Abstract
Background
Aging is associated with increased inflammation and risk for community-acquired pneumonia (CAP). Streptococcus pneumoniae co-opts the NFkB-regulated proteins Polymeric immunoglobulin receptor (pIgR) and Platelet-activating factor receptor (PAFr) to attach and invade cells. We sought to determine if aging and chronic inflammation was associated with increased pIgR & PAFr in the lungs and increased susceptibility to S. pneumoniae.
Methods
Lung protein and mRNA levels were quantitated using Western blot and quantitative PCR. NFkB activation was measured by electrophoretic mobility shift assay. Cytokine levels were measured by cytometric bead analysis. To model chronic inflammation mice were implanted with osmotic pumps that delivered tumor necrosis factor (TNF)α.
Results
Aged mice and those infused with TNFα had increased levels of pIgR & PAFr in their lungs and were more susceptible to S. pneumoniae. During pneumonia, aged mice had reduced levels of pIgR & PAFr and less NFkB activation despite greater bacterial burden. We determined that aged mice had decreased amounts of lung Toll-like receptors (TLR)-1, 2, and 4 and reduced capacity to respond to S. pneumoniae with pro-inflammatory cytokine production.
Conclusions
Aged mice, and potentially elderly humans, are more susceptible to pneumonia because of a priming effect of chronic inflammation and TLR dysfunction.
Keywords: Streptococcus pneumoniae, aging, inflammation, pneumonia, platelet activating factor receptor, PAFr, polymeric immunoglobulin receptor, pIgR, Toll-like receptor, TLR
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is the most frequent microorganism associated with community-acquired pneumonia (CAP), up to 58% of all cases [1]. It is also a leading cause of invasive bacterial disease (i.e. bacteremia, sepsis, and meningitis) [2–4]. In developed countries, despite aggressive vaccination policies, S. pneumoniae remains a major medical problem for the elderly. In the United States it is estimated that >130,000 elderly (≥65) are affected by pneumococcal pneumonia annually [4–7]. Importantly, despite access to intensive medical care and appropriate antimicrobial therapy, the case-fatality rate among the elderly is 15–25% [2, 4, 7, 8]. Thus, the elderly are at risk for severe pneumonia that frequently results in death.
Cellular inflammation is an integral component of the pneumococcal disease process. Pneumococcal cell wall and the toxin pneumolysin cause inflammation by binding to Toll-like receptor (TLR)-1/2 and TLR-4, respectively, on the surface of cells [9, 10]. Binding of these molecules initiates a cell-signaling cascade that activates the transcription factor Nuclear Factor Kappa β (NFkB). NFkB is a major regulator of innate immunity and its activation results in the expression of genes encoding acute phase proteins, production and secretion of pro-inflammatory cytokines, and increased surface expression of the proteins Polymeric Immunoglobulin receptor (pIgR) and Platelet Activating Factor receptor (PAFr) among others [11, 12]. S. pneumoniae attachment is mediated by the bacterial adhesin Choline binding protein A (CbpA) and the cell wall component phosphorylcholine (ChoP). CbpA has been shown to bind to pIgR on nasopharyngeal and bronchial epithelial cells, while ChoP binds to PAFr on mucosal epithelial and vascular endothelial cells. Because pIgR & PAFr are positively regulated by NFkB, cells in acute and chronic states of inflammation express greater amounts of pIgR & PAFr and are bound by S. pneumoniae 5 to 50-fold more than resting cells [13–16].
It is now known that aging is associated with low-grade chronic inflammation. Studies have demonstrated that levels of TNFα and Interleukin (IL)-6 are 2- to 4-fold higher in blood of the elderly compared to middle-aged adults [17, 18]; that tissues from senescent animals exhibit elevated levels of activated NFkB [19–21]; and that inflammatory markers such as C-reactive protein are higher in plasma and bronchoalveolar lavage fluid isolated from the elderly [18]. The cause of age-associated inflammation (AAI) is multifaceted: a combination of underlying medical conditions, exposures to infectious and environmental agents, cell damage, dysregulation of senescent cells, increased fat tissue, and a reduction in sex steroids. Considerable evidence now indicates that persistent low-grade inflammation is present in the elderly population and that AAI occurs in the lungs [19, 20, 22–25].
Given that aging is associated with increased inflammation and risk for CAP, we sought to determine if aging was associated with increased lung pIgR and PAFr levels; if chronic inflammation altered pIgR and PAFr levels in the lungs and was sufficient to increase susceptibility to S. pneumoniae; and to determine if AAI initiated a positive feedback loop during pneumococcal pneumonia (AAI → increased pIgR and PAFr → S. pneumoniae adhesion and invasion → inflammation) that contributed to the greater disease severity that is observed among the elderly. Based on our experimental findings, we conclude that the susceptibility of aged mice, and potentially elderly humans, is due in part to the priming effect of chronic inflammation and its effect on pIgR and PAFr levels, as well as age-dependent TLR dysfunction which impairs the ability of mice to respond to pneumococcal components in their lungs.
METHODS
Invasive pneumococcal disease
Young (4–5 months) and aged (19–20 months) Balb/cBy mice were obtained from the National Institute on Aging (NIA) Aged Rodent Colony at Harlan Sprague Dawley, Inc. (Indianapolis IN). S. pneumoniae serotype 4, strain TIGR4 [26], was grown in Todd-Hewitt broth or on blood agar plates at 37° C in 5% CO2. Exponential phase cultures of TIGR4 were centrifuged, washed with sterile phosphate buffered saline (PBS) and suspended in PBS to a final concentration of 5 × 108 cfu/ml. Mice were anesthetized with 2.5% vaporized isoflurane and 20 µl of the pneumococcal suspension instilled into the left nostril (107 cfu). At the designated times, mice were sacrificed and tissue samples were collected. Blood was obtained by heart puncture and bacterial titers were determined by serial dilution and plating. Bacterial titers in the lungs were assessed per gram of homogenized tissue. For pathological analyses, the 4th lobe of the right lung was embedded in cryoprotectant and sectioned using a cryostat. Lung sections/slides were stained with hemoxylin & eosin (H&E) and scored in a blind manner on the amount of infiltrates, consolidation, and necrosis observed: 1=no damage, 2=mild, 3=moderate, 4=severe, 5=maximum damage. For all experiments the challenge dose was confirmed by serial dilution and plating of the bacterial suspension used for infection. TIGR4 was used in an effort to avoid age-dependent serotype bias. The incidence of serotype 4 disease in humans being the same for children, adults, and the elderly [27]. We used an infectious dose of 107 cfu because Balb/cJ mice, a closely related mouse strain, reproducibly developed pneumococcal disease when challenged with TIGR4 at this dose [28].
Quantitative analysis of pIgR, PAFr, and TLR protein levels in the lungs
Relative levels of protein were determined by comparative densometric analysis of Western blot bands using a Molecular Imager Gel Doc XR System (BioRad, Hercules, CA). Quarter sections of whole lung were homogenized in RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M NaH2PO4) containing 1% (vol/vol) protease inhibitor cocktail (Sigma, St. Louis MO). For each lung sample, 10 µg of protein was boiled in SDS-sample buffer and separated b y SDS-PAGE. Proteins were transferred to PVDF membrane, and immunoblot analysis performed using commercially available polyclonal antibodies (pIgR: Cat# RSC-25-1, Immunology Consultants Laboratory Inc., Newberg OR; PAFr: Cat# sc-8744, Santa Cruz Biotechnology, Santa Cruz CA; TLR1: Cat# ab37068; Abcam, Cambridge MA; TLR2: Cat# ab47840, Abcam; TLR4: Cat# ab13867, Abcam). Detection was performed using horse-radish peroxidase conjugated secondary antibodies and Super-Signal West Pico Chemiluminescent Substrate (Pierce). To ensure that equal amounts of protein had been loaded, the immunoblots were stripped and the amount of actin confirmed to be equal using rabbit anti-actin antibodies (Cat# A2668; Sigma).
TNFα infusion
Alzet osmotic pumps (model #1007D, Durect, Cupertino, CA) were infused with recombinant mouse TNFα (Sigma) or saline and surgically implanted subcutaneously on the back slightly posterior to the scapulae of young Balb/cBy adult mice. TNFα (20 µg/ml) was dissolved in PBS with 0.25% mouse serum albumin and a 0.5% non-toxic protease inhibitor cocktail designed for use in tissue culture (Cat# P1860, Sigma). Albumin and the protease inhibitor were included to increase protein stability and prevent cytokine degradation. Control mice were implanted with pumps that delivered the same PBS/albumin/protease cocktail without TNFα. Alzet 1007D osmotic pumps have an infusion rate of 0.5 µl/hour and a 6-day pumping capacity; thus the pumps subcutaneously delivered 10 ng/hour of TNFα for the duration of the experiments. In experiments with uninfected mice, pump mice were sacrificed 5 days after surgery. In experiments involving infection with S. pneumoniae, pump mice were infected 4 days after surgery and tissue samples were collected at the end of day 6. All experiments involving mice were performed using Institutional Animal Care and Use Committee approved protocols.
Measurement of activated NFkB
Levels of NFkB activation in the lungs were determined using an electrophoretic mobility-shift assay (EMSA) [29]. Nuclear extracts were prepared from freshly excised lung sections using a Nuclear Extraction Kit (Chemicon, Temecula, CA). To determine specificity of the EMSA for p65, excess unlabeled oligonucleotides were added to some samples. Samples were separated on 5% polyacrylamide gels with Tris/borate-EDTA running buffer, the gels transferred onto Whatman paper and the bound complexes visualized and quantitated using a Molecular Dynamics Storm-840 Phosphorimager (Piscataway, NJ).
Cytokine analysis of bronchoalveolar lavage
Mice were infected intratracheally with 100 µl of PBS containing heat killed bacteria (equivalent to 107 cfu), recombinant pneumolysin (1 µg/ml; a gift from Tim Mitchell, Glasgow, United Kingdom), or purified pneumococcal cell wall (108 cfu equivalents). Six hours later mice were sacrificed and bronchoalveolar lavage fluid (BALF) was collected by lung lavage with 3.0 ml PBS [30]. Cellular debris was removed from the BALF by centrifugation at 14,000 X G for 10 minutes and the supernatants were aliquoted and frozen at −80° C. A cytometric bead array, (BD™ CBA Mouse Inflammation Kit, BD Biosciences, San Diego, CA) was used to measure IL-6 and TNFα in the samples. Flow cytometry was performed using a FACSAria (BD Biosciences) cell sorter/analyzer. Pneumococcal cell wall was collected and purified by the method described by Tuomanen [31]. To ensure that the samples were endotoxin free, samples were tested for endotoxin with a Limulus test (Pyrosate, Associates of Cape Cod Inc., East Falmouth, MA) immediately prior to their use. Samples were collected at 6 hours post-challenge to preclude measurement of TNFα and IL-6 produced by infiltrating neutrophils. Neutrophil influx has been shown to peak 6–12 hours after intratracheal challenge with bacterial components [32, 33].
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from lung tissue using a Qiagen RNeasy Mini Kit (Alameda, CA). RNA was DNased using the Turbo DNA-free Kit (Ambion, Austin, TX) and quantitated using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Quality and purity of the RNA was confirmed by visual inspection on a Tris-Borate EDTA 1.5% agarose gel. Using a High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA) 1µg of RNA was converted into cDNA. qRT-PCR was performed on a BioRad Chromo4 Real-Time PCR Detector (BioRad) using Sybr Green Master Mix (Applied Biosystems), 5–10ng cDNA, and gene specific primers at a concentration of 300nM each. A no template control was included for each primer set to detect false-positive results arising from the amplification of primer-dimers. Primers were designed using Primer3 software and checked for single gene amplification using BLAT [34, 35]. Primer sequences were as follows: GAPDH_F:5’-CTCATGACCACAGTCCATGC; GAPDH_R: 5’-CACATTGGGGGTAGGAACAC; PAFr_F: 5’-AGCAGAGTTGGGCTACCAGA; PAFr_R: 5’-TGCGCATGCTGTAAAACTTC; pIgR_F: 5’-CCTCTCCAGACACACAGCAA; pIgR_R” 5’-CAGCTATTGTGCTGGACTGA; TLR1_F: 5’-GGACCTACCCTTGCAAACAA; TLR1_R: 5’-GGTGGCACAAGATCACCTTT; TLR2_F 5’-AAGAGGAAGCCCAAGAAAGC; TLR2_R: 5’-CGATGGAATCGATGATGTTG; TLR4_F 5’-GGCAGCAGGTGGAATTGTAT; TLR4_R: 5’- AGGCCCCAGAGTTTTGTTCT.
Repeat measures and statistical analyses
For each experiment at least 2 groups of mice were tested, each with experimental procedures performed at least 1 week apart. Differences in mortality were examined for significance using a Kaplan-Meier Log-Rank Test. For all other comparisons a Student’s t-test was used.
RESULTS
Aged mice are more susceptible to S. pneumoniae and express elevated levels of pIgR and PAFr
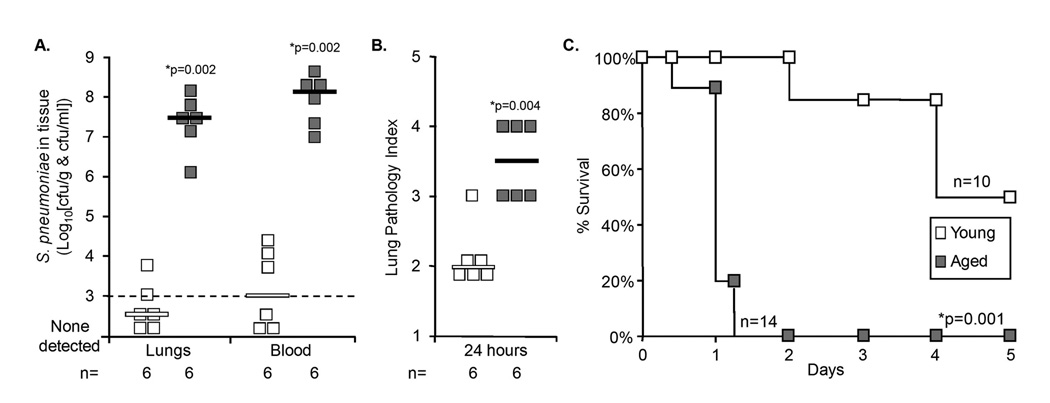
Twenty four hours following intranasal infection with S. pneumoniae, aged mice had >10,000-fold more bacteria in their lungs and blood than young mice (Figure 1A). Pathological examination of lung sections demonstrated that aged mice had significantly greater lung damage characterized by vascular congestion, alveolar edema, and infiltration of neutrophils and red blood cells (Figure 1B). Increased bacterial blood titers corresponded with an increased rate of mortality in the aged mouse (Figure 1C). Thirty six hours post-infection all aged mice infected with S. pneumoniae had died, in contrast all young mice remained alive and greater than 50% of the young mice survived the study. Thus aged mice, like elderly humans, were more susceptible to pneumococcal pneumonia than their young counterparts.
Figure 1. Aged mice are highly susceptible to S. pneumoniae infection.
A) Bacterial titers in the lungs and blood of young and aged Balb/cBy mice one day after intranasal challenge with 107 cfu of S. pneumoniae. B) Scoring of lung pathology in the same mice where 1 is no pathology on histological cross-section and 5 is extensive vascular congestion, hemorrhage and alveolar edema. C) Kaplan-Meier plot illustrating 100% mortality in aged mice at 36 hours compared to a 50% survival of young mice post-challenge. For A) and B) squares represent values obtained from individual mice. Horizontal bars indicate the median value. Statistical analysis was performed using a Student’s t-test. For C) statistical analysis was performed using a Kaplan-Meier Log-Rank Test.
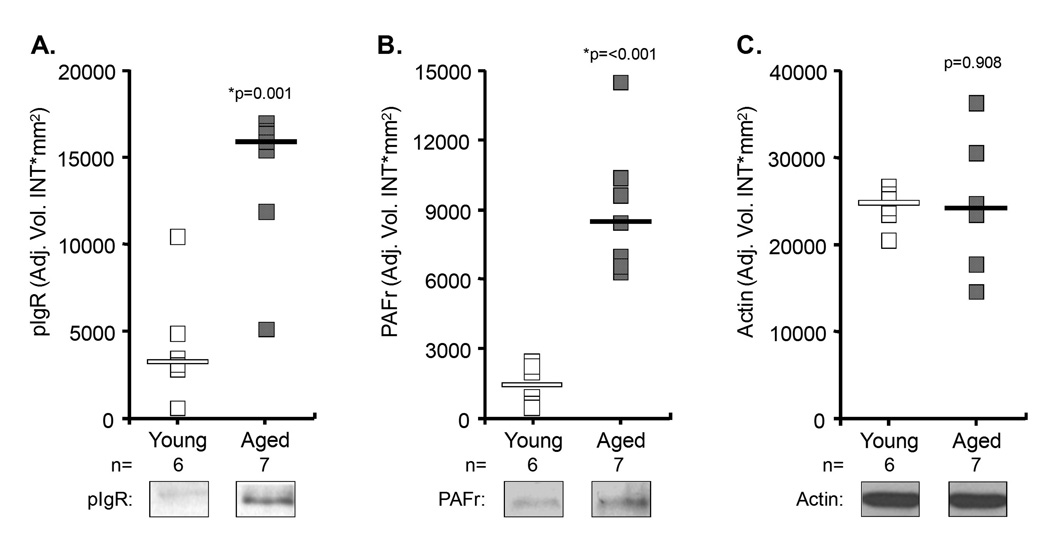
Because aging is associated with chronic low-grade inflammation, we tested for age-dependent changes in the NFkB-regulated proteins pIgR and PAFr. Quantitative immunoblots using whole lung extracts determined that aged mice had 4.7- and 5.8-fold more pIgR and PAFr in their lungs than young mice, respectively (Figure 2). Although we observed no difference in pIgR mRNA (aged n=5, young n=6, p=0.66), using qRT-PCR we determined that PAFr mRNA levels were increased 2.2-fold in aged mice (aged n=6, young n=6, p=0.04). Thus, aging was associated with increased lung transcript and protein for PAFr, but only protein for pIgR.
Figure 2. Elevated levels of pIgR and PAFr in the lungs of aged mice.
Relative amounts of A) pIgR and B) PAFr in the lungs of individual young (open square) and aged (closed square) mice as determined by quantitative chemiluminescent analysis of Western blots. C) Protein loads were determined to be equal based on subsequent blots for actin. Horizontal bars indicate the median value. Statistical analysis was performed using a Student’s t-test.
Young mice infused with TNFα express elevated levels of pIgR and PAFr
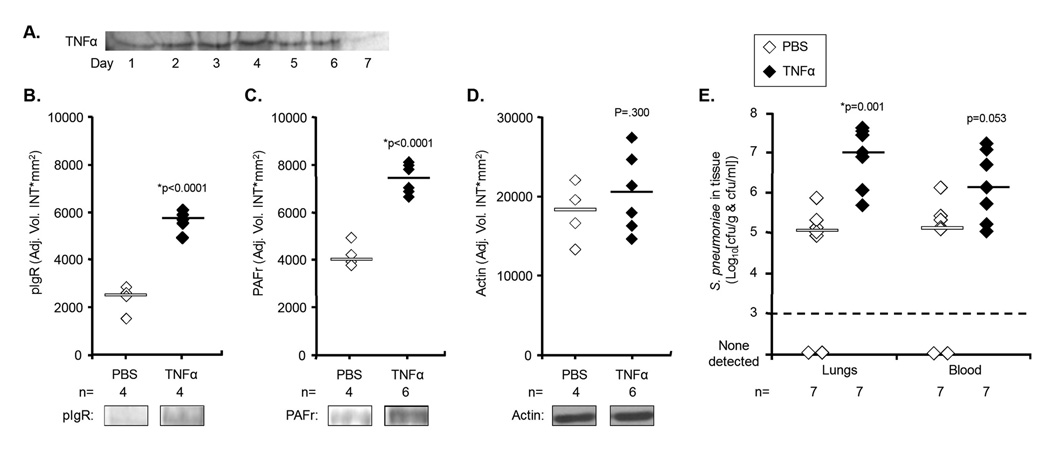
To test if systemic low-grade inflammation was sufficient to alter lung pIgR and PAFr levels, osmotic pumps were implanted subcutaneously that delivered 10 ng/hour of TNFα or saline continuously. Figure 3A demonstrates consistent delivery of intact TNFα by an osmotic pump for up to 6 days, the duration of our longest experiment. Mice with saline pumps (n=4) had undetectable levels of TNFα (<20 pg/mL) at day 5, whereas those receiving TNFα (n=6) had an average serum value of 27 pg/mL (p=0.008). Prolonged delivery of TNFα increased lung pIgR and PAFr protein levels 2.4- and 1.9-fold versus saline controls (Figure 3B–D). pIgR mRNA levels remained unchanged (p=0.197), however, PAFr mRNA levels increased 1.5-fold (p=0.02). Mice infused with TNFα were determined to be more susceptible to pneumococcal pneumonia. Two days post-challenge, mice infused with TNFα had 60-fold more bacteria in their lungs and 10-fold more bacteria in their blood (p=0.053) than saline controls (Figure 3E). Thus low-grade inflammation resulting from TNFα infusion was sufficient to increase lung pIgR and PAFr protein levels and increase susceptibility to pneumococcal pneumonia.
Figure 3. Young mice infused with TNFα express more pIgR and PAFr and are more susceptible to pneumonia.
A) Silver stain demonstrating continuous delivery of intact TNFα by an osmotic pump in vitro. Relative levels of B) pIgR and C) PAFr in the lungs of mice receiving osmotic pump delivery of TNFα (closed diamond) or a saline loading control (open diamond) after five days; as determined by quantitative chemiluminescent analysis of Western blots. D) Protein loads were determined to be equal based on subsequent blots for actin. E) Bacterial titers in the lungs and blood of young Balb/cBy mice implanted with osmotic pumps that delivered TNFα or PBS two days after intranasal challenge with 107 cfu of S. pneumoniae. Horizontal bars indicate the median value. Statistical analysis was performed using a Student’s t-test.
pIgR and PAFr levels are lower in aged mice during infection
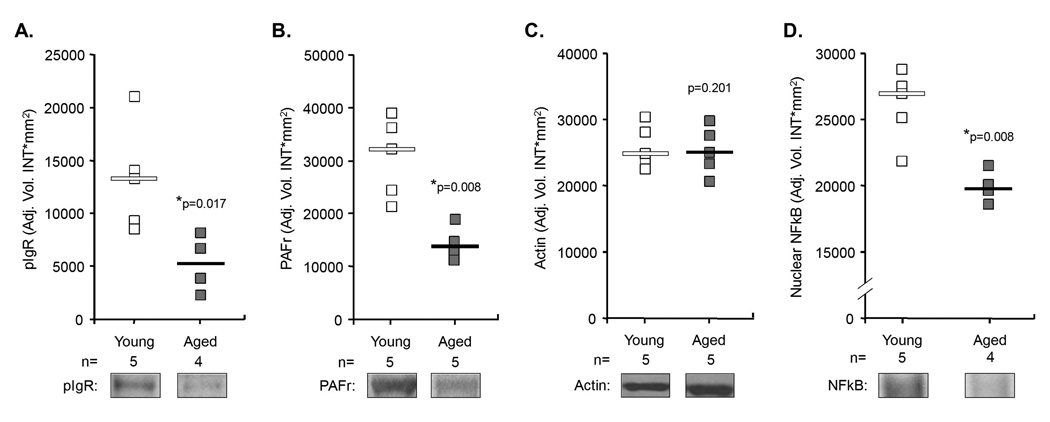
Having shown that lung pIgR and PAFr levels were elevated in aged mice, we examined whether increased levels of these proteins were also present during pneumonia. Surprisingly, aged mice infected with S. pneumoniae had 40% of pIgR and 42% of PAFr protein levels observed in the infected young mice (Figure 4A–C). This reduction coincided with diminished levels of activated NFkB (Figure 4D) and no differences in pIgR and PAFr mRNA levels between young and aged infected mice (young n=6; aged n=6; pIgR p=0.29; PAFr p=0.08) These findings were unexpected as aged mice had >10,000-fold more bacteria in their lungs at time of tissue collection (Figure 1A), and we expected to observe significant increases in pIgR and PAFr protein and mRNA levels as well as activated NFkB in aged mice versus young.
Figure 4. Levels of activated NFkB, pIgR and PAFr are lower in aged mice during an infection when compared to young mice.
A) Relative levels of pIgR, B) PAFr, C) actin, and D) activated NFkB in young versus aged mice 2 days following infection with 107 cfu of S. pneumoniae. Protein levels were determined by quantitative immunoblot analyses, activated NFkB levels determined by EMSA. Squares and diamonds indicate the individual protein expression level for each mouse tested. Horizontal bars indicate the median value. Statistical analysis was performed using a Student’s t-test.
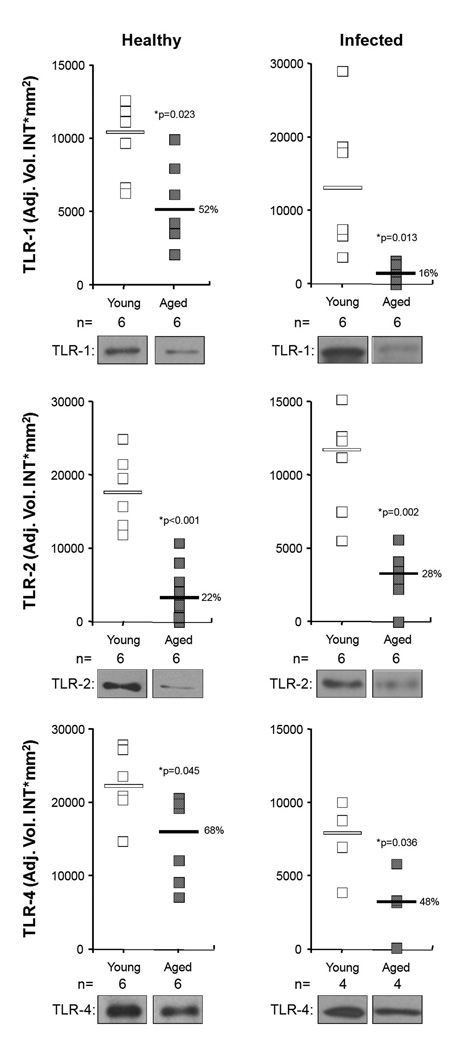
Aging is associated with decreased TLR protein levels in the lungs
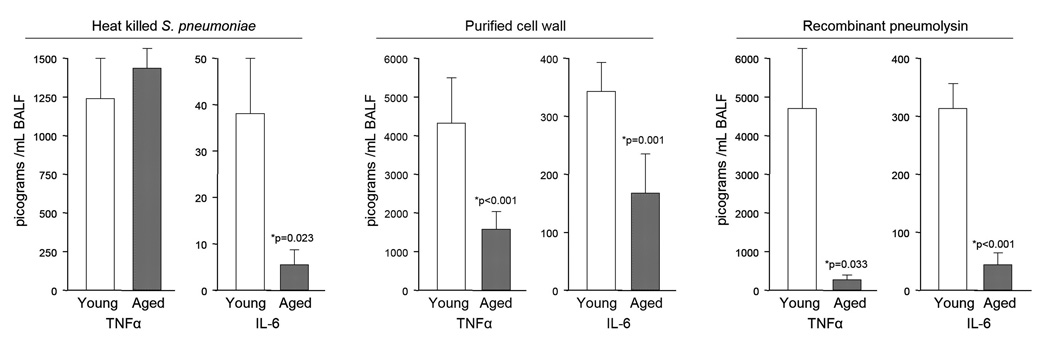
To determine if reduced TLR levels might be responsible for the anergic NFkB response, we examined TLR levels in the lungs of young and aged mice. Although no differences were observed between uninfected young and aged mice in mRNA levels for TLR-1, 2, and 4, we determined that aged mice had 52%, 22%, and 68% the levels of these proteins, respectively, than young mice (Figure 5A). This reduction was also observed in infected aged mice having 16%, 28%, and 48% the TLR levels of young mice, respectively (Figure 5B). We subsequently determined that aged mice produced significantly less TNFα and IL-6 when intratracheally challenged with heat killed bacteria, recombinant pneumolysin, and purified pneumococcal cell wall (Figure 6). Heat killed bacteria was the least inflammatory component tested and age-dependent differences were observed only in regards to IL-6 (aged mice: 16% of young IL-6 levels). Recombinant pneumolysin was a potent TLR stimulus in young mice but not aged mice. Aged mice produced 5% and 13% of TNFα and IL-6 levels that young mice produced, respectively. Purified cell wall gave similar results; aged mice produced 41% and 52% the levels of TNFα and IL-6 than young mice, respectively. Collectively, these experiments demonstrate that aged mice respond to S. pneumoniae with a muted cytokine response. Combined with the previously observed age-dependent decrease in TLR protein levels, these results suggest age-dependent TLR dysfunction (ADTD) occurs in the lungs and helps to explain the increased susceptibility of aged mice to pneumococcal infection.
Figure 5. TLRs are decreased only in aged mice prior to and during infection.
Relative levels of TLR-1, TLR-2, and TLR-4 proteins measured in the lungs of healthy and infected young and aged mice two days after infection. Squares indicate the individual protein expression level for each mouse tested. Horizontal bars indicate the median value. Protein loads were confirmed to be equal by probing for actin (not shown). Statistical analysis was performed using a Student’s t-test.
Figure 6. Aged mice produce less TNFα and IL-6 when challenged with S. pneumoniae components.
Levels of TNFα and IL-6 in BALF of young and aged mice 6 hours after intratracheal challenge with 100 µl of PBS containing either 107 cfu equivalents of whole heat killed S. pneumoniae (young n=4, aged n=6), purified pneumococcal cell wall (108 cfu equivalents; young n=7, aged n=7) or recombinant pneumolysin (1µg/mL; young n=5, aged n=5). Statistics was performed using a Student’s t-test.
DISCUSSION
In the elderly pneumococcal pneumonia is characterized by its rapid onset, severity, and high case-fatality rate [4–7]. Consistent with reports describing increased levels of activated NFkB in aged tissues [19–21], elevated levels of pro-inflammatory cytokines in serum and BALF of healthy seniors [22, 23], and positive regulation of pIgR and PAFr by nuclear localized NFkB [11, 12], we determined that aged mice had increased levels of pIgR and PAFr in their lungs, moreover were more susceptible to infection with S. pneumoniae. In agreement with findings by Yende et al., which have shown that elevated levels of TNFα and IL-6 are alone sufficient to increase the risk for CAP [36], we determined that mice infused with TNFα were more susceptible to S. pneumoniae than those that received saline. Our observation of increased pIgR and PAFr protein in these mice provides a molecular mechanism that helps to explain how aging and pre-infection inflammation, such as that which is frequently observed in individuals with underlying morbidities (e.g. cardiovascular disease), increases susceptibility to CAP.
Because of the inflammatory nature of pneumococcal disease, we hypothesized that during pneumonia elevated levels of pIgR and PAFr would be present in the lungs of aged mice. Unexpectedly, we observed the opposite, and determined that despite considerably greater bacterial burden, aged mice had lower levels of pIgR and PAFr in the lungs and less NFkB activation. Importantly, young mice infused with TNFα had normal pIgR and PAFr expression/production as well as NFkB activation during infection (data not shown) suggesting that this muted response was not the result of prolonged exposure to pro-inflammatory cytokines. TLRs-1, 2 and 4 detect pneumococcal PAMPs and initiate a cell signaling cascade that activates NFkB. Further experiments determined that aged mice had significantly reduced protein levels of TLRs 1, 2, and 4 in their lungs and produced less TNFα and IL-6 when challenged with purified pneumococcal components. These observations help to explain the reduced levels of activated NFkB observed in infected aged mice. It is plausible that diminished TLR levels would lead to reduced cell-signaling, activation of NFkB and subsequent cytokine production. Reduced cytokine production would further diminish autocrine and paracrine NFkB activation through the TNFα and IL-6 receptor pathways.
Multiple age-related immune defects have been reported that increase susceptibility to pneumonia including reduced mucociliary clearance of air-borne particles, diminished macrophage function and T-cell activation, and decreased antibody avidity [37–39]. While ADTD has already been described for peritoneal macrophages from aged C57/Bl6 mice, as well as peripheral monocytes from healthy elderly humans [40–42], this is the first report to indicate that age-dependent TLR dysfunction occurs in the lungs and that it may contribute towards susceptibility to pneumonia. Interestingly, elderly with pneumonia often have disease presentation that would be considered atypical for mature adults. One or more of the 3 classic symptoms for pneumonia (cough, fever, dyspnea) are absent in >50% of the elderly with pneumonia and ~10% show no signs of infection with exception to confusion or delirium [43]. TLR dysfunction is a possible explanation for why these individuals fail to show overt symptoms of infection such as fever. Importantly, age-related defects in TLR-signaling may be occurring simultaneously with diminished TLR protein levels. Studies have shown a 50% reduction in the expression of protein kinases (e.g. p38 and JNK) and their phosphorylation in peritoneal macrophages isolated from aged mice versus young controls [41].
At this time it is unclear which cells in the lungs experience ADTD. While published data has shown that macrophages experience ADTD [40–42], the possibility of epithelial cell TLR-dysfunction is supported by the fact that we observed these age-dependent changes using whole lung extracts which are composed primarily of non-lymphoid cells. Which lung cells experience ADTD is an important question that remains to be answered. Another important consideration is that changes in pIgR and TLR protein levels were not mirrored by changes in the corresponding mRNAs. However, multiple studies have shown that the amount of mRNA detected does not always correlate with the observed protein levels [44, 45]. Possible explanations include regulation of protein production by post-translational modification and age-dependent differences in protein degradation.
Multiple respiratory tract pathogens including Haemophilus influenzae, N eisseria meningitidis, and Pseudomonas aeroginosa express ChoP on their surface and use it to bind PAFr [46–48]. Increased lung expression of PAFr as a result of aging/chronic inflammation would facilitate the ability of these bacteria to cause respiratory disease. Conflicting reports exist regarding the ability of S. pneumoniae CbpA to attach to mouse pIgR [49, 50], however it is undisputed that CbpA binds to human pIgR. Increased levels of pIgR in the lungs of the elderly would also most likely increase their susceptibility to pneumonia. Finally, age-dependent TLR-1, 2, and 4 defects would impair the ability of aged animals to detect a variety of microorganisms and delay activation of the immune response, again increasing susceptibility to pneumonia. It remains undetermined if other TLRs are also negatively affected by age in the lungs.
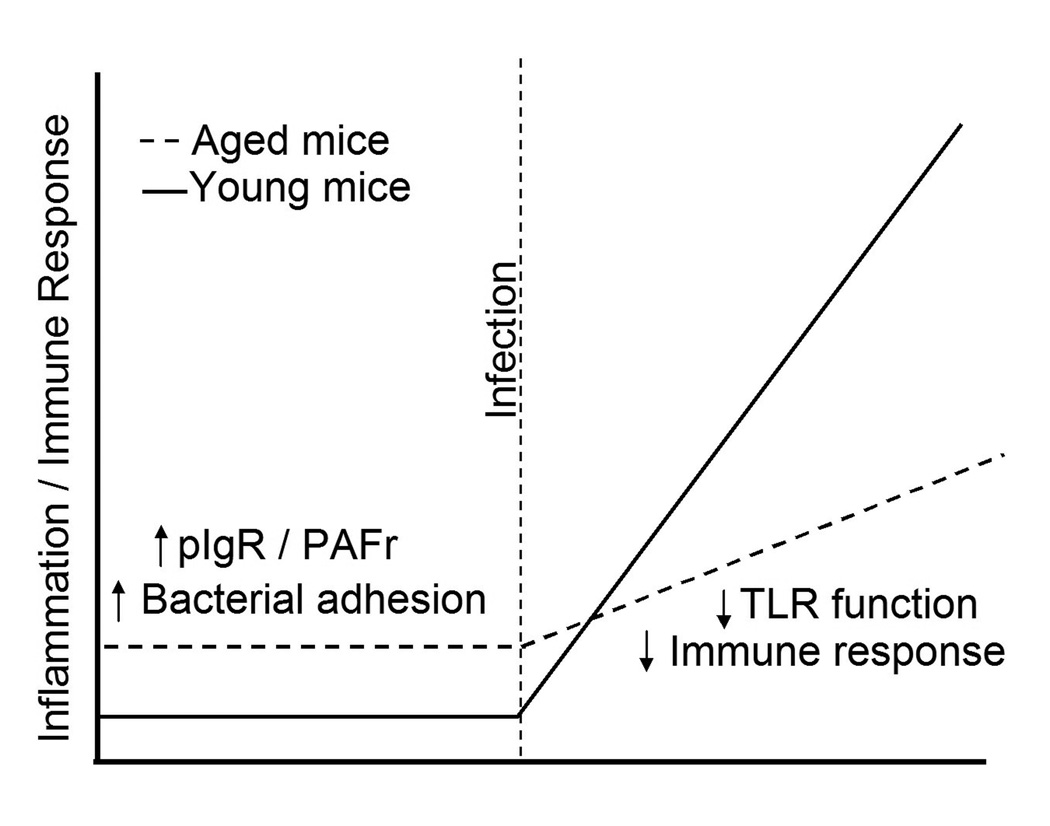
At first glance AAI and TLR dysfunction appear to be paradoxical. However, AAI occurs in uninfected aged animals; a very different physiological condition than during infection. In Figure 7, we propose a model that suggests AAI primes the lungs for S. pneumoniae attachment and facilitates the establishment of a lower respiratory tract infection. Subsequently, ADTD inhibits detection of the bacteria and results in a delayed/muted immune response. Our finding that AAI primes the lungs for infection through pIgR and PAFr, combined with the finding that aged mice experience lung TLR dysfunction suggests that in addition to the already described age-associated immune defects, conditions are favorable for the development of S. pneumoniae infection. Because these events occur before the innate immune system is fully engaged, these studies suggest that an infection can become well established before an adequate immune response is initiated.
Figure 7. Illustration explaining how age-associated inflammation and Toll-like receptor dysfunction increases the susceptibility of the elderly to pneumococcal pneumonia.
Underlying disease and chronic age-associated inflammation cause expression of the host proteins pIgR and PAFr in the lungs which the pneumococcus uses to attach to and invade lung cells. Once the infection is established, TLR dysfunction results in a muted immune response to the bacteria and development of fulminate pneumonia with a high mortality rate.
Acknowledgments
Financial support: This research was made possible by support from the National Institutes of Health, National Institute on Aging grants AG29313 and AG013319-14S2.
Footnotes
Potential conflicts of interest: The authors do not have commercial or other associations that might pose a conflict of interest.
Presented in part: American Thoracic Society 2008 International Conference; Toronto, Canada, May 16–21, 2008: abstract A587.
CITATIONS
- 1.Janssens JP. Pneumonia in the elderly (geriatric) population. Curr Opin Pulm Med. 2005;11:226–230. doi: 10.1097/01.mcp.0000158254.90483.1f. [DOI] [PubMed] [Google Scholar]
- 2.Pneumococcal vaccines. WHO position paper. Wkly Epidemiol Rec. 1999;74:177–183. [PubMed] [Google Scholar]
- 3.Edwards KM. Pneumococcal infections: Therapeutic strategies and pitfalls. In: Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG, editors. The Pneumococcus. Washington D.C.: ASM Press; 2004. pp. 314–330. [Google Scholar]
- 4.Feikin DR, Schuchat A, Kolczak M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90:223–229. doi: 10.2105/ajph.90.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Active Bacterial Core Surveillance (ABCs) Centers for Disease Control and Prevention; 2007. Report Emerging Infections Program Network Streptococcus pneumoniae, 2006. [Google Scholar]
- 6.Kinsell K, Velkoff VA. In: An Aging World: 2001. Bureau USC, editor. Vol. P95/01-1. U.S. Government Printing Office; 2001. [Google Scholar]
- 7.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. Jama. 2005;294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 8.Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. Jama. 2001;285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 9.Malley R, Henneke P, Morse SC, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber JR, Freyer D, Alexander C, et al. Recognition of pneumococcal peptidoglycan: an expanded, pivotal role for LPS binding protein. Immunity. 2003;19:269–279. doi: 10.1016/s1074-7613(03)00205-x. [DOI] [PubMed] [Google Scholar]
- 11.Bruno ME, Kaetzel CS. Long-term exposure of the HT-29 human intestinal epithelial cell line to TNF causes sustained up-regulation of the polymeric Ig receptor and proinflammatory genes through transcriptional and posttranscriptional mechanisms. J Immunol. 2005;174:7278–7284. doi: 10.4049/jimmunol.174.11.7278. [DOI] [PubMed] [Google Scholar]
- 12.Mutoh H, Ishii S, Izumi T, Kato S, Shimizu T. Platelet-activating factor (PAF) positively auto-regulates the expression of human PAF receptor transcript 1 (leukocyte-type) through NF-kappa B. Biochem Biophys Res Commun. 1994;205:1137–1142. doi: 10.1006/bbrc.1994.2784. [DOI] [PubMed] [Google Scholar]
- 13.Cundell DR, Gerard C, Idanpaan-Heikkila I, Tuomanen EI, Gerard NP. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv Exp Med Biol. 1996;416:89–94. doi: 10.1007/978-1-4899-0179-8_16. [DOI] [PubMed] [Google Scholar]
- 14.Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JR, Mostov KE, Lamm ME, et al. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell. 2000;102:827–837. doi: 10.1016/s0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 16.Miller ML, Gao G, Pestina T, Persons D, Tuomanen E. Hypersusceptibility to invasive pneumococcal infection in experimental sickle cell disease involves platelet-activating factor receptor. J Infect Dis. 2007;195:581–584. doi: 10.1086/510626. [DOI] [PubMed] [Google Scholar]
- 17.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 20.Helenius M, Hanninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318(Pt 2):603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan ZQ, Sirsjo A, Bochaton-Piallat ML, Gabbiani G, Hansson GK. Augmented expression of inducible NO synthase in vascular smooth muscle cells during aging is associated with enhanced NF-kappaB activation. Arterioscler Thromb Vasc Biol. 1999;19:2854–2862. doi: 10.1161/01.atv.19.12.2854. [DOI] [PubMed] [Google Scholar]
- 22.Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996;153:1072–1079. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- 23.Meyer KC, Rosenthal NS, Soergel P, Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech Ageing Dev. 1998;104:169–181. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 24.Meyer KC, Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax. 1999;54:697–700. doi: 10.1136/thx.54.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer NF, Poynter ME, Im SY, Daynes RA. Constitutive activation of NF-kappa B in an animal model of aging. Int Immunol. 1997;9:1581–1588. doi: 10.1093/intimm/9.10.1581. [DOI] [PubMed] [Google Scholar]
- 26.Tettelin H, Masignani V, Cieslewicz MJ, et al. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc Natl Acad Sci U S A. 2002;99:12391–12396. doi: 10.1073/pnas.182380799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster D, Knox K, Walker AS, et al. Invasive pneumococcal disease: epidemiology in children and adults prior to implementation of the conjugate vaccine in the Oxfordshire region, England. J Med Microbiol. 2008;57:480–487. doi: 10.1099/jmm.0.47690-0. [DOI] [PubMed] [Google Scholar]
- 28.Orihuela CJ, Gao G, McGee M, Yu J, Francis KP, Tuomanen E. Organ-specific models of Streptococcus pneumoniae disease. Scand J Infect Dis. 2003;35:647–652. doi: 10.1080/00365540310015854. [DOI] [PubMed] [Google Scholar]
- 29.Hilgendorff A, Muth H, Parviz B, et al. Statins differ in their ability to block NF-kappaB activation in human blood monocytes. Int J Clin Pharmacol Ther. 2003;41:397–401. doi: 10.5414/cpp41397. [DOI] [PubMed] [Google Scholar]
- 30.Moussa K, Michie HJ, Cree IA, et al. Phagocyte function and cytokine production in community acquired pneumonia. Thorax. 1994;49:107–111. doi: 10.1136/thx.49.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuomanen E, Hengstler B, Zak O, Tomasz A. Induction of meningeal inflammation by diverse bacterial cell walls. Eur J Clin Microbiol. 1986;5:682–684. doi: 10.1007/BF02013304. [DOI] [PubMed] [Google Scholar]
- 32.Maus UA, Srivastava M, Paton JC, et al. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J Immunol. 2004;173:1307–1312. doi: 10.4049/jimmunol.173.2.1307. [DOI] [PubMed] [Google Scholar]
- 33.Ulich TR, Watson LR, Yin SM, et al. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol. 1991;138:1485–1496. [PMC free article] [PubMed] [Google Scholar]
- 34.Rozen S, Skaletsky HJ. Primer3 on teh WWW for gereneral users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 35.Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yende S, Tuomanen EI, Wunderink R, et al. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005;172:1440–1446. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chelvarajan RL, Collins SM, Doubinskaia IE, et al. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J Leukoc Biol. 2004;75:982–994. doi: 10.1189/jlb.0403179. [DOI] [PubMed] [Google Scholar]
- 38.Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77:503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 39.Finkelstein MS. Unusual features of infections in the aging. Geriatrics. 1982;37:65–67. 71–75, 78. [PubMed] [Google Scholar]
- 40.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 41.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 42.van Duin D, Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc. 2007;55:1438–1444. doi: 10.1111/j.1532-5415.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- 43.Fein AM. Pneumonia in the elderly. Special diagnostic and therapeutic considerations. Med Clin North Am. 1994;78:1015–1033. doi: 10.1016/s0025-7125(16)30117-1. [DOI] [PubMed] [Google Scholar]
- 44.Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- 45.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swords WE, Ketterer MR, Shao J, Campbell CA, Weiser JN, Apicella MA. Binding of the non-typeable Haemophilus influenzae lipooligosaccharide to the PAF receptor initiates host cell signalling. Cell Microbiol. 2001;3:525–536. doi: 10.1046/j.1462-5822.2001.00132.x. [DOI] [PubMed] [Google Scholar]
- 47.Swords WE, Buscher BA, Ver Steeg Ii K, et al. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37:13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- 48.Weiser JN, Goldberg JB, Pan N, Wilson L, Virji M. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1998;66:4263–4267. doi: 10.1128/iai.66.9.4263-4267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elm C, Braathen R, Bergmann S, et al. Ectodomains 3 and 4 of human polymeric Immunoglobulin receptor (hpIgR) mediate invasion of Streptococcus pneumoniae into the epithelium. J Biol Chem. 2004;279:6296–6304. doi: 10.1074/jbc.M310528200. [DOI] [PubMed] [Google Scholar]
- 50.Rosenow C, Ryan P, Weiser JN, et al. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]