Abstract
We have characterized a 17-residue peptide, MgtL, which is translated specifically in high Mg2+ from an open reading frame (ORF) embedded in the Mg2+ riboswitch domain, previously identified in the 5′ leader region of Mg2+ transporter gene mgtA in Salmonella. We demonstrate that mgtL translation is required to prematurely terminate mgtA transcription. Abrogation of mgtL translation by mutation of its start codon results in transcription of the mgtA-coding region in high Mg2+, suggesting that ribosome stalling is not required for preventing premature transcription termination. Consistently, the Mg2+ riboswitch responds to cytoplasmic Mg2+, but not to proline or arginine, both repeatedly present in the MgtL sequence, to mediate mgtL translation-coupled regulation. RNA structural probing and nucleotide substitution analysis show that the riboswitch loop A region alters base pairing in response to Mg2+, and favours stem-loop A1 in high Mg2+, subsequently opening the ribosome-binding sequence for mgtL translation. Presumably, mgtL ORF directs translation to localize a ribosome in cis to act on downstream RNA in a manner similar to some upstream ORFs in prokaryotes and eukaryotes.
Keywords: Mg2+ riboswitch, premature termination of transcription, the 5′ leader region, the leader peptide
Introduction
In Mg2+-depleted conditions, bacteria facilitate uptake of this divalent cation by inducing synthesis of specific Mg2+ transporters (Snavely et al, 1991). The mgtA gene in the Gram-negative bacteria Salmonella typhimurium and Escherichia coli, which encodes a P-type ATPase to mediate Mg2+ influx (review see ref. Moncrief and Maguire (1999)), has served as a prototype in studies of both inducible regulation and biochemical function of genetic loci encoding Mg2+ transporters. It is suggested that two independent mechanisms are involved in Mg2+-dependent transcriptional regulation of mgtA. (i) The PhoP/PhoQ two-component system responds to micro-molar levels of environmental Mg2+ and activates transcription initiation; or to milli-molar levels of Mg2+ and represses transcription initiation (Garcia Vescovi et al, 1996). (ii) Once transcription is initiated, the 5′ untranslated region (here, called the 5′ leader region or 5′LR) of nascent mgtA transcripts functions as an alternative Mg2+-sensing system. If the Mg2+ concentration increases in the bacterial cytoplasm, the latter system interrupts mgtA transcription before it is extended to the downstream coding region (Cromie et al, 2006).
In Salmonella, the mgtA transcript initiated from the QJ;PhoP-activated promoter contains a 264-nt 5′LR (Lejona et al, 2003), which contains a cis-acting regulatory element responsive to Mg2+. This element is similar to other riboswitches that are able to interact with a small molecule, normally a specific metabolite, and modify the RNA structures through stem-loop switching, subsequently exerting their regulatory effects (review see ref. Tucker and Breaker (2005)). A structural probing of the 5′LR showed that a high Mg2+ condition (3.5 mM) induced regions containing nucleotides 56–125 and 136–159 to form stem-loops A and B (see ref. Cromie et al (2006) and illustrated in Figure 1A). Whereas, a low Mg2+ condition (0.35 mM) caused a stem-loop switching via alternative base pairing between nucleotides 118–125 located in the right arm of stem A, and 140–147 in the left half of stem-loop B, resulting in formation of stem-loop C. Stem-loop B is a prerequisite for initiating premature termination of mgtA transcription mediated by the mgtA Mg2+ riboswitch (Cromie et al, 2006). The stem-loop C, favoured in low Mg2+, may have prevented formation of stem-loop B, and thus allowed mgtA transcription to be extended into the coding region (Cromie et al, 2006). The E. coli mgtA 5′LR also responds to Mg2+ similarly as its Salmonella homologue (Cromie et al, 2006). A truncated RNA representing the mgtA 5′LR was characterized from E. coli cells (Kawano et al, 2005), providing direct evidence for premature termination of mgtA transcription in vivo. Notably, the truncated transcripts are different in length from in vivo (∼240-nt; Kawano et al, 2005) and in vitro (220-nt; Cromie et al, 2006) samples. As the mgtA 5′LR does not have sequences consistent with a Rho-independent terminator, the 220-nt transcript is unlikely a product generated in vitro through transcription termination, but a product from the strong pausing of the RNA polymerase in high Mg2+. The mechanism of termination or pausing, however, is not known. It is possible that mgtA transcription is paused at nucleotide 220, probably by an RNA conformation induced in high Mg2+, and subsequently terminated near nucleotide 240 in vivo by additional cellular components.
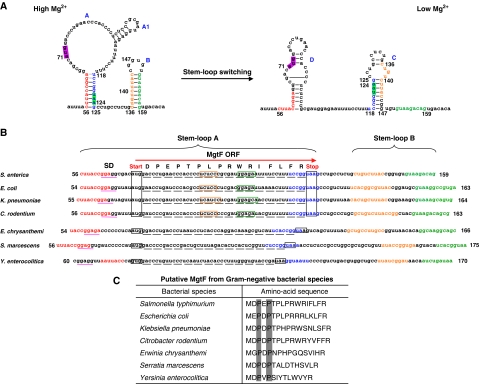
Figure 1.
Prediction of the MgtL leader peptide encoded by the 5′LR of mgtA homologues in Gram-negative bacteria. (A) A schematic representation of the Mg2+ riboswitch domain in Salmonella mgtA 5′LR (5′LR). Coloured letters are the nucleotide sequences involved in the stem-loop switching in different Mg2+ concentrations. Numbering represents the positions of nucleotides in the mgtA 5′LR. Uppercase letters are the stem-loop structures formed in different Mg2+ concentrations. Highlighted sequences are the start and stop codons of the mgtL open reading frame (ORF). The dotted lines indicate that base pairing is possible, however, our data does not support it. (B) Sequence alignment of the stem-loops A and B in the 5′LR region of the mgtA gene. Sequences in colour and underlined in red are stem-loop structures A and B, and the ribosome-binding sites (SD). Three-letter sequences black framed, and underlined in black are start and stop codons, and codons in the mgtL ORF. Orange and green frames are the sequences to form stem A1. Amino-acid residues in the MgtL peptide from Salmonella are shown. Numbering represents the positions of nucleotides in the mgtA 5′LR. (C) MgtL peptides predicted from Gram-negative bacterial species. Highlighted residues are conserved in these MgtL peptide sequences.
Stem-loop A is critical for Mg2+ sensing in the riboswitch because Mg2+-promoted conformational changes in stem-loops B and C depend on the presence of the stem-loop A sequence (Cromie et al, 2006). As the stem-loop A region is transcribed, it might trap the 5′LR RNA into distinct structures depending on the Mg2+ concentration that ultimately determine whether transcription is prematurely terminated. Interestingly, a transition mutation in loop A, that substituted nucleotide 98 from C to U, resulted in uncharacteristic expression of Salmonella mgtA in high Mg2+ concentrations (O’Connor et al, 2009). While the significance of stem-loop A has been implicated, it remained unknown what regulatory element embedded in this region contributed to the 5′LR function.
Importantly, previous results suggested that additional cellular factors could have a role in transcriptional regulation of mgtA via the 5′LR. (i) When transcribed in E. coli, mgtA transcript from Salmonella was degraded more in a high Mg2+ condition in an RNase E-dependent manner (Spinelli et al, 2008). Mutations at the 5′LR eliminated the degradation, suggesting that this nuclease degrades the mgtA mRNA by targeting the 5′LR. (ii) A transcriptional regulator, Rob, can bind to the Salmonella sequence: 5′-accgccaTaattgccacaaa-3′, which includes the PhoP-dependent transcription start (shown in uppercase) (Barchiesi et al, 2008). When overexpressed, Rob initiates transcription from nucleotide 44 of the 5′LR in a PhoP/PhoQ-independent manner.
An Mg2+-responsive RNA element was also characterized in Gram-positive bacteria. The 5′LR of the Mg2+ transporter gene, mgtE from Bacillus subtilis, harbours a metal-sensing domain (M box), which is able to bind Mg2+ and enhance formation of the downstream Rho-independent terminator structure (Dann et al, 2007). Interestingly, these regulatory RNAs from Gram-negative and Gram-positive bacteria do not share homologous sequences, suggesting that they employ different mechanisms to sense Mg2+ and mediate transcription regulation.
In this study, we identify a novel component that controls the regulatory function of the Mg2+ riboswitch in mgtA. Our results demonstrate that the stem-loop A region in the 5′LR comprises a translational unit, which encodes the 17-residue peptide, MgtL, in Salmonella. We show that Mg2+ facilitates modification of the stem-loop A conformation through stem switching to allow mgtL translation from the integral open reading frame (ORF), resulting in premature termination of mgtA transcription. This mechanism seems to be adopted by the MgtA-type Mg2+ transporters in many other Gram-negative species, providing an example in which a small molecule ligand stimulates regulatory function of its cognate riboswitch to initiate premature termination of transcription by coupling translation of a leader peptide. During the submission of our manuscript, a publication became available online which reported an ORF encoding a putative peptide MgtL in the Salmonella mgtA 5′LR presumably responsive to proline (Park et al, 2010). While the presence of the mgtL ORF is undisputed, our model of the Mg2+-dependent/proline-independent mgtL translation via a novel stem-loop switch does not support their conclusions.
Results
An ORF embedded in the stem-loop A region of the mgtA 5′LR
To study the regulatory function of stem-loop A, we analysed the phylogenetically conserved sequences from several Gram-negative species that harbour mgtA homologues, including S. typhimurium, E. coli, Klebsiella pneumoniae, Citrobacter rodentium, Erwinia chrysanthemi, Serratia marcescens, and Yersinia enterocolitica. These stem-loop A sequences greatly varied, but contained three highly conserved regions that could form an ORF: (i) a consensus sequence for ribosome-binding (SD) located upstream of a start codon, (ii) a start codon, AUG (GUG in Yersinia), and (iii) a stop codon, UAA, lying in the same reading frame (Figure 1B). In Salmonella, this ORF encodes a 17-residue peptide (referred to as MgtL, hereafter) from the start codon 71AUG73, which is located 4-nt downstream of a putative SD sequence 62GGAGG66, to the stop codon 122UAA124 (Figure 1B). MgtL homologues can also be predicted from stem-loop A sequences of other Gram-negative species (Figure 1B). MgtL in E. coli, K. pneumoniae, and C. rodentium are also 17-residue peptides sharing high identity with that in Salmonella (70.6, 76.5, and 64.7%, respectively, Figure 1C). On the other hand, MgtL from E. chrysanthemi, S. marcescens, and Y. enterocolitica are shorter peptides, and merely share proline residues at positions 3 and 5 (amino acid residues highlighted in Figure 1C), and arginine at the C terminus, as with the peptides from other species. Regardless of the varied sequence and length in these species, the stop codon UAA is always located at the end of the right arm of stem A (Figure 1B, except Y. enterocolitica whose UAA is located just before the right arm). The right arm is the switching sequence in the riboswitch structure, that base pairs with alternative sequences to form stem-loop C in low Mg2+ and stem-loop A in high Mg2+ (Cromie et al, 2006). We presume that this architectural design of the mgtL ORF is important for the regulatory function of the mgtA 5′LR. Similar to our observation, an 18 codon ORF, predicted to encode a peptide whose suggested sequence is the same as MgtL, was identified from Salmonella mgtA 5′LR in a recent study (Park et al, 2010).
Characterization of MgtL peptide encoded by the stem-loop A sequence in Salmonella mgtA 5′LR
The MgtL peptide is probably either highly unstable or produced at very low levels in the conditions used in this study. We were unable to detect MgtL peptide expressed from the 5′LR in the chromosomal location in vivo using western blot. Therefore, we constructed a plasmid, pYS1475, which carries the full-length mgtA 5′LR with an inserted 21-nt sequence encoding the FLAG-epitope to generate MgtL tagged by FLAG at the N terminus (hereafter MgtL–FLAG; Figure 2A). In this plasmid, the Plac1−6 promoter (Liu et al, 2004), which is independent of Mg2+ and the PhoP/PhoQ system (Cromie et al, 2006; Kong et al, 2008), initiates transcription of the 5′LR and a downstream lacZ gene. Notably, the Rob regulator does not control this transcription because the Rob-binding site is partially deleted in this plasmid (data not shown). β-Galactosidase activity in wild-type Salmonella harbouring pYS1475 and its parent plasmid pYS1010 (i.e., Plac1−6-mgtA 5′LR-lacZ) (Cromie et al, 2006) grown in N minimal medium (Snavely et al, 1989) supplemented with 0.01 mM (low) Mg2+ are 13.3- and 18-fold higher than those with 10 mM (high) Mg2+, respectively. This suggests that the engineered mgtA 5′LR responds similarly to Mg2+ as wild-type 5′LR. Because the Salmonella 5′LR can also function in E. coli (β-galactosidase activity from MC4100 harbouring pYS1010 grown in low Mg2+ is ∼15-fold higher than in high Mg2+), we introduced pYS1475 into an E. coli Maxicell mutant, CSR603. MgtL–FLAG was produced in UV-irradiated bacterial cells in which protein synthesis directed by chromosomal loci, but not by plasmid, was generally inhibited due to extensive degradation of the chromosomal DNA (Sancar et al, 1979). Affinity chromatography was carried out to isolate MgtL–FLAG (MW 3164 da) from bacterial cultures grown in low and high Mg2+. The peptide sample was separated through electrophoresis and a band was detected from the bacterial cells grown in high Mg2+ (Figure 2B), which migrated to a position slightly slower than a control peptide, magainin 2 (MW 2465 da). However, this peptide could not be detected from the bacterial cells grown in low Mg2+, suggesting that MgtL–FLAG is synthesized only in high Mg2+. We then carried out a parallel experiment using a plasmid, pYS1475–A71C, which carries an A–C substitution at nucleotide 71 of the 5′LR that changes 71AUG73 to 71CUG73, resulting in deletion of the start codon. The MgtL–FLAG peptide could not be detected from the cells harbouring this plasmid grown in low and high Mg2+ (Figure 2B). Furthermore, when MgtL was overexpressed in a Salmonella wild type harbouring an IPTG-inducible plasmid pUHE-mgtL, we were able to detect an m/z 2171.38 peak in a MALDI-TOF mass spectrum analysis from an eluent derived from bacteria cells grown in the presence of IPTG and 10 mM Mg2+ (Figure 2C, bottom). This peak is specific because it could not be detected from a wild-type cell lysate harbouring control vector (Figure 2C, top).
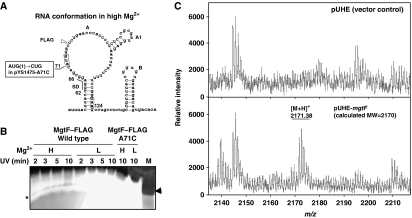
Figure 2.
Characterization of the MgtL leader peptide encoded by the 5′LR of Salmonella mgtA gene. (A) A schematic representation of the FLAG insertion site in plasmid pYS1475 containing Salmonella mgtA 5′LR. A site-directed substitution is marked in the small frame, and (1) is the mutated start codon in the MgtL sequence. (B) Silver staining of Salmonella MgtL peptides. Peptide preparations were derived from E. coli Maxicell mutant (CSR603) harbouring pYS1475 and pYS1475-A71C. Bacterial cultures were subjected to UV irradiation (50 J/m2) for 2, 3, 5, and 10 min to enhance MgtL–FLAG synthesis. H and L represent N medium supplemented with 10 and 0.01 mM Mg2+, respectively. Arrow indicates the position of magainin 2. Asterisk represents MgtL–FLAG bands. (C) MALDI-TOF mass spectrum analysis of MgtL from Salmonella harbouring vector pUHE (top) and pUHE-mgtL (bottom) grown for 4 h in N medium with 0.5 mM IPTG. m/z represents the mass-to-charge ratio, and MgtL peptides carry one positive charge.
Premature termination of Salmonella mgtA transcription in high Mg2+ is coupled to mgtL translation initiation
The observation that mgtL translation and premature termination of mgtA transcription both occur in the 5′LR in high Mg2+ suggests that these convergent phenomena are coordinated in response to Mg2+. Thus, we hypothesize that MgtL synthesis is a prerequisite for the premature termination of mgtA transcription. We constructed a set of pYS1010 derivatives with site-directed substitutions inside the mgtL-coding region (Figure 3A and C), and determined lacZ expression in Salmonella wild-type cells harbouring these plasmids. In contrast to the result from parental pYS1010, lacZ expression in cells carrying pYS1010-A71C, in which MgtL could not be synthesized due to disruption of the start codon (Figure 2A and B) remained activated in high Mg2+ because β-galactosidase activity was only 1.9-fold lower in high Mg2+ than in low Mg2+ (Figure 3B). On the other hand, lacZ transcription from cells harbouring pYS1010-A71G, which also carried a substitution at nucleotide 71, but changed 71AUG73 to another start codon 71GUG73 (Figure 3A and C), was repressed in high Mg2+ because β-galactosidase activity was 12.2-fold lower when grown in high Mg2+ than in low Mg2+ (Figure 3B). To further determine the importance of the start codon, we tested pYS1010-G73C in which the start codon was disrupted by a substitution at nucleotide 73 (Figure 3A and C). Comparable to pYS1010-A71C, β-galactosidase activity from cells with pYS1010-G73C was only two-fold lower in high Mg2+ than in low Mg2+ (Figure 3B). Apparently, if mgtL fails to be translated, high Mg2+ is not sufficient to prematurely terminate mgtA transcription. The 5′LR in another plasmid, pYS1010-G74C, which contains a substitution at nucleotide G74 that changes the second amino acid from Asp to His without interfering with mgtL translation (Figure 3A and C) remained responsive to Mg2+ because β-galactosidase activity in high Mg2+ was 14.9-fold lower than in low Mg2+ (Figure 3B). Collectively, these observations demonstrate that mgtL translation is essential for the premature termination of mgtA transcription in high Mg2+.
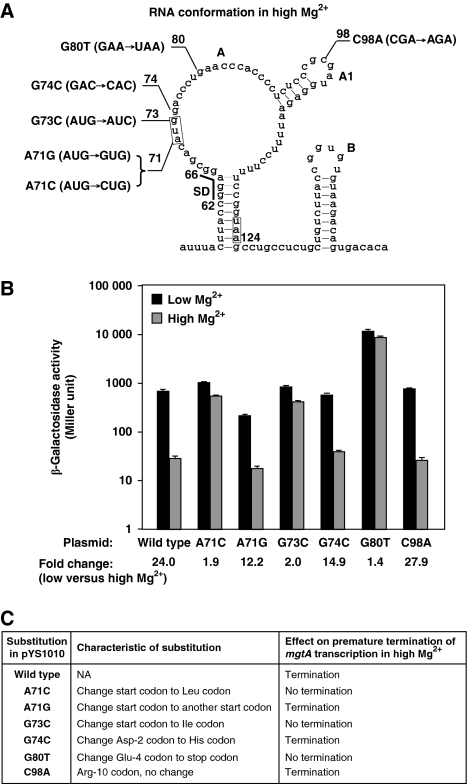
Figure 3.
Genetic evidence demonstrates that MgtL translation is coupled to premature termination of mgtA transcription in the mgtA 5′LR. (A) Illustration of the substituted nucleotides in pYS1010 derivatives. Numbering represents the positions of the nucleotides in the Salmonella mgtA 5′LR. Framed sequences represent the start and stop codons. The SD sequence is underlined. (B) β-Galactosidase activity was determined in Salmonella wild-type 14028s, which harboured wild-type plasmid, pYS1010, or one of the substituted derivatives shown in (A). Bacteria were grown for 4 h in N medium supplemented with 0.01 mM (low) or 10 mM (high) Mg2+. Fold change was determined by β-galactosidase activity from low Mg2+ divided by activity from high Mg2+. Assays were conducted in triplicate. Error bars correspond to the standard deviation. (C) Effect of the substitutions of the mgtL ORF on premature termination of mgtA transcription based on the results from (B).
Disruption of mgtL translation elongation prevents the premature termination of mgtA transcription in high Mg2+
We created a stop codon within the mgtL ORF to determine whether interference of its translation elongation could inhibit premature termination of mgtA transcription in high Mg2+. The plasmid, pYS1010-G80T, harbours a substitution that replaces the fourth codon, 80GAA82 (Glu), with stop codon 80UAA82 (Figure 3A and C), in which mgtL translation should be stopped prematurely. β-Galactosidase activity from cells harbouring this plasmid in high Mg2+ was only 1.4-fold lower than that in low Mg2+ (Figure 3B), indicating that mgtA transcription could not be prematurely terminated due to the nonsense point mutation. Furthermore, a recent study showed that a substitution, C98U, in the mgtA 5′LR resulted in mgtA expression in high Mg2+ (O’Connor et al, 2009). This mutation changes the tenth codon, 98CGA100 (Arg), to a stop codon 98UGA100, thus causing a premature stop of mgtL translation at a codon far downstream of 80GAA82 (fourth codon). We constructed a plasmid pYS1010-C98A, which carried a substitution at the same nucleotide, C98, and generated a silent mutation (Figure 3A and C) and found that β-galactosidase activity was 27.9-fold lower in high Mg2+ than in low Mg2+ (Figure 3B), indicating that the 5′LR carrying a substitution of C98A, unlike C98U, remained responsive to Mg2+. With these results and the observation that the full-length MgtL peptide is detected specifically in high Mg2+ (Figure 2B), we propose that mgtL translation should be completed in high Mg2+ to prematurely terminate mgtA transcription.
Mg2+ concentration modulates a stem switching within stem-loop A that determines conformation of the ribosome-binding site for mgtL translation
We synthesized the full-length 264-nt mgtA 5′LR and probed the stem-loop A structure in different Mg2+ conditions using dimethyl sulphate (DMS) which modifies adenosine, cytidine, and guanosine when located in single-stranded regions. A primer extension assay, in which the reverse transcription reaction is disrupted at the modified nucleotides in RNA templates, showed that 62GGAGG66, proposed to be the SD sequence here (Figure 1B), was located in a double-stranded region in low Mg2+, however, in a single-stranded region in high Mg2+ (Figure 4A). The nucleotides G63 and A64 in the SD sequence were modified 2.7- and 2.4-fold more in high Mg2+ (3 mM) than in low Mg2+ (0.1 mM), respectively (Figure 4A), indicating their locations in a single-stranded region in high Mg2+ regardless of simulated base pairs (Cromie et al, 2006). Furthermore, G73, G74, C85, A86, C96, and G97 were modified 2.9-, 2.4-, 2.6-, 4.7-, 2.1-, and 2.6-fold more in high Mg2+ than in low Mg2+, respectively, implying that they are base paired or protected in a stem (named D, Figure 1A) formed in low Mg2+. Based on these observations, we proposed that the sequence, 91UCUCC95 (named anti-SD), can form part of stem D by base pairing with the SD sequence in low Mg2+; and can alternatively form stem A1 with the sequence, 102GGAGA106 (named anti-anti-SD), in high Mg2+ in which the SD site is accessible for mgtL translation (summarized in Figure 6). Consistent with this, substitution of the anti-SD sequence in stem A1 with 91AGAGG95 enhanced premature transcription termination regardless of Mg2+ because β-galactosidase activity in a wild-type strain harbouring this substituted plasmid (pYS1010-A1-sub) grown in low Mg2+ was as low as that in wild-type strain harbouring the wild-type plasmid grown in high Mg2+ (Figure 4B, also see ref. Cromie et al (2006)). To determine the role of the anti-SD sequence, we used DMS to map the full-length RNA carrying this substituted sequence. We found that G63 and A64 in the SD sequence was modified regardless of Mg2+ at a similar level as wild type in high Mg2+ (Figure 4C), indicating that without the anti-SD sequence, the SD sequence remained single stranded in low and high Mg2+, causing constitutive repression of mgtA transcription likely due to the continuous translation of mgtL. Introduction of a second substitution to pYS1010-A1-sub, which replaced 102GGAGA106 with 102CCUCU106 to form pYS1010-A1-rev, complemented the first substitution by creating a modified stem-loop A1 and restored a wild-type-like response to Mg2+ (Figure 4B, also see ref. Cromie et al (2006)). It is likely that 102CCUCU106 forms a new anti-SD sequence that base pairs with the SD sequence, thus resulting in inhibition of mgtL translation similar to wild type in low Mg2+; whereas the 91AGAGG95 becomes a new anti-anti-SD sequence in the double substituted 5′LR and turns on mgtL translation in high Mg2+. This was supported by a DMS probing assay using this double substituted full-length RNA in which, like wild-type RNA, the nucleotides G63 and A64 in the SD sequence were protected from DMS modification in low Mg2+ (Figure 4C). Additional mapping of the full-length wild-type RNA with RNase T1, which cleaves unpaired G residues, revealed that high Mg2+ facilitates the accessibility of this nuclease to G65 and G66 located in the SD sequence because they were cleaved 3.4-fold more in high Mg2+ than in low Mg2+ (Supplementary Figure S2), suggesting that the SD site was localized in a single-stranded region in high Mg2+ making it more accessible. In contrast, G105 in the anti-anti-SD sequence was cleaved 3.7-fold more in low Mg2+ than in high Mg2+, implying that it should be located in double-stranded region by base paring with the anti-SD sequence in high Mg2+, however, located in a single-stranded region when the anti-SD sequence is switched to form stem-loop D in low Mg2+ (Figure 6). Collectively, these results provide evidence that Mg2+ controls the accessibility of the mgtL SD sequence via a stem-loop switching that determines formation of stem-loop A1 and D by which it modulates mgtL translation.
Figure 4.
Mg2+ modifies the secondary structure of stem-loop A in the full-length 264-nt mgtA 5′LR. (A) Primer extension of DMS-treated stem-loop A following incubation with 0.1, 0.3, 1, and 3 mM Mg2+. In both (A) and (C), 6% polyacrylamide gel was used to separate the products. Lane M corresponds to Maxam–Gilbert reaction using DNA fragment amplified from pYS1010 with primers 220 and 32P-labeled 201. Lane C corresponds to a reaction with untreated RNA. Quantification in both (A) and (C) was conducted using Quantity One software (Bio-Rad). After all bands in a lane were normalized by an unchanged band at different Mg2+, the DMS modification ratio was calculated and shown on the right of (A) by comparing with the corresponding band from the sample incubated with 0.1 mM Mg2+. The x-axis represents the DMS modification ratio at a nucleotide as calculated while its position is shown on the y-axis. (B) The mgtA 5′LR with substitution at 91–95 does not respond to Mg2+ and the transcription is significantly reduced. The SD and stem-loop A1 sequences of plasmid pYS1010 and derivatives with the substitutions are shown. Numbering represents position of nucleotide in the mgtA 5′LR. β-Galactosidase activity was determined in Salmonella wild-type 14028s, which harboured wild-type plasmid pYS1010 or derivatives. Bacteria were grown for 4 h in N medium supplemented with 0.01 mM (low) or 10 mM (high) Mg2+. (C) DMS modification of the full-length 264-nt 5′LR with wild-type or substituted sequences shown in (B). The RNA was incubated with 0.1 mM (L) and 3 mM (H) Mg2+ before DMS treatment and primer extension. After all bands in a lane were normalized by an unchanged band at different Mg2+, the DMS modification ratio was calculated and shown on the bottom of (C) by comparing with the corresponding band from wild-type sample incubated with 0.1 mM Mg2+.
Similar stem-switching domains that determine the accessibility of the SD site for mgtL translation were also found in additional species, such as E. coli, K. pneumoniae, and C. rodentium (Figure 1B). It remains to be investigated how the SD site is modulated in E. chrysanthemi, S. marcescens, and Y. enterocolitica.
Investigation of premature termination of mgtA transcription influenced by amino acids conserved in the MgtL sequence
To determine whether the four Pro residues in Salmonella MgtL sequence (Figure 1B) are involved in mgtL translation-coupled transcriptional regulation of mgtA, we constructed four plasmids pYS1010-C77G, pYS1010-C83G, pYS1010-C89G, and pYS1010-C95G which harbour single substitutions at nucleotides 77, 83, 89, and 95, respectively, and therefore substitute the individual Pro codons to Ala codons. β-Galactosidase activity from wild-type cells with the substituted plasmids remained responsive to Mg2+ similar to the wild-type plasmid (Figure 5A). In addition, we constructed a plasmid that replaced the Pro-3 and Pro-5 codons, which are conserved in the mgtA 5′LRs from various enteric bacteria (listed in Figure 1C), with Ala and found that this double substitution still retained an Mg2+ response similar to the wild-type plasmid. Furthermore, there are three Arg residues in the Salmonella MgtL sequence (Figure 1B). Therefore, we assayed the function of the 5′LR by depleting the amino acids from the medium in proline and arginine auxotrophs. It was presumed that reduction of the cytoplasmic concentration of proline or arginine may slow down or prematurely stop mgtL translation due to a possible ribosome stalling and subsequent inhibition of premature termination of mgtA transcription. However, β-galactosidase activity from the auxotrophs (constructed using one-step gene disruption strategy; Datsenko and Wanner, 2000) carrying pYS1010 grown with low and high Mg2+, and 0 and 10 mM proline (for proC mutant, Deutch et al, 1982; YS14029) or 10 mM arginine (for argA mutant, Marvil and Leisinger, 1977; YS12014) was similar to that from wild-type strain harbouring pYS1010 (Figure 5B), suggesting that starvation of these amino acids does not affect the 5′LR-mediated premature termination. Similar to the results from Salmonella, β-galactosidase activity from E. coli proline auxotrophic mutants, proB (Smith et al, 1984) and proC, as well as arginine auxotrophic mutants, argB (Parsot et al, 1988) and argE (Meinnel et al, 1992), harbouring pYS1010 was similar to wild type in each Mg2+ condition regardless of the proline or arginine concentration (Supplementary Figure S3D).
Figure 5.
Clarification of the effect of amino acids on premature termination of mgtA transcription. (A) β-Galactosidase activity was determined in Salmonella wild-type 14028s, which harboured wild-type plasmid, pYS1010, or one of the derivatives with substituted Pro codons. Bacteria were grown for 4 h in N medium supplemented with 0.01 mM (low) or 10 mM (high) Mg2+. Fold change was determined by β-galactosidase activity from low Mg2+ divided by activity from high Mg2+. (B) β-Galactosidase activity determined in 14028s, proC mutant (YS14029), and argA mutant (YS12014) harbouring pYS1010. β-Galactosidase activity was determined from two groups: one is 14028s and proC grown for 4 h in N medium supplemented with 0 or 10 mM proline, and 0.01 mM (low) or 10 mM (high) Mg2+; and the other is 14028s and argA grown for 4 h in N medium supplemented with 0 or 10 mM arginine, and 0.01 mM (low) or 10 mM (high) Mg2+. (C) Intracellular proline concentration determined in 14028s and proC grown as described in (B). Assays were conducted in triplicate. Error bars correspond to the standard deviation.
A recent study compared the level of mgtA mRNA transcribed from its native promoter and proposed that the 5′LR could respond to cytoplasmic concentration of proline (Park et al, 2010). On the contrary, we found that in high Mg2+ without supplemented proline the 5′LR-dependent mgtA transcription in the proC mutant was similar to that in wild type (Figure 5B), although the cytoplasmic proline level was much higher in the Salmonella wild type than in the proC mutant (∼3.7 and 2.4 mmol/g protein, respectively, Figure 5C). When proC mutant was grown in high Mg2+ with supplemented proline (10 mM), which significantly raised the cytoplasmic proline concentration (from ∼2.4 to 3.4 mmol/g protein), β-galactosidase activity remained similar to that without proline (Figure 5B). These results indicate that intracellular proline does not influence 5′LR-dependent mgtA expression. Noteworthy, the proline levels in wild type grown in high Mg2+ are actually lower than that grown in low Mg2+, ruling out the possibility that high Mg2+ facilitates premature transcription termination by raising the cytoplasmic proline levels to enhance mgtL translation.
Furthermore, we repeated our assays using the conditions in a recent study investigating the proline effect on mgtA transcription (Park et al, 2010). In a time (i.e., 15 min) sufficient to see an ∼7-fold Mg2+-dependent response of the 5′LR-dependent mgtA transcription in wild-type and proC strains, a proline effect was not observed. In fact, the cytoplasmic proline concentration was lower in the proC mutant than the wild type in all the tested conditions. Previous results indicate that supplementing proline reduces mgtA-coding mRNA levels within 15 min, we found that this time period is too short for the accumulation of cytoplasmic proline because supplementation of proline cannot significantly increase the cytoplasmic concentration (Supplementary Figure S3C). Thus, we prolonged the incubation for 2 h and found that β-galactosidase activity in the proC mutant supplemented with proline had similar expression levels as wild type grown in low Mg2+ without proline supplemented, while the proC mutant expression without proline was significantly reduced (Supplementary Figure S3A) due to the significant reduction of colony forming units (CFUs) (Supplementary Figure S3B). Although the cytoplasmic proline levels from cultures grown in low Mg2+ with proline supplemented are lower in the proC mutant (3.7 mmol/g protein) than the wild type (6.0 mmol/g protein), the β-galactosidase activity from both remained similar (Supplementary Figure S3A). In addition, we constructed two pYS1010 derivatives harbouring substituted Pro codons at three (5, 7, 9) and four (3, 5, 7, 9) Pro residues, similar to the introduced substitutions at the chromosomal mgtA 5′LR in the recent study in which they report a loss in its ability to respond to proline (Park et al, 2010). Although β-galactosidase activity from the proC mutant harbouring these plasmids was generally lower, overall, the 5′LR activity was similar in wild-type and proC mutant strains harbouring wild-type, triple-substituted, and tetra-substituted plasmids in the conditions (Figure 5A; Supplementary Figure S3A). Taken together, our data does not support the conclusion that the 5′LR responds to proline.
Investigation of the mgtL translation-coupled premature termination of mgtA transcription in vitro
Full-length mgtA 5′LR is sufficient to direct mgtL translation in vivo (Figure 2C), but not in vitro (data not shown). To explore the possibility that mgtL may be translated from a transcribing mgtA 5′LR, we carried out a transcription–translation-coupled in vitro reaction using DNA fragments in which the Plac1−6 promoter controls the transcription initiation of the mgtA 5′LR. We detected the full-length transcript (264-nt) and the truncated transcript (220-nt), which was produced at higher levels in high Mg2+ (Supplementary Figure S1A) similarly as a previous in vitro transcription result (Cromie et al, 2006). However, we could not detect the MgtL peptide in this reaction that could detect a truncated LacZ (∼14.8 kDa) in low and high Mg2+ at similar protein levels using a control DNA fragment with the Plac1−6-promoted 390-bp lacZ coding region (Supplementary Figure S1B). A template containing the Plac1−6-promoted mgtA 5′LR followed by the 390-bp lacZ coding region could direct synthesis of truncated LacZ at a lower amount from the reaction without supplemented EDTA (i.e., high Mg2+) than that with EDTA (i.e., low Mg2+), yet, MgtL could not be detected (Supplementary Figure S1B). The 5′LR carrying mutation in the anti-SD sequence (A1-sub, 91–95), which resulted in constitutive mgtL translation in vivo, could produce truncated transcripts, in vitro, in high Mg2+, similar to wild type (Supplementary Figure S1A). Thus, it is likely that the in vitro system allows the 5′LR to mediate pausing of the downstream lacZ transcription in high Mg2+, independent of mgtL translation. As MgtL peptide could not be detected from in vitro reactions, it is very likely that translation of mgtL requires additional cellular factors.
Discussion
A Mg2+-dependent translation of leader peptide within a riboswitch is coupled to the premature termination of transcription
This study presents an example in which a translational unit comprises an integral part of a riboswitch to mediate premature termination of transcription. An ORF is characterized within the 5′LR of the Salmonella mgtA gene, which encodes a 17-residue peptide, MgtL. The mgtL ORF is embedded in stem-loop A region, which has been regarded as the Mg2+-sensing domain in the Mg2+ riboswitch (Cromie et al, 2006). We found that this translation occurs specifically in high Mg2+, a condition known to facilitate premature termination of mgtA transcription, thus indicating that high Mg2+ should stimulate mgtL translation that triggers this transcription termination. A typical model of translation-coupled transcription attenuation is the 5′LR that harbours a coding region for the 14-residue TrpL in the E. coli trp operon, which includes two tandem tryptophan codons. Lack of tryptophan caused the TrpL-translating ribosome to stall due to the uncharged tRNATrp at the 5′LR, which triggers modification of the leader RNA to eliminate a downstream terminator structure (a review see Henkin and Yanofsky (2002)). Different from trp regulation (i) mgtA transcription will not be prematurely terminated in high Mg2+ if mgtL translation is unable to be initiated due to start codon mutations, in which no ribosome can enter the mgtL ORF region (Figure 3A and B); and (ii) cytoplasmic levels of amino acids present repeatedly in the MgtL sequence, such as proline and arginine, are irrelevant to the regulatory activity of the mgtA 5′LR because premature transcription termination takes place similarly in wild-type and isogenic auxotrophs (Figure 5A–C; Supplementary Figure S3A, C, and D). These observations rule out the possibility that a stalled ribosome would be required to induce a specific RNA conformation in order to prevent premature transcription termination. The mgtL translation has only been detected in vivo so far, possibly because this intricate process requires additional cellular component(s) in the translation system. An ongoing project was initiated to identify chromosomal loci that are required for mgtL translation in high Mg2+ that has led to the identification of a mutant that abrogates premature termination of mgtA transcription in high Mg2+ (data not shown).
Mg2+ induces a conformation change in the stem-loop A RNA region that determines initiation of mgtL translation
Alternative strategy must be adapted for regulation of mgtL translation since ribosome stalling does not appear to prematurely terminate translation. Our results suggest that mgtL translation is not constitutive, but Mg2+ dependent (Figure 2B and C). mgtL translation unlikely modulates the mgtA 5′LR stem switching because, in vitro, Mg2+ is sufficient to induce formation of stem-loops A and B (in high concentrations); or alternative stem-loop C (Cromie et al, 2006), and likely stem-loop D (in low concentrations). According to the model in Figure 6, we conclude that, in low Mg2+, the SD sequence, 62GGAGG66, is base paired with the anti-SD sequence, 91UCUCC95, to form stem-loop D and prevent access of the ribosome, thus inhibiting translation of MgtL and subsequent premature termination of mgtA transcription. However, in high Mg2+, along with the formation of stem-loop A, the anti-SD sequence base pairs with the anti-anti-SD sequence, 102GGAGA106, to form stem-loop A1, thus opening the SD site for mgtL translation. Stem-loop D cannot be formed in low Mg2+ when the anti-SD sequence is substituted, which leaves the SD site single stranded even in low Mg2+ (Figure 4C), resulting in mgtL translation and significantly reduced mgtA transcription (Figure 4B, and also ref. Cromie et al (2006)). Our model suggests that it is the Mg2+ signal that determines the mgtL translation, not the mgtL translation that leads to the Mg2+ sensing. That is to say, mgtL translation is an intermediate step that relays the Mg2+ signal from the Mg2+-sensor domain to the far downstream region in which the premature termination of mgtA transcription takes place.
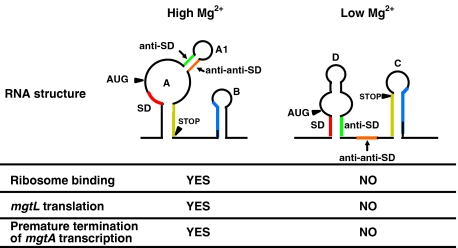
Figure 6.
Illustration of the mgtA 5′LR conformational changes that influence mgtL translation. In high Mg2+, the SD site is accessible (in loop A), while the anti-SD and anti-anti-SD sequences are base paired (in stem A1), resulting in mgtL translation and premature termination of mgtA transcription once translation is completed. In low Mg2+, the SD sequence is base paired to the anti-SD sequence (in stem D) inhibiting mgtL translation.
A possible role of mgtL translation in the Mg2+ riboswitch function
Premature termination of mgtA transcription is abrogated in high Mg2+ if the ribosome is either absent due to the lack of mgtL translation initiation, or is released early by a nonsense mutation in the mgtL ORF at the fourth codon (Figure 3B) or even at the tenth and thirteenth codons located downstream of the four Pro codons (O’Connor et al, 2009; Park et al, 2010). Thus, it is plausible that completion of mgtL translation is a key step that determines premature termination in high Mg2+. This is probably the reason why MgtL peptide can only be detected from bacterial cells grown in high Mg2+ (Figure 2B). In addition, mgtA transcription cannot be prematurely terminated if the stem-loop B structure is disrupted (Cromie et al, 2006), suggesting that both mgtL translation and stem-loop B formation are essential for premature transcription termination. However, it is unlikely that mgtL translation is required for formation of stem-loop B because it can be induced in high Mg2+ in vitro without any protein component (Cromie et al, 2006). Accordingly, these two regulatory elements likely exert their effects simultaneously. Two possible mechanisms might facilitate premature transcription termination when mgtL translation is completed: (i) mgtL translation may bring the ribosome to a position that facilitates termination of mgtA transcription. This is in a manner comparable to the function of some eukaryotic upstream ORFs (uORF) in which the post-translation release of the ribosome subunit(s) can cause destabilization of the downstream coding region (Vilela and McCarthy, 2003). Thus, the mgtL ORF sequence may provide a ‘track’ that, once opened in high Mg2+, allows a ribosome to reach a region to the mgtL stop codon or a far downstream and enables premature transcription termination. This is why the ribosome has to be introduced by the mgtL translation in cis because a ribosome supplemented in an in vitro transcription system could not facilitate premature transcription termination in low Mg2+ (Supplementary Figure S1C). The premature termination of transcription takes place at a region far downstream of the mgtL stop codon (Kawano et al, 2005; Cromie et al, 2006). It has been suggested that high Mg2+ reduced the 5′LR mgtA transcript stability in an RNase E-dependent manner (Spinelli et al, 2008). Furthermore, it is shown that RNase E cleaves the ferric uptake regulator fur mRNA when the ribosome cannot initiate translation of the upstream ORF due to its SD site base paired with a trans-acting regulatory RNA RyhB (Veæerek et al, 2007). We propose that, in high Mg2+ when the cis-acting anti-SD sequence is unpaired to its target, the SD site of mgtL, translation confers a novel function to bring a ribosome on site, probably to interact with stem-loop B, subsequently facilitating RNase E to bind and degrade mgtA 5′LR. (ii) The MgtL peptide could function as a trans-acting factor and regulate transcription of the mgtA downstream region. However, the translation machinery, but not the MgtL peptide itself, most likely has a role in premature termination of mgtA transcription because the MgtL sequence from various species is not well conserved (Figure 1C), and because MgtL synthesized in trans from a plasmid cannot prematurely terminate mgtA transcription in mgtL translation initiation mutants (i.e., A71C and G73C) in high Mg2+ (data not shown).
Our conclusions that the Mg2+-dependent synthesis of MgtL is required for premature termination of mgtA transcription contradicts a recent report in which they found that mgtL translation was Mg2+ independent (Park et al, 2010). Their conclusions, however, are misleading because (i) their engineered mgtL–lacZ fusion contained a deleted mgtL stop codon, which disrupted the stem A required for formation of the Mg2+-sensing domain of the 5′LR. (ii) Their ‘low Mg2+’ condition used to test the mgtL–lacZ fusion was indeed a high Mg2+ condition (Cromie et al, 2006; Cromie and Groisman, 2010) and therefore could not be distinguished by the 5′LR whose Mg2+ sensing had been disrupted anyway. (iii) Different from the low-copy number plasmid (pYS1010) that we used to study regulatory function of the mgtA 5′LR in which transcription is only regulated by the 5′LR, they determined mgtA transcription, particularly its response to proline, from its chromosomal locus, which, in addition to the 5′LR (Cromie et al, 2006), is regulated by at least two independent promoters controlled by PhoP, in response to the extracytoplasmic Mg2+ (Garcia Vescovi et al, 1996) and Rob (Barchiesi et al, 2008). It is shown that when the 5′LR is located in its native chromosomal location, it appears to have an additional regulatory function because the C98T substitution in the 5′LR unexpectedly led to constitutive mgtA transcription even in high Mg2+ (O’Connor et al, 2009), in which the transcription initiation is supposed to be repressed by the PhoP/PhoQ system. This result cannot be explained by the premature termination of mgtL translation which takes place after transcription is initiated, simply because transcription initiation does not occur. (iv) The regulatory activity of Rob might be changed in altered nutrient conditions they used, such as proline, which should mediate transcription initiated from nucleotide 44 of the 5′LR (Barchiesi et al, 2008). Their real-time PCR assay can measure the transcripts of the mgtA-coding region, but not the 5′LR due to a primer (Park et al, 2010) which corresponds to nucleotides 7–31 of the 5′LR absent in Rob-stimulated transcripts, resulting in biased ratios of the 5′LR to other RNAs.
In summary, our results suggest a model in which mgtL translation should function as an intermediate to transduce the Mg2+ signal from the upstream signal sensing domain to the far downstream effector domain, thus providing an integral component for the riboswitch function to facilitate premature termination of transcription, such as in mgtA, while the Rho-independent terminator and ribozyme structures seem to be absent.
Materials and methods
Bacterial strains and growth conditions
All Salmonella enterica serovar Typhimurium strains were derived from the wild-type strain 14028s. Bacteria were grown at 37°C in Luria–Bertani broth or N minimal medium (Snavely et al, 1989), pH 7.4, supplemented with 0.1% casamino acids and 38 mM glycerol. When necessary, antibiotics were added at final concentrations of 50 μg/ml for ampicillin and 20 μg/ml for chloramphenicol. E. coli DH5α and DH5α T1 (Invitrogen) were used as host for the preparation of plasmid DNA. χ2680 (recA uvrA phr-1) was an E. coli Maxicell mutant CSR603 (Sancar and Rupert, 1978) used to express MgtL–FLAG from plasmid pYS1475. Amino-acid auxotrophic mutants proB, proC, argB, and argE were derived from an E. coli wild-type BW25113 in the Keio Knockout Collection (Baba et al, 2006). Oligonucleotides used in this study are described in Table I.
Table 1. Primers used in this study.
| No. | Sequence (from 5′ to 3′) |
|---|---|
| 201 | AGGTAATCCCTCCGCGCCG |
| 220 | TAATACGACTCACTATAGTAATTGCCACAAAACTTATGG |
| 241 | GGCCTGCTTCTCGCCGAAACGTTTGG |
| 298 | GCGTCGACCTTTACACTTTAAGCTTTTTATGTTTATGTTGTGTGGATAATTGCCACAAAACTTATGGATTTATGC |
| 299 | CCGCTCGAGGTAATCCCTCCGCGCCGAAGTCAGGCG |
| 768 | TCGAATAATAATTCACTAGTGGGGCGCGCACATATGAATATCCTCCTTAG |
| 769 | GATAAGCGCAGCGCCATCAGGCCCCCCTTG GTGTAGGCTGGAGCTGCTTC |
| 794 | TCTCCATCGCGGGAGAGGGGTGGGTTCAGGCTTGTCATCGTCGTCCTTGTAGTCCATGTCGCCTCCGGT |
| 811 | CCCGTGGCGTGACGCTGATGGTGATGAAAACATATGAATATCCTCCTTAG |
| 812 | CCATTGCCCTTCGCTTGAGTAAAGTTACTCGTGTAGGCTGGAGCTGCTTC |
| 903 | CCGGATCCTAATTGCCACAAAACTTATG |
| 904 | CCCAAGCTTAGGTAATCCCTCCGCGCCGAAG |
| 912 | TACTTACCGGAGGCGACCTGGACTACAAGGACGAC |
| 913 | GTCGCCTCCGGTAAGTA |
| 1154 | AACCCACCCCTCTCC |
| 1243 | ATGTCGCCTCCGGTAAG |
| 1244 | CTTACCGGAGGCGACATCGACCCTGAACCCACCCCTCTCC |
| 1245 | CTTACCGGAGGCGACATGCACCCTGAACCCACCCCTCTCC |
| 1361 | CGGGAGAGGGGTGGGTTCAG |
| 1362 | GAACCCACCCCTCTCCCGAGATGGAGAATTTTCCTTTTCC |
| 1553 | ACTTACCGGAGGCGACCTGGACCCTGAACCCAC |
| 1554 | ACTTACCGGAGGCGACGTGGACCCTGAACCCAC |
| 1555 | GGGAGAGGGGTGGGTTAAGGGTCCATGTCGCCTC |
| 1557 | GGGAGAGGGGTGGGTTCAGCGTCCATGTCGCCTCCG |
| 1559 | TTCAGGGTCCATGTCGCCTC |
| 1560 | GCGACATGGACCCTGAAGCCACCCCTCTCCCGCGAT |
| 1562 | GCGACATGGACCCTGAACCCACCGCTCTCCCGCGATGGAGAAT |
| 1564 | GCGACATGGACCCTGAACCCACCCCTCTCGCGCGATGGAGAATTTTCCTT |
| 1578 | GTCCATGTCGCCTCCGGTAAG |
| 1579 | CGGAGGCGACATGGACGCTGAAGCCACCCCTCTCCCGCGATG |
| 1600 | GGGGTGGGTTCAGGGTCC |
| 1601 | GACCCTGAACCCACCCCAGAGGCGCGATGGAGAATTTTCC |
| 1602 | GACCCTGAACCCACCCCAGAGGCGCGATCCTCTATTTTCCTTTTCCGGTAAGCC |
| 1607 | CCATAACACACAAACATAGGGAGTGACGAGCATATGAATATCCTCCTTAG |
| 1608 | CGAAGTGGCGGCATGACGTCCAGCCGGGCTGTGTAGGCTGGAGCTGCTTC |
| 1609 | TTCAGGGTCCATGTCGCC |
| 1610 | GGCGACATGGACCCTGAAACCACCCATCTCCTGCGATGGAGAATTTTCC |
| 1611 | GTCCATGTCGCCTCCGGTAAG |
| 1612 | ACCGGAGGCGACATGGACCTTGAAACCACCCATCTCCTGCGATGGAGAATTTTCC |
| 1613 | TATTCATTAAGGTAATCCCTCCGCGCCG |
| 1614 | TATTCATTATTCATCAACATTAAATGTGAGC |
β-Galactosidase assay
β-Galactosidase assays were carried out in triplicate using a VERSAmax plate reader (Molecular Device) and the activity (Miller unit) was determined as described (Miller, 1972). Data correspond to three independent assays conducted in duplicate, and all values are mean±s.d.
Preparation and analysis of MgtL peptide
The MgtL–FLAG peptide was expressed in bacteria as follows: an E. coli Maxicell mutant CSR603 (χ2680) (recA uvrA phr-1), carrying plasmid pYS1475 or pYS1475-A71C was grown in N medium with 0.01 and 10 mM Mg2+ at 37°C to mid-log phase. After subjecting the culture to UV irradiation (50 J/m2), cultures were further incubated with shaking for 20 h. Then, bacterial cells were harvested by centrifugation and resuspended in phosphate-buffered saline (PBS) buffer, and opened by ultrasonication. Cell lysates with a same amount of total protein (protein concentration was determined using the BCA Protein Assay Kit from Pierce) were loaded onto columns each containing 2 ml Red anti-FLAG affinity gel (Sigma). The peptide was eluted with 0.1 M glycine HCl at pH 3.5 and lyophilized. The sample was treated with SDS loading buffer, separated in SDS–PAGE (18% total acrylamide–bisacrylamide monomer, acrylamide:bisacrylamide=15:1) and then visualized by silver staining using Color Silver Stain Kit (Pierce). The overexpressed MgtL peptide from Salmonella harbouring pUHE-mgtL was determined as follows: Salmonella wild-type 14028s carrying pUHE-mgtL was grown with shaking in N medium with 10 mM Mg2+ and 0.5 mM IPTG at 37°C for 4 h. Bacterial cells were harvested by centrifugation and resuspended in PBS buffer, and opened by ultrasonication. Cell lysate was ultracentrifuged at 38 000 r.p.m. for 1 h, and the supernatant was passed through a Microcon YM-3 filter (cutoff, 3000 da; Millipore). The pass-through was lyophilized, resuspended in PBS, desalted using ZipTip C18 (Millipore), and analysed by a Bruker Ultraflex MALDI-TOF-MS operated in the positive ion reflector mode.
Enzymatic and chemical probing of the mgtA 5′ leader RNA structure
The mgtA full-length 5′LR RNA was synthesized with T7 RNA Polymerase (New England Biolabs) using PCR product as template. The PCR product was generated using wild-type plasmid pYS1010 or derivatives and primers 220 and 201. Probing of the mgtA 5′LR RNA with DMS and RNase T1 was carried out as described in a previous study (Cromie et al, 2006).
Intracellular proline quantification
Two kinds of growth conditions were used: (i) Bacteria were grown overnight in 2 ml of an N minimal medium (38 mM glycerol, 0.1% casamino acid, and 10 mM MgCl2). Bacteria were harvested, washed in N medium without MgCl2, and resuspended in 2 ml of the same medium. The cell suspension was used to inoculate 15 ml of medium containing 10 mM MgCl2 and 10 mM proline, 10 mM MgCl2 and no proline, 0.01 mM MgCl2 and 10 mM proline, or 0.01 mM MgCl2 and no proline (1:50 dilution). Bacteria were grown for 4 h and harvested to determine β-galactosidase activity, CFUs, and intracellular proline concentration. (ii) Bacteria were grown overnight in 2 ml of a modified N medium (0.2% glucose, 10 mM MgCl2, and 1 mM proline). The overnight culture was used to inoculate 60 ml of the same medium (1:50 dilution) and grown for 3 h at 37°C with shaking. The harvested cells were washed in this modified N medium containing 0.5 mM MgCl2 and grown in 60 ml of the same medium with 1 mM proline for 1 h. The harvested bacteria were washed in the modified N medium containing 0.5 mM MgCl2 and suspended in 0.6 ml of the same medium. This cell suspension was used to inoculate 15 ml of medium containing 0.5 mM MgCl2 and 1 mM proline, 0.5 mM MgCl2 and no proline, no MgCl2 and 1 mM proline, or no MgCl2 and no proline (1:100 dilution). Bacteria were grown for 15 min and 2 h and harvested to determine β-galactosidase activity, CFU, and intracellular proline concentration. For the intracellular proline determination, bacterial cells (∼5 × 109) were washed in cold PBS, suspended in 0.25 ml of PBS, and disrupted by sonication. The lysate was centrifuged (30 000 g, 15 min) and protein concentration in supernatant was determined using the BCA Protein Assay Kit (Pierce). Then the proteins were precipitated from the supernatant using trichloroacetate, followed by centrifugation at 30 000 g for 15 min. The intracellular proline concentration was determined by measuring the remaining supernatant solution as described (Bates et al, 1973). Briefly, 0.2 ml of the supernatant was incubated with 0.2 ml of acid-ninhydrin (0.25 g ninhydrin dissolved in 6 ml glacial acetic acid and 4 ml 6 M phosphoric acid) and 0.2 ml of glacial acetic acid for 1 h at 100°C. The tubes were then transferred to an ice bath to stop the reaction and the mixtures were extracted with 0.8 ml of toluene. The toluene phase was separated and the absorbance was read at 520 nm with a spectrophotometer. The absorbance was converted to the proline concentration by comparing with a standard curve plotted with a set of known concentrations of proline (Supplementary Figure S4), and normalized by the total protein concentration above.
Supplementary Material
Acknowledgments
We thank Roy Curtiss III for thoughtful discussion and E. coli strain χ2680 and Chad Borges for technical support on MgtL mass spectrum analysis. This study was supported by research funds from the Center for Infectious Diseases and Vaccinology in the Biodesign Institute (for GZ), and Arizona State University (for YS).
Footnotes
The authors declare that they have no conflict of interest.
References
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchiesi J, Castelli ME, Soncini FC, Vescovi EG (2008) mgtA Expression is induced by rob overexpression and mediates a Salmonella enterica resistance phenotype. J Bacteriol 190: 4951–4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207 [Google Scholar]
- Cromie MJ, Groisman EA (2010) Promoter and riboswitch control of the Mg2+ transporter MgtA from Salmonella enterica. J Bacteriol 192: 604–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie MJ, Shi Y, Latifi T, Groisman EA (2006) An RNA sensor for intracellular Mg2+. Cell 125: 71–84 [DOI] [PubMed] [Google Scholar]
- Dann CE III, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC (2007) Structure and mechanism of a metal-sensing regulatory RNA. Cell 130: 878–892 [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97: 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AH, Smith CJ, Rushlow KE, Kretschmer PJ (1982) Escherichia coli Δ1-pyrroline-5-carboxylate reductase: gene sequence, protein overproduction and purification. Nucleic Acids Res 10: 7701–7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Vescovi E, Soncini FC, Groisman EA (1996) Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84: 165–174 [DOI] [PubMed] [Google Scholar]
- Henkin TM, Yanofsky C (2002) Regulation by transcription attenuation in bacteria: how RNA provides instructions for transcription termination/antitermination decisions. Bioessays 24: 700–707 [DOI] [PubMed] [Google Scholar]
- Kawano M, Reynolds AA, Miranda-Rios J, Storz G (2005) Detection of 5′- and 3′-UTR-derived small RNAs and cis-encoded antisense RNAs in Escherichia coli. Nucleic Acids Res 33: 1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Weatherspoon N, Shi Y (2008) Molecular mechanism for establishment of signal-dependent regulation in the PhoP/PhoQ system. J Biol Chem 283: 16612–16621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejona S, Aguirre A, Cabeza ML, Garcia Vescovi E, Soncini FC (2003) Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. J Bacteriol 185: 6287–6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Tolstorukov M, Zhurkin V, Garges S, Adhya S (2004) A mutant spacer sequence between -35 and -10 elements makes the Plac promoter hyperactive and cAMP receptor protein-independent. Proc Natl Acad Sci USA 101: 6911–6916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvil DK, Leisinger T (1977) N-acetylglutamate synthase of Escherichia coli: purification, characterization, and molecular properties. J Biol Chem 252: 3295–3303 [PubMed] [Google Scholar]
- Meinnel T, Schmitt E, Mechulam Y, Blanquet S (1992) Structural and biochemical characterization of the Escherichia coli argE gene product. J Bacteriol 174: 2323–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH (1972) Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Moncrief MB, Maguire ME (1999) Magnesium transport in prokaryotes. J Biol Inorg Chem 4: 523–527 [DOI] [PubMed] [Google Scholar]
- O’Connor K, Fletcher SA, Csonka LN (2009) Increased expression of Mg2+ transport proteins enhances the survival of Salmonella enterica at high temperature. Proc Natl Acad Sci USA 106: 17522–17527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Cromie MJ, Lee EJ, Groisman EA (2010) A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell 142: 737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C, Boyen A, Cohen GN, Glansdorff N (1988) Nucleotide sequence of Escherichia coli argB and argC genes: comparison of N-acetylglutamate kinase and N-acetylglutamate-γ-semialdehyde dehydrogenase with homologous and analogous enzymes. Gene 68: 275–283 [DOI] [PubMed] [Google Scholar]
- Sancar A, Hack AM, Rupp WD (1979) Simple method for identification of plasmid-coded proteins. J Bacteriol 137: 692–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Rupert CS (1978) Determination of plasmid molecular weights from ultraviolet sensitivities. Nature 272: 471–472 [DOI] [PubMed] [Google Scholar]
- Smith CJ, Deutch AH, Rushlow KE (1984) Purification and characteristics of a γ-glutamyl kinase involved in Escherichia coli proline biosynthesis. J Bacteriol 157: 545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely MD, Florer JB, Miller CG, Maguire ME (1989) Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol 171: 4761–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely MD, Gravina SA, Cheung TT, Miller CG, Maguire ME (1991) Magnesium transport in Salmonella typhimurium. Regulation of mgtA and mgtB expression. J Biol Chem 266: 824–829 [PubMed] [Google Scholar]
- Spinelli SV, Pontel LB, Garcia Vescovi E, Soncini FC (2008) Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol Lett 280: 226–234 [DOI] [PubMed] [Google Scholar]
- Tucker BJ, Breaker RR (2005) Riboswitches as versatile gene control elements. Curr Opin Struct Biol 15: 342–348 [DOI] [PubMed] [Google Scholar]
- Moll I, Bläsi U (2007) Control of Fur synthesis by the non-coding RNA RyhB and iron-responsive decoding. EMBO J 26: 965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela C, McCarthy JE (2003) Regulation of fungal gene expression via short open reading frames in the mRNA 5′untranslated region. Mol Microbiol 49: 859–867 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.