Abstract
Lipolysis in adipocytes is associated with phosphorylation of hormone sensitive lipase (HSL) and translocation of HSL to lipid droplets. In this study, adipocytes were cultured in a high-throughput format (96-well dishes), exposed to lipolytic agents, and then fixed and labeled for nuclei, lipid droplets, and HSL (or HSL phosphorylated on serine 660 [pHSLser660]). The cells were imaged via automated digital fluorescence microscopy, and high-content analysis (HCA) methods were used to quantify HSL phosphorylation and the degree to which HSL (or pHSLser660) colocalizes with the lipid droplets. HSL:lipid droplet colocalization was quantified through use of Pearson's correlation, Mander's M1 Colocalization, and the Tanimoto coefficient. For murine 3T3L1 adipocytes, isoproterenol, Lys-γ3-melanocyte stimulating hormone, and forskolin elicited the appearance and colocalization of pHSLser660, whereas atrial natriuretic peptide (ANP) did not. For human subcutaneous adipocytes, isoproterenol, forskolin, and ANP activated HSL phosphorylation/colocalization, but Lys-γ3-melanocyte stimulating hormone had little or no effect. Since ANP activates guanosine 3′,5′-cyclic monophosphate (cGMP)-dependent protein kinase, HSL serine 660 is likely a substrate for cGMP-dependent protein kinase in human adipocytes. For both adipocyte model systems, adipocytes with the greatest lipid content displayed the greatest lipolytic responses. The results for pHSLser660 were consistent with release of glycerol by the cells, a well-established assay of lipolysis, and the HCA methods yielded Z′ values >0.50. The results illustrate several key differences between human and murine adipocytes and demonstrate advantages of utilizing HCA techniques to study lipolysis in cultured adipocytes.
Introduction
Obesity is an overwhelming problem in the United States and worldwide,1–3 has enormous health and economic impact,4 and is an important underlying risk factor for coronary artery disease, fatty liver disease, and diabetes. Fat is stored predominantly in lipid droplets within adipocytes and to a lesser extent in hepatocytes and skeletal muscle fibers. The lipid droplets contain triglycerides, an important store of metabolic energy. A variety of proteins associate with lipid droplets and are involved in the regulation of triglyceride storage and metabolism. Lipid droplets in adipocytes are coated with perilipin, the founding member of the PAT family of proteins (named for Perilipin, ADRP, TIP47).5 In a well-defined pathway of hormone-regulated lipolysis, activation of β-adrenergic receptors increases adenosine3′,5′-cyclic monophosphate (cAMP) levels, and activates protein kinase A (PKA). PKA, in turn, phosphorylates hormone sensitive lipase (HSL) and perilipin,6,7 leading to translocation of HSL to the outer edge of the lipid droplets.
An additional lipase, adipocyte triglyceride lipase (ATGL), is also critical for lipolysis.8 ATGL may initiate metabolism of the lipid droplet triglycerides, releasing fatty acid from triglyceride to form diacylglycerol. HSL then acts on diacylglycerol (its preferred substrate) to release fatty acid and monoacylglycerol. ATGL and HSL perform enzymatic reactions along the same lipolytic path; thus, hormonal pathways that regulate HSL activity are also likely to regulate ATGL. In addition to processing tri- and di-glycerides, HSL is also a neutral cholesteryl ester hydrolase and is found in adrenal glands, testes, ovaries, placenta, and macrophages, where it participates in cholesterol metabolism and steriodogenesis.9–11 Pancreatic β-cells express a distinct isoform of HSL, and β-cell HSL activity is involved in glucose-mediated insulin secretion.12,13 Thus, HSL activity is critical to steroid metabolism, macrophage foam cell development, and pancreatic function, in addition to the metabolism of triglycerides.
Three serines within HSL are phosphorylated by PKA (serine 563, 659, and 660—by convention, the serine numbering nomenclature originally developed with rat HSL will be used in the present report14) and phosphorylation of these sites activate HSL.15–18 Serine 553, 555, and 651 in human HSL are highly analogous to serine 563, 565, and 660 in rat HSL. HSL activity is also activated by a variety of hormones via extracellular signal-regulated kinase (ERK)-mitogen-activated protein kinase (MAPK),15,19,20 and this is likely due to ERK-MAPK-mediated phosphorylation of HSL on serine 600.15 In contrast, AMP-activated protein kinase phosphorylates HSL on serine 565, which prevents activation of HSL.21,22 A longer splice variant of HSL, which features an additional 300 amino acid sequence at the amino terminus, is expressed within the testes.18
The hormonal and signal transduction pathways that regulate HSL continue to be elucidated. Lys-γ3-melanocyte stimulating hormone (Lys-γ3-MSH) regulates lipolysis in adipocytes and other tissues23,24 and stimulates phosphorylation of HSL on serine 660 in 3T3L1 cells.23 Receptors for Lys-γ3-MSH are found in adipose tissue, skeletal muscle, testes, heart, ovary, and spleen, as well as the adrenal gland, which correlates well with the distribution of HSL.24 Lys-γ3-MSH increases HSL activity in the rat adrenal cortex, but may not activate adenylyl cyclase activity in that tissue.25,26 Thus, the mechanism via which Lys-γ3-MSH regulates HSL in various tissues remains to be fully elucidated. Additionally, atrial natriuretic peptide (ANP) increases lipolysis, and this likely involves increased cGMP levels and phosphorylation of HSL by cGMP-dependent protein kinase27,28; however, the phosphorylation sites involved the activation of HSL by ANP have not been reported.
In addition to the many hormonal pathways that acutely regulate HSL activity, lipolysis may also be regulated by genetic variations within the HSL gene or by conditions that influence expression of HSL. For example, β-adrenergic stimulation of lipolysis is sometimes blunted in adipocytes isolated from obese humans or from their first-degree relatives.29,30 Polymorphisms within introns 6 and 7 of the HSL gene are also linked to blunted metabolic responses to isoproterenol (ISO)31 and polymorphisms in the 5′ noncoding promoter region are linked to variations in waist circumference.32 In contrast, increased expression of HSL occurs in patients afflicted with cancer and AIDS, and this may contribute to involuntary weight loss (cachexia).33,34
High-content analysis (HCA) is an emerging technique in which cells are exposed to test compounds or genomic constructs, and observed for one or multiple protein or organelle biomarkers via fluorescence or bright-field microscopy; the cells are then digitally photographed, and the images analyzed in an automated manner. The term “high-content” refers to the wealth of information available from cell images.35–39 HCA methods can be used in a high-throughput mode to screen chemical or genomic libraries, or to further characterize lead compounds for potential beneficial or toxic effects. Multi-gigabyte data sets containing thousands of images are generated in typical HCA experiments, and image analysis algorithms are needed to extract information relevant to the cellular process of interest. HCA-based high-throughput screens of genomic constructs have identified Coat Protein Complex I proteins as important mediators of lipid droplet formation,40,41 and certain microRNAs that regulate hepatocyte lipid droplet content.42 HCA was also used to identify a lipid droplet-regulating function for seipin, a protein mutated in patients with Beradinelli-Seip Congential Lipodystrophy, a condition in which adipose tissue is reduced.43
Recently, methods have been developed that allow HSL, or phosphorylated forms of HSL (e.g., pHSLser660), to be selectively labeled and observed.14 Further, algorithms have been developed for analysis of the expression and lipid droplets and the distribution of cellular proteins with respect to the lipid droplets.35 Although previous studies have described hormone-induced translocation of HSL to the lipid droplets in qualitative terms, a goal of the present study was to explore the possibility of quantifying HSL translocation via analysis of the colocalization of HSL with the lipid droplets. To do this, a general strategy was utilized, in which nuclei, lipid droplets, and HSL (or HSL phosphorylated on serine 660) were observed in separate fluorescence channels, and the two channels corresponding to lipid droplets and HSL analyzed via correlation and colocalization analyses. Lipid droplet/HSL colocalization was quantified via the Pearson's correlation (Pr), which is widely used for analyzing correlations in a variety of contexts, the Mander's M1 colocalization coefficient, which was developed to quantify colocalization of cellular proteins or structures in digital images obtained via fluorescence microscopy,44 and the Tanimoto coefficient (Tan), which is widely used in analyzing chemical fingerprint data45,46 but has not previously been applied to images of cells.
Methods
Cell Culture
3T3L1 cells were obtained from ATCC and maintained in growth medium (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin). Cells were passed a maximum of 17 times before use in experiments. To set up the 3T3L1 cells for differentiation and experimentation, the cells were seeded in 75 cm2 flasks and allowed to grow to confluence. The cells were then exposed to the differentiation medium, which was the growth medium additionally supplemented with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 0.25 μM dexamethasone, and 1 μg/mL insulin (see protocol in Table 1). After 3 or 4 days in the differentiation medium, the cells were harvested, seeded in 96-well glass-bottomed dishes (Nunc Cat. no. 73520-168; two 96-well dishes/flask) precoated with gelatin that was cross-linked with glutaraldehyde35 at a density of 67,000 cells/well, and refed with the growth medium. With this protocol, a high degree of the cells typically featured clusters of lipid droplets and experiments were typically performed 2 to 5 days after seeding into the 96-well dishes. Human subcutaneous preadipocytes, harvested via liposuction procedures (Lot No. S0031; a mixture of preadipocytes obtained via liposuction procedures from 6 female donors; average age 39; average body mass index (BMI) = 27.3; BMI range = 25.2–29.4) by Zen-Bio were plated (13,000 cells/well; passage 2) on glass-bottomed 96-well dishes (Nunc). Cells were exposed to the peroxisome proliferator-activated receptor γ (PPARγ) agonist rosiglitazone (300 nM) to induce adipogenesis for 17 days, before experimentation.
Table 1.
Overview of the Cell Culture and Assay Protocols
| A. 3T3L1 Adipocytes | |||
|---|---|---|---|
| Step | Parameter | Value | Description |
| 1 | Cell maintenance | 75 cm2 flask | Split 1:2, weekly, into flasks for maintaining the cell line and flasks for adipogenesis. |
| 2 | Adipogenesis | 75 cm2 flask | Medium supplemented with 0.5 mM IBMX, 0.25 μM dexamethasone, and 1 μg/mL insulin for 3 days. |
| 3 | Seeding for HTS | 96-well dish | Seed 30,000 to 60,000 differentiated adipocytes/well in maintenance medium. |
| 4 | High-content assay | 96-well dish | 2–5 days postplating, cells were exposed to lipolytic agents, and then fixed and labeled for nuclei, lipid droplets, and protein. |
| B. Human Subcutaneous Adipocytes | |||
|---|---|---|---|
| Step | Parameter | Value | Description |
| 1 | Adipogenesis | 96-well dish | Preadipocytes (passage 2) were seeded at 13,000 cells/well and exposed to 300 nM rosiglitazone for 17 days. |
| 2 | High-content assay | 96-well dish | Cells were exposed to lipolytic agents, and then fixed and labeled for nuclei, lipid droplets, and protein. |
To maintain the 3T3L1 line, cells were cultured before reaching confluence. To prepare for adipogenesis, cells were cultured 2–3 days after achieving confluence to insure they were postmitotic.
Precoating of the 96-well glass-bottomed dishes with either gelatin (cross linked with glutaraldehyde) or Matrigel (diluted 1/50) is necessary to insure adipocyte adherence. A large number of adipocytes were plated per well, as confluent cell cultures are best analyzed by CyteSeer®.
Experiments were performed in the absence of rosiglitazone (cells were switched to rosiglitazone-free medium, 2 h before experimentation).
IBMX, 3-isobutyl-1-methylxanthine; HTS, high-throughput screening.
Lipid Staining and Protein Labeling
After exposure to test chemicals, cells were rinsed with phosphate-buffered saline (PBS), and fixed with 4% paraformaldehyde (15 min, room temperature), followed by 15 min permeabilization with 0.1% Triton-X prepared in PBS. Permeabilization and all other steps were performed with rotation at 37°C. After fixation and permeabilization, the cells were incubated in blocking buffer (10% normal goat serum, 3% bovine serum albumin, and 0.02% sodium azide, in PBS) for 60 min, and then with labels that are specific for either HSL or for HSL that has been preferentially phosphorylated on serine 660 (pHSLser660) (components of Vala Sciences's HSL and HSL Activity kit). After labeling, the samples were rinsed three times with PBS and incubated with fluorescent secondary reagents, which included Vala Sciences's lipid staining reagent to label the lipid droplets in the green fluorescent channel and HSL in the red fluorescence channel. Finally, the samples were rinsed and stained for nuclei by incubating for 20 min in 4′,6-diamidino-2-phenylindole (DAPI; 250 ng/mL DAPI prepared in 10 mM Tris, 10 mM ethylenediaminetetraacetic acid, 100 mM NaCl, and 0.02% sodium azide, buffered to pH 7.4).
Image Acquisition
Images were acquired with a Beckman Coulter IC 100. This instrument includes a Nikon Eclipse microscope with an automated stage interfaced to a fluorescence light source and filter wheel and cubes with filters for UV (DAPI), fluorescein isothiocyanate, and rhodamine fluorescence. The workstation features a Windows computer, which controls stage positioning and data acquisition. Images were acquired with a Hamamatsu Orca ERG progressive scan 1,344 × 1,024 cooled interline CCD camera, utilizing 2 × 2 binning. Typically, four images (representing a 2 × 2 contiguous image set) were acquired in the center of each well with either a 20 × 0.5 NA (resulting in 0.6848 × 0.6848 μm2/pixel) or a 40 × 0.75 NA “dry” objective (resulting in 0.344 × 0.344 μm2/pixel). Images were stored as gray-scale bit-mapped images (*.bmp).
Lipolysis Assay
Lipolysis was measured in 3T3L1 and human adipocytes using Zen-Bio's Lipolysis Assay kit reagents. The medium was aspirated and the cells washed twice with 200 μL Wash Buffer before adding 10 μM ISO, 0.01 and 0.1 μM ANP, 0.1 μM Lys-γ3-MSH, or 6 μM forskolin (FSK) in 100 μL of Assay Buffer. Lipolysis proceeded for either 3.5 or 16 h at 37°C, after which 50 μL of the conditioned assay buffer was transferred to an assay plate. Fifty microliters of Glycerol Reagent A was added to each well and incubated at room temperature for 15 min before measuring the optical density at 540 nm. Accumulated glycerol in each sample was determined by comparison with a glycerol standard curve.
Quantification of Lipid Droplets and HSL Activation from Cell Images
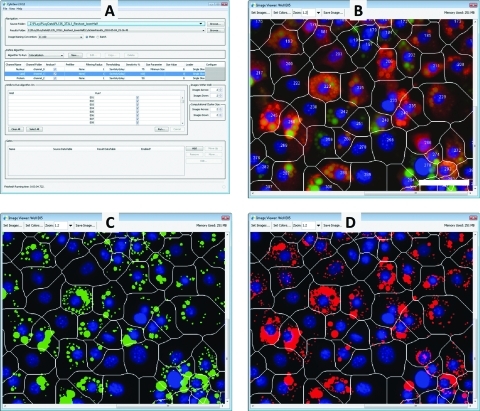
As described previously, Vala Sciences's has developed CyteSeer®, a Java-based, PC/Mac/Linux-compatible cell image analysis program designed specifically for HCA.35,47 The Lipid Droplet Algorithm, one of several cell analysis algorithms incorporated into CyteSeer, quantifies lipid droplet number and size. The Colocalization Algorithm performs the same analysis as the Lipid Droplet Algorithm, and additionally quantifies the degree of colocalization of a protein of interest, with the lipid droplets. CyteSeer's main interface (Fig. 1) enables users to direct the program toward a folder containing images to be analyzed, and also to an output directory, to which CyteSeer will write results in text files that are compatible with Excel. After cell images are loaded into CyteSeer, and the analysis initiated, cells are uniquely identified within each field of view by identifying the individual nuclei within the nuclear image, and estimating the boundaries of each cell (the whole cell mask) via a tessellation procedure (Fig. 1B). Next, the Lipid mask (Lm) for each cell is identified from the Lipid image (Fig. 1C), and the Protein mask (Pm) is identified from the Protein image (Fig. 1D). The sensitivity of the algorithm can be adjusted for each channel, independently, to compensate for differences in image exposure.
Fig. 1.
High-content analysis of lipid droplets and associated proteins by CyteSeer®. (A) CyteSeer's main interface allows users to navigate to directories that contain images to analyze and to directories to which mask and tabular data will be stored. (B) CyteSeer's Image Viewer allows images from each fluorescence channel to be displayed independently, or overlaid in any combination. 3T3L1 adipocytes treated with ISO are shown observed for nuclei (blue), lipid droplets (green), and pHSLser660 (red); estimated boundaries (white lines) that define the whole cell mask for each cell are also shown along with cell identification numbers. The image was obtained with a 40 × objective. Scale bar = 50 μm. (C) and (D) are the Lipid (green) and Protein (red) masks, respectively, derived by the Colocalization Algorithm, shown overlaid on the nuclear image. ISO, isoproterenol.
The Colocalization Algorithm extracts 91 data parameters from the Nuclear, Lipid, and Protein images. Data parameters featured in this report include the area of the Protein mask (Area Pm) and the mean Lipid Droplet area (Mean LD Area), which is the average area of the lipid droplets/cell (Table 2). Next is the Average Pixel Intensity of the Protein image for the Protein mask (API Pi Pm), which is calculated by summing the pixel intensities for pixels within the Protein mask of the Protein image, then dividing by the number of pixels within the Protein mask. Since the images collected were 8-bit, API values ranged from 0 to 255. The API for the Lipid image, Lipid mask, (API Li Lm) is calculated in an analogous manner. Note that CyteSeer projects a mask developed with one image to the other images acquired for the same field of view. For example, the API of the Protein image over the Lipid mask (API Pi Lm) is calculated by first identifying the x,y coordinates of pixels within the Lipid image that correspond to the Lipid mask (the lipid droplets); the intensities of pixels at the same x,y coordinates within the Protein image are then sampled to obtain API Pi Lm. API Pi Lm is therefore the average intensity of the protein that directly overlays and colocalizes to the lipid droplets.
Table 2.
Data Parameters Calculated By Cyteseer® from Images of Adipocytes
| Data parameter | Definition |
|---|---|
| Area Pm | Area of the Protein mask |
| Mean LD Area | Average area of the lipid droplets |
| API Pi Pm | Average Pixel Intensity of the Protein image for pixels that correspond to the Protein mask |
| API Li Lm | Average Pixel Intensity of the Lipid image for pixels that correspond to the Lipid mask |
| API Pi Lm | Average Pixel Intensity of the Protein image for pixels that correspond to the Lipid mask |
| Pr | Pearson's correlation coefficient calculated between the Lipid and the Protein images over the Whole cell mask |
| M Pr | Masked Pearson's correlation coefficient calculated between Lipid image, and the Protein image over the Whole cell mask |
| M1 | Masked Manders' M1 colocalization coefficient calculated between the Lipid and the Protein images over the Whole cell mask |
| Tan | Tanimoto coefficient, calculated between the Lipid mask and the Protein mask over the Whole cell mask |
The data parameters illustrated are a subset of the 91 different data parameters derived from cell images by Vala Sciences's Lipid Droplet and Colocalization Algorithms.
The Pr between the lipid and the protein images over the whole cell mask Pr was calculated using published equations.35 A second version of the Pr is the “M Pr” for the whole cell mask. To calculate M Pr, the algorithm first identifies the pixels within each cell that make up the Lipid and Protein masks, respectively. Nonmask pixels in both the Lipid and Protein images are then assigned a “0” intensity value and the Pr is calculated. Thus, M Pr provides an index of the colocalization that is selective for the regions within the cell where the lipid droplets and HSL are localized. The M Pr is only calculated for cells that contain both lipid droplets and protein (i.e., for cells in which both Area Lm and Area Pm are >0). For Pr and M Pr, the theoretical range is from −1.0 to +1.0; a value of −1 means that the two labels distribute in a perfectly exclusionary manner, a value of 0 means that the two labels are distributed randomly with respect to one another, and a value of 1.0 means that there is perfect correlation (colocalization).
The Manders' colocalization coefficient (M1) is calculated by CyteSeer in a manner similar to M Pr; after identification of the Lipid and Protein masks, nonmask pixels within the images are assigned a “0” intensity value, and the M1 coefficient is calculated for each cell utilizing published equations.44 M1 ranges from 0 to 1.0, and represents the fraction of fluorescence associated with the lipid droplets that is colocalized with protein. M1 is only calculated for cells that have lipid droplets (Area Lm > 0).
The Tan is defined as: Tan = c/(a + b − c), where a = the number of pixels in the Lipid mask, b = the number of pixels in the Protein mask, and c = the number of pixels common to both the Lipid and Protein masks. Tan is the fraction of overlap between the Lipid and Protein masks, and ranges from 0 to 1.00 and is thus conceptually related to M1. However, unlike M1, Tan does not take into account variations in pixel intensity within the masks. Tan is calculated by CyteSeer for any cell that exhibits either lipid droplets or protein (i.e., Tan is calculated for cells in which Area Lm > 0 or Area Pm >0).
CyteSeer calculates all data parameters on a true cell-by-cell basis. Furthermore, CyteSeer also provides population statistics (mean, standard deviation [SD], maximum, and minimum) derived from all cells in a sample (e.g., all cells observed in four images obtained from a single well of a 96-well dish). For the figures in this report, mean values/well are presented.
Statistical Analysis
GraphPad Prizm was used to test for statistically significant differences between controls and experimental groups (analysis of variance followed by Dunnett's test, or analysis of variance followed by Tukey's test), and to calculate EC50 and Hill coefficient values for agonist dose–response curves.
To quantitatively assess the suitability of the various data parameters for use in HCA, Z′ values were calculated. Z′ is an index of the dynamic range of an assay, which is a function of the ratio of the variability of the data to the difference between the means of treatments that result in maximum and minimum values,48
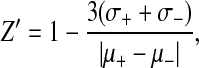 |
where μ+ and μ− are the SDs of the positive and negative controls, respectively, and μ+ and μ− are the corresponding means, respectively. Z′ has a theoretic range of negative infinity to 1.0 (an ideal assay in which there is no SD and finite separation between means would yield Z′ = 1.0). Z′ is conceptually much more stringent than a Student's t-test (Z′ can be negative, yet P can be <0.001 by Student's t-test, for example). The Z′ statistic goes beyond the assessment of whether or not there is a statistically significant difference between data sets to provide a quantitative index of assay performance. Z′ values >0.2 are considered very good for an assay, and assays in which Z′ >0.50 are considered excellent for potential high-throughput screening applications involving hundreds of thousands of test compounds, as such high Z′ values insure that the frequency of occurrence of false-positives in the data set will be nil (see Appendix for additional discussion of Z′).
Results
Quantification of Isoproterenol-Induced HSL Translocation and Phosphorylation in 3T3L1 Cells
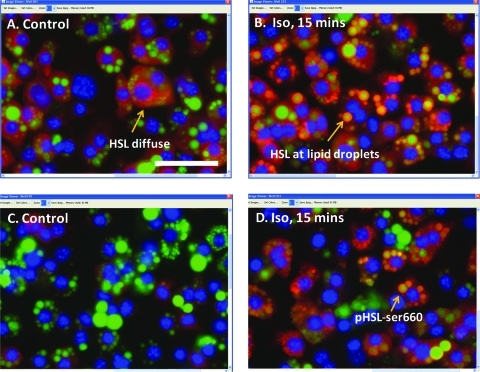
In an initial experiment to test if HSL activation could be studied via HCA, 3T3L1 adipocytes were exposed to the control medium or medium supplemented with 10 μM ISO for 15 min. The cells were then fixed, permeabilized, and labeled for nuclei, lipid droplets, and additionally for either HSL or pHSLser660. For cells exposed to the control medium, HSL was distributed throughout the cytoplasm (Fig. 2A); exposure to 10 μM ISO, however, led to translocation of HSL to the edges of the lipid droplets (Fig. 2B), which is consistent with previous observations that HSL translocates to the droplets, upon activation. Control cells labeled for pHSLser660 were very dim (Fig. 2C). In contrast, for cells exposed to ISO, the label for pHSLser660 was very apparent and highly localized to the edges of the lipid droplets (Fig. 2D).
Fig. 2.
HSL activation in 3T3-L1 adipocytes. Cells plated in a 96-well dish were exposed to the control medium (A) and (C) or 10 μM ISO for 15 min (B) and (D), and then fixed and labeled for nuclei, lipid droplets (green), and protein (red). (A, B) Cells observed for HSL. (C, D) Cells observed for pHSL-ser660. Images were collected with a 40 × objective. Scale bar = 50 μm. HSL, hormone sensitive lipase.
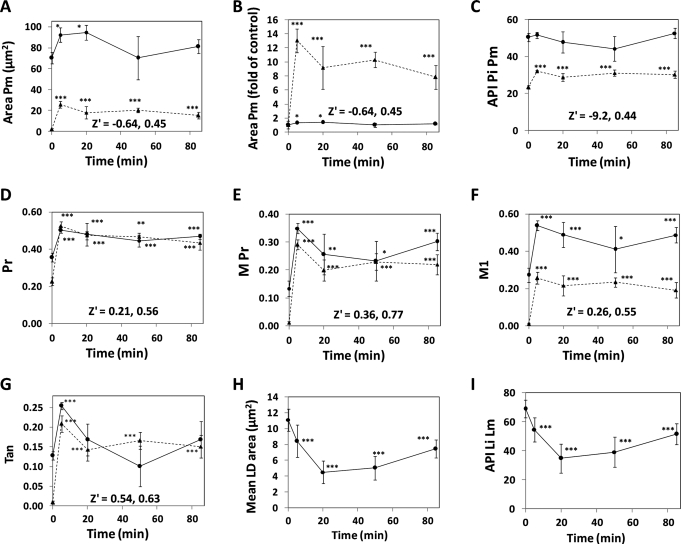
To quantify the effects of ISO on HSL, a second-time course experiment was performed, in which cells were exposed to 1 μM ISO, and images obtained from the cells were analyzed via CyteSeer's Colocalization Algorithm. For control cells observed for HSL, Area Pm averaged 70.2 μm2 and this increased 92.2 μm2 after 5 min of ISO, a small, but statistically significant effect (P < 0.05, Figure 3A, solid line), likely due to increased overall recognition of HSL by the Colocalization Algorithm after the HSL has translocated to the lipid droplets. In contrast, for pHSLser660, the Area Pm for control cells averaged just 1.97 μm2, and this was increased to 25.6 μm2 by 5 min of ISO (P < 0.001, Figure 3A, dotted line). To further illustrate this relationship, data from Figure 3A were normalized to the time zero control value for each label (Fig. 3B); thus, ISO induced a 1.3-fold increase in HSL labeling, and a 13-fold increase in labeling for pHSLser660. For the effect of ISO on HSL for Area Pm, Z′ = −0.64; in contrast, for pHSLser660 for Area Pm, Z′ = 0.45. Thus, there is much better discrimination between the control and ISO-treated cells for pHSLser660 than for HSL. The effect of ISO on Area Pm for pHSLser660 was maximal at the 5 min time point, but it remained well above the control values, throughout the 90 min time course.
Fig. 3.
Time course of HSL activation by ISO. 3T3L1 cells were plated in a 96-well dish, exposed to 1 μM ISO for 0–85 min, and then fixed and labeled for either HSL or pHSLserine660. The plate was scanned at 40 × , four images/well, and the images analyzed with CyteSeer. (A–G) represent Area Pm, Area Pm normalized to the t = 0 time point, API Pi Pm, Pr, M Pr, M1, and Tan, respectively. For (A–G), circles and solid lines represent HSL; triangles and dotted lines represent pHSLser660; each symbol represents the mean ± SD for n = 4 wells (an average of 285 cells/well were analyzed). Z′ values calculated between the control time point and the 5 min time point are shown for HSL and for pHSLser660. (H) mean LD area. (I) API Li Lm. For (H, I), each symbol represents the mean ± SD for n = 16 wells. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001 versus 0 time point. API Pi Pm, Average Pixel Intensity of the Protein image for the Protein mask; Pr, Pearson's correlation; M Pr, Masked Pearson's correlation; M1, Manders' coefficient; Tan, Tanimoto coefficient; API Li Lm, API of the Lipid image for the Lipid mask; SD, standard deviation; mean LD area, mean lipid droplets area.
Another data parameter that is related to the amount of protein label that is present is API Pi Pm, which is the average intensity of the protein image, for pixels corresponding to the protein mask (Table 2). API Pi Pm for HSL averaged 49.5 for controls and did not change with addition of ISO (Fig. 3C). In contrast, API Pi Pm for pHSLser660 averaged 23.5 for controls and increased to 32.0 for the 5 min time point, an increase of 1.36-fold; since there was very little variability in the API Pi Pm data for pHSLser660, the effect of ISO was very significant (P < 0.001), and Z′ = 0.44.
Regarding quantification of colocalization of HSL with the lipid droplets, a series of relationships were calculated, which included the Pr, the M Pr, the M1, and the Tan. Pr is calculated utilizing the original unprocessed Lipid and Protein images as input, for all pixel locations within the whole cell mask. For control cells, Pr averaged 0.36 (Fig. 3D), suggesting a certain degree of association even in the absence of hormone; addition of ISO led to an abrupt increase in Pr, to 0.53, that was highly significant (P < 0.001, Z′ = 0.21). For pHSLser660, Pr averaged 0.23 for controls and 0.50 after 5 min of ISO; this response was also highly significant (P < 0.001, Z′ = 0.56). For both HSL and pHSLser660, Pr diminished, somewhat, after 5 min of ISO, but remained elevated compared to the controls throughout the time course. Notably, lipolytic responses mediated by β-adrenergic stimulation desensitize in the continued presence of agonist;49 such desensitization may have occurred in this experiment. In limited experiments, ISO also activated the appearance and colocalization of pHSLser563 to the lipid droplets, to a degree similar to that obtained with pHSLser660, but this was not extensively investigated (data not shown).
It was hypothesized that modifying the Pr analysis, to take advantage of CyteSeer's ability to identify lipid droplets and areas of elevated protein expression, would yield improved resolution between basal and activated cells. Accordingly, the M Pr was developed to selectively calculate colocalization between the identified Lipid and Protein masks (see Methods section). For HSL, M Pr averaged 0.13 for control cells, and this was increased to 0.35 after a 5 min exposure to ISO (P < 0.001, Z′ = 0.36, Fig. 3E). For pHSLser660, M Pr for control cells averaged 0.01, and this increased to 0.29 after 5 min of ISO (P < 0.001, Z′ = 0.77, which was the largest Z′ value in the experiment). Thus, compared to the Pr, the M Pr provided a greater degree of discrimination between control and ISO-treated cells, as demonstrated by the greater Z′ values achieved for M Pr. The M1, similar to M Pr, is also calculated on the images after identification of the Lipid and Protein masks (see Methods section). For control cells observed for HSL, M1 averaged 0.27 and this increased to 0.54 (twofold) in the presence of ISO (P < 0.001, Z′ = 0.26, Fig. 3F). For pHSLser660, M1 averaged 0.0100 for controls and 0.256 for cells treated with ISO for 5 min (25.6-fold increase, P < 0.001, Z′ = 0.55).
For HSL, Tan averaged 0.127 for control cells and this increased to 0.254 for cells treated with ISO for 5 min (twofold increase, Figure 3G, P < 0.001). For cells labeled for pHSLser660, Tan increased from 0.00850 to 0.209 after 5 min with ISO (24.6-fold increase, P < 0.001). For both HSL and pHSLser660, Tan provided excellent discrimination between the control and ISO treated cells at the early time point (Z′ values >0.50 for both labels).
Notably, all of the colocalization coefficients were maximal at the 5 min time point, which was consistent with the Area Pm data for pHSLser660. These data suggest that the phosphorylation of HSL at serine 660 in response to ISO may partially desensitize upon prolonged exposure to the agonist.
With the HCA assay, it was possible to examine the effect of ISO on the lipid droplets, themselves, along with observation of HSL. For control cells, lipid droplets averaged 11.1 μm2/droplet. However, the average area diminished to 4.48 μm2/droplet after a 20 min exposure to ISO (Fig. 3H). Similarly, API Li Lm, which is the average intensity of the lipid droplets as observed in the lipid image, diminished from 69.0 to 34.8 at the 20 min time point (Fig. 3I). Thus, exposure to ISO led to diminished size and brightness of the lipid droplets, consistent with the activation of the HSL. For longer time points, lipid droplet size and API Li Lm partially recovered; these results, obtained by analysis of the Lipid image, are also consistent with the hypothesis that the lipolytic response to ISO may desensitize, to some extent.
Dose–Response Relationships for the Phosphorylation and Lipid Droplet Colocalization of HSL in 3T3L1 Adipocytes
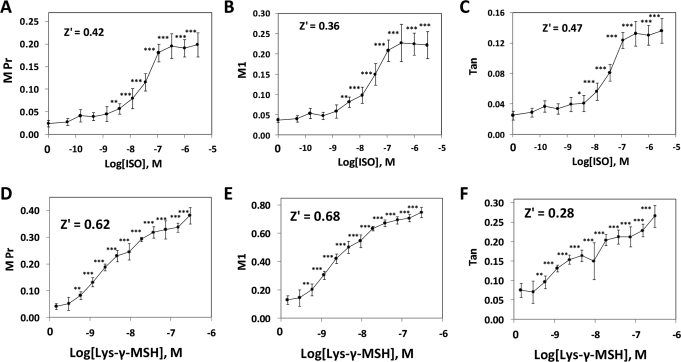
It was of interest to more fully characterize the response to ISO, and to compare it with the responses of other agents known to be lipolytic in 3T3L1 cells, such as Lys-γ3-MSH and FSK. For ISO, a dose–response experiment was conducted (threefold dilution series; highest concentration was 3 μM), and the cells were exposed to ISO for 15 min. For control cells, M Pr averaged 0.024, and this increased to 0.20 at 3 μM ISO (Fig. 4A), with EC50 = 31 nM and Hill Slope = 1.13. Similarly, M1 increased from 0.037 in the control cells to 0.22 at 3 μM ISO (Fig. 4B), with EC50 = 25 nM and Hill Slope = 1.05. For Tan, EC50 = 34 nM, and the Hill Slope = 1.32 (Fig. 4C). The dose–response relationship for ISO in this system is very similar to that reported for ISO-induced lipolysis in primary human subcutaneous adipocytes by other researchers.50
Fig. 4.
Quantification of pHSLser660:lipid droplet colocalization in 3T3L1 cells in response to ISO and Lys-γ3-MSH. 3T3L1 adipocytes were exposed to test compounds for 15 min, fixed, labeled, and observed for nuclei, lipid droplets, and pHSLser660. (A–C), the dose–response relationship ISO is shown, for M Pr, M1, and Tan, respectively. A 40 × objective was used, and each symbol represents the mean ± SD for n = 8 wells (an average of 413 cells were analyzed/well). (D–F) represent the same series of colocalization coefficients obtained for cells treated with Lys-γ3-MSH, utilizing a 20 × objective. Each symbol represents the mean ± SD for n = 8 wells (an average of 845 cells/well were analyzed). Z′ values were calculated between the controls and the highest concentration of agonist. *P < 0.05 versus controls; **P < 0.01; ***P < 0.001. Lys-γ3-MSH, Lys-γ3-melanocyte stimulating hormone.
The peptide hormone Lys-γ3-MSH stimulates lipolysis in 3T3L1 cells and other cell types.23,24 Accordingly, Lys-γ3-MSH was tested at concentrations ranging from 0.3 to 300 nM. Images of Lys-γ3-MSH-treated cells observed for pHSLser660 (not shown) appeared very similar to those for ISO-treated cells. Lys-γ3-MSH increased M Pr from 0.041 to 0.38 (Fig. 4D), with EC50 = 2.1 nM and Hill Slope = 0.576. M1 increased from 0.13 to 0.75 in response to Lys-γ3-MSH (Fig. 4E), with EC50 = 2.2 nM and Hill Slope = 0.76. Over the same concentration range, Tan increased from 0.075 to 0.266 (Fig. 4F), with a good Z′ value (Z′ = 0.28), but curve fitting on the original data yielded ambiguous results; however, excluding the results for 9.4 nM and 300 nM from the data set yielded an EC50 = 1.6 nM, with a Hill Slope of 0.77, which is consistent with the curve fits to M Pr and M1. The dose–response relationships shown here for the colocalization of pHSLser660 with lipid droplets are virtually identical to the dose–response relationship found for Lys-γ3-MSH-induced lipolysis in 3T3L1 cells.23,24
FSK activates adenylyl cyclase to increase cAMP production.51 FSK was found to be a very strong activator of pHSLser660 formation (see Appendix Table A1), with a maximal effect on M Pr, M1, and Tan at 6.67 μM. The effect of FSK was immediate (maximal or near-maximal at 5 min), but unlike the response to ISO, the response to FSK was more sustained, with little drop-off, throughout a 2 h time course. Since the response to FSK was strong and sustained, FSK was used as a positive control for the pHSLser660 response in subsequent experiments.
Analysis of pHSLser660 in Subpopulations 3T3L1 Adipocytes
3T3L1 murine adipocytes are heterogeneous with respect to expression of lipid droplets and protein markers of adipogenesis and it has been suggested that c-AMP-mediated lipolysis occurs to a greater extent in adipocytes that feature the greatest lipid droplet content.52 To test this hypothesis, an experiment was conducted in which 3T3L1 cells were exposed to a panel of potentially lipolytic agents for 20 min, which included 0.01 and 0.1 μM ANP, 10 μM ISO, 6 μM FSK, and 0.1 μM Lys-γ3-MSH. The cells were then observed for nuclei, lipid droplets, and pHSLser660, and imaged with a 20 × objective. For this experiment, the number of cells analyzed averaged 481 cells/well, which is below the 845 cells/well analyzed in the earlier experiment in which a 20 × objective was utilized for image acquisition (the dose–response experiment for Lys-γ3-MSH, reported in Fig. 4). The two experiments were performed with different preparations of 3T3L1 cells, and even though great pains were taken to insure that identical numbers of cells were seeded per well, there was still a variation in the final number of cells that were present at the time images were acquired. In general, treatment with the lipolytic agents did not change the cell number per well, within the experimental time frame, since exposure time to agonists was relatively brief.
After analysis of the images from the experiment, the cell-by-cell data readout from four control wells (a total of 1,898 cells) was examined to estimate the population distribution of Area Lm. On the basis of this distribution, gating tools within CyteSeer were used to prepare data tables from the experiment specific for subpopulations of adipocytes that featured either a small (“Small Lm”) or large (“Large Lm”) complement of lipid droplets. Cells with Area Lm between 5.2 and 46.9 μm2 were assigned to the “Small Lm” subpopulation (mean value = 17.8 μm2, see Table 3 for further details); the lower limit was chosen so that cells without lipid droplets were excluded from the population, whereas the choice of the upper limit yielded a subpopulation (20.1% of the Total population) whose Area Lm averaged just 40% of that for the Total population. In contrast, cells within the Large Lm subpopulation represent the upper 11.6% of the Total population, sorted by the Area Lm data parameter, and featured Area Lm values that averaged 6-fold and 15-fold greater than mean Area Lm values for the Total population and Small Lm subpopulation, respectively. The population distributions for the 3T3L1 cells were reproducible; for a second preparation of 3T3L1 cells, prepared a few weeks earlier, the population distribution using the same criteria was as follows: Total population = 100%, Small Area Lm = 20.0%, and Large Area Lm = 15.1%, which are similar to the values in Table 3. Thus, cells representing the Small Lm and Large Lm subpopulations did not overlap, and there was considerable separation between the two groups regarding the degree of expression of the lipid droplets. The data parameters considered were Area Pm, API PI Pm, API Pi Lm, M Pr, M1, and Tan. To test for statistically significant effects of the agonists within a cell population, Dunnett's test was utilized. To test for statistically significant differences between the cell populations, Tukey's test was used, which performed pair-wise comparisons across all subgroups in the experiment.
Table 3.
Selection Criteria Utilized to Compare Adipocytes with Different Degrees of Lipid Droplet Content
| Selection Criteria | Area Lm, Mean | ||
|---|---|---|---|
| Murine 3T3L1 adipocytes: | |||
| Total population | 100% | 46 μm2 | |
| Small Lm | 20.1% | 5.2 μm2 < Lm < 46.9 μm2 | 17.8 μm2 |
| Large Lm | 11.6% | Lm > 141 μm2 | 265 μm2 |
| Human subcutaneous adipocytes: | |||
| Total population | 100% | 410 μm2 | |
| Small Lm | 35% | 59 < Lm ≤ 355 μm2 | 186 μm2 |
| Large Lm | 41% | Lm > 355 μm2 | 801 μm2 |
Adipocytes were exposed to lipolytic agents, and then fixed and observed for nuclei, lipid droplets, and pHSLser660. Images from the cells were then analyzed by the Colocalization Algorithm. Gates, as defined above, were then applied by CyteSeer to the cell-by-cell data to identify subpopulations of cells with a high degree (large lipid mask) or low degree (small lipid mask) of lipid droplet expression. Data resulting from the procedure are shown in Figure 5 (3T3L1 adipocytes) and Figure 7 (human adipocytes).
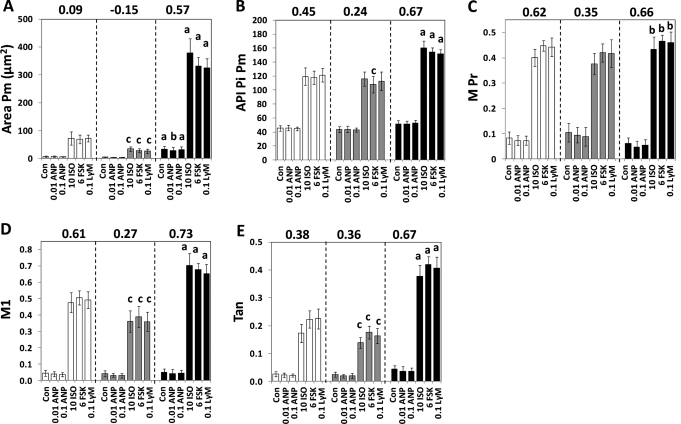
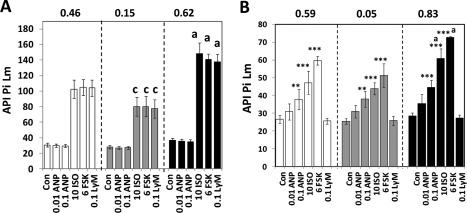
Area Pm for the Total population averaged 7.2 μm2/cell control cells and neither concentration of ANP had an effect (Fig. 5A, white bars). In contrast, ISO, FSK, and Lys-γ3-MSH similarly stimulated 10-fold increases in Area Pm and the effects were all significant at P < 0.001. In fact, ISO, FSK, and Lys-γ3-MSH stimulated increases in all data parameters in all of the cell populations with a significance of P < 0.001. For control cells in the Small Lm subpopulation (Fig. 5A, gray bars), Area Pm averaged 4.9 μm2/cell and this was increased seven-, six-, and fivefold, by ISO, FSK, and Lys-γ3-MSH, respectively; Area Pm values resulting from exposure to ISO, FSK, and Lys-γ3-MSH were significantly less than the analogous values from the Total Population (“c” designation in Fig. 5A). For control cells from the Large Lm subpopulation, Area Pm averaged 33 μm2 and exposure to ISO, FSK, or Lys-γ3-MSH increased Area Pm by 12-, 10-, and 10-fold (dark bars). Further, the Area Pm values for the control, ISO, FSK, and Lys-γ3-MSH-treated cells were greater than the values obtained from the Total population or the Small Lm subpopulations (the “a” designation). Thus, the Area Pm values for pHSLser660 after treatment with the lipolytic agents were proportional to the Area Lm values, which is consistent with the observation that expression of lipid droplets and HSL increase in a correlated manner during 3T3L1 cell adipogenesis.52
Fig. 5.
Effects of lipolytic agents on pHSLser660 in subpopulations of 3T3L1 cells. 3T3L1 adipocytes were exposed to the indicated concentrations (μM) of ANP, ISO, FSK, and Lys-γ3-MSH and assayed for pHSLser660. The cells were then fixed and observed for nuclei, lipid droplets, and pHSLser660 and scanned using a 20 × objective (four images per well, an average of 481 cells/well). Data from the total population of cells are shown as white bars. Subpopulations of cells with a small degree of lipid droplet content (Small Lm, gray bars) or a high degree of lipid droplet content (Large Lm, black bars) were identified by CyteSeer utilizing the criteria of Table 3. (A–E) represent Area Pm, API Pi Pm, M Pr, M1, and Tan, respectively, and each bar is the mean ± SD for n = 16 wells. Within each population of cells the effects of ISO, FSK, and Lys-γ-MSH were significant versus control at P < 0.001 (asterisks were omitted for clarity). aDifferent from the Total population and the Small Lm subpopulation (P < 0.05); bdifferent from the Small Lm subpopulation (P < 0.05); cdifferent from the Total population (P < 0.05). ANP, atrial natriuretic peptide; FSK, Forskolin.
For API Pi Pm, control cells for the Total population averaged 45.2, and ISO, FSK, and Lys-γ3-MSH increased API Pi Pm by ∼2.6-fold (Fig. 5B, white bars). In general, the data for the Small Lm subpopulation were very similar to that for the Total population, though the results for FSK were slightly reduced. For control cells from the Large Lm subpopulation, API Pi Pm averaged 51.6, and exposure to ISO, FSK, and Lys-γ3-MSH increased API Pi Pm by approximately threefold. Further, the FSK, ISO, and Lys-γ3-MSH-stimulated API Pi Pm values were elevated compared to both the Total population and the Small Lm subpopulation. Thus, for the Large Lm cells, the labeling of pHSLser660 was brighter than for the other cell populations.
The API Pi Lm values obtained from this experiment are also of interest (see Appendix). In general, the pattern of the API Pi Lm results was similar to results obtained for API Pi Pm. Notably, for API Pi Lm, the values obtained for the Small Lm subpopulation were significantly different from the Total population for ISO, FSK, and Lys-γ3-MSH; in contrast, as discussed above for API Pi Pm, statistical significance was only achieved between these groups for FSK. Thus, API Pi Lm correlated with the degree of expression of the lipid droplets to an even greater extent than API Pi Pm.
Regarding M Pr, values for the Total population averaged 0.084 for control medium, and this increased to 0.40, 0.45, and 0.44, after exposure to ISO, FSK, or Lys-γ3-MSH (Fig. 5C). Data obtained from the Small Lm subpopulation were not statistically different from the results from the Total population. Data from the Large Lm population were also not different from the Total Population values. However, for ISO, FSK, and Lys-γ3-MSH, data obtained from the Large Lm population were increased relative to the data from the Small Lm population; overall, though, the difference was slight. In general, M Pr was more consistent between the different cell populations than the other data parameters.
M1 (Fig. 5D) values for control cells of the Total population averaged 0.045 and this was increased 10.5- to 11-fold by ISO, FSK, and Lys-γ3-MSH. For the Small Lm control cells, M1 averaged 0.042, and this was increased 8.5 to 9-fold, and the values were reduced compared the Total population. For control cells in the Large Lm subpopulation, M1 averaged 0.052; ISO, FSK, or Lys-γ3-MSH increased the values by ∼13-fold; and the data points were significantly elevated compared to both the Total population and Small Lm subpopulation. Thus, M1 displayed a marked dependence on Area Lm value.
Tan for control cells from the Total population (Fig. 5E) averaged 0.026, and this was increased 6.6, 8.5, and 8.6-fold by ISO, FSK, and Lys-γ3-MSH. For control cells from the Small Lm subpopulation, Tan averaged 0.024, and ISO, FSK, and Lys-γ3-MSH led to 5.7-, 7.2-, and 6.7-fold increases, which were reduced compared to the analogous data from the Total population. For control cells from the Large Lm subpopulation, Tan averaged 0.044 and exposure to ISO, FSK, or Lys-γ3-MSH led to increases of 8.6-, 9.76-, and 9.2-fold, which were significantly elevated compared to both the Total population and the Small Lm subpopulation; indeed, the Tan values for the Large Lm population were twofold greater than that derived from the Total population. Thus, among the colocalization coefficients, Tan displayed the greatest dependency on the degree of expression of the lipid droplets.
Overall, the data developed via gating on Area Lm are consistent with the hypothesis that the cells with the largest lipid droplet content were more responsive to ISO, FSK, and Lys-γ3-MSH. Additionally, Z′ values, calculated between the control medium and the FSK-containing medium, were highest for the cells with extensive expression of lipid droplets, and lowest for the cells with the least expression of lipid droplets for every data parameter. Thus, not only are the larger cells more responsive to the agonists, but also the results are overall less variable with regard to the effect of the agonist.
Observation of HSL and pHSLser660 in Primary Human Adipocytes
Human subcutaneous adipocytes are a widely used model system for examining the pathways controlling lipid droplet formation and lipolysis, and results obtained from these cells are likely to be very applicable to applications relevant to human health. To determine if HSL and pHSLser660 could be quantified in this cell type, human subcutaneous adipocytes were exposed to ANP, ISO, FSK, and Lys-γ3-MSH, and labeled for HSL or pHSLser660 utilizing techniques similar to those used for the 3T3L1 adipocytes.
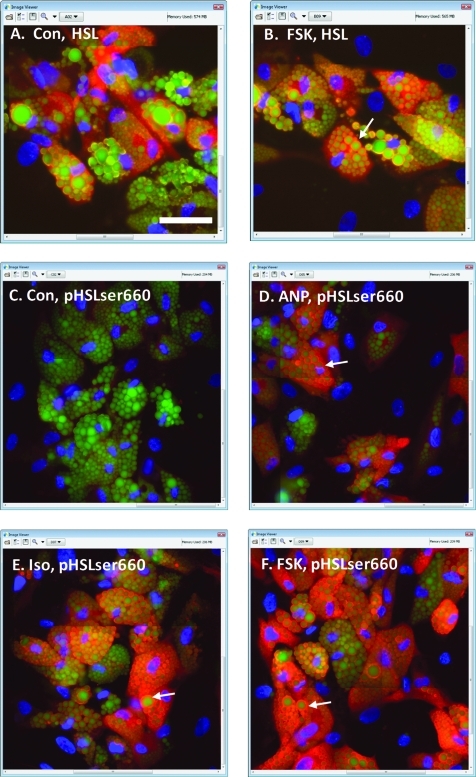
For control human adipocytes observed for lipid droplets and HSL, HSL was apparent between the lipid droplets (Fig. 6A), and FSK induced a discernable increase in brightness at the edges of the droplets (Fig. 6B). However, the effect was not visually as dramatic as that seen in the 3T3L1 cells. This may be because the human adipocytes featured a high degree of expression of lipid droplets relative to the overall cell size, which made it somewhat difficult to distinguish between HSL within the cytoplasm versus HSL localized at the rims of the lipid droplets. For human adipocytes observed and imaged for HSL, Pr averaged 0.33 ± 0.020 for controls and 0.47 ± 0.066 for FSK-treated cells (mean ± SD, n = 4 wells, P < 0.01), confirming that FSK elicited an increase in HSL:lipid droplet colocalization in this cell type.
Fig. 6.
Lipid droplets, HSL, and pHSLser660 observed in primary human adipocytes. The cells were then treated with agonists for 20 min, fixed, and labeled for nuclei (blue), lipid droplets (green), or either HSL or pHSLser660 (red). (A, B) Cells exposed to control or 6 μM FSK, respectively, and labeled for HSL; white arrow in (B) points to HSL at the outer edge of a lipid droplet. (C–F) Cells exposed to control, 0.1 μM ANP, 10 μM ISO, or 6 μM FSK, respectively, and labeled for pHSLser660; white arrows in (D–F) point to pHSLser660 at the outer edge of a lipid droplet. Images in (C–F) are displayed at identical brightness and contrast optimized for observation of pHSLser660 for cells treated with ANP; the original images for (E) and (F) were not saturated in the red channel. Images were collected with a 40 × objective. Scale bar = 50 μm.
For human adipocytes exposed to the control medium and labeled for pHSLser660, very little signal was observed (Fig. 6C). Exposure to ANP at 0.1 μM discernibly increased the label for pHSLser660 at the edges of the droplets (Fig. 6D). The label for pHSLser660 was also increased by ISO and FSK, to a greater extent than that observed with ANP (Fig. 6E, F). In contrast, 100 nM Lys-γ3-MSH did not increase pHSLser660 labeling (images not shown).
Analysis of pHSLser660 in Subpopulations of Human Adipocytes
To compare the effects of the potential lipolytic agents and to test the hypothesis that the lipolytic responses in the human adipocytes might also depend upon the degree of lipid droplet content, an experiment was conducted for cells plated on a 96-well dish, in which four wells were observed for pHSLser660 per condition, and the wells were scanned at 40 ×. Similarly to the procedure utilized above for the 3T3L1 adipocytes, data from the analysis were sorted into three groups (Total population, Small Lm subpopulation, and Large Lm subpopulation; see Table 3). Different sorting criteria were used for human adipocytes versus those used with the 3T3L1 cells because the human adipocytes and the associated lipid droplets are generally larger than those for the 3T3L1 cells. For the Total population, the average Area Lm was 410 μm2. Cells assigned to the Small Lm and Large Lm subpopulations averaged 0.5-fold and twofold of the average Area Lm for the Total Population, respectively, and the subpopulations do not overlap.
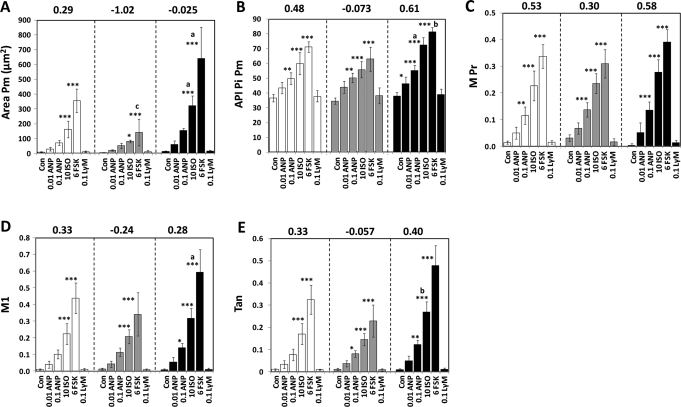
Area Pm values (Fig. 7A) for control cells for the Total population averaged 8.14 μm2, and ISO and FSK induced increases in Area Pm of 20-fold and 44-fold, respectively (P < 0.001, for each). For Small Lm control cells, Area Pm averaged 5.19 μm2 and ISO and FSK stimulated increases of 15-fold (P < 0.05) and 27-fold (P < 0.001). For the Large Lm control cells, Area Pm averaged 11.3 μm2 and ISO and FSK increased Area Pm by 28-fold and 57-fold, respectively (P < 0.001 for both). Also, for cells treated with either ISO or FSK, the Area Pm values for the Large Lm cells were significantly greater than for the Total population and the Small Lm subpopulation. Relatedly, Area Pm values for the FSK-treated Small Lm cells were significantly different from the FSK-treated Total population. Thus, the appearance of pHSLser660 after ISO or FSK treatment was correlated with the degree of expression of lipid droplets in the human adipocytes in a manner similar to that observed with the 3T3L1 adipocytes.
Fig. 7.
Quantification of pHSLser660 in subpopulations of human adipocytes. Cells were incubated with the indicated concentrations (micromolar) of either ANP, ISO, FSK, or Lys-γ3-MSHfor 20 min, fixed, and observed for nuclei, lipid droplets, and pHSLser660; the cells were then scanned using a 40 × objective and the images analyzed with CyteSeer. (A–E) represent Area Pm, API Pi Pm, M Pr, M1, and Tan, respectively, for the total population (open bars), adipocytes with a Small Lm (gray bars) or a Large Lm (black bars), as defined in Table 3. Each bar is the mean ± SD for n = 4 wells (9 images/well, an average of 150 cells/well). *P < 0.05 versus control; **P < 0.01 versus control; ***P < 0.001 versus control. aDifferent from the Total population and the Small Lm subpopulation; bdifferent from the Small Lm subpopulation; cdifferent from the Total population. Z′ values were calculated between control and FSK.
API Pi Pm for the control cells of the Total population average 37.6 intensity units (Fig. 7B), and this was increased 1.36-fold (P < 0.01), 1.63-fold (P < 0.001), and 1.94-fold (P < 0.001) by 0.1 μM ANP, ISO, and FSK, respectively. For the Small Lm subpopulation API Pi Pm values were generally similar to the data obtained from the Total Population. For the Large Lm subpopulation API Pi Pm for the control cells averaged 37.9, and this was increased 1.22-fold (P < 0.05), 1.46-fold (P < 0.001), 1.91-fold (P < 0.001), and 2.14-fold (P < 0.001) by 0.01 μM ANP, 0.1 μM ANP, ISO, and FSK, respectively; API Pi Pm for ISO-treated cells of the Large Lm subpopulation was greater than for ISO-treated cells of either the Total population or the Small Lm subpopulation, and the effect of FSK was greater for the Large Lm vs. the Small Lm subpopulation. Overall, the results suggest that the cells representing the Large Lm subpopulation are more responsive to ANP, ISO, and FSK than the Small Lm subpopulation.
For API Pi Lm (see Appendix Fig. A1), the overall pattern of results obtained for the human adipocytes was very similar to the results for API Pi Pm; notably, a Z′ value of 0.83 was obtained for the effect of FSK for the Large Lm subpopulation, which was the highest Z′ value of the experiment.
For M Pr, control cells of the total population averaged 0.0139, and this was significantly increased to 0.115, 0.227, and 0.337 by 0.1 μM ANP, ISO, and FSK, respectively (Fig. 7C). Also, the response to each agonist was very similar for the Total population and the Small Lm and Large Lm subpopulations. Thus, M Pr did not significantly vary with the degree of expression of lipid droplets, which is consistent with the results obtained with 3T3L1 cells.
For M1, control cells from the Total population control cells averaged 0.0103, and this was increased 21.6-fold (P < 0.001) and 42.5-fold (P < 0.001) by ISO and FSK, respectively (Fig. 7D). For the Small Lm subpopulation, controls averaged 0.126, and this was increased 16.4 (P < 0.001) and 26.9 (P < 0.001) by ISO and FSK, respectively. For the Large Lm subpopulation, controls averaged 0.009 and this was increased 15.6-fold (P < 0.05), 35.0-fold, and 65.6-fold by 0.1 μM ANP, ISO, and FSK; the values for the FSK-treated cells of the Large Lm subpopulation were greater than those obtained for the Total population and Small Lm subpopulation.
For Tan, controls cells for the Total population averaged 0.0098, and this was increased 17.4-fold (P < 0.001) and 33.3-fold (P < 0.001) by ISO and FSK, respectively (Fig. 7E). For control cells from the Small Lm subpopulation, Tan averaged 0.0117, and this was increased 6.86-fold (P < 0.05), 12.4-fold (P < 0.001), and 19.6-fold (P < 0.001) by 0.1 μM ANP, ISO, and FSK, respectively. For the Large Lm subpopulation, control cells averaged 0.0097, and this was increased 12.6-fold (P < 0.01), 27.8-fold (P < 0.001), and 49.5-fold (P < 0.001) by 0.1 μM ANP, ISO, and FSK, respectively. For cells treated with ISO, Tan was greater for cells of the Large Lm subpopulation than for cells of the Small Lm subpopulation.
For all of the data parameters discussed, Z′ values were lower for the Small Lm subpopulation than for the Total and Large Lm subpopulations. Thus, the variability in the data was greater for the Small Lm subpopulation regarding the effect of FSK. For API Pi Pm, API Pi LM, and Tan, the Z′ values were higher for the Large Lm subpopulation vs. the Total population. In general, the results are consistent with the hypothesis that the lipolytic response to ANP, ISO, and FSK is more marked in the Large Lm subpopulation.
In this experiment, Lys-γ3-MSH did not increase any of the data parameters in any of the cell populations for the human subcutaneous adipocytes. However, in a follow-up experiment utilizing an additional aliquot of human adipocytes from the same original cell lot, 0.1 μM Lys-γ3-MSH slightly increased M Pr for pHSLser660; the effect was ∼50% of the response to 0.1 μM ANP for cells in the Large Lm subpopulation (data not shown).
Glycerol Release from Adipocytes Induced by ANP, ISO, FSK, and Lys-γ3-MSH
Glycerol is produced from triglycerides during lipolysis, and the accumulation of glycerol in the culture medium is an established assay for determining the rate of lipolysis. Thus, experiments were performed to quantify glycerol release from human subcutaneous and 3T3L1 adipocytes exposed to the lipolytic agents, employed above, to examine the relationship between the immediate phosphorylation of HSL and the subsequent lipolytic response.
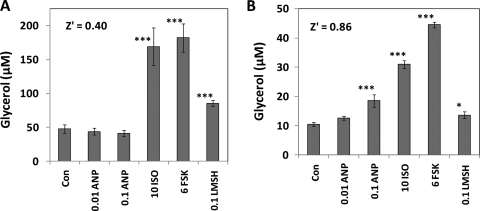
For 3T3L1 adipocytes, ANP did not elicit an increase in glycerol content in the culture medium, whereas ISO, FSK, and Lys-γ3-MSH increased glycerol by 3.5-, 3.8-, and 1.8-fold, respectively (Fig. 8A). Thus, the results for ANP, ISO, and FSK regarding glycerol accumulation are similar to the results obtained for these agents at inducing phosphorylation of HSL. Furthermore, the Z′ value for the effect of FSK was 0.40, which is below the Z′ values found for the microscopy-based data parameters derived from pHSLser660, especially for the Large Lm subpopulation of 3T3L1 cells. However, Lys-γ3-MSH was less efficacious than FSK in the glycerol release assay, whereas these agents elicited similar increases in pHSLser660. One possibility for this discrepancy is that the lipolytic response to Lys-γ3-MSH might desensitize over a time course of several hours, but this hypothesis was not further explored.
Fig. 8.
Glycerol release from murine 3T3L1 (A) or human subcutaneous (B) adipocytes elicited by ANP, ISO, FSK, and Lys-γ3-MSH. Adipocytes were exposed to the indicated concentrations (μM) of ANP, ISO, FSK, and Lys-γ3-MSH for either 15 h (A) or 3.5 h (B) and aliquots of the culture medium were assayed for glycerol. Bars represent the mean ± SD for n = 8 or n = 3 wells for (A) and (B), respectively. *P < 0.05 versus controls; **P < 0.01 versus control; ***P < 0.001 versus control. Z′ values were calculated between the control and the FSK-treated cells.
For human subcutaneous adipocytes, a time point of 3.5 h was chosen, which was known from previous work with this cell type to be an optimal time point to assay the accumulation of glycerol in the culture medium. For cells exposed to the control medium, glycerol averaged 10.5 μM (Fig. 8B), whereas exposure to 0.1 μM ANP, ISO, and FSK increased glycerol release by 1.8-fold, 3-fold, and 4-fold, respectively (P < 0.001 for all responses). The pattern of glycerol release induced by ANP, ISO, and FSK was remarkably similar to the pattern of results obtained for the HCA assay, particularly those data obtained for the Large Lm subpopulation of cells for API Pi Pm, API PI Lm, M Pr, M1, and Tan. Exposure to Lys-γ3-MSH led to a 30% increase in glycerol release from the human adipocytes (P < 0.05); the small effect of Lys-γ3-MSH on glycerol release is consistent with the effect of Lys-γ3-MSH on pHSLser660, which was small and inconsistently observed.
Discussion
The present results demonstrate that HCA methods can quantify hormone-induced phosphorylation of HSL and colocalization of HSL with lipid droplets in both 3T3L1 and human primary subcutaneous adipocytes. Utilizing cells plated in a high-throughput mode, labels specific for HSL or pHSLser660, and algorithms specifically designed to quantify lipid droplets and lipid droplet-associated proteins,35 the HCA assay defined time course and dose–response relationships for the activation of HSL by a variety of lipolytic agents. Furthermore, the assay defined key differences between the murine and human adipocyte cell models, phenotypic heterogeneity within the cell cultures, and identified a likely site of phosphorylation of HSL by c-GMP-activated protein kinase.
Colocalization of HSL with lipid droplets was quantified utilizing a variety of correlation and colocalization coefficients (Pr, M1, and the Tan). The Tan is a general tool for comparing the similarity of sample sets and this study is likely the first in which it has been applied to analysis of colocalization of cellular structures in digital images acquired via fluorescence microscopy. Under basal conditions, the Pr was positive (∼0.35) for both 3T3L1 and the human adipocytes, implying that there was a partial association of HSL with the lipid droplets even under the basal conditions. A moderate degree of association of HSL with large lipid droplets has previously been demonstrated for 3T3L1 cells under basal conditions, via immunogold labeling and high-resolution electron microscopy.14 Addition of lipolytic agents led to rapid translocation of HSL to the lipid droplets, which yielded consistent increases in the colocalization coefficients.
Phosphorylation of HSL on serine 660 is a key step in the activation of HSL. In our study, treatment of cells with lipolytic agents for just 5 min (the briefest time point tested) led to maximal labeling of the cells for pHSLser660. Our data are consistent with the observation by Martin et al. that HSL is maximally phosphorylated on serine 660 within 30 s of exposure to ISO.14 HCA quantification of the appearance of pHSLser660 (as exemplified by the Area Pm, API Pi Pm, and API Pi Lm data parameters) and the colocalization of pHSLser660 with lipid droplets (as exemplified by the Pr, M Pr, M1, and Tan data parameters) provided excellent discrimination between the control and activating treatments. The Z′ statistic provides a numerical reference for this property (e.g., assay “quality”48), and higher Z′ values were typically achieved with the pHSLser660-specific label as opposed to the general label for HSL.
Cultured 3T3L1 adipocytes are heterogeneous with respect to the number and size of the lipid droplets found within each cell. During adipogenesis, lipid droplets and HSL accumulate within 3T3L1 cells with a similar time course52–54 and the cells with the greatest amount of lipid droplets feature the highest degree of HSL expression.52 Previous workers incubated 3T3L1 cells with FSK for 24 h, observed that the number of cells with a large degree of lipid droplet content was preferentially reduced, and suggested that cells with a large complement of lipid droplets are likely to be more sensitive to the activation of lipolysis.52 The results of the present study support this hypothesis, since data parameters that report changes in the levels of pHSLser660 (e.g., Area Pm, API Pi Pm, and API Pi Lm) were increased after stimulation with ISO, FSK, or Lys-γ3-MSH in a manner that correlated with the degree of lipid droplet expression. Further, cells with large lipid masks treated with these lipolytic agents also featured elevated M1 and Tan values relative to cells with small lipid masks, supporting the hypothesis that the lipolytic machinery can be activated more fully in such cells.
HSL and pHSLser660 were also well observed in primary human subcutaneous adipocytes, and prompted a comparison of the two adipocyte model systems to ANP, ISO, FSK, and Lys-γ3-MSH. ANP increased the appearance of pHSLser660 in human adipocytes but not in 3T3L1 adipocytes. The lack of effect of ANP on the murine 3T3L1 cells is consistent with the lack of lipolytic response to ANP previously reported in primary adipocytes from mouse and rat. This likely relates to the expression pattern of receptors for natriuretic peptides on these cell types55; whereas human adipocytes express the GC-A receptor, which couples to c-GMP-dependent protein kinase, the rodent adipocytes do not. Notably, in humans, ANP promotes lipolysis in both subcutaneous and visceral fat,56 and exercise-induced lipolysis in subcutaneous fat has been linked to increased plasma levels of ANP in overweight men.57 ANP is known to elicit HSL phosphorylation by activating cGMP-dependent protein kinase rather than cAMP-dependent protein kinase.27 Our results therefore suggest that HSL serine 660 is phosphorylated by cGMP-dependent protein kinase in human subcutaneous adipocytes. The amino acid sequence neighboring the serine660 site corresponds to a consensus phosphorylation site for both cAMP- and cGMP-dependent protein kinases58; thus, phosphorylation of HSL serine660 can likely serve as an activation step for both signaling pathways in human adipocytes.
Consistent with results obtained by others,23,24 Lys-γ3-MSH strongly induced HSL phosphorylation and lipolysis in 3T3L1 cells. However, in our hands, Lys-γ3-MSH had only a small and inconsistent effect on pHSLser660 and a small effect on glycrerol release in human subcutaneous adipocytes. The regulation of lipolysis in adipose and other tissues by the various forms of MSHs and MSH receptors is complex and not fully elucidated, and likely to be relevant to many areas of physiology and metabolism.
Human adipocytes with the large lipid masks were more responsive to the lipolytic agents than the cells with small lipid droplets, in a manner similar to that found with the 3T3L1 cells. Thus, Area Pm for pHSLser660 following FSK correlated with the degree of lipid droplet content, and cells with a large lipid mask yielded the highest values for API PI Pm, API PI LM, and M1. Additionally, cells with large lipid masks were most responsive to ANP, since as little as 0.01 μM ANP stimulated a significant increase in API PI PM in such cells.
The possibility was also tested that expression of lipid droplets and the appearance of pHSLser660 might depend upon DNA content of the adipocytes; however, no such trend was identified for either cell type (data not shown), which is consistent with the postmitotic state of the differentiated adipocytes. Relatedly, there was no overt fragmentation of the nuclei and no relationship between nuclear size and lipid load within the adipocytes, suggesting that lipid droplet expression is not related to apoptosis.
Overall, the sensitivity of pHSLser660 to the agonists matched very well against the glycerol-release assay, a standard assay used to quantify lipolysis. Importantly, the data from the HCA assay were obtained after just a 15 min exposure to the agonists, whereas glycerol release was assayed several hours later. The close correlation between these results is consistent with the key role played by HSL in lipolysis. Currently, ATGL appears to be a less suitable biomarker for HCA analysis of the lipolytic state of adipocytes, as labels are not yet available that are specific for activated ATGL. For the hMADs adipocyte model system, exposure to FSK leads to translocation of ATGL to the periphery of relatively small lipid droplets, and increased colocalization of ATGL with HSL,59 but this has not been extensively quantified.
In summary, our data demonstrate that HSL activation can be accurately quantified via HCA techniques, particularly with regard to phosphorylation of HSLser660, and the colocalization of pHSLser660 with lipid droplets. The methods developed will likely have application to conducting screens to identify small molecule and genomic regulators of lipolysis, and also for use in studies to fully characterize the responses to lead candidates or introduced genetic constructs. With the HCA technique, results pertinent to activation of the lipolytic response can be obtained with much shorter incubation times than is possible with the glycerol release assay, and the information obtained is specific to a crucial step in lipolysis activation. Further, while the results from the HCA assay were generally consistent with the glycerol release assay, the HCA provided additional, more detailed information. For example, using gating techniques, the HCA assay was able to selectively examine HSL phosphorylation in the most mature fraction of the adipocytes. In contrast, the glycerol release assay in its current configuration cannot examine lipolysis in selected subpopulations of the cells, and heterogeneity is a very common feature of all cultured adipocyte preparations. Thus, the HCA strategy can be utilized to provide data that will likely be most relevant to mature adipocytes in intact animals and human patients.
It has been recently established that there are complexities in the spatial-temporal pattern of HSL phosphorylation. Thus, pHSLser660 appears within seconds of agonist addition, and its initial appearance may precede translocation of HSL to the lipid droplets; in contrast, pHSLser563 appears at a later time, after HSL translocation.14 Vala Sciences has recently developed monoclonal antibodies that specifically recognize perilipin that has been phosphorylated on either serine 497, or serine 522 (human sequence values are referenced, which are equivalent to serine 492 or serine 517 of rat perilipin). Preliminary work with these antibodies suggest that perilipin may be differentially phosphorylated on these two sites, in a manner that is reminiscent of the situation with HSL phosphorylation (manuscript in preparation). The results of the present study and the studies in progress underscore that our understanding of the regulatory processes that regulate lipolysis is evolving, and illustrate the utility of HCA methods in elucidating the mechanisms that control this key metabolic process.
Abbreviations
- ANP
atrial natriuretic peptide
- API
average pixel intensity
- API Li Lm
API of the Lipid image for the Lipid mask
- API Pi Lm
API of the Protein image for the Lipid mask
- API Pi Pm
API of the Protein image for the Protein mask
- ATGL
adipocyte triglyceride lipase
- cAMP
adenosine 3′,5′-cyclic monophosphate
- cGMP
guanosine 3′,5′-cyclic monophosphate
- DAPI
4′,6-diamidino-2-phenylindole
- FSK
Forskolin
- HCA
high-content analysis
- HSL
hormone sensitive lipase
- ISO
isoproterenol
- Lys-γ3-MSH
Lys-γ3-melanocyte stimulating hormone
- M Pr
Masked Pearson's correlation
- M1
Manders' colocalization coefficient
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- Pr
Pearson's correlation
- SD
standard deviation
- Tan
Tanimoto coefficient
Appendix
Additional Discussion of Z′ Versus P Values
The relationship between the Z′ statistic and statistical tests to determine whether or not a difference between two groups is significant is sometimes confusing. The Z′ value, as defined by Zhang, et al.,48 can be thought of as a statistic that takes things one step further than a test for a significant difference between two (or more) data sets, as Z′ provides a numerical index of the robustness of the assay. As discussed in the main text, Z′ has a theoretical range from negative infinity to positive 1, and Z′ values >0.20 designate an assay that are practical for high-throughput screening applications, and assays with Z′ values >0.50 are considered excellent for such purposes. However, what is somewhat counter-intuitive is that there can be a negative Z′ value between the control and experimental data sets, and the P value (the probability that the classic null hypothesis is true) can still be very low. For example, consider the hypothetical data sets in which the control values = 23, 28, 30, and 20 (mean = 25.25, standard deviation [SD] = 4.57), and the stimulated data values = 40, 42, 45, and 50 (mean = 44.25, SD = 4.35). If one uses a t-test to test for a significant difference between these data sets, P = 0.000947 (two-tailed t-test), indicating that the two data sets are different, statistically, to a high degree (P < 0.001). However, the Z' value for this set = −0.41. So, it is possible to have a P-value < 0.001 but a Z' value that is negative. To further illustrate the relationship between Z′ and P, consider a data set in which controls = 20, 15, 15, 25, and 25, and in which the stimulated data values are 80, 77, 81, 87, and 74. The mean ± SD values are therefore 20 ± 5 for controls, and 80 ± 5 for the stimulated data; the Z′ value for this comparison is 0.50, which is the benchmark criteria for assays with excellent potential for high-throughput screening involving hundreds of thousands to millions of test compounds; the P value is 0.00000268 for the data set (two-tailed, t-test).
Additional Results
Forskolin
FSK increases intracellular cAMP, via direct activation of adenylyl cyclase. In experiments designed to define the dose–response and time-course relationships for activation of HSL phosphorylation, 3T3L1 adipocytes were exposed to FSK, fixed, and labeled for pHSLser660, and the cells analyzed for M Pr, M1, and Tan. FSK was found to maximally to have maximal effects on pHSLser660 at 6.66 μM, and the effect was immediate (Table A1).
Appendix Table A1.
Colocalization of pHSLser660 with Lipid Droplet Induced by Forskolin
| FSK (μM) | M Pr | M1 | Tan |
|---|---|---|---|
| 0 | 0.0764 ± 0.0154 | 0.0475 ± 0.0113 | 0.0293 ± 0.0067 |
| 0.74 | 0.299 ± 0.026 | 0.296 ± 0.03 | 0.176 ± 0.02 |
| 2.22 | 0.37 ± 0.0197 | 0.393 ± 0.0334 | 0.23 ± 0.0186 |
| 6.66 | 0.404 ± 0.0346 (0.54) | 0.466 ± 0.0427 (0.61) | 0.265 ± 0.0354 (0.47) |
| 20 | 0.395 ± 0.0254 | 0.428 ± 0.0475 | 0.249 ± 0.025 |
| 60 | 0.303 ± 0.1397 | 0.315 ± 0.1586 | 0.191 ± 0.097 |
| Time (min) | M Pr | M1 | Tan |
|---|---|---|---|
| 0 | 0.0864 ± 0.02 | 0.0629 ± 0.0107 | 0.0324 ± 0.0058 |
| 5 | 0.504 ± 0.0283 (0.65) | 0.758 ± 0.0522 (0.73) | 0.239 ± 0.0422 (0.3) |
| 15 | 0.497 ± 0.0526 | 0.775 ± 0.101 | 0.217 ± 0.0685 |
| 30 | 0.477 ± 0.0356 | 0.755 ± 0.0738 | 0.189 ± 0.0432 |
| 60 | 0.452 ± 0.0415 | 0.732 ± 0.0537 | 0.196 ± 0.0601 |
| 120 | 0.368 ± 0.0647 | 0.665 ± 0.113 | 0.113 ± 0.0374 |
3T3L1 adipocytes plated in 96-well dishes were exposed to FSK, and then fixed, labeled for pHSLser660, imaged at 20 × , and analyzed with CyteSeer®. The dose–response relationships for M Pr, M1, and Tan are shown in the upper section (Z′ values, calculated between control medium and 6.66 μM FSK, are shown in parentheses), and the time-course relationship for 20 μM FSK is shown below (Z′ was calculated between the 0 and 5 min time points). Each symbol represents the mean ± standard deviation for n = 16 wells. All of the data parameters obtained with FSK were significantly different from the corresponding control values (P < 0.001, Dunnett's test).
FSK, Forskolin.
Average Pixel Intensity of the Protein image for the Lipid Mask
In experiments in which the heterogeneity of the lipolytic response was examined (see Figs. 5 and 7 of the main text), API of the Protein image for the Lipid mask (API Pi Lm) for pHSLser660 was also calculated. API Pi Lm is the API for the protein in regions of the cell that correspond to the lipid droplets. For murine 3T3L1 cells, API Pi Lm showed a strong dependence on the degree of lipid droplet expression, as the values for cells from the Small Lm subpopulation were significantly different from both the Total population and Large Lm subpopulation for FSK, isoproterenol, and Lys-γ3-melanocyte stimulating hormone (Fig. A1). For both the murine and human adipocytes, API Pi Lm values displayed relatively little variation, and excellent Z′ values were obtained, particularly for the Large Lm subpopulation.
Appendix Fig. A1.
API Pi Lm values obtained from subpopulations of 3T3L1 or human adipocytes. (A) API Pi Lm values obtained from 3T3L1 adipocytes are shown, which were obtained from the same experiment described in Figure 5 of the main text. (B) API Pi Lm values obtained from human adipocytes are shown, which were obtained from the same experiment described in Figure 7 of the main text. Data obtained from the Total population, the Small Lm subpopulation, or the Large Lm subpopulation are depicted as white, gray, or black bars, respectively. API Pi Lm, API of the Protein image for the Lipid mask.
Acknowledgments
We wish to thank Dr. Andrew Bicknell for generously providing us with Lys-γ3-MSH. This research was supported, in part, by NIH 5R44DK074333 “HT Image Assay of Lipid Droplet Formation in Human Adipocytes.”
Disclosure Statement
No competing financial interests exist.
References
- 1.Ford ES. Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S1–S8. doi: 10.1210/jc.2008-1356. [DOI] [PubMed] [Google Scholar]
- 2.Misra A. Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 3.Brown WV. Fujioka K. Wilson PW. Woodworth KA. Obesity: why be concerned? Am J Med. 2009;122(4 Suppl 1):S4–S11. doi: 10.1016/j.amjmed.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Trasande L. Chatterjee S. The impact of obesity on health service utilization and costs in childhood. Obesity (Silver Spring) 2009;17:1749–1754. doi: 10.1038/oby.2009.67. [DOI] [PubMed] [Google Scholar]
- 5.Londos C. Sztalryd C. Tansey JT. Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Wang S. Soni KG. Semache M, et al. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab. 2008;95:117–126. doi: 10.1016/j.ymgme.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Watt MJ. Triglyceride lipases alter fuel metabolism and mitochondrial gene expression. Appl Physiol Nutr Metab. 2009;34:340–347. doi: 10.1139/H09-019. [DOI] [PubMed] [Google Scholar]
- 8.Zechner R. Kienesberger PC. Haemmerle G. Zimmermann R. Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Yeaman SJ. Hormone sensitive lipase—new roles for an old enzyme. Biochem J. 2004;379(Pt 1):11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraemer FB. Shen WJ. Hormone sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer FB. Adrenal cholesterol utilization. Mol Cell Endocrinol. 2007;265–266:42–45. doi: 10.1016/j.mce.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Fex M. Mulder H. Lipases in the pancreatic beta-cell: implications for insulin secretion. Biochem Soc Trans. 2008;36(Pt 5):885–890. doi: 10.1042/BST0360885. [DOI] [PubMed] [Google Scholar]
- 13.Fex M. Haemmerle G. Wierup N, et al. A beta cell-specific knockout of hormone sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia. 2009;52:271–280. doi: 10.1007/s00125-008-1191-9. [DOI] [PubMed] [Google Scholar]
- 14.Martin S. Okano S. Kistler C. Fernandez-Rojo MA. Hill MM. Parton RG. Spatiotemporal regulation of early lipolytic signaling in adipocytes. J Biol Chem. 2009;284:32097–32107. doi: 10.1074/jbc.M109.002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg AS. Shen WJ. Muliro K, et al. Stimulation of lipolysis and hormone sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- 16.Anthonsen MW. Ronnstrand L. Wernstedt C. Degerman E. Holm C. Identification of novel phosphorylation sites in hormone sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 17.Garton AJ. Yeaman SJ. Identification and role of the basal phosphorylation site on hormone sensitive lipase. Eur J Biochem. 1990;191:245–250. doi: 10.1111/j.1432-1033.1990.tb19116.x. [DOI] [PubMed] [Google Scholar]
- 18.Mairal A. Melaine N. Laurell H, et al. Characterization of a novel testicular form of human hormone sensitive lipase. Biochem Biophys Res Commun. 2002;291:286–290. doi: 10.1006/bbrc.2002.6427. [DOI] [PubMed] [Google Scholar]
- 19.Jiang B. Yang Y. Jin H, et al. Astragaloside IV attenuates lipolysis and improves insulin resistance induced by TNFalpha in 3T3-L1 adipocytes. Phytother Res. 2008;22:1434–1439. doi: 10.1002/ptr.2434. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y. Ju D. Zhang M. Yang G. Interleukin-6 stimulates lipolysis in porcine adipocytes. Endocrine. 2008;33:261–269. doi: 10.1007/s12020-008-9085-7. [DOI] [PubMed] [Google Scholar]
- 21.Daval M. Foufelle F. Ferre P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574(Pt 1):55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daval M. Diot-Dupuy F. Bazin R, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 23.Bicknell KA. Harmer SC. Yiangson S. Lockwood W. Bicknell AB. Lys-gamma(3)-MSH: a global regulator of hormone sensitive lipase activity? Mol Cell Endocrinol. 2008;300:71–76. doi: 10.1016/j.mce.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Harmer SC. Pepper DJ. Cooke K. Bennett HP. Bicknell AB. Evidence of a possible role for Lys-gamma3-MSH in the regulation of adipocyte function. J Endocrinol. 2008;196:149–158. doi: 10.1677/JOE-07-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen RC. Brownie AC. Ling N. Pro-adrenocorticotropin/endorphin-derived peptides: coordinate action on adrenal steroidogenesis. Science. 1980;208:1044–1046. doi: 10.1126/science.6246578. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen RC. Brownie AC. Lys-gamma 3-melanotropin binds with high affinity to the rat adrenal cortex. Endocrinology. 1983;112:1279–1287. doi: 10.1210/endo-112-4-1279. [DOI] [PubMed] [Google Scholar]
- 27.Sengenes C. Bouloumie A. Hauner H, et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278:48617–48626. doi: 10.1074/jbc.M303713200. [DOI] [PubMed] [Google Scholar]
- 28.Lafontan M. Moro C. Berlan M. Crampes F. Sengenes C. Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–137. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Large V. Reynisdottir S. Langin D, et al. Decreased expression and function of adipocyte hormone sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res. 1999;40:2059–2066. [PubMed] [Google Scholar]
- 30.Langin D. Dicker A. Tavernier G, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54:3190–3197. doi: 10.2337/diabetes.54.11.3190. [DOI] [PubMed] [Google Scholar]
- 31.Jocken JW. Blaak EE. van der Kallen CJ. van Baak MA. Saris WH. Blunted beta-adrenoceptor-mediated fat oxidation in overweight subjects: a role for the hormone sensitive lipase gene. Metabolism. 2008;57:326–332. doi: 10.1016/j.metabol.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Carlsson E. Johansson LE. Strom K, et al. The hormone sensitive lipase C-60G promoter polymorphism is associated with increased waist circumference in normal-weight subjects. Int J Obes (Lond) 2006;30:1442–1448. doi: 10.1038/sj.ijo.0803299. [DOI] [PubMed] [Google Scholar]
- 33.Ryden M. Agustsson T. Laurencikiene J, et al. Lipolysis—not inflammation, cell death, or lipogenesis—is involved in adipose tissue loss in cancer cachexia. Cancer. 2008;113:1695–1704. doi: 10.1002/cncr.23802. [DOI] [PubMed] [Google Scholar]
- 34.Agustsson T. Ryden M. Hoffstedt J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67:5531–5537. doi: 10.1158/0008-5472.CAN-06-4585. [DOI] [PubMed] [Google Scholar]
- 35.McDonough PM. Agustin RM. Ingermanson RS. Loy PA. Buehrer BM. Nicoll JB. Prigozhina NL. Mikic I. Price JH. Quantification of lipid droplets and associated proteins in cellular models of obesity via high content;/high throughput microscopy and automated image analysis. Assay Drug Dev Technol. 2009;7:440–460. doi: 10.1089/adt.2009.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denner P. Schmalowsky J. Prechtl S. High-content analysis in preclinical drug discovery. Comb Chem High Throughput Screen. 2008;11:216–230. doi: 10.2174/138620708783877780. [DOI] [PubMed] [Google Scholar]
- 37.Dragunow M. High-content analysis in neuroscience. Nat Rev Neurosci. 2008;9:779–788. doi: 10.1038/nrn2492. [DOI] [PubMed] [Google Scholar]
- 38.Gasparri F. Ciavolella A. Galvani A. Cell-cycle inhibitor profiling by high-content analysis. Adv Exp Med Biol. 2007;604:137–148. doi: 10.1007/978-0-387-69116-9_13. [DOI] [PubMed] [Google Scholar]
- 39.Gasparri F. Sola F. Bandiera T. Moll J. Galvani A. High-content analysis of kinase activity in cells. Comb Chem High Throughput Screen. 2008;11:523–536. doi: 10.2174/138620708785204126. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y. Walther TC. Rao M, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beller M. Sztalryd C. Southall N, et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whittaker R. Loy PA. Sisman E, et al. Identification of microRNAs that control lipid droplet formation and growth in hepatocytes via high-content screening. J Biomol Screen. 2010;15:798–805. doi: 10.1177/1087057110374991. [DOI] [PubMed] [Google Scholar]
- 43.Szymanski KM. Binns D. Bartz R, et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manders EMM. Verbeek FJ. Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J Microsc. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen TG. Nielsen J. Pedersen CN. A tree-based method for the rapid screening of chemical fingerprints. Algorithms Mol Biol. 2010;5:9. doi: 10.1186/1748-7188-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y. Bajorath J. Development of a compound class-directed similarity coefficient that accounts for molecular complexity effects in fingerprint searching. J Chem Inf Model. 2009;49:1369–1376. doi: 10.1021/ci900108d. [DOI] [PubMed] [Google Scholar]
- 47.Prigozhina NL. Zhong L. Hunter EA, et al. Plasma membrane assays and three-compartment image cytometry for high content screening. Assay Drug Dev Technol. 2007;5:29–48. doi: 10.1089/adt.2006.024. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JH. Chung TD. Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 49.Mori S. Nojiri H. Yoshizuka N. Takema Y. Rapid desensitization of lipolysis in the visceral and subcutaneous adipocytes of rats. Lipids. 2007;42:307–314. doi: 10.1007/s11745-007-3034-8. [DOI] [PubMed] [Google Scholar]
- 50.Morisset AS. Huot C. Legare D. Tchernof A. Circulating IL-6 concentrations and abdominal adipocyte isoproterenol-stimulated lipolysis in women. Obesity (Silver Spring) 2008;16:1487–1492. doi: 10.1038/oby.2008.242. [DOI] [PubMed] [Google Scholar]
- 51.Insel PA. Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol. 2003;23:305–314. doi: 10.1023/A:1023684503883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loo LH. Lin HJ. Singh DK. Lyons KM. Altschuler SJ. Wu LF. Heterogeneity in the physiological states and pharmacological responses of differentiating 3T3-L1 preadipocytes. J Cell Biol. 2009;187:375–384. doi: 10.1083/jcb.200904140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masson O. Chavey C. Dray C, et al. LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue. PLoS One. 2009;4:e7422. doi: 10.1371/journal.pone.0007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rayalam S. Yang JY. Ambati S. Della-Fera MA. Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 55.Sengenes C. Zakaroff-Girard A. Moulin A, et al. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R257–R265. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 56.Dicker A. Astrom G. Wahlen K, et al. Primary differences in lipolysis between human omental and subcutaneous adipose tissue observed using in vitro differentiated adipocytes. Horm Metab Res. 2009;41:350–355. doi: 10.1055/s-0028-1112135. [DOI] [PubMed] [Google Scholar]
- 57.Moro C. Pillard F. de Glisezinski I, et al. Exercise-induced lipid mobilization in subcutaneous adipose tissue is mainly related to natriuretic peptides in overweight men. Am J Physiol Endocrinol Metab. 2008;295:E505–E513. doi: 10.1152/ajpendo.90227.2008. [DOI] [PubMed] [Google Scholar]
- 58.Pearce LR. Komander D. Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 59.Bezaire V. Mairal A. Ribet C, et al. Contribution of adipose triglyceride lipase and hormone sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]