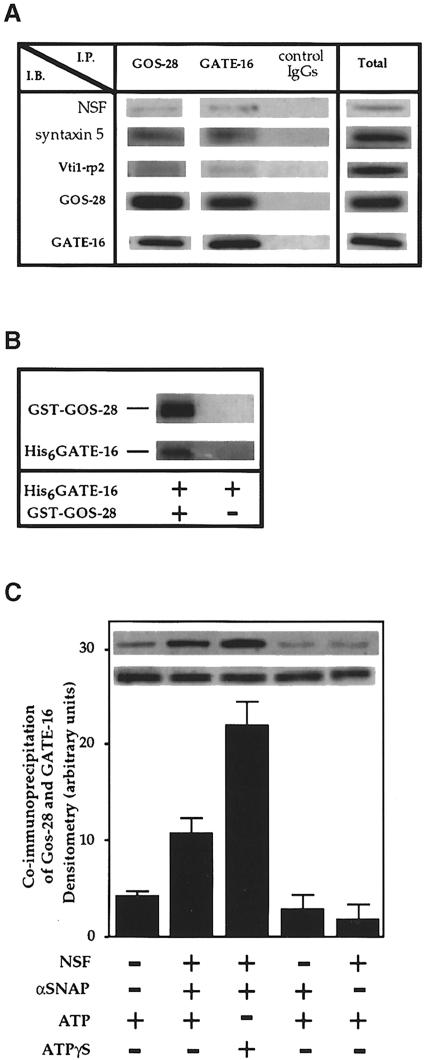
To characterize further the mechanism by which GATE–16 stimulates intra-Golgi transport, we searched for proteins that interact specifically with it. Protein A–Sepharose beads coupled to anti-GATE–16 antibodies were mixed with crude bovine brain cytosol, then washed, and the eluted material was subjected to Western blot analysis with different antibodies. Anti-GATE–16 antibodies specifically co-precipitated GATE–16 and significant amounts of NSF (Figure 4A). Similarly, when we used anti-NSF antibodies to precipitate NSF from the cytosol, GATE–16 co-immunoprecipitated (Figure 4A) while other factors, such as αSNAP, p115 and the ATPase p97, did not (data not shown). These results indicate that GATE–16 interacts specifically with NSF in the cytosol, although they do not exclude an indirect interaction between the two. To address this issue, we incubated His6-tagged GATE–16 with recombinant Myc-tagged NSF. As shown in Figure 4B, anti-Myc monoclonal antibodies co-precipitated NSF with significant amounts of GATE–16, indicating that GATE–16 and NSF interact directly.
Fig. 4. GATE–16 interacts specifically with NSF. (A) Bovine brain cytosol (1.2 mg) was immunoprecipitated (IP) with anti-GATE–16 and anti-NSF antibodies, or with pre-immune IgGs as control. Immunoprecipitates were washed (see Materials and methods), and proteins were eluted with 2% SDS at 95°C for 2 min and analyzed by Western blots (IB) using the indicated antibodies. The right panel (cytosol) represents 150 ng of total cytosolic proteins. (B) His6GATE–16 (200 ng) was mixed with agarose beads coupled to anti-Myc epitope monoclonal antibodies (20 μl) in the presence or absence of Myc-tagged NSF (300 ng) for 120 min at 4°C. The immunoprecipitates were immunoblotted and reacted with anti-GATE–16 and anti-NSF antibodies. (C) ATPase activity of NSF was measured in 50 μl of reaction buffer (10 mM PIPES–KOH pH 6.8, 200 mM sucrose, 150 mM MnCl2, 1 mM ATP and 2 mM DTT) in the presence of 1.5 μg/ml His6NSF and the indicated concentration of His6GATE–16 (•), or in the presence of heat-inactivated His6GATE–16 (65°C, 30 min) (▴). Samples were incubated for 2 h at 30°C and ATPase activity was determined as described (Lill et al., 1990). The dashed line indicates ATPase activity measured in the absence of NSF. The background signal from reactions incubated with NSF and ATP on ice was subtracted.
GATE-16 activates NSF ATPase activity
NSF is a member of the AAA protein family of ATPases (Patel and Latterich, 1998). The very low ATPase activity of NSF is stimulated ∼2–fold by αSNAP molecules attached to plastic (Morgan et al., 1994). Within the complex of NSF and SNAREs, such ATPase activity leads to the dissociation of the complex (Söllner et al., 1993a). We tested the effect of increasing concentrations of recombinant GATE–16 on the ATPase activity of His6-Myc-NSF. As shown in Figure 4C, GATE–16, which by itself lacks ATPase activity (dashed line), stimulated the NSF ATPase activity by up to 3.5–fold, whereas heat-inactivated GATE–16 had no effect on the assay. Similar results were obtained when endogenous GATE–16 was used to stimulate the NSF ATPase activity (data not shown). Notably, maximal stimulation of NSF ATPase activity was observed at a molar ratio of ∼2:1 NSF to GATE–16. Evidently, the direct interaction between GATE–16 and NSF has a functional role.
GATE-16 is localized on the Golgi apparatus
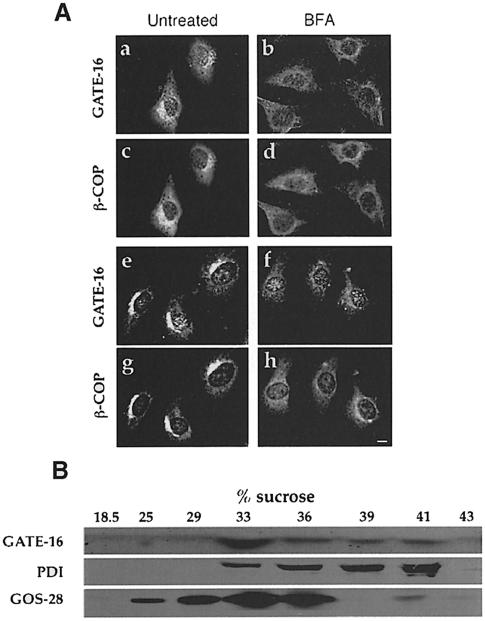
Affinity-purified anti-GATE–16 polyclonal antibodies were used to determine the intracellular localization of GATE–16 in NRK and NIH 3T3 cells by indirect immunofluorescence. Using methanol to fix and acetone to permeabilize the cells, a clear juxtanuclear labeling was observed with anti-GATE–16 antibodies (Figure 5A). No labeling was observed when anti-GATE–16 antibodies were incubated with excess His6GATE–16 (data not shown). To identify the subcellular localization of GATE–16, we performed a double labeling experiment using both monoclonal antibodies directed against the β–subunit of COPI (β–COP) that label the Golgi, and anti-GATE–16 antibodies. We showed by confocal microscopy that labeling with anti-GATE–16 antibodies overlapped that of β–COP, indicating that GATE–16 is localized in these cells on the Golgi (Figure 5A, a, c, e and g). Brefeldin A (BFA) previously was shown specifically to disassemble the Golgi complex in vivo (Lippincott–Schwartz et al., 1989). Here we showed that in the presence of BFA, the labeling observed by both anti-GATE–16 and anti-β–COP was significantly reduced (Figure 5A, b, d, f and h). Similar labeling by anti-GATE–16 antibodies was observed in CHO cells and in hippocampal neurons (data not shown). These results clearly demonstrate that GATE–16 is localized in vivo predominantly on the Golgi complex.
Fig. 5. GATE–16 is localized on the Golgi. (A) NRK (a–d) and NIH 3T3 cells (e–h) were incubated in the absence (a, c, e and g) or presence (b, d, f and h) of 15 μM BFA for 1 h before fixation. Cells were incubated with both anti-GATE–16 antibodies and mouse monoclonal anti-β–COP antibodies. Rhodamine-conjugated goat anti-rabbit IgGs were used to detect GATE–16 (a, b, e and f); FITC-conjugated goat anti-mouse IgGs were used to detect β–COP (c, d, g and h). Bar = 10 μm. (B) Bovine brain post-nuclear supernatant in 0.5 M sucrose was overlaid on top of a sucrose step gradient composed of 0.86 and 1.25 M sucrose layers. Following centrifugation, the 0.86/1.25 sucrose interface was adjusted to 1.6 M sucrose and loaded at the bottom of a second step gradient of 1.25, 1.0, 0.86 and 0.5 M sucrose. The gradients were centrifuged, and the indicated fractions were analyzed by immunoblotting with either anti-GATE–16 antibodies, anti-GOS–28 (a Golgi marker) or anti-PDI (an ER marker) antibodies.
The intracellular localization of GATE–16 was also determined by subcellular fractionation of bovine liver post-nuclear supernatant in two sequential equilibrium density sucrose gradients. Membranes concentrated at the 0.86/1.25 interface of the first gradient were harvested (see Materials and methods), adjusted to 1.6 M sucrose and loaded at the bottom of a second gradient (Figure 5B). Fractions were analyzed by immunoblotting with affinity-purified anti-GATE–16 antibodies and with antibodies that recognize either the Golgi marker GOS–28, or PDI, an ER marker. Immunoblot analysis of the second gradient showed that GATE–16 co-localized predominantly with GOS–28, indicating that it is associated mainly with the Golgi. GATE–16 could be dissociated from the Golgi membrane by 1 M KCl (data not shown), indicating that it is a peripheral membrane protein.
GATE-16 interacts with the Golgi v–SNARE GOS–28
As shown above, GATE–16 has been identified as a Golgi peripheral membrane protein. We then tested the ability of GATE–16 to interact with Golgi membrane proteins, attempting to identify the membrane target of this protein. As shown in Figure 6A, anti-GATE–16 antibodies specifically co-immunoprecipitated GATE–16 with GOS–28, a Golgi-specific v–SNARE implicated in ER to Golgi and intra-Golgi transport (Nagahama et al., 1996; Subramaniam et al., 1996). In addition, anti-GOS–28 antibodies specifically immunoprecipitated significant amounts of GATE–16 (Figure 6A). Other Golgi SNAREs such as Vti1–rp2 (Figure 6A) or GS–15 (data not shown) did not co-precipitate with GATE–16, nor did other Golgi membrane proteins such as p23 and p24 (data not shown). In comparison with anti-GOS–28 antibodies, only a small fraction of the Golgi t–SNARE syntaxin 5 was co-immunoprecipitated by anti-GATE–16 antibodies, suggesting that GATE–16 interacts mostly with GOS–28 and to a much lesser extent with syntaxin 5. Notably, small amounts of NSF found in the membrane extract co-immunoprecipitated with anti-GATE–16 antibodies as well as with GOS–28 antibodies.
Fig. 6. GATE–16 interacts with GOS–28. (A) Detergent extracts (40 μg) of rat liver Golgi membranes and His6-GATE–16 (1 μg) were immunoprecipitated with either anti-GATE–16, anti-GOS–28 antibodies, or pre-immune IgGs as control. Immunoprecipitates were analyzed by Western blots using the indicated antibodies. Total (right panel) represents 10% of the membrane detergent extract used for each experiment in the presence of His6-GATE–16. (B) Glutathione–agarose beads were incubated with His6GATE–16 in the presence of recombinant GST–GOS–28 (left) or GST alone (right). The glutathione–agarose beads were washed and the bound material was eluted with free glutathione and analyzed by Western blots. (C) Detergent extracts were immunoprecipitated with anti-GATE–16 antibodies in the presence of 5 mM MgCl2 and with either NSF (0.3 μg), αSNAP (0.8 μg), 1 mM ATP or 1 mM ATPγS, as indicated. The immunoprecipitates were analyzed by Western blots to detect co-immunoprecipitated GOS–28. Panels show immunoblots of GOS-28 (top) and GATE-16 (bottom) of the respective samples.
To determine whether GATE–16 interacts directly with GOS–28, we used a fusion protein between GST and the cytosolic tail of GOS–28 (GST–GOS–28). His6GATE–16 was incubated with glutathione–agarose in the presence of GST–GOS–28 fusion protein or GST alone. The presence of GATE–16 and GOS–28 in the eluted material was determined by specific anti–GATE–16 and anti-GOS–28 antibodies (Figure 6B). His6GATE–16 did not interact with recombinant GST–syntaxin 5 fusion protein (data not shown). These results confirmed a direct and specific interaction between GATE–16 and GOS–28.
Since our working hypothesis is that GATE–16 first interacts with NSF and then is transferred to GOS–28, we tested the ability of NSF and SNAP to enhance the association between GATE–16 and GOS–28. Golgi membrane extracts were incubated with recombinant Myc-NSF and His6SNAP in the presence of ATP—conditions that stimulate the dissociation of SNARE complexes (Söllner et al., 1993a). As depicted in Figure 6C, NSF, SNAP and ATP significantly increased the interaction between GATE–16 and GOS–28. This interaction does not require ATP hydrolysis because in the presence of ATPγS, the non-hydrolyzable analog of ATP, the interaction between GATE–16 and GOS–28 was stimulated further. Addition of either NSF in the absence of SNAP, or SNAP in the absence of NSF had no stimulatory effect on this interaction. The molar ratio of GATE–16 to GOS–28 found in the immunoprecipitate in the presence of ATPγS was ∼1:1. Apparently, GATE–16 interacts with GOS–28 in an NSF- and SNAP-dependent manner.
GATE-16 protects GOS-28 and syntaxin 5 from proteolysis
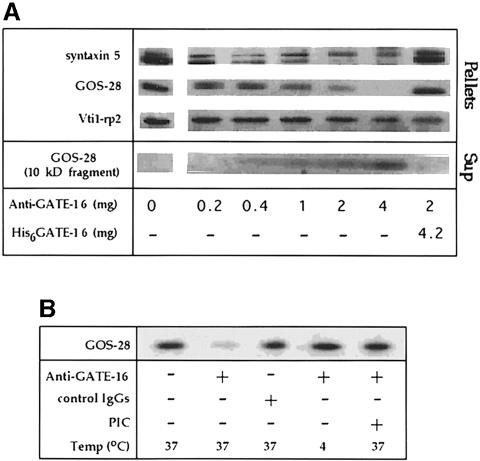
As shown previously, the interaction between GATE–16 and GOS–28 requires NSF, SNAP and ATP (Figure 6C). We also demonstrated that the participation of GATE–16 in intra-Golgi transport is blocked specifically in vitro by anti-GATE–16 antibodies (Figure 3). To elucidate the nature of the linkage between the effect of GATE–16 on intra-Golgi transport and its interaction with SNARE molecules, we tested the influence of these affinity-purified anti-GATE–16 antibodies on Golgi-associated SNARE molecules under transport conditions. As shown in Figure 7A, addition of the anti-GATE–16 antibodies to the transport reaction was accompanied by a dramatic depletion from the membrane of both GOS–28 and its t–SNARE partner, the low molecular weight isoform of syntaxin 5 (Hay et al., 1998). Pre-immune IgGs serving as a control in these experiments had no effect. The effect of anti-GATE–16 antibodies on the Golgi SNAREs was blocked specifically by recombinant GATE–16, indicating that the antibodies act by associating with the endogenous GATE–16 (Figure 7A). Anti-GATE–16 antibodies had no influence on the Golgi SNAREs when the reaction mixture was kept on ice, nor when a protease inhibitor cocktail was present (Figure 7B), indicating that the loss of the SNARE molecules from the membrane pellets resulted from proteolysis. Furthermore, a fragment of ∼10 kDa corresponding to GOS–28 appeared in the supernatant in the presence of anti-GATE–16 antibodies (Figure 7A). This proteolysis is specific because other Golgi SNAREs such as Vti1–rp2, the high molecular weight isoform of syntaxin 5 (Figure 7A) and Gs15 (data not shown), none of which interact with GOS–28, remained intact. Notably, anti-GATE–16 antibodies did not affect the amount of the Golgi membranes in the different pellets, as judged by their mannosidase activity (data not shown). A good correlation was found between the inhibition of transport observed in the presence of the anti-GATE–16 antibodies (Figure 3A) and their effect on the SNARE molecules (Figure 7A), suggesting that this is the mechanism by which the antibodies block transport. Indeed, Golgi membranes treated with anti-GATE–16 antibodies irreversibly lost ∼70% of their ability to mediate intra-Golgi transport.
Fig. 7. GATE–16 protects the membrane-bound GOS–28 from proteolysis. (A) Golgi membranes (12.5 μg) were incubated in a final volume of 100 μl under standard transport conditions (see Materials and methods) with the indicated amounts of anti-GATE–16 antibodies and recombinant GATE–16 for 15 min at 37°C. Membranes were then mounted on top of a 15% sucrose cushion and pelleted by ultracentrifugation. The membrane pellets and the supernatant trichloroacetic acid precipitates were subjected to Western blot analysis. To detect the 10 kDa fragment corresponding to the GOS–28 degradation product, the image was sharpened using the Adobe Photoshop program. (B) Golgi membranes were incubated at the indicated temperature with 1 μg of anti-GATE–16 antibodies or with 1 μg of pre-immune IgGs, in the presence or absence of a protease inhibitor cocktail (PIC), and analyzed by Western blots as described above. The PIC consisted of leupeptin (0.5 μg/ml), pepstatin A (1.4 μg/ml), aprotinin (2 μg/ml) and PMSF (1 mM).
Discussion
Protein transport between two organelles is a multistep process that requires formation of vesicles and their targeting to a recipient membrane, followed by docking and fusion processes. In this study, we describe a soluble transport factor, GATE–16, which is involved in late stages of intra-Golgi protein transport. We suggest that GATE–16 specifically interacts with NSF, which in turn presents it to the Golgi-specific v–SNARE GOS–28. Recombinant GATE–16 significantly stimulated intra-Golgi transport in vitro provided that other known transport factors such as SNAP and p115 were present. Moreover, affinity-purified anti-GATE–16 antibodies specifically inhibited the transport assay reconstituted with crude cytosol, ruling out cytosol fractionation as a prerequisite for GATE–16 activity. Immunofluorescence and subcellular fractionation showed that GATE–16 is localized mainly on the Golgi complex, suggesting that it functions within this organelle in vivo.
The amino acid sequence of GATE–16 is highly conserved in evolution, implying a fundamental physiological role for this protein. GATE–16 is found in secretory organs such as brain, liver, kidney, spleen and intestine. In some tissues, another minor 18 kDa polypeptide is recognized, which might be a related protein, such as GABA–RAP (Wang et al., 1999) or LC3 of the neuronal MAPs (Mann and Hammarback, 1994, 1996).
The requirement for GATE–16 in intra-Golgi transport in vitro suggests that it is an integral part of the protein trafficking machinery. We have shown previously that GATE–16 isolated from bovine brain was active in the absence of budding factors such as ARF1 and coatomer and resistant to BFA or GTPγS (Legesse–Miller et al., 1998). This raises the possibility that GATE–16 is required only for uncoupled fusion (Orci et al., 1991; Elazar et al., 1994b). However, here we demonstrate that GATE–16 is also required for transport reconstituted with crude cytosol, i.e. conditions that allow the formation of coated vesicles.
NSF and αSNAP are general transport factors participating in almost all intracellular fusion events. We report here that within the cytosol, NSF is found in a complex with GATE–16. However, only a small portion of NSF is interacting with GATE–16, as indicated by our immuno– precipitation experiments. GATE–16, and possibly other proteins such as LMA1 and p13 (Xu et al., 1998), may enhance the specificity of NSF towards different subcellular targets. In this study, we demonstrated that GATE–16 interacts with NSF and activates its ATPase activity. Maximum activation of NSF ATPase activity was observed when GATE–16 was added to NSF in a molar ratio of 1:2. A similar ratio was observed for αSNAP to NSF (Morgan et al., 1994) and for p47 to p97 (Kondo et al., 1997). It is well established that αSNAP is responsible for the actual attachment of NSF to the different SNAREs. Here we have shown that GATE–16 interacts with the Golgi v–SNARE, GOS–28, in an NSF- and SNAP-dependent manner. It is feasible that αSNAP removes GATE–16 from its NSF-binding site, freeing it to bind GOS–28. Other experiments are required to establish whether such transfer takes place.
The similarity between GATE–16 and the yeast LMA1 is striking: LMA1 first interacts with the yeast homolog of NSF, Sec18p, and upon ATP hydrolysis is transferred to Vam3p, a vacuolar t–SNARE, and is then released during fusion (Xu et al., 1998; Ungermann et al., 1999). Although there is no clear amino acid sequence homology between the two factors, GATE–16 may represent the first mammalian functional analog of LMA1 that interacts specifically with the Golgi v–SNARE GOS–28. Our experiments indicate that the interaction between GATE–16 and GOS–28 takes place in the presence of ATP, NSF and SNAP, conditions that allow the dissociation of the SNARE complex (Söllner et al., 1993a). Apparently GATE–16 interacts with the free form of GOS–28, an idea supported by the finding that recombinant GATE–16 and GST–GOS–28 interact directly. Our data also indicate that the interaction between GATE–16 and GOS–28, mediated by NSF and SNAP, is stimulated further in the presence of the non-hydrolyzable analog of ATP, ATPγS; evidently, ATP hydrolysis is not required for this process.
NSF and SNAP induce the dissociation of the SNARE complex in a reaction that requires ATP hydrolysis. According to Mayer et al. (1996), NSF and SNAP dissociate v–SNARE–t–SNARE complexes, activating them prior to the docking stage. It is thus feasible that factors such as LMA1 and GATE–16 are involved in maintaining the dissociated SNARE molecules in an active form, possibly preventing their re-association. The function of a SNARE ‘protector’ was also assigned to Sec1p and to its mammalian homologs, n–Sec1, mun18 and rbSec1, which interact with free t–SNAREs (Pevsner et al., 1994; Pevsner, 1996). GATE–16, however, represents the first soluble factor that interacts specifically with a Golgi v–SNARE. Furthermore, in contrast to the SNARE protectors that function as negative regulators of the fusion process, GATE–16 has a positive role, exerting an active, promoting effect on the overall transport process. We therefore propose that the interaction between GATE–16 and GOS–28 maintains the SNARE molecule in a conformational state ready for the next round of fusion.
Support for the role of GATE–16 in maintaining the proper conformational state of SNAREs comes from the surprising effect of the anti-GATE–16 antibodies on the Golgi SNAREs. The experiments presented in Figure 7 suggest that under transport conditions, GATE–16 specifically protects the SNARE molecules with which it interacts from proteolysis. Accordingly, in the absence of a functional GATE–16, GOS–28 and syntaxin 5 change their conformational state significantly, rendering them susceptible to proteases. The protease inhibitory activity of GATE–16 therefore does not reflect its physiological role but rather its effect on the state of the SNARE molecules with which it interacts.
Recently, Wang et al. (1999) suggested that the GABA receptor-associated protein GABAA-RAP, which exhibits high sequence homology to GATE–16, is involved in linking GABA receptors to the cytoskeleton. GABA-RAP co-localizes with GABA receptors in a punctated structure throughout the cell membrane, whereas GATE–16 is localized specifically in the Golgi. It is thus feasible that GABA-RAP functions specifically in neuronal cells, mediating the function of membrane receptors such as the GABAA receptor, whereas GATE–16 functions in intracellular protein transport, regulating the activity of membrane-bound SNAREs. We propose that the GATE–16 protein family has a pleiotropic effect, acting as chaperones for membrane proteins that undergo functional conformational changes. That molecules involved in vesicular transport may act in other systems has been shown, for example, for NSF and SNAP, which also affect GluR2, a postsynaptic membrane receptor, altering its channel activity (Nishimune et al., 1998; Osten et al., 1998).
The GATE–16 homolog in S.cerevisiae recently was reported to be involved in autophagic processes (Lang et al., 1998; Kirisako et al., 1999). Lang and co-workers suggested that this protein acts together with Aut2 in the delivery of autophagosome to the vacuole (Lang et al., 1998); Kirisako and co-workers suggested that this protein plays an important role in autophagosome formation (Kirisako et al., 1999). It is clear, however, that membrane fusion is essential for either the formation of an autophagosome or its delivery to the vacuole. Based on the data obtained for GATE–16, we suggest that Aut7p/Apg8 mediates membrane fusion in the autophagic pathway. We found that Aut7p/Apg8 can partially replace GATE–16 in the cell-free intra-Golgi transport assay and interact specifically with v–SNAREs involved in early secretion stages as well as with a vacuolar v–SNARE (Z.Elazar and A.Legesse-Miller, to be published elsewhere). We cannot rule out the possibility that these two proteins play different roles in yeast versus mammalian cells. However, it is conceivable that GATE–16 is involved in constitutive transport under normal growth conditions, whereas under stress conditions, such as starvation, it is essential for the enhanced transport of autophagosomes from the cytoplasm to the lysosomes. AUT7 is the only relative of GATE–16 in the yeast genome. It appears that under normal growth conditions, this gene is non-essential (Lang et al., 1998; Kirisako et al., 1999). Our data suggest that GATE–16 exerts its action by interacting with the Golgi v–SNARE, GOS–28. Consistent with this, Gos1p, the yeast homolog of GOS–28, is also a non-essential protein involved in multiple transport steps, in particular ER to Golgi and intra-Golgi transport (McNew et al., 1998). Moreover, in the aut7 null strain, other factors such as LAM1 or p13 may substitute its function.
The following model for the function of GATE–16 during late stages of membrane transport is suggested. GATE–16 binds to and forms a complex with NSF in the cytosol, which in turn interacts with SNAP molecules bound to SNARE complexes on the membrane. It has been shown recently that αSNAP is associated with a GOS–28–syntaxin 5 complex via direct interaction with GOS–28 (Subramaniam et al., 1997). The attachment of NSF to SNAP molecules bound to GOS–28 may facilitate the release of GATE–16 from NSF, enabling its interaction with GOS–28, a reaction that precedes the hydrolysis of ATP by NSF. Next, ATP hydrolysis by NSF catalyzes the disassembly of the SNARE complex, leaving GATE–16 bound to GOS–28. We speculate that this interaction is essential for keeping GOS–28 in a stable conformation that allows the next step of docking and fusion.
Materials and methods
Preparation of cytosolic factors
Bovine brain cytosol was prepared by the method of Malhotra et al. (1989). Fraction Iβ, recombinant His6NSF and His6αSNAP were prepared as described (Legesse–Miller et al., 1998). P115 was purified from bovine liver cytosol as described (Waters et al., 1992; Elazar et al., 1994a).
Intra-Golgi transport assay
The standard assay mixture (25 μl) contained 0.4 μCi of UDP-[3H]N-acetylglucosamine (America Radiolabeled Chemical), 5 μl of a 1:1 mixture of donor and acceptor CHO Golgi membrane, and crude bovine brain cytosol. Transport reactions were incubated at 30°C for 2 h and the incorporation of [3H]N-acetylglucosamine into vesicular stomatitis virus (VSV)-G protein was determined as described previously (Balch et al., 1984). The GATE–16-dependent assay was performed as described previously (Legesse-Miller et al., 1998). Briefly, each assay contained 0.4 μCi of UDP-[3H]N-acetylglucosamine, 5 μl of a 1:1 mixture of donor and acceptor CHO Golgi membrane, 100 μg of Iβ, 0.5 μg of p115, 5 ng of His6NSF, 60 ng of His6SNAP, 10 μM palmitoyl-CoA, and ATP and UTP regeneration systems, unless otherwise indicated in the figure legends. Salt-washed membranes (1 M KCl) were prepared as described previously (Waters et al., 1992).
Peptide sequencing
GATE–16 was purified from bovine brain cytosol as described previously (Legesse–Miller et al., 1998). About 2 μg of isolated GATE–16 was subjected to SDS–PAGE followed by blotting onto nitrocellulose. The GATE–16 polypeptide was excised, digested by trypsin, and the obtained peptides were separated by reverse-phase HPLC chromatography on a C18 column. The amino acid sequence of five different peptides was determined using an automated sequencer.
Screening for the GATE-16 gene
Degenerate oligonucleotides were synthesized based on the amino acid sequence of two GATE–16 tryptic peptides (VSGSQIVDIDK and YVAYSGENTFGF). PCR was performed using bovine brain cDNA (Stratagen) as a template, resulting in a 150 bp fragment. This fragment, radiolabeled with [α-32P]dATP (Stratagen, ‘Primeit’ kit), was used to screen ∼1 × 106 bacteriophage plaques of a λZAP bovine brain cDNA library (Stratagen). Thirteen positive plaques were subcloned and re-screened, giving rise to 10 positive colonies. The nucleotide sequence of six of these colonies was determined on the automated sequencer at the Weizmann Institute of Science sequencing unit. All colonies contained the full-length GATE–16 ORF of 117 amino acids.
Expression and purification of recombinant GATE–16
The ORF encoding GATE–16 was amplified by PCR using oligonucleo– tides containing an NcoI site for the N–terminus and an EcoRI site for the C–terminus of GATE–16. The PCR product was digested and subcloned into the pRSET–C bacterial expression vector (Invitrogen) to obtain a His6 N–terminal-tagged recombinant GATE–16 protein. The pRSET–C plasmid containing GATE–16 was transformed into E.coli BL–21. Cells were grown to an OD600 of 0.4–0.6 and induced with 1 mM isopropyl–1–thio–β–d–galactopyranoside (IPTG) for 3 h at 37°C. Following centrifugation in a Sorvall GS3 rotor at 3000 r.p.m. for 15 min, the pellet was re-suspended and sonicated in a breaking buffer [25 mM Tris–HCl pH 7.4, 250 mM KCl, 2 mM β–mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μg/ml leupeptin and 2 μM pepstatin]. The lysate was cleared by 30 min centrifugation at 40 000 r.p.m. in a Beckman Ti60 rotor and then loaded onto a nickel beads column (Ni2+-NTA, Qiagen). The column was washed with 20 mM imidazole in a breaking buffer, and elution was carried out in a gradient of 50–300 mM imidazole in the same buffer without KCl. For further purification, His6GATE–16 was dialyzed against 10 mM phosphate buffer pH 6.8, 50 mM KCl, 10% glycerol and 1 mM dithiothreitol (DTT), and loaded onto a Mono–S column (Pharmacia). The column was washed with 100 mM KCl, and His6GATE–16 was eluted with a KCl gradient (0.1–0.5 M). Samples were detected on SDS–PAGE, and the protein concentration was determined by the Bradford assay (Bio-Rad). Fractions containing pure His6GATE–16 were equilibrated to 25 mM Tris–HCl pH 7.4, 250 mM KCl, 2 mM DTT by gel filtration on a G–25 Sephadex column and stored at –70°C until use.
Antibody production
His6GATE–16 was purified as described above and used to raise polyclonal antibodies in rabbits by the animal service unit at the Weizmann Institute of Science. Polyclonal antibodies were affinity purified on nitrocellulose strips containing pure His6GATE–16.
Tissue distribution
To prepare rat tissue extracts, frozen organs were washed in cold phosphate-buffered saline (PBS) and lysed with homogenizer (PCU Kinematica) in ice-cold protein extraction buffer containing 0.5 M β–glycerophosphate, 15 mM EGTA, 10 mM EDTA, 1 mM orthovanadate, 1 mM benzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 μg/ml pepstatin A and 1 mM DTT, at pH 7.4. The lysate was cleared by a 15 min spin at 20 000 r.p.m. at 4°C. Supernatants were mixed with sample buffer, heated to 95°C for 2 min, and equal amounts of tissue extracts (42 μg) were subjected to SDS–PAGE (15% acrylamide). The resolved proteins were transferred to nitrocellulose (Sartorius) and probed with anti-GATE–16 antibodies using horseradish peroxidase (HRP)-coupled secondary antibodies and ECL reagent (Amersham).
Indirect immunofluorescence microscopy
NRK and NIH 3T3 cells were seeded on coverslips incubated in the absence or presence of 15 μM BFA for 1 h before fixation. For immunofluorescence, cells were fixed with cold methanol for 10 min at 20°C and then permeabilized with cold acetone for 1 min at room temperature. The coverslips were blocked by incubation with 10% fetal calf serum in PBS for 1 h at room temperature, followed by 2 h incubation with two primary antibodies: polyclonal anti-GATE–16 and M3A5 anti-βCOP monoclonal antibodies (Sigma). Cells were then incubated with rhodamine-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Jackson Immunoresearch Laboratories) for 2 h at room temperature. Stained cells were analyzed by MRC1024 confocal microscopy (Bio-Rad).
Subcellular fractionation
Fresh rat liver (25 g) was minced and added to 150 ml of ice-cold lysis buffer (0.5 M sucrose, 0.1 M KPi pH 6.8, 5 mM MgCl2, 1 mM DTT, 1 mM PMSF, 0.5 μg/ml leupeptin, 2 μM pepstatin A and 2 μg/ml aprotinin). The tissue was mildly homogenized by pressing over a metal grid. Rat liver lysates were fractionated over sucrose gradients as described for bovine brain (Walter et al., 1998). Briefly, the homogenate was centrifuged for 10 min at 1366 g, and 18 ml of the post-nuclear supernatant were overlaid on top of each of four 0.86 M sucrose (10 ml) and 1.25 M sucrose (10 ml) step gradients. Gradients were centrifuged at 4°C in an SW-28 rotor (Beckman) at 25 000 r.p.m. for 90 min with slow acceleration and deceleration. The four 0.86/1.25 M interfaces were collected with Pasteur pipets, adjusted to 1.6 M sucrose, and 10 ml were placed in the bottom of each of two SW-28 tubes, followed by overlaying with 1.25 M (7 ml), 1.0 M (7 ml), 0.86 M (7 ml) and 0.5 M (7 ml) sucrose solutions. The gradients were centrifuged at 4°C in an SW-28 rotor (Beckman) at 25 000 r.p.m. for 2.5 h with slow acceleration and deceleration. Fractions were collected continuously from the top of the gradient. From each fraction, 300 μl were diluted 1:1 with 0.1 M KPi and 5 mM MgCl2, and centrifuged at 100 000 r.p.m. in a TLA-100.1 rotor at 4°C for 10 min. Supernatants were removed and the pellets were analyzed by Western blot.
Immunoprecipitation
To prepare Golgi detergent extracts, 1.3 mg of rat liver Golgi membranes, obtained as described above, were centrifuged at 14 000 g for 10 min at 4°C, resuspended in 8 ml of buffer A (20 mM HEPES pH 7.4, 200 mM KCl, 1% Triton X-100, 0.5 μg/ml leupeptin, 2 μM pepstatin A and 2 μg/ml aprotinin) and incubated at 4°C for 30 min with gentle stirring. Insoluble material was pelleted by centrifugation at 14 000 g for 30 min at 4°C, and the supernatant (0.2 mg/ml) was kept at –70°C.
For the immunoprecipitation experiments, anti-GATE–16 antibodies were covalently coupled to protein A–agarose with dimethylpimelimidate, while anti-GOS–28 or anti-NSF monoclonal antibodies were coupled to protein G–agarose. The coupled antibodies were mixed gently with either 200 μl of bovine brain cytosol (6 mg/ml) or with 200 μl of rat liver Golgi extract (0.2 mg/ml) to which 1 μg of His6-GATE–16 was added, and incubated at 4°C for 16 h. The beads were washed five times with buffer A supplemented with 50 mM NaCl. The bound material was eluted with 30 μl of 2% SDS at 95°C for 3 min and analyzed by Western blotting.
Accession number
The nucleotide sequence data for the bovine GATE–16 appear in the DDBJ/EMBL/GenBank database under the accession No. AF20262.
Acknowledgments
Acknowledgements
We thank J.E.Rothman for providing His6αSNAP, His6-Myc-NSF, GST–GOS–28, anti-SNAP serum and anti-NSF monoclonal antibodies; W.Hong for anti-GOS–28, anti-syntaxin 5, anti-Vti1-rp2 and anti-GS15 antibodies; B.Balch for anti-Sec22 antibodies; J.Hay for GST–syntaxin 5; and F.Wieland for anti-p23 antibodies. We also thank Dan Cassel, Bernd Helms and Tony Futerman for critical reading of the manuscript. Z.E. is an incumbent of the Shloimo and Michla Tomarin Career Development Chair of Membrane Physiology. This work was supported in part by the Israel Science Foundation, the Israel Cancer Foundation and the Weizmann Institute Minerva Center.
References
- Balch W.E., Dunphy, W.G., Braell, W.A. and Rothman, J.E. (1984) Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell, 39, 405–416. [DOI] [PubMed] [Google Scholar]
- Barlowe C. (1997) Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol., 139, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M.R., Glick, B.S., Wilcox, C.A., Wieland, F.T. and Rothman, J.E. (1988) Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl Acad. Sci. USA, 85, 7852–7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Ballew, N. and Barlowe, C. (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J., 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D.O., Griff, I.C. and Rothman, J.E. (1990) SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell, 61, 709–721. [DOI] [PubMed] [Google Scholar]
- Elazar Z., Mayer, T. and Rothman, J.E. (1994a) Removal of Rab GTP-binding proteins from Golgi membranes by GDP dissociation inhibitor inhibits inter-cisternal transport in the Golgi stacks. J. Biol. Chem., 269, 794–797. [PubMed] [Google Scholar]
- Elazar Z., Orci, L., Ostermann, J., Amherdt, M., Tanigawa, G. and Rothman, J.E. (1994b) ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J. Cell Biol., 124, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Otto, H., Eliason, W.K., Jahn, R. and Brunger, A.T. (1997) Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein–attachment protein receptor complex formation. J. Biol. Chem., 272, 28036–28041. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S. and Jahn, R. (1994) Vesicle fusion from yeast to man. Nature, 370, 191–193. [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Roth, R., Morisaki, H., Jahn, R. and Heuser, J.E. (1997) Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell, 90, 523–535. [DOI] [PubMed] [Google Scholar]
- Hay J.C., Klumperman, J., Oorschot, V., Steegmaier, M., Kuo, C.S. and Scheller, R.H. (1998) Localization, dynamics and protein interactions reveal distinct roles for ER and Golgi SNAREs. J. Cell Biol., 141, 1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T., Baba, M., Ishihara, N., Miyazawa, K., Ohsumi, M., Yoshimori, T., Noda, T. and Ohsumi, Y. (1999) Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J. Cell Biol., 147, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Rabouille, C., Newman, R., Levine, T.P., Pappin, D., Freemont, P. and Warren, G. (1997) p47 is a cofactor for p97-mediated membrane fusion. Nature, 388, 75–78. [DOI] [PubMed] [Google Scholar]
- Lang T., Schaeffeler, E., Bernreuther, D., Bredschneider, M., Wolf, D.H. and Thumm, M. (1998) Aut2p and Aut7p, two novel microtubule-associated proteins are essential for delivery of autophagic vesicles to the vacuole. EMBO J., 17, 3597–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse-Miller A., Sagiv, Y., Porat, A. and Elazar, Z. (1998) Isolation and characterization of a novel low molecular weight protein involved in intra-Golgi traffic. J. Biol. Chem., 273, 3105–3109. [DOI] [PubMed] [Google Scholar]
- Lill R., Dowhan, W. and Wickner, W. (1990) The ATPase activity of SecA is regulated by acidic phospholipids, SecY and the leader and mature domains of precursor proteins. Cell, 60, 271–280. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan, L.C., Bonifacino, J.S. and Klausner, R.D. (1989) Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell, 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin V.V. and Waters, M.G. (1997) t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science, 276, 1255–1258. [DOI] [PubMed] [Google Scholar]
- Lupashin V.V., Hamamoto, S. and Schekman, R.W. (1996) Biochemical requirements for the targeting and fusion of ER-derived transport vesicles with purified yeast Golgi membranes. J. Cell Biol., 132, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V., Serafini, T., Orci, L., Shepherd, J.C. and Rothman, J.E. (1989) Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell, 58, 329–336. [DOI] [PubMed] [Google Scholar]
- Mann S.S. and Hammarback, J.A. (1994) Molecular characterization of light chain 3. A microtubule binding subunit of MAP1A and MAP1B. J. Biol. Chem., 269, 11492–11497. [PubMed] [Google Scholar]
- Mann S.S. and Hammarback, J.A. (1996) Gene localization and developmental expression of light chain 3: a common subunit of microtubule-associated protein 1A (MAP1A) and MAP1B. J. Neurosci. Res., 43, 535–544. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner, W. and Haas, A. (1996) Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- McNew J., Coe, J., Sogaard, M., Zemelman, B., Wimmer, C., Hong, W. and Sollner, T. (1998) Gos1p, a Saccharomyces cerevisiae SNARE protein involved in Golgi transport. FEBS Lett., 435, 89–95. [DOI] [PubMed] [Google Scholar]
- Morgan A., Dimaline, R. and Burgoyne, R.D. (1994) The ATPase activity of N-ethylmaleimide-sensitive fusion protein (NSF) is regulated by soluble NSF attachment proteins. J. Biol. Chem., 269, 29347–29350. [PubMed] [Google Scholar]
- Nagahama M., Orci, L., Ravazzola, M., Amherdt, M., Lacomis, L., Tempst, P., Rothman, J.E., Söllner, T.H. (1996) A v-SNARE implicated in intra-Golgi transport. J. Cell Biol., 133, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Lowe, M., Levine, T.P., Rabouille, C. and Warren, G. (1997) The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell, 89, 445–455. [DOI] [PubMed] [Google Scholar]
- Nickel W., Weber, T., McNew, J.A., Parlati, F., Söllner, T.H. and Rothman, J.E. (1999) Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc. Natl Acad. Sci. USA, 96, 12571–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune A., Isaac, J.T., Molnar, E., Noel, J., Nash, S.R., Tagaya, M., Collingridge, G.L., Nakanishi, S. and Henley, J.M. (1998) NSF binding to GluR2 regulates synaptic transmission. Neuron, 21, 87–97. [DOI] [PubMed] [Google Scholar]
- Orci L., Tagaya, M., Amherdt, M., Perrelet, A., Donaldson, J.G., Lippincott, S.J., Klausner, R.D. and Rothman, J.E. (1991) Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell, 64, 1183–1195. [DOI] [PubMed] [Google Scholar]
- Osten P. et al. (1998)The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and α- and β-SNAPs. Neuron, 21, 99–110. [DOI] [PubMed] [Google Scholar]
- Otto H., Hanson, P.I. and Jahn, R. (1997) Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl Acad. Sci. USA, 94, 6197–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. (1975) Intracellular aspects of the process of protein synthesis (secretion). Science, 189, 347–358. [DOI] [PubMed] [Google Scholar]
- Parlati F., Weber, T., McNew, J.A., Westermann, B., Söllner, T.H. and Rothman, J.E. (1999) Rapid and efficient fusion of phospholipid vesicles by the α-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl Acad. Sci. USA, 96, 12565–12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. and Latterich, M. (1998) The AAA team: related ATPases with diverse functions. Trends Cell Biol., 8, 65–71. [PubMed] [Google Scholar]
- Paul K.S., Bogan, A.A. and Waters, M.G. (1998) Phosphatidylinositol transfer protein (PITPα) stimulates in vitro intra-Golgi transport. FEBS Lett., 431, 91–96. [DOI] [PubMed] [Google Scholar]
- Peters C. and Mayer, A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Pevsner J. (1996) The role of Sec1p-related proteins in vesicle trafficking in the nerve terminal. J. Neurosci. Res., 45, 89–95. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Hsu, S.C. and Scheller, R.H. (1994) n-Sec1: a neural-specific syntaxin-binding protein. Proc. Natl Acad. Sci. USA, 91, 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S.R. (1996) Transport vesicle docking: SNAREs and associates. Annu. Rev. Cell. Dev. Biol., 12, 441–461. [DOI] [PubMed] [Google Scholar]
- Rossi G., Kolstad, K., Stone, S., Palluault, F., Ferro-Novick, S. (1995) BET3 encodes a novel hydrophilic protein that acts in conjunction with yeast SNAREs. Mol. Biol. Cell, 6, 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E. (1994) Mechanisms of intracellular protein transport. Nature, 372, 55–63. [DOI] [PubMed] [Google Scholar]
- Sacher M. et al. (1998)TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J., 17, 2494–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein S.K., Lupashin, V.V., Schmitt, H.D. and Waters, M.G. (1996) Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol., 132, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Bennett, M.K., Whiteheart, S.W., Scheller, R.H. and Rothman, J.E. (1993a) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation and fusion. Cell, 75, 409–418. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart, S.W., Brunner, M., Erdjument, B.H., Geromanos, S., Tempst, P. and Rothman, J.E. (1993b) SNAP receptors implicated in vesicle targeting and fusion. Nature, 362, 318–324. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B., Lowe, M., Levine, T., Jamsa, E., Dirac-Svejstrup, B. and Warren, G. (1998) A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol., 140, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam V.N., Peter, F., Philp, R., Wong, S.H. and Hong, W. (1996) GS28, a 28-kilodalton Golgi SNARE that participates in ER–Golgi transport. Science, 272, 1161–1163. [DOI] [PubMed] [Google Scholar]
- Subramaniam V.N., Loh,E. and Hong,W. (1997) N-Ethylmaleimide-sensitive factor (NSF) and α-soluble NSF attachment proteins (SNAP) mediate dissociation of GS28–syntaxin 5 Golgi SNAP receptors (SNARE) complex. J. Biol. Chem., 272, 25441–25444. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Sato, K. and Wickner, W. (1998) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Wickner, W. and Xu, Z. (1999) Vacuole acidification is required for trans-SNARE pairing, LMA1 release and homotypic fusion. Proc. Natl Acad. Sci. USA, 96, 11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter D.M., Paul, K.S. and Waters, M.G. (1998) Purification and characterization of a novel 13S hetero-oligomeric protein complex that stimulates in vitro Golgi transport. J. Biol. Chem., 273, 29565–29576. [DOI] [PubMed] [Google Scholar]
- Wang H., Bedford, F.K., Brandon, N.J., Moss, S.J. and Olsen, R.W. (1999) GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature, 397, 69–72. [DOI] [PubMed] [Google Scholar]
- Waters M.G., Clary, D.O. and Rothman, J.E. (1992) A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol., 118, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T.H. and Rothman, J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Xu Z. and Wickner, W. (1996) Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. J. Cell Biol., 132, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Mayer, A., Muller, E. and Wickner, W. (1997) A heterodimer of thioredoxin and I (B)2 cooperates with Sec18p (NSF) to promote yeast vacuole inheritance. J. Cell Biol., 136, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Sato, K. and Wickner, W. (1998) LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex and is released during fusion. Cell, 93, 1125–1134. [DOI] [PubMed] [Google Scholar]