Abstract
Vascular endothelial growth factor (VEGF) binding to the kinase domain receptor (KDR/FLK1 or VEGFR–2) mediates vascularization and tumor-induced angiogenesis. Since there is evidence that KDR plays an important role in tumor angiogenesis, we sought to identify peptides able to block the VEGF–KDR interaction. A phage epitope library was screened by affinity for membrane-expressed KDR or for an anti-VEGF neutralizing monoclonal antibody. Both strategies led to the isolation of peptides binding KDR specifically, but those isolated by KDR binding tended to display lower reactivities. Of the synthetic peptides corresponding to selected clones tested to determine their inhibitory activity, ATWLPPR completely abolished VEGF binding to cell-displayed KDR. In vitro, this effect led to the inhibition of the VEGF-mediated proliferation of human vascular endothelial cells, in a dose-dependent and endothelial cell type-specific manner. Moreover, in vivo, ATWLPPR totally abolished VEGF-induced angiogenesis in a rabbit corneal model. Taken together, these data demonstrate that ATWLPPR is an effective antagonist of VEGF binding, and suggest that this peptide may be a potent inhibitor of tumor angiogenesis and metastasis.
Keywords: angiogenesis/antagonist peptides/KDR/phage-display/vascular endothelial cell growth factor
Introduction
Angiogenesis, the formation of blood vessels by sprouting from pre-existing ones, is essential for the growth of solid tumors beyond 2–3 mm in diameter and for tumor metastasis (Folkman, 1995a,b; reviewed in Bouck et al., 1996). The generation of new capillaries involves a multistep process, which includes dissolution of the membrane of the originating vessel, endothelial cell migration and proliferation, and formation of a new vascular tube (Cliff, 1963; Schoefl, 1963; Ausprunck and Folkman, 1977). Suppression of any one of these steps would inhibit the formation of new vessels and therefore affect tumor growth and generation of metastases. Indeed, it has been estimated that the elimination of a single endothelial cell could inhibit the growth of 100 tumor cells (Thorpe and Burrows, 1995). Moreover, endothelial cells are genetically stable and therefore unlikely to mutate into drug-resistant variants (Young, 1989; Kerbel, 1991; Boehm et al., 1997). Since they line the inside of blood vessels, they are easily accessible to circulating drugs. This feature suggests that anti-angiogenic therapies targeting endothelial cells may provide a promising mechanism for cancer treatment.
So far, several angiogenic factors have been identified (reviewed in Folkman, 1995a; Hanahan and Folkman, 1996), including the particularly potent vascular endothelial growth factor (VEGF), also known as VPF or vasculotropin (reviewed in Ferrara, 1993; Ferrara and Davis-Smyth, 1997). Unlike other angiogenic factors, VEGF acts as an endothelial cell-specific mitogen during angiogenesis (Terman et al., 1992; Ferrara, 1993). Antibodies raised against VEGF have been shown to suppress tumor growth in vivo (Kim et al., 1993), indicating that VEGF antagonists could have therapeutic applications as inhibitors of tumor-induced angiogenesis.
VEGF was purified initially from the conditioned media of folliculostellate cells and from a variety of tumor cell lines (Ferrara and Henzel, 1989; Plouët et al., 1989; Myoken et al., 1991). It is a member of the cystine-knot family of growth factors, which also includes platelet-derived growth factor (PDGF). Recently, a number of VEGF structural homologs have been identified: VEGF–B, VEGF–C, VEGF–D and placenta growth factor (PlGF) (Klagsbrun and D'Amore, 1996; reviewed in Ferrara, 1999). The human gene encoding VEGF is organized into eight exons, separated by seven introns. Alternative splicing of mRNAs for the VEGF gene results in the generation of five different molecular species, having 121, 145, 165, 189 or 206 amino acid residues in the mature monomer (Houck et al., 1991; Tisher et al., 1991). Only VEGF165, which lacks the residues encoded by exon 6, is the mature and active form of VEGF. It binds to heparin and cell surface heparan sulfate proteoglycans, and can be expressed as a free or a cell membrane-bound form (Houck et al., 1992). Two tyrosine kinase receptors have been identified for which VEGF acts as a high affinity ligand: an fms-like tyrosine kinase–1 (Flt–1 orVEGFR–1) and a kinase domain receptor (KDR/Flk–1 or VEGFR–2) (Matthews et al., 1991; Terman et al., 1991; De Vries et al., 1992; Millauer et al., 1993). Although Flt–1 binds VEGF with 50–fold higher affinity than KDR (De Vries et al., 1992), most of the VEGF angiogenic properties (mitogenicity, chemotaxis and induction of morphological changes) are mediated by interaction with KDR (Waltenberger et al., 1994). Therefore, the interaction between VEGF and KDR is the most appropriate to interrupt in order to inhibit angiogenesis.
The screening of phage-displayed libraries is a powerful technique for identifying peptides mimicking protein surfaces (Smith, 1985; Hoess, 1993; Felici et al., 1995). Since each peptide is physically linked to a genetic particle, clones specifically binding a target molecule can be selected by consecutive cycles of in vitro ‘biopanning’ and in vivo ‘amplification’. New agonists and antagonists for cell membrane receptors have been identified successfully using this process (Cwirla et al., 1990; Cortese et al., 1996), for example, RGD-containing peptides that bind either the GPIIb/IIIa receptor on platelets (O'Neil et al., 1992) or the α5β1 integrin (Koivunen et al., 1993). The selected peptides were able to antagonize integrin-mediated cell adhesion.
In this study, we have attempted to identify peptides blocking the binding of VEGF to KDR. A random peptide library displayed on filamentous phages (Cortese et al., 1996) was screened using two parallel strategies. In the first, the peptide repertoire was screened with cells expressing recombinant KDR (Plouët et al., 1997), and in the second with a monoclonal antibody raised against VEGF. Since this antibody blocked VEGF-dependent endothelial cell proliferation, we postulated that its antigen-binding site mimics all or part of the VEGF interaction surface with KDR. Both strategies led to the isolation of peptides that compete with VEGF binding to KDR, including a peptide, ATWLPPR, which specifically inhibited human endothelial cell proliferation in vitro. Moreover, it totally abolished VEGF-induced angiogenesis in vivo. ATWLPPR, as a specific antagonist of VEGF–KDR interaction, may represent an effective anti-tumor agent.
Results
CHO–KDR cells express a VEGF-binding KDR
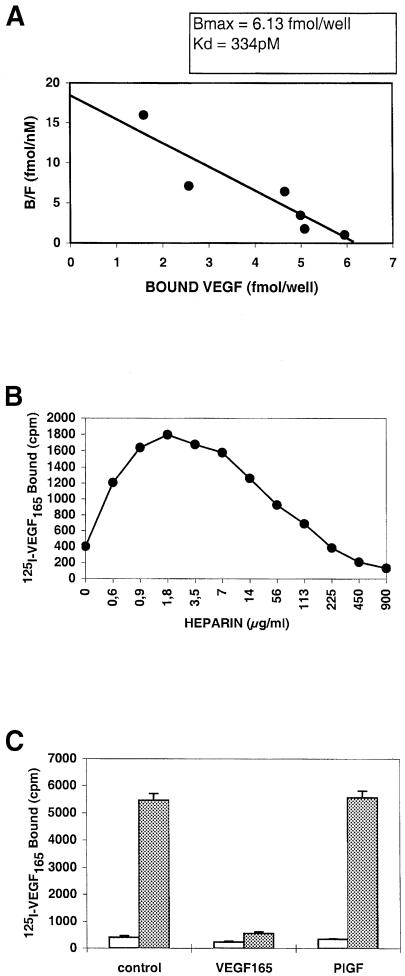
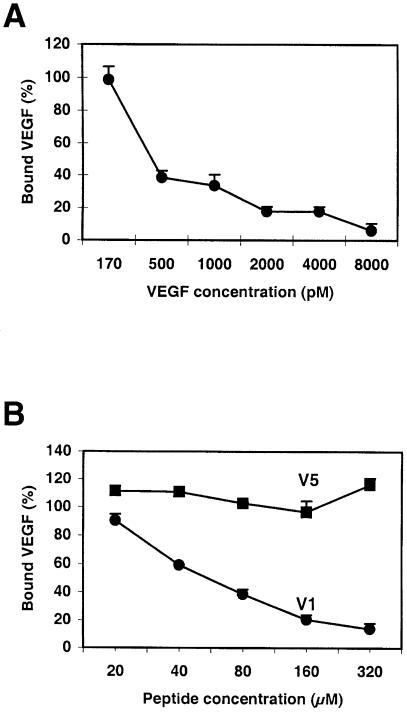
In order to find peptides binding KDR, we first looked for a receptor that we could use to perform panning techniques. Chinese hamster ovary (CHO) cells expressing a recombinant KDR at their membrane surface were tested for their ability to bind VEGF in a variety of conditions. Figure 1A shows that the dissociation constant of VEGF on CHO–KDR cells was 334 pM. Heparin was able to increase the binding of VEGF to CHO–KDR cells, the optimal concentration being 1.8 μg/ml (Figure 1B), and PlGF did not modify VEGF binding to CHO–KDR cells (Figure 1C). Taken together, all these data suggested that the KDR expressed by recombinant CHO cells exhibited the same characteristics as the endothelial receptor for binding to VEGF.
Fig. 1. CHO–KDR cells express a functional KDR. (A) Scatchard analysis of VEGF binding. The ratio of bound to free VEGF molecules (B/F) was plotted against bound VEGF concentration. (B) Effect of heparin. VEGF binding to CHO–KDR cells was measured in the presence of various amounts of heparin. (C) Effect of PlGF. VEGF (100 ng/ml) binding to CHO–KDR cells was tested in the absence (white bars) or presence (gray bars) of heparin (1.8 μm/ml), and compared with PlGF (50 ng/ml) or PBS (control). Data correspond to the mean and standard deviations of triplicate samples. All binding experiments were performed twice and gave similar results.
Identification of peptides binding specifically to KDR
Since this receptor was able to bind VEGF, we tried to find peptides able to mimic this interaction and that bind KDR in the same conditions. A random 7mer library composed of 2 × 109 independent clones was screened by binding to CHO–KDR cells. At the end of the selection, 24 clones were isolated and analyzed. DNA sequencing showed that seven independent peptides had been selected (K1–K7), with no sequence homology (Table I).
Table I. DNA sequence of the peptides selected by binding to CHO–KDR cells or to the anti-VEGF antibody.
| Selection on CHO–KDR cells | Selection on anti-VEGF antibody | ||
|---|---|---|---|
| K1 | YLTMPTP | V1 | ATWLPPR |
| K2 | WPTPPYA | V2 | NPRALNY |
| K3 | TPHNTVS | V3 | ANLFKAK |
| K4 | SLPAHAR | V4 | YHSSFQA |
| K5 | HSSLQTP | V5 | ILDNYKL |
| K6 | YSIPKSS | V6 | LPPNPTK |
| K7 | ALQPRYL | V7 | YAIMPLV |
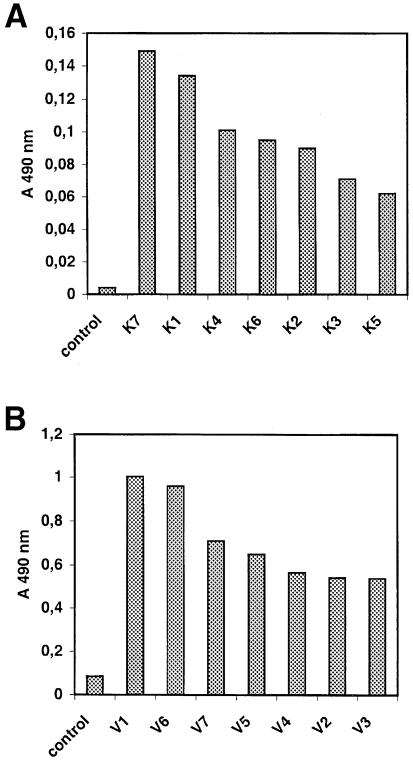
The binding of each selected clone to KDR was tested by enzyme-linked immunosorbent assay (ELISA) on CHO–KDR cells. All the clones gave an ELISA signal significantly higher than that of the control bacteriophage (Figure 2A). However, ELISA signals were detectable only for phage concentrations >1013/ml, suggesting that the selected peptides could bind KDR specifically, but with low affinity.
Fig. 2. Selected phage-displayed peptides bind to KDR specifically in ELISA. Clones selected by KDR binding (1013 p.f.u./ml) (A) or by anti-VEGF antibody binding (1012 p.f.u./ml) (B) were compared with M13 phage particles (control). Results are representative of three independent assays.
An anti-VEGF antibody blocking VEGF–KDR interaction
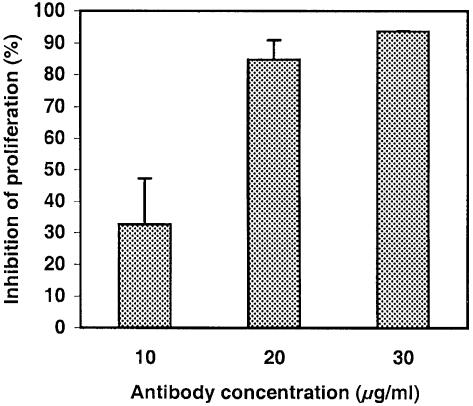
In order to find peptides binding KDR with a higher affinity, we decided to screen the peptide library using an alternative strategy. Peptides mimicking the antigen-binding site of many monoclonal antibodies have been isolated successfully from phage-displayed libraries (reviewed in Felici et al., 1995). Screening our repertoire with an anti-VEGF antibody neutralizing its biological activity on endothelial cells would allow us to identify peptides mimicking the KDR-binding site on VEGF, and therefore able to block the VEGF–KDR interaction. We first checked if the human anti-VEGF antibody V–4758 could inhibit the proliferation of endothelial cells. Calf pulmonary aortic endothelial (CPAE) cells were used as model cells for VEGF-dependent proliferation. As shown in Figure 3, the anti-VEGF antibody exerted a dose-dependent inhibitory activity on the proliferation of these cells, and a complete abolition of cell division was achieved in the presence of 30 μg/ml antibody. At the same concentration, this antibody had no inhibitory effect on the growth of NIH 3T3 fibroblast cells (not shown). These results confirmed that the anti-VEGF antibody could block VEGF–KDR interaction by binding VEGF at the KDR-binding site.
Fig. 3. The anti-VEGF antibody blocks CPAE cell growth. CPAE cell growth was measured in cultures supplemented with various concentrations of anti-VEGF antibody and compared with untreated cultures. Data represent means and standard deviations of proliferation inhibition for triplicate samples and are representative of two independent experiments.
Identification of peptides specifically binding the anti-VEGF antibody
The peptide library was then screened by binding to the anti-VEGF antibody. At the end of the selection, 24 clones were isolated and analyzed. DNA sequencing showed that seven independent peptides had been selected (V1–V7) with no consensus motifs, although an LPP motif was found in two of them (V1 and V6) (Table I).
When tested for binding to KDR by ELISA on CHO–KDR cells, all the selected clones gave strong ELISA signals, confirming that they were able to bind the cell receptor (Figure 2B). Furthermore, they showed higher reactivity than the peptides selected by KDR binding, using phage concentrations 10 times lower. This suggests that they had a higher affinity for the receptor. Interestingly, the best reactivities were observed for V1 and V6.
DNA sequence analysis of the selected clones highlights a common LPP/A motif
In order to identify the residues responsible for this increased affinity for KDR, a multiple alignment analysis on all selected sequences was performed. Table II shows that three consensus groups, corresponding to the underlined residues, could be identified. Group A was composed of four clones and was characterized by the motif YX(I/T) (M/P)P, the tyrosine residue always occurring in the first position. Two peptides of this group, K1 and V7, shared a strong sequence homology, with three identical residues that are particularly hydrophobic. Group B was characterized by a longer motif, HSSLQPRXL. Interestingly, two pairs of two clones obtained from different selection strategies showed significant homologies: K5 and V4 containing HSSXQ, and K7 and V2 with the PRXL motif. A shorter consensus LP(A/P) was found in group C. Interestingly, this group contained the two clones with the LPP motif, extended with the K4 clone that has a similar motif LPA. The peptide sequences were also compared with the primary sequence of VEGF (Table II). Alignment of the individual selected clones or of the consensus motifs with VEGF did not produce any signifi– cant homology, suggesting that the selected peptides mimicked a discontinuous binding site.
Table II. DNA sequence analysis of the selected clones.
| Multiple alignment of the selected clones | |||||
|---|---|---|---|---|---|
| (A) | (B) | (C) | |||
| K1 | YLTMPTP | K5 | HSSLQTP | V1 | ATWLPPR |
| V7 | YAIMPLV | V4 | YHSSFQA | K4 | SLPAHAR |
| K6 | YSIPKSS | K7 | ALQPRYL | V6 | LPPNPTK |
| K2 | WPTPPYA | V2 | NPRALNY | ||
| Alignment with the VEGF primary sequence | ||||||
| 10 | 20 | 30 | 40 | 50 | 60 | |
| APMAEGGGQNHHEVVKFMDVYQRSYCHPIETLVDIFQEYPDEIEYIFKPSCVPLMRCGGC | ||||||
| 70 | 80 | 90 | 100 | 110 | ||
| CNDEGLECVPTEESNITMQIMRIKPHQGQHIGEMSFLQHNKCECRPKKDR | ||||||
V1 inhibits the binding of VEGF to KDR
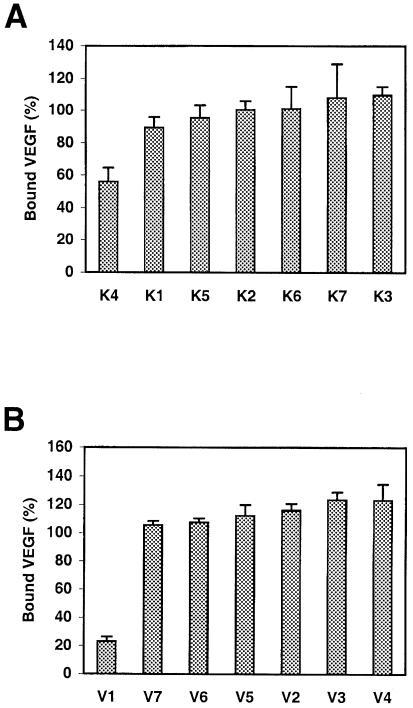
Since the selected peptides were all able to bind KDR, we examined whether they were able to block VEGF interaction with the receptor. Synthetic peptides based on the amino acid sequence of the selected clones were synthesized. They were then tested in competition with VEGF for binding to CHO–KDR cells, using a fixed peptide concentration (2.1 × 10–4 M). Two peptides only, V1 and to a lesser extent K4, showed an inhibitory effect on VEGF binding to KDR at this concentration (Figure 4). Figure 5 compares the dose-dependent inhibition of VEGF binding to CHO–KDR cells by VEGF itself (A) or by V1 (B). V1 could nearly block VEGF binding at a 320 μM concentration, its IC50 being estimated at 8 × 10–5 M. On the contrary, V5 tested in the same conditions did not modify VEGF binding. The other peptides binding to the anti-VEGF antibody did not show any inhibitory effect of VEGF binding to KDR at this concentration (data not shown).
Fig. 4. Synthetic peptides compete with VEGF for KDR binding. Peptides selected by KDR binding (A) or by anti-VEGF binding (B) were tested in competition with VEGF for binding to CHO–KDR cells at the concentration of 2.1 × 10–4 M and in the presence of heparin (1.8 μg/ml). Data represent the means and standard deviations of triplicate samples. Similar results were obtained in three independent experiments.
Fig. 5. V1 can abolish VEGF binding to KDR. Various concentrations of VEGF (A) or of V1 peptide (B) were tested in competition with radioactively labeled VEGF for binding to CHO–KDR cells. As an uninhibitory control, V5 was tested in the same conditions. Data represent the mean and standard deviations of triplicate samples. Similar results were obtained in two different experiments.
V1 specifically inhibits the proliferation of vascular endothelial cells
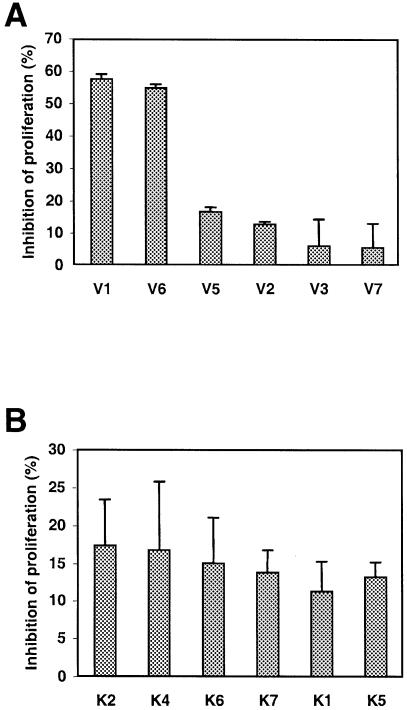
Since the selected peptides could block VEGF binding to KDR, and since endothelial cell proliferation is dependent on VEGF, we tested whether they could inhibit proliferation of endothelial cells in vitro. CPAE cell cultures supplemented with 2.1 × 10–4 M synthetic peptides were compared with untreated cultures for cell growth (Figure 6). After 24 h of incubation, CPAE cell proliferation in the presence of V1 was reduced by 60%. Surprisingly, the V6 peptide, which had no effect on VEGF binding to KDR, showed a similar effect. The other peptides also had an inhibitory effect on cell division, but exhibited lower reactivity (∼15%). For all peptides, the inhibitory effects on cell proliferation could be maintained at similar levels in 48 and 72 h cultures by daily supplementation of the peptides (data not shown).
Fig. 6. V1 inhibits CPAE cell proliferation. CPAE cell growth was measured after 24 h of incubation in the presence of synthetic peptides selected by antibody binding (A) or by KDR binding (B) and compared with untreated cultures. Data represent means and standard deviations of proliferation inhibition for triplicate samples and are representative of three independent experiments.
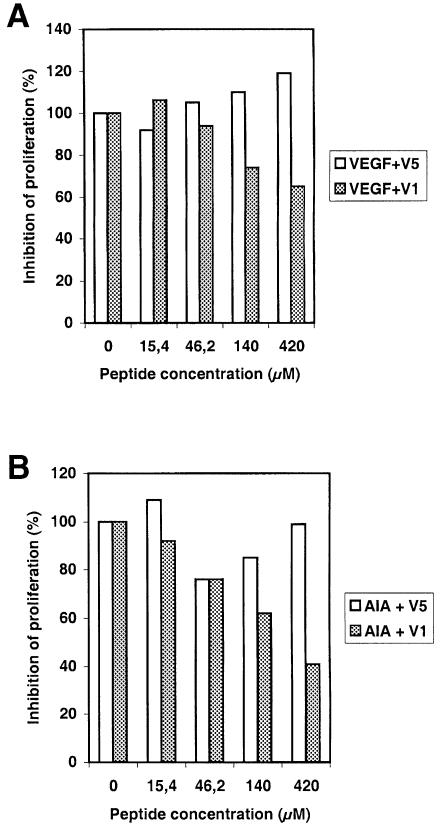
The ability of V1 to block VEGF-induced proliferation of human endothelial cells was also examined. As shown in Figure 7A, V1 suppressed the mitogenic activity of VEGF in a dose-dependent manner, whereas V5 had no inhibitory effect. To check the specificity of the interaction between V1 and KDR, the same experiment was repeated using KDR anti-idiotypic antibodies (AIAs). AIAs were generated and found to be selective agonists for KDR, since they promoted endothelial cell proliferation in vitro (data not shown). Figure 7B shows that unlike V5, V1 could suppress the AIA-dependent cell proliferation, indicating that the V1 effect was mediated by a direct interaction with KDR.
Fig. 7. V1 inhibits the proliferation of human endothelial cells induced by VEGF or by AIA in a dose-dependent manner. HUAE cell cultures were grown in the presence of VEGF (A) or anti- idiotypic antibodies (B), and were supplemented daily with various concentrations of V1 or V5. Cells were counted after 5 days. Data are means of proliferation inhibition percentages for triplicate samples.
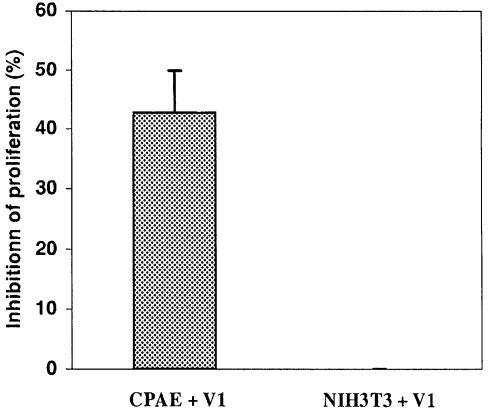
To see if this effect was specific for endothelial cells, we tested the effect of V1 on NIH 3T3 fibroblast growth. V1 peptide did not modify the proliferation of these cells, confirming that it blocks VEGF-dependent cell growth only (Figure 8).
Fig. 8. V1 acts specifically on endothelial cells. CPAE and NIH 3T3 fibroblasts were cultured with or without V1 peptide, and the changes in cell proliferation were measured after 24 h. Data represent the means and standard deviations of proliferation inhibition percentages for triplicate samples, and similar results were obtained in two independent experiments.
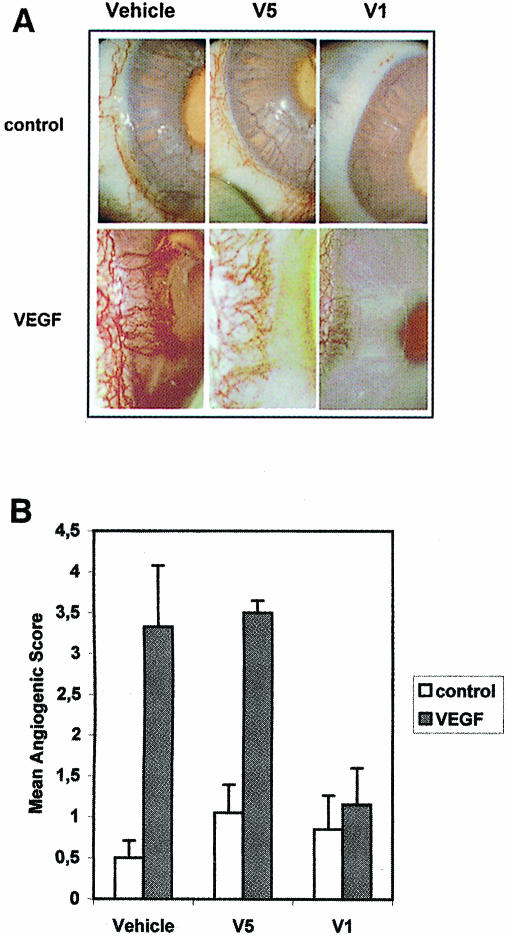
V1 inhibits corneal angiogenesis in vivo
A rabbit corneal pocket assay was used to determine whether V1 could inhibit angiogenesis in vivo. The neovascularization in corneal implants containing VEGF in the presence or absence of peptides was measured (Figure 9A and B). When compared with the vehicle alone, V1 and V5 did not improve the generation of new blood vessels. VEGF induced a significant angiogenic response, which was not modified by the addition of V5. This stimulatory effect was totally abolished by V1, demonstrating that V1 could inhibit VEGF-induced corneal angiogenesis.
Fig. 9. V1 inhibits corneal angiogenesis in vivo. The neovascu- larization in implants containing V1, V5 or PBS (vehicle), in the presence or absence of VEGF was assessed 12 days after insertion in rabbit corneal pockets. (A) A representative photograph of each implant group. (B) Angiogenic score means and standard errors measured for eight implant groups.
Discussion
Previous studies have demonstrated that anti-angiogenic therapy is a promising approach for the treatment of cancer (Folkman, 1995a,b). Solid tumor growth and tumor metastasis are dependent on tumor vascularization. Moreover, clinical studies of patients with a variety of solid tumors have shown a direct correlation between the density of tumour vessels and an adverse prognosis (Weidner et al., 1991; Albo et al., 1994). VEGF and its signal transducing tyrosine kinase receptor VEGFR–2 (KDR/flk–1) are major mediators of tumor-induced angiogenesis (Terman et al., 1992; Ferrara, 1993). Also, VEGF is different from other growth factors because it acts as an endothelial cell-specific mitogen during angiogenesis (Leung et al., 1989; Plouët et al., 1989; Ferrara, 1993). Previous studies have shown that blocking the VEGF–VEGF receptor pathway using antibodies results in murine and human tumor regression (Kim et al., 1993; Cheng et al., 1996). We report here the identification of peptides antagonistic for VEGF by use of a phage-displayed peptide library.
There are only limited examples of the direct screening of peptide libraries with a cellular receptor, the main difficulty being the removal of clones binding non-specific determinants. To select peptides binding cell-displayed uPAR (urokinase plasminogen activator receptor), Goodson et al. (1994) performed multiple rounds of biopanning using either recombinant Sf9 insect cells or COS–7 cells. In this study, we performed two rounds of negative selection against non-recombinant cells at the end of the selection process. This resulted in an efficient removal of non-specific clones since all the isolated phage clones could bind KDR specifically on ELISA.
Another possible approach is to screen peptide libraries using antibodies blocking the interaction between receptor and ligand. This assumes that the antibody-binding site on the ligand contains residues interacting with the receptor. Using anti-basic fibroblast factor (bFGF) antibodies blocking the binding to its receptor, Yayon et al. (1993) successfully isolated peptides able to inhibit bFGF-induced proliferation of vascular endothelial cells. However, the use of bFGF as a therapeutic target is limited by the fact that it acts on a broad spectrum of cell types and that the in vivo expression of its receptor by endothelial cells is controversial. In contrast, VEGF is a secreted endothelial cell-specific mitogen whose receptors are expressed almost exclusively on vascular endothelial cells and is therefore of greater therapeutic interest (Millauer et al., 1993; Peters et al., 1993). To isolate VEGF antagonists, we used a 7mer random peptide library displayed on bacteriophage M13 and performed two selections, one based on binding to KDR and the other on binding to an anti-VEGF blocking antibody. This allowed us to compare the sequences selected by the two strategies, and to identify residues responsible for the antagonist activity.
Library screening for binding to KDR was performed on CHO cells expressing a recombinant receptor at the membrane surface, and we demonstrated that this molecule could bind VEGF similarly to the natural receptor. Indeed, the affinity of the recombinant receptor was comparable to the constant measured on endothelial cells (Terman et al., 1992; Klasgsbrun and D'Amore, 1996). Also, heparin was able to increase VEGF binding to CHO–KDR cells. This phenomenon was bimodal, lower heparin concentrations improving the binding of VEGF and higher concentrations having an inhibitory effect, and reproduces the effect of heparin on VEGF binding to natural KDR (Gitay-Goren et al., 1992, 1993). In accordance with previous work, PlGF did not modify VEGF binding to KDR-expressing cells (Terman et al., 1994). The reactivity of the peptides selected for KDR binding was relatively low, and only one of them could compete with VEGF for KDR binding at concentrations in the 10–4 M range. Furthermore, none were potent at suppressing the growth of endothelial cells in vitro, suggesting that the selected peptides bound KDR in regions distant from the VEGF-binding site and therefore interfered poorly with the VEGF–KDR interaction.
Library screening for antibody binding was an indirect way to isolate peptides antagonistic for VEGF–KDR interactions, but nevertheless led to the identification of the most potent peptide. The V1 peptide (ATWLPPR) showed the highest reactivity in ELISA and the highest inhibitory effect on endothelial cell growth. Importantly, V1 inhibited VEGF-induced proliferation of human endothelial cells without influencing the growth of non-endothelial cells, and was able to inhibit VEGF-induced angiogenesis in vivo in a rabbit corneal model. This suggests that the effect of V1 was mediated by the direct binding to KDR. Recently, Soker et al. (1998) have identified a new receptor for VEGF that is expressed by endothelial and tumor cells. This receptor is identical to human NRP–1, a receptor for the collapsin/semaphorin family that mediates neuronal cell guidance. When co-expressed in cells with KDR, NRP–1 enhanced the binding of VEGF to KDR. Conversely, inhibition of VEGF binding to NRP–1 inhibited its binding to KDR and its subsequent mitogenic activity on endothelial cells. In fact, NRP–1 might be acting as a co-receptor that enhances the VEGF-induced activities mediated by KDR. To see whether V1 reacts specifically with KDR, we tested the binding of V1 to KDR in competition with KDR AIAs. We found that V1 was able to block the proliferation of endothelial cells induced by these antibodies, showing that its inhibitory effect was due to a specific interaction with KDR.
Interestingly, V6, which shared the LPP motif in common with V1, significantly inhibited the proliferation of endothelial cells but did not affect the binding of VEGF to KDR. In contrast, the K4 peptide contained an LPA motif and could partially inhibit VEGF binding to KDR, but had a minor effect on endothelial growth. This suggests that the presence of an alanine residue instead of a proline may account for its lack of reactivity, and that the LPP motif is essential for peptide antagonist activity.
Alignment of the individual selected clones with the primary sequence of the VEGF did not produce any homology. In particular, no LPP motif could be found. This suggests that the KDR-binding site on VEGF is discontinuous and that the selected peptides may contain residues distant in the VEGF primary sequence, but in close proximity in the folded molecule. This is in accordance with the crystal structure of VEGF that has been resolved recently (Muller et al., 1997a). Mutational analysis revealed that two spots of residues involved in KDR binding are located at each pole of the VEGF monomer, which may constitue a functional binding site in the dimer (Muller et al., 1997b). We are currently investigating whether residues corresponding to the selected motifs are accessible at the surface of the VEGF dimer and in close proximity in the folded molecule.
Angiogenesis is involved in a variety of human diseases. The VEGF–KDR interaction has been shown to play a role in cancer, but also in diabetic retinopathy (Pierce et al., 1995), psoriasis (Detmar et al., 1994), hemangioblastoma (Wizigmann-Voos et al., 1995) and Kaposi's sarcomas in AIDS patients (Albini et al., 1996). Thus, identification of VEGF antagonists may have potential applications in the treatment of a variety of human diseases. Moreover, for therapeutic use, small molecules are preferred since their reduced size makes them more amenable to translation into organic molecules. Peptides provide leading molecules for the design of inexpensive drugs that can be administered orally.
Our results demonstrate that the ATWLPPR peptide is an effective antagonist of VEGF and an inhibitor of angiogenesis. This peptide could be a potent inhibitor of tumor angiogenesis, and could be of a more general interest in diseases in which angiogenesis is involved. In this context, in vivo anti-tumor chemotherapy assays using this peptide must be investigated.
Materials and methods
Cell lines
KDR-expressing cells (CHO–KDR) were obtained by transfecting glycosaminoglycan–deficient pgsA 745 CHO cells (Esko, 1991) with the psV–7d expression vector (Plouët et al., 1997). CHO cells and the CHO–KDR cell line were grown routinely in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal calf serum (FCS), 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mM l–glutamine. CPAE cells were kindly provided by Dr Binétruy (CNRS UPR 9079, Villejuif, France) and grown in minimum Eagle's medium (MEM) supplemented with 20% (v/v) FCS, 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mM l–glutamine. Human umbilical artery endothelial (HUAE) cells were grown routinely on gelatinized dishes in EGM supplemented with antibiotics and 15% (v/v) FCS. Stock HUAE cultures were maintained by addition of 1 ng/ml VEGF every other day. The NIH 3T3 fibroblast cell line was grown routinely in MEM supplemented with antibiotics and 10% (v/v) FCS.
Library screening with anti-VEGF antibody
Biopanning was adapted from the Ph.D.–7 kit standard procedure (New England Biolabs, Beverly, MA). Anti-human VEGF monoclonal antibody (V–4758; Sigma, St Louis, MO) was used to coat microtiter plates at 10 μg/ml. Three rounds of selection were performed with non-specific elution of bound phages in pH 2.2 acidic buffer. To analyze the selected clones, overnight cultures of Escherichia coli (strain ER2537) were diluted 1:100, infected with single clones, grown for 5 h with shaking at 37°C, and the culture supernatant containing phage particles was harvested. This phage stock was used to perform ELISA binding assays and to determine the peptide-encoding sequence as described in the Ph.D.–7 kit guidelines.
Library screening with CHO–KDR cells
The selection procedure was adapted from Watters et al. (1997). Four rounds of biopanning were performed by incubating 1011 phage par– ticles with 2 × 106 CHO–KDR cells. The last amplified eluate was then absorbed twice on 2 × 106 non-recombinant CHO cells to enrich for phage clones specifically binding KDR.
DNA and amino acid sequence analysis
Peptide sequences were analyzed with the GCG software package (Wisconsin). A multiple sequence alignment performed by ‘Pileup’ analysis was used to determine the groups of related peptides. ‘Gap’ analysis was used to find an optimal alignment between the consensus motifs and the VEGF primary sequence.
ELISA of the phage-displayed peptide binding to CHO–KDR cells
Exponentially growing CHO–KDR cells were seeded in 96–well microtiter plates at 7 × 104 cells/well and left overnight at 37°C. Cells were fixed with paraformaldehyde [4% in phosphate-buffered saline (PBS)] for 15 min at room temperature and washed with PBS. Wells were then filled with PBS–glycine 0.2%, kept for 15 min at room temperature and then washed with PBS. Phage particles (1012 or 1013/ml) were added to each well and incubated for 2 h at room temperature. Wells were washed with PBS and the amount of bound phage was detected with peroxidase-conjugated anti-M13 phage serum (Pharmacia Biotech, Uppsala, Sweden).
Competition assay of binding to CHO–KDR cells
CHO–KDR cells were seeded in 24–well plates at a density of 5 × 105 cells/well. After 24 h, subconfluent plates were transferred to 4°C, and all subsequent operations were performed at 4°C. Cells were washed twice with PBS and incubated with [125I]VEGF (30 000 c.p.m.; Amersham, Buckinghamshire, UK) in binding buffer (DMEM supplemented with 20 mM HEPES pH 7.4 and 2 mg/ml gelatin). Various concentrations of unlabeled human recombinant VEGF (Pharmingen, San Diego, CA), heparin (Sigma, St Louis, MO), PlGF (R&D Systems, Minneapolis, MN) or peptides (Neosystem, Strasbourg, France) were added in a final volume of 0.3 ml. After 3 h, cells were washed five times with binding buffer and solubilized by the addition of 0.5 M NaOH. The amount of radioactivity bound to the cells was counted in a gamma counter (LKB 1261 Multigamma). The receptor affinity and the number of binding sites per cell were determined by Scatchard's analysis (Scatchard, 1986).
Cell proliferation assay
To test the anti-VEGF antibody neutralizing effect, CPAE cells were plated into 96–well tissue culture plates at a density of 500 cells/well. After 24 h, various concentrations of anti-VEGF antibody were added. The cells were cultured for an additional day and cell proliferation was measured using the cell proliferation ELISA kit from Boerhinger (Indianapolis, IN). Cells were incubated with bromodeoxyuridine (BrdU) for 4 h at 37°C, and the incorporation of BrdU into newly synthesized DNA was quantified by immunoassay as described by the manufacturer. To investigate the peptide effect, after 24 h of CPAE cell growth, 2.1 × 10–4 M synthetic peptides were added daily to the culture medium. Cell proliferation was assessed after 24, 48 or 72 h as described above. HUAE cells were seeded at 5000 cells/well in 12–well plates in medium containing 2 ng/ml VEGF or 100 ng/ml immunopurified KDR anti-idiotypic antibodies (Ortega et al., 1997), and supplemented daily with various concentrations of V1 or V5 peptide. Cells were trypsinized and counted after 5 days using a Coulter counter.
Rabbit corneal pocket assay
Slow releasing implants of hydrogel (2 × 1 mm) were rehydrated with 2 μl of PBS containing 25 μg of bovine serum albumin and 2 pmol of VEGF in the presence or absence of 30 nmol of peptide. These implants were inserted in New Zealand rabbit (Elevage du Trottis, Esperce, France) corneal stroma 2 mm away from the limbus (Favard et al., 1991). Neovascularization was assessed on day 12 by direct examination with a slit lamp and scored according to a four-grade scale (grade 1, <1 mm long neovessels; grade 2, 1 mm long neovessels; grade 3, 1–2 mm long neovessels; grade 4, neovessels extending to the implant). Means and standard deviations were determined on eight implant groups for each condition.
Acknowledgments
Acknowledgements
We thank Dr Binétruy (CNRS UPR 9079, Villejuif, France) for the gift of CPAE cells and helpful discussions. We gratefully acknowledge Dr A.G.D.Bean (CSIRO, Geelong, VIC, Australia) for reviewing this manuscript. This work was supported by grants from the Ligue Nationale contre le Cancer (Equipe Labellisée) and the Association pour la Recherche Contre le Cancer (ARC).
References
- Albini A., et al. (1996)The angiogenesis induced by HIV–1 tat protein is mediated by the Flk–1/KDR on endothelial cells. Nature Med., 2, 1371–1375. [DOI] [PubMed] [Google Scholar]
- Albo D., Granick, M.S., Jhala, N., Atkinson, B. and Solomon, M.P. (1994) The relationship of angiogenesis biological activity in human squamous cell carcinomas of the head and neck. Ann. Plastic Surg., 32, 588–594. [DOI] [PubMed] [Google Scholar]
- Ausprunk D.H. and Folkman, J. (1977) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during angiogenesis. Microvasc. Res., 14, 53–65. [DOI] [PubMed] [Google Scholar]
- Boehm T., Folkman, J., Browder, T. and O'Reilly, M.S. (1997) Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature, 390, 404–407. [DOI] [PubMed] [Google Scholar]
- Bouck N., Stellmach, V. and Hsu, S.C. (1996) How tumors become angiogenic. Adv. Cancer Res., 69, 135–174. [DOI] [PubMed] [Google Scholar]
- Cheng S.Y., Huang, H.J.S., Nagane, M., Ji, X.D., Wang, D., Shih, C.C.Y., Arap, W., Huang, C.M. and Cavenee, W.K. (1996) Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc. Natl Acad. Sci. USA, 93, 8502–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W.J. (1963) Observations on healing tissues: a combined light and electron microscopic investigation. Philos. Trans. R. Soc., 246, 305–325. [Google Scholar]
- Cortese R., et al. (1996) Selection of biologically active peptides by phage display of random peptides libraries. Curr. Opin. Biotechnol., 7, 616–621. [DOI] [PubMed] [Google Scholar]
- Cwirla S.E., Peters, E.A., Barrett, R.W. and Dower, W.J. (1990) Peptides of phage: a vast library of peptides for identifying ligands. Proc. Natl Acad. Sci. USA, 87, 6378–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmar M., Brown, L.F., Claffey, K.P., Yeo, K.T., Kocher, O., Jackman, R.W., Berse, B. and Dvorak, H.F. (1994) Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptor in psoriasis. J. Exp. Med., 180, 1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries C., Escobedo, J.A., Ueno, H., Houck, K., Ferrara, N. and Williams, L.T. (1992) The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science, 255, 989–991. [DOI] [PubMed] [Google Scholar]
- Esko J.D. (1991) Genetic analysis of proteoglycan structure, function and metabolism. Curr. Opin. Cell Biol., 3, 805–816. [DOI] [PubMed] [Google Scholar]
- Favard C., Moukadiri, H., Dorey, C., Praloran, V., Plouët, J. (1991) Purification and biological properties of vasculotropin, a new angiogenic cytokine. Biol. Cell, 73, 1–6. [DOI] [PubMed] [Google Scholar]
- Felici F., Luzzago,A., Monaci,P., Nicosia,A., Sollazo,M. and Traboni,C. (1995) Peptide and protein display on the surface of filamentous bacteriophage. In Raafat El–Gewely,M. (ed.), Biotechnology Annual Review. Elsevier, Amsterdam, The Netherlands, pp. 149–183. [DOI] [PubMed] [Google Scholar]
- Ferrara N. (1993) Vascular endothelial growth factor. Trends Cardiovasc. Med., 3, 244–250. [DOI] [PubMed] [Google Scholar]
- Ferrara N. (1999) Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med., 77, 527–543. [DOI] [PubMed] [Google Scholar]
- Ferrara N., and Davis-Smyth, T. (1997) The biology of vascular endothelial growth factor. Endocr. Rev., 18, 4–25. [DOI] [PubMed] [Google Scholar]
- Ferrara N. and Henzel, W.J. (1989) Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun., 161, 851–858. [DOI] [PubMed] [Google Scholar]
- Folkman J. (1995a) Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nature Med., 1, 27–30. [DOI] [PubMed] [Google Scholar]
- Folkman J. (1995b) Clinical applications of research on angiogenesis. N. Engl. J. Med., 333, 1757–1763. [DOI] [PubMed] [Google Scholar]
- Gita-Goren H., Soker, S., Vlodavsky, I. and Neufeld, G. (1992) The binding of vascular endothelial growth factor to its receptor is dependent on cell surface-associated heparin-like molecules. J. Biol. Chem., 267, 6093–6098. [PubMed] [Google Scholar]
- Gita-Goren H., Halaban, R. and Neufeld, G. (1993) Human melanoma cells but not normal melanocytes express vascular endothelial growth factor receptors. Biochem. Biophys. Res. Commun., 190, 702–709. [DOI] [PubMed] [Google Scholar]
- Goodson R.J., Doyle, M.V., Kaufman, S.E. and Rosenberg, S. (1994) High-affinity urokinase receptor antagonists identified with bacteriophage peptide display. Proc. Natl Acad. Sci. USA, 91, 7129–7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. and Folkman, J. (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell, 86, 353–364. [DOI] [PubMed] [Google Scholar]
- Hoess R.H. (1993) Phage display of peptide and protein domains. Curr. Opin. Struct. Biol., 3, 572–579. [Google Scholar]
- Houck K., Ferrara, N., Winer, J., Cachianes, G., Li, B. and Leung, D.W (1991) The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol., 5, 1806–1814. [DOI] [PubMed] [Google Scholar]
- Houck K., Leung, D.W., Rowland, A.M., Winer, J. and Ferrara, N. (1992) Dual regulation of vascular endothelial growth factor biovailability by genetic and proteolytic mechanisms. J. Biol. Chem., 267, 26031–26036. [PubMed] [Google Scholar]
- Kerbel R.S. (1991) Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioassays, 13, 31–36. [DOI] [PubMed] [Google Scholar]
- Kim K.J., Li, B., Winer, J., Armanini, M., Gillett, N., Philips, H.S. and Ferrara, N. (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo.Nature, 362, 841–844. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. and D'Amore, P.A. (1996) Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev., 7, 259–270. [DOI] [PubMed] [Google Scholar]
- Koivunen E., Gay, D.A. and Ruoslahti, E. (1993) Selection of peptides binding to the α5β1 integrin from phage display library. J. Biol. Chem., 268, 20205–20210. [PubMed] [Google Scholar]
- Leung D.L., Cachianes, G., Kuang, W.J., Goeddel, D.V. and Ferrara, N. (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science, 246, 1306–1309. [DOI] [PubMed] [Google Scholar]
- Matthews W., Jordan, C.T., Gavin, M., Jenkins, N.A., Copeland, N.G. and Lemischka, I.R. (1991) A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c–kit.Proc. Natl Acad. Sci. USA, 88, 9026–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos, S., Schnürch, H., Martinez, R., Moller, N.P.H., Risau, W. and Ullrich, A. (1993) High affinity VEGF binding and developmental expression suggest Flk–1 as a major regulator of vasculogenesis and angiogenesis. Cell, 72, 835–846. [DOI] [PubMed] [Google Scholar]
- Muller Y.A., Christinger, H.W., Bruce, A.K. and De Vos, A.M. (1997a) The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 Å resolution: multiple copy flexibility and receptor binding. Stucture, 5, 1325–1338. [DOI] [PubMed] [Google Scholar]
- Muller Y.A., Li, B., Christinger, H.W., Wells, J.A., Cunningham, B.C. and De Vos, A.M. (1997b) Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc. Natl Acad. Sci. USA, 94, 7192–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoken Y., Kayada, Y., Okamoto, T., Kan, M., Sato, G.H. and Sato, J.D. (1991) Vascular endothelial cell growth factor (VEGF) produced by A–431 human epidermoid carcinoma cells and identification of VEGF membrane binding sites. Proc. Natl Acad. Sci. USA, 88, 5819–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K.T., Hoess, R.H., Jackson, S.A., Ramachandran, N.S., Mousa, S.A. and Degrado, W.F. (1992) Identification of novel peptide antagonists for gpIIB/IIIa from a conformationally constrained phage peptide library. Proteins, 14, 509–515. [DOI] [PubMed] [Google Scholar]
- Ortéga N., Jonca, F., Vincent, S., Favard, C., Malavaud, B., Ruchoux, M.–M. and Plouët, J. (1997) Systemic activation of the vascular endothelial growth factor receptor flk–1 selectively triggers angiogenic endothelial cells. Am. J. Pathol., 151, 1215–1224. [PMC free article] [PubMed] [Google Scholar]
- Peters K.G., De Vries, C. and Williams, L.T. (1993) Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc. Natl Acad. Sci. USA, 90, 8915–8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce E.A., Avery, R.L., Foley, E.D., Aiello, L.P. and Smith, L.R.H. (1995) Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl Acad. Sci. USA, 92, 905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouët J., Schilling, J. and Gospodarowicz, D. (1989) Isolation and characterization of newly identified endothelial cell mitogen produced by AtT–20 cells. EMBO J., 8, 3801–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouët J., Moro, F., Bertagnolli, S., Coldeboeuf, N., Mazarguil, H., Clamens, S. and Bayard, F. (1997) Extracellular cleavage of the vascular endothelial growth factor 189-amino acid form by urokinase is required for its mitogenic effect. J. Biol. Chem., 272, 13390–13396. [DOI] [PubMed] [Google Scholar]
- Scatchard G. (1986) The attraction of proteins for small molecules and ions. Ann. NY Acad. Sci., 261, 4660–4662. [Google Scholar]
- Schoefl G.I. (1963) Studies on inflammation. III. Growing capillaries: their structure and permeability. Virchows Arch. Pathol. Anat., 337, 97–141. [PubMed] [Google Scholar]
- Smith G.P. (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science, 228, 1315–1317. [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima, S., Miao, H.Q., Neufeld, G. and Klagsbrun, M. (1998) Neuropilin–1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell, 92, 735–745. [DOI] [PubMed] [Google Scholar]
- Terman B.I., Carrion, M.E., Kovacs, E., Rasmussen, B.A., Eddy, R.L. and Shows, T.B. (1991) Identification of a new endothelial growth factor receptor tyrosine kinase. Oncogene, 6, 1677–1683. [PubMed] [Google Scholar]
- Terman B.I., Dougher–Vermazen, M., Carrion, M., Dimitrov, D., Armellino, D., Gospodarowicz, D. and Bohlen, P. (1992) Identification of the KDR tyrosine kinase as a receptor for vascular endothelial growth factor. Biochem. Biophys. Res. Commun., 187, 1579–1586. [DOI] [PubMed] [Google Scholar]
- Terman B.I., et al. (1994)VEGF receptor subtypes KDR and FLT1 show different sensitivities to heparin and placenta growth factor. Growth Factors, 11, 187–195. [DOI] [PubMed] [Google Scholar]
- Tisher E., Mitchell, R., Hartman, T., Silva, M., Gospodarowicz, D., Fiddes, J.C. and Abraham, J.A. (1991) The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem., 266, 11947–11954. [PubMed] [Google Scholar]
- Thorpe P.E and Burrows, F.J. (1995) Antibody-directed targeting of the vasculature of solid tumors. Breast Cancer Res. Treat., 36, 237–251. [DOI] [PubMed] [Google Scholar]
- Waltenberger J., Claesson–Welsh, L., Siegbahn, M. and Heldin, C.H. (1994) Different signal transduction properties of KDR and FLT–1, two receptors for vascular endothelial growth factor. J. Biol. Chem., 269, 26988–26995. [PubMed] [Google Scholar]
- Watters J.M., Telleman, P. and Junghans, R.P. (1997) An optimized method for cell-based phage displayed panning. Immunotechnology, 3, 21–29. [DOI] [PubMed] [Google Scholar]
- Weidner N., Semple, J.P., Welch, W.R. and Folkman, J. (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N. Engl. J. Med., 324, 1–8. [DOI] [PubMed] [Google Scholar]
- Wizigmann-Voos S., Breier, G., Risau, W. and Plate, K.H. (1995) Up-regulation of vascular endothelial growth factor and its receptor in von Hippel–Lindau disease-associated and sporadic hemangioblastomas. Cancer Res., 55, 1358–1364. [PubMed] [Google Scholar]
- Yayon A., Aviezer, D., Safran, M., Gross, J.L., Heldman, Y., Cabilly, S., Givol, D. and Katchalski-Katzir, E. (1993) Isolation of peptides that inhibit binding of basic fibroblast growth factor to its receptor from a random phage-epitope library. Proc. Natl Acad. Sci. USA, 90, 10643–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C. (1989) Drug resistance: the clinical problem. Cancer Treat. Res., 48, 1–12. [DOI] [PubMed] [Google Scholar]