Abstract
Objective
Post-cardiac arrest therapeutic hypothermia (TH) improves outcomes in comatose cardiac arrest survivors. This study tests the hypothesis that the efficacy of post-cardiac arrest TH is dependent on the onset and duration of therapy.
Design
Prospective randomized laboratory investigation
Setting
University research laboratory
Subjects
268 male Long Evans rats
Interventions
Post-cardiac arrest therapeutic hypothermia
Measurements and Main Results
Adult male Long Evans rats that achieved return of spontaneous circulation (ROSC) after a 10-min asphyxial cardiac arrest were block randomized to normothermia (37±1°C) or TH (33±1°C) initiated 0, 1, 4, or 8 hrs after ROSC and maintained for 24 or 48 hrs. TH initiated 0, 1, 4, and 8 hours after ROSC resulted in 7-day survival rates of 45%*, 36%*, 36%*, and 14% respectively compared to 17% for normothermic controls, and survival with good neurologic function rates of 24%*, 24%*, 19%*, and 0% respectively compared to 2% for normothermic controls (*p<0.05 vs. normothermia). These outcomes were not different when TH was maintained for 24 vs. 48 hours. In contrast, hippocampal CA1 pyramidal neuron counts were 53±27%*, 53±19%*, 51±24%*, and 65±16%* of normal respectively when TH initiated 0, 1, 4, or 8 hrs after ROSC compared to 9% in normothermic controls (*p<0.01 vs. normothermia). Furthermore, surviving neuron counts were greater when TH was maintained for 48 hrs compared to 24 hrs (68%±15%* vs. 42%±22%, *p<0.0001)
Conclusions
In this study, post-cardiac arrest TH resulted in comparable improvement of survival and survival with good neurologic function when initiated within 4-hours after ROSC. However, histological assessment of neuronal survival revealed a potentially broader therapeutic window and greater neuroprotection when TH was maintained for 48 vs. 24 hours.
Keywords: heart arrest, resuscitation, prognosis, survival, brain, hypothermia, targeted temperature management
Introduction
Mild therapeutic hypothermia (TH) is the first and only therapy clinically demonstrated to improve survival and neurologic outcome in patients that achieve return of spontaneous circulation (ROSC) after cardiac arrest. Two prospective randomized clinical trials have demonstrated improved outcome of comatose survivors of witnessed out-of-hospital ventricular fibrillation cardiac arrest who were treated with prolonged, mild TH (1, 2). In the study by Bernard et al, target temperature (33°C) was achieved within 2 hours after ROSC and maintained for 12 hours (1). In the study by the Hypothermia after Cardiac Arrest Study Group, the median time to achieve target temperature (32-34°C) was 8 hours (interquartile range was 4-16 hours), and hypothermia was maintained for 24 hours (2). Improved functional outcome was demonstrated in both studies and survival in the latter. Based on these and subsequent studies, the American Heart Association currently recommends TH for comatose survivors of out-of-hospital cardiac arrest when the initial rhythm is ventricular fibrillation (VF), and consideration of TH in patients with other presenting rhythms or after in-hospital cardiac arrest (3). Although there is growing adoption of post-cardiac arrest TH, the substantial differences in time to achieve target temperature and duration of therapy in the two major clinical trials have left clinicians guessing as to how this therapeutic strategy should be optimally implemented. In addition, exclusion of non-VF rhythms in these studies has left uncertainty as to whether therapeutic hypothermia is beneficial if the presenting rhythm is pulseless electrical activity (PEA) or asystole.
Animal studies have thus far failed to systematically optimize therapeutic hypothermia after cardiac arrest. Published studies have used small numbers of animals with varied models, temperatures, onsets and durations of therapy. Importantly, when post-cardiac arrest TH is maintained for ≤1 hour, the beneficial effect is lost if the onset is delayed by more than 10-20 minutes (4-6). These studies support the concept that TH should be initiated as soon as possible after ROSC. However, this concept is challenged by subsequent work in which TH was maintained for ≥24 hours after cardiac arrest. For example, Hicks et al demonstrated equivalent improvement of outcomes when TH sustained for 24 hours was initiated either immediately or one hour after ROSC following asphyxial cardiac arrest in rats (7). To the best of our knowledge, there have been no animal cardiac arrest studies that have directly compared different hypothermia onset times beyond 1 hour after ROSC, or compared different durations of TH. However, in rodent models of transient forebrain ischemia both the onset time and duration of TH have an impact on neuroprotection (8-12).
The overall goal of this study was to determine if the benefit of prolonged post-cardiac arrest TH was dependent on onset and duration of therapy. To this end we compared TH initiated at 0, 1, 4, or 8 hours after ROSC and maintained for 24 or 48 hours in a rat model of PEA/asystole cardiac arrest. Outcomes included 7-day survival, 7-day survival with good neurologic function, and survival of selectively vulnerable hippocampal CA1 sector pyramidal neurons.
Materials and Methods
Cardiac Arrest Model
This study was approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Male Long Evans rats (Harlan Laboratories, Inc., Indianapolis, IN) weighing 325–375g (approximately 12-14 weeks old) were anesthetized with 4% isoflurane, 66% N2O and 33% O2, orotracheally intubated, and mechanically ventilated using a pressure-controlled ventilator (Kent Scientific, Torrington, CT, USA). Ventilation was titrated to achieve partial pressure end-tidal CO2 (PetCO2) of 40mmHg (rate ∼40 breaths/min; peak inspiration pressure ∼17cm H20; tidal volume ∼9mL/kg) with a positive end-expiratory pressure of 5cm H2O. Anesthesia was maintained with 2% isoflurane and inspired gas was 66% N2O and 33% O2. Intraperitoneal ampicillin (50mg/kg) was administered before initial incision. The left femoral vein and artery were cannulated (PE-50 tubing; Harvard Apparatus, Holliston, MA) for arterial and central venous pressure monitoring and intravenous administration of drugs and fluids. Temperature was initially monitored with a needle thermocouple probe placed between the temporalis muscle and skull. Prior to cardiac arrest, temperature was maintained between 37.0°C and 37.5°C. ECG monitoring was performed with electrocardiographic limb leads. Oxygenation was continuously monitored by pulse oximetry (Nonin Medical Inc, Plymouth, MN, USA). Hemodynamic, respiratory, and temperature data were continuously recorded (Chart5 for Windows, ADInstruments, Inc., Colorado Springs, CO, USA).
Cardiac arrest was induced by asphyxia as previously described with minor modifications (13,14). Rats were chemically paralyzed using intravenous vecuronium (2mg/kg), isoflurane was discontinued, and inspired gas changed to room air. Asphyxia was induced by disconnecting the ventilator for 10 minutes. Circulatory arrest was confirmed by cessation of arterial pulse pressure and reduction of mean arterial pressure to < 20 mmHg. Typically, circulatory arrest occurred within 3-4 minutes. After 10 minutes of asphyxia, mechanical ventilation was re-initiated with no positive end-expiratory pressure using 100% inspired oxygen at a respiratory rate of 40 breaths/min. Intravenous epinephrine (0.005 mg/kg) and bicarbonate (1.0 mEq/kg) were administered, and external chest compressions performed at a rate of 350-400 compressions/minute. Rats without ROSC within 2 minutes received continued CPR and up to two additional doses of epinephrine (0.01mg/kg). Rats without ROSC after 4 minutes of CPR were considered out of protocol. Following ROSC, inspired oxygen was titrated to maintain a pulse oximetry reading of 94-98%. A total of 20cc/kg 0.9% saline was infused intravenously during the first hour after ROSC. Sixty minutes after ROSC, a telemetric thermoprobe was placed into the peritoneal cavity for subsequent temperature regulation and the vascular cannulas were removed. Rats were then weaned from mechanical ventilation, extubated and transferred to a specialized temperature regulation cubicle. Supplemental oxygen was provided for the first 6 hours after extubation as needed to maintain a pulse oximetry reading above 94%.
Study Protocol
Rats that achieved ROSC after a 10-minute asphyxial cardiac arrest were block randomized to normothermia (N: 37±1°C, n=42) or TH (33±1°C) initiated 0, 1, 4, or 8 hrs after ROSC and maintained for 24 or 48 hrs (8 groups, n=21 per group). Sham injured rats underwent surgical instrumentation under general anesthesia without asphyxiation or CPR (n=6).
Induction of TH in 0-hour and 1-hour onset groups was achieved with an electric fan (Holmes HFH111T-U), hand-held spray bottle and thermopad during mechanical ventilation. After extubation, rats were transferred to the temperature regulation cubicles where core body temperature was monitored via a telemetric intraperitonal thermoprobe and regulated using a computerized feedback system that controls a fan, mister, and heating lamp (175W) as originally described by Colbourne et al (15). For the TH 4-hour and 8-hour onset groups, normothermia was actively maintained until the time of TH initiation, and TH was induced in the temperature regulation cubicles. Target temperature was achieved within 30 minutes of initiating cooling, and rats were rewarmed at a rate of 0.5°C/hr. Subcutaneous fluid (5% dextrose in 0.45% saline) and orogastric feeding (20% PMI Micro-Stabilized Rodent Liquid Diet LD101) were given twice daily.
Neurologic function score was measured daily. The neurologic function score (NFS, Table 1) is based on our previously described neurologic deficit score that has been modified to measure function (500 best to 0 worst) rather than deficit (500 worst to 0 best) (13, 14). Good neurologic function (GNF) was defined as a neurologic function score ≥450 out of 500. Primary outcomes were 7-day survival, and 7-day survival with GNF.
Table 1. Neurologic Function Scoring System.
| General | Consciousness | Unresponsive, depressed, normal | 0, 50,100 |
| Respiration | Abnormal (<60 or > 120), normal | 0,100 | |
| Cranial Nerve | Olfactory | Orient to smell | no = 0, yes = 20 |
| Vision | Startle response to stimulus | no = 0, yes = 20 | |
| Corneal reflex | Blink response to corneal stimulus | no = 0, yes = 20 | |
| Whisker movement | Spontaneous | no = 0, yes = 20 | |
| Hearing | Startle response to loud noise | no = 0, yes = 20 | |
| Motor | Left forepaw | Spontaneous or withdraw from pain | no = 0, yes = 10 |
| Right forepaw | Spontaneous or withdraw from pain | no = 0, yes = 10 | |
| Left hindpaw | Spontaneous or withdraw from pain | no = 0, yes = 10 | |
| Right hindpaw | Spontaneous or withdraw from pain | no = 0, yes = 10 | |
| Tail | Spontaneous or withdraw from pain | no = 0, yes = 10 | |
| Sensory | Left forepaw | React to pain | no = 0, yes = 10 |
| Right forepaw | React to pain | no = 0, yes = 10 | |
| Left hindpaw | React to pain | no = 0, yes = 10 | |
| Right hindpaw | React to pain | no = 0, yes = 10 | |
| Tail | React to pain | no = 0, yes = 10 | |
| Coordination | Ledge traverse | no = 0, yes = 25 | |
| Righting reflex | no = 0, yes = 25 | ||
| Placing test | no = 0, yes = 25 | ||
| Stop at table edge | no = 0, yes = 25 | ||
| Total Score | 500 |
Histology
At 7 days post-cardiac arrest, surviving rats were euthanized by transcardial perfusion fixation [PBS flush followed by 4% paraformaldehyde (PFA)] under general anesthesia. Brains were then postfixed in 4% PFA at 4°C for overnight, cryoprotected in serial 10%, 20%, and 30% sucrose solutions in 0.1M PB, and snap-frozen in chilled isopentane. Coronal sections (50μm thick) were generated using a freezing sliding microtome (Microtome HM 440E Germany). Beginning at the rostral end of the hippocampus, every 10th brain section was selected with randomization of the first section for immunolabeling and stereological quantification of CA1 pyramidal neurons. Sections were immunolabeled with primary antibody targeting the neuron-specific marker protein NeuN (MAB 377, Millipore, 1:1,000) and a goat anti-mouse secondary antibody (Alexa Fluor 488, Molecular Probes, 1:500). Sections were counterstained with Hoechst 33342 (5.0μg/ml, Sigma), and mounted with Fluoromount medium (Electron Microscopy Sciences).
Quantification of CA1 pyramidal neurons was performed using fluorescence microscopy and stereologic techniques (Nikon microphot–SA). Section thickness was measured at high (200×) magnification. 10% of thickness was defined as guard (buffer) zone above and below the counting region. 10% of the area of the outlined CA1 pyramidal layer was selected in an unbiased manner by the stereology software to perform neuronal counting. Only pyramidal neuron nuclei with normal morphology were counted using 200 × magnification with 20μm×20μm counting frame. The total number of CA1 pyramidal neurons was calculated using the formula N=ΣQ-×t/h×1/asf ×1/ssf where Q-= the number of cells actually counted, h = height of dissector, tsf = thickness sampling fraction (height of dissector/section thickness), asf = area sampling fraction (area of the counting frame/area associated with each step of the stepping motor) and ssf = section sampling fraction (1/10). Sections adjacent to those used for stereology were selected for standard hematoxylin and eosin staining to confirm the presence of ischemic neuronal pathology.
Data Analysis
Pre-arrest, intra-arrest and post-arrest hemodynamic and ventilatory parameters were compared using one-way ANOVA with Scheffe post-hoc analysis for intergoup comparison. A logrank test was used for between group comparisons of survival time. A Pearson's chi-square test was used for between group comparisons of 7-day survival with GNF, and a Fisher's exact test was used for contingency tables with expected cell values less than 10. An analysis of variance was used for group comparisons of post-ischemic neurodegeneration, indexed by the percentages of hippocampal CA1 pyramidal neuron counts compared to normal rats. The percentages were arcsine transformed to normalize the data. It should be noted the post-ischemic neurodegeneration was conditioned on subject survival at Day 7. Mean percentages were reported with their standard deviation. Survival times were presented by Kaplan-Meier curves. Values of p<0.05 were considered significant.
Results
Out of a total of 268 rats subjected to cardiac arrest, ROSC was achieved in 222 (83%). Block randomization after ROSC resulted in 21 rats in each of the 8 TH treatment groups, and 42 rats in the normothermia group. Twelve rats that achieved ROSC were out of protocol after randomization due to malfunction of the temperature regulation system. When this occurred, the rat was excluded from analysis and an additional subject with the same treatment parameters was added to the randomization block. Pre-arrest, intra-arrest, and post-arrest hemodynamic and ventilatory parameters did not differ between treatment groups (Table 2). Temperature curves for each treatment group are presented in Figure 1. The interaction between onset and duration was not significant for any outcome parameter. Therefore, only main effects are reported.
Table 2. Pre-arrest and Post-ROSC Hemodynamic and Respiratory Parameters.
| HR | MAP | CVP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Arrest | Post-ROSC | Pre-Arrest | Post-ROSC | Pre-Arrest | Post-ROSC | |||||||
| Group | Baseline | 10 Min | 30 Min | 60 Min | Baseline | 10 Min | 30 Min | 60 Min | Baseline | 10 Min | 30 Min | 60 Min |
| Sham | 367±34 | 381±40 | 360±44 | 354±41 | 111±10 | 100±16 | 108±13 | 88±15 | 3.5±0.7 | 3.9±0.6 | 3.7±1.0 | 3.9±0.6 |
| Normo | 354±39 | 374±38 | 366±38 | 321±33 | 97±17 | 152±38 | 93±15 | 85±14 | 3.9±0.9 | 4.5±1.0 | 3.9±0.9 | 4.2±1.1 |
| TH 0-24 | 369±28 | 355±30 | 319±33a | 335±27 | 101±16 | 140±38 | 120±16b | 94±19 | 4.4±1.1 | 4.4±0.9 | 4.3±1.0 | 4.5±1.8 |
| TH 0-48 | 369±38 | 354±30 | 313±26a | 344±27 | 98±19 | 146±38 | 122±17b | 96±14 | 3.7±1.1 | 4.4±1.1 | 4.5±0.9 | 3.9±1.2 |
| TH 1-24 | 374±35 | 378±28 | 380±33 | 334±33 | 97±14 | 156±36 | 97±18 | 83±17 | 3.9±0.9 | 4.6±1.0 | 4.2±1.1 | 4.1±1.1 |
| TH 1-48 | 373±34 | 377±23 | 371±26 | 336±32 | 97±17 | 161±26 | 96±14 | 85±12 | 3.8±1.0 | 4.3±0.9 | 4.0±0.6 | 3.9±1.4 |
| TH 4-24 | 367±39 | 381±31 | 371±35 | 336±38 | 99±17 | 147±41 | 94±21 | 84±15 | 4.2±0.9 | 4.7±1.1 | 4.5±1.1 | 3.7±1.0 |
| TH 4-48 | 368±39 | 378±31 | 372±23 | 333±33 | 100±17 | 149±43 | 90±17 | 88±15 | 4.2±1.2 | 4.4±0.9 | 4.2±1.3 | 4.0±1.3 |
| TH 8-24 | 357±31 | 385±24 | 367±37 | 321±29 | 95±20 | 148±41 | 98±20 | 86±19 | 4.1±0.9 | 4.8±0.7 | 4.4±0.9 | 4.5±1.3 |
| TH 8-48 | 376±28 | 381±23 | 377±38 | 330±31 | 99±18 | 151±33 | 98±17 | 86±16 | 3.9±1.2 | 4.6±0.9 | 4.0±0.9 | 4.2±1.3 |
| PetCO2 | SaO2 | |||||||||||
| Pre-Arrest | Post-ROSC | Pre-Arrest | Post-ROSC | |||||||||
| Group | Baseline | 10 Min | 30 Min | 60 Min | Baseline | 10 Min | 30 Min | 60 Min | ||||
| Sham | 40±2 | 40±0 | 40±3 | 41±2 | 98±2 | 98±1 | 97±1 | 98±2 | ||||
| Normo | 40±2 | 45±5 | 45±5 | 41±3 | 99±2 | 97±3 | 96±3 | 96±3 | ||||
| TH 0-24 | 40±2 | 41±5 | 41±2 | 39±3 | 99±2 | 99±1 | 97±2 | 97±2 | ||||
| TH 0-48 | 40±2 | 43±5 | 42±4 | 40±2 | 98±2 | 98±2 | 96±3 | 96±3 | ||||
| TH 1-24 | 40±2 | 45±5 | 45±4 | 40±2 | 99±1 | 98±3 | 96±3 | 97±3 | ||||
| TH 1-48 | 40±2 | 45±5 | 45±4 | 41±3 | 99±1 | 96±3 | 96±2 | 97±3 | ||||
| TH 4-24 | 39±2 | 44±5 | 45±5 | 40±2 | 99±1 | 98±2 | 97±3 | 97±2 | ||||
| TH 4-48 | 40±2 | 45±6 | 45±6 | 40±3 | 99±2 | 98±3 | 96±4 | 97±3 | ||||
| TH 8-24 | 40±2 | 45±6 | 47±5 | 41±3 | 99±1 | 97±3 | 96±3 | 97±3 | ||||
| TH 8-48 | 40±3 | 42±4 | 45±4 | 43±6 | 99±1 | 97±3 | 97±3 | 97±3 | ||||
TH = Therapeutic hypothermia, HR = heart rate, MAP = mean arterial pressure, CVP = central venous pressure, PetCO2 = partial pressure of end-tidal CO2, SaO2 = arterial oxyhemoglobin saturation measured by pulse oximetry
= Statistically different from other groups (p<0.05).
= Statistically different from other groups (p<0.05) except the Sham group.
Values expressed and mean ± standard deviation
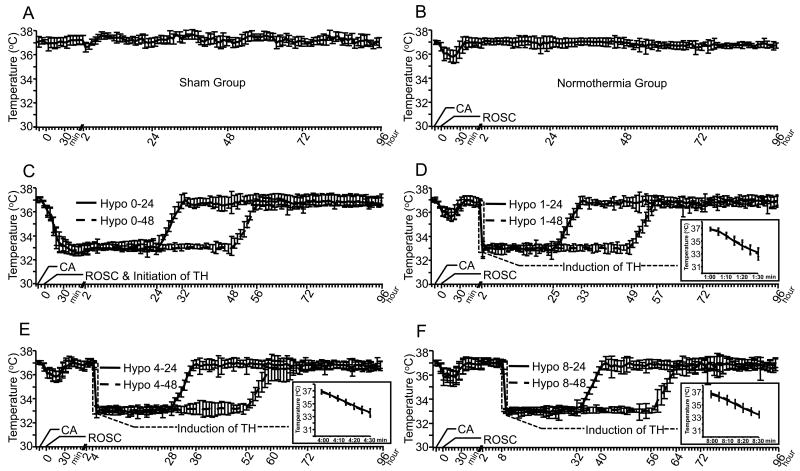
Figure 1. Temperature Regulation.
Temperature curves (mean ± SD) for sham injured rats (A), normothermic post-cardiac arrest rats (B) and post-cardiac arrest rats treated with TH beginning 0 (C), 1, (D), 4 (E), or 8 (F) hours after ROSC. The time scale in minutes shows asphyxia onset (-10 min), CPR onset (0 min) and the first 60 minutes after ROSC. The remainder of the time scale in hours shows the time course of therapeutic hypothermia. All cardiac arrest rats developed a spontaneous hypothermia from 37.0° (at -15 min) to 35.5° (at 15 min), and were rewarmed to normothermia except for those in the immediate cooling groups (C). The induction of TH in all the hypothermia groups was achieved in 30 minutes (see insert graphs). After 24 hours (solid line) or 48 hours (dash line) of hypothermia, rats were rewarmed to normothermia over 8 hours at a rate of 0.5°C/hour.
Impact of Post-Cardiac Arrest Therapeutic Hypothermia on Survival
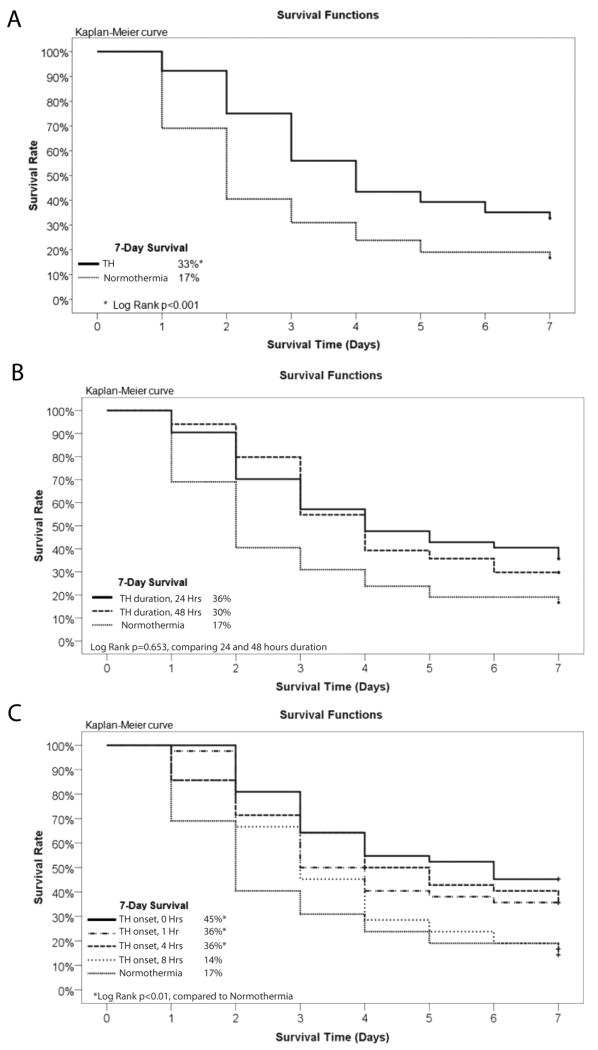
7-day survival rate was 17% in the normothermia group and 33% for all TH groups combined (Log rank p <0.001; Figure 2A). There was no statistical difference in 7-day survival between rats treated with TH for 24 hours compared to 48 hours (36% vs. 30%, Log rank p=0.65; Figure 2B). Hypothermia initiated 0, 1, 4, and 8 hours after ROSC resulted in 7-day survival rates of 45%, 36%, 36%, and 14% respectively (Figure 2C). Only TH initiated at 0, 1 and 4 hours after ROSC resulted in a statistically significant improvement in survival (Log Rank p <0.01 vs. normothermia). 7-day survival was not statistically different between the TH 0, 1 and 4 groups.
Figure 2. Impact of Post-Cardiac Arrest Therapeutic Hypothermia on Survival.
A Kaplan-Meier curve demonstrating improved survival with post-cardiac arrest TH compared to normothermia. B. Kaplan-Meier curve demonstrating equivalent improvement in survival with 24-hour and 48-hour duration TH compared to normothermia. C. Kaplan-Meier curve demonstrating improved survival with TH initiated 0, 1 or 4 hours after ROSC but not 8 hours after ROSC.
Impact of Post-Cardiac Arrest Therapeutic Hypothermia on Neurologic Outcome
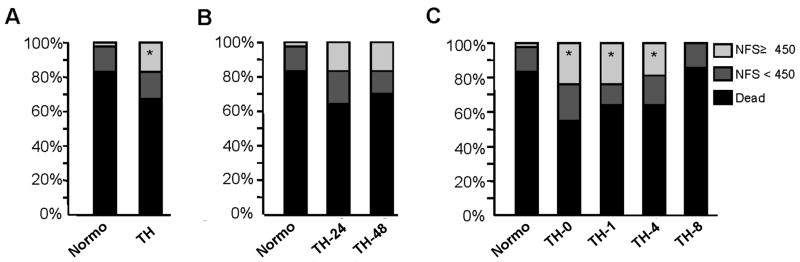
To examine the effect of post-cardiac arrest TH on neurologic outcome, we compared the percentage of rats that survived with good neurologic function (GNF) between treatment groups. Survival with GNF was defined as a neurologic function score (NFS) ≥ 450 on day 7 after cardiac arrest. The rate of survival with GNF was higher in rats treated with post-cardiac arrest TH compare to those maintained at normothermia (17% vs. 2%, p<0.05 Fisher's Exact) (Figure 3A). There was no statistical difference in survival with GNF between rats treated with TH for 24 hours compared to 48 hours (17% vs. 17%) (Figure 3B). Hypothermia initiated 0, 1, 4, and 8 hours after ROSC resulted 24%, 24%, 19%, and 0% survival with GNF respectively (Figure 3C). Only TH initiated at 0, 1 and 4 hours after ROSC resulted in a statistically significant improvement in survival with GNF (Fisher's exact p <0.05 vs. normothermia). 7-day survival with GNF was not statistically different between the TH 0, 1 and 4 hour onset groups.
Figure 3. Impact of Post-Cardiac Arrest Therapeutic Hypothermia on Neurologic Function.
A. Post-cardiac arrest TH significantly improves survival with good neurologic function (GNF) (16.67% NFS≥450) compared to normothermia (2.3% NFS≥450; Fisher's exact test, *p<0.05). B. 48-hour duration TH (16.67% NFS≥450) is equivalent to 24-hour duration TH (16.67% NFS≥450) in improving survival with GNF (Pearson's chi-square test, p=1). C. TH initiated at 0, 1, and 4 hours (23.81%, 23.81% and 19.05% NFS≥450) but not 8 hours (0% NFS≥450) after ROSC significantly improved survival with GNF compared to normothermia (2% NFS≥450; Fisher's Exact Test, *p<0.05).
Impact of Post-Cardiac Arrest Therapeutic Hypothermia on Neurodegeneration
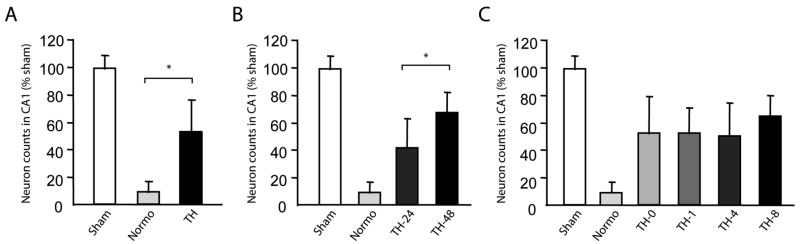
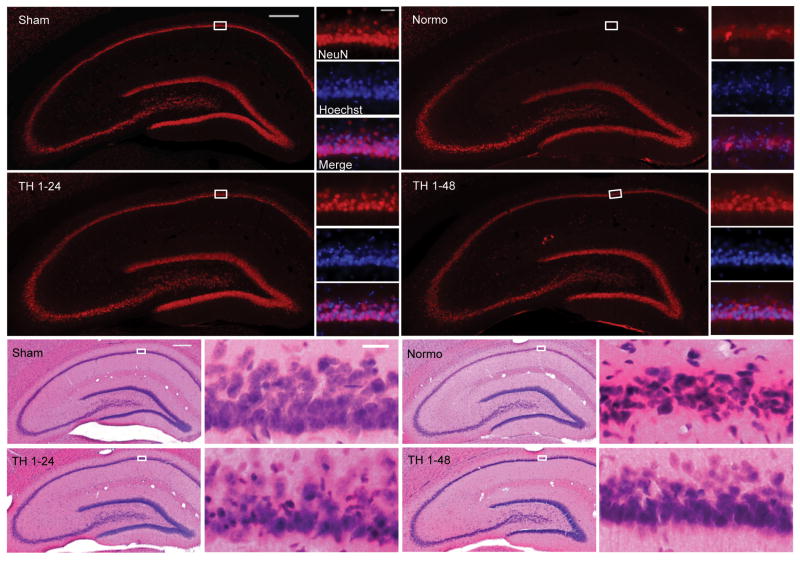
To examine the effect of TH on post-cardiac arrest neurodegeneration, we compared the number of hippocampal sector CA1 pyramidal neurons surviving at 7 days after ROSC between treatment groups. Stereologically quantified hippocampal CA1 sector pyramidal neuron counts are reported as a percentage of counts obtained from sham-injured rats. CA1 pyramidal neuron counts were 9% ± 8% of normal in brains from normothermic rats and 53% ± 23% of normal in brains from rats treated with TH (One way ANOVA, p<0.0001 (Figure 4A). CA1 pyramidal neuron counts were greater in rats treated with 48-hour TH compared to 24–hour TH (68%± 15% vs. 42%± 22%, One way ANOVA, p<0.0001) (Figure 4B). However, there was no statistical difference in CA1 pyramidal neuron counts based on time of hypothermia onset (0-hour 53%± 27%; 1-hour 53%± 19%; 4-hour 51%± 24%; 8 hour 65%± 16%, Two way ANOVA, p=0.5) (Figure 4C). Images from representative brain sections stained with NeuN/Hoechst and H&E are presented in Figure 5.
Figure 4. Neuroprotection with Post-Cardiac Arrest Therapeutic Hypothermia.
Stereologic quantification of hippocampal sector CA1 pyramidal neurons in sham injured rats and post-cardiac arrest rats at 7 days after ROSC. The results are displayed on the original scale while the comparisons were performed after arcsine transforming the proportions, which was used to normalize the data and stabilize the variances. A. Comparison of sham (100%), normothermia (8.81%) and combined hypothermia (53.4%) groups demonstrates improved neuron survival with post-cardiac arrest TH compared to rats maintained at normothermia (One way ANOVA, p<0.0001). B. Comparison of sham (100%), normothermia (8.8%), combined 24-hour duration TH group (41.6%) and combined 48-hour duration TH (67.6%) group demonstrates 48-hour duration TH results in superior neuroprotection compared to 24-hour TH (One way ANOVA, p<0.0001). C. Comparison of sham (100%), normothermia (8.8%) and 0-hour (52.6%), 1-hour (52.6%), 4-hour (50.5%), or 8-hour (65%) post-ROSC TH onset indicates that TH initiated between 0-8 hours after ROSC results in equivalent neuroprotection among 7-day survivors (Two way ANOVA, p=0.5058). Two-way ANOVA was adopted because that the hypothermia duration was significant.
Figure 5. Hippocampal Histology after Post-Cardiac Arrest Therapeutic Hypothermia.
Representative hippocampal sections from sham injured rats or rats 7 days after 10-minute asphyxial cardiac arrest followed by normothermia or TH initiated 1 hour after ROSC and maintained for 24 or 48 hours. A. Sections immunolabeled for NeuN (red) and counterstained with Hoechst (blue). Degeneration of CA1 pyramidal neurons is characterized by loss of NeuN immunolabeling (red) and condensed shrunken nuclei (blue). B. Sections stained by the H&E method. Classic ischemic neuronal change in the CA1 pyramidal layer is characterized by shrunken dark-staining pyknotic nuclei (blue) and eosinophilic cytoplasm (pink) see in the high power images. Scale bar 500μm for low power images and 50μm for high power images.
Discussion
This study systematically examined how the beneficial effects of post-cardiac arrest TH are impacted by onset time and duration of therapy. Most importantly, our results suggest that the impact of TH on survival with good neurologic function is comparable when TH is initiated between 0 and 4 hours after ROSC, but completely lost when TH is initiated 8 hours after ROSC. Although the duration of TH had no detectable impact on survival or survival with GNF, we did observe significantly greater protection of hippocampal CA1 pyramidal neurons when TH was maintained for 48 hours compared to 24 hours. The potential mechanisms and implications of this differential effect are discussed below.
Optimal Timing for Initiation of Post-Cardiac Arrest Therapeutic Hypothermia
Our results question the concept that TH should be initiated as soon as possible after ROSC. If post-cardiac arrest TH affords comparable outcome benefit when initiated between 0 and 4 hours after ROSC in humans, the implications for clinical implementation are significant. However, it should be noted that target temperature was achieved 30 minutes after ROSC in our earliest treatment group. Therefore, this study does not address the potential benefit of achieving target temperature within <30 minutes of ROSC or during cardiac arrest. In fact, short-duration cooling studies suggest that achieving target temperature during cardiac arrest or within <10-20 minutes of ROSC could tap into a therapeutic mechanism that is distinct from and potentially additive to the benefits of prolonged post-cardiac arrest TH (4-6).
Our results also suggest that there is a therapeutic window after which the benefit of TH on survival and survival with GNF is lost. Although we found this to occur between 4 and 8 hours after ROSC in our model, the therapeutic window in humans remains to be determined, and is likely to vary based in individual circumstances. Furthermore, among survivors, TH appeared to protect hippocampal CA1 pyramidal neurons even when initiated 8 hours after ROSC. This observation is consistent with evidence that degeneration of hippocampal CA1 pyramidal neurons is delayed for 24-48 hours after global brain ischemia in both rats (16, 17) and humans (18, 19). These results are also consistent with data from our laboratory demonstrating equivalent neuroprotection when hypothermia (33°C) is initiated 1, 2, 4, or 8 hours after simulated ischemia in organotypic hippocampal slices and maintained for 24 hours (20). Using a gerbil model of transient forebrain ischemia, Colbourne et al, reported hippocampal CA1 neuroprotection even when hypothermia was initiated 12 hours after reperfusion, although the protection was less than when initiated 1 or 6 hours after reperfusion (12).
There are a number of possible explanations for the apparently broader therapeutic window for the neuroprotective effect of TH compared to the effect on survival and neurologic function. First, we do not expect the mechanisms for improved survival with TH to be solely based on neuroprotection. Therapeutic hypothermia has well documented effects on the cardiovascular and immune system that likely to contribute to post-cardiac arrest survival independent of neuroprotection, and these independent mechanisms are likely to have their own therapeutic window. Additionally, the neurologic function score used in this study assesses sensorimotor function and level of consciousness, but not cognitive function. Loss of hippocampal CA1 pyramidal neurons causes learning and memory deficits that are most reliably measured by behavioral cognitive testing. However, in rodents, these tests rely heavily on intact motor function and would be confounded in this study by presence of significant motor deficits in surviving animals. Finally, it is important to note that the therapeutic window for protection of other selectively vulnerable neuron populations in other brain regions could be different than that of hippocampal CA1 pyramidal neurons.
Existing clinical data provides limited insight into how the time to target temperature affects outcome in humans. In the two major positive clinical trials, target temperature was reportedly achieved within 2 hours (1) or at a median time of 8 hours (interquartile range was 4-16 hours)(2) after ROSC. The latter study suggests a potentially broader therapeutic window than we observed in our rat model. However, it is important to note that cooling was initiated at a median 105 minutes (IQR 61-192 minutes) after ROSC, the median temperature was <36°C by 2 hours after ROSC, and half of the patients did reach target temperature in less than 8 hours (2). A recent analysis of data from a prospective clinical registry of 975 post-cardiac arrest patients treated with TH revealed the median time to achieving target temperature was 4.3 hours (interquartile range 3.0 to 6.7 hours), and no association between time to target temperature and outcome was detected (21). Several clinical studies have reported on the feasibility of early post-cardiac arrest cooling and intra-arrest cooling in the out-of-hospital setting (22-25). Among these, the only study powered to detect an outcome benefit compared out-of-hospital and in-hospital initiation of cooling after out-of-hospital cardiac arrest, and found that out-of-hospital initiation of cooling did not improve outcomes (25).
Optimal Duration of Post-Cardiac Arrest Therapeutic Hypothermia
Our data suggest comparable survival and survival with GNF when TH is maintained for either 24 or 48 hours after ROSC, and there appear to be no significant interaction between onset and duration of therapy. However, neuroprotection based on hippocampal CA1 pyramidal neuron counts was significantly greater when TH was maintained for 48 hours compared to 24 hours. As stated previously, our NFS does not assess memory and therefore is not expected to detect differences in hippocampal neuron injury. Since degeneration of hippocampal CA1 pyramidal neurons is delayed for 24-48 hours after transient global brain ischemia in rodents, it is not unexpected that extending hypothermia beyond 24 hours provides additional neuroprotection (16, 17).
Existing clinical data provides limited insight into how the duration of TH affects patient outcome. TH was maintained for either 12 or 24 hour in the two major prospective randomized clinical trials (1, 2). Recent analysis of data from a prospective clinical registry of 975 post-cardiac arrest patients reported that TH was maintained for 24 hours in 93% of patients (21). Pediatric clinical trials are underway comparing the effectiveness of 48-hour TH vs. normothermia after cardiac arrest (ClinicalTrials.gov Identifiers: NCT0087864 and NCT00880087), and comparing 24-hour vs. 72-hour TH after cardiac arrest (ClinicalTrials.gov Identifier: NCT00797680). Cleary, more clinical studies are needed to discern optimal duration of post-cardiac arrest TH.
Study Limitations
While the results presented here provide important and clinically relevant insights into the optimization of post-cardiac arrest TH, this study has a number of limitations. Most importantly, we were not able to evaluate cognitive function as an outcome parameter. This was not attempted because surviving rats in this model have motor deficits of variable severity, which confound interpretation of behavioral studies used to assess cognitive function. Another limitation is that we studied a single duration of cardiac arrest caused by asphyxia resulting in PEA/asystole. It is possible that the optimal timing and duration of TH is different for other presenting rhythms (e.g. ventricular fibrillation) or durations of cardiac arrest. Furthermore, we did not determine if there is a duration of cardiac arrest beyond which TH does not improve outcome. This is a clinically important knowledge gap that should be the subject of additional animal and human studies.
Our cardiac arrest model is without co-morbidities such as acute coronary syndrome and acute myocardial infarction that are commonly associated with cardiac arrest in humans. It is possible that the therapeutic window for the survival benefit of TH could be altered with such co-morbidities. Species differences in the time course of pathophysiologic mechanisms could also result in a different therapeutic window for rats and humans
In terms of TH parameters, we did not evaluate intra-arrest TH or TH achieved within <30 minutes of ROSC, and we did not evaluate TH for durations longer than 48 hours. We also did not address the issues of optimal temperature or rewarming rate. All of these parameters have the potential to impact the efficacy of post-cardiac arrest TH and should be the subject of additional studies.
This translational study does not address the mechanisms by which TH improves survival, improves neurologic function, and reduces post-ischemic neurodegeneration. Future studies to systematically elucidate these protective mechanisms are essential to achieve optimal implementation of post-cardiac arrest TH in the greatest number of patients.
Conclusions
In this rat model of PEA/asystole cardiac arrest, hypothermia initiated between 0 and 4 hours of ROSC and maintained for 24 to 48 hours resulted in comparable improvement in survival and survival with GNF compared to normothermia. However, histologic evidence of neuroprotection was evident when hypothermia was initiated up to 8 hours after ROSC, and was significantly greater when TH was maintained for 48 hours compared to 24 hours. Overall these results provide important insights into the optimization of post-cardiac arrest TH, and suggest potential differences in the optimal parameters when targeting survival versus neuroprotection.
Acknowledgments
Fred Colbourne served as a consultant for establishing the automated telemetric temperature regulation system and provided the Thermoreg software.
Financial support used for the study: NIH R21-NS054654 (Robert W. Neumar)
Dr. Guo and Dr. Neumar received funding from NIH.
Footnotes
Performance Site: Center for Resuscitation Science, University of Pennsylvania School of Medicine
The remaining authors have not disclosed any potential conflicts of interest.
References
- 1.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 2.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 4.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Takata K, Takeda Y, Sato T, Nakatsuka H, Yokoyama M, Morita K. Effects of hypothermia for a short period on histologic outcome and extracellular glutamate concentration during and after cardiac arrest in rats. Crit Care Med. 2005;33:1340–5. doi: 10.1097/01.ccm.0000166351.19369.d3. [DOI] [PubMed] [Google Scholar]
- 6.Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Circulation. 2004;109:2786–91. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 7.Hicks SD, DeFranco DB, Callaway CW. Hypothermia during reperfusion after asphyxial cardiac arrest improves functional recovery and selectively alters stress-induced protein expression. J Cereb Blood Flow Metab. 2000;20:520–530. doi: 10.1097/00004647-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Chopp M, Chen H, Dereski MO, Garcia JH. Mild hypothermic intervention after graded ischemic stress in rats. Stroke. 1991;22:37–43. doi: 10.1161/01.str.22.1.37. [DOI] [PubMed] [Google Scholar]
- 9.Carroll M, Beek O. Protection against hippocampal CA1 cell loss by post-ischemic hypothermia is dependent on delay of initiation and duration. Metab Brain Dis. 1992;7:45–50. doi: 10.1007/BF01000440. [DOI] [PubMed] [Google Scholar]
- 10.Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 11.Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colbourne F, Sutherland GR, Auer RN. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J Neurosci. 1999;19:4200–4210. doi: 10.1523/JNEUROSCI.19-11-04200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A, Katz L, Ebmeyer E, Safar P. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation. 1995;29:249–263. doi: 10.1016/0300-9572(94)00827-3. [DOI] [PubMed] [Google Scholar]
- 14.Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15:1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 15.Colbourne F, Sutherland GR, Auer RN. An automated system for regulating brain temperature in awake and freely moving rodents. J Neurosci Methods. 1996;67:185–190. [PubMed] [Google Scholar]
- 16.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–98. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 17.Kirino T, Tamura A, Sano K. Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol. 1984;64:139–147. doi: 10.1007/BF00695577. [DOI] [PubMed] [Google Scholar]
- 18.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987 Aug;37(8):1281–6. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 19.Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence EJ, Dentcheva E, Curtis KM, Roberts VL, Siman R, Neumar RW. Neuroprotection with delayed initiation of prolonged hypothermia after in vitro transient global brain ischemia. Resuscitation. 2005;64:383–388. doi: 10.1016/j.resuscitation.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen N, Hovdenes J, Nilsson F, Rubertsson S, Stammet P, Sunde K, Valsson F, Wanscher M, Friberg H Hypothermia Network. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926–34. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim F, Olsufka M, Longstreth WT, Jr, Maynard C, Carlbom D, Deem S, Kudenchuk P, Copass MK, Cobb LA. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation. 2007;115:3064–70. doi: 10.1161/CIRCULATIONAHA.106.655480. [DOI] [PubMed] [Google Scholar]
- 23.Bruel C, Parienti JJ, Marie W, Arrot X, Daubin C, Du Cheyron D, Massetti M, Charbonneau P. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care. 2008;12:R31. doi: 10.1186/cc6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castrén M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, Eichwede F, Mols P, Schwab T, Vergnion M, Storm C, Pesenti A, Pachl J, Guérisse F, Elste T, Roessler M, Fritz H, Durnez P, Busch HJ, Inderbitzen B, Barbut D. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness) Circulation. 2010;122:729–3. doi: 10.1161/CIRCULATIONAHA.109.931691. [DOI] [PubMed] [Google Scholar]
- 25.Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, Kelly AM, Silvester W Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122:737–742. doi: 10.1161/CIRCULATIONAHA.109.906859. [DOI] [PubMed] [Google Scholar]