SUMMARY
The ribosome is a major target in the bacterial cell for antibiotics. Here we dissect the effects that the thiopeptide antibiotics thiostrepton (ThS) and micrococcin (MiC) as well as the orthosomycin antibiotic evernimicin (Evn) have on translational GTPases. We demonstrate that, like ThS, MiC is a translocation inhibitor, and that the activation by MiC of the ribosome-dependent GTPase activity of EF-G is dependent on the presence of the ribosomal proteins L7/L12 as well as the G′ subdomain of EF-G. In contrast, Evn does not inhibit translocation, but is a potent inhibitor of back-translocation as well as IF2-dependent 70S initiation complex formation. Collectively, these results shed insights not only into fundamental aspects of translation, but also into the unappreciated specificities of these classes of translational inhibitors.
Keywords: antibiotics, elongation factor G (EF-G), evernimicin, initiation factor 2 (IF2), LepA (EF4), micrococcin, protein synthesis, ribosome, thiostrepton, translation inhibition
INTRODUCTION
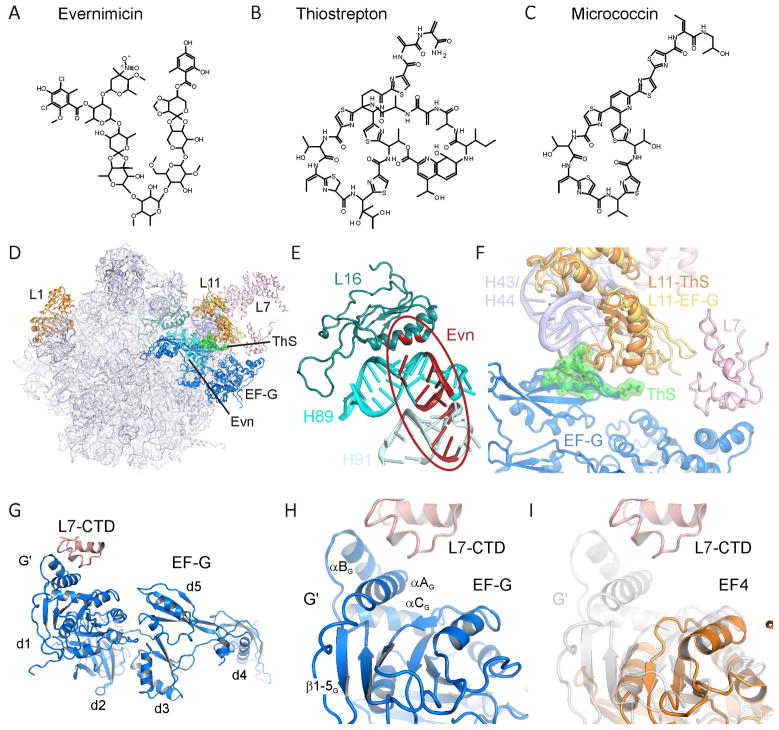
Protein synthesis occurs on large macromolecular particles called ribosomes, which are composed of RNA and protein. In bacteria, the 70S ribosome can be split into a small (30S) and large (50S) subunit. The bacterial translational machinery represents a major target within the cell for antibiotics (reviewed by Blanchard et al., 2010; Wilson, 2009). Many clinically important classes of antibiotics inhibit translation by binding to the active centers of ribosome: For example, the tetracyclines and aminoglycosides bind at the decoding site on the small subunit, and the chloramphenicols, macrolides/ketolides, oxazolidinones and lincosamides bind at the peptidyltransferase center (PTC) on the large subunit (Sohmen et al., 2009a; Sohmen et al., 2009b). Despite the potency of many of these drug classes, antibiotic resistance among clinically relevant pathogens is an increasing problem and thus the need for new antibiotics is more urgent than ever before. Ideally, the new antibiotics should have non-overlapping sites with the currently used antimicrobial agents, so that the occurrence of cross-resistance is reduced or prevented. Two such classes are the thiopeptides and orthosomycins (Figure 1A–C), which bind to distinct sites on the large ribosomal subunit that are located far from the PTC (Figure 1D, E).
Figure 1. Chemical structures and ribosomal binding sites of thiopeptide and orthosomycin antibiotics.
Chemical structures of the (A) orthosomycin evernimicin (Evn), and the thiopeptide antibiotics (B) thiostrepton (ThS) and (C) micrococcin (MiC). (D) Overview of the binding sites of orthosomycins and thiopeptides on the large subunit relative to EF-G. R-proteins L1, L11 and L7 are shown for reference. (E) Putative binding site of orthosomycins spanning from H89 and H91 of the 23S rRNA. Residues highlighted in red have been associated biochemically with Evn or avilamycin (reviewed by Wilson, 2009). (F) Binding site of thiostrepton (green) in the cleft between H43 and H44 of the 23S rRNA and the N-terminal domain of L11 (L11-ThS) (Harms et al., 2008). The relative positions of EF-G (blue), C-terminal domain of L7/L12 (L7-CTD) and of a different conformation of L11 (L11-EF-G) are from (Gao et al., 2009). (G) Overview of domain arrangement of EF-G with contact between the L7-CTD and the G′ domain of EF-G as observed in the 70S-EF-G crystal structure (Gao et al., 2009). (H) Expansion of (G) highlighting the secondary structure elements of the G′ subdomain. (I) juxtaposition of the G′ subdomain of EF-G (grey transparency) with the G-domain of EF4 (orange) (Evans et al., 2008) that lacks a G′ subdomain. See also Figure S1.
The orthosomycins, such as evernimicin (Evn), are oligosaccharide antibiotics (Figure 1A) that display excellent antimicrobial activity against a broad range of Gram-positive bacteria, both in vivo and in vitro. Although attempts to introduce Evn, marketed as Ziracin by Schering-Plough, have so far been unsuccessful, a related compound, Avilamycin (Avn), is used as a growth promoter in animal feeding. A multitude of resistance mutation/modification and chemical footprinting studies indicate that the orthosomycin binding site is located at the base of the L7/L12 stalk (Figure 1D, E), ~50 Å from the PTC. Mutations in ribosomal protein L16 (Aarestrup and Jensen, 2000; Adrian et al., 2000b; McNicholas et al., 2001; Zarazaga et al., 2002), and in helix 89 (H89) and H91 of the 23S rRNA, as well as methylation of G2470 (E. coli numbering used throughout) in H89 (Mann et al., 2001), confer resistance to Evn and Avn (Adrian et al., 2000a; Belova et al., 2001). In addition, Evn and Avn protect 23S rRNA nucleotides, for example, A2482 in H89 and A2534 in H91, from chemical modification (Belova et al., 2001; Kofoed and Vester, 2002). It is also noteworthy that mutations in rplP (L16 gene) confer relatively low level resistance (MIC <12 μg ml−1), whereas higher level resistance (MIC >256 μg ml−1) is obtained by EmtA-mediated methylation or rRNA mutations (Belova et al., 2001; Mann et al., 2001). Taken together, these results suggest that the orthosomycin binding site spans from the minor groove of H89 to the loop region of H91 (Figure 1E), and that mutations in L16 confer resistance indirectly via perturbation of the 23S rRNA. In agreement with this novel location, Evn does not inhibit peptide-bond formation (Belova et al., 2001), nor compete with several other ribosomal antibiotics for ribosome binding (McNicholas et al., 2000). Although some effect of Avn on aa-tRNA binding to ribosomes has been observed (Wolf, 1973), Evn is better known as an initiation inhibitor; Evn inhibits the formation of fMet-puromycin in an IF2-dependent manner (Belova et al., 2001), although the exact step of inhibition remains unclear. Moreover, the effects of orthosomycins on translation factors other than IF2 and EF-Tu have not yet been addressed.
In contrast, thiopeptides, such as thiostrepton (ThS), have been extensively studied (reviewed by Bagley et al., 2005; Nicolaou et al., 2009; Wilson, 2009). Although ThS is already in veterinary usage, its low water solubility and poor bioavailability has so far precluded its use in human medicine. Nevertheless, the thiopeptide class of antibiotics has received renewed interest in the recent years because (i) of their effectiveness against Gram-positive bacteria, in particular, methicillin-resistant Staphylococcus aureus (MRSA), and against the malarial parasite Plasmodium falciparum (McConkey et al., 1997), as well as (ii) recent successes in the total synthesis of a number of thiopeptides (reviewed by Hughes and Moody, 2007; Nicolaou et al., 2009), including amongst others, ThS (Nicolaou et al., 2005a; Nicolaou et al., 2005b) and micrococcin (MiC) (Lefranc and Ciufolini, 2009). Thiopeptide antibiotics, such as ThS and MiC, are composed of oxazoles and thiazoles, as well as non-natural amino acids that are linked together to form complex macrocyclic frameworks (Figure 1B, C).
Both ThS and MiC have been crystallized in complex with the large ribosomal subunit, revealing their binding site to be located in a cleft formed by the N-terminal domain (NTD) of ribosomal protein L11 and H43/H44 of the 23S rRNA (Figure 1D, F) (Harms et al., 2008), consistent with a vast wealth of prior biochemical studies (reviewed by Wilson, 2009). This region is part of the GTPase-associated center (GAC), so named because it is involved in binding of translation factors and stimulation of their GTPase activities: Consistently, thiopeptide antibiotics have been shown to inhibit IF2-dependent 70S initiation complex (70SIC) formation (Brandi et al., 2004; Grigoriadou et al., 2007), EF-Tu-dependent delivery of aminoacyl-tRNAs to the ribosome (Brandi et al., 2004; Gonzalez et al., 2007; Modelell et al., 1971; Otaka and Kaji, 1974), translocation of the tRNA2-mRNA complex through the ribosome (Munro et al., 2010; Pan et al., 2007; Pestka, 1970; Pestka and Brot, 1971; Rodnina et al., 1997), and stringent factor RelA-dependent synthesis of ppGpp (Cundliffe and Thompson, 1981; Jenvert and Schiavone, 2005). Surprisingly, however, ThS and MiC exhibit differential effects on the uncoupled ribosome-dependent EF-G GTPase activities: ThS strongly inhibits multiple-turnover GTP hydrolysis of EF-G (Pestka, 1970; Weisblum and Demohn, 1970) by preventing Pi release and thus trapping EF-G on the ribosome (Rodnina et al., 1999; Seo et al., 2006). The overlap between the ThS and EF-G binding sites on the ribosome (Figure 1D, F) (Harms et al., 2008) suggests that ThS stabilizes an initial binding state of EF-G (Rodnina et al., 1999; Seo et al., 2006), which has weaker affinity than a subsequently formed, accommodated state (Cameron et al., 2002; Seo et al., 2006). In contrast, MiC does not prevent Pi release (Starosta et al., 2009) and actually stimulates the multiple turnover GTP hydrolysis activity of EF-G (Cameron et al., 2002; Cundliffe and Thompson, 1981; Lentzen et al., 2003).
The G domains of translational GTPases have a well conserved architecture, with the exception of a region located between the G4 and G5 motifs, which in EF-G is termed the G′ subdomain (Figure S1). In EF-G, the G′ subdomain consists of ~90 amino acids that form four consecutive β-strands (2G-5G) followed by three α-helices (AG-CG) (Figure 1G, H, S1). In contrast, translational GTPases, such as elongation factor EF-Tu, initiation factor IF2, the ribosome-associated stress response factor BipA (deLivron et al., 2009; deLivron and Robinson, 2008; Owens et al., 2004) and the back-translocation factor LepA (EF4) (Liu et al., 2010; Qin et al., 2006) are completely lacking the G′ subdomain (Figure 1I), while the ribosomal protection protein TetM (Connell et al., 2003a) has a partial G′ subdomain, lacking three β-strands (3G-5G) (Figure S1). Interaction of the G′ subdomain of EF-G with the C-terminal domain of ribosomal protein L7/L12 (L7-CTD) is observed structurally (Connell et al., 2007; Datta et al., 2005; Gao et al., 2009; Harms et al., 2008; Helgstrand et al., 2007) (Figure 1G, H) and is required for efficient GTP hydrolysis (Diaconu et al., 2005; Nechifor et al., 2007; Savelsbergh et al., 2005; Savelsbergh et al., 2000) and Pi release (Diaconu et al., 2005; Savelsbergh et al., 2005), leading to the suggestion that enhanced recycling of EF-G by MiC results from stabilization of this interaction (Harms et al., 2008).
Here we show that although MiC stimulates the multiple-turnover ribosome-dependent EF-G GTPase, it inhibits the GTPase activities of other translational GTPases, such as TetM, EF4, BipA and IF2, which have reduced, or completely absent, G′ subdomains. Furthermore, deletion of the G′ subdomain from EF-G removes the stimulatory effect of MiC, as does the absence of L7/L12 on the ribosome. Despite the differential effects of MiC and ThS on EF-G GTPase, we show that MiC, like ThS, is a potent inhibitor of the EF-G catalyzed translocation process. In contrast, the orthosomycin Evn, while not interfering with EF-G GTPase and translocation activities, is a potent inhibitor of the ribosome-dependent IF2 and EF4 GTPase activities, as well as of EF4-mediated back-translocation and IF2-dependent 70SIC formation. Collectively, our results delineate the specific steps of interference and reveal the differential effects that these inhibitors have on translocation factor function - an important step for the development of new, improved antimicrobial agents.
EXPERIMENTAL PROCEDURES
Component preparation
ThS was purchased from Sigma, micrococcin P1 was a kind gift of Dr. Torsten Stachelhaus and gDNA from S. faecalis was kindly provided by Dr Vincent Perreten. E. coli fusA and bipA, S. faecalis tetM as well as T. thermophilus (HB8) fusA full length genes were cloned into pET-46 Ek/LIC vector, and E. coli infB gene was cloned into pET-14b, in accordance with the manufacturer’s instructions (Novagen). EF-GΔG′ mutants were prepared using QuikChange Site-Directed Mutagenesis Kit (Stratagene). E. coli EF4 was expressed in pET14b as described previously (Qin et al., 2006). Recombinant proteins were expressed in BL21 (DE3) cells, at 20 °C with 0.2 mM IPTG, then purified with a Ni2+-NTA affinity column (Qiagen), followed by gel-filtration chromatography on a HiLoad 16/60 Superdex 75 prep grade column (Amersham-Pharmacia) in a buffer containing 10 mM Tris pH 7.8, 100 mM NaCl and 10 mM 2-mercaptoethanol. E. coli 70S ribosomes lacking L7/L12 were prepared as described by (Hamel et al., 1972; Wystup et al., 1979). Tight-coupled E. coli ribosomes, cloned E. coli His-tagged proteins EF-G, EF-Tu, IF1, IF2, and IF3, and E. coli [35S]fMet-tRNAfMet; E. coli [3H]Phe-tRNAPhe were prepared as described (Liu et al., 2010). MFK-mRNA was purchased from Dharmacon (Lafayette, CO) with sequences 5′-GGG AAG GAG GUA AAA AUG UUU AAA CGU AAA UCU ACU-3′ (initiator codon underlined).
IF2-dependent 70SIC formation light scattering assay
This assay was performed as described (Grigoriadou et al., 2007): 30SIC was formed by mixing 0.3 μM 30S, 0.45 μM IF1, 0.45 μM IF3; 0.45 μM fMet-tRNAfMet, 0.15 μM IF2, 0.9 μM AUG022-mRNA (Grigoriadou et al., 2007) and 100 μM GTP, premixed with various concentrations of Evn (0–5 μM) and then rapidly mixed with 50S subunits (0.3 μM) in a Kintek stopped flow spectrophotometer. Excitation was at 436 nm and light scattering was determined using a 455 nm cut-off filter. All concentrations are final after mixing.
Back-translocation assay
All of the following complexes were made up in buffer A (20 mM Hepes–KOH, pH 7.5, at 0 °C, 150 mM NH4Ac, 4.5 mM MgAc2, 4 mM β-mercaptoethanol, 0.05 mM spermine, and 2 mM spermidine) at 37 °C. Initiation complex was formed by incubating WT ribosomes (2 μM) with mRNA MFK (8 μM), IF1 (3 μM), IF2 (3 μM), IF3 (3 μM), GTP (1 mM) and [35S]-fMet-tRNAfMet (3 μM) for 25 min. Ternary complex was formed by incubating EF-Tu (6 μM) with labeled Phe-tRNAPhe (3 μM), GTP (1 mM), phosphoenolpyruvate (Roche Diagnostics) (1.5 mM), pyruvate kinase (Roche Diagnostics) (0.015 mg/mL) for 15 min. POST complexes were formed by incubating ternary complex and initiation complex at 37 °C briefly for 45 sec and then in the presence of EF-G (molar ratio of EF-G: ribosome was 0.2:1) and GTP (1 mM) at 37 °C for 10 min. Then they were purified by ultracentrifugation through a 1.1 M Sucrose cushion in buffer A (450,000 g, 40 min, 4 °C). POST complex concentration was calculated from the amount of ribosome-bound fMet-[3H]-Phe-tRNAPhe. Stopped-flow fluorescence experiments were performed using an SX.18MV stopped-flow spectrofluorometer (Applied Photophysics). POST complex (0.1 μM) containing fMetPhe-tRNAPhe(Prf) in the P site and tRNAfMet in the E site was rapidly mixed with 0.15 μM tRNAfMet, 3 μM EF4•GDPNP and various concentrations of Evn (0–5 μM). Proflavin was excited at 460 nm and fluorescence was monitored using a 495 nm long-pass filter. Lines through the data are fit to triple-exponential equations using the program Igor-Pro (Wavemetrics).
Translocation assay
PRE complexes were formed by incubating initiation complex and ternary complex at 37 °C for 45 sec. Then they were purified by ultracentrifugation through a 1.1 M Sucrose cushion in buffer A with 20 mM Mg2+ (450,000 g, 40 min, 4 °C). PRE complex concentration was calculated from the amount of ribosome-bound fMet-[3H]-Phe-tRNAPhe. Stopped-flow fluorescence experiments were performed using an SX.18MV stopped-flow spectrofluorometer. Proflavin was excited at 460 nm and fluorescence was monitored using a 495-nm long-pass filter. Data are fit to double-exponential equations using the program Igor-Pro (Wavemetrics).
Malachite green GTPase activity assays
GTPase activity was measured using the Malachite Green Phosphate Kit (BioAssay) that quantifies the green complex formed between Malachite Green, molybdate and free orthophosphate. Unless otherwise mentioned, all reactions contained 30 nM E. coli 70S ribosomes, 20 μM GTP and 60 nM protein in the presence or absence of antibiotics as necessary. Reactions were transferred into 96-well microtiter plates and color formation was measured on Tecan - Infinite M1000 microplate reader at 650 nm. Reactions performed in the absence of ribosomes were used as a background signal to account for the intrinsic GTPase activity of the translation factor.
In vitro transcription-translation assay
All coupled transcription-translation experiments were performed using an E. coli lysate-based system in the presence and absence of antibiotics as described previously (Starosta et al., 2010; Starosta et al., 2009). Reactions were transferred into 96-well microtiter plates and the GFP fluorescence was measured with a Typhoon Scanner 9400 (Amersham Bioscience) using a Typhoon blue laser module (Amersham Bioscience). Images were then quantified using ImageQuantTL (GE Healthcare) and represented graphical using SigmaPlot (Systat Software, Inc.).
Figure preparation
Chemical structures for the precursor compounds were drawn using ChemDraw (Advanced Chemistry Development, Inc. Toronto, Canada) and all structural figures were prepared with PyMol (http://www.pymol.org).
RESULTS
Differential effects of thiopeptide antibiotics on GTPase activities of translational factors
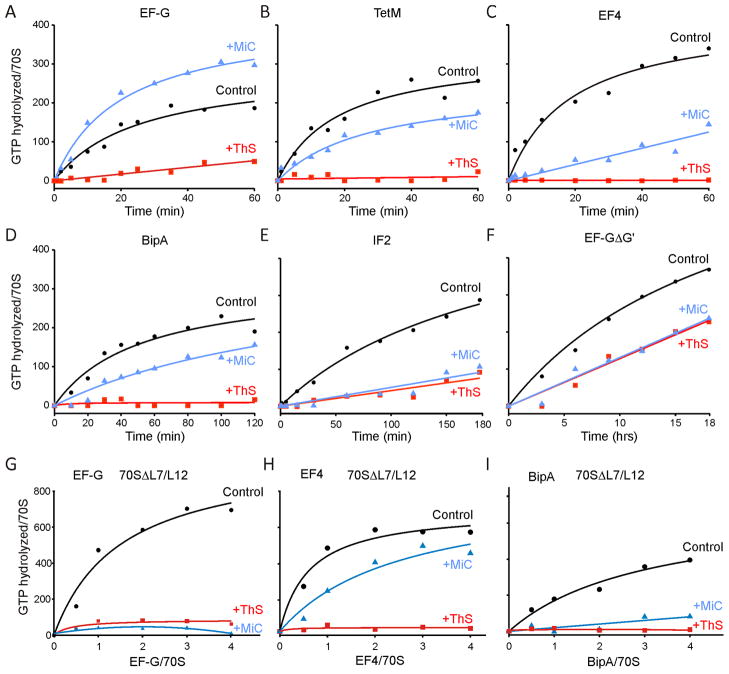
The suggestion that MiC stimulates the uncoupled ribosome-dependent GTPase (rdGTPase) of EF-G by stabilizing the interaction of L7-CTD with the G′ subdomain of EF-G (Harms et al., 2008) prompted us to investigate the effect of MiC on the uncoupled rdGTPase activities of other translational GTPases that have reduced or completely absent G′ subdomains (such as TetM, EF4 and BipA), as determined using the malachite-green assay (Starosta et al., 2009). We found rdGTPase activity of EF-G to be inhibited by ThS (Figure 2A), as expected from previous reports (Pan et al., 2007; Pestka, 1970; Rodnina et al., 1999; Weisblum and Demohn, 1970). The rdGTPase activities of TetM, EF4 and BipA were also inhibited by ThS (1 μM), (Figures 2B–D), as was the rdGTPase activity of IF2 (Figure 2E), as reported previously (Grunberg-Manago et al., 1972). Similar trends were found for all factors independent of the excess of the factor over the ribosome (Figure S2) or the concentration of antibiotics used (data not shown). Consistent with these results, ThS has been shown previously to inhibit the rdGTPase activity of a related ribosome protection protein, TetO (Connell et al., 2003b), the formation of a stable complex between TetM and the ribosome in the presence of GDPNP and GTP (Dantley et al., 1998), and the IF2-dependent formation of 70S initiation complex (70SIC) (Grigoriadou et al., 2007). However, we could not reproduce the recently reported stimulatory effects of ThS on IF2 GTPase (Brandi et al., 2004; Cameron et al., 2002).
Figure 2. Effect of thiostrepton and micrococcin on GTPase activity of various translation factors.
(A–F) The rdGTPase activities of translation factors (A) E. coli EF-G, (B) TetM, (C) E. coli EF4, (D) E. coli BipA, (E) E. coli IF2 and (F) E. coli EF-GΔG′, using E. coli 70S ribosomes in the absence (black circles) and presence of 1 μM thiostrepton (ThS, red squares) or 5 μM micrococcin (MiC, blue triangles). (G–I) The rdGTPase activities of E. coli translation factors (G) EF-G, (H) EF4 and (I) BipA, using E. coli 70S ribosomes lacking L7/L12. Reactions in (G–I) were incubated for 12 hours at 20 °C. In all cases, background hydrolysis due to the intrinsic GTPase activity of each factor has been subtracted. See also Figure S2.
Guided by a comparison of the crystal structures of T. thermophilus EF-G (PDB1FNM, Laurberg et al., 2000) with E. coli EF4 (PDB3CB4, Evans et al., 2008), we also generated an E. coli EF-G lacking the G′ subdomain (EF-GΔG′): EF-GΔG′ has a deletion of amino acids 172-265, thus truncating the G′ subdomain before β3G and after β61 (Figure S1). In contrast to previous attempts to produce an EF-GΔG′ protein (Nechifor et al., 2007), soluble protein was obtained under native conditions, and therefore refolding or purification under denaturing conditions was unnecessary (see Materials and methods). The purified E. coli EF-GΔG′ had an intrinsic GTPase activity comparable with that of wildtype E. coli EF-G (data not shown), suggesting that the protein was not misfolded. Moreover, although the rdGTPase was significantly slower (>10×) than wildtype E. coli EF-G, it was nevertheless inhibited by ThS (Figure 2F), albeit more weakly than for wildtype EF-G. As expected from previous studies (Cameron et al., 2002; Cundliffe and Thompson, 1981; Lentzen et al., 2003), we also observed that MiC enhanced the rdGTPase activity of EF-G (Figure 2A). Conversely, we could show that MiC inhibited the rdGTPase of all other translational GTPases that were tested, namely TetM, EF4, BipA and IF2 (Figures 2B–E). Additionally, deletion of the G′ subdomain of EF-G also produced a change in the activity of MiC, since MiC was seen to inhibit, rather than stimulate, the rdGTPase of EF-GΔG′ (Figure 2F). We note that deletion of the G′ subdomain of EF-G greatly reduced (>10-fold) the rdGTPase activity of the factor, similar to the previously reported introduction of mutations within αAG of the G′ subdomain (Nechifor et al., 2007). Similarly, ThS and MiC also inhibited the rdGTPase activities of EF-G, EF4 and BipA when E. coli 70S ribosomes were used that lacked L7/L12 (70SΔL7/L12) (Figure 2G–I). Defects in rdGTPase of EF-G have also been seen when the ribosomal proteins L7/L12 are selectively removed from the ribosome (Diaconu et al., 2005; Kischa et al., 1971; Nechifor et al., 2007) or mutations are made within the L7-CTD (Diaconu et al., 2005).
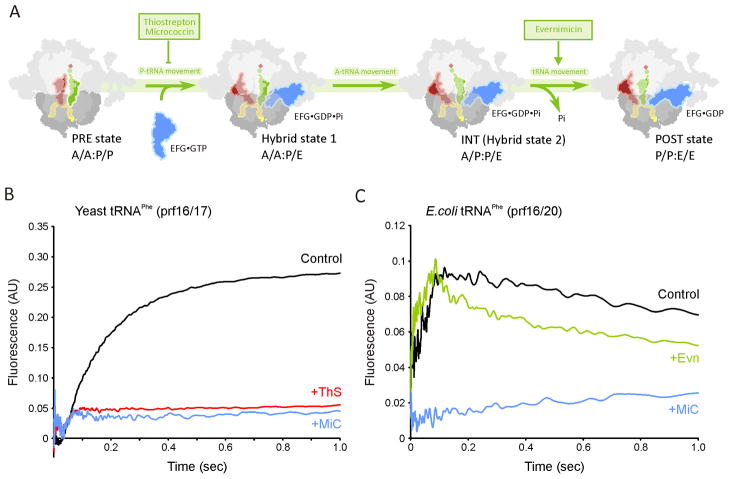
Inhibition of translocation by thiopeptide antibiotics, but not by evernimicin
The translocation reaction occurs after peptide-bond formation and involves the EF-G catalyzed movement of the peptidyl- and deacylated-tRNAs in the A- and P-sites into the P- and E-sites, respectively (Figure 3A) (reviewed by Schmeing and Ramakrishnan, 2009). Conversion of the pre-translocational (PRE) complex into a post-translocational (POST) complex proceeds through (A/P and P/E) hybrid states, where the CCA 3′ ends of the tRNAs move with respect to the large subunit while remaining relatively fixed with respect to the small subunit (Blanchard et al., 2004; Moazed and Noller, 1989; Ratje et al., 2010). To monitor translocation rates, PRE complexes were assembled containing proflavin (prf) labeled tRNAs, thus enabling tRNA movement to be followed by stopped-flow monitoring of the fluorescence change following delivery of EF-G•GTP (Pan et al., 2007; Rodnina et al., 1997; Savelsbergh et al., 2003). In Figure 3B, rapid addition of EF-G•GTP to PRE complexes formed with unlabelled E. coli tRNAfMet in the P-site and yeast fMetPhe-tRNAPhe (prf16/17) in the A-site leads to an apparent monophasic increase in fluorescence as A-site tRNA is translocated to the P site (Pan et al., 2007; Rodnina et al., 1997; Savelsbergh et al., 2003). Pre-incubation of the PRE complex with (10 μM) MiC or ThS completely abolished fluorescence, as reported previously for ThS (Pan et al., 2007; Rodnina et al., 1997).
Figure 3. Effect of the micrococcin and evernimicin on translocation.
(A) Scheme for EF-G catalysed translocation with sites of antibiotic inhibition. (B) Isolated PRE complex (0.1 μM) containing yeast fMetPhe-tRNAPhe(Prf16/17) in the A-site and tRNAfMet in the P–site, either in the absence of antibiotic (black trace) or in the presence of thiostrepton (10 μM ThS; red trace) or micrococcin (10 μM MiC; blue trace), were rapidly mixed in a stopped-flow spectrophotometer with 5 μM EF-G and 1 mM GTP. (C) Isolated PRE complex (0.1 μM) containing E.coli fMetPhe-tRNAPhe(Prf16/20) and tRNAfMet in the P–site, in the absence of antibiotic (black trace) or in the presence of evernimicin (10 μM Evn; green trace) or micrococcin (10 μM MiC; blue trace), were rapidly mixed in a stopped-flow spectrophotometer with 5 μM EF-G and 1 mM GTP. All concentrations are final after mixing. The traces in the control and + Evn traces are each fit to a two-step process (Pan et al., 2007) yielding the following rate constants: Control: 20.7 ± 0.6 s−1, 1.3 ± 0.1 s−1; + Evn: 28.1 ± 0.7 s−1, 3.5 ± 0.2 s−1. See also Figure S3.
By contrast, translocation clearly proceeds via a two-step reaction for PRE complexes containing E. coli tRNAfMet in the P-site and E. coli fMetPhe-tRNAPhe (Prf16/20) in the A-site (Figure 3C; Pan et al., 2007). In this case, an initial rapid increase in fluorescence intensity is followed by a gradual decrease, with respective apparent rate constants of 20.7 ± 0.6 s−1 and 1.3 ± 0.1 s−1, The presence of MiC abolishes almost all fluorescent change (Figure 3C), strongly inhibiting step 1 and thus step 2, as reported previously for ThS (Pan et al., 2007). In contrast, Evn does not inhibit the translocation reaction. In fact, the apparent rate constants (Step1: 28.1 ± 0.7 s−1; Step 2: 3.5 ± 0.2 s−1) suggest that the drug actually accelerates the process, particularly the second step (Figure 3C).
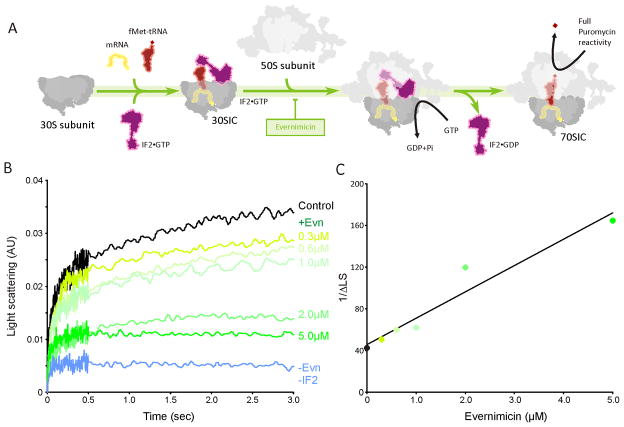
Evernimicin inhibits IF2-dependent 70SIC formation
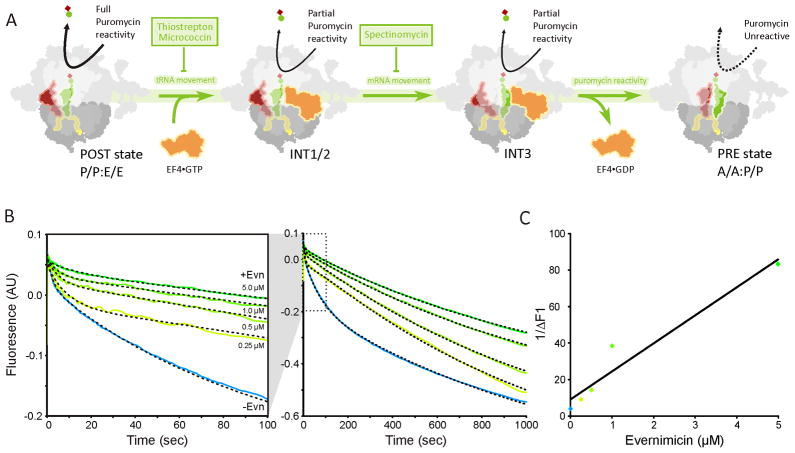
In bacteria, formation of 70S initiation complex (70SIC) involves the association of the large 50S subunit with a 30S initiation complex (30SIC) comprising the 30S subunit, mRNA; initiator fMet-tRNA and three initiation factors IF1, IF2 and IF3 (Figure 4A) (reviewed by Laursen et al., 2005; Simonetti et al., 2009). Binding of the 50S subunit to the 30SIC stimulates the GTPase activity of IF2, leading to release of IF2•GDP and resulting in a puromycin reactive 70SIC (Figure 4A) (Grigoriadou et al., 2007).
Figure 4. Evernimicin inhibits IF2-dependent 70SIC formation.
(A) Scheme for 70S initiation complex formation with site of Evn inhibition. (B) 30S initiation complex (0.3 μM) was premixed with various concentrations of Evn (0–5 μM) and then rapidly mixed with 50S subunits (0.3 μM) in a Kintek stopped flow spectrophotometer. The sample from which IF2 was omitted demonstrates the dependence of 70S initiation complex formation on IF2. (B) A plot of the reciprocal of IF2-dependent light scattering increase at 1 sec vs. Evn concentration, allowing calculation of an apparent Ki for Evn of 1.8 ± 0.2 μM.
Evn has previously been shown to inhibit IF2-dependent formation of fMet-puromycin manner (Belova et al., 2001), leading to its classification as a translation initiation inhibitor. However, the exact step of inhibition has not been determined. To examine this further, 70SIC formation has been monitored kinetically using light scattering as described previously (Grigoriadou et al., 2007): 30SIC programmed with 022AUG mRNA were rapidly mixed with 50S subunits and the increase in light scattering due to 70SIC formation (black control trace in Figure 4B) was monitored using stopped flow spectrophotometry. In the absence of IF2, no increase in light scattering was observed (blue trace in Figure 4B), illustrating the IF2-dependence for 70SIC reported previously (Antoun et al., 2006; Grigoriadou et al., 2007). Pre-incubation of the 30SIC with increasing concentrations of Evn before 50S addition led to a corresponding decrease in IF2-dependent light scattering (green traces in Figure 4B). At 5 μM Evn, almost all IF2-dependent light scattering was abolished, indicating that Evn inhibits the IF2-dependent association of the 30SIC with the 50S subunit. A plot of the reciprocal of the increase of IF2-dependent light scattering at 1 sec versus Evn concentration (Figure 4C) yields an apparent Ki for Evn of 1.8 ± 0.2 μM.
Differential effects of evernimicin on GTPase activity of translational GTPases
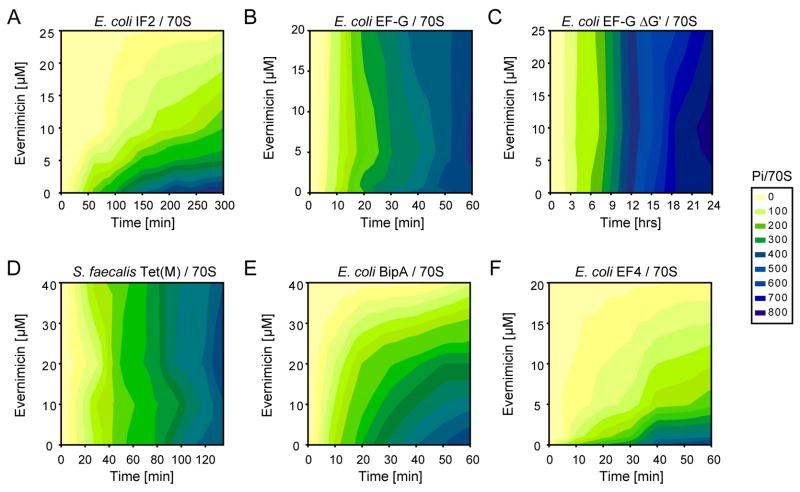
Evn strongly inhibits the rdGTPase activity of IF2 (Figure 5A), but has little or no effect on the rdGTPase activities of EF-G (Figure 5B), or EF-GΔG′ (Figure 5C), or of the EF-G paralog Tet(M) (Figure 5D), consistent with the potency of Evn in inhibiting 70SIC formation (Figure 4B) and with the lack of effect of Evn on EF-G-dependent translocation (Figure 3C). Surprisingly, Evn was also found to inhibit the rdGTPase of BipA (Figure 5E) and especially EF4 (Figure 5F). Indeed, the inhibitory activity of Evn toward EF4 (IC50 = ~3 μM) was higher than that toward IF2 (IC50 = ~7 μM), and much higher than toward BipA (IC50 = ~20 μM).
Figure 5. Effect of evernimicin on the GTPase activity of various translation factors.
(A–F) Activation of uncoupled ribosome-dependent GTPases of translation factors (A) E. coli IF2, (B) E. coli EF-G, (C) E. coli EF-GΔG′, (D) S. faecalis TetM, (E) E. coli BipA, and (F) E. coli EF4, in the presence of increasing concentrations of evernimicin (Evn). The inset shows the colour gradient scale from 0 (yellow) to 800 pmol (blue) of inorganic phosphate (Pi) produced per pmol of 70S ribosomes, following subtraction of back-ground intrinsic GTPase activities.
EF4-dependent back-translocation is inhibited by evernimicin
EF4 catalyzes partial back-translocation, i.e. the movements of mRNA and tRNAs from POST toward the PRE state (Figure 6A) (Liu et al., 2010; Qin et al., 2006). As described previously (Liu et al., 2010), EF4-mediated partial back-translocation was monitored using proflavin (prf) labelled tRNAs: POST state ribosomes containing fMetPhe-tRNAPhe(Prf16/20) in the P-site and tRNAfMet in the E-site were rapidly mixed in a stopped-flow spectrophotometer with EF4 and GDPNP, with fluorescence change being monitored over time (Figure 6B). In the absence of antibiotic, back-translocation proceeds via a three-step process (blue trace in Figure 6B) consistent with movement through a series of three intermediate states as reported (Liu et al., 2010) (INT1-3 in Figure 6A). In the presence of increasing concentrations of Evn, both the fluorescence change and rates of each step in the partial back-translocation were inhibited by Evn, (Figure 6B), leading to an apparent Ki for Evn binding to the POST complex of 0.6 μM (Figure 6C). High concentrations of Evn (5 μM in Figure 6B) completely abolished the EF4-catalyzed component of the back-translocation, and only the two-step spontaneous reverse translocation process that occurs in the absence of EF4 was observed (Liu et al., 2010). Evn inhibition of EF4-dependent partial back-translocation is similar to that reported earlier using spectinomycin (Liu et al., 2010), a well-characterized translocation inhibitor (Wilson, 2009), and is consistent with the observation that Evn is a potent inhibitor of the EF4 rdGTPase activity (Figure 5F).
Figure 6. Evernimicin inhibits EF4-mediated back-translocation.
(A) Scheme for EF4-catalyzed back-translocation with sites of antibiotic inhibition. (B) Fluorescence changes over different time scales. Isolated POST complex (0.1 μM) containing fMetPhe-tRNAPhe(Prf) in the P-site and tRNAfMet in the E-site, premixed with 0.15 μM tRNAfMet, to increase E-site occupancy, were rapidly mixed in a stopped-flow spectrophotometer with 3 μM EF4, 0.5 mM GDPNP and different concentrations of Evn as indicated on the figure. All concentrations are final after mixing. Lines through the traces are fit to a kinetic model (Liu et al., 2010) in which back translocation proceeds via a three step process in the absence of Evn (the first two of which are catalyzed by EF4) and via a two-step process at saturating Evn. (C) A plot of the reciprocal of the apparent magnitude of the fluorescence change for the second, EF4-catalyzed step versus Evn concentration, giving an apparent Ki for Evn binding to the POST complex of 0.6 ± 0.1 μM.
DISCUSSION
Influence of thiopeptides on translocation and translation factor GTPase activities
Although both ThS and MiC inhibit the multiple-turnover rdGTPase activities of IF2, TetM, EF4 and BipA (Figure 2B–E), MiC differs from ThS in stimulating the rdGTPase of EF-G, an activity that is strongly inhibited by ThS (Figure 2A). Our results suggest that ThS inhibition and MiC stimulation of EF-G rdGTPase arises ultimately from differential effects on the interactions between the G′ subdomain of EF-G and L7-CTD.
ThS allows ribosome-binding and single-turnover GTPase activity of EF-G, but prevents the stable accommodation of EF-G on the ribosome, which is necessary for tRNA translocation (Rodnina et al., 1999; Seo et al., 2006). Part of the accommodation of EF-G encompasses the movement of EF-G towards L11-NTD, which is inhibited by ThS (Seo et al., 2006), consistent with the structural overlap between the binding site of ThS and domain V of EF-G, both locating to the cleft formed by H43/44 and L11-NTD (Figure 1F) (Harms et al., 2008). Because ThS also prevents multiple-turnover GTPase activity of EF-G by inhibiting Pi release (Savelsbergh et al., 2003; Seo et al., 2006), EF-G remains trapped on the ribosome but in an unaccommodated state. In contrast, MiC allows Pi release from EF-G (Starosta et al., 2009) and thus stimulates the multiple-turnover rdGTPase activity of EF-G, as observed here (Figure 2A) and reported previously (Cameron et al., 2002; Cundliffe and Thompson, 1981; Lentzen et al., 2003). In agreement with the idea that MiC stimulates the rdGTPase of EF-G by stabilization of the interaction between the L7-CTD and the G′ subdomain of EF-G (Harms et al., 2008), we could show that the MiC-dependent stimulation of EF-G rdGTPase activity was lost when the G′ subdomain of EF-G was removed (Figure 2F) or the ribosomes lacked L7/L12 (Figure 2G–I). Moreover, the rdGTPase activities of translation factors that naturally lack or have a reduced G′ subdomain (TetM, EF4, BipA, IF2) were also inhibited by MiC (Figure 2B–E).
Although many translation factors lack the complete G′ subdomain, NMR studies indicate that IF2, EF-Tu, RF3 interact with the same conserved region of L7-CTD as EF-G (Helgstrand et al., 2007). L7/L12 has been proposed to interact with helix αD1 of domain I of EF-Tu. However, this interaction is more important for initial binding of EF-Tu•GTP•aa-tRNA to the ribosome, rather than for subsequent steps, such as A-site binding and GTPase activation (Kothe et al., 2004). Nevertheless, like EF-G, the rdGTPase activity of EF-Tu is dramatically reduced when ribosomes are depleted of L7/L12 (Diaconu et al., 2005; Mohr et al., 2002). In contrast, depletion of L7/L12 reduces the rate of association of IF2 with the ribosome, rather than directly affecting GTP hydrolysis and Pi release (Huang et al., 2010). We observed that the rdGTPase of LepA and BipA with 70SΔL7/L12 was significantly reduced, but also inhibited by both MiC and ThS (Figure 2H–I). Further work will be needed to distinguish between factor binding versus GTPase activity defects.
Despite the contrasting effects of MiC and ThS on the GTPase activities of EF-G (Figure 2A) (Cameron et al., 2002; Cundliffe and Thompson, 1981; Lentzen et al., 2003), our kinetic analysis demonstrates that MiC, like ThS, is a potent inhibitor of the translocation reaction (Figure 3B). The finding that MiC targets translocation is in agreement with original conclusions of Pestka and Brot (Pestka and Brot, 1971), which were based upon its inhibition of poly(U)-dependent poly(Phe) synthesis, but its lack of affect on either aa-tRNA binding or peptide-bond formation. Given the similarity in binding site between MiC and ThS, it thus appears likely that both MiC and ThS inhibit translocation analogously – namely, by preventing the transition of EF-G from an initially weaker binding state to a fully accommodated state on the ribosome, which, we would suggest, is necessary for translocation (Seo et al., 2006). We note that despite their diverse effects on the rdGTPase of EF-G, the inhibitory potency of MiC and ThS with respect to in vitro transcription-translation systems is comparable (both have an IC50 of ~3 μM) (Figure S3).
Influence of the orthosomycin evernimicin on translation factor activities
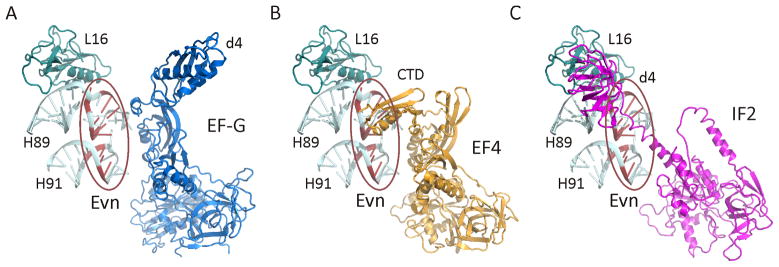
Unlike thiopeptides, we find that the orthosomycin Evn has no inhibitory effect on rbGTPase activity of EF-G (Figure 5B), nor on the EF-G-mediated translocation reaction (Figure 3C). This is consistent with the lack of overlap between the putative Evn binding site and the binding position of EF-G determined by structural studies (Connell et al., 2007; Gao et al., 2009; Ratje et al., 2010) (Figure 7A). Similarly, Evn does not inhibit the rdGTPase of Tet(M) (Figure 5D) or of Tet(O) (data not shown), which interacts with the ribosome is an analogous manner to EF-G (Spahn et al., 2001). In contrast, we find that Evn is a potent inhibitor of the rdGTPase activity of IF2 (Figure 5A), BipA and EF4 (Figure 5E, F). While little is known about the structure or function of BipA on the ribosome (deLivron et al., 2009), structures of EF4 alone (Evans et al., 2008) and bound to the ribosome (Connell et al., 2008) reveal an overall similarity with EF-G. One exception is the unique CTD of EF4 (Evans et al., 2008), which on the ribosome is oriented back towards the large subunit (Connell et al., 2008) and encroaches upon the Evn binding site (Figure 7B). Such overlap is consistent with our finding that Evn is a potent inhibitor EF4-mediated back-translocation reaction (Figure 6B). Our results demonstrating potent Evn inhibitory effects on non-initiation translation factors, such as BipA and EF4, suggest that Evn can no longer be considered exclusively as an initiation inhibitor, as it has been heretofore. Indeed, Evn is a slightly stronger inhibitor of the rdGTPase activity of EF4 compared with IF2 and the Ki (0.6 μM) for Evn binding to the POST complex (Figure 6C) is a little lower than the Ki (1.8 μM) for Evn inhibition of IF2-dependent 70SIC formation.
Figure 7. Putative binding sites of orthosomycin antibiotics relative to translation factors EF-G, EF4 and IF2.
(A–C) Relative position of (A) EF-G (blue) (Connell et al., 2007; Gao et al., 2009), (B) EF4 (orange) (Connell et al., 2008) and (C) IF2 (purple) (Allen et al., 2005; Marzi et al., 2003) to the putative Evn binding site (encircled in red) (Belova et al., 2001; Wilson, 2009); Nucleotides associated with Evn binding in H89 and H91 are colored red and L16 (teal) is shown for reference. Note the overlap in positions of the CTD of EF4 and domain 4 (d4) of IF2 with the putative Evn binding site.
Nevertheless, since the gene for EF4 is not essential for survival in E. coli (Dibb and Wolfe, 1986), the principal antimicrobial target of Evn is most likely IF2. Here we demonstrate that Evn can inhibit the rdGTPase activity of IF2 (Figure 5A) as well as prevent the IF2-dependent association of the 30SIC with the large ribosomal subunit (Figure 4B). These findings support an earlier suggestion that Evn inhibits 70SIC formation, which was based on the ability of Evn to prevent the formation of fMet-puromycin in an IF2-dependent manner (Belova et al., 2001). Models for IF2 bound to the ribosome derived from biochemical (Marzi et al., 2003) and cryo-EM data (Allen et al., 2005; Myasnikov et al., 2005) suggest that domain 4 of IF2 and the associated linker region encroach on the Evn binding site (Figure 7C). Thus, we believe that Evn sterically interferes with IF2 binding to the large ribosomal subunit, accounting for the Evn-dependent reduction in rdGTPase activity of IF2 with 70S ribosomes (Figure 5A) as well as the reduction in 70SIC as observed using light scattering (Figure 4B). The similarity between the Ki (1.8 μM) of Evn inhibition for 70SIC formation and the half-inhibitory concentration (IC50 = ~2 μM) of Evn for synthesis of GFP as measured in an E. coli in vitro coupled transcription-translation system (Figure S3), also supports the claim that Evn targets predominantly the initiation phase of protein synthesis (Belova et al., 2001).
Significance
Insight into the mechanism of action of diverse classes of antibiotics, such as the thiopeptides and orthosomycins, to inhibit distinct steps during translation can provide insight into the fundamental process of translation. Here we demonstrate that although the thiopeptides MiC and ThS have contrasting effects on the rdGTPase activity of EF-G, both antibiotics are potent inhibitors of EF-G-dependent translocation reaction. Our results demonstrate that the MiC-dependent stimulation of the rdGTPase of EF-G requires the presence of the G′ subdomain of EF-G as well as ribosomal proteins L7/L12. This finding supports the idea that recycling of EF-G from the ribosome, which occurs upon release of Pi, is mediated via the interaction of L7-CTD with the G′ subdomain of EF-G. In contrast, we can demonstrate that Evn does not influence EF-G rdGTPase, nor EF-G dependent translocation, but is a potent inhibitor of EF4-dependent back-translocation reaction as well as IF2-dependent 70S initiation complex formation. These findings are in agreement with the predicted binding site of the orthosomycins relative to the binding sites of EF-G, EF4 and IF2 on the ribosome. Understanding mechanistically how antibiotics perturb the translational apparatus is an important step for the future development of new improved antimicrobial agents to overcome the emerging resistant bacterial pathogens.
Supplementary Material
Acknowledgments
We would like to thank Torsten Stachelhaus for preparation of the Micrococcin P1 and Dr Vincent Perreten for providing S. faecalis gDNA. This work was financed by the EMBO young investigator program (D.N.W.), Deutsche Forschungsgemeinschaft (WI3285/1-1 to D.N.W.) and by the National Institutes of Health (GM071014 to B.S.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarestrup FM, Jensen LB. Presence of variations in ribosomal protein L16 corresponding to susceptibility of enterococci to oligosaccharides (Avilamycin and evernimicin) Antimicrob Agents Chemother. 2000;44:3425–3427. doi: 10.1128/aac.44.12.3425-3427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian PV, Mendrick C, Loebenberg D, McNicholas P, Shaw KJ, Klugman KP, Hare RS, Black TA. Evernimicin (SCH27899) inhibits a novel ribosome target site: analysis of 23S ribosomal DNA mutants. Antimicrob Agents Chemother. 2000a;44:3101–3106. doi: 10.1128/aac.44.11.3101-3106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian PV, Zhao W, Black TA, Shaw KJ, Hare RS, Klugman KP. Mutations in ribosomal protein L16 conferring reduced susceptibility to evernimicin (SCH27899): implications for mechanism of action. Antimicrob Agents Chemother. 2000b;44:732–738. doi: 10.1128/aac.44.3.732-738.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, Zavialov A, Gursky R, Ehrenberg M, Frank J. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell. 2005;121:703–712. doi: 10.1016/j.cell.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors tune the rate of initiation of protein synthesis in bacteria. EMBO J. 2006;25:2539–2550. doi: 10.1038/sj.emboj.7601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley M, Dale J, Merritt E, Xiong X. Thiopeptide antibiotics. Chem Rev. 2005;105:685–714. doi: 10.1021/cr0300441. [DOI] [PubMed] [Google Scholar]
- Belova L, Tenson T, Xiong LQ, McNicholas PM, Mankin AS. A novel site of antibiotic action in the ribosome: Interaction of evernimicin with the large ribosomal subunit. Proc Natl Acad Sci USA. 2001;98:3726–3731. doi: 10.1073/pnas.071527498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard SC, Cooperman BS, Wilson DN. Probing translation with small-molecule inhibitors. Chem Biol. 2010;17:633–645. doi: 10.1016/j.chembiol.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard SC, Kim HD, Gonzalez RL, Jr, Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci USA. 2004;101:12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi L, Marzi S, Fabbretti A, Fleischer C, Hill W, Lodmell J, Gualerzi C. The translation initiation functions of IF2: Targets for thiostrepton inhibition. J Mol Biol. 2004;335:881–894. doi: 10.1016/j.jmb.2003.10.067. [DOI] [PubMed] [Google Scholar]
- Cameron DM, Thompson J, March PE, Dahlberg AE. Initiation factor IF2, thiostrepton and micrococcin prevent the binding of elongation factor G to the Escherichia coli ribosome. J Mol Biol. 2002;319:27–35. doi: 10.1016/S0022-2836(02)00235-8. [DOI] [PubMed] [Google Scholar]
- Connell SR, Takemoto C, Wilson DN, Wang H, Murayama K, Terada T, Shirouzu M, Rost M, Schuler M, Giesebrecht J, et al. Structural basis for interaction of the ribosome with the switch regions of GTP-bound elongation factors. Mol Cell. 2007;25:751–764. doi: 10.1016/j.molcel.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Connell SR, Topf M, Qin Y, Wilson DN, Mielke T, Fucini P, Nierhaus KH, Spahn CM. A new tRNA intermediate revealed on the ribosome during EF4-mediated back-translocation. Nat Struct Mol Biol. 2008;15:910–915. doi: 10.1038/nsmb.1469. [DOI] [PubMed] [Google Scholar]
- Connell SR, Tracz DM, Nierhaus KH, Taylor DE. Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob Agents Chemother. 2003a;47:3675–3681. doi: 10.1128/AAC.47.12.3675-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell SR, Trieber CA, Dinos GP, Einfeldt E, Taylor DE, Nierhaus KH. Mechanism of Tet(O)-mediated tetracycline resistance. EMBO J. 2003b;22:945–953. doi: 10.1093/emboj/cdg093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E, Thompson J. Concerning the mode of action of micrococcin upon bacterial protein synthesis. Eur J Biochem. 1981;118:47–52. doi: 10.1111/j.1432-1033.1981.tb05484.x. [DOI] [PubMed] [Google Scholar]
- Dantley K, Dannelly H, Burdett V. Binding interaction between Tet(M) and the ribosome: Requirements for binding. J Bacteriol. 1998;180:4089–4092. doi: 10.1128/jb.180.16.4089-4092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta PP, Sharma MR, Qi L, Frank J, Agrawal RK. Interaction of the G′ domain of elongation factor G and the C-terminal domain of ribosomal protein L7/L12 during translocation as revealed by cryo-EM. Mol Cell. 2005;20:723–731. doi: 10.1016/j.molcel.2005.10.028. [DOI] [PubMed] [Google Scholar]
- deLivron MA, Makanji HS, Lane MC, Robinson VL. A novel domain in translational GTPase BipA mediates interaction with the 70S ribosome and influences GTP hydrolysis. Biochemistry. 2009;48:10533–10541. doi: 10.1021/bi901026z. [DOI] [PubMed] [Google Scholar]
- deLivron MA, Robinson VL. Salmonella enterica serovar Typhimurium BipA exhibits two distinct ribosome binding modes. J Bacteriol. 2008;190:5944–5952. doi: 10.1128/JB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconu M, Kothe U, Schlunzen F, Fischer N, Harms JM, Tonevitsky AG, Stark H, Rodnina MV, Wahl MC. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell. 2005;121:991–1004. doi: 10.1016/j.cell.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Dibb NJ, Wolfe PB. lep operon proximal gene is not required for growth or secretion by Escherichia coli. J Bacteriol. 1986;166:83–87. doi: 10.1128/jb.166.1.83-87.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RN, Blaha G, Bailey S, Steitz TA. The structure of LepA, the ribosomal back translocase. Proc Natl Acad Sci U S A. 2008;105:4673–4678. doi: 10.1073/pnas.0801308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YG, Selmer M, Dunham CM, Weixlbaumer A, Kelley AC, Ramakrishnan V. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science. 2009;326:694–699. doi: 10.1126/science.1179709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RL, Jr, Chu S, Puglisi JD. Thiostrepton inhibition of tRNA delivery to the ribosome. RNA. 2007;13:2091–2097. doi: 10.1261/rna.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadou C, Marzi S, Kirillov S, Gualerzi CO, Cooperman BS. A quantitative kinetic scheme for 70 S translation initiation complex formation. J Mol Biol. 2007;373:562–572. doi: 10.1016/j.jmb.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg-Manago M, Dondon J, Graffe M. Inhibition by thiostrepton of the IF-2-dependent ribosomal GTPase. FEBS Lett. 1972;22:217–221. doi: 10.1016/0014-5793(72)80049-8. [DOI] [PubMed] [Google Scholar]
- Hamel E, Koka M, Nakamoto T. Requirement of an E. coli 50S ribosomal protein component for effective interaction of the ribosome with T and G factors and with guanosine triphosphate. J Biol Chem. 1972;247:805–814. [PubMed] [Google Scholar]
- Harms JM, Wilson DN, Schluenzen F, Connell SR, Stachelhaus T, Zaborowska Z, Spahn CM, Fucini P. Translational regulation via L11: molecular switches on the ribosome turned on and off by thiostrepton and micrococcin. Mol Cell. 2008;30:26–38. doi: 10.1016/j.molcel.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Helgstrand M, Mandava CS, Mulder FA, Liljas A, Sanyal S, Akke M. The ribosomal stalk binds to translation factors IF2, EF-Tu, EF-G and RF3 via a conserved region of the L12 C-terminal domain. J Mol Biol. 2007;365:468–479. doi: 10.1016/j.jmb.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Huang C, Mandava CS, Sanyal S. The ribosomal stalk plays a key role in IF2-mediated association of the ribosomal subunits. J Mol Biol. 2010;399:145–153. doi: 10.1016/j.jmb.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Moody CJ. From amino acids to heteroaromatics--thiopeptide antibiotics, nature’s heterocyclic peptides. Angew Chem Int Ed Engl. 2007;46:7930–7954. doi: 10.1002/anie.200700728. [DOI] [PubMed] [Google Scholar]
- Jenvert R-M, Schiavone L. Characterization of the tRNA and ribosome-dependent pppGpp-synthesis by recombinant stringent factor from Escherichia coli. FEBS J. 2005;272:685–695. doi: 10.1111/j.1742-4658.2004.04502.x. [DOI] [PubMed] [Google Scholar]
- Kischa K, Möller W, Stöffler G. Reconstitution of a GTPase activity by a 50S ribosomal protein from E. coli. Nature New Biol. 1971;233:62–63. doi: 10.1038/newbio233062a0. [DOI] [PubMed] [Google Scholar]
- Kofoed CB, Vester B. Interaction of avilamycin with ribosomes and resistance caused by mutations in 23S rRNA. Antimicrob Agents Chemother. 2002;46:3339–3342. doi: 10.1128/AAC.46.11.3339-3342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothe U, Wieden HJ, Mohr D, Rodnina MV. Interaction of helix D of elongation factor Tu with helices 4 and 5 of protein L7/12 on the ribosome. J Mol Biol. 2004;336:1011–1021. doi: 10.1016/j.jmb.2003.12.080. [DOI] [PubMed] [Google Scholar]
- Laurberg M, Kristensen O, Martemyanov K, Gudkov AT, Nagaev I, Hughes D, Liljas A. Structure of a mutant EF-G reveals domain III and possibly the fusidic acid binding site. J Mol Biol. 2000;303:593–603. doi: 10.1006/jmbi.2000.4168. [DOI] [PubMed] [Google Scholar]
- Laursen BS, Sorensen HP, Mortensen KK, Sperling-Petersen HU. Initiation of protein synthesis in bacteria. Microbiol Mol Biol Rev. 2005;69:101–123. doi: 10.1128/MMBR.69.1.101-123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc D, Ciufolini MA. Total synthesis and stereochemical assignment of micrococcin P1. Angew Chem Int Ed Engl. 2009;48:4198–4201. doi: 10.1002/anie.200900621. [DOI] [PubMed] [Google Scholar]
- Lentzen G, Klinck R, Matassova N, Aboul-ela F, Murchie A. Structural basis for contrasting activities of ribosome binding thiazole antibiotics. Chem Biol. 2003;10:769–778. doi: 10.1016/s1074-5521(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Pan D, Pech M, Cooperman BS. Interrupted catalysis: the EF4 (LepA) effect on back-translocation. J Mol Biol. 2010;396:1043–1052. doi: 10.1016/j.jmb.2009.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PA, Xiong L, Mankin AS, Chau AS, Mendrick CA, Najarian DJ, Cramer CA, Loebenberg D, Coates E, Murgolo NJ, et al. EmtA, a rRNA methyltransferase conferring high-level evernimicin resistance. Mol Microbiol. 2001;41:1349–1356. doi: 10.1046/j.1365-2958.2001.02602.x. [DOI] [PubMed] [Google Scholar]
- Marzi S, Knight W, Brandi L, Caserta E, Soboleva N, Hill WE, Gualerzi CO, Lodmell JS. Ribosomal localization of translation initiation factor IF2. RNA. 2003;9:958–969. doi: 10.1261/rna.2116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey GA, Rogers MJ, McCutchan TF. Inhibition of Plasmodium falciparum protein synthesis. Targeting the plastid-like organelle with thiostrepton. J Biol Chem. 1997;272:2046–2049. doi: 10.1074/jbc.272.4.2046. [DOI] [PubMed] [Google Scholar]
- McNicholas PM, Mann PA, Najarian DJ, Miesel L, Hare RS, Black TA. Effects of mutations in ribosomal protein L16 on susceptibility and accumulation of evernimicin. Antimicrob Agents Chemother. 2001;45:79–83. doi: 10.1128/AAC.45.1.79-83.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas PM, Najarian DJ, Mann PA, Hesk D, Hare RS, Shaw KJ, Black TA. Evernimicin binds exclusively to the 50S ribosomal subunit and inhibits translation in cell-free systems derived from both Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother. 2000;44:1121–1126. doi: 10.1128/aac.44.5.1121-1126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D, Noller HF. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Modelell J, Cabrer B, Parmeggiani A, Vazquez D. Inhibition by siomycin and thiostrepton of both aminoacyl-tRNA and factor G binding to ribosomes. Proc Natl Acad Sci USA. 1971;68:1796–1800. doi: 10.1073/pnas.68.8.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr D, Wintermeyer W, Rodnina MV. GTPase activation of elongation factors Tu and G on the ribosome. Biochemistry. 2002;41:12520–12528. doi: 10.1021/bi026301y. [DOI] [PubMed] [Google Scholar]
- Munro JB, Wasserman MR, Altman RB, Wang L, Blanchard SC. Correlated conformational events in EF-G and the ribosome regulate translocation. Nat Struct Mol Biol. 2010 doi: 10.1038/nsmb.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasnikov A, Marzi S, Simonetti A, Giuliodori A, Gualerzi C, Yusupova G, Yusupov M, Klaholz B. Conformational transition of initiation factor 2 from the GTP- to GDP-bound state visualized on the ribosome. Nat Struct Mol Biol. 2005;12:1145–1149. doi: 10.1038/nsmb1012. [DOI] [PubMed] [Google Scholar]
- Nechifor R, Murataliev M, Wilson KS. Functional interactions between the G′ subdomain of bacterial translation factor EF-G and ribosomal protein L7/L12. J Biol Chem. 2007;282:36998–37005. doi: 10.1074/jbc.M707179200. [DOI] [PubMed] [Google Scholar]
- Nicolaou K, Safina M, Zak M, Lee S, Nevalainen M, Bella M, Estrada A, Funke C, Zécri F, Bulat S. Total synthesis of thiostrepton. Retrosynthetic analysis and construction of key building blocks. J Am Chem Soc. 2005a;127:1159–1175. doi: 10.1021/ja0529337. [DOI] [PubMed] [Google Scholar]
- Nicolaou K, Zak M, Safina M, Estrada A, Lee S, Nevalainen M. Total synthesis of thiostrepton. Assembly of key building blocks and completion of the synthesis. J Am Chem Soc. 2005b;127:11176–11183. doi: 10.1021/ja052934z. [DOI] [PubMed] [Google Scholar]
- Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA. Recent advances in the chemistry and biology of naturally occurring antibiotics. Angew Chem Int Ed Engl. 2009;48:660–719. doi: 10.1002/anie.200801695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaka T, Kaji A. Micrococcin: acceptor-site-specific inhibitor of protein synthesis. Eur J Biochem. 1974;50:101–106. doi: 10.1111/j.1432-1033.1974.tb03876.x. [DOI] [PubMed] [Google Scholar]
- Owens RM, Pritchard G, Skipp P, Hodey M, Connell SR, Nierhaus KH, O’Connor CD. A dedicated translation factor controls the synthesis of the global regulator Fis. EMBO J. 2004;23:3375–3385. doi: 10.1038/sj.emboj.7600343. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pan D, Kirillov SV, Cooperman BS. Kinetically competent intermediates in the translocation step of protein synthesis. Mol Cell. 2007;25:519–529. doi: 10.1016/j.molcel.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Thiostrepton: a ribosomal inhibitor of translocation. Biochem Biophys Res Commun. 1970;40:667–674. doi: 10.1016/0006-291x(70)90956-3. [DOI] [PubMed] [Google Scholar]
- Pestka S, Brot N. Studies on the formation of transfer ribonucleic acid-ribosome complexes. IV. Effect of antibiotics on steps of bacterial protein synthesis: some new ribosomal inhibitors of translocation. J Biol Chem. 1971;246:7715–7722. [PubMed] [Google Scholar]
- Qin Y, Polacek N, Vesper O, Staub E, Einfeldt E, Wilson DN, Nierhaus KH. The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosome. Cell. 2006;127:721–733. doi: 10.1016/j.cell.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Ratje A, Loerke J, Mikolajka A, Brünner M, Hildebrand P, Starosta A, Dönhöfer A, Connell S, Fucini P, Mielke T, et al. Head swivel on the ribosome facilitates translocation via intra-subunit tRNA hybrid sites. Nature. 2010 doi: 10.1038/nature09547. accepted manuscript number 2010-09999A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina MV, Savelsbergh A, Katunin VI, Wintermeyer W. Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature. 1997;385:37–41. doi: 10.1038/385037a0. [DOI] [PubMed] [Google Scholar]
- Rodnina MV, Savelsbergh A, Matassova NB, Katunin VI, Semenkov YP, Wintermeyer W. Thiostrepton inhibits the turnover but not the GTPase of elongation factor G on the ribosome. Proc Natl Acad Sci USA. 1999;96:9586–9590. doi: 10.1073/pnas.96.17.9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelsbergh A, Katunin VI, Mohr D, Peske F, Rodnina MV, Wintermeyer W. An elongation factor G-induced ribosome rearrangement precedes tRNA-mRNA translocation. Mol Cell. 2003;11:1517–1523. doi: 10.1016/s1097-2765(03)00230-2. [DOI] [PubMed] [Google Scholar]
- Savelsbergh A, Mohr D, Kothe U, Wintermeyer W, Rodnina MV. Control of phosphate release from elongation factor G by ribosomal protein L7/12. EMBO J. 2005;24:4316–4323. doi: 10.1038/sj.emboj.7600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelsbergh A, Mohr D, Wilden B, Wintermeyer W, Rodnina MV. Stimulation of the GTPase activity of translation elongation factor G by ribosomal protein L7/12. J Biol Chem. 2000;275:890–894. doi: 10.1074/jbc.275.2.890. [DOI] [PubMed] [Google Scholar]
- Schmeing TM, Ramakrishnan V. What recent ribosome structures have revealed about the mechanism of translation. Nature. 2009;461:1234–1242. doi: 10.1038/nature08403. [DOI] [PubMed] [Google Scholar]
- Seo H, Abedin S, Kamp D, Wilson DN, Nierhaus KH, Cooperman BS. EF-G-dependent GTPase on the ribosome. Conformational change and fusidic acid inhibition. Biochemistry. 2006;45:2504–2514. doi: 10.1021/bi0516677. [DOI] [PubMed] [Google Scholar]
- Simonetti A, Marzi S, Jenner L, Myasnikov A, Romby P, Yusupova G, Klaholz BP, Yusupov M. A structural view of translation initiation in bacteria. Cell Mol Life Sci. 2009;66:423–436. doi: 10.1007/s00018-008-8416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohmen D, Harms JM, Schlunzen F, Wilson DN. Enhanced SnapShot: Antibiotic inhibition of protein synthesis II. Cell. 2009a;139:212–212 e211. doi: 10.1016/j.cell.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Sohmen D, Harms JM, Schlunzen F, Wilson DN. SnapShot: Antibiotic inhibition of protein synthesis I. Cell. 2009b;138:1248–e1241. doi: 10.1016/j.cell.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Spahn CM, Blaha G, Agrawal RK, Penczek P, Grassucci RA, Trieber CA, Connell SR, Taylor DE, Nierhaus KH, Frank J. Localization of the ribosomal protection protein Tet(O) on the ribosome and the mechanism of tetracycline resistance. Mol Cell. 2001;7:1037–1045. doi: 10.1016/s1097-2765(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Starosta A, Karpenko V, Shishkina A, Mikolajka A, Sumbatyan N, Schluenzen F, Korshunova G, Bogdanov A, Wilson D. Interplay between the ribosomal tunnel, nascent chain, and macrolides influences drug inhibition. Chem Biol. 2010;17:1–10. doi: 10.1016/j.chembiol.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Starosta AL, Qin H, Mikolajka A, Leung GY, Schwinghammer K, Nicolaou KC, Chen DY, Cooperman BS, Wilson DN. Identification of distinct thiopeptide-antibiotic precursor lead compounds using translation machinery assays. Chem Biol. 2009;16:1087–1096. doi: 10.1016/j.chembiol.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B, Demohn V. Inhibition by thiostrepton of the formation of a ribosome-bound guanine nucleotide complex. FEBS Lett. 1970;11:149–152. doi: 10.1016/0014-5793(70)80515-4. [DOI] [PubMed] [Google Scholar]
- Wilson DN. The A-Z of bacterial translation inhibitors. Crit Rev Biochem Mol Biol. 2009;44:393–433. doi: 10.3109/10409230903307311. [DOI] [PubMed] [Google Scholar]
- Wolf H. Avilamycin, an inhibitor of the 30S ribosomal subunits function. FEBS Lett. 1973;36:181–186. doi: 10.1016/0014-5793(73)80364-3. [DOI] [PubMed] [Google Scholar]
- Wystup G, Teraoka H, Schulze H, Hampl H, Nierhaus KH. 50S subunit from E coli ribosomes. Isolation of active ribosomal proteins and protein complexes. Eur J Biochem. 1979;100:101–113. doi: 10.1111/j.1432-1033.1979.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Zarazaga M, Tenorio C, Del Campo R, Ruiz-Larrea F, Torres C. Mutations in ribosomal protein L16 and in 23S rRNA in Enterococcus strains for which evernimicin MICs differ. Antimicrob Agents Chemother. 2002;46:3657–3659. doi: 10.1128/AAC.46.11.3657-3659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.