Abstract
The forces that drive conversion of nascent protein to major histocompatibility complex (MHC) class I-restricted peptides remain unknown. We explored the fundamental property of overt hydrophobicity as such a driver. Relocation of a membrane glycoprotein to the cytosol via signal sequence ablation resulted in rapid processing of nascent protein not because of the misfolded luminal domain but because of the unembedded transmembrane (TM) domain, which serves as a dose-dependent degradation motif. Dislocation of the TM domain during the natural process of endoplasmic reticulum-associated degradation (ERAD) similarly accelerated peptide production, but in the context of markedly prolonged processing that included nonnascent species. These insights into intracellular proteolytic pathways and their selective contributions to MHC class I-restricted peptide supply, may point to new approaches in rational vaccine design.
Keywords: degradation signal, ERAD, hydrophobicity, MHC class I peptides, transmembrane domain
Introduction
CD8+ T cells (TCD8+) of the adaptive immune system limit viral spread in the host by killing infected cells prior to the release of progeny. Identification of infected cells is based upon cell surface display of virus-derived peptides (epitopes), usually 8–10 amino acids in length, in combination with major histocompatibility complex (MHC) class I molecules (Yewdell, 2007; Peaper and Cresswell, 2008). One remarkable aspect of this antigen presentation system is the speed with which peptides can be generated by the intracellular proteolytic machinery following infection. Using peptide appearance at the cell surface or peptide transport into the ER as a readout, several studies have concluded that MHC class I-bound peptides are derived primarily from proteins that are degraded within ∼30 min of their synthesis (Reits et al, 2000; Princiotta et al, 2003; Yewdell, 2007; Dolan et al, 2010), a rate that maximizes the opportunity for TCD8+ to carry out their effector functions before production and release of viral progeny. Indeed, the implied half-life of ⩽10 min approaches that of so-called ‘N-end rule substrates’, artificially constructed polypeptides with destabilizing N-terminal amino acids (N-degrons) (Varshavsky, 1992), whose destruction commences even before synthesis has been completed (Turner and Varshavsky, 2000). These indirect measures of nascent protein turnover have been supported by biochemical studies, demonstrating that a fraction of the nascent polypeptide pool rapidly disappears (Wheatley, 1984; Schubert et al, 2000; Vabulas and Hartl, 2005).
Despite intensive study, the bases by which certain copies of nascent protein are selected for immediate turnover remain poorly understood. In previous studies, we observed that appreciable misfolding imposed by genetic manipulation does not substantially alter degradation rate and presentation efficiency (Golovina et al, 2005). This suggested to us that immediate peptide supply is not driven by conventional protein folding/quality control decisions. The relatively slow pace by which misfolded proteins are targeted for degradation lends additional support to this notion (Eisenlohr et al, 2007). In searching for a more potent signal of degradation, we focused on overt hydrophobicity, exemplified by an unembeded transmembrane (TM) domain, as a feature that might require immediate and decisive action. This was based on the well-known threat that such species pose to cell viability via aggregate formation and nonspecific association with functional proteins (Stefani and Dobson, 2003) and the notion that their intractability would be readily recognized. The experiments reported here support the concept that a high degree of overt hydrophobicity acts as a signal for immediate degradation and, consequently, peptide presentation.
The line of investigation that we chose also enabled us to investigate the extent to which class I peptides are derived from immediately degraded proteins. Of particular interest in this regard are proteins targeted to the ER, comprising ∼25% of the proteome (Istrail et al, 2004). These species undergo cotranslational translocation, evaluation by the quality control machinery and, if rejected, retrotranslocation to the cytosol for degradation by the proteasome (Vembar and Brodsky, 2008). Collectively, these steps appear to preclude rapid peptide supply. Our results show substantially prolonged peptide production from ER-targeted proteins compared with their cytosolic counterparts.
In the final series of experiments reported here, overt hydrophobicity and ER targeting as determinants of peptide supply were concurrently explored in investigating whether the TM domain of a dislocated endoplasmic reticulum-associated degradation (ERAD) substrate also influences degradation rate and presentation efficiency. Results not only reinforced the strong effects of these two attributes, but also provided new insights into the process of ERAD.
Results
TM domain drives cytoTac degradation by the proteasome and enhanced presentation
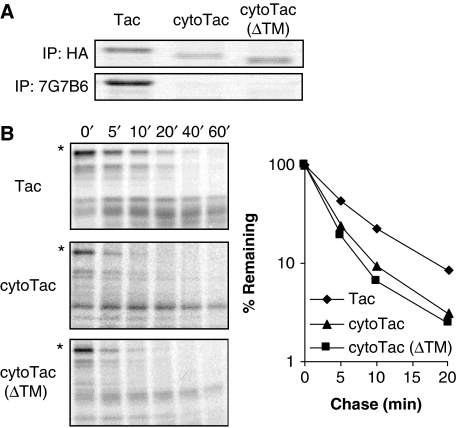
A straightforward means of generating unresolvable, overt hydrophobicity is by ablating the signal sequence of a membrane glycoprotein. This results in an ‘orphaned’ TM domain and default delivery to the cytosol where the rest of the protein is not configured to shield. The base construct for these experiments was the human IL-2 receptor α subunit (Tac), a type I membrane glycoprotein to which we appended a triple hemagglutinin (HA) tag and the OVA257−264 (SIINFEKL) epitope between the signal sequence and N-terminus of the mature protein (Figure 1A). These additions do not interfere with proper folding of the ER-directed luminal domain as reactivity with the conformation-dependent monoclonal Ab 7G7B6 is intact (Golovina et al, 2005). This construct and all its variants were expressed by recombinant vaccinia viruses. Cells were infected at 3 p.f.u./cell for 5 h, with equivalent virus infection that was verified by anti-vaccinia Ab staining (Supplementary Figures S1A and 2). Consistent with earlier reports (Townsend et al, 1986; Tobery and Siliciano, 1997; Golovina et al, 2005), elimination of the signal sequence (creating ‘cytoTac’) substantially reduced half-life, as assessed by pulse-chase analysis (Figure 1A and B), and this corresponded with a marked increase in OVA257−264 presentation as assessed by flow cytometry using the 25-D1.16 OVA257−264/Kb-specific monoclonal Ab (Porgador et al, 1997) (Figure 1C).
Figure 1.
Impact of the orphaned TM domain on cytoTac degradation and MHC class I epitope presentation. (A) Schematic representation of Tac-based model antigens. SS, signal sequence; HA, HA-tag; S, OVA257−264; TM, transmembrane. Right panel, metabolic pulse chase of infected cells followed by immunoprecipitation with anti-HA Ab to determine the stability of Tac-based proteins. (B) Stability of the model antigens quantified by phosphoroimaging analysis. Data represent the mean and s.d. from three independent experiments. (C) OVA257−264 presentation from Tac-based antigens in a 5-h infection assay, as assessed by staining of surface OVA257−264/Kb complexes with 25-D1.16 Ab. Error bars represent the s.d. of triplicate samples. This experiment was repeated at least three times with similar results. (D) Metabolic pulse assay in the absence or presence of proteasome inhibitor epoxomicin (1 μM). Right panel, phosphoroimager quantification of relative amounts of proteins. Data represent the mean and s.d. from three independent experiments.
To determine whether the exposed TM domain, rather than the luminal domain, drives rapid degradation of cytoTac, we eliminated the TM domain from cytoTac protein, generating cytoTac (ΔTM). This manipulation resulted in an appreciable increase in stability (Figure 1A and B) and, consequently, a marked decrease in epitope presentation compared with cytoTac. These differences were detectable by indirect 25-D1.16 Ab staining (Figure 1C) and also by direct Alexa 647-conjugated 25-D1.16 Ab staining (Supplementary Figure S2). This effect was observed at many time points post-infection (Supplementary Figure S1B and C) and also with other cell types (Supplementary Figure S3). The stability of cytoTac (ΔTM) is somewhat lower than wild-type Tac protein (Figure 1A) and this does appear to correlate with presentation level (Figure 1C). However, the qualitative differences between the processing of cytosolic and ER-targeted proteins (Golovina et al, 2002 and below) confounds this kind of comparison. Rapid degradation of cytoTac was mediated by the proteasome as demonstrated by a short-time pulse experiment in the presence of the proteasome inhibitor epoxomicin (Figure 1D). After metabolic labelling, cytoTac protein accumulated in L-Kb cells treated with 1 μM epoxomicin for 30 min, accounting for ∼40 and 90% increase compared with untreated controls at 5 and 10 min pulse, respectively, reflecting a half-life of <10 min. In comparison, no significant accumulation of cytoTac (ΔTM) protein follows treatment with epoxomicin during the short-time pulse, further demonstrating the relative stability of cytoTac (ΔTM).
As anticipated from previously reported results (Townsend et al, 1986), both cytoTac and cytoTac (ΔTM) failed to react with the conformation-sensitive anti-Tac Ab 7G7B6, suggesting that both species are misfolded (Figure 2A). This was reinforced by proteolytic digestion of immunoprecipitated cytoTac and cytoTac (ΔTM). Both forms were similar in terms of trypsin sensitivity and digestion patterns (Figure 2B), and these patterns were clearly different from that of wild-type, ER-targeted Tac. In addition, both are completely degraded within a few minutes by proteinase K while Tac is not (results not shown). Thus, misfolding of the luminal domain is not a powerful driver of rapid protein degradation.
Figure 2.
Folding status of the model antigens. (A) The folding status was evaluated in virus-infected L-Kb cells by metabolic labelling and immunoprecipitation with conformation-insensitive (anti-HA) and conformation-sensitive (anti-Tac clone 7G7B6) Abs. (B) Limited proteolysis to study protein conformation. Virus-infected L-Kb cells were metabolically labelled, and the constructs were immunoprecipitated with anti-HA Ab prior to proteolysis with 5 μg/ml trypsin for 0–60 min on ice. The resulting digests were analysed using 12% SDS–PAGE. The stars indicate the position of the full-length model antigens. Right panel, stability of the different full-length species quantified by phosphoroimaging analysis. All images are representative of two independent experiments.
Confocal immunofluorescence was employed to visualize subcellular location of the various constructs 6 h post-infection (Supplementary Figure S4). While cytoTac (ΔTM) is diffusely distributed throughout the cell, cytoTac appears to localize to the ER membrane. This is not unexpected since it has been reported that access to the cytosolic face of the ER is required for the degradation stimulated by hydrophobicity (Metzger et al, 2008). Notably, there is no evidence of aggregate formation, a potentially complicating factor, by any of the proteins. The proteasome sensitivity of cytoTac also argues against the disappearance of the protein in biochemical studies being due to the formation of detergent-insoluble aggregates.
cytoTac and cytoTac (ΔTM) are processed via the conventional pathway
Conventional class I-restricted antigen processing involves degradation by the proteasome and transport into the ER via the transporter of antigenic peptides (TAP) (Yewdell et al, 2003). Since alternatives to both steps have been described (Snyder et al, 1997; Gil-Torregrosa et al, 1998; Lautscham et al, 2001), it was important to determine whether the difference between the processing of cytoTac and cytoTac (ΔTM) could be due to processing via distinct pathways. Treatment with 3 μM proteasome inhibitor epoxomicin prior to a 5-h infection substantially reduced presentation of the OVA257−264 epitope from either substrate (Figure 3A) but not presentation of a minigene product, which does not require processing. In addition, TAP−/− B6 cells were completely unable to present the OVA257−264 epitope from any of the three Tac-based contexts 5 h post-infection (Figure 3B). In contrast, a minigene product that is targeted to the ER via signal sequence-mediated translocation (ss-m/S), thereby obviating TAP function, was presentable by TAP−/− cells. Thus, both TM-intact and TM-ablated constructs are processed via the classical MHC class I pathway.
Figure 3.
OVA257−264 presentation from Tac-based proteins via the classical MHC class I pathway. (A) OVA257−264 presentation from L-Kb cells infected with the indicated viruses at 3 p.f.u./cell for 5 h in the presence or absence of epoxomicin (3 μM). A vaccinia virus that expresses the SIINFEKL-encoding minigene at limiting amounts via a 5′ UTR hairpin technique (m/S-HP18) (Wherry et al, 1999) was used as a toxicity control to confirm that there was no effect upon proteasome-independent presentation. Data are representative of three independent experiments. (B) OVA257−264 presentation from TAP-deficient B6 fibroblast cells (H-2b haplotype) infected with the indicated viruses at 3 p.f.u./cell for 5 h. A vaccinia virus that expresses the SIINFEKL-encoding minigene targeted to the ER independent of TAP function via signal sequence-mediated translocation, ss-m/S, was used as a control to show processing and presentation capability via a TAP-independent pathway. Data are representative of three independent experiments.
cytoTac is processed with the same kinetics as cytoTac (ΔTM)
Given its relative stability, cytoTac (ΔTM) represents a typical cytosolic protein that is subjected to only the enigmatic system of basal antigen processing (which we propose to be driven by processes other than standard quality control decisions). Thus, peptides were expected to be generated from the cohort of cytoTac (ΔTM) that is degraded within 30 min of production. This was tested according to established methods (Princiotta et al, 2003); cells were infected with recombinant vaccinia expressing cytoTac (ΔTM) and the accumulation of OVA257−264/Kb complexes at the cell surface was documented by flow cytometry. At 3 h post-infection, the protein synthesis inhibitors cycloheximide and emetine were added at 25 μg/ml each, resulting in depletion of processing substrate and a subsequent leveling off of surface complexes. The delay between addition of inhibitors and cessation of complex accumulation was ∼60 min (Figure 4A), consistent with an ∼30 min lifespan after taking post-proteasomal steps into account (Princiotta et al, 2003). When the TM domain containing cytoTac was similarly analysed, the cycloheximide delay time was identical (Figure 4B). Thus, peptides from both cytosolic proteins are derived from rapid degradation of nascent proteins, with the additional product from cytoTac being stimulated by overt hydrophobicity of the orphaned TM domain that drives antigen processing at the same high rate as the basal system.
Figure 4.
Kinetics of OVA257−264 presentation. OVA257−264 presentation from L-Kb cells infected with antigen-expressing viruses and treated with protein synthesis inhibitors (PSI) at 3 h post-infection. Data represent the mean and s.d. from three independent experiments. (A) cytoTac (ΔTM). (B) cytoTac. (C) membrane-bound Tac. (D) Soluble Tac (ΔTM). The slopes for the two ER-targeted constructs (C, D) are essentially identical after 1.5 h but statistically distinct up to 1.5 h post-PSI treatment (P<0.0001).
The orphaned TM domain is a dose-dependent degradation signal
Exposed hydrophobicity in other systems has been reported to act as a degradation signal (Gilon et al, 1998; Johnson et al, 1998; Arteaga et al, 2006). The results thus far suggested to us that the orphaned TM domain acts in the same capacity. We tested the notion that the orphaned TM domain acts as a bona fide degradation signal (‘degron’) in three different ways. First, we speculated that the degree of hydrophobicity, as governed by the length of the domain, might determine the strength of signal. Thus, we progressively truncated the TM domain by removing 6 or 13 amino acids from the C-terminus of the 19-residue domain (Figure 5A). Indeed, protein stability was proportional to the length of the TM domain (Figure 5A and B). Likewise, a graded diminution in OVA257−264 epitope presentation was also observed (Figure 5C). Second, we expected that the TM domain would act independently of its position within Tac. Accordingly, we moved the TM domain from its original location near the C-terminus to the N-terminus. This resulted in a level of protein degradation and OVA257−264 presentation that was comparable to cytoTac (Figure 5D–F). Finally, we anticipated that the TM domain should have a similar effect when transferred to a different cytosolic protein. We therefore appended the Tac TM domain to the N- or C-terminus of an HA-tagged, OVA257−264-containing cytosolic version of influenza nucleoprotein (NP13−498) (Figure 6A). Addition in either location resulted in rapid degradation of the NP-based protein (Figure 6A and B) and much higher levels of surface OVA257−264/Kb complexes compared with the unmodified NP13−498 protein (Figure 6C). In previous work we deduced that sequences could be added to the N-terminus of NP13−498 without disturbing folding (Golovina et al, 2005). This also appeared to be the case with TM added to the N-terminus of NP13−498 since this construct is immunoprecipitated by two different NP-specific monoclonal Abs (Supplementary Figure S5). Thus, misfolding appears not to be mandatory for rapid peptide supply. Due to the availability of a previously generated recombinant vaccinia (Golovina et al, 2005), these NP-based substrates provided the opportunity to assess the relative strengths of the TM domain and an N-degron, the latter being well known for its potency. Indeed, the N-end rule substrate was most rapidly degraded (Figure 6D) and generated the highest levels of epitope (Figure 6E), but the TM degron was also well above the control construct.
Figure 5.
The orphaned TM domain as a degradation signal in cytoTac variants. (A) cytoTac variants with a truncated TM domain. HA, HA-tag; S, OVA257−264; TM, transmembrane. Right panel, metabolic pulse chase of cytoTac variants with a truncated TM domain. (B) Stability of the model antigens quantified by phosphoroimaging analysis. Data represent the mean and s.d. from three independent experiments. (C) OVA257−264 presentation from cytoTac proteins with a truncated TM domain in a 5-h infection assay. Error bars represent the s.d. of triplicate samples. This experiment was repeated three times with similar results. (D) cytoTac variant with a relocated TM domain. HA, HA-tag; S, OVA257−264; TM, transmembrane. Right panel, metabolic pulse chase of cytoTac protein with a relocated TM domain. (E) Stability of the model antigens quantified by phosphoroimaging analysis. Data represent the mean and s.d. from three independent experiments. (F) OVA257−264 presentation from cytoTac proteins with a relocated TM domain in a 5-h infection assay. Error bars represent the s.d. of triplicate samples. This experiment was repeated three times with similar results.
Figure 6.
Transfer of the Tac TM domain to another model antigen. (A) NP13−498-based model antigens. HA, HA-tag; S, OVA257−264; TM, Tac transmembrane. Right panel, metabolic pulse chase of NP13−498-based proteins. (B) Stability of the model antigens quantified by phosphoroimaging analysis. Data represent the mean and s.d. from three independent experiments. (C) OVA257−264 presentation from NP13−498-based proteins in a 5-h infection assay. Error bars represent the s.d. of triplicate samples. This experiment was repeated three times with similar results. (D) Metabolic pulse chase of N-end rule substrate of NP13−498 protein, Ub-R-NP13−498. Bottom panel, stability of Ub-R-NP13−498 quantified by phosphoroimaging analysis. Data represent the mean and s.d. from two independent experiments. (E) OVA257−264 presentation from NP13−498 proteins appended with a degradation signal (TM or N-degron) in a 5-h infection assay. Error bars represent the s.d. of triplicate samples. This experiment was repeated three times with similar results.
Vaccinia infection is profoundly disruptive to cellular organization, ultimately leading to cell death. To determine whether such disruption in some way contributes to the drive toward immediate degradation, we expressed the Tac-based model antigens via transfection. L-Kb cells were transfected with the same amount of various pIRES2-EGFP constructs and then cultured in the absence or presence of the proteasome inhibitor MG132 (Supplementary Figure S6). In the absence of MG132, the cytoTac band is barely visible, whereas both Tac and cytoTac (ΔTM) bands are easily detected. However, cytoTac accumulated to the same extent as cytoTac (ΔTM) after proteasome inhibition. Thus, the impact of overt hydrophobicity is not peculiar to the virally infected cell.
Peptide production from an ER-targeted protein is substantially delayed
Experiments thus far concentrated upon cytosolic proteins from which immediate peptide supply was readily appreciable (Figure 4A and B). Considering the various steps involved in ERAD (Meusser et al, 2005), we imagined that slower kinetics might apply to ER-targeted proteins. Thus, we carried out the same experiments with wild-type (ER translocated) Tac that had been carried out for the cytosolic counterparts, measuring the delay between addition of protein synthesis inhibitors at 3 h post-infection and the termination of peptide/MHC complex delivery to the cell surface. As anticipated, peptide production was substantially more prolonged (Figure 4C). These kinetics data are consistent with the reduced levels of surface epitope from Tac versus cytoTac (Figure 1C).
TM domain is a driver for ERAD and membrane protein presentation
In light of our findings with cytosolic proteins, we considered the possibility that the destruction rates of membrane glycoproteins undergoing ERAD are influenced by the TM domain that is orphaned upon retrotranslocation. To investigate this, we generated Tac (ΔTM), an ER-targeted, untethered protein, and similarly analysed peptide production kinetics (Figure 4D). While peptide display from wild-type Tac plateaued at 2–2.5 h post-cycloheximide treatment, peptide from Tac (ΔTM) continued to accumulate at 3 h post-treatment.
These kinetics experiments were complemented with biochemical analysis. We first ascertained that such ERAD intermediates of our nominal antigens could be visualized by immunoblotting analysis. Following their retrotranslocation, ERAD substrates are deglycosylated prior to proteasomal destruction (Romisch, 2005). Cells expressing wild-type Tac in the presence of the reversible proteasome inhibitor MG132 accumulated a species with mobility similar to that of cytoTac (Supplementary Figure S6) and mature Tac treated with endoglycosidase H (Endo H) (Figure 7B), consistent with removal of the signal sequence and absence of sugar groups. Because this species could be either nascent Tac that had not yet been glycosylated (unglycosylated) or retrotranslocated Tac that had been deglycosylated, the protein synthesis inhibitor emetine was added 10 min prior to lysis in a detergent-containing buffer to clear any unglycosylated Tac. A similar species was observable when we expressed Tac (ΔTM). Clearing the majority of glycoproteins from the cell lysates with ConA sepharose 4B aided in visualization of these minor deglycosylated degradation intermediates (Figure 7A, right panel). Thus, retrotranslocated and deglycosylated ERAD substrates were readily visualized with addition of proteasome inhibitor.
Figure 7.
Contribution of the orphaned TM domain to ERAD. (A) Immunoblotting analysis of proteasome substrates from ER-targeted Tac proteins before (left panel) and after (right panel) removing the majority of glycosylated species by Con A sepharose 4B beads. (B) Endo H sensitivity of MG132-treated samples after clearance of most glycosylated species by Con A separation, illustrating the shift of the residual glycosylated species to proteasome substrates. (C) Degradation of accumulated proteasome substrates following 10 min emetine treatment and withdrawal of MG132. Cells were incubated for varying times prior to lysis, clearance of glycosylated species, and analysis via immunoblotting. Stars indicate the positions of the ERAD intermediates. Right panel, stability of the ERAD substrates quantified by densitometric analysis. Data represent the mean and s.d. from two independent experiments. (D) Proteasome substrates from HA-based proteins in L-Kb cells infected with virus recombinants for 4 h and detected by immunoblotting with anti-HA S1 Ab. (E) Degradation of accumulated proteasome substrates from two HA proteins in L-Kb cells infected with virus recombinants for 18 h in the presence of MG132. Following 10 min emetine treatment and withdrawal of MG132, cells were incubated for varying times prior to immunoblotting analysis. Stars indicate the positions of the ERAD intermediates for HA and HA (ΔTM). Right panel, stability of the ERAD substrates quantified by densitometric analysis. Data represent the mean and s.d. from two independent experiments. All blots are representative of 2–3 independent experiments.
In order to determine relative degradation rates of these intermediates, similar conditions were employed but then MG132 was withdrawn and the cells were incubated for varying times prior to lysis and removal of glycosylated species by Con A beads. Due to limited sensitivity of metabolic pulse for visualization of the minor ERAD species (Supplementary Figure S7), the protein synthesis inhibitor-chase assay followed by immunoblotting analysis was employed for tracking the fate of the deglycosylated species following withdrawal of reversible proteasome inhibitor. As shown in Figure 7C, the proteasome substrate fraction derived from Tac protein disappeared more rapidly than that from Tac (ΔTM) protein following MG132 withdrawal. Indeed, the rate difference was comparable to that observed for the cytosolic substrates (Figure 1A). This observation was not peculiar to viral infection as comparable results were obtained from a transfection system (Supplementary Figure S8). In contrast, the glycosylated species, representing mature folded proteins that have passed quality control, are quite stable during the period of 60 min chase (Supplementary Figure S9). As with cytoTac, stabilization of the ERAD substrates by proteasome inhibitor (Figure 7A) argues against the possibility that disappearance of the Tac ERAD intermediate is due to formation of detergent-insoluble aggregates. This conclusion is also supported by the confocal images (Supplementary Figure S4) that show no evidence of aggregate formation. Interestingly, high molecular weight polyubiquitin species were present in the ERAD fractions from both Tac and Tac (ΔTM) proteins (Supplementary Figure S10), suggesting that the orphaned TM domain, not the process of polyubiquitination, is a more potent determinant of rapid degradation.
We further explored the influence of the dislocated TM domain on ERAD with another type I membrane protein, influenza HA, which was expressed in wild-type, TM-ablated and cytosolic (signal sequence-ablated) forms. As observed previously (Townsend et al, 1986), cytoHA is subject to rapid turnover and, as with cytoTac, this form of HA is considerably stabilized by proteasome inhibition (Figure 7D). Further, an ERAD intermediate of wild-type HA with mobility identical to that of cytoHA (Figure 7D) and deglycosylated HA (Supplementary Figure S11) became apparent with exposure to proteasome inhibitor. Most important, this intermediate was turned over at a faster rate compared with its ΔTM counterpart, as indicated by its relatively greater accumulation in the presence of MG132 (Figure 7D) and faster degradation following withdrawal of MG132 (Figure 7E). Thus, orphaned TM domains resulting from ERAD can also increase the degradation rate, thereby enhancing peptide supply.
Discussion
It has been known for several decades that a fraction of the nascent protein pool is subjected to immediate degradation (Wheatley et al, 1980), perhaps as a way of ensuring immediate amino-acid availability for rapid adjustment of the proteome in response to environmental changes (Wheatley, 1984). An obvious benefit of immediate turnover is the rapid display of MHC/peptide complexes at the surface of the infected cell, allowing for CD8+ T-cell recognition and effector function prior to the release of progeny. While there is some debate about the size of nascent protein pool that is subjected to this fate (Wheatley et al, 1980; Schubert et al, 2000; Vabulas and Hartl, 2005), even lower estimates are more than sufficient for CD8+ T-cell recognition, which is extremely sensitive (Wherry et al, 1999; Zook et al, 2006).
Despite intensive investigation, the basis for rapid peptide supply has remained elusive. Indeed, to this point only N-end rule substrates have been known to approximate the speed with which the first wave of peptides are produced following viral infection. However, N-degrons appear to be utilized under selective conditions (Varshavsky, 1992) and are generally nonfunctional in eukaryotic cells (Gueguen and Long, 1996; Wong et al, 2004). Thus, the bulk of rapid peptide supply is likely driven by other signals. Here, we provide evidence that overt hydrophobicity of sufficient density can provide such a signal.
The favoured explanation for rapid peptide supply is the defective ribosomal products (DRiP) model, which proposes that production of any protein is an imperfect process, and that recognition of defective copies drives immediate degradation (Yewdell et al, 1996). We have expressed some reservations with the original iteration of this model (Eisenlohr et al, 2007) because most, if not all, quality control decisions that have been investigated appear to operate with kinetics that are slower than those associated with rapid peptide supply. Further, we have made several mutations to nominal antigens that cause misfolding and these do not appreciably alter the level of antigen processing (Golovina et al, 2005). Consequently, we have proposed an alternative model in which copies of nascent cytosolic protein are selected for a basal level of degradation independent of quality control decisions (Eisenlohr et al, 2007). Specifically, we have suggested that the minor fraction of the nascent protein pool that is produced by ribosomes lacking their associated chaperones (Raue et al, 2007) is immediately degraded since unshielded hydrophobicity represents a substantial danger to the cell (Wickner et al, 1999). This model is supported by the results reported here and may also provide the mechanistic basis for an updated version of the DRiP model, which has proposed the existence of ‘immunoribosomes’, ribosome variants that generate a high proportion of DRiPs (Yewdell and Nicchitta, 2006). The key point in our model is that most nascent polypeptides are not inherently defective (the linear sequence is correct) and that defectiveness might be considered to lie more with the ribosome and its associated chaperones. In practical terms, this could strongly influence strategies for enhancing rapid peptide production.
Our results shed new light on the landmark publication of Townsend et al (1986), who reported that redirecting influenza HA to the cytosol by genetic ablation of the signal sequence resulted in accelerated turnover and substantially enhanced peptide presentation, a finding that initiated focus on rapid peptide supply (Townsend et al, 1988). The default explanation for the result is misfolding of cytosolic HA due to lack of glycosylation and disulfide bonding. However, our results indicate that the unembedded TM domain is the more potent driver. The targeting of viral or tumour antigens for rapid cytosolic degradation has been reported to enhance CD8+ CTL responses (Tobery and Siliciano, 1997, 1999). Thus, the novel degradation signal we have identified is expected to impact CD8 T-cell responses in vivo.
Exposed hydrophobicity has been previously reported to stimulate degradation (Gilon et al, 1998; Johnson et al, 1998; Arteaga et al, 2006), but our study appears to be the first report of a TM domain acting in this capacity and of the impact on peptide supply. While we first made this observation using an artificial cytosolic protein with an ablated signal sequence, we subsequently demonstrated the impact upon ERAD. We are currently testing two additional mechanisms by which orphaned hydrophobicity may drive the rapid turnover of cytosolic proteins. First is the unchaperoned nascent polypeptide model described above. Second is the mistargeting of a membrane glycoprotein to the cytosol due to lack of signal recognition particle engagement. This is not necessarily a rare event, depending upon the particular signal sequence and level of cellular stress (Kang et al, 2006). Indeed, it has been reported that an epitope, which contains the cleavage site of the ER signal peptidase, is efficiently presented to MHC class I molecules, implying a pathway for processing ER-targeted proteins prior to translocation into the ER lumen (Schlosser et al, 2007). Other potential mechanisms for the generation of overt hydrophobicity include endoproteolysis that separates a hydrophobic segment from the domain that normally shields it and premature termination of translation that could achieve the same effect.
The observation that degradation rate and, consequently, peptide supply is proportional to the length of the TM domain suggests a triaging system that may be essential for cell survival. Certain acute stresses cause immediate and widespread protein unfolding to which there is a rapid, programmed response (Hartl et al, 1994). Our results suggest that those unfolded proteins with the greatest degree of overt hydrophobicity, representing the greatest threat, would be selected for most rapid disposal.
The ability of a TM domain to accelerate ERAD was not necessarily predictable considering the fundamental topological differences between ERAD and cytosolic degradation. An important aspect of ERAD revealed by our results is that degradation rate can be impacted by properties that are distinct from those that initiate ERAD, such as folding state of the luminal domain (Carvalho et al, 2006). Our results are seemingly at odds with a recent report that two canonical TM-anchored ERAD substrates are turned over less quickly than their untethered counterparts (Bernasconi et al, 2010). One potential explanation is that we focused on only the latter stages of ERAD, and the TM domain might have a larger negative impact on other stages of ERAD. However, the relative rates of peptide production from Tac and Tac (ΔTM), as determined by all stages of ERAD, suggest that this is not the case for the Tac-based proteins that we studied. Another consideration is that our model proteins are likely subjected to ERAD based upon a variety of defects. The model proteins of Bernansconi et al are essentially uniform in their defectiveness and may not represent most stochastically generated Tac defects. Further, in the earlier study, 100% of the model substrates expressed at high levels were subjected to ERAD. This pressure on the system may impact the steps in the process that are rate limiting. Further exploration of these issues could have important ramifications for therapeutic approaches to the many inherited human diseases that are ERAD based (Gregersen et al, 2006).
A second point emerges from our ERAD studies; while peptides are certainly generated from very short-lived (<30 min) proteins, this need not be the case as evidenced by the prolonged processing of our ER-targeted substrates. This is contrary to previous reports that estimate a lifetime of ⩽30 min for all or the bulk of proteins that contribute to peptide supply (Reits et al, 2000; Princiotta et al, 2003). The timing we observed is consistent with conventional ERAD, which features a quality control step prior to retrotranslocation (Vembar and Brodsky, 2008) and is the embodiment of the DRiP model in any form. This notion is supported by an earlier report on the ER-targeted protein tyrosinase, whose efficiency of presentation was enhanced by misfolding mutations and decreased by treatment with chemical chaperones (Ostankovitch et al, 2005). We speculate that the more limited time windows deduced by earlier reports were due, in one case, to the processing substrates chosen for analysis (Princiotta et al, 2003; Qian et al, 2006; Dolan et al, 2010) and, in the other, to relative insensitivity of the assay employed (Reits et al, 2000). Indeed, the current DRiP model recognizes the heterogeneous nature of processing substrates and their wide range of degradation rates (Yewdell, 2007). Our results suggest that immediate peptide supply is limited to cytosolic processing substrates, which may, therefore, contribute to the earliest targets for CD8+ T-cell recognition of the infected cell.
These studies add insight into fundamental aspects of intracellular proteolysis, thereby shedding new light on the forces involved in MHC class I-restricted peptide supply. In turn, this information could provide new approaches to the design of vaccines that are intended to optimize CD8+ T-cell-based protection.
Materials and methods
Cell lines, antibodies and reagents
L-Kb (L929 stably transfected with H-2Kb), TAP−/− B6 fibroblast cells, MC57G, RMA, RMA-S and HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS). Anti-HA tag Ab (clone 12CA5) was purchased from Roche; anti-actin Ab from Sigma-Aldrich; anti-BiP Ab from Abcam. Anti-B5R Ab and Alexa 647-conjugated 25-D1.16 Ab were generously provided by the laboratory of Drs JW Yewdell and JR Bennink (NIAID). CM1-1.2 is a mouse Ab that is specific for HA S1 (Chianese-Bullock et al, 1998), and 7G7B6 is a conformation-sensitive mouse Ab that is specific for Tac protein (Golovina et al, 2005). General chemical supplies were obtained from Sigma-Aldrich unless otherwise specified.
Recombinant vaccinia viruses
The recombinant vaccinia viruses encoding Tac, cytoTac, NP13−498, m/OVA257−264, a control construct without OVA257−264 and PR8 HA have been previously described (Eisenlohr et al, 1992; Golovina et al, 2005). Other constructs were generated by standard PCR to manipulate the TM domain or the signal sequence in the base constructs. Generation and titration of recombinant vaccinia virus was conducted as described (Eisenlohr et al, 1992). Cells were infected for 1 h at 37°C with vaccinia viruses in balanced salt solution containing 0.1% BSA. After 1 h, DMEM plus 5% FBS was added and the cells were incubated for the reminder of the assay.
Metabolic labelling and immunoprecipitation
L-Kb cells were infected with antigen-expressing viruses at 3 p.f.u./cell for 3 h, and then pulse labelled with 35S-labelling mix (MP Biomed) for 10 min. In some experiments, the pulse assay was carried out in the presence of the irreversible proteasome inhibitor epoxomicin (Boston Biochem) at 1 μM for 30 min before 35S-labelling. To chase protein degradation, the labelling medium was removed and the cells were cultured in normal medium. At the indicated time points, aliquots were washed with PBS and lysed in a lysis buffer containing 20 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride (PMSF) and the protease inhibitor cocktail. Cell extracts were incubated with specific Abs bound to protein A agarose (Thermo) at 4°C for 4 h. Precipitated proteins were resolved by 10% SDS–PAGE and analysed using Typhoon 9400 scanner (Amersham). Band intensities were quantified using ImageQuant software (Amersham).
Antigen presentation analysis
Cells infected with antigen-expressing viruses at 3 p.f.u./cell for 5 h were stained for OVA257−264/Kb complexes on the surface with 25-D1.16 Ab (Porgador et al, 1997) followed by fluorescein-conjugated anti-mouse IgG Ab (Vector Lab), and then fixated in 2% paraformaldehyde (Electron Microscopy Sciences) in PBS. In some experiments, cells were treated with proteasome inhibitor epoxomicin (3 μM) for 30 min prior to virus infection. The mean fluorescence intensity (MFI) was measured using Epics XL (Beckman Coulter) or FACSCalibur (BD Biosciences) flow cytometers.
Limited proteolysis
The cells were metabolically labelled and disrupted with lysis buffer (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100). The constructs were immunoprecipitated with anti-HA Ab. Protease susceptibility of model antigens was determined by incubation with 5 μg/ml trypsin. After the indicated time of incubating on ice, aliquots were removed and 5 mM PMSF was added to stop the reactions. Proteolytic fragments were separated by 12% SDS–PAGE gel and visualized by autoradiography.
Kinetics of peptide display after antigen synthesis
L-Kb cells were infected with antigen-expressing viruses at 3 p.f.u./cell. After 3 h, infected cells were treated with protein synthesis inhibitors cycloheximide and emetine (25 μg/ml each). Aliquots were taken at the indicated time points and subjected to antigen presentation analysis using 25-D1.16 Ab.
Immunoblotting
Cells were lysed in Tris-buffered saline containing 1% Triton X-100, protease inhibitor cocktail and PMSF. Cell lysates were separated by SDS–PAGE and transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, the protein bands were detected using primary Abs, peroxidase-labelled secondary Abs (Vector Lab) and a chemiluminescence detection kit (Kirkegaard & Perry Laboratories), according to the manufacturer's instructions. All blots were reprobed with loading (anti-actin Ab).
Endoglycosidase digestion
Cell extracts were divided into two equal portions, which were then incubated with or without Endo H (New England Biolabs) for 2 h at 37°C. The reaction mixtures were analysed by immunoblotting using the indicated Abs.
ERAD substrate degradation
L-Kb cells were infected with recombinant vaccinia viruses for 1 h and then for 3 h in the presence of the reversible proteasome inhibitor MG132 (10 μM, Sigma-Aldrich) to allow the minor ERAD substrate fraction to accumulate. Ten minutes prior to MG132 withdrawal, the irreversible protein synthesis inhibitor emetine (50 μg/ml) was added to shut down additional protein synthesis. After washing with normal medium, cells were cultured in the absence of MG132. At the indicated time points, aliquots were washed with PBS and the cells were solubilized with lysis buffer containing 20 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 5 mM N-ethylmaleimide to prevent deubiquitination of proteins, 0.5 mM PMSF and the protease inhibitor cocktail. Cell extracts were incubated with Con A sepharose 4B (Amersham) overnight at 4°C to remove the glycoproteins. The supernatants were resolved by SDS–PAGE and subsequent immunoblotting.
Expression plasmids and transfection
The Tac-based model antigens were subcloned from the pSC11-based vaccinia virus recombination vectors into the eukaryotic expression vector pIRES2-EGFP (Clontech). Transient transfection of L-Kb cells was performed using Lipofectamine 2000 (Invitrogen). The cells were incubated for 16 h before treatment with proteasome inhibitor MG132 (10 μM) for an additional 8 h.
Statistical analysis
Data are expressed as means±s.d. and evaluated by Student's t-test. To identify the differences in the presentation kinetics, a first-order autocorrelation covariance structure was used to model the correlation among repeated measures over time. Analysis was performed using SAS software version 9.2 (SAS Institute). The threshold P-value required for significance was 0.05.
Supplementary Material
Acknowledgments
We thank the Yewdell/Bennink laboratory (NIAID) for providing anti-B5R and Alexa 647-conjugated 25-D1.16 Abs and BE Leiby (Thomas Jefferson University) for assistance with statistical analysis. We also thank N Siciliano, K Kandler and S Apcher for helpful comments on this manuscript. This work was supported by a grant from the National Institutes of Health to LCE (AI39501).
Footnotes
The authors declare that they have no conflict of interest.
References
- Arteaga MF, Wang L, Ravid T, Hochstrasser M, Canessa CM (2006) An amphipathic helix targets serum and glucocorticoid-induced kinase 1 to the endoplasmic reticulum-associated ubiquitin-conjugation machinery. Proc Natl Acad Sci USA 103: 11178–11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi R, Galli C, Calanca V, Nakajima T, Molinari M (2010) Stringent requirement for HRD1, SEL1L, and OS-9/XTP3-B for disposal of ERAD-LS substrates. J Cell Biol 188: 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho P, Goder V, Rapoport TA (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126: 361–373 [DOI] [PubMed] [Google Scholar]
- Chianese-Bullock KA, Russell HI, Moller C, Gerhard W, Monaco JJ, Eisenlohr LC (1998) Antigen processing of two H2-IEd restricted epitopes is differentially influenced by the structural changes in a viral glycoprotein. J Immunol 161: 1599–1607 [PubMed] [Google Scholar]
- Dolan BP, Li L, Takeda K, Bennink JR, Yewdell JW (2010) Defective ribosomal products are the major source of antigenic peptides endogenously generated from influenza A virus neuraminidase. J Immunol 184: 1419–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr LC, Huang L, Golovina TN (2007) Rethinking peptide supply to MHC class I molecules. Nat Rev Immunol 7: 403–410 [DOI] [PubMed] [Google Scholar]
- Eisenlohr LC, Yewdell JW, Bennink JR (1992) Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med 175: 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Torregrosa BC, Raul Castano A, Del Val M (1998) Major histocompatibility complex class I viral antigen processing in the secretory pathway defined by the trans-Golgi network protease furin. J Exp Med 188: 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon T, Chomsky O, Kulka RG (1998) Degradation signals for ubiquitin system proteolysis in Saccharomyces cerevisiae. EMBO J 17: 2759–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina TN, Morrison SE, Eisenlohr LC (2005) The impact of misfolding versus targeted degradation on the efficiency of the MHC class I-restricted antigen processing. J Immunol 174: 2763–2769 [DOI] [PubMed] [Google Scholar]
- Golovina TN, Wherry EJ, Bullock TN, Eisenlohr LC (2002) Efficient and qualitatively distinct MHC class I-restricted presentation of antigen targeted to the endoplasmic reticulum. J Immunol 168: 2667–2675 [DOI] [PubMed] [Google Scholar]
- Gregersen N, Bross P, Vang S, Christensen JH (2006) Protein misfolding and human disease. Ann Rev Genomics Human Genet 7: 103–124 [DOI] [PubMed] [Google Scholar]
- Gueguen M, Long EO (1996) Presentation of a cytosolic antigen by major histocompatibility complex class II molecules requires a long-lived form of the antigen. Proc Natl Acad Sci USA 93: 14692–14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F-U, Hlodan R, Langer T (1994) Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biochem Sci 19: 20–25 [DOI] [PubMed] [Google Scholar]
- Istrail S, Florea L, Halldorsson BV, Kohlbacher O, Schwartz RS, Yap VB, Yewdell JW, Hoffman SL (2004) Comparative immunopeptidomics of humans and their pathogens. Proc Natl Acad Sci USA 101: 13268–13272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Swanson R, Rakhilina L, Hochstrasser M (1998) Degradation signal masking by heterodimerization of MATalpha2 and MATa1 blocks their mutual destruction by the ubiquitin-proteasome pathway. Cell 94: 217–227 [DOI] [PubMed] [Google Scholar]
- Kang SW, Rane NS, Kim SJ, Garrison JL, Taunton J, Hegde RS (2006) Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127: 999–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautscham G, Mayrhofer S, Taylor G, Haigh T, Leese A, Rickinson A, Blake N (2001) Processing of a multiple membrane spanning Epstein-Barr virus protein for CD8(+) T cell recognition reveals a proteasome-dependent, transporter associated with antigen processing-independent pathway. J Exp Med 194: 1053–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger MB, Maurer MJ, Dancy BM, Michaelis S (2008) Degradation of a cytosolic protein requires endoplasmic reticulum-associated degradation machinery. J Biol Chem 283: 32302–32316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T (2005) ERAD: the long road to destruction. Nature Cell Biol 7: 766–772 [DOI] [PubMed] [Google Scholar]
- Ostankovitch M, Robila V, Engelhard VH (2005) Regulated folding of tyrosinase in the endoplasmic reticulum demonstrates that misfolded full-length proteins are efficient substrates for class I processing and presentation. J Immunol 174: 2544–2551 [DOI] [PubMed] [Google Scholar]
- Peaper DR, Cresswell P (2008) Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol 24: 343–368 [DOI] [PubMed] [Google Scholar]
- Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN (1997) Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6: 715–726 [DOI] [PubMed] [Google Scholar]
- Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW (2003) Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity 18: 343–354 [DOI] [PubMed] [Google Scholar]
- Qian SB, Reits E, Neefjes J, Deslich JM, Bennink JR, Yewdell JW (2006) Tight linkage between translation and MHC class I peptide ligand generation implies specialized antigen processing for defective ribosomal products. J Immunol 177: 227–233 [DOI] [PubMed] [Google Scholar]
- Raue U, Oellerer S, Rospert S (2007) Association of protein biogenesis factors at the yeast ribosomal tunnel exit is affected by the translational status and nascent polypeptide sequence. J Biol Chem 282: 7809–7816 [DOI] [PubMed] [Google Scholar]
- Reits EA, Vos JC, Gromme M, Neefjes J (2000) The major substrates for TAP in vivo are derived from newly synthesized proteins [see comments]. Nature 404: 774–778 [DOI] [PubMed] [Google Scholar]
- Romisch K (2005) Endoplasmic reticulum-associated degradation. Annu Rev Cell Dev Biol 21: 435–456 [DOI] [PubMed] [Google Scholar]
- Schlosser E, Otero C, Wuensch C, Kessler B, Edelmann M, Brunisholz R, Drexler I, Legler DF, Groettrup M (2007) A novel cytosolic class I antigen-processing pathway for endoplasmic-reticulum-targeted proteins. EMBO Rep 8: 945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes [see comments]. Nature 404: 770–774 [DOI] [PubMed] [Google Scholar]
- Snyder HL, Bacik I, Bennink JR, Kearns G, Behrens TW, Bachi T, Orlowski M, Yewdell JW (1997) Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing. J Exp Med 186: 1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani M, Dobson CM (2003) Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med 81: 678–699 [DOI] [PubMed] [Google Scholar]
- Tobery T, Siliciano RF (1999) Induction of enhanced CTL-dependent protective immunity in vivo by N- end rule targeting of a model tumor antigen. J Immunol 162: 639–642 [PubMed] [Google Scholar]
- Tobery TW, Siliciano RF (1997) Targeting of HIV-1 antigens for rapid intracellular degradation enhances cytotoxic T lymphocyte (CTL) recognition and the induction of de novo CTL responses in vivo after immunization. J Exp Med 185: 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A, Bastin J, Gould K, Brownlee G, Andrew M, Coupar B, Boyle D, Chan S, Smith G (1988) Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med 168: 1211–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend ARM, Bastin J, Gould K, Brownlee GG (1986) Cytotoxic T lymphocytes recognize influenza hemagglutinin that lacks a signal sequence. Nature 324: 575–577 [DOI] [PubMed] [Google Scholar]
- Turner GC, Varshavsky A (2000) Detecting and measuring cotranslational protein degradation in vivo. Science 289: 2117–2120 [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Hartl FU (2005) Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 310: 1960–1963 [DOI] [PubMed] [Google Scholar]
- Varshavsky A (1992) The N-end rule. Cell 69: 725–735 [DOI] [PubMed] [Google Scholar]
- Vembar SS, Brodsky JL (2008) One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9: 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley DN (1984) Intracellular protein degradation: basis of a self-regulating mechanism for the proteolysis of endogenous proteins. J Theor Biol 107: 127–149 [DOI] [PubMed] [Google Scholar]
- Wheatley DN, Giddings MR, Inglis MS (1980) Kinetics of degradation of ‘short-’ and ‘long-lived’ proteins in cultured mammalian cells. Cell Biol Int Rep 4: 1081–1090 [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC (1999) The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol 163: 3735–3745 [PubMed] [Google Scholar]
- Wickner S, Maurizi MR, Gottesman S (1999) Posttranslational quality control: folding, refolding, and degrading proteins. Science 286: 1888–1893 [DOI] [PubMed] [Google Scholar]
- Wong SB, Buck CB, Shen X, Siliciano RF (2004) An evaluation of enforced rapid proteasomal degradation as a means of enhancing vaccine-induced CTL responses. J Immunol 173: 3073–3083 [DOI] [PubMed] [Google Scholar]
- Yewdell JW (2007) Plumbing the sources of endogenous MHC class I peptide ligands. Curr Opin Immunol 19: 79–86 [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Antón LC, Bennink JR (1996) Defective ribosomal products (DRIPs): a major source of antigenic peptides for MHC class I molecules? J Immunol 157: 1823–1826 [PubMed] [Google Scholar]
- Yewdell JW, Nicchitta CV (2006) The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol 27: 368–373 [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Reits E, Neefjes J (2003) Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat Rev Immunol 3: 952–961 [DOI] [PubMed] [Google Scholar]
- Zook MB, Howard MT, Sinnathamby G, Atkins JF, Eisenlohr LC (2006) Epitopes derived by incidental translational frameshifting give rise to a protective CTL response. J Immunol 176: 6928–6934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.