Abstract
The pro-apoptotic activity of the Bcl–2 family member Bax has been shown to be facilitated by homodimerization. However, it is unknown whether Bcl–2 or Bcl–xL have to homodimerize to protect cells from apoptosis. Here we show by co-immunoprecipitation and FPLC analyses that while Bax multimerizes and forms heterodimers with Bcl–2, there is no evidence for Bcl–2 homodimerization, even in conditions under which Bcl–2 protects cells from apoptosis. Immunofluorescence studies confirmed that Bax can attract active, soluble Bcl–2 to mitochondrial membranes, but that nuclear/ER membrane-bound Bcl–2 was incapable of dislocating soluble Bcl–2. The failure of Bcl–2 to homodimerize is due to structural constraints as versions of Bcl–2 deleted or mutated in the BH1 and BH2 domains effectively dimerized with wild-type Bcl–2 and were dislocated by Bcl–2 inside cells. These data indicate that naturally occurring Bcl–2 does not expose protein domains that mediate homodimerization and therefore most likely acts as a monomer to protect cells from apoptosis.
Keywords: apoptosis/Bax/Bcl-2/BH3 domain/dimerization
Introduction
Apoptosis is a tightly controlled, physiological mechanism to eliminate superfluous and damaged cells from a multi– cellular organism. At the center stage of this process are caspases, a family of cysteine-dependent, aspartate-directed proteases, which dismantle cells by cleaving survival factors, cytoskeletal and nuclear scaffold proteins and components of the replication, transcription, splicing or repair machineries (Earnshaw et al., 1999). Inhibition of caspases and hence interference with apoptosis occurs either directly through a pseudosubstrate strategy (p35, IAPs) (Clem et al., 1996) or indirectly by sequestering and inactivating caspase-activating adaptor proteins (Thome et al., 1997). Some members of the Bcl–2 family have emerged as prototypes for the latter mechanism (Reed, 1997).
Bcl–2 family members can be grouped into antiapoptotic survival factors such as Bcl–2 or Bcl–xL, which block caspase activation, and pro-apoptotic killer proteins such as Bax and Bak, which facilitate apoptosis via caspase-dependent and -independent mechanisms (Reed, 1997). Sequence analysis has not provided any clue as to how these proteins may work. However, they share one to four α–helical Bcl–2 homology domains, called the BH domains, whose subtle differences in primary sequence and spatial arrangements may determine pro- and anti-apoptotic functions (Muchmore et al., 1996; Aritomi et al., 1997).
The anti-apoptotic proteins Bcl–2 and Bcl–xL possess four BH domains (BH1–4), which are all required for death protection (Adams and Cory, 1998). Based on the crystal structure of Bcl–xL, these domains cooperate to form a hydrophobic face on one side of the molecule to which numerous proteins, including caspase activators, may bind (Muchmore et al., 1996; Aritomi et al., 1997). An additional, amphipathic C–terminus allows the proteins to be tail-anchored to intracellular membranes (mainly mitochondria and nuclear/ER) (Nguyen et al., 1993; Lithgow et al., 1994) and thus to sequester apoptosis regulatory molecules from the cytoplasm to specific membrane sites. This ‘docking effect’ may, however, not be the only activity of Bcl–2 or Bcl–xL (Reed, 1997). Structurally, Bcl–xL resembles the bacterial, pore-forming toxins colicin or diphtheria toxin, especially in an α–helical region that mediates the membrane insertion of the toxins (Muchmore et al., 1996). This region corresponds to BH1 and part of the BH2 domains of Bcl–2 and Bcl–xL, and is called the α5/α6 region. As recombinant Bcl–2 and Bcl–xL can form ion channels on artificial membranes, it was proposed that, like the bacterial toxins, these proteins use their α5/α6 region for membrane insertion and/or channel formation (Schendel et al., 1998).
Apart from lacking an N–terminal BH4 domain, the pro-apoptotic family members Bax and Bak contain BH3, BH1 and BH2 domains that are highly homologous and similarly spaced as in Bcl–2 or Bcl–xL (Adams and Cory, 1998). Studies on the structure of the pro-apoptotic family member Bid (Chou et al., 1999; McDonnell et al., 1999) as well as molecular modeling of Bax (Aritomi et al., 1997; Schendel et al., 1998) suggest that the overall structure of Bax or Bak may be very similar to that of Bcl–xL. In addition, like Bcl–xL and Bcl–2, Bax can form ion channels on artificial membranes, and this also depends on the α5/α6 membrane spanning region encompassing the BH1/BH2 domains (Schendel et al., 1998). However, a major difference between Bax/Bak and Bcl–2/Bcl–xL is that the former undergo conformational changes inside cells. While Bcl–2 and Bcl–xL are mostly membrane bound, Bax and Bak are often found in the cytoplasm of normal cells. In response to apoptotic stimuli, the pro-apoptotic proteins change their conformation, expose an N–terminal epitope and translocate to mitochondria where they perform their cytotoxic action (Wolter et al., 1997; Goping et al., 1998; Griffiths et al., 1999). Part of this action involves the release of cytochrome c into the cytoplasm (Jürgensmeier et al., 1998; Rossé et al., 1998) where it binds to the adapter protein Apaf–1 to activate caspases–9 and -3 (Green and Reed, 1998). Bax may itself form a channel through which cytochrome c can pass or regulate an outer mitochondrial channel, such as the voltage-dependent anion channel (VDAC), to make it more permeable for cytochrome c (Shimizu et al., 1999).
In addition to the α5/α6 membrane spanning regions, Bax and Bak depend on the BH3 domain for cytotoxicity (Kelekar and Thompson, 1998). Two modes of action have been proposed for this domain. First, it can interact with Bcl–2 and Bcl–xL and inhibit their death protective activities (Chittenden et al., 1995; Zha et al., 1996; Holinger et al., 1999). Structural analysis revealed that the BH3 domain of Bak nestles into a shallow groove in the hydrophobic face of Bcl–xL because it changes from a random coil to an α–helical structure and rotates outward to expose its hydrophobic surface (Sattler et al., 1997). Secondly, the BH3 domain is used for Bax or Bak homodimerizations (Simonen et al., 1997; Zha and Reed, 1997; Kelekar and Thompson, 1998). While there is still some disagreement whether such homodimerizations are required for pro-apoptotic activities (Simonian et al., 1996), enforced dimerization of Bax was shown to facilitate mitochondrial translocation, mitochondrial dysfunction and apoptosis induction (Gross et al., 1998). Moreover, purified Bax can form heptamers in solution (Lewis et al., 1998).
It has remained even more controversial whether Bcl–2 and Bcl–xL require homodi- or multimerization for their survival activity. This is an important issue if one wants to know whether these proteins form channels within cells. Although the crystal structure of soluble, anchorless Bcl–xL is a monomer (Muchmore et al., 1996), it could still form dimers after conformational changes and/or insertion into membranes. Bcl–2 was shown to interact with another Bcl–2 molecule in a head-to-tail fashion in the yeast two-hybrid system (Hanada et al., 1995) and in co-immunoprecipitates (Yin et al., 1994). However, these analyses were often done with tagged [glutathione S-transferase (GST), LexA, hemagglutinin (HA), etc.] versions of Bcl–2 where the native structure of Bcl–2 may have been disrupted. Here we show that while Bax dimerizes in a BH3-dependent manner, Bcl–2 does not readily form homodimers both in vitro and within cells. This is not because the BH3 domain cannot participate at dimerization reactions, but because this domain is not accessible and/or recruitable in the wild-type Bcl–2 molecule.
Results
Bax homodimerizes and heterodimerizes with Bcl–2 in a BH3-dependent manner
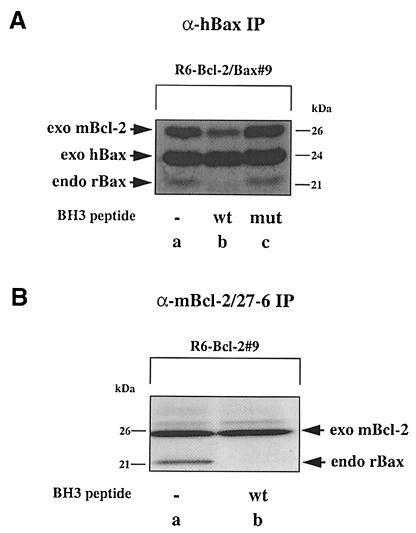
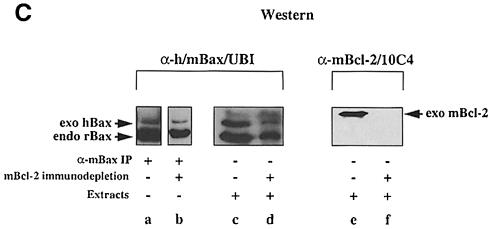
We previously showed homodimerization between exogenous and endogenous Bax and heterodimerization between exogenous Bax and Bcl–2 in anti-Bax immuno– precipitates from rat 6 (R6) embryo fibroblasts stably co-overexpressing Bax and Bcl–2 (R6-Bcl–2/Bax#9) (Otter et al., 1998). As these interactions are thought to be mediated via the BH3 domain of Bax, we performed competition experiments using a 19 amino acid Bax–BH3 peptide either in its wild-type (56TKKLSECLKRIGDELDSNM74) or mutant (D68A) configuration. When mixed at a concentration of 100 μM into a [35S]methionine/cysteine-labeled R6-Bcl–2/Bax#9 cell lysate, the wild-type, but not the mutant Bax–BH3 peptide prevented the co-immunoprecipitation of endogenous rat Bax (rBax) with exogenous human Bax (hBax) (Figure 1A). The same peptide also interfered with the co-immunoprecipitation of endogenous rat Bax with exogenous mouse Bcl–2 (mBcl–2) from an R6-Bcl–2#9 cell lysate (Figure 1B). To rule out the possibility that Bax formed homodimers via Bcl–2, we immunodepleted the R6-Bcl–2/Bax#9 cell extract of Bcl–2 prior to anti-Bax immunoprecipitations. Although this procedure also diminished the levels of Bax (due to its binding to Bcl–2) (Figure 1C, lanes c and d), we could still detect the co-immunoprecipitation of exogenous hBax with endogenous rBax (lane b) in the absence of any detectable Bcl–2 (lane f). Thus, Bax can form either Bcl–2-independent homodimers or heterodimers with Bcl–2 in a BH3-dependent manner.
Fig. 1. BH3-dependent interaction between Bax and Bax/Bcl–2 in R6 cell extracts. (A) α-hBax immunoprecipitates (IP) from radiolabeled R6 cell extracts co-overexpressing hBax and mBcl–2 (R6-Bcl–2/Bax#9), performed in the absence (lane a) or presence of a wild-type (lane b) or a mutant (D68A) (lane c) Bax–BH3 peptide. The location of the immunoprecipitated overexpressed hBax (exo hBax) [24 kDa; see Otter et al., (1998)], the co-precipitating endogenous rBax (endo rBax) (21 kDa) and overexpressed mBcl–2 (exo mBcl–2) (26 kDa) are indicated. (B) α–mBcl–2/27–6 immunoprecipitates from R6 cell extracts overexpressing mBcl–2 (R6-Bcl–2#9), performed in the absence (lane a) or presence of the wild-type Bax–BH3 peptide (lane b). (C) α–h/mBax/UBI Western blot of α–mBax immuno- precipitates from R6-Bcl–2/Bax#9 cell extracts before (lane a) and after (lane b) three rounds of mBcl–2 immunodepletion using the α–mBcl–2/10C4 antibody. We also show α–h/mBax/UBI and α–mBcl–2/10C4 Western blots of the cell extracts before (lanes c and e) and after (lanes d and f) mBcl–2 immunodepletion to prove the total disappearance of Bcl–2 (compare lanes e and f) and the partial disappearance of exo hBax and endo rBax (compare lanes c and d).
No evidence for Bcl–2 homodimers in anti-Bcl–2 and anti-Flag immunoprecipitates
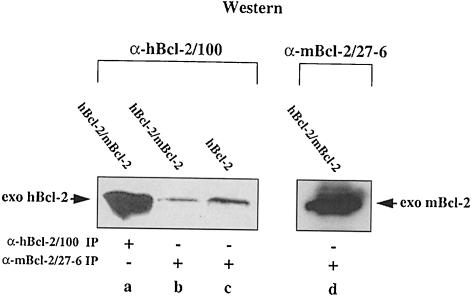
Next, we wanted to know whether Bcl–2 formed homodimers under the same extraction/immunoprecipitation conditions. For that purpose we transiently co-overexpressed hBcl–2 and mBcl–2 in HEK cells. Following an immunoprecipitation with a Bcl–2 antibody against one species, the immunoprecipitate was immunoblotted and probed with a Bcl–2 antibody against the other species. As shown in Figure 2, the mouse Bcl–2-specific antibody α-mBcl–2/27–6 immunoprecipitated high amounts of mBcl–2 from an HEK-hBcl–2/mBcl–2 cell extract (lane d). This antibody slightly crossreacted with hBcl–2 as seen in an α–mBcl–2/27–6 immunoprecipitate from HEK-hBcl–2 lysates (Figure 2, lane c). The amount of crossreactive hBcl–2 was, however, far below that immunoprecipitated with the human Bcl–2-specific antibody α-hBcl–2/100 (Figure 2, compare lanes a and c). Moreover, an α–mBcl– 2/27–6 immunoprecipitate from an HEK-hBcl–2/mBcl–2 lysate did not contain more hBcl–2 than an α–mBcl–2/27–6 immunoprecipitate from an HEK-hBcl–2 lysate (Figure 2, compare lanes b and c), indicating that the two Bcl–2 species did not interact.
Fig. 2. No interaction between Bcl–2 molecules in HEK cell extracts. α–hBcl–2/100 Western blot of unlabeled α–hBcl–2/100 (lane a) and α–mBcl–2/27–6 (lanes b and c) immunoprecipitates from extracts of HEK cells transfected with hBcl–2 alone or co-transfected with hBcl–2 and mBcl–2. Lane d shows an α–mBcl–2/27–6 Western blot of an α–mBcl–2/27–6 immunoprecipitate from extracts of co-transfected cells. Note that there is not more hBcl–2 in lane b than in lane c, indicating that hBcl–2 does not co-precipitate with mBcl–2.
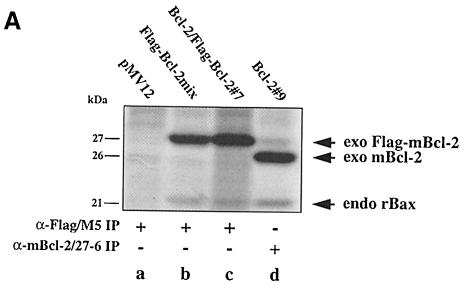
To confirm the absence of Bcl–2 dimers in vitro, we investigated the interaction of mBcl–2 with an N–terminally Flag-tagged version of mBcl–2 (Flag–mBcl–2) in lysates from R6 cells stably overexpressing the two proteins individually (R6-Bcl–2#9, R6-Flag–Bcl–2mix) or in combination (R6-Bcl–2/Flag–Bcl–2#7). Flag–mBcl–2 was as efficient in protecting cells from apoptosis as mBcl–2 (Otter et al., 1998; also see Supplementary material available in The EMBO Journal Online). Thus, if dimer formation was crucial for Bcl–2 function it should also occur between Flag–mBcl–2 and mBcl–2. When we compared α–Flag immunoprecipitates from extracts of [35S]methionine/cysteine-labeled R6-Bcl–2/Flag–Bcl–2#7 and R6-Flag–Bcl–2mix cells, we found in both cases a 27 kDa protein corresponding to Flag–mBcl–2 as well as a 21 kDa protein corresponding to co-precipitated endogenous rBax (Figure 3A, lanes b and c). However, there was no additional 26 kDa mBcl–2 that co-immuno-precipitated from the R6-Bcl–2/Flag–Bcl–2#7 cell extract (Figure 3A, lane c), indicating that Flag–mBcl–2 and mBcl–2 did not interact.
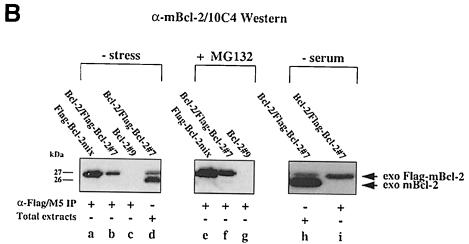
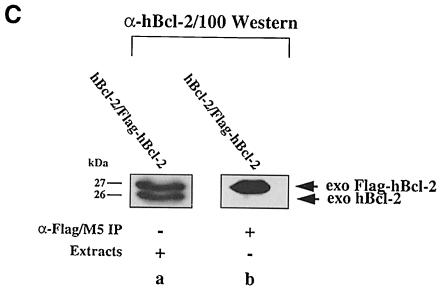
Fig. 3. No interaction between Bcl–2 and Flag-tagged Bcl–2 in R6 extracts. (A) α–Flag/M5 (lanes a–c) or α–mBcl–2/27–6 (lane d) immunoprecipitates from radiolabeled R6 cell extracts harboring either the transfer vector (pMV12) (lane a), Flag–mBcl–2 (R6-Flag–Bcl–2mix) (lane b), mBcl–2 (R6-Bcl–2#9) (lane d) or both (R6-Bcl–2/Flag–Bcl–2#7) (lane c). Note the absence of a band at 26 kDa in lane c, indicating that 26 kDa mBcl–2 does not co-precipitate with 27 kDa Flag–mBcl–2. (B) α–mBcl–2/10C4 Western blot of α–Flag/M5 immunoprecipitates from unlabeled extracts of R6 cells overexpressing Flag–mBcl–2 (R6-Flag–Bcl–2mix) (lanes a and e) or mBcl–2 (R6-Bcl–2#9) (lanes c and g) or both (R6-Bcl–2/Flag–Bcl–2#7) (lanes b, f and i) before (– stress) and after treatment with 1 μM MG132 (+ MG132) or depletion of serum for 48 h (– serum). Total extracts from R6-Bcl–2/Flag–Bcl–2#7 cells in the presence or absence of serum are shown in lanes d and h, respectively. Note that neither lane b nor lanes f and i contain co-precipitating 26 kDa exo mBcl–2 although it is clearly present in the total extracts (lanes d and h). (C) α–hBcl–2/100 Western blot of an extract (lane a) or an α–Flag/M5 immunoprecipitate of this extract (lane b) prepared from HEK cells co-transfected with Flag–hBcl–2 and hBcl–2. Note that the human forms of Bcl–2 also do not interact.
To increase the sensitivity of immunodetection, we probed the α–Flag immunoprecipitates from unlabeled R6-Bcl–2/Flag–Bcl–2#7 and R6-Flag–Bcl–2mix lysates on an α–mBcl–2/10C4 Western blot. In addition, we considered the possibility that Bcl–2 homodimers would only form when the cells were exposed to apoptotic stimuli, i.e. when Flag–mBcl–2 and mBcl–2 acted as survival factors. For that purpose, we treated the cells with 1 μM MG132 or removed the serum for 48 h. Under these conditions most of the R6 cells underwent apoptosis while the Bcl–2 overexpressing counterparts were effectively protected (Otter et al., 1998; our unpublished data). α–Flag immunoprecipitates from both unstressed and stressed R6–Bcl–2/Flag–Bcl–2#7 lysates still contained only the 27 kDa Flag–mBcl–2 and no co-precipitated 26 kDa mBcl–2 when tested on an α–mBcl–2/10C4 Western blot (Figure 3B, lanes b, f and i). This was not due to the degradation or lack of expression of the 26 kDa mBcl–2 as this protein was clearly immunodetected in a total extract of unstressed (Figure 3B, lane d) or stressed (lane h) R6-Bcl–2/Flag–Bcl–2#7 cells. In addition, the lack of Bcl–2 homodimerization was not species specific since the human forms of Flag–Bcl–2 and Bcl–2 also did not interact in α–Flag immunoprecipitates (Figure 3C, lane b). Taken together, our data show that even under apoptotic stress conditions when Bcl–2 effectively acts as a survival factor, it does not form homodimers.
Purified recombinant Bax multimerizes, while Bcl–2 stays monomeric
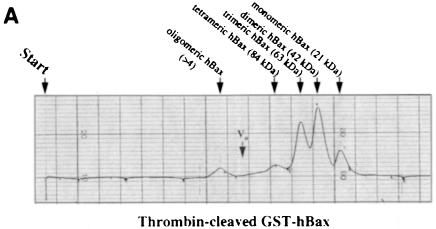
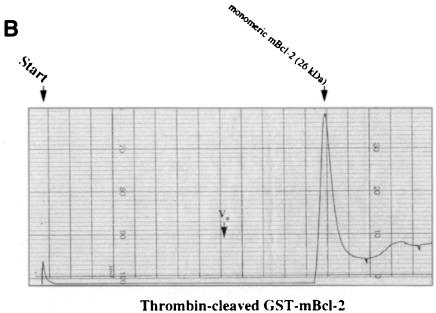
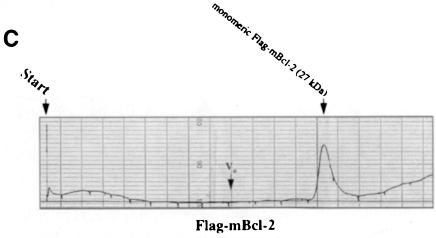
We embarked on yet another approach to study di- or multimer formation of Bcl–2 and Bax in vitro. Both proteins were generated as GST fusions in bacteria, attached to a glutathione–Sepharose column and cleaved on the column by thrombin to release the recombinant mBcl–2 or hBax proteins. Since we worked with the full-length molecules, detergent had to be included at all steps of the purification. However, to minimize micelle formations on the FPLC column, elutions of mBcl–2 and hBax from the glutathione column were performed in the presence of 0.05% N,N–dimethyldodecylamine-N–oxide (LDAO) instead of 1% Triton X–100. After passing through an FPLC Superose 12 sizing column and comparing with the elution of standard proteins (see figure legends), the two recombinant proteins exhibited a strikingly different elution profile. While hBax eluted in several peaks that were multiples of ∼21 kDa (Figure 4A), mBcl–2 eluted in a single peak of ∼26 kDa (Figure 4B). These peaks all contained the respective hBax or mBcl–2 proteins as tested by Western blotting of the eluted fractions (our unpublished data). Moreover, when Flag–mBcl–2 was affinity purified from an R6-Flag–Bcl–2mix extract and applied to the Superose column, it also eluted as a single peak at ∼27 kDa (Figure 4C). These data show that Bax, but not Bcl–2, can multimerize in vitro.
Fig. 4. Recombinant Bax, but not Bcl–2 or Flag–Bcl–2, forms multimers in vitro. On-line 280 nm measurements of glutathione affinity-purified, thrombin-cleaved GST–hBax (A), GST–mBcl–2 (B) or α–Flag/M2-purified Flag–mBcl–2 (C) eluting from an FPLC Superose 12 column. The time of sample application (Start) and the void volume of the column (Vo) are indicated. While hBax elutes in multiple peaks corresponding to the molecular masses of its multimers (21, 42, 63, 84 kDa and higher), mBcl–2 and Flag–mBcl–2 are recovered in one single peak each (monomeric 26 or 27 kDa forms, respectively). The molecular masses were calculated according to the elutions of the standard proteins carbonic anhydrase (29 kDa), ovalbumin (45 kDa), bovine serum albumin (66 kDa) and β–galactosidase (115 kDa).
Bax, but not Bcl–2, dislocates a soluble form of Bcl–2 inside cells
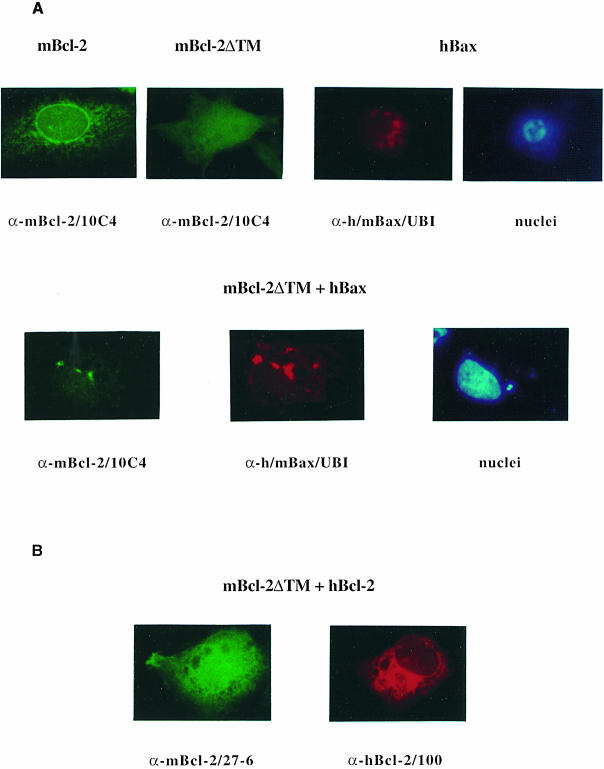
It has been reported that detergent can either promote or disrupt Bcl–2 family protein interactions (Hsu and Youle, 1997; Otter et al., 1998). We therefore needed to confirm our Bcl–2–Bax interaction studies in an experimental system that does not require detergent extraction. We chose an immunofluorescence analysis on fixed, permeabilized R6 cells and investigated how Bax and Bcl–2 molecules recruit each other to different subcellular compartments. Our previous data showed that while Bax was mainly associated with mitochondria, a high portion of Bcl–2 was bound to the nuclear envelope and the associated ER membrane (Borner et al., 1994a; Rossé et al., 1998). Upon co-overexpression, Bax could attract Bcl–2 to the mitochondrial membrane, indicating that the two proteins interacted inside cells (Otter et al., 1998; Rossé et al., 1998); however, it was difficult to monitor Bcl–2–Bcl–2 interactions as mBcl–2, hBcl–2 and Flag–mBcl–2 all localized to the same nuclear/ER site (our unpublished data). We therefore took advantage of a Bcl–2 mutant that was devoid of its C–terminal membrane anchor and thus localized to the cytoplasm (mBcl–2ΔTM). This mutant was previously shown to retain its function to protect neurons from NGF-deprived apoptosis (Borner et al., 1994a). We first validated our strategy by testing whether Bax could attract mBcl–2ΔTM similarly to the way in which it attracted wild-type mBcl–2. As expected, while wild-type Bcl–2 was detected on nuclear/ER membranes, mBcl–2ΔTM exhibited a diffuse cytoplasmic and intranuclear staining after transfection into R6 cells (Figure 5A). The reason for a presumed nuclear staining of mBcl–2ΔTM is unknown but the lack of its membrane anchor and its low molecular mass (24 kDa) may allow a free passage into the nucleus. In contrast, transfected hBax showed a punctated, mitochondrial pattern as previously reported (Rossé et al., 1998), and triggered nuclear fragmentation (Figure 5A). Upon co-transfection with Bax, mBcl–2ΔTM lost most of its cytoplasmic/nuclear association, became co-localized with Bax in punctated mitochondria and inhib– ited Bax-induced nuclear fragmentation (Figure 5A). Thus, cytosolic mBcl–2ΔTM behaved like wild-type membrane-bound Bcl–2 in that it was attracted by Bax to mitochondria and inhibited the pro-apoptotic activity of the latter. This set the stage to investigate whether mBcl–2ΔTM inter– acted similarly with wild-type Bcl–2. To distinguish between the two proteins, we co-transfected R6 cells with mBcl–2ΔTM and a human form of wild-type Bcl–2 and used species-specific antibodies for immunofluorescence. As shown in Figure 5B, while hBcl–2 was mainly detected on the nuclear envelope/ER network, mBcl–2ΔTM remained diffuse, cytoplasmic and intranuclear after co-transfection into R6 cells. This was also the case when the cells were exposed to the apoptotic stimulus MG132 (our unpublished data). Thus, unlike Bax, hBcl–2 cannot significantly recruit mBcl–2ΔTM to the ER/nuclear membrane, suggesting that Bcl–2 homodimers do not readily form within cells.
Fig. 5. Membrane recruitment of cytoplasmic Bcl–2ΔTM by Bax, but not by Bcl–2. (A) α–mBcl–2/10C4 (fluorescein), α–h/mBax/UBI (Texas Red) and Hoechst 33342 (UV, nuclei) fluorescence analysis on R6 cells transfected with the C–terminally deleted mBcl–2ΔTM mutant, hBax or both at 24 h post-transfection. For comparison a wild-type mBcl–2 transfectant is shown. (B) α–mBcl–2/27–6 (fluorescein) and α–hBcl–2/100 (Texas Red) fluorescence analysis on R6 cells co-transfected with mBcl–2ΔTM and hBcl–2 at 24 h post-transfection. Note that hBax attracts mBcl–2ΔTM from a diffuse to a punctated (mitochondrial) staining, accompanied by a block in nuclear condensation/fragmentation. Both hBcl–2 and mBcl–2 localize to the ER/nuclear membrane and do not attract mBcl–2ΔTM.
Bcl–2 lacking BH1/BH2 domains dimerizes with wild-type Bcl–2 in a BH3-dependent manner
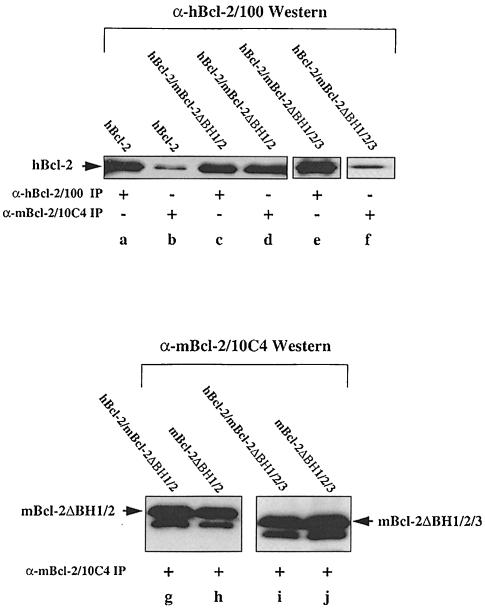
Our data so far indicate that despite a high sequence homology, Bcl–2 and Bax may be structurally different. An attractive possibility is that Bax can form di- or multimers because its BH3 domain is exposed or at least easily recruitable. In Bcl–2 this domain would be hidden inside the molecule. Indeed, based on the crystal structure of Bcl–xL, the BH3 domain of Bcl–2 is in close contact with the BH1/BH2 domains (Muchmore et al., 1996; Aritomi et al., 1997). Thus, by breaking this intramolecular interaction, we may obtain a Bcl–2 molecule that now has its BH3 domain exposed for dimerizations. We therefore generated two Bcl–2 deletion mutants: an mBcl–2 lacking BH1/BH2 (mBcl–2ΔBH1/2) and an mBcl–2 lacking BH1/BH2 and BH3 (mBcl–2ΔBH1/2/3). The purpose of the latter mutant was to test whether an eventual interaction between mBcl–2ΔBH1/2 and Bcl–2 was BH3 mediated. Both mBcl–2ΔBH1/2 and mBcl–2ΔBH1/2/3 were stable proteins of 19 and 17 kDa, respectively, which could be highly expressed in HEK, R6 and other cell types (Figure 6, lanes g–j; our unpublished data). To see whether mBcl– 2BHΔ1/2, but not mBcl–2ΔBH1/2/3, was capable of dimerizing with hBcl–2, we first performed the above described Western blot/immunoprecipitation analysis on HEK cells transiently transfected with hBcl–2, each of the mutants, or in combinations. As shown in Figure 6, the α–hBcl–2/100 antibody detected similar amounts of 26 kDa hBcl–2 in HEK-hBcl–2 (lane a), HEK-hBcl–2/mBcl–2ΔBH1/2 (lane c) and HEK-hBcl–2/mBcl–2ΔBH1/ 2/3 lysates (lane e). Also, the α–mBcl–2/10C4 antibody detected similar amounts of 19 kDa mBcl–2ΔBH1/2 or 17 kDa mBcl–2ΔBH1/2/3 in HEK-mBcl–2ΔBH1/2 (lane h), HEK-mBcl–2ΔBH1/2/3 (lane j), HEK-hBcl–2/mBcl2ΔBH1/2 (lane g) and HEK-hBcl–2/mBcl–2ΔBH1/ 2/3 lysates (lane i). This showed that the respective proteins were similarly expressed in single and double transfectants. As previously seen with α–mBcl–2/27–6 (Figure 2, lane c), the α-mBcl–2/10C4 antibody slightly crossreacted with hBcl–2 and immunoprecipitated a low amount of the protein from an HEK-hBcl–2 extract (Figure 6, lane b). The amount of hBcl–2 was, however, 5–10 times higher in an α–mBcl–2/10C4 immmunoprecipitate from HEK-hBcl–2/mBcl–2ΔBH1/2 cells (Figure 6, lane d). This was not the case when the immunoprecipitation was performed on an HEK-hBcl–2/mBcl–2ΔBH1/2/3 extract (Figure 6, lane f), indicating that hBcl–2 and mBcl– 2ΔBH1/2 interacted in a BH3-dependent fashion.
Fig. 6. BH3-dependent interaction between Bcl–2 and Bcl–2ΔBH1/2 in HEK extracts. α–hBcl–2/100 Western blot of α–hBcl–2/100 (lanes a, c and e) or α–mBcl–2/10C4 immunoprecipitates (lanes b, d and f) from unlabeled extracts of HEK cells transfected with hBcl–2 alone (lanes a and b) or together with mBcl–2ΔBH1/2 (lanes c and d) or mBcl–2ΔBH1/2/3 (lanes e and f). Note that there is more 26 kDa hBcl–2 in lane d than b or f, indicating that hBcl–2 co-precipitates with mBcl–2ΔBH1/2, but not with mBcl–2ΔBH1/2/3. As a control for equal expression/immunoprecipitation of the mutant proteins, we show an α–mBcl–2/10C4 Western blot of α–mBcl–2/10C4 immunoprecipitates from extracts of HEK cells transfected with mBcl–2ΔBH1/2 (lane h) or mBcl–2ΔBH1/2/3 (lane j) alone or together with hBcl–2 (lanes g and i).
Bcl–2 dislocates mitochondrial Bcl–2ΔBH1/2 to the ER/nuclear membrane
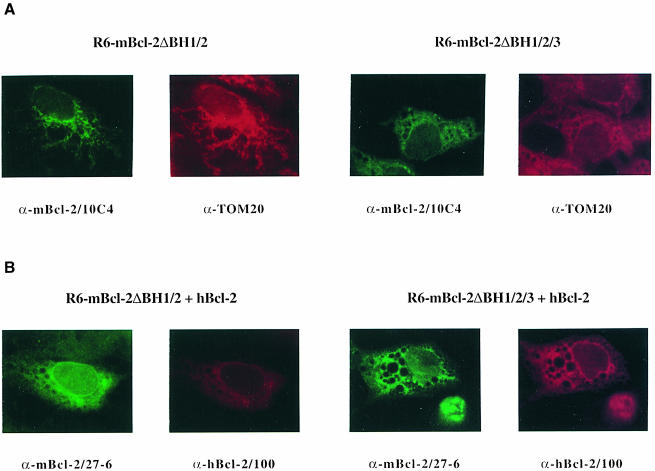
To substantiate our in vitro findings, we used immuno– fluorescence to see whether Bcl–2 could dislocate mBcl–2ΔBH1/2, but not mBcl–2ΔBH1/2/3, inside cells. Strikingly, when mBcl–2ΔBH1/2 was transiently expressed in R6 cells, it displayed a ‘spaghetti-like’ immunodetection pattern that co-localized with the mitochondrial marker Tom20 (Figure 7A). This was detected with both the α-mBcl–2/10C4 (Figure 7A) and α-mBcl–2/27–6 (our unpublished data) antibodies. In contrast, as shown in Figure 5B, hBcl–2 was mainly associated with ER/nuclear membrane structures (Figure 7B). Upon co-transfection with hBcl–2, mitochondrial mBcl–2ΔBH1/2 dislocated to the nuclear/ER membrane, indicating that the two proteins could interact within cells (Figure 7B). This was not the case with the mBcl–2ΔBH1/2/3 mutant as it did not show co-localization with Bcl–2 on the nuclear envelope after co-transfection with Bcl–2 (Figure 7B). Thus, our data show that the deletion of the BH1/BH2 domains converts Bcl–2 into a molecule that can interact with wild-type Bcl–2 in a BH3-dependent manner, both in vitro and within cells.
Fig. 7. Dislocation of mitochondria-associated Bcl–2ΔBH1/2, but not Bcl–2ΔBH1/2/3, to the ER/nuclear membrane by Bcl–2 in R6 cells. (A) α–mBcl–2/10C4 (fluorescein) and α–Tom20 (Texas Red) fluorescence analysis on R6 cells transfected with mBcl–2ΔBH1/2 or mBcl–2ΔBH1/2/3 at 24 h post-transfection. (B) α–mBcl–2/27–6 (fluorescein) and α–hBcl–2/100 (Texas Red) fluorescence analysis on R6 cells co-transfected with mBcl–2ΔBH1/2 and hBcl–2 or mBcl–2ΔBH1/2/3 and hBcl–2 at 24 h post-transfection. In this co-localization experiment α–mBcl–2/10C4 cannot be used because it is a monoclonal mouse antibody like α–hBcl–2/100. However, α–mBcl–2/27–6 and α–mBcl–2/10C4 exhibit the same immunostaining of mBcl–2ΔBH1/2 and mBcl–2ΔBH1/2/3 (our unpublished data). Note that mBcl–2ΔBH1/2 dislocates from its original mitochondrial to a nuclear envelope/ER pattern upon co-transfection with hBcl–2. In contrast, mBcl–2ΔBH1/2/3 does not co-localize with hBcl–2 on the nuclear envelope. The vacuoles are due to the transient transfection with Superfect.
A Bcl–2 mutated in BH2 can attract wild-type Bcl–2 to mitochondria
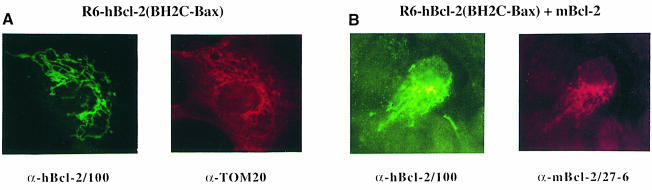
It is known that blatant deletions in proteins can produce structural changes that may be artefactual. Although the mBcl–2ΔBH1/2 mutant is a perfect homolog of the naturally occurring Bcl–xs splice variant (the variant of Bcl–xL that lacks BH1 and BH2 domains) (Boise et al., 1993), we were still worried that it might not fold correctly inside cells and promote an aberrant interaction with wild-type Bcl–2. We therefore created a Bcl–2 variant that had retained a BH2 domain but in a mutated form. To avoid the introduction of unnatural mutations, we exchanged the BH2 domain and C–terminus of Bcl–2 with the corresponding regions of Bax. The resulting hBcl–2(BH2C–Bax) construct was expressed as a stable 25 kDa protein in HEK cells (our unpublished data) and exhibited a pre- dominantly mitochondrial immunolocalization after transfection into R6 cells (Figure 8A). Like mBcl–2ΔBH1/2, this mutant Bcl–2 seemed to interact with wild-type Bcl–2 but at another intracellular site. Instead of being passively attracted by hBcl–2 to the ER/nuclear membrane (Figure 7B), the hBcl–2(BH2C–Bax) variant actively dislodged Bcl–2 from the ER/nuclear membrane to mitochondria (Figure 8B). This dislocation again depended on the BH3 domain, as an hBcl–2(BH2C–Bax) deleted in BH3 did not recruit Bcl–2 (our unpublished data). Thus, our findings indicate that not only blatant deletions but also subtle mutations in the BH2 region convert Bcl–2 into a molecule that can now interact with Bcl–2 in a BH3-dependent fashion.
Fig. 8. Attraction of ER-localized Bcl–2 to mitochondria by Bcl–2(BH2C–Bax). (A) α–hBcl–2/100 (fluorescein) and α–Tom20 (Texas Red) fluorescence analysis on R6 cells transfected with hBcl–2(BH2C–Bax) at 24 h post-transfection. (B) α–hBcl–2/100 (fluorescein) and α-mBcl–2/27–6 (Texas Red) fluorescence analysis on R6 cells co-transfected with hBcl–2(BH2C–Bax) and mBcl–2 at 24 h post-transfection. Note that mBcl–2, which usually associates with nuclear/ER structures (Figure 5A), localizes to ‘spaghetti-like’ mitochondria upon co-expression with hBcl–2(BH2C–Bax).
Discussion
It has been proposed that Bcl–2 family members regulate apoptosis like a rheostat (Oltvai et al., 1993). If pro-apoptotic factors such as Bax prevail, they form homodimers and cell death ensues. If anti-apoptotic factors such as Bcl–2 are more abundant, it is their turn to form homodimers and cells are protected from apoptotic insults. In between these two extremes is the formation of Bax–Bcl–2 heterodimers that keep additional cytotoxic and survival activities in check. Although various mutagenesis and expression studies have confirmed the existence and role of Bax–Bax and Bax–Bcl–2 dimerizations (Adams and Cory, 1998; Reed, 1998), those of Bcl–2 homodimers have remained hypothetical. In this study we challenge this issue and show by several methods that Bcl–2 does not form homodimers when it sets the rheostat to ‘survival’.
The crystal structure of Bcl–xL in the absence or presence of the Bak–BH3 peptide shows that Bcl–2 family members with several BH domains can be in two conformational states (Muchmore et al., 1996; Aritomi et al., 1997; Sattler et al., 1997): a ‘receptor state’, where all BH domains join to form a hydrophobic face to which other proteins can bind, and a ‘ligand state’, where the BH3 domain of this hydrophobic face can turn outwards to interact with the hydrophobic face of another Bcl–2-like molecule. This nicely explains the formation of heterodimers between anti-apoptotic and pro-apoptotic partners, especially when the pro-apoptotic partner is a so-called ‘BH3-only’ protein. Such proteins have the BH3 domain as their only Bcl–2 homology domain and use non-homologous regions to localize to different subcellular compartments (Kelekar and Thompson, 1998). This has been elegantly demonstrated with the protein Bim, which usually binds to the microtubule-associated dynein motor complex via regions outside of BH3 but uses the BH3 domain to translocate to mitochondrial Bcl–2 in apoptotic cells (Puthalakath et al., 1999).
An unresolved issue is whether Bax or Bcl–2 homodi- or multimers form via the same mechanism as Bax–Bcl–2 heterodimers. In favor of such a model is that homodimerization of Bax depends on both the BH3 and BH1 domains (Simonian et al., 1996; Simonen et al., 1997; Zha and Reed, 1997; Kelekar and Thompson, 1998), so that one Bax molecule would use its BH3 domain as a ‘ligand’, the other as part of the hydrophobic face ‘receptor’. However, a previous study (Lewis et al., 1998) and our study here show that Bax forms high-order structures (up to heptamers) that are inconsistent with such a dimerization scheme. Alternatively, Bax may not be able to maintain a hydrophobic face ‘receptor’ structure, but exposes its BH3 domain for homotypic interactions. There is, however, no reported evidence that BH3 peptides, either in their isolated form or when present in ‘BH3-only’ proteins have the tendency to dimerize or even multimerize. It therefore remains unknown how Bax multimers form.
Part of the reason for our lack of insight into the Bax structure is that this molecule undergoes a conformational change when it moves from an inactive state in the cytoplasm to an active state on the mitochondrial membrane (Wolter et al., 1997; Goping et al., 1998; Griffiths et al., 1999). Analogous to diphtheria toxin or colicin, this change may disrupt the hydrophobic face of Bax and expose its pore-forming BH1/BH2 (α5/α6) regions for membrane insertion. As a consequence, the Bax structure would open up like an umbrella (as proposed for the bacterial toxins) to allow other protein regions, such as the BH3 domain, to bind other proteins or to participate in multimerization reactions (Schendel et al., 1998). In this way, Bax could form a multimerized ion- or cytochrome c-releasing channel on the mitochondrial membrane. Although our data do not prove such a mechanism, they show that Bax can form homodimers in immunoprecipitates of membrane extracts and even multimers as full-length recombinant protein in vitro. These Bax–Bax interactions are clearly mediated by the BH3 domain and not by regions that are artificially exposed due to the presence of detergent (NP-40 or LDAO), as recently suggested (Hsu and Youle, 1997).
The novel and quite surprising finding of this study is that Bcl–2 behaves differently from Bax under the same experimental conditions. Despite the fact that immunoprecipitations and FPLC analysis were also performed in detergent, neither cell-based, cell-extracted nor recombinant Bcl–2 homodi- or multimerized. Even in response to apoptotic stimuli such as MG132 or serum removal Bcl–2 remained in a monomeric form. These findings strongly indicate that Bcl–2 is structurally different from Bax and does not require homodimerization for its survival activity. Indeed, when Aritomi et al. built a homology model of Bax from the Bcl–xL structure, they noted that Bax exhibited more hydrophobic patches and a better exposure of its α5/α6 helices than Bcl–xL (Aritomi et al., 1997). They suggested that Bax has a greater potential for membrane insertion and is more likely to form membrane pores than either Bcl–2 or Bcl–xL. Moreover, there is no reported evidence that Bcl–2, like Bax, changes its conformation and translocates from the cytoplasm to membranes in response to apoptotic stimuli. Bcl–2 is rarely cytoplasmic, but if it is due to the removal of its hydrophobic C–terminus (e.g. mBcl–2ΔTM), it is still partially active (Borner et al., 1994a) and looks like the known structure of C–terminally deleted Bcl–xL. We therefore propose that Bcl–2 retains its hydrophobic face structure to bind crucial death-regulatory proteins (including BH3-containing proteins) both in the cytoplasm and on membranes. In this respect the C–terminal sequence would serve to anchor Bcl–2 to various different membranes to increase its access to crucial binding proteins (the ‘docking activity’ model) (Reed, 1997).
However, this model implies, as previously suggested (Aritomi et al., 1997), that Bcl–2 would not necessarily form multimeric channels like Bax within cells. How can this be reconciled with the reported in vitro channel activity of Bcl–2 (Schendel et al., 1998)? First, there is currently no proof that any member of the Bcl–2 family forms channels under physiological conditions. In fact, one report shows that Bcl–2 blocks the channel activity of Bax rather than being a channel itself (Antonsson et al., 1997). Secondly, the non-physiological amounts of pure, recombinant Bcl–2 protein and phospholipid vesicles could force Bcl–2 into a conformation that facilitates membrane insertion and dimerizations like Bax. Thirdly, most of the recombinant Bcl–2 proteins used to measure channel activities in vitro were C–terminally deleted and/or tagged at their N–termini (His, GST, etc.) (Minn et al., 1997; Schendel et al., 1997). Although some fusion proteins such as Flag–Bcl–2 are active and stay in a monomeric form as shown here, others such as GST–Bcl–2 lose their capacity to protect cells from apoptosis and tend to dimerize (our unpublished data). Similarly, homodimerization of Bcl–2 in the yeast two-hybrid system may have been due to the presence of the LexA protein at the N–terminus of the Bcl–2 bait (Hanada et al., 1995). By using untagged, full-length Bcl–2 proteins, we studied this molecule in its most natural form.
Our mutagenesis analyses indeed show that Bcl–2 needs to be in a correct conformation to perform its survival action. If the BH1 and BH2 regions of Bcl–2 are deleted, or the BH2 region is replaced by that of Bax, the hydrophobic face most likely changes its conformation in a way that Bcl–2 now homodimerizes in a BH3-dependent manner. That the dimerizations between the Bcl–2 mutants and Bcl–2 are not an artefact due to detergents can be shown by immunofluorescence analysis where the proteins are mutually attracted to mitochondria or ER/nuclear membrane sites. Why the Bcl–2 mutants are predominantly localized to mitochondria, as compared with the ER/nuclear association of wild-type Bcl–2, is not yet known. Perhaps there are exposed regions that now have a higher affinity for mitochondria than for other membranes, just as seen with Bax (Rossé et al., 1998). Strikingly, both the Bcl–2ΔBH1/2 and Bcl–2(BH2C–Bax) mutants have lost their death-protective activity (Borner et al., 1994a), indicating that the BH1/BH2 regions of Bcl–2 are indeed crucial for its function. Moreover, these mutants, but not the Bcl–2ΔBH1/2/3 version, can block the survival activity of Bcl–2 in a dominant-negative fashion (see Supplementary material available in The EMBO Journal Online), suggesting that their BH3 domains bind to the hydrophobic face of Bcl–2 like the BH3 domain of Bax. This reinforces the notion that the hydrophobic face of Bcl–2 is different from that of Bcl–2(BH2C–Bax) because only the former is maintained in a death-protective state while the latter is disrupted to expose its BH3 domain. It remains to be seen which region of Bcl–2 confers the stability of the hydrophobic face, and by what mechanism pro-apoptotic molecules such as Bax, Bak or the Bcl–2(BH2C–Bax) mutant change their conformation to di- or multimerize. A candidate region for regulation is the BH2 domain, in particular at its C–terminal half where it differs in sequence between Bcl–2 and Bax. This region, which we have called domain X, is absolutely crucial for the survival activity of Bcl–2 (Borner etal., 1994b) but its role in homodimerization and cytotoxic activity of Bax is not yet known. Additional mutagenesis and crystallographic analysis will provide further insights into the structural differences between Bcl–2 and Bax and why they regulate apoptosis in an opposing fashion.
Materials and methods
Antibodies and cDNAs
The anti-Bcl–2 and anti-Bax antibodies used for immunoprecipitations and Western blots are described in the Supplementary material available in The EMBO Journal Online. The cDNAs for human Bax (hBax), human Bcl–2 (hBcl–2), mouse Bcl–2 (mBcl–2), mBcl–2 devoid of its C–terminus (mBcl–2ΔTM, formerly T3) and mBcl–2 devoid of the BH1/BH2 domains (mBcl–2ΔBH1/2, formerly DEL2) were generated and/or subcloned into the pcDNA3 or pcDNAamp vectors (Invitrogen) as previously described (Borner et al., 1994a). The cDNAs for Flag-tagged hBcl–2, the mBcl–2 mutant lacking the BH1, BH2 and BH3 domains (mBcl–2ΔBH1/2/3) and the hBcl–2 mutant in which the BH2 and C–terminus were replaced by those of Bax [hBcl–2(BH2C–Bax)] were generated by PCR as described in the Supplementary material available in The EMBO Journal Online.
Cells
Human embryonic kidney 293 cells (HEK) and rat 6 (R6) embryo fibroblasts overexpressing Bcl–2, Flag–Bcl–2 and/or Bax were generated and cultured as described in the Supplementary material available in The EMBO Journal Online.
Immunoprecipitations
HEK or R6 cell derivatives (3 × 106) were either non-labeled or radiolabeled with [35S]methionine/cysteine (Tran35S-label, Hartmann Analytic) and lysed for immunoprecipitations as previously described (Otter et al., 1998). To disrupt Bax–Bax and Bax–Bcl–2 interactions, the cell lysate was supplemented with 100 μM wild-type (56TKKLSECLKRIGDELDSNM74) or mutant (56TKKLSECLKRIGAELDSNM74) Bax–BH3 peptide (kindly provided by Markus Wartmann and Carlos Garcia-Echeverria, Novartis Pharma Ltd, Basle, Switzerland). The lysates were then subjected to immunoprecipitation using 5 μl of α–mBcl–2/27–6, 3 μl of α–hBcl–2/100, 3 μl of α–mBcl–2/10C4, 6 μl of α–mBax or 2.5 μl of α–Flag/M5. Following antibody incubation for 2 h at 4°C, 50 μl of 50% (v/v) protein A–Sepharose (Sigma) or protein G–Sepharose (Amersham Pharmacia) were added, and the immunocomplexes were captured on an end-over-end wheel at 4°C for 1 h. Immunocomplexes were applied to 15% SDS–polyacrylamide gels and either blotted to a polyvinylidene difluoride membrane (PVDF) (Immobilon-P, Millipore) for Western blotting (unlabeled extracts) or analyzed by fluorography as described (Otter et al., 1998). For Bcl–2 immunodepletion, cell extracts were subjected to three rounds of immunoprecipitations using 5 μl of α–mBcl–2/10C4 and 50 μl of protein G–Sepharose for each round.
Western blotting
Western blot analysis of immunoprecipitates from unlabeled cell extracts was performed as described previously (Olivier et al., 1997), using either α–hBcl–2/100 at a titer of 1:200, α–mBcl–2/10C4 at 1:500, α–mBcl–2/27–6 at 1:5000 or α–h/mBax/UBI at 1:10000. Secondary antibodies were Fcγ-specific, peroxidase-coupled goat anti-rabbit or anti-mouse antibodies (Jackson ImmunoResearch Laboratories).
Transient transfections
HEK cells were grown on 100 mm plates until ∼80% confluence and transfected with 5 μg of one cDNA plus 5 μg of the pcDNA3 vector (single transfections) or 5 μg of each of two cDNAs (double transfections) using 25 μl of Superfect (Qiagen) as described by the manufacturer. After 3 h the Superfect–DNA complex was removed, and the cells were cultured for another 21 h in fresh medium before lysis for immunoprecipitation analysis.
Immunofluorescence analysis
Cells grown on 12 mm glass coverslips were transfected with 0.8 μg of one cDNA in 2.4 μl Superfect (single transfections) or 0.8 μg of each of two cDNAs in 4.8 μl Superfect as described above. At 24 h post-transfection, cells were washed twice in phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde, and permeabilized with 0.05% saponin and acetone. The cells were treated with α–mBcl–2/27–6 (1:100), α–h/mBax/UBI (1:100), α–mBcl–2/10C4 (1:100) or α–hBcl–2/100 (1:100) in the absence or presence of the mitochondria marker antibody α-Tom20 (1:300) for 1 h followed by incubation with Texas Red- and fluorescein-conjugated goat anti-rabbit or anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratories). After another fixation in 4% paraformaldehyde containing 2 μg/ml Hoechst 33342 dye (Molecular Probes), the anti-fading agent Slowfade (Molecular Probes) was added, and the cells were viewed under a Zeiss Axiovert fluorescence microscope at a magnification of 1000×. Pictures were taken with a Contax 167 MT camera.
FPLC analysis of Bcl–2/Bax multimers
mBcl–2 and hBax were expressed as GST fusion proteins in DH5α bacteria using the pGEX-based expression vectors as previously described (Olivier et al., 1997). After protein induction with 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG; Life Technologies) at 30°C for 4 h, bacterial extracts were prepared and applied to glutathione–Sepharose beads according to the manufacturer's protocol (Amersham Pharmacia). After washing in PBS plus 1% Triton X–100, the attached GST–mBcl–2 and GST–hBax were cleaved by 0.6 U of thrombin at 25°C for 1 h to release the mBcl–2 and hBax proteins into the supernatant. One hundred microliters (100 μg) of the cleaved proteins were applied to FPLC Superose 12 chromatography using 0.05% LDAO (Sigma) as detergent. Eluted protein was measured at OD280 on-line. For the analysis of Flag–mBcl–2, a cell extract of R6-Flag–Bcl–2mix cells was prepared in buffer A plus 0.2% NP–40 as described above for immunoprecipitations. The extract was applied to an anti-Flag M2 affinity column (Sigma), the column washed in buffer A and Flag–mBcl–2 eluted with 0.1 M glycine at pH 3.5. The eluted sample was immediately neutralized with NaOH and 100 μl (100 μg) subjected to FPLC chromatography as described for GST–mBcl–2.
Acknowledgments
Acknowledgements
We are grateful to Brendon Hanson and Nick Hoogenraad for the α–Tom20 antibody, Markus Wartmann and Carlos Garcia-Echeverria for the Bax–BH3 peptides and Laurent Monney and Lotti Egger for critically reading the manuscript. This work was supported by grants from the Swiss National Science Foundation (31-46930.96 and 31-34600.92).
References
- Adams J.A. and Cory, S. (1998) The Bcl–2 protein family: arbiters of cell survival. Science, 281, 1322–1326. [DOI] [PubMed] [Google Scholar]
- Antonsson B. et al. (1997)Inhibition of Bax channel-forming activity by Bcl–2. Science, 277, 370–372. [DOI] [PubMed] [Google Scholar]
- Aritomi M., Kunishima, N., Inohara, N., Ishibashi, Y., Ohta, S. and Morikawa, K. (1997) Crystal structure of Rat Bcl–xL: implications for the function of the Bcl–2 protein family. J. Biol. Chem., 272, 27886–27892. [DOI] [PubMed] [Google Scholar]
- Boise L.H., Gonzalez-Garcia, M., Postema, C.E., Ding, L., Lindsten, T., Turka, L.A., Mao, X., Nunez, G. and Thompson, C.B. (1993) Bcl–x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell, 74, 597–608. [DOI] [PubMed] [Google Scholar]
- Borner C., Martinou, I., Mattmann, C., Irmler, M., Schaerer, E., Martinou, J.-C. and Tschopp, J. (1994a) The protein Bcl–2α does not require membrane attachment, but two conserved domains to suppress apoptosis. J. Cell Biol., 126, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C., Olivier, R., Martinou, I., Mattmann, C., Tschopp, J. and Martinou, J.-C. (1994b) Dissection of functional domains in Bcl–2α by site-directed mutagenesis. Biochem. Cell Biol., 72, 463–469. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Flemington, C., Houghton, A.B., Ebb, R.G., Gallo, G.J., Elangovan, B., Chinnadurai, G. and Lutz, R.J. (1995) A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J., 14, 5589–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J.J., Li, H., Salvesen, G.S., Yuan, J. and Wagner, G. (1999) Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell, 96, 615–624. [DOI] [PubMed] [Google Scholar]
- Clem R.J., Hardwick, J.M. and Miller, L.K. (1996) Anti-apoptosis genes of baculoviruses. Cell Death Differ., 3, 9–16. [PubMed] [Google Scholar]
- Earnshaw W.C., Martins, L.M. and Kaufmann, S.H. (1999) Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu. Rev. Biochem., 68, 383–424. [DOI] [PubMed] [Google Scholar]
- Goping I.S., Gross, A., Lavoie, J.N., Nguyen, M., Jemmerson, R., Roth, K., Korsmeyer, S.J. and Shore, G.C. (1998) Regulated targeting of BAX to mitochondria. J. Cell Biol., 143, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R. and Reed, J.C. (1998) Mitochondria and apoptosis. Science, 281, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Griffiths G.J., Dubrez, L., Morgan, C.P., Jones, N.A., Whitehouse, J., Corfe, B.M., Dive, C. and Hickman, J.A. (1999) Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol., 144, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A., Jockel, J., Wei, M.C. and Korsmeyer, S.J. (1998) Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J., 17, 3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M., Aimé-Sempé, C., Sato, T. and Reed, J.C. (1995) Structure–function analysis of Bcl–2 protein: identification of conserved domains important for homodimerization with Bcl–2 and heterodimerization with Bax. J. Biol. Chem., 270, 11962–11969. [DOI] [PubMed] [Google Scholar]
- Holinger E.P., Chittenden, T. and Lutz, R.J. (1999) Bak BH3 peptides antagonize Bcl–xL function and induce apoptosis through cytochrome c-independent activation of caspases. J. Biol. Chem., 274, 13298–13304. [DOI] [PubMed] [Google Scholar]
- Hsu Y.-T. and Youle, R.J. (1997) Nonionic detergents induce dimerization among members of the Bcl–2 family. J. Biol. Chem., 272, 13829–13834. [DOI] [PubMed] [Google Scholar]
- Jürgensmeier J.M., Xie, Z., Deveraux, Q., Ellerby, L., Bredesen, D. and Reed, J.C. (1998) Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl Acad. Sci. USA, 95, 4997–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar A. and Thompson, C.B. (1998) Bcl–2 family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol., 8, 324–330. [DOI] [PubMed] [Google Scholar]
- Lewis S., Bethell, S.S., Patel, S., Martinou, J.C. and Antonsson, B. (1998) Purification and biochemical properties of soluble recombinant human Bax. Protein Expr. Purif., 13, 120–126. [DOI] [PubMed] [Google Scholar]
- Lithgow T.R., van Driel, R., Bertram, J.F. and Strasser, A. (1994) The protein product of the oncogene Bcl–2 is a component of the nuclear envelope, the endoplasmic reticulum and the outer mitochondrial membrane. Cell Growth Differ., 5, 411–417. [PubMed] [Google Scholar]
- McDonnell J.M., Fushman, D., Milliman, C.L., Korsmeyer, S.J. and Cowburn, D. (1999) Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell, 96, 625–634. [DOI] [PubMed] [Google Scholar]
- Minn A.J., Vélez, P., Schendel, S.L., Liang, H., Muchmore, S.W., Fesik, S.W., Fill, M. and Thompson, C.B. (1997) Bcl–xL forms an ion channel in synthetic lipid membranes. Nature, 385, 353–357. [DOI] [PubMed] [Google Scholar]
- Muchmore S.W., et al. (1996)X-ray and NMR structure of human Bcl–xL, an inhibitor of programmed cell death. Nature, 381, 335–341. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Millar, D.G., Yong, W.V., Korsmeyer, S.J. and Shore, G.C. (1993) Targeting of Bcl–2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J. Biol. Chem., 268, 25265–25268. [PubMed] [Google Scholar]
- Olivier R., Otter, I., Monney, L., Wartmann, M. and Borner, C. (1997) Bcl–2 does not require Raf kinase activity for its death-protective function. Biochem. J., 324, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z.N., Milliman, C.L. and Korsmeyer, S.J. (1993) Bcl–2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell, 74, 609–619. [DOI] [PubMed] [Google Scholar]
- Otter I., Conus, S., Ravn, U., Rager, M., Olivier, R., Monney, L., Fabbro, D. and Borner, C. (1998) The binding properties and biological activities of Bcl–2 and Bax in cells exposed to apoptotic stimuli. J. Biol. Chem., 273, 6110–6120. [DOI] [PubMed] [Google Scholar]
- Puthalakath H., Huang, D.C.S., O'Reilly, L.A., King, S.M. and Strasser, A. (1999) The proapoptotic activity of the Bcl–2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell, 3, 287–296. [DOI] [PubMed] [Google Scholar]
- Reed J.C. (1997) Double identity for proteins of the Bcl–2 family. Nature, 387, 773–776. [DOI] [PubMed] [Google Scholar]
- Reed J.C. (1998) Bcl–2 family proteins. Oncogene, 17, 3225–3236. [DOI] [PubMed] [Google Scholar]
- Rossé T., Olivier, R., Monney, L., Rager, M., Conus, S., Fellay, I., Jansen, B. and Borner, C. (1998) Bcl–2 prolongs cell survival after Bax-induced release of cytochrome c. Nature, 391, 496–499. [DOI] [PubMed] [Google Scholar]
- Sattler M. et al. (1997)Structure of Bcl–xL–Bak peptide complex: recognition between regulators and apoptosis. Science, 275, 983–986. [DOI] [PubMed] [Google Scholar]
- Schendel S.L., Xie, Z., Montal, M.O., Matsuyama, S., Montal, M. and Reed, J.C. (1997) Channel formation by antiapoptotic protein Bcl–2. Proc. Natl Acad. Sci. USA, 94, 5113–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel S.L., Montal, M. and Reed, J.C. (1998) Bcl–2 family proteins as ion-channels. Cell Death Differ., 5, 372–380. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Narita, M. and Tsujimoto, Y. (1999) Bcl–2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature, 399, 483–487. [DOI] [PubMed] [Google Scholar]
- Simonen M., Keller, H. and Heim, J. (1997) The BH3 domain of Bax is sufficient for interaction of Bax with itself and with other family members and it is required for induction of apoptosis. Eur. J. Biochem., 249, 85–91. [DOI] [PubMed] [Google Scholar]
- Simonian P.L., Grillot, D.A.M., Andrews, D.W., Leber, B. and Nunez, G. (1996) Bax homodimerization is not required for bax to accelerate chemotherapy-induced cell death. J. Biol. Chem., 271, 32073–32077. [DOI] [PubMed] [Google Scholar]
- Thome M. et al. (1997)Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature, 386, 517–521. [DOI] [PubMed] [Google Scholar]
- Wolter K.G., Hsu, Y.-T., Smith, C.L., Nechushtan, A., Xi, X.-G. and Youle, R.J. (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol., 139, 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X.-M., Oltvai, Z.N. and Korsmeyer, S.J. (1994) BH1 and BH2 domains of Bcl–2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature, 369, 321–323. [DOI] [PubMed] [Google Scholar]
- Zha H. and Reed, J.C. (1997) Heterodimerization-independent functions of cell death regulatory proteins Bax and Bcl–2 in yeast and mammalian cells. J. Biol. Chem., 272, 31482–31488. [DOI] [PubMed] [Google Scholar]
- Zha H., Aimé-Sempé, C., Sato, T. and Reed, J.C. (1996) Proapoptotic protein Bax heterodimerizes with Bcl–2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem., 271, 7440–7444. [DOI] [PubMed] [Google Scholar]