Abstract
Juvenile myoclonic epilepsy is the most frequent idiopathic generalized epilepsy syndrome. It is characterized by predominant myoclonic jerks of upper limbs, often provoked by cognitive activities, and typically responsive to treatment with sodium valproate. Neurophysiological, neuropsychological and imaging studies in juvenile myoclonic epilepsy have consistently pointed towards subtle abnormalities in the medial frontal lobes. Using functional magnetic resonance imaging with an executive frontal lobe paradigm, we investigated cortical activation patterns and interaction between cortical regions in 30 patients with juvenile myoclonic epilepsy and 26 healthy controls. With increasing cognitive demand, patients showed increasing coactivation of the primary motor cortex and supplementary motor area. This effect was stronger in patients still suffering from seizures, and was not seen in healthy controls. Patients with juvenile myoclonic epilepsy showed increased functional connectivity between the motor system and frontoparietal cognitive networks. Furthermore, we found impaired deactivation of the default mode network during cognitive tasks with persistent activation in medial frontal and central regions in patients. Coactivation in the motor cortex and supplementary motor area with increasing cognitive load and increased functional coupling between the motor system and cognitive networks provide an explanation how cognitive effort can cause myoclonic jerks in juvenile myoclonic epilepsy. The supplementary motor area represents the anatomical link between these two functional systems, and our findings may be the functional correlate of previously described structural abnormalities in the medial frontal lobe in juvenile myoclonic epilepsy.
Keywords: juvenile myoclonic epilepsy, functional MRI, connectivity, supplementary motor area
Introduction
Juvenile myoclonic epilepsy (JME) is the most frequent idiopathic generalized epilepsy syndrome, accounting for 5–10% of all epilepsies (Janz and Christian, 1957; Janz, 1985). The characteristic feature of JME is myoclonic jerks of the proximal upper extremities, particularly in the hour after waking. Patients with JME are particularly susceptible to seizure facilitation through sleep deprivation, alcohol consumption or photic stimulation. Furthermore, there are specific seizure triggers, not commonly observed in other epilepsy syndromes, such as cognitive activities like reading, calculation or decision making (da Silva Sousa et al., 2005a, b), and induction by praxis, i.e. the ideation or execution of complicated movements, including sequential spatial processing, such as drawing, writing, playing games or instruments (Inoue et al., 1994).
These findings are in line with electrophysiological studies on provocative effects of cognitive tasks on epileptiform discharges. In a large group of 480 patients with epilepsy, an increased spike frequency during neuropsychological activation was found in 38 patients, 22 of whom had JME (Matsuoka et al., 2005). A similar provocative effect was found in 38% of patients with JME (Guaranha et al., 2009). Both studies reported that the combination of a cognitive task with a manual motor response was a stronger precipitant of EEG discharges than a purely cognitive task.
The predominance of myoclonic jerks in JME has led to the motor circuitry hyperexcitability hypothesis, which has been addressed by transcranial magnetic stimulation. Using paired pulse paradigms these studies have documented increased cortical excitability in the motor cortex of patients with JME (Manganotti et al., 2004); accentuated after sleep deprivation (Badawy et al., 2006) and more pronounced in the morning than in the afternoon (Badawy et al., 2009). The crucial role of motor cortex hyperexcitability in the generation of myoclonic jerks is supported by a study that used jerk-locked back averaging of EEG and found a focal cortical generation of myoclonic jerks in JME, with frontocentral polyspikes (but not single spikes) preceding the jerks by 10 ms (Panzica et al., 2001).
It is not clear how myoclonic jerks are facilitated by cognitive effort. Motor circuitry hyperexcitability has not yet been linked to the predominantly frontal changes reported in JME consisting of: (i) generalized spike and polyspike wave complexes with frontocentral maximum; (ii) neuropsychological deficits in frontal lobe executive and memory functions (Devinsky et al., 1997; Pascalicchio et al., 2007; Piazzini et al., 2008; Wandschneider et al., 2010); and (iii) subtle neuroimaging abnormalities in the medial and dorsolateral prefrontal lobes (Woermann et al., 1999; Koepp, 2005; O'Muircheartaigh et al., 2010; Vulliemoz et al., 2010).
The aims of this study were to investigate cognitive activation and functional connectivity patterns of the motor and prefrontal cortex and its modulation through cognitive interactions in patients with JME and healthy controls.
Patients and methods
This study was approved by the Research Ethics Committee of the UCL Institute of Neurology and UCL Hospitals. Written informed consent was obtained from all participants.
Study population
Thirty patients with JME [17 females, mean (SD) age 32.8 (9.9) years] and 26 age- and gender-matched healthy controls [14 females, mean (SD) age 31.4 (8.2) years] performed a working memory functional MRI task. All patients had a typical clinical history of JME with onset of myoclonic jerks and generalized seizures in adolescence, at least one EEG showing generalized spike wave or polyspike wave complexes and normal clinical MRI. All patients except one were taking anti-epileptic medication. Fourteen patients had been seizure free for at least 1 year. Healthy controls had no history of neurological disease or family history of epilepsy and had normal structural neuroimaging. Analysis of structural MRI and neuropsychology data from 28 of the patients with JME has been published recently (O'Muircheartaigh et al., 2010).
Data acquisition
MRI data were acquired on a GE Excite HDx 3 T scanner, using a multichannel head coil and parallel imaging with a SENSE factor of two. We used a 50 slice gradient echo planar imaging sequence in axial orientation with 2.4-mm thickness and 0.1-mm gap, providing full brain coverage. Slices had a 64 × 64 matrix with 3.75 × 3.75 mm voxel size. Repetition time was 2500 ms and echo time 25 ms. The first four scans were discarded to ensure magnetization equilibrium.
Functional MRI paradigm
The spatial working memory paradigm used was described by Kumari et al. (2009). The task presented randomly appearing dots and subjects had to respond to the dots sequentially with a joystick at their right hand. They were instructed to move the joystick to the position of the current dot in the ‘0-back’ condition, to the position of the previous dot in ‘1-back’ and to the position of the dot two presentations back in ‘2-back’ blocks. These three 30-s active conditions were repeated five times, in pseudorandom order and alternated with 15-s rest blocks. Total duration of the experiment was 11 min and 20 s, during which 272 echo planar imaging volumes were acquired. Participants underwent task familiarization before scanning.
Functional MRI processing and analysis
Functional MRI analysis was performed using Statistical Parametric Mapping-5, (http://www.fil.ion.ucl.ac.uk/spm). Images were realigned for movement correction, normalized to an acquisition-specific echo planar imaging template in Montreal Neurological Institute space, resampled to isotropic 3 × 3 × 3 mm voxels and smoothed with an 8 × 8 × 8 mm kernel. Functional MRI results were rendered on a 3D surface created from the MNI152_T1 data set.
Single-subject statistical analysis of functional MRI data was carried out, using a full factorial block design, including movement parameters as regressors. Task conditions were modelled as 30-s blocks and convolution with Statistical Parametric Mapping’s default canonical haemodynamic response function was applied.
Contrasts were defined, comparing task conditions against the resting period, and comparing task with memory load (‘1-back’ and ‘2-back’) against the control task (‘0-back’). For example, the ‘2-back minus 0-back’’ contrast controls for the visual input and for the motor response, and reveals selectively the additional cortical responses to the increasing working memory load.
To identify task-negative areas, i.e. areas increasingly deactivated with increasing task difficulty, we defined an additional contrast with values −1, −2 and −3 for the three conditions ‘0-back’, ‘1-back’ and ‘2-back’, modelling such deactivation compared with the resting periods.
Group comparisons were carried out as two sample t-tests or using a full factorial design. Group statistics were corrected for multiple comparisons across the whole brain, using the false discovery rate (FDR) method. As group results vary in statistical strength, the threshold was adjusted between P < 0.05 and P < 0.0001 for visual clarity of figures. The data have also been tested at a lower, uncorrected threshold, when no differences between groups are reported and the lack of difference indicates that P remained >0.05. An extent threshold with minimum cluster size of 20 voxels was applied to all analyses. For correlation analyses, each subject’s extracted functional MRI response was correlated against a variable of interest, e.g. the seizure-free interval.
Functional connectivity
To assess functional connectivity between activated areas, regions of interest were defined by activation in the combined group activation maps from patients with JME and controls. Two spherical 56 voxel regions of interest were defined at local maxima of the group activation location from the ‘0-back’ condition: one in the left sensorimotor cortex and one in the left supplementary motor area (SMA). Two additional regions of interest were defined from the group activation map of the ‘2-back minus 0-back’ contrast, in the left and right dorsolateral prefrontal cortex (locations of regions of interest are shown as crosshairs in Fig. 3). The average time series were extracted from all four regions of interest for each individual and were used as regressor for new general linear model functional MRI analyses in Statistical Parametric Mapping. Correlation with this signal time series reveals areas functionally coupled with the regions of interest.
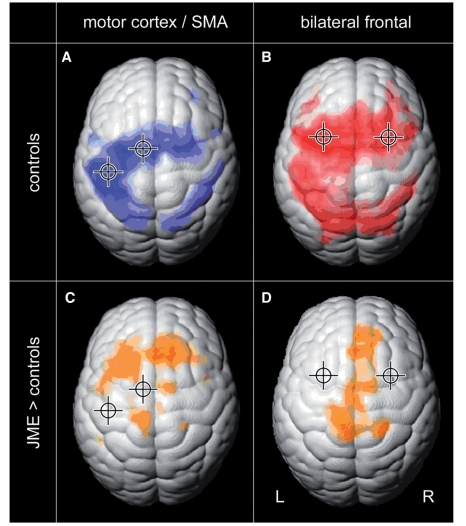
Figure 3.
Functional connectivity is increased in JME. Functional connectivity of the motor system and of the cognitive working memory system were analysed by two seed regions each (crosshair shows location of the seed region). Group connectivity maps of healthy controls show functionally connected cortical areas for the left motor cortex and SMA in blue (A, FDR, P < 10−6), and for the bilateral frontal working memory network in red (B, FDR, P < 10−6). Group difference maps in orange show increased functional connectivity in JME compared with controls: motor cortex and SMA are increasingly connected to prefrontal cortex (C, FDR, P < 0.001), and the bilateral working memory network shows increased connectivity to the medial central region, SMA and medial prefrontal areas (D, FDR, P < 0.005). There were no areas of increased connectivity in healthy controls.
Independent component analysis
Independent component analysis was carried out at a group level using MELODIC from FMRIB software library (http://www.fmrib.ox.ac.uk/fsl/). A 4D file of the realigned, normalized, smoothed images was created for each subject, and image data were prefiltered with a high-pass filter with a cut-off at 100 s. The algorithm was constrained to identify 32 independent components, common across all subjects in each group and characterized with regard to their location, spatial extent and signal time course. Components were ranked according to their relative contribution to overall signal variance, no manual selection or rejection of components was carried out.
Results
Both groups performed equally during the three conditions (‘0-back’, ‘1-back’, ‘2-back’) with an average success rate of 88, 81 and 70% in controls, and 87, 86 and 68% in JME, respectively (two-sample t-test, P = 0.88).
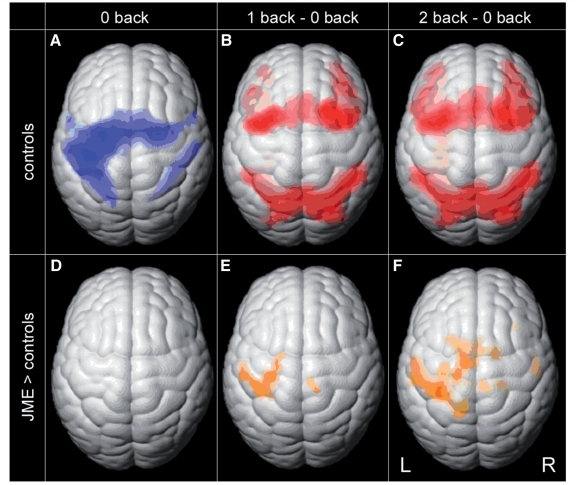
In the ‘0-back’ condition, all subjects showed left central and bilateral SMA activation, reflecting the right-hand motor response (Fig. 1A, motor response shown in blue, FDR, P < 0.0001). By subtracting ‘0-back’ from ‘1-back’ and ‘2-back’ we controlled for this motor component, the resulting contrast maps showed significant bilateral frontal and parietal activation of the working memory network (Fig. 1B and C, cognitive response shown in red, FDR, P < 0.001). Group differences are shown in orange in Fig. 1D–F. There was no group difference for the ‘0-back’ task (Fig. 1D, P > 0.05) but patients with JME showed increased motor cortex and SMA activation in the ‘1-back minus 0-back’ contrast compared with controls (Fig. 1E, FDR, P < 0.05) and this increased with additional task load for the ‘2-back minus 0-back’ contrast (Fig. 1F, FDR, P < 0.05). There were no areas of higher activation in controls compared with JME for any contrast (P > 0.05).
Figure 1.
Functional MRI activation from working memory task in controls and group differences. Group functional MRI activation maps of healthy controls (A–C) show cortical activation for the three different task conditions: motor cortex and SMA activation for ‘0-back’ shown in blue (A, FDR, P < 0.0001). Bilateral frontal and parietal activation for ‘1-back minus 0-back’ and ‘2-back minus 0-back’ shown in red (B and C, FDR P < 0.001). Increased activation in JME compared with controls is shown in orange in the lower row (D–F): no difference for ‘0-back’ (D, uncorrected, P > 0.05), but higher activation in motor cortex and SMA was seen in JME with increasing task difficulty in ‘1-back minus 0-back’ (E, FDR, P < 0.05) and ‘2-back minus 0-back’ (F, FDR, P < 0.05).
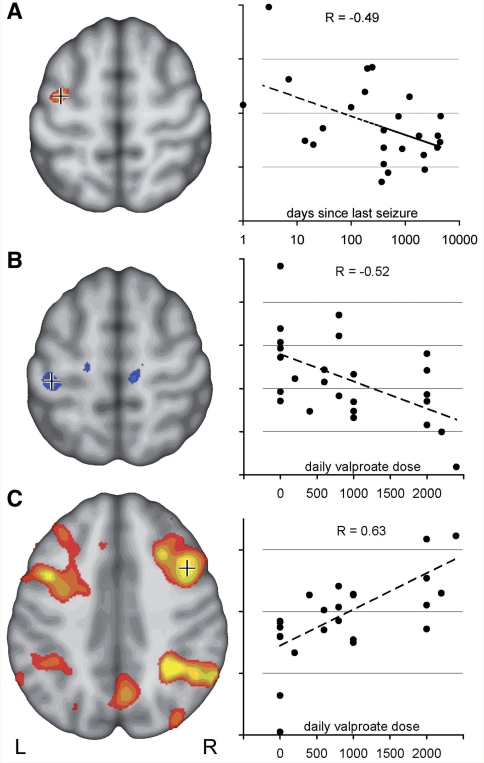
The interval between the last seizure and the functional MRI scan ranged from 1 to 4500 days. Left motor cortex activation correlated negatively with duration of the seizure-free interval (Fig. 2A, voxels overlaid in sectional image thresholded at P < 0.05 uncorrected, correlation coefficient R = −0.49); i.e. greater motor cortex activation was seen in those with more recent seizures (no patient reported any myoclonic jerks during the experiments).
Figure 2.
Motor cortex coactivation correlates with disease activity and treatment. (A) Activation in the left central region in ‘2-back minus 0-back’ contrast was stronger in patients with JME with more active disease. The section on the left shows voxels, negatively correlated with time since last seizure (uncorrected, P < 0.05). Crosshair indicates voxel for which correlation is plotted on the right (R = −0.49). (B) Post hoc analysis of drug effects indicates a specific effect of valproate in JME. Left central activation decreased with increasing daily valproate dose (uncorrected, P < 0.05, R = −0.52). (C) Activity within the typical bilateral frontal and parietal working memory network, on the other hand, correlated positively with valproate dose, indicating a normalizing effect of valproate on the cortical activation pattern in JME (C, uncorrected, P < 0.05, R = 0.63).
Additional post hoc analyses were carried out to assess the effect of medication in JME. Twenty-one of the 30 patients with JME were treated with valproate (400–2400 mg/day). Increased left motor cortex activation correlated negatively (Fig. 2B, R = −0.52), and activation of bilateral frontal and parietal working memory network correlated positively with valproate dosage (Fig. 2C, R = 0.63).
There was no significant correlation between these two variables of interest, the seizure-free interval and the daily valproate dose (R = −0.17).
Group analysis of functional connectivity maps in controls showed that the left motor cortex and SMA signal was highly correlated with bilateral motor cortex, SMA and parietal regions (Fig. 3A, FDR, P < 10−8). Group functional connectivity maps from the two frontal regions of interest activated by the ‘2-back’ condition, largely resembled the bilateral frontal and parietal working memory network (Fig. 3B, FDR, P < 10−8).
Compared with healthy controls, patients with JME showed increased functional connectivity of the left motor cortex and SMA within the motor system and mainly to bilateral prefrontal cortex, including areas activated by the working memory task (Fig. 3C, FDR, P < 0.001). Functional connectivity analysis of the two dorsolateral prefrontal regions showed greater connectivity to the medial central region, SMA and medial prefrontal areas in JME (Fig. 3D, FDR, P < 0.005). There were no areas of higher connectivity in controls in any of the functional connectivity maps (uncorrected, P > 0.05).
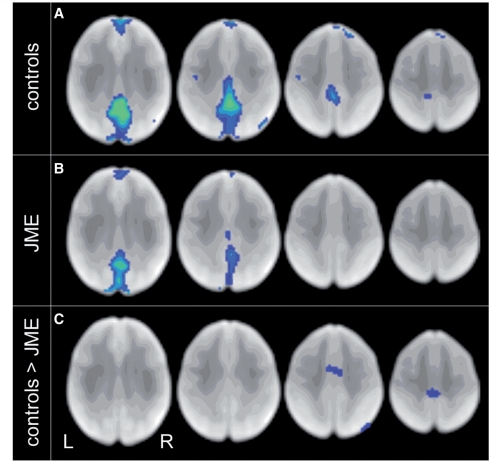
The task-negative contrast showed large clusters of deactivation with increasing task difficulty in the known default mode networks in controls, namely the precuneus, and anterior medial prefrontal cortex (Fig. 4A, FDR, P < 0.003). In patients with JME, we observed similar but less-extensive deactivations (Fig. 4B, FDR, P < 0.003). Direct group comparison revealed clusters that were less deactivated, i.e. remained persistently active, in JME in the medial central and frontal regions (Fig. 4C, uncorrected P < 0.01, peak voxel survived FDR correction within a 20 mm spherical region of interest in the SMA, PFDR-corr = 0.025).
Figure 4.
Deactivation of default mode network is reduced in JME. The task-negative contrast shows that precuneus and medial prefrontal areas (default mode network) are deactivated during the working memory task in controls (A, FDR, P < 0.003). Deactivation is weaker in JME (B, FDR, P < 0.003). Most significant group difference: during the task, controls deactivate more than JME in medial central and frontal areas, including the SMA (C, uncorrected, P < 0.01).
The 32 independent components identified by independent component analysis described 92% of the total functional MRI signal variance in controls and 91% in JME. In both groups, the first component (explaining 7.4 and 7.0% of the total signal variance in controls and JME, respectively) was located in the left central region, representing the motor response of the task (controls: Fig. 5A; JME: Fig. 5C). This component’s signal changes were time-locked to the task timing (frequency 1/45 s), and the response showed the same amplitude across all three task conditions. The second most relevant component identified in controls and JME (explaining 6.6 and 5.3% signal variance) comprised the working memory network, comprising bilateral frontal and parietal clusters (Fig. 5B and D). This component’s response showed a strong correlation with the cognitive load from the task, with higher amplitude in more difficult conditions.
Figure 5.
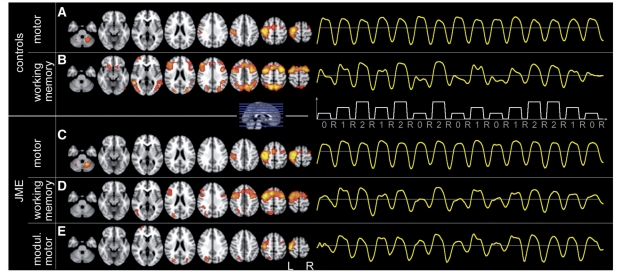
Independent component analysis shows additional task-modulated subcomponents of the motor system in JME. Group-independent component analysis: image shows location of major components common to all subjects on the left hand side and the corresponding group average signal time course during the experiment on the right (onset and duration of the three task conditions ‘0-back’, ‘1-back’, ‘2-back’ and rest are indicated by the white plot). The two most relevant components identified in controls were: motor cortex (A), which shows constant response amplitude throughout all task conditions. The working memory network (B) is modulated by task difficulty: stronger activation during more demanding task conditions. Similar main components were found in JME: motor cortex (C) and working memory network (D). Patients with JME showed additional subcomponent of the motor system, not seen in controls (E): motor cortex response was modulated by task difficulty, similar to modulation of the working memory network.
Patients with JME showed additional components within the left motor area, whose response was also modulated by task difficulty (Fig. 5E). No such modulated motor components were found in controls.
Discussion
We showed increased coactivation of the motor system during a highly demanding cognitive task and increased functional coupling between the motor cortex and prefrontal and parietal cognitive areas in patients with JME, compared with healthy controls. Hyperexcitability within the motor cortex has previously been described in JME, mainly by transcranial magnetic stimulation studies. In our study, we demonstrate motor cortex coactivation triggered by cognitive effort in functional MRI. Our findings provide an explanation for the particular phenotype of JME, the myoclonic jerk and its facilitation through cognitive stressors. This is clinically relevant, as such cognitive trigger factors are not always recognized by patients and physicians (da Silva Sousa et al., 2005b), but are associated with poor seizure control (Matsuoka et al., 2002).
Seizure facilitation through interaction between cognitive systems and the motor system
Our spatial working memory task is cognitively demanding, requiring continuous attention throughout the task. The motor cortex coactivation seen in JME clearly depended on the task difficulty and occurred only under a high cognitive load. This may explain why a previous functional MRI study, which used an easier working memory paradigm, failed to find differences between patients with JME and controls (Roebling et al., 2009). Recent neuropsychological–EEG studies have shown that the combination of a cognitive task with a motor response had the strongest provocative effect on epileptiform discharges or myoclonic jerks (Matsuoka et al., 2005; Guaranha et al., 2009). Our paradigm design was therefore well-suited to trigger provocative effects.
The fast polyspike component on EEG back-averaging preceding myoclonic jerks (Panzica et al., 2001) and increases of EEG activity by cognitive performance are all in the same frequency band of 16–27 Hz (Papanicolaou et al., 1986), which is also similar to fast central movement-related rhythms. We hypothesize a trigger mechanism of cognitive activity with increasing beta rhythms generated in cognitively activated areas, spreading into the motor system, where resonance effects with intrinsic motor cortex frequencies can ultimately result in polyspikes and myoclonic jerks. More demanding tasks recruit larger neuronal networks and therefore are more likely to reach the critical mass. The increased provocative effect of combined cognitive and motor tasks could stem from the fact that such tasks elicit activation with similar frequencies in both, the cognitive and the motor networks, which in turn increases the likelihood of interferences between them.
It is important to note that there were no differences in performance between the two groups. While this may reflect a positive selection bias of relatively good performing patients, it is important to note that only in the case of matched performance is it possible to compare functional MRI activation patterns without performance bias.
Other seizure-facilitating mechanisms
In our study, we refer to praxis induction as the seizure-facilitating mechanism, because the functional MRI paradigm included such a combination of a cognitive task and a motor response. In JME, there are additional provocative factors, such as photic stimulation, which may be based on a similar mechanism, i.e. an increased functional connectivity between visual systems and the motor system. Unfortunately, we have no reliable information about current state of photosensitivity, as nearly half of our patient group was seizure free, and investigations involving such provocation mechanisms are not performed unless clinically indicated.
There is also a significant overlap between JME and reading epilepsy, the most prominent example of seizures triggered by cognitive effort. Most interestingly, in a recent series of simultaneous EEG-functional MRI studies in reading epilepsy, we observed bilateral blood oxygen level-dependent increases ictally, time-locked with reading-induced seizures, in the hand area of a patient with reading epilepsy, who was previously diagnosed with JME (Patient MH in Salek-Haddadi et al., 2009). Spike-triggered functional MRI changes induced by cognitive activation were first reported in a 16-year-old female patient with reading epilepsy showing increased activity, related to individual spikes in the left posterior middle frontal gyrus, that co-localized with the brain regions activated by the working memory component of the reading task, and also bilateral cortical and subcortical motor activity in the inferior central sulcus and globus pallidus (Archer et al., 2003). We observed similar ictal functional MRI activations within cortical (right medial frontal gyrus) and subcortical (left putamen) areas during reading-induced seizures (Salek-Haddadi et al., 2009), but no gross abnormalities in cognitive or motor organization.
These ictal observations in reading epilepsy not only support our finding of motor cortex hyperconnectivity, but also suggest a functional link for the often noticed association of reading epilepsy and JME in the same individual, and the recently reported observation of orofacial reflex myocloni triggered by reading and talking in JME (Mayer et al., 2006). Orofacial reflex myocloni also occur in JME where they are always precipitated by talking and by reading in ∼40% (Mayer and Wolf, 1997).
Effects of medication and seizures
To further investigate the effects of medication, we carried out additional post hoc analyses. Twenty-one of our 30 patients with JME were being treated with valproate, while the number of patients treated with other drugs was too small to investigate a specific drug or dose-related effect. The observed negative correlation of left motor cortex coactivation with increasing daily valproate dose suggests that valproate has a specific effect in reducing this increased activation in patients with JME. Furthermore, the positive correlation of valproate dosage with activation in the bilateral frontal and parietal working memory network may indicate a possible normalizing effect of valproate on the cognitive activation pattern. In a different cohort of patients with focal epilepsies, an opposite effect of valproate was found: higher valproate dosages were associated with a widely reduced cortical activation of the working memory network (unpublished data). This matches the clinical observation that valproate is less likely to cause cognitive side effects in patients with JME than in other epilepsy syndromes. However, our study cannot separate drug effects from subject effects, and these exploratory results require confirmation in subsequent studies, specifically designed to assess drug effects in more detail.
Motor cortex coactivation was higher in patients with JME with more active disease, i.e. with a shorter seizure-free interval. However, most of our patients with JME had well-controlled epilepsy with about half of them free of all seizures for >1 year. This strongly argues against our findings being a secondary effect caused by frequent seizures. It is interesting that the observed hyperconnectivity effect can be seen in such a well-controlled cohort and we hypothesize that the hyperconnectivity might be even stronger in a drug-naïve cohort of patients with JME without the inhibitory, ‘normalizing’ effect of medication. Post hoc, we also observed greater hyperconnectivity in those patients examined in the morning compared with those examined in the afternoon, in keeping with the known circadian clustering of seizures in JME.
Increased functional connectivity in juvenile myoclonic epilepsy
We observed increased functional connectivity between the motor system and higher cognitive systems of the frontal and parietal lobe. No such increase in functional coupling between two independent neuronal systems had been previously described in epilepsy research. Most functional connectivity studies to date have shown disruption of known functional networks in epilepsy, such as the attention (Zhang et al., 2009) or memory networks (Bettus et al., 2009), and reduced connectivity often correlated with an associated cognitive impairment. Here the increased functional connectivity in JME seems to reflect the mechanism underlying cognitively triggered epileptiform discharges and seizures. Compared with the block design functional MRI analysis, functional connectivity takes into account each subject’s individual variation in onset, duration and amplitude of the blood oxygen level-dependent response. It is independent from the block design specification and can therefore reveal changes in the absence of differences from traditional functional MRI analysis. In our study, we assessed functional connectivity across the whole duration of the task and there may be differences in functional connectivity between rest and cognitive activity. However, the resting periods in our task were short (15 s) and we aimed specifically to investigate effects driven by a cognitive task. Resting state functional connectivity was therefore not analysed.
Modulated motor components in independent component analysis
Independent component analysis provides a method to identify distinct clusters of functional activation, completely independent of any prior specification of the experimental design or any region of interest. Components are identified solely based on correlations of their signal time course, location and commonality throughout the group of subjects. This provides an independent method, complementary to model-based functional MRI analysis or region-based functional connectivity analysis. In our study, independent component analysis reliably identified the motor system and the working memory system components in our patients with JME and controls. Most importantly, independent component analysis identified additional subcomponents of the motor system in JME, whose response amplitude is modulated by task difficulty (Fig. 5E), and these were not found in healthy controls. This finding was consistent across several iterations of independent component analysis and is consistent with our results from block design and functional connectivity analyses.
Impaired deactivation of the default mode network
The task-negative contrast was modelled to identify areas deactivated during task performance. It showed deactivation in areas of the default mode network in both groups, but these were less extensive and deactivation was weaker in JME. Patients with JME appeared to be less able to deactivate their default mode network during the task, indicating a less efficient reallocation of neuronal resources to task-relevant areas, which may interfere with cognitive processing (Sonuga-Barke and Castellanos, 2007). Fransson (2006) used the same functional MRI paradigm comparing intrinsic activity during rest and the ‘2-back’ task. They showed that spontaneous intrinsic activity in the default mode network was not extinguished but rather attenuated and reorganized during a working memory task in healthy controls. Some of our observations in JME could therefore also be interpreted as an imbalance between the task relevant cognitive network and the opposing default mode network. An ‘overload’ of the task-positive cognitive network during a highly demanding task, together with impaired deactivation of the default mode network, could lead to hyperexcitability and hyperconnectivity across systems, including the motor cortex, and cause myoclonic jerks. Alterations of the default mode network modulation are not specific to JME. Similar changes have recently been described in a range of neuropsychiatric disorders (Broyd et al., 2009), including anxiety, attention-deficit hyperactivity disorder (Bush, 2010), and schizophrenia (Calhoun et al., 2008; Whitfield-Gabrieli et al., 2009). Voon et al. (2010) described increased functional connectivity between the amygdala and SMA under emotional load in patients with motor conversion disorder. Impaired default mode network deactivation altered mutual modulation of functional subsystems and increased connectivity between higher order cognitive systems and the motor system may therefore be common mechanisms in the generation of motor symptoms, triggered by emotional or cognitive stressors.
Role of the supplementary motor area
Independent analysis techniques have consistently shown alterations in the SMA in JME. We found a load-dependent co-activation of the motor cortex and SMA during the cognitive task in block design analysis. This was an effect of increased functional connectivity between the frontal and parietal cognitive networks and the motor system. The areas of greatest difference in task-related deactivation between JME and controls were located in medial central and frontal regions of the SMA (see Fig. 6 for spatial relationship between findings). Our findings corroborate morphometric structural changes in JME shown by previous studies (Woermann et al., 1999; Bernhardt et al., 2009; O'Muircheartaigh et al., 2010).
Figure 6.
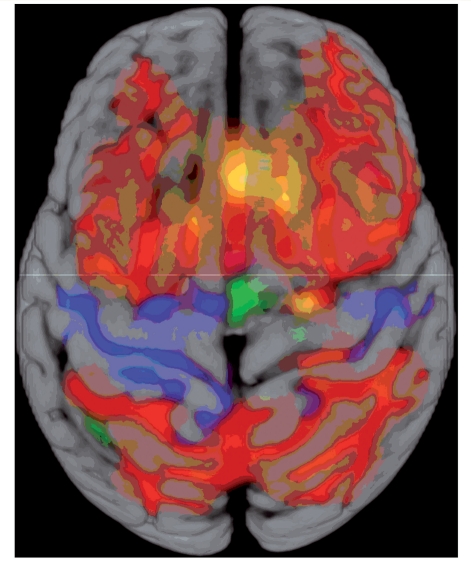
Spatial relationship between findings. This figure summarizes some of the results, to aid the interpretation of their spatial relationship. Activation from the working memory task in controls is shown in red (2-back minus 0-back, as in Fig. 1C). Areas of increased activation in JME, compared with controls are shown in blue—mainly in the pre- and postcentral gyrus and SMA (as in Fig. 1F). The orange clusters show areas of increased functional connectivity to the motor cortex and SMA (as in Fig. 3C)—mainly in the medial prefrontal cortex, overlapping with the task-induced activation. The green cluster shows the area of impaired deactivation during the cognitive task in JME (as in Fig. 4C). Gyral anatomy is overlaid from the MNI152_T1 template. The horizontal line indicates y coordinate 0 in Montreal Neurological Institute space.
Even though the detailed anatomical and functional parcellation of the SMA is still ongoing (Fink et al., 1997; Chouinard and Paus, 2006), it is clear that the SMA is well connected to both the primary motor cortex and prefrontal cognitive networks (Zilles et al., 1995). Impaired task-dependent deactivation of certain SMA areas may indicate a specific role as relay for the abnormal functional connectivity between the cognitive and the motor system we observed in JME and this could reflect the functional correlate of previously described structural abnormalities in the SMA.
Our findings document a dynamic modulatory effect of cognitive processes on the motor system, explaining the provocative effect of cognitive activity on myoclonic jerks in JME.
Funding
Wellcome Trust (Project Grant No 079474); The Big Lottery Fund, Wolfson Trust and the Epilepsy Society (ES MRI scanner). Part of this work was undertaken at University College London Hospitals who received a proportion of funding from the NIHR, Biomedical Research Centres funding scheme.
Acknowledgements
The authors acknowledge infrastructure support from the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and the Institute of Psychiatry, King’s College London.
Glossary
Abbreviations
- FDR
false discovery rate
- JME
juvenile myoclonic epilepsy
- SMA
supplementary motor area
References
- Archer JS, Briellmann RS, Syngeniotis A, Abbott DF, Jackson GD. Spike-triggered fMRI in reading epilepsy: involvement of left frontal cortex working memory area. Neurology. 2003;60:415–21. doi: 10.1212/wnl.60.3.415. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Neurology. 2006;67:1018–22. doi: 10.1212/01.wnl.0000237392.64230.f7. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Macdonell RA, Jackson GD, Berkovic SF. Why do seizures in generalized epilepsy often occur in the morning? Neurology. 2009;73:218–22. doi: 10.1212/WNL.0b013e3181ae7ca6. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage. 2009;46:373–81. doi: 10.1016/j.neuroimage.2009.01.055. [DOI] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–91. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29:1265–75. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–52. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- da Silva Sousa P, Lin K, Garzon E, Ceiki Sakamoto A, Yacubian EM. Language- and praxis-induced jerks in patients with juvenile myoclonic epilepsy. Epileptic Disord. 2005a;7:115–21. [PubMed] [Google Scholar]
- da Silva Sousa P, Lin K, Garzon E, Sakamoto AC, Yacubian EM. Self-perception of factors that precipitate or inhibit seizures in juvenile myoclonic epilepsy. Seizure. 2005b;14:340–6. doi: 10.1016/j.seizure.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Gershengorn J, Brown E, Perrine K, Vazquez B, Luciano D. Frontal functions in juvenile myoclonic epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:243–6. [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–74. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–45. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Guaranha MS, da Silva Sousa P, de Araujo-Filho GM, Lin K, Guilhoto LM, Caboclo LO, et al. Provocative and inhibitory effects of a video-EEG neuropsychologic protocol in juvenile myoclonic epilepsy. Epilepsia. 2009;50:2446–55. doi: 10.1111/j.1528-1167.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Seino M, Kubota H, Yamakaku K, Tanaka M, Yagi K. Epilepsy with praxisinduced seizures. In: Wolf P, editor. Epileptic seizures and syndromes. London: John Libbey; 1994. pp. 81–91. [Google Scholar]
- Janz D. Epilepsy with impulsive petit mal (juvenile myoclonic epilepsy) Acta Neurol Scand. 1985;72:449–59. doi: 10.1111/j.1600-0404.1985.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Janz D, Christian W. Impulsive petit mal. Dtsch Z Nervenheilk. 1957;176:348–86. Translated into English by Genton P. In: Malafosse A, Genton P, Hirsch E, Marescaux C, Broglin D, Bernasconi R, editors. Idiopathic generalized epilepsies. London: John Libbey; 1994. p. 229–51. [Google Scholar]
- Koepp MJ. Juvenile myoclonic epilepsy–a generalized epilepsy syndrome? Acta Neurol Scand Suppl. 2005;181:57–62. doi: 10.1111/j.1600-0404.2005.00511.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Peters ER, Fannon D, Antonova E, Premkumar P, Anilkumar AP, et al. Dorsolateral prefrontal cortex activity predicts responsiveness to cognitive-behavioral therapy in schizophrenia. Biol Psychiatry. 2009;66:594–602. doi: 10.1016/j.biopsych.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Tamburin S, Bongiovanni LG, Zanette G, Fiaschi A. Motor responses to afferent stimulation in juvenile myoclonic epilepsy. Epilepsia. 2004;45:77–80. doi: 10.1111/j.0013-9580.2004.21003.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Nakamura M, Ohno T, Shimabukuro J, Suzuki T, Numachi Y, et al. The role of cognitive-motor function in precipitation and inhibition of epileptic seizures. Epilepsia. 2005;46(Suppl 1):17–20. doi: 10.1111/j.0013-9580.2005.461006.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Takahashi T, Sasaki M, Yoshida S, Numachi Y, Sato M. The long-term course of seizure susceptibility in two patients with juvenile myoclonic epilepsy. Seizure. 2002;11:126–30. doi: 10.1053/seiz.2002.0591. [DOI] [PubMed] [Google Scholar]
- Mayer TA, Schroeder F, May TW, Wolf PT. Perioral reflex myoclonias: a controlled study in patients with JME and focal epilepsies. Epilepsia. 2006;47:1059–67. doi: 10.1111/j.1528-1167.2006.00575.x. [DOI] [PubMed] [Google Scholar]
- Mayer TA, Wolf PT. Reading epilepsy: related to juvenile myoclonic epilepsy? Epilepsia. 1997;38(Suppl 3):18–19. [Google Scholar]
- O’Muircheartaigh J, Vollmar C, Barker G, Kumari V, Symms M, Thompson P, et al. Focal changes and their association with cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. 2011;76:34–40. doi: 10.1212/WNL.0b013e318203e93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzica F, Rubboli G, Franceschetti S, Avanzini G, Meletti S, Pozzi A, et al. Cortical myoclonus in Janz syndrome. Clin Neurophysiol. 2001;112:1803–9. doi: 10.1016/s1388-2457(01)00634-4. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Loring DW, Deutsch G, Eisenberg HM. Task-related EEG asymmetries: a comparison of alpha blocking and beta enhancement. Int J Neurosci. 1986;30:81–5. doi: 10.3109/00207458608985658. [DOI] [PubMed] [Google Scholar]
- Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, Lin K, Caboclo LO, Vidal-Dourado M, et al. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007;10:263–7. doi: 10.1016/j.yebeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Piazzini A, Turner K, Vignoli A, Canger R, Canevini MP. Frontal cognitive dysfunction in juvenile myoclonic epilepsy. Epilepsia. 2008;49:657–62. doi: 10.1111/j.1528-1167.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- Roebling R, Scheerer N, Uttner I, Gruber O, Kraft E, Lerche H. Evaluation of cognition, structural, and functional MRI in juvenile myoclonic epilepsy. Epilepsia. 2009;50:2456–65. doi: 10.1111/j.1528-1167.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Mayer T, Hamandi K, Symms M, Josephs O, Fluegel D, et al. Imaging seizure activity: a combined EEG/EMG-fMRI study in reading epilepsy. Epilepsia. 2009;50:256–64. doi: 10.1111/j.1528-1167.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–86. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC, Jr, et al. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–36. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliemoz S, Vollmar C, Koepp MJ, Yogarajah M, O'Muircheartaigh J, Carmichael DW, et al. Connectivity of the supplementary motor area in juvenile myoclonic epilepsy and frontal lobe epilepsy. Epilepsia. 2010;52:507–14. doi: 10.1111/j.1528-1167.2010.02770.x. [DOI] [PubMed] [Google Scholar]
- Wandschneider B, Kopp UA, Kliegel M, Stephani U, Kurlemann G, Janz D, et al. Prospective memory in patients with juvenile myoclonic epilepsy and their healthy siblings. Neurology. 2010;75:2161–7. doi: 10.1212/WNL.0b013e318202010a. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122(Pt 11):2101–8. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, et al. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci Lett. 2009;458:97–101. doi: 10.1016/j.neulet.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qu M, et al. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat. 1995;187(Pt 3):515–37. [PMC free article] [PubMed] [Google Scholar]