Abstract
Ovarian cancer is a significant malignancy for women in the western world, and its death rate has remained unchanged over the past 50 years, leaving room for proper chemoprevention. Kaempferol is a natural flavonoid widely distributed in fruits and vegetables, and epidemiological studies have found a negative correlation between kaempferol consumption and ovarian cancer risk. To understand the mechanism behind this negative correlation, we investigated kaempferol’s ability to induce apoptosis in A2780/CP70, A2780/wt, and OVCAR-3 ovarian cancer cell lines. Kaempferol inhibited cell proliferation but did not cause necrosis in all 3 cell lines. For the apoptosis, caspase 3/7 levels were induced in a concentration-dependent manner by kaempferol treatment, with A2780/wt cells being the most responsive. This induction can be diminished by pre-treatment with a caspase-9 inhibitor, indicating an intrinsic apoptosis pathway. Western blot analysis revealed that protein levels of Bcl-xL were decreased in ovarian cancer cells, while p53, Bad, and Bax proteins were up-regulated by kaempferol treatment. Our data indicate that kaempferol induces apoptosis in ovarian cancer cells through regulating pro-apoptotic and anti-apoptotic protein expressions in the intrinsic apoptosis pathways, and is a good candidate for the chemoprevention of ovarian cancers in humans. Further studies in animal models and clinical trials are therefore warranted.
Keywords: Kaempferol, Ovarian Cancer, Apoptosis
1. Introduction
It is estimated that 13,850 women in the United States will die from ovarian cancer in 2010, marking 5% of the total cancer deaths in females (Jemal et al., 2010). Prevention of ovarian cancer is challenging, because no specific carcinogen is known to cause this disease (Banks, 2000) and no specific biomarker is clinically available for screening and early diagnosis (Skates et al., 2000). Prevention of ovarian cancer is, however, possible, because migration studies found that this disease is more related to environmental factors than to genetic background (Banks, 2000). The question is which environmental factors or life styles can reduce the risk of ovarian cancers.
It is a common belief that a diet rich in fruits and vegetables will help reduce the risk of various chronic diseases, including cancers. More specifically, low intake of vegetables has been consistently associated with an increased risk of ovarian cancer (Banks, 2000). Kaempferol is a natural flavonoid that is widely distributed in fruits and vegetables, and prospective studies revealed that over decades, consumption of kaempferol dramatically and significantly reduced the risk of ovarian cancer in American female nurses (Gates et al., 2007). This finding suggests that kaempferol is a promising agent for the chemoprevention of ovarian cancers, because it is a dietary component, relatively non-toxic, inexpensive, and consumption of kaempferol can be easily adopted into the lifestyles of most women. The chemopreventive mechanism, however, is unclear or incomplete, and some basic mechanistic studies are needed before designating kaempferol as a real chemoprevention agent.
Our earlier studies have demonstrated that kaempferol inhibits expression of vascular epithelial growth factor (VEGF) angiogenesis in ovarian cancer cells (Luo et al., 2009), and this effect will indirectly prevent ovarian cancer cells from demonstrating unlimited proliferation. However, kaempferol’s direct effects on ovarian cancer cells are still unknown. While short-term exposure to kaempferol does not cause any necrosis in ovarian cancer cells (Luo et al., 2008), long-term effects are unknown for both necrosis and apoptosis. Meanwhile, kaempferol has been reported to induce apoptosis in some cells (Huang et al., 2010; Sharma et al., 2007; Nguyen et al., 2003), but inhibit apoptosis in other cells (Ruiz E et al., 2005). To promote kaempferol toward a chemoprevention agent, kaempferol’s effects on ovarian cancer cells need to be better characterized and kaempferol’s underlying mechanisms of action need to be examined. In this study, we investigated whether kaempferol treatment will cause necrosis and/or apoptosis in ovarian cancer cells, and the pathway involved for these effects.
2. Materials and methods
2.1. Cell culture and treatment
OVCAR-3 and A2780/CP70 ovarian cancer cell lines were provided by Dr. Jiang at the West Virginia University, and the A2780/wt ovarian cancer cell line was kindly provided by Dr. Kenneth Tew at the Medical University of South Carolina. IOSE 364, normal ovarian surface epithelial cells from healthy women, but immortalized with SV40 T/t, were courtesy of Dr. Auersperg at University of British Columbia, Canada. All cells were maintained in RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) at 37 °C with 5% CO2. A stock solution of kaempferol (Sigma) and cisplatin (Sigma) were prepared in dimethyl sulfoxide (DMSO) at 100 mM and stored at -20 °C. Different concentrations of kaempferol and cisplatin were prepared in a RPMI 1640 medium with FBS for cell treatments, and DMSO was included in the preparations to ensure equal concentrations of DMSO in each treatment.
2.2. Cell proliferation assay
As kaempferol has a yellow colour, a colour gradient appeared in serial dilutions of kaempferol in the RPMI 1640 medium, which interferes with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS)-based colour absorbance assays (unpublished data). Repeated washing of cells with PBS, effectively removed colourful treatments, but caused an appreciable and an uneven loss of cells (unpublished data). For this reason, genomic DNA abundance was measured to estimate cell numbers after each treatment. Ovarian cancer cells were seeded in 96-well plates at 2000 cells/well and incubated overnight before treatment with 0-160 μM kaempferol for 24 hours in triplicates. The medium was removed, and the plates were freeze-thawed to lyse cells. Each well was added with 200 μl 1x CyQUANT cell lysis buffer (Invitrogen) containing 5x SYBR Green I (Invitrogen) and incubated at room temperature (RT) for 5 minutes. The reaction (50 μl) was transferred to PCR strip tubes and the fluorescent signal was measured at 90 °C with a real-time Chromo4™ PCR instrument (Bio-Rad, Hercules, CA). To ensure that cell proliferation assays were performed within a linear range of cell numbers, a standard curve was generated by seeding different amount of OVCAR-3 cells (based on counting with a hemacytometer) in a 96-well plate, and measuring genomic DNA abundance after overnight incubation. Three independent experiments were performed and data was pooled for statistical analysis.
2.3. Cytotoxicity assay
Ovarian cancer cells were seeded in 96-well plate at 5000 cells/well, incubated overnight, and treated in triplicates with 100 μl kaempferol for 24 hours. Culture medium (20 μl) was sampled to measure free lactate dehydrogenase (LDH) levels. Lysis Solution (10x) was then added into cells and incubated at 37 °C for 1 hour to release all LDH into culture medium, which is sampled again (20 μl) to measure total LDH levels. A CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI) was deployed to measure the free and total LDH levels from each well, and non-cell wells were measured as a medium for background control. LDH levels contained in intact cells were derived by subtracting the free LDH levels from total LDH levels, and normalized to the total LDH of the control samples. Two to three independent experiments were carried out and the results pooled for statistical analysis.
2.4. Apoptosis assay
To time apoptosis in ovarian cancer cells, A2780/CP70 Ovarian cancer cells were seeded in 96-well plates at 10,000 cells/well, incubated overnight, and treated in triplicates with 0- or 80-μM kaempferol for 0, 2, 4, and 8 hours. No-cell wells were included for background correction. At each time point, a plate of the culture medium was removed and frozen in -80 °C until final analysis. Cells were thawed, lyzed with Passive Lysis Buffer (Promega), and analyzed for caspase 3/7 activities with a Caspase-Glo 3/7 Assay (Promega) and the total protein levels with a BCA assay (Pierce) as per the instructions. Caspase 3/7 activities were normalized by total protein levels, and the levels of kaempferol-treated cells were expressed as percentages of controls for statistics. To analyze kaempferol-induced apoptosis in ovarian cells, all 3 lots of ovarian cancer cells and 1 lot of immortalized normal cells were seeded in 96-well plates, incubated overnight, and treated with various concentrations of kaempferol for 2 hours. A positive control, cisplatin, was also included in the cancer cell treatments for confirmation and comparison. Caspase 3/7 activities and total protein levels were analyzed as described above.
2.5. Caspase-9 inhibition experiment
OVCAR-3 cells were seeded in 96-well plates at 10,000 cells/well and incubated overnight. Cells were treated with a caspase-9 inhibitor (Z-LEHD-FMK) (0-10 μM) or 10 μM negative control (Z-FA-FMK) (Biovision, Mountain View, CA) in triplicates for 24 hours, and treated with 0 or 80 μM kaempferol for 2 hours. Caspase 3/7 activities were measured with a Caspase-Glo 3/7 Assay (Promega) and normalized by cell numbers, which were measured with a CellTiter 96 Aqueous One Solution Cell proliferation Assay (Promega) following the manufacturer’s instructions. Data from two independent experiments were pooled for statistical analysis.
2.6. Western Blot
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with 0-80 μM kaempferol for 24 hours. The cells were harvested with M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, IL) supplemented with Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (Pierce) as per the instructions, subject to SDS-PAGE, transferred to nitrocellulose membranes, blocked with 5% non-fat milk in TBST, and probed with different primary antibodies targeting human p53, Bad, Bax, or Bcl-xL (Santa Cruz, Santa Cruz, CA). Membranes were incubated with Goat-anti-Mouse-Poly-HRP (Pierce) and visualized with x-ray film exposure. Membranes were further stripped with Restore PLUS Western Blot Stripping Buffer (Pierce) and re-probed with a GAPDH antibody (Santa Cruz) to normalize target protein abundances. Film images were quantitated with NIH “ImageJ” software and 2-3 independent experiments were pooled for statistical analysis.
2.7. Statistical analysis
All replicates within an experiment were averaged and the mean values from different experiments were pooled for statistical analysis. One-way ANOVA followed by a Dunnett’s test or t-test was performed, as appropriate, to determine the differences between groups using SPSS software and a p-value of less than 0.05 is considered to be significant.
3. Results and discussion
3.1. Kaempferol inhibits proliferation of ovarian cancer cells at 40-μM or higher concentrations
After a 24-hour treatment, kaempferol caused a significant and concentration-dependent inhibition of proliferation in all 3 ovarian cancer cells tested (Figure 1A). This inhibition was observed at 40-μM or higher concentrations of treatment. In validation of measuring the genomic DNA abundance at 90 °C as a substitute for cell numbers, a standard curve was generated by seeding a different number of cells (based on counting on hemocytometer) for this assay. Linearity is maintained over a range of 0-20,000 cells/well seeded (Figure 1B), and our proliferation assay stayed within the linear range only 2,000 cells/well were seeded for kaempferol treatment. Measuring genomic DNA abundance at 90 °C with SYBR Green I chemistry avoids possible interference by kaempferol’s colour in MTT- or MTS-based cell proliferation assays, yet can be an overestimation because DNA dead cells will add to the signal in this assay. However, our microscopic observation reveals that no death of ovarian cancer cells is noticeable at concentrations of 20-μM kaempferol or lower. Furthermore, if death of a small number of cells occurs, the dead cells should have detached from the culture plate and removed with the culture medium. As a natural dietary component, kaempferol is not expected to exhibit strong inhibition of ovarian cancer cell proliferation under physiologically relevant concentrations, and 10- to 20-μM kaempferol in the blood is reachable in humans under a plant-rich diet (Gates et al., 2007). In agreement with this observation, our data suggests that kaempferol does not directly inhibit proliferation of ovarian cancer cells at physiologically relevant concentrations.
Figure 1. Kaempferol inhibits proliferation of ovarian cancer cells at 40-uM or higher concentrations.
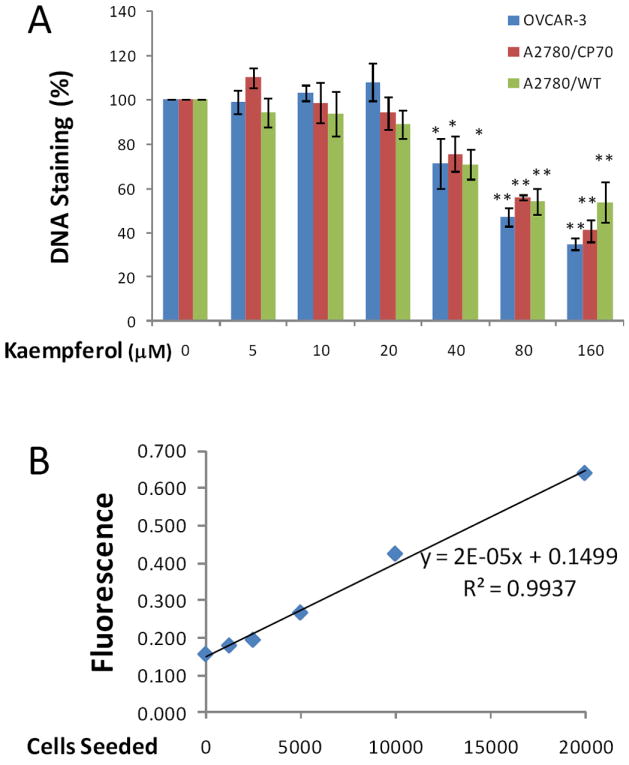
A. OVCAR-3, A2780/wt, and A2780/CP70 cells were seeded into 96-well plates at 2000 cells/well and incubated overnight before treatment with various concentration of kaempferol in triplicate for 24 hours. After removing medium, the plate was frozen at -20°C for 30 minutes, thawed at room temperature, and cells were lysed with 200 μl 1x CyQUANT cell lysis buffer containing 5X SYBR Green I dye. After 5 minutes incubation at room temperature, cell lysates (50 μl) were transferred to PCR strip tubes and the fluorescence were measured at 90 °C in a real-time PCR instrument. Data represent Means ± SE from 3 independent experiments. * p<0.05, **p<0.01 as compared to control. B. A standard curve was generated by seeding different numbers of OVCAR-3 cells in 96-well plate, incubating them overnight, and measuring DNA abundance as described above in A.
3.2. Kaempferol causes no cytotoxicity or necrosis in ovarian cancer cells
Although our DNA-based cell proliferation assay showed that kaempferol has no inhibition of proliferation with ovarian cancer cells at 20-μM or lower concentrations, there is a possibility of overestimation of cell proliferation because dead cells, if any, might add to the signal in the DNA measurement. To further confirm our results from the cell proliferation assays and investigate kaempferol’s cytotoxicity on ovarian cancer cells, we performed LDH-based cytotoxicity assays on ovarian cancer cells and defined cell necrosis here by rupture of the cell membrane and release of LDH into the culture medium. Treatment with kaempferol for 24 hours caused no more release of LDH into the culture medium (free LDH), especially at 20-μM and lower concentrations, in all 3 ovarian cancer cells (Figure 2). The relatively high level (15-16%) of cell rupture and necrosis in controls is possibly caused by natural death or DMSO treatment. These data confirmed that kaempferol treatment does not lead to further cell necrosis and that the DNA-based cell proliferation assay is not an overestimation in our experiments.
Figure 2. Kaempferol has no cytotoxicity on ovarian cancer cells.
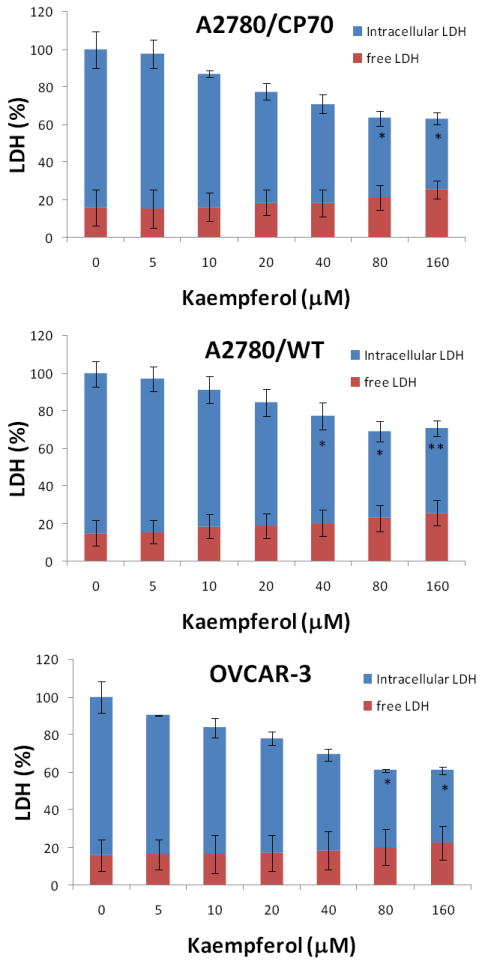
Ovarian cancer cells were seeded in 96-well plate at 5000 cells/well and incubated overnight before treatment with kaempferol for 24 hours. Culture medium was sampled for measurement of free LDH levels. Cell culture were then added with Lysis Solution and incubated at 37°C for 1 hour before being sampled again to measure total LDH levels. LDH levels were analyzed with a CytoTox 96 Non-Radioactive Cytotoxicity Assay from Promega. None-cell culture medium was assayed to the control background, and contained LDH levels were derived by subtracting free LDH levels from total LDH levels. All LDH levels within a single experiment were standardized to the total LDH level of control for statistical analysis. Data represent Mean ± SE from 2 or 3 independent experiments.
It is interesting to notice that although free LDH is not different among kaempferol treatments, the intracellular LDH levels (representing live cells) showed concentration-dependent decreases starting from 5-μM of kaempferol, where the differences became more prominent and significant at concentrations of 40-μM or higher (Figure 2). This significant decrease in intracellular LDH levels agrees well with our DNA-based cell proliferation assay. However, a disparity arises with the 5-20 μM kaempferol treatments where the DNA-based cell proliferation assay showed no difference or trend at all (Figure 1A), but a concentration-dependent decrease and steady trend appeared in the LDH-based assays. The constant genomic DNA levels coupled with reduced intracellular enzyme (LDH) levels in ovarian cancer cells suggest that kaempferol causes apoptosis, where cellular DNA (even fragmented) contributes to assay signals but cellular metabolism and production of LDH is compromised.
3.3. Kaempferol induces apoptosis in ovarian cancer cells
To test our hypothesis that kaempferol induces apoptosis in ovarian cancer cells, we analyzed the key apoptosis execution enzymes, caspase 3 and/or caspase 7, in all 3 ovarian cancer cell lines with kaempferol treatment. We first timed caspase 3/7 activity in A2780/CP70 cells induced by kaempferol treatment and the time course suggests that a 2-hour treatment would provide the best response in caspase 3/7 activities (Figure 3). Expanded experiments found that activities of caspase 3/7 increased in all 3 ovarian cancer cell lines after kaempferol treatment for 2 hours (Figure 4). Significantly raised levels of caspase 3/7 were observed at 80-μM kaempferol for all 3 cancer cell lines, and a concentration-response was observed from 0- to 80-μM kaempferol in all 3 ovarian cancer cells. A 160-μM kaempferol treatment also induced significant elevations in caspase 3/7 levels, however this high concentration is not any better than 40- or 80-μM kaempferol treatments and is of much less physiological relevance. Cisplatin is a commonly used chemotherapy agent and is known to induce apoptosis in ovarian cancer cells (Asselin et al., 2001). Inclusion of cisplatin in our experiments confirmed the positive effects of kaempferol treatment and they demonstrated comparable effectiveness in ovarian cancer cells in terms of raising caspase 3/7 activities. To learn whether kaempferol can distinguish between cancerous cells and normal cells, we also tested kaempferol in an immortalized normal ovarian epithelial cell line IOSE 364 (Figure 5). Unfortunately kaempferol does not discriminate those normal/cancerous cells in a perfect manner and high concentrations of kaempferol treatment (160 μM) also significantly induced caspase 3/7 activities in IOSE 364 cells. However, at more physiologically relevant concentrations (40- or 80-μM), kaempferol’s effect in inducing caspase 3/7 activities is less potent and non-significant in IOSE 364 cells. It is also worth noting that IOSE 364 cells, although originated from normal ovarian epithelial cells, have been transformed to a cell line by immortalizing with SV40 T/t. This process itself confers certain cancerous attributes to these cells, and thus far, results from these normal ovarian cells are at best a reference, rather than any conclusions. Overall, these data support our hypothesis about kaempferol-induced apoptosis in ovarian cancer cells and helps explain the disparity raised above. It is also interesting to notice that the increase in caspase 3/7 is relatively small, mostly less than 200% of the control in ovarian cancer cells. This is within our anticipated range, because a strong effect is normally not expected from a natural dietary component. However, this apoptotic effect, although small in size, is affirmative, quick, and only requires a low concentration that is only slightly above the amount normally physiologically available. These characteristics make kaempferol an appropriate agent for chemoprevention of ovarian cancers.
Figure 3. Time course of kaempferol-induced apoptosis in A2780/CP70 ovarian cancer cells.
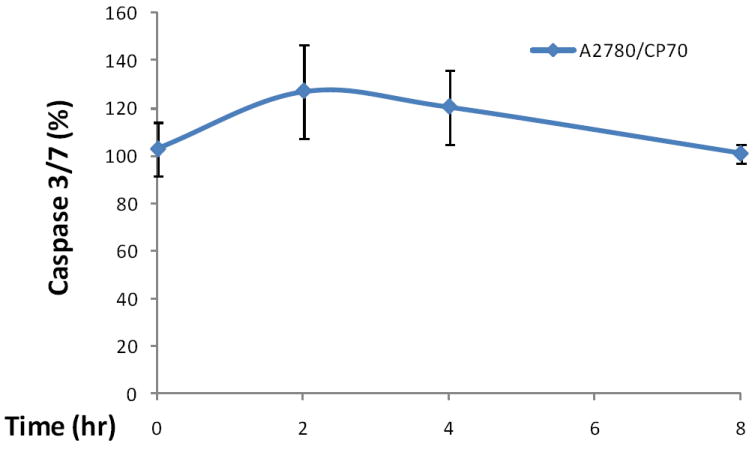
A2780/CP70 ovarian cancer cells were seeded in 96-well plates at 10,000 cells/well and incubated overnight. The cells were treated with 0- or 80-μM kaempferol in triplicates for 0, 2, 4, or 8 hours. No-cell wells were included for background correction. At each time point, a plate was removed of culture medium and frozen in -80 °C until final analysis. Cells were thawed, lyzed with Passive Lysis Bufer, and analyzed for caspase 3/7 activities with a Caspase-Glo 3/7 Assay and total protein levels with a BCA assay. Caspase 3/7 activities were normalized by total protein levels, and the levels of kaempferol-treated cells were expressed as percentages of the controls for statistics. Data represent Means ± SE from 2 independent experiments.
Figure 4. Kaempferol induces apoptosis in ovarian cancer cells.
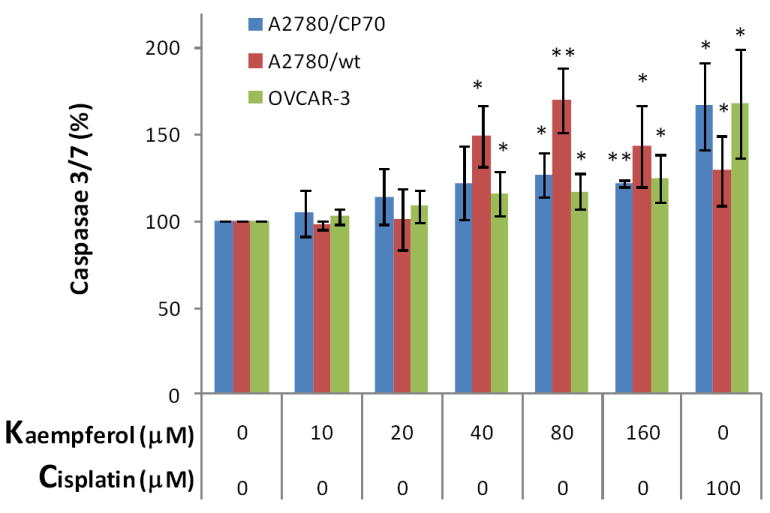
Ovarian cancer cells were seeded in two 96-well plates at 10,000 cells/well and incubated overnight before treatment with kaempferol in triplicate for 2 hours. Plates were removed of culture medium and frozen at -80 °C. For apoptosis analysis, cells were thawed and lyzed with Passive Lysis Buffer, and measured for caspase 3/7 activities and total protein levels. No-cell wells were included for background correction. Caspase 3/7 activities were adjusted by total protein levels and expressed as percentages of the controls. Data represent Means ± SE from 6 or 7 independent experiments. *p<0.05, **p<0.01 as compared to control.
Figure 5. Kaempferol induces apoptosis in immortalized normal ovarian cells only at high concentration.
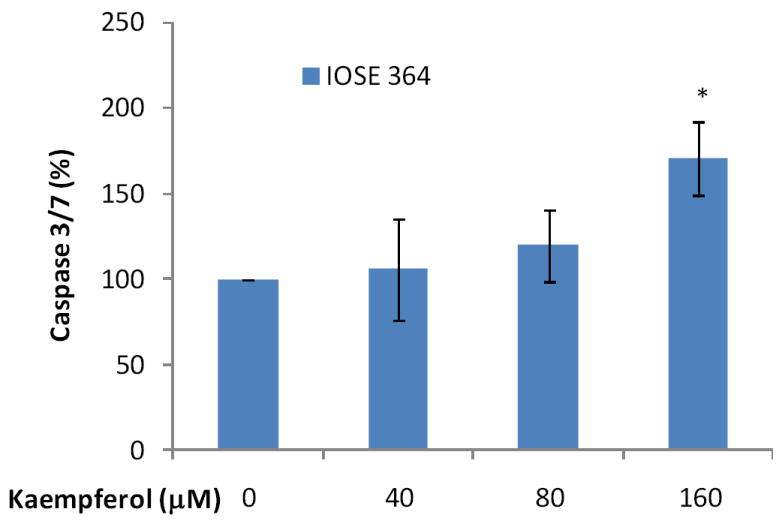
Immotalized normal ovarian epithelial cell line IOSE 364 were seeded in 96-well plates at 10,000 cells/well and incubated overnight. The cells were treated with various concentrations of kaempferol in triplicates for 2 hours and no-cell wells were included for background correction. Plates were then removed of culture medium and frozen at -80 °C. For apoptosis analysis, cells were thawed and lyzed with Passive Lysis Buffer, and measured for caspase 3/7 activities and total protein levels. Caspase 3/7 activities were normalized by total protein levels and expressed as percentages of controls. Data represent Means ± SE from 3 independent experiments. *p<0.05 as compared to control.
3.4. Kaempferol induces apoptosis through an intrinsic pathway in ovarian cancer cells
Recent studies demonstrated that kaempferol induces apoptosis through an intrinsic pathway in various human cells (Nguyen et al., 2003; Sharma et al., 2007; Huang et al., 2010), so this pathway was also investigated in our study. Caspase 9 is unique to the intrinsic pathway in apoptosis, and pre-treatment with a caspase-9 inhibitor significantly diminished kaempferol-induced apoptosis in OVCAR-3 cells (Figure 6), suggesting that kaempferol induces apoptosis through an intrinsic pathway in ovarian cancer cells.
Figure 6. Kaempferol induces apoptosis through intrinsic pathway in OVCAR-3 ovarian cancer cells.
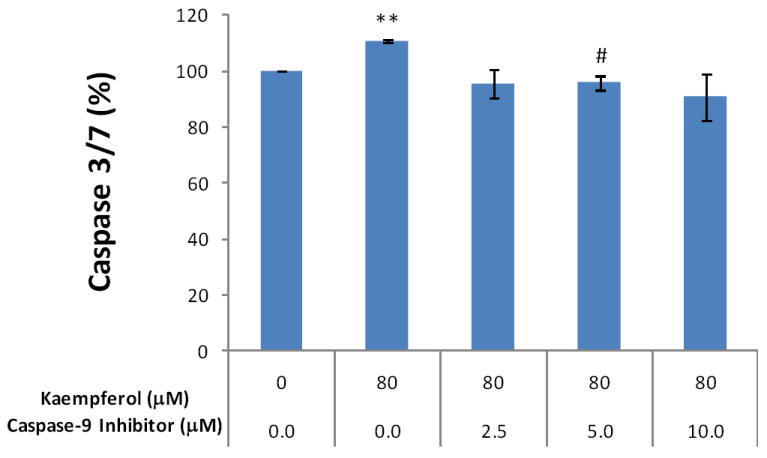
OVCAR-3 cells were seeded at 10,000 cells/well in 96-well plates and incubated overnight. The cells were pre-treated with various concentrations of a caspase-9 inhibitor (Z-LEHDFMK) or negative control (Z-FA-FMK) in triplicate for 24 hours, and treated with 0 or 80 μM of kaempferol for 2 hours. For the caspase 3/7 assay, 100 μl freshly prepared reagent was added to each well, incubated for 1 hour at RT, and 60 μl of the reaction mixture was transferred to glass tubes to measure luminescence. For cell number assay, 100 μl of freshly prepared AqueousOne reagent was added, incubated for 1 hour at RT, and measured at OD 560 with a microplate reader. Non-cell wells were included to measure background values for both assays. Caspase 3/7 values were adjusted by cell number values. Data represent Means ± SE from 2 independent experiments. **p<0.01 as compared to control. #p<0.05 as compared to the kaempferol treated control.
3.5. Kaempferol modulates expression of pro-apoptotic and anti-apoptotic genes toward apoptosis in ovarian cancer cells
Protein expression of three pro-apoptotic genes and one anti-apoptotic gene in the intrinsic pathway was examined by western blots in ovarian cancer cells treated with kaempferol. As seen in Figure 7-9, 24-hour kaempferol treatment concentration-dependently and significantly induced protein levels of p53, Bad, and Bax, in all 3 ovarian cancer cell lines. Meanwhile, the anti-apoptotic gene, Bcl-xL, was down-regulated by kaempferol treatment (Figure 10). Clearly, the balance between pro-apoptosis and anti-apoptosis genes in ovarian cancer cells was modulated by kaempferol treatment, and the new profile of gene expressions within the intrinsic pathway favors apoptosis in ovarian cancer cells. In addition to confirming kaempferol’s effect in apoptosis induction, these data further revealed the underlying mechanism for kaempferol-induced apoptosis in ovarian cancer cells, making it a promising chemoprevention agent for ovarian cancers.
Figure 7. Kaempferol increases “p53” protein levels in ovarian cancer cells.
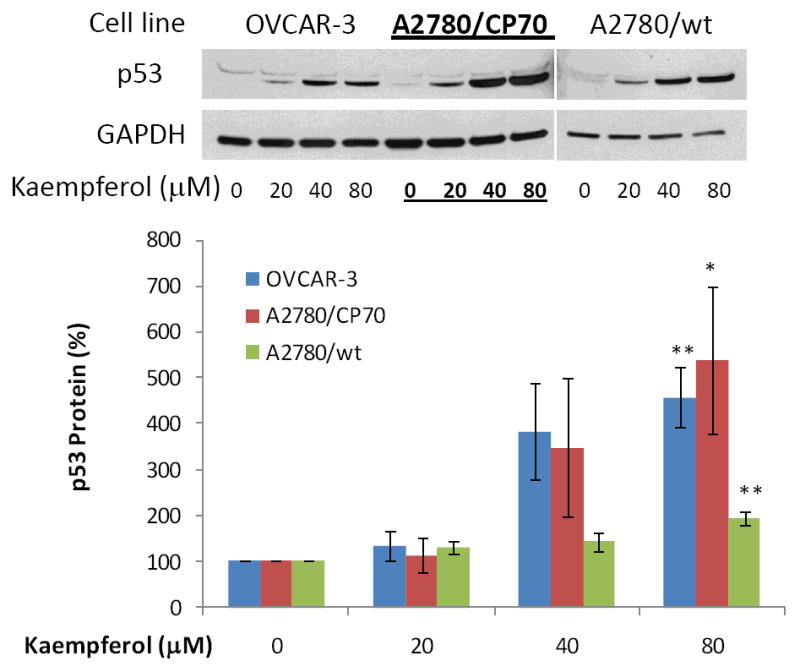
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “p53” protein in western blots. Images shown are typical western blot bands for “p53” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “p53” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
Figure 9. Kaempferol increases “Bax” protein levels in ovarian cancer cells.
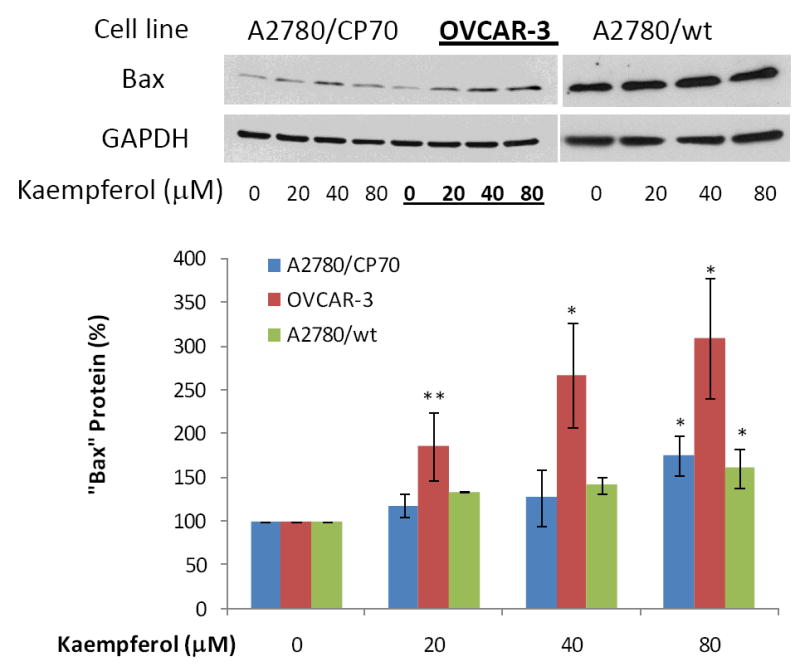
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “Bax” protein in western blots. Images shown are typical western blot bands for “Bax” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “Bax” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 2-3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
Figure 10. Kaempferol decreases “Bcl-xL” protein levels in ovarian cancer cells.
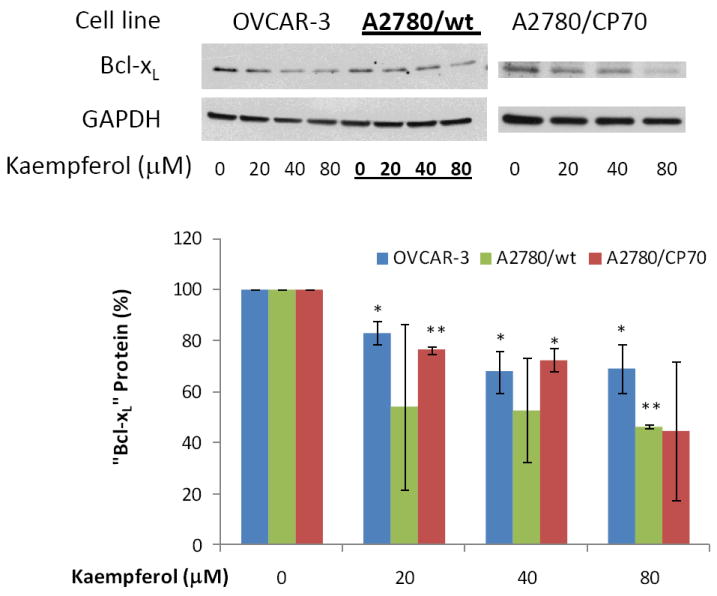
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “Bcl-xL” protein in western blots. Images shown are typical western blot bands for “BcL-xL” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “Bcl-xL” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 2-3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
4. Conclusion
The rate of ovarian cancer deaths in the United States has not seen any changes since 1960 (Jemal et al., 2010), challenging ovarian cancer treatment from primary prevention to late-stage cancer treatment. The chemoprevention of ovarian cancer has to be applied to the whole female population, with a agent that is relatively nontoxic, effective, inexpensive, readily available and acceptable, because women at higher risk of ovarian cancer cannot be identified due to a lack of known carcinogens (Banks, 2000) and appropriate biomarkers (Skates et al., 2000). Kaempferol, a dietary flavonoid, has shown promise in meeting these requirements, though further experimentation is necessary for this purpose.
Our early experiments found that kaempferol inhibits Akt phosphorylation in ovarian cancer cells (Luo et al., 2009), which in turn regulates expression of p53, Bad, Bax, and Bcl-xL genes that are studied in this report (Wang et al., 2007; Majewski at al., 2004; Flacke et al., 2009). A mechanism/pathway for kaempferol-induced apoptosis in ovarian cancer cells is proposed here (Figure 11) and our study demonstrates that, in addition to its angioprevention effects (Luo et al., 2009), kaempferol also directly, but mildly, induces apoptosis in ovarian cancer cells. These studies could possibly provide a plausible mechanism to explain earlier epidemiological findings that kaempferol reduces the risk of ovarian cancers in American registered nurses (Gates et al., 2007). Overall, these data suggest that a diet rich in fruits and vegetables will have continuous “kaempferol treatment” on early ovarian cancer cells possibly hidden in a woman, and could prevent/inhibit its development through constant angioprevention and apoptosis induction mechanisms.
Figure 11. Proposed mechanism/pathways for kaempferol-induced apoptosis in ovarian cancer cells.
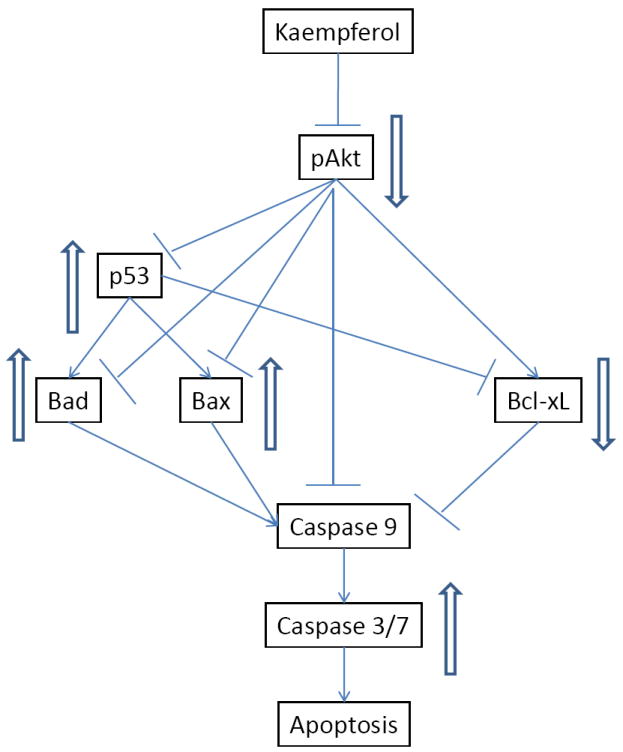
Kaempferol treatment in ovarian cancer cells inhibits Akt phosphorylation, which in turn inhibits expression of Bcl-xL gene but up-regulates expression of p53, Bad, and Bax genes, leading to an imbalance between pro-apoptosis and anti-apoptotic factors and in favour of activated Caspase 3/7 and intrinsic apoptosis.
We conclude from this and earlier studies that kaempferol represents a class of natural, dietary components that may be appropriate for chemoprevention of ovarian cancers, through various mechanisms, for every woman. Kaempferol should be further examined in animal models and clinical trials toward a possible application in humans.
Figure 8. Kaempferol increases “Bad” protein levels in ovarian cancer cells.
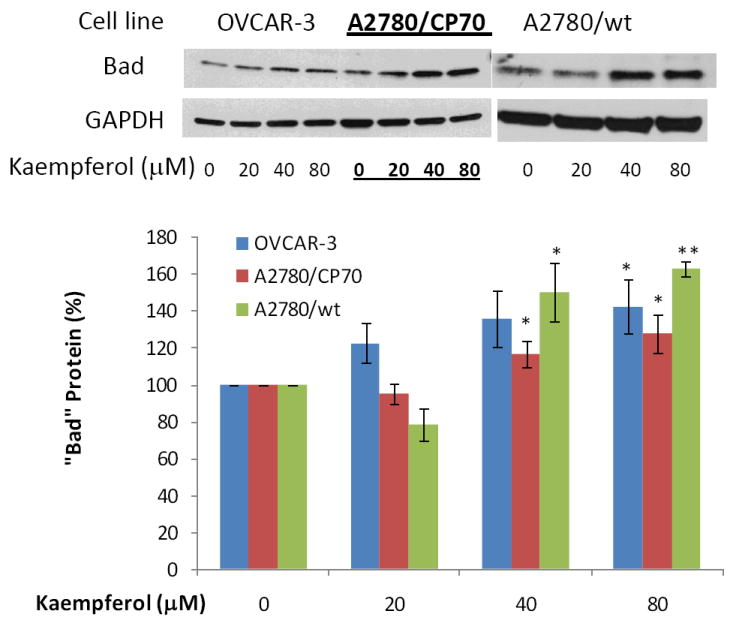
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “Bad” protein in western blots. Images shown are typical western blot bands for “Bad” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “Bad” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
Acknowledgments
We thank Dr. Jiang at the West Virginia University, Dr. Tew at the Medical University of South Carolina, and Dr. Auersperg at University of British Columbia, Canada for providing ovarian cancer or normal cell lines in this study. We also thank Shannon Straley and Whitney Reger for their participation. This research was supported by NIH grant P20 RR016477 from the National Center for Research Resources awarded to the West Virginia IDeA Network of Biomedical Research Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asselin E, Mills GB, Tsang BK. XIAP Regulates Akt Activity and Caspase-3-dependent Cleavage during Cisplatin-induced Apoptosis in Human Ovarian Epithelial Cancer Cells. Cancer Research. 2001;61:1862–1868. [PubMed] [Google Scholar]
- Banks E. The Epidemiology of Ovarian Cancer. In: Bartlett JMS, editor. Ovarian Cancer Methods and Protocols. Totowa: Humana Press; 2000. pp. 3–11. [Google Scholar]
- Flacke JP, Kumar S, Kostin S, Reusch HP, Ladilov Y. Acidic Preconditioning Protects Endothelial Cells against Apoptosis through p38- and Akt-dependent Bcl-xL Overexpression. Apoptosis. 2009;14:90–96. doi: 10.1007/s10495-008-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Tworoger SS, Hecht JL, Vivo ID, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. International Journal of Cancer. 2007;121(10):2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF, Li KH, Chen PY, Chung JG, Yang JS. Kaempferol Induced Apoptosis via Endoplasmic Reticulum Stress and Mitochondria-dependent Pathway in Human Osteosarcoma U-2 OS Cells. Mol Nutr Food Res. 2010;54:1–11. doi: 10.1002/mnfr.201000005. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:000–000. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Luo H, Jiang BH, King SM, Chen YC. Inhibition of Cell Growth and VEGF Expression in Ovarian Cancer Cells by Flavonoids. Nutrition and Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- Luo H, Rankin GO, Liu L, Daddysman MK, Jiang BH, Chen YC. Kaempferol Inhibits Angiogenesis and VEGF Expression through both HIF Dependent and Independent Pathways in Human Ovarian Cancer Cells. Nutrition and Cancer. 2009;61:554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Robey RB, Hay N. Akt Inhibits Apoptosis Downstream of BID Cleavage via a Glucose-Dependent Mechanism Involving Mitochondrial Hexokinases. Molecular and Cellular Biology. 2004;24(2):730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TTT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, Huynh H. Kaempferol-induced Growth Inhibition and Apoptosis in A549 Lung Cancer Cells is Mediated by Activation of MEK-MAPK. Journal of Cellular Physiology. 2003;197:110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- Ruiz E, Padilla E, Redondo S, Gordillo-Moscoso A, Tejerina T. Kaempfeorl Inhibits Apoptosis in Vascular Smooth Muscle Induced by a Component of Oxidized LDL. European Journal of Pharmacology. 2006;529:79–83. doi: 10.1016/j.ejphar.2005.10.061. [DOI] [PubMed] [Google Scholar]
- Sharma V, Joseph C, Ghosh S, Agarwal A, Mishra MK, Sen E. Kaempferol Induces Apoptosis in Glioblastoma Cells through Oxidative Stress. Mol Caner Ther. 2007;6:2544–2553. doi: 10.1158/1535-7163.MCT-06-0788. [DOI] [PubMed] [Google Scholar]
- Skates SJ, Jacobs IJ, Knapp RC. Tumor Markers in Screening for Ovarian Cancer. In: Bartlett JMS, editor. Ovarian Cancer Methods and Protocols. Totowa: Humana Press; 2000. pp. 61–73. [DOI] [PubMed] [Google Scholar]
- Wang L, Dutta SK, Kojima T, Xu X, Khosravi-Far R, Ekker SC, Mukhopadhyay D. Neuropilin-1 Modulates p53/Caspase Axis to Promote Endothelial Cell Survivial. PLoS ONE. 2007;2(11):e1161. doi: 10.1371/journal.pone.0001161. [DOI] [PMC free article] [PubMed] [Google Scholar]


