Abstract
Recent evidence suggests that age-related impairments in cognition may be mediated by a specific deficit in the ability to maintain goal-relevant information, a critical component of cognitive control dependent on the dorsolateral prefrontal cortex, although the underlying neural mechanism of these deficits remains unclear. To examine white matter hyperintensities as a neurobiological mechanism of these impairments, older individuals with severe white matter hyperintensity burden, older individuals with low white matter hyperintensity burden, and young adults were assessed in an event-related functional imaging scan while performing the ‘AX’-continuous performance task. Individuals with severe white matter hyperintensity burden showed a significant reduction in dorsolateral prefrontal cortex activity during the high cognitive control cue condition relative to the low white matter hyperintensity group and young individuals. Conversely, those with severe white matter hyperintensity burden showed greater activity in rostral anterior cingulate cortex compared to young individuals. These results are consistent with impaired cognitive control and a possible failure to deactivate default-mode regions in these subjects. Additionally, those with severe white matter hyperintensity burden showed reduced functional connectivity between dorsolateral prefrontal cortex and task-relevant brain regions including middle frontal gyrus, and supramarginal gyrus relative to young subjects and those with minimal white matter hyperintensity burden. These results suggest that age-related goal maintenance impairments and associated dorsolateral prefrontal cortex dysfunction may partly reflect incipient white matter disease of interconnected cognitive networks.
Keywords: ageing, functional connectivity, functional MRI, white matter hyperintensities
Introduction
A number of cognitive and biological changes occur in the brains of healthy older adults. Behavioural studies of older adults reveal striking cognitive decline in measures of cognitive control (Hasher and Zacks, 1988; Light, 1996). It has recently been suggested that specific impairments in goal maintenance may be the hallmark deficit underlying these age-related differences (Rush et al., 2006). Goal maintenance, the ability to actively maintain goals in order to accomplish a task, is involved in many cognitive tasks and is thought to involve a network of regions dependent on the dorsolateral prefrontal cortex (Braver et al., 2001). Impairment in this network is consistent with hypotheses that attribute differences with cognitive ageing to prefrontal cortex function (West, 1996; Raz et al., 1997), volumetric studies that indicate that prefrontal cortex volume is selectively decreased in older adults (Raz et al., 1997), and functional imaging studies that suggest age-related changes in the recruitment of prefrontal cortex resources (Reuter-Lorenz et al., 2000; Cabeza, 2002).
Though age-related prefrontal dysfunction has been widely reported, the underlying mechanisms of this impairment remain poorly understood (West, 1996; Raz et al., 1997). White matter hyperintensities increase in frequency with age and have been linked with a selective impairment of the frontal lobe structure and function across a wide range of studies including magnetic resonance spectroscopy, PET and diffusion tensor imaging (O'Sullivan et al., 2004; Tullberg et al., 2004; Schuff et al., 2011). In a recent functional MRI study, increasing white matter hyperintensity burden was associated with reduced prefrontal cortical activity during episodic memory retrieval and working memory (Nordahl et al., 2006). White matter hyperintensity burden was also associated with reduced activity in anterior cingulate cortex, a region functionally linked to the prefrontal cortex (Nordahl et al., 2006). The objective of this study was to extend this previous work by investigating cerebrovascular disease as represented by white matter hyperintensity as a potential mechanism of age-related cognitive control deficits and prefrontal cortex dysfunction, possibly through altered connectivity of task-relevant cortical processing.
In the present study, 18 older adults with severe white matter hyperintensity burden, 15 older adults with low white matter hyperintensity burden and 15 young adults were assessed using structural and functional MRI while performing the ‘AX’ continuous performance task, a task that allows comparison of multiple conditions with varying loads of cognitive control. Given previous findings of reduced prefrontal activity associated with white matter hyperintensity in normal elderly during working and episodic memory tasks (Nordahl et al., 2006), we hypothesized that individuals with severe white matter hyperintensity burden would have reduced dorsolateral prefrontal cortex activity and reduced dorsolateral prefrontal cortex correlated activity during high cognitive control conditions relative to older individuals with minimal white matter hyperintensity and young individuals.
Materials and methods
Participants
Eighteen normal older adults with severe white matter hyperintensity burden, 15 normal older adults with low white matter hyperintensity burden and 15 young adults were recruited for participation in the study. All subjects gave informed consent to participate in the study. Two older subjects with severe white matter hyperintensity burden were excluded due to functional interference on the functional imaging and the presence of a silent stroke, respectively. An additional older subject with minimal white matter hyperintensity burden was excluded due to task non-compliance resulting in a total of 16 subjects with severe white matter hyperintensity burden and 15 subjects with minimal white matter hyperintensity for study. Subject demographics are shown in Table 1.
Table 1.
Subject demographics
| Variable | Young | Low white matter hyperintensity | High white matter hyperintensity |
|---|---|---|---|
| n | 15 | 14 | 16 |
| Age | 24.1 + 3.1a,* | 75.3 + 4.6 | 77.4 + 5.5 |
| Education (years) | 16 + 2.0 | 15.9 + 2.1 | 14.3 + 2.6 |
| Gender (F/M) | 6/9 | 12/2 | 10/6 |
| MMSE | N/A | 29.2 + 0.8 | 28.6 + 1.1 |
| White matter hyperintensity volume (%TCV) | 0.06 + 0.02 | 0.15 + 0.07 | 2.1 + 1.3* |
| Category fluency | 27.1 + 5.9 | 21.4 + 6.5** | 15.6 + 3.2** |
| Digit span forward | 11.0 + 1.9 | 10.6 + 1.5 | 9.6 + 2.1 |
| Digit span backward | 7.9 + 2.1 | 7.1 + 2.3 | 5.7 + 2.1*** |
a Values displayed as mean + SD.
*Differs from all groups, P < 0.0001; **Differs from all groups, P < 0.02; ***Differs from young, P < 0.02. MMSE = Mini-Mental State Examination; TCV = total cranial volume.
N/A = young subjects did not receive MMSE testing.
Elderly participants were normal controls from a pool of individuals seen at the University of California Davis Alzheimer’s Disease Centre and recruited from the community through advertising or from spouses of patients seen at the Alzheimer’s Disease Centre. Participants received clinical neuropsychological testing at the University of California Davis Alzheimer’s Disease Centre. The testing was administered as part of a clinical work-up used to determine the clinical diagnosis of cognitively normal. The neuropsychological battery included Mini-Mental State Exam, Wechsler Memory Scale-Revised, Logical Memory I and II, Memory Assessment Scales List Learning, Boston Naming, Block Design, Digit Span, Animal Fluency and the Geriatric Depression Scale (Yesavage, 1988). All elderly participants received a clinical diagnosis of normal cognition through the Alzheimer’s Disease Centre based on neurological exams and extensive neuropsychological evaluations. The diagnosis of normal was adjudicated at a multidisciplinary case conference, based upon all available clinical information. All elderly subjects were aged 66–89 years and in stable health, and exclusion criteria were limited to diagnosis of dementia or mild cognitive impairment, major medical illness, history of cortical strokes, medications thought to affect cognition and white matter hyperintensity burden outside the range defined in the study. Severe white matter hyperintensity burden was defined as the highest 75th percentile of white matter hyperintensity burden in the normal subjects seen at the Alzheimer’s Disease Centre, and low white matter hyperintensity burden was defined as those in the lowest 25th percentile of normal subjects seen at the Alzheimer’s Disease Centre. Young subjects aged 19–29 years were recruited from the university by advertisement.
Cognitive tasks
Subjects performed the ‘AX’-continuous performance task in the scanner as this task previously demonstrated impairment in cognitive control for older individuals with severe white matter hyperintensity burden (A.B.V. Mayda et al. manusript under review). In this task, randomly chosen letters were presented sequentially on the computer screen. The cue letter appeared for 500 ms, followed by a 3500 ms delay. A probe letter was then shown for 500 ms duration. Subjects were instructed to make a target response (with a right index finger response) to the letter ‘X’ only when it followed the letter ‘A’. All other stimuli required a non-target response (with a right middle finger response). A 9500 ms inter-trial interval followed. The frequency of targets was high (70%), with the remaining 30% of trials divided among three distracter conditions: a ‘non-A’ followed by an ‘X’ (‘BX’), an ‘A’ followed by a ‘non-X’ (‘AY’) and a ‘non-A’ followed by a ‘non-X’ (‘BY’). The letters ‘K’ and ‘Y’ were excluded due to the similarity to the letter ‘X’. A total of 160 trial pairs were run.
Imaging acquisition
All magnetic resonance images were collected on a GE 1.5 T scanner. Functional MRI (T2weighted echo planar functional imaging: repetition time 2000 ms, echo time 40 ms, flip angle 90°, field of view 22 cm) were acquired prior to structural images. A fluid attenuated inversion recovery sequence (repetition time 11 000 ms, echo time 144 ms, field of view 25 cm, 48 slices, slice thickness 3 mm, matrix 256 × 192) and a coplanar T1-weighted sequence (repetition time 450 ms, echo time 20 ms, field of view 22, 24 slices, slice thickness 4 mm, matrix 256 × 256) were also obtained.
Quantification of white matter hyperintensity volume was performed using a custom-written computer program operating on a Unix, Solaris platform as previously described (DeCarli et al., 1992). In brief, all images had non-brain tissue removed and an image intensity non-uniformity correlation applied (DeCarli et al., 1996). Once brain matter segmentation was achieved, a segmentation threshold for white matter hyperintensity volume was determined a priori as 3.5 SDs in pixel intensity above the mean of the fitted distribution of brain parenchyma. Repeated estimates using inter-class correlations consistently find inter-class correlation values around 0.98 for brain segmentation (Decarli et al., 2005). Morphometric erosion of two exterior image pixels was also applied to the brain matter image before modelling to remove the effects of partial volume CSF pixels on white matter hyperintensity determination (DeCarli et al., 1992, 1996).
Image preprocessing
The first eight volumes of each run were discarded to allow the magnetic resonance signal to reach steady state. Preprocessing of functional images was implemented using SPM5 (http://www.fil.ion.ucl.ac.uk/spm) and included: (i) slice timing correction; (ii) realignment; (iii) coregistration; (iv) segmentation; (v) normalization to the Montreal Neurological Institute template and (vi) spatial smoothing with a Gaussian 8-mm full-width half-maximum kernel. One subject with severe white matter hyperintensity burden was excluded for >4 mm of within-run movement. All functional MRI analyses were performed on scan data collected from correct trials only. In addition, all analyses were modelled using the Canonical haemodynamic function standard to SPM. CueA, CueB, ProbeAX, ProbeBX, ProbeAY and ProbeBY (i.e. all possible cue and probe onsets) were statistically modelled for the analyses.
Statistical analysis
Dorsolateral prefrontal cortex
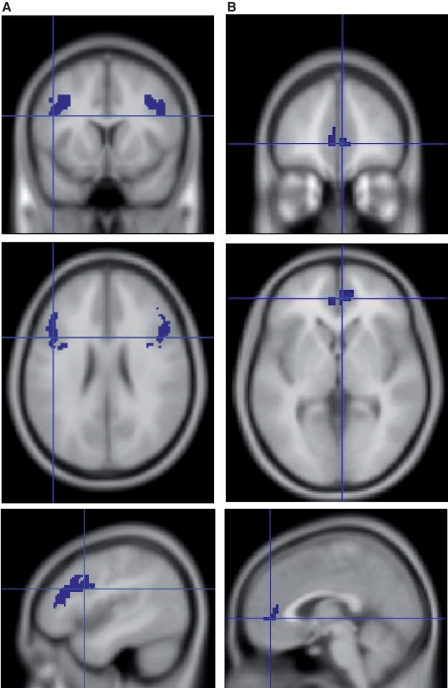
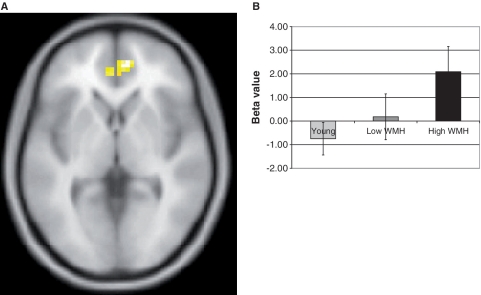
A region of interest was used for this study in which analyses were restricted to the dorsolateral prefrontal cortex given our a priori hypothesis. This was done by creating an anatomical dorsolateral prefrontal cortex mask using the Pick Atlas tool (Maldjian et al., 2003). From this mask, we identified clusters of five or more voxels that showed greater ‘B’ cue activity relative to ‘A’ cue activity in each group (P < 0.05, uncorrected). A mask of the resulting regions from each group were then combined using an AND function to form a single region of interest (Fig. 1A) to compare activity across groups.
Figure 1.
(A) Functionally derived dorsolateral prefrontal cortex and (B) rostral anterior cingulate regions of interest.
A second-stage analysis was performed to test for group differences. This analysis was conducted by averaging signal across all voxels in the functionally defined region of interest, and extracting contrast estimates in the ‘B’ cue versus ‘A’ cue condition for each subject. These data were then subjected to analysis of variance and Tukey’s honestly significant difference tests to test for group differences. An additional three subjects with severe white matter hyperintensity burden were excluded from this analysis due to the lack of a sufficient number of correct ‘B’ trials, defined as <50% correct trials.
Anterior cingulate cortex
A region of interest analysis was also conducted for the anterior cingulate cortex, given the recent evidence of the association between white matter hyperintensity and anterior cingulate activity (Nordahl et al., 2006) and studies indicating age-related increases in the region’s sensitivity to the presence of competing streams of information (Milham et al., 2002). The same procedure used in the dorsolateral prefrontal cortex analysis was used in the anterior cingulate analysis (Fig. 1B), again using the Pick Atlas tool (Maldjian et al., 2003) to identify regions of activation in anterior cingulated cortex.
Dorsolateral prefrontal cortex correlated activity
We measured functional connectivity of the dorsolateral prefrontal cortex using the beta series correlation method (Rissman et al., 2004; Yoon et al., 2008) implemented in SPM5 (http://www.fil.ion.ucl.ac.uk/spm) with additional scripts designed for this study. The standard SPM haemodynamic response function was used to convolve trial specific covariates modelled activity during the component stages of each individual trial in order to generate beta series. Voxel-wise bivariate Pearson’s correlations between a left dorsolateral prefrontal cortex seed region and the rest of the brain were computed for each task component. Given our a priori approach based on review of the prior literature, our initial analysis was based on locating an area of activation near to a previously described region in the dorsolateral prefrontal cortex related to cognitive control (MacDonald et al., 2000). The location of maximal group differences under the contrast analysis of ‘B’ cue versus ‘A’ cue conditions was used to identify the area for the seed region. A 5 mm sphere was placed at this location for the connectivity analysis.
In addition, we performed a second-confirmatory analysis using the MacDonald et al. (2000) region to replicate the initial findings. Between-group random effects analysis was then performed for group-averaged correlations. We identified clusters of five or more voxels that showed greater functional connectivity in the ‘B’ cue relative to the ‘A’ cue in each group (P ≤ 0.001, uncorrected). Signal was averaged across all voxels in significant areas, and contrast estimates were extracted in the ‘B’ cue > ‘A’ cue condition for each subject. These data were then subjected to analysis of variance and Tukey’s honestly significant difference tests to test for group differences. The three same subjects with severe white matter hyperintensity burden noted above were also excluded from this analysis due to the lack of a sufficient number of correct ‘B’ trials, defined as <50% correct trials.
Results
‘AX’-continuous performance task in-scanner performance
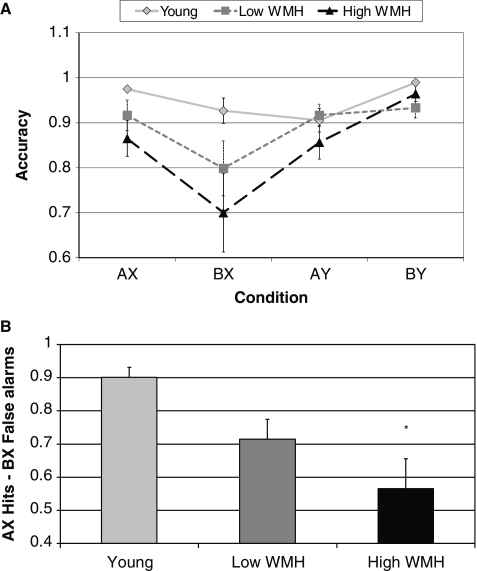
We observed significant between group differences in accuracy of the ‘AX’-continuous performance task, as shown in Fig. 2A. On the ‘AX’ and ‘BX’ conditions, older individuals with severe white matter hyperintensity burden performed significantly worse than young individuals (‘AX’: F = 3.27, P = 0.05, ‘BX’: F = 3.12, P = 0.05). Older individuals with minimal white matter hyperintensity burden did not differ from the young or the severe white matter hyperintensity burden group on either condition. No group differences were found on the ‘AY’ or ‘BY’ condition (‘AY’: F = 1.13, P = 0.33, ‘BY’: F = 2.90, P = 0.07). In another measure of performance, we analysed the accuracy of hits on the ‘AX’ trials minus the false alarms on the ‘BX’ trials. As shown in Fig. 2B, we found a significant group difference such that subjects with severe white matter hyperintensity burden performed worse than young subjects (F = 5.80, P = 0.006). Older individuals with minimal white matter hyperintensity burden did not differ from the high white matter hyperintensity or young subjects on this measure.
Figure 2.
(A) Accuracy for all conditions of the ‘AX’ continuous performance task; ‘AX’ and ‘BX’: older subjects with severe white matter hyperintensity (WMH) burden impaired relative to young group, P < 0.05. (B) Corrected recognition rate (‘AX’ hits − ‘BX’ false alarms) by group, differs from young, *P = 0.004. Low white matter hyperintensity denotes older subjects with minimal white matter hyperintensity, High white matter hyperintensity denotes older subjects with severe white matter hyperintensity.
We also observed significant group differences in reaction times such that reaction times on the ‘AX’, ‘BX’ and ‘AY’ conditions of the ‘AX’-continuous performance task were affected by age, but not white matter hyperintensity burden (‘AX’: F = 9.08, P = 0.0005; ‘BX’: F = 11.60, P < 0.0001; ‘AY’: F = 8.09, P = 0.001).
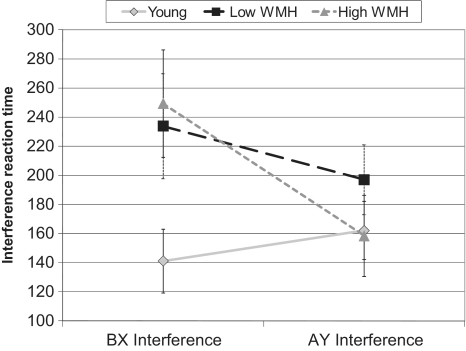
In addition to these analyses, two reaction time interference scores were calculated: ‘AY’ interference (mean ‘AY’ reaction time − mean ‘AX’ reaction time) and ‘BX’ interference (mean ‘BX’ reaction time − mean ‘AX’ reaction time). These measures show the influence of goal maintenance on reaction time in ‘AY’ and ‘BX’ trials relative to ‘AX’ trials. As shown in Fig. 3, in a multiple analysis of variance including the conditions of ‘BX’ and ‘AY’ interference and group, there was a significant group by condition interaction (F = 3.68, P = 0.03). The ‘BX’ interference measure differed by group (F = 3.31, P = 0.05). Older individuals with severe white matter hyperintensity burden had more ‘BX’ interference than the young group (P = 0.05). The groups did not differ in ‘AY’ interference.
Figure 3.
Reaction time to interference; ‘BX’ interference = ‘BX’ reaction time − ‘AX’ reaction time. Older individuals with severe white matter hyperintensity (WMH) burden differ from young subjects, P = 0.05; ‘AY’ interference = ‘AY’ reaction time − ‘AX’ reaction time.
Functional MRI of dorsolateral prefrontal cortex
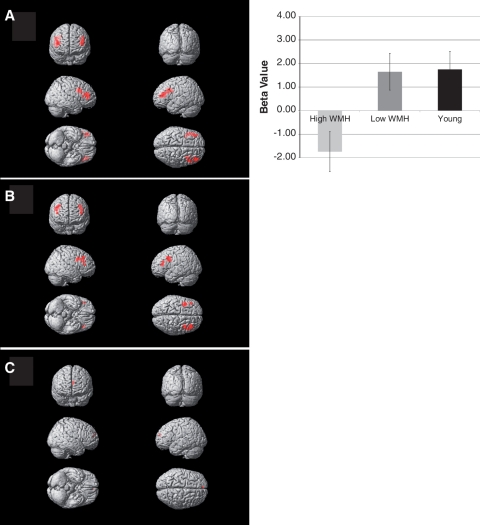
Group differences in activity within the functionally defined region of interest are shown in Fig. 4. There was a significant group difference of the mean contrast estimates in the dorsolateral prefrontal cortex region of interest during the ‘B’ cue condition relative to the ‘A’ cue condition (−1.73 ± 3.49, 1.65 ± 2.71, 1.75 ± 2.63 for severe white matter hyperintensity burden, minimal white matter hyperintensity burden and young subject groups, respectively; F = 5.8, P = 0.006). Post hoc analysis using Tukey–Kramer honest significant differences found that the severe white matter hyperintensity burden group had reduced activation relative to the minimal white matter hyperintensity burden and young individuals groups, which did not differ from one another. This finding remained essentially unchanged when examined only in the left hemisphere portion of the region of interest (F = 5.8, P = 0.006) or the right hemisphere portion of the region of interest (F = 4.9, P = 0.01).
Figure 4.
Dorsolateral prefrontal cortex region of interest analysis in (A) young subjects, (B) older subjects with minimal white matter hyperintensity and (C) older subjects with severe white matter hyperintensity. Chart shows group differences in mean beta values over dorsolateral prefrontal cortical region of interest (shown in Fig. 1) during ‘B’ cue relative to ‘A’ cue. Young and older subjects with minimal white matter hyperintensity had significant activation relative to older subjects with severe white matter hyperintensity (P < 0.05, post hoc t-test).
Functional MRI of anterior cingulate cortex
There was a significant group difference of the mean contrast estimates in the anterior cingulated cortex region of interest during the ‘B’ cue condition relative to the ‘A’ cue condition such that the severe white matter hyperintensity burden group had increased activation relative to the group of young subjects (F = 4.10, P = 0.02), and a trend towards greater activity than the minimal white matter hyperintensity burden group (P = 0.12). The location of the region within our functionally defined region of interest for the severe white matter hyperintensity burden group is shown in the slice in Fig. 5. The co-ordinates for the centre of mass of the region of interest were (x = 0, y = 42, z = 5). We note that this region is within the rostral subdivision of the anterior cingulated cortex. Group differences in beta values are shown in the inset. It is interesting to note that the betas in the healthy young group and the minimal white matter hyperintensity burden group are negative for this region, but are positive for the severe white matter hyperintensity burden group.
Figure 5.
(A) Anterior cingulate cortex region of interest activity in subjects with severe white matter hyperintensity burden. (B) Young subjects and older subjects with minimal white matter hyperintensity had significantly less activation relative to older subjects with severe white matter hyperintensity burden (P < 0.05, post hoc t-test).
Dorsolateral prefrontal cortex-correlated brain network
Previous studies of age-related differences in both cognitive control performance and activation (Braver et al., 2001; Braver and Barch, 2002) found significant age-related differences in performance as well as differences in activation of the left dorsolateral prefrontal cortex. Our a priori approach, therefore, sought to replicate these findings based on our functional data as well as to confirm the findings based on a region previously shown to be associated with the cognitive control network (MacDonald et al., 2000). Contrast analysis of ‘B’ cue versus ‘A’ cue comparing young individuals to subjects with severe white matter hyperintensity burden using small volume correction based on the dorsolateral prefrontal region of interest identified a region centred at −42, 12, 34 (P = 0.021, corrected at cluster level, T = 3.22, Z = 3.03, false discovery rate corrected = 0.007, voxel level) very near the MacDonald and colleagues published region [−41, 12, 24; (MacDonald et al., 2000)]. Results of correlation analyses are summarized in Table 2. In young subjects, the right angular gyrus, left supramarginal gyrus and left medial frontal gyrus exhibited enhanced functional connectivity with the dorsolateral prefrontal cortex in the ‘B’ cue condition relative to the ‘A’ cue condition. In older subjects with minimal white matter hyperintensity burden, the right and left middle frontal gyrus showed greater functional connectivity with the dorsolateral prefrontal cortex in the ‘B’ cue condition relative to the ‘A’ cue condition. Among subjects with severe white matter hyperintensity burden, there were no regions that showed greater functional connectivity within the dorsolateral prefrontal cortex in the ‘B’ cue condition greater than the ‘A’ cue condition. Contrast analysis comparing connectivity between the young and older individuals with severe white matter hyperintensity burden, using small volume correction, failed to identify any regions that remained significant after adjustment for multiple comparisons.
Table 2.
Dorsolateral prefrontal cortex functional connectivity analysis
| Group and brain region (Brodmann area) | MNI co-ordinates (x, y, z) | Analysis |
|
|---|---|---|---|
| T | Z | ||
| Young group | |||
| Right angular gyrus (39) | 30, −57, 24 | 6.12 | 4.20 |
| Left supramarginal (40) | −39, −48, 30 | 4.75 | 3.61 |
| Left middle frontal (46) | −39, 21, 21 | 4.29 | 3.37 |
| Low white matter hyperintensity | |||
| Right middle frontal (9) | 24, 33, 21 | 5.15 | 3.73 |
| Left middle frontal (8) | −39, 39, 51 | 3.90 | 3.12 |
These results were essentially confirmed by a repeat connectivity analysis using the MacDonald and colleagues (2000) published region (data not shown). Contrast analysis comparing connectivity between healthy young and older individuals with severe white matter hyperintensity burden using small volume correction also failed to identify any regions that remained significant after adjustment.
Discussion
Though it is well established that older adults exhibit age-related cognitive control decline as well as prefrontal cortex dysfunction, the underlying neural mechanisms are unknown. In this study, we show that white matter hyperintensities contribute not only to impairments in goal maintenance, but also to prefrontal cortex impairments manifested as reduced activity in dorsolateral prefrontal cortex and reduced connectivity of the dorsolateral prefrontal cortex with its anatomical targets. In addition, we found that white matter hyperintensities are associated with increased activity in the anterior cingulate. Although our a priori hypothesis sought to examine the anterior cingulated cortex region previously shown to be involved with monitoring for the presence of conditions that can lead to erroneous responses (Carter et al., 1998), our results identified a more rostral region known to be part of the default mode network (Greicius et al., 2003). As in our previous behavioural study, older individuals with severe white matter hyperintensity burden exhibited impaired goal maintenance with poor performance on the ‘BX’ trials, but spared performance on ‘AY’ trials relative to the young subjects (A.B.V. Mayda et al. manusript under review). Older subjects with low white matter hyperintensity burden did not differ from young subjects or older subjects with severe white matter hyperintensity burden. Though the older subjects with minimal white matter hyperintensity burden exhibited a level of performance intermediate to the young and severe white matter hyperintensity burden groups, they did not display impaired goal maintenance relative to the young comparison subjects. These behavioural results, coupled with white matter hyperintensity-associated impairments in prefrontal cortex activity and preliminary connectivity analyses, suggest that white matter hyperintensity may play a role in producing these impairments.
White matter hyperintensity and dorsolateral prefrontal cortex dysfunction
It has been suggested that the dorsolateral prefrontal cortex is important during task preparation and the implementation of control (MacDonald et al., 2000). Thus, in order to successfully complete the present task, activity in dorsolateral prefrontal cortex would be expected to increase in response to the high cognitive control ‘B’ cue in preparation for the upcoming task demands. Our results show that older subjects with severe white matter hyperintensity burden are unable to upregulate activity in dorsolateral prefrontal cortex during the high cognitive control ‘B’ cues in order to accomplish the task. Additionally, task relevant regions functionally connected to dorsolateral prefrontal cortex failed to engage.
Our results both confirm and contrast with results from prior studies of age-related differences in ‘AX’-continuous performance task and task performance (Braver et al., 2001, 2009; Braver and Barch, 2002; Paxton et al., 2008). These studies (Braver and Barch, 2002) show that older individuals are significantly less accurate on ‘BX’ trials, but perform superior to young on the ‘AY’ trails. Our performance data found similar results, although the overall high performance of the young could not detect a significant difference in the ‘AY’ condition. In contrast, our functional MRI study found no significant differences between young and older individuals with minimal white matter hyperintensity burden in task-related activation of the dorsolateral prefrontal cortex. While these results may reflect, in part, differences in study design [our delay was 3.5 s; intermediate to the short and long studied by Paxton et al. (2008)], this group comparison suggests that age alone may not fully explain differences in dorsolateral prefrontal cortex activation patterns with the ‘AX’-continuous performance task. Conversely, older individuals with severe white matter hyperintensity burden were unable to activate the dorsolateral prefrontal cortex during this task. While we dichotomized our groups to enhance power to detect differences, prior evidence shows that the influence of white matter hyperintensity on cognitive performance and cognitive activation is continuous (Nordahl et al., 2006). These results, therefore, suggest that various amounts of clinically silent cerebrovascular disease as manifest by white matter hyperintensity—common to advancing age—may explain at least some of the age-related differences in task activation patterns noted by Paxton and colleagues (2008).
White matter hyperintensity, age and cerebral connectivity
The cortical regions identified in the functional connectivity analysis have established functions that support task-relevant processing during the ‘AX’-continuous performance task (Paxton et al., 2008; Braver et al., 2009). Both young and older subjects with minimal white matter hyperintensity burden exhibited greater dorsolateral prefrontal functional connectivity in the ‘B’ cue relative to the ‘A’ cue. As noted by Paxton et al. (2008), ‘… the service of control is a commonly ascribed function of the PFC’ and our results support this assertion.
In young subjects, the left supramarginal and right angular gyrus similarly exhibited greater connectivity in ‘B’ cues relative to ‘A’ cues. Located in the inferior parietal lobule with extensive connections to prefrontal cortex (Miller and Asaad, 2002), these areas are important to multimodal sensory processing and may support task-relevant process during the ‘AX’-continuous performance task by facilitating sustained attention (Husain and Nachev, 2007). This parietal region has also been suggested to be an important component of the network of brain areas that mediate the short-term storage and retrieval of verbal material (Jonides et al., 1998; Honey et al., 2000). Conversely, the older group with minimal white matter hyperintensity burden showed greater connectivity in bilateral middle frontal gyrus. This interesting, although preliminary, difference from the young group suggests the possibility for age-related differences in cognitive processing streams where older individuals rely more heavily on dorsolateral prefrontal cortical systems, consistent with the HAROLD model (Cabeza, 2002), as discussed below.
We hypothesized that white matter hyperintensity would lead to prefrontal cortex dysfunction and the associated cognitive impairments by disrupting white matter tracts that connect prefrontal cortex to the rest of the brain (Nordahl et al., 2006), explaining at least some of previously identified age-related differences seen with this task (Braver and Barch, 2002). Relating our findings to previous functional MRI studies is difficult given that the extent of white matter hyperintensity in older study populations is generally not taken into account. Our results, however, may be interpreted in light of current literature. For example, Cabeza (2002) has reported that prefrontal activity during cognitive processes tends to be less lateralized in older adults than younger adults, and supported by functional neuroimaging evidence from domains of episodic memory, semantic memory, working memory, perception and inhibitory control. Age-related increase in neural recruitment, therefore, may play a compensatory role in the brain or reflect a difficulty in recruiting specialized neural systems, known as dedifferentiation or non-selective recruitment. Though these theories are not mutually exclusive, evidence of improved cognitive performance with increased bilateral involvement in older adults suggests that this is a compensatory mechanism. For example, Reuter-Lorenz and colleagues (2000) reported that older adults who display a pattern of bilateral activation were faster in verbal working memory tasks than those who did not display the pattern. Our results are potentially consistent with the compensatory account as evidence by the fact that older subjects with minimal white matter hyperintensity burden show improved performance compared to older subjects with severe white matter hyperintensity burden while showing both increased activity in dorsolateral prefrontal cortex and increased correlations within right and left middle frontal cortices. Conversely, older individuals with severe white matter hyperintensity burden performed significantly more poorly than the young group, showed no significant activation or correlations within frontal or parietal lobes in our preliminary connectivity analysis. Recent work by Braver and colleagues (2009), however, suggests an interesting alternative hypothesis. Older individuals may have reduced ‘flexibility’ in neural networks resulting in transition from a ‘proactive’ to ‘reactive’ response to situations demanding high level cognitive control. Both our behavioural and functional MRI results are compatible with this hypothesis in that older individuals with severe white matter hyperintensity burden were unable to modulate cognitive systems necessary for successful performance of the task. Interestingly, it is possible that older subjects with minimal white matter hyperintensity burden may have retained better neuromodulation. Unfortunately, the study was not designed to test the premise of the Braver et al. (2009) model and further studies will be necessary to investigate this most interesting hypothesis.
Independent of the exact underlying systems biology, these results strongly suggest that disruption of white matter tracts may impair compensatory recruitment and are consistent with our earlier work (Nordahl et al., 2006) as well as other preliminary data on age-related differences in white matter integrity (Andrews-Hanna et al., 2007) and connectivity. Unfortunately, our small sample size was insufficient for definite conclusions and therefore, further work will be necessary to confirm or refute these hypotheses.
White matter hyperintensity and anterior cingulate cortex
In this study, we also found that older individuals with severe white matter hyperintensity burden had significantly increased activation in rostral anterior cingulate cortex during high cognitive control conditions relative to the young group, with a trend towards increased activity over the minimal white matter hyperintensity burden group as well. In both the young and minimal white matter hyperintensity burden groups the betas were negative or near zero while in the severe white matter hyperintensity burden group they were positive.
The rostral anterior cingulate cortex is associated with the default mode network (Greicius et al., 2003). First described by Raichle and colleagues (2001), this network is believed to be the ‘default’ or ‘resting state’ network of the brain. In addition, deactivation or ‘anti-correlation’ of this network occurs commonly during task-related brain activation (Fox et al., 2005). Importantly, anti-correlations between default network activity and performance is diminished with advancing age (Grady et al., 2006). Moreover, age associated increases in anterior cingulate cortex activity during a verbal decision task were found in Brodmann area 32 near the location of the findings seen with our study (Sharp et al., 2006) and reduced anti-correlation is associated with diminished performance with increasing load on a verbal task in older individuals (Persson et al., 2007).
An interesting implication of the present result is that intact prefrontal cognitive control systems may be needed to mediate default mode network suppression, although the age-related differences in anterior cingulate cortex activity without age-related differences in dorsolateral prefrontal cortex activity for older individuals with minimal white matter hyperintensity burden suggest that other factors may also be involved. It is clear, however, that extensive white matter hyperintensity, as seen in the severe white matter hyperintensity burden group, is associated with both reduced task-related dorsolateral prefrontal cortex activation and increased rostral anterior cingulate cortex activity, suggesting the possibility that white matter hyperintensity may alter the relation between resting-state and task-related cognitive networks. This hypothesis is also consistent with diminished neuromodulation as suggested by Braver and colleagues (Braver and Barch, 2002; Braver et al., 2009). Additional studies will be needed to confirm this interesting hypothesis.
Behavioural performance and group differences in activation patterns
Group differences in task performance often confound biological inferences from studies of cognitive activation. It is important to note, however, that the reduced dorsolateral prefrontal cortex activity and altered connectivity in older individuals with severe white matter hyperintensity burden was not simply due to reduced accuracy on the ‘AX’-continuous performance task (and the associated lower number of correct ‘BX’ trials). For example, ‘AX’ performance for the older individuals with minimal white matter hyperintensity burden did not differ significantly from performance of older individuals with severe white matter hyperintensity burden. Yet, older individuals with minimal white matter hyperintensity exhibited activation of dorsolateral prefrontal cortex similar to the young group and associated functional connectivity during the high cognitive control cues. Also, imaging analyses included only correct trials; therefore, for the purpose of these investigations, subjects with severe white matter hyperintensity burden performed equally on task as the other two groups.
Limitations
One possible limitation to the present study is the inclusion of subjects with known vascular risk factors in a functional imaging study that uses the blood oxygen level dependant signal. However, the available evidence argues against vascular risk factors as the major contributor to the reduction in the blood oxygen level-dependent signal in dorsolateral prefrontal cortex. Prior studies have shown that the blood oxygen level-dependent signal is not reduced universally across the brain in subjects with severe white matter hyperintensity burden (Nordahl et al., 2006) and in the present study, we found increased activity in anterior cingulate cortex among subjects with severe white matter hyperintensity burden. Although we cannot say whether vascular risk factors affect different regions of the brain differentially, we can say that vascular risk factors do not contribute to global reductions in brain activity. The limited number of subjects is a second limitation to this study. While there was sufficient power to detect differences in behaviour and task-related activations, the power to detect significant associations in the connectivity analysis was low and these results should be viewed as only preliminary. In addition, given that our results are observed in a set of hypothesis driven region of interest analyses rather than a whole-brain analysis, there may be additional regions of the brain that show similar effects to those reported above. Finally, we acknowledge that the use of white matter hyperintensity as our stratification variable is likely to underestimate age-related differences in white matter integrity that may be better imaged using diffusion tensor imaging and could further explain age-related differences in performance and functional connectivity associated with this task. Further work with diffusion tensor imaging is clearly necessary.
Conclusion
The functional imaging results of this study support the disconnection hypothesis of ageing that suggests that injury to white matter tracts, as manifest by white matter hyperintensity, contributes to age-related alterations in prefrontal cortex function. Although inferential power is somewhat limited due to the small sample size, the functional connectivity results strengthen this argument by offering further evidence that white matter hyperintensities are associated with reduced correlations among nodes within brain networks activated during a cognitive control task. It is clear that cerebrovascular disease, as manifest by white matter hyperintensity, contributes to age-related changes in brain activation and connectivity, though not all age-related differences in our study were explained by white matter hyperintensity and other age-related effects in the brain are likely. A unique consequence of altered prefrontal function associated with severe white matter hyperintensity burden is the possibility that white matter hyperintensity may adversely affect the ability of the anterior cingulate to normally deactivate during evaluatory control or the modulation of task-related systems activation and default network deactivation. It is, therefore, important to consider the role of cerebrovascular disease in age-related cognitive performance, given that it may be one of the underlying mechanisms of these changes and risk factors for cerebrovascular disease are modifiable.
Funding
The National Institutes of Health (P30 AG10129, R01 AG021028 and R01 AG10220).
Acknowledgements
The authors would like to thank the subject volunteers and the staff at the UC Davis Alzheimer’s Disease Centre without whom this research would be impossible.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, et al. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. J Exp Psychol Gen. 2001;130:746–63. [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–17. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci USA. 2009;106:7351–6. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomo. 1992;16:274–84. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- Decarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996;6:519–28. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–41. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Honey GD, Bullmore ET, Sharma T. Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. Neuroimage. 2000;12:495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–6. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, et al. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–34. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light LL. Memory and aging. In: Bjork EL, Bjork RA, editors. Memory. 2nd edn. San Diego: Academic Press; 1996. pp. 443–90. [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, et al. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–96. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Miller EK, Asaad WF. The prefrontal cortex, conjunction and cognition. In: Grafman J, editor. Handbook of neuropsychology. St Louis: Elsevier; 2002. [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–29. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry. 2004;75:441–7. doi: 10.1136/jnnp.2003.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18:1010–28. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–32. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–63. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rush BK, Barch DM, Braver TS. Accounting for cognitive aging: context processing, inhibition or processing speed? Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. Neuroimage. 2011;54(Suppl 1):S62–8. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Mehta MA, Wise RJ. The neural correlates of declining performance with age: evidence for age-related changes in cognitive control. Cereb Cortex. 2006;16:1739–49. doi: 10.1093/cercor/bhj109. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–53. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–11. [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–14. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]